Abstract
Charcot-Marie-Tooth (CMT) disease 4A is an autosomal-recessive polyneuropathy caused by mutations of ganglioside-induced differentiation-associated protein 1 (GDAP1), a putative glutathione transferase, which affects mitochondrial shape and alters cellular Ca2+ homeostasis. Here, we identify the underlying mechanism. We found that patient-derived motoneurons and GDAP1 knockdown SH-SY5Y cells display two phenotypes: more tubular mitochondria and a metabolism characterized by glutamine dependence and fewer cytosolic lipid droplets. GDAP1 interacts with the actin-depolymerizing protein Cofilin-1 and beta-tubulin in a redox-dependent manner, suggesting a role for actin signaling. Consistently, GDAP1 loss causes less F-actin close to mitochondria, which restricts mitochondrial localization of the fission factor dynamin-related protein 1, instigating tubularity. GDAP1 silencing also disrupts mitochondria-ER contact sites. These changes result in lower mitochondrial Ca2+ levels and inhibition of the pyruvate dehydrogenase complex, explaining the metabolic changes upon GDAP1 loss of function. Together, our findings reconcile GDAP1-associated phenotypes and implicate disrupted actin signaling in CMT4A pathophysiology.
Subject terms: Mechanisms of disease, Molecular medicine, Peripheral neuropathies, Peripheral neuropathies
GDAP1 mutations effect Charcot-Marie-Tooth disease 4A by inhibiting the pyruvate dehydrogenase complex and restricting mitochondrial localization of dynamin-related protein 1 through alterations of the actin cytoskeleton.
Introduction
Charcot-Marie-Tooth (CMT) disease is the most frequently inherited peripheral neuropathy in humans and affects one in 2500 people. Clinically, this group of diseases can be distinguished by mode of inheritance, age of onset, and by electrophysiological characteristics that distinguish demyelinating and axonal forms. Mutations in the gene GDAP1 (ganglioside-induced differentiation-associated protein 1) cause various forms of CMT: the most frequent recessively inherited demyelinating subtype CMT4A1, the axonal-recessive (AR)-CMT22, the intermediate-recessive subtype CMTRIA3, and the dominant subtype CMT2K4.
GDAP1 is located in the mitochondrial outer membrane facing the cytosol. It possesses two glutathione transferase (GST)-like domains2,5,6 and a C-terminal hydrophobic anchor which crosses the outer mitochondrial membrane7. Whether GDAP1 is a catalytically active GST remains controversial6,8–10, but it appears clear that GDAP1 can bind GST substrates9,10.
One potential mechanism of GDAP1 action that has been connected to CMT4A disease is mitochondrial dynamics. Over-expression of GDAP1, but not over-expression of GDAP1 bearing recessive disease-causing mutations, results in more fragmented mitochondria, whereas a GDAP1 knockdown (KD) results in mitochondrial elongation11. This GDAP1-mediated mitochondrial fragmentation depends on the activity of dynamin-related protein 1 (DRP1), the major mitochondrial fission factor12,13. The mechanism behind this is unknown. DRP1 is a cytosolic protein which, when recruited to mitochondria, promotes fission by forming ring-like oligomers that constrict and divide mitochondria14. The recruitment of DRP1 to mitochondria depends on the presence of ER tubules15 and filamentous actin (F-actin)16 at the sites of constriction. The ER-protein inverted formin 2 (INF2) promotes actin polymerization which occurs before DRP1-driven constriction17. INF2-mediated actin polymerization also increases mitochondria-ER contact sites (MERCS)18. This further connects actin polymerization and GDAP1 function as mitochondrial and ER marker proteins colocalize less frequently in GDAP1 KD cells19. Interestingly, INF2 mutations also cause CMT disease (CMTDIE)20, implying that actin polymerization is important for the survival of peripheral nervous system neurons, similar to GDAP1 function. Another protein at the interface of F-actin polymerization and DRP1 recruitment is Cofilin-1. Cofilin-1 binds to monomeric actin and F-actin and controls cytoskeletal dynamics mostly by actin depolymerization. Cofilin-1 deletion results in DRP1 accumulation at mitochondria and fragmentation21. In summary, defective actin polymerization affects the same mitochondrial fission pathway upstream of DRP1 and results in a clinical phenotype similar to GDAP1 mutation. Whether F-actin polymerization and its regulation play a role in CMT4A is still poorly understood.
Another process that is affected by loss of GDAP1 is cellular Ca2+ homeostasis. Neuronal GDAP1 KD reduces Ca2+ influx from the extracellular space that follows depletion of the ER Ca2+ stores, so-called store-operated Ca2+ entry (SOCE), possibly due to an impaired mitochondrial localization at subplasmalemmal microdomains19. GDAP1 KD also blunts the Ca2+-dependent increase of mitochondrial respiration upon SOCE22. Mitochondrial Ca2+ levels increase the activity of several enzymes of the tricarboxylic acid cycle (TCA) like pyruvate dehydrogenase (PDH)23, the key enzyme of the pyruvate dehydrogenase complex (PDC) which catalyzes the conversion of pyruvate to acetyl-CoA and links glycolysis to the TCA. This connection between Ca2+ and TCA activity is thought to connect mitochondrial activity to ATP demand. Changes in the activity of the PDC have not been studied yet in CMT4A or in cells with perturbed GDAP1 expression but, interestingly, a pathogenic mutation of pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) that inhibits the PDC also causes CMT disease, CMTX624. How GDAP1-mediated changes of the mitochondrial Ca2+ homeostasis are connected to its fission activity is still unclear.
In this study, we used motoneurons obtained from CMT4A-patient-derived induced-pluripotent stem cells and neuronal GDAP1 KD cells to study the pathophysiology of CMT4A. We found that GDAP1 interacts with actin-binding Cofilin-1. Loss of GDAP1 results in a reduction of F-actin fibers in mitochondrial proximity, which restricts DRP1 access to mitochondrial constriction sites and disrupts mitochondria-ER contact sites. This reduces mitochondrial Ca2+ levels and inhibits the PDC resulting in a rewired cellular metabolism characterized by glutamine dependence and increased consumption of fatty acids. Together, these findings implicate disrupted F-actin signaling in CMT4A pathophysiology.
Results
More tubular mitochondria and an increased mitochondrial membrane potential in CMT4A patient-derived neuronal cells and GDAP1 knockdown cells
To establish a model for CMT4A, we compared GDAP1 KD SH-SY5Y cells19,25 with neuronal cells derived from CMT4A patients. Patient CMT#1 is a 25-year-old ambulant male with a compound heterozygosity (L239F/R273G) of mutations in the C-terminal GST domain of GDAP1 and patient CMT#2 is a 40-year-old wheelchair-bound male with a homozygous mutation of the intron 4 splice donor site (c.579 + 1G>A). This mutation causes skipping of exon 4 leading to a frameshift and a truncated protein lacking the C-terminal GST and the transmembrane domain of GDAP1 (Fig. 1a).
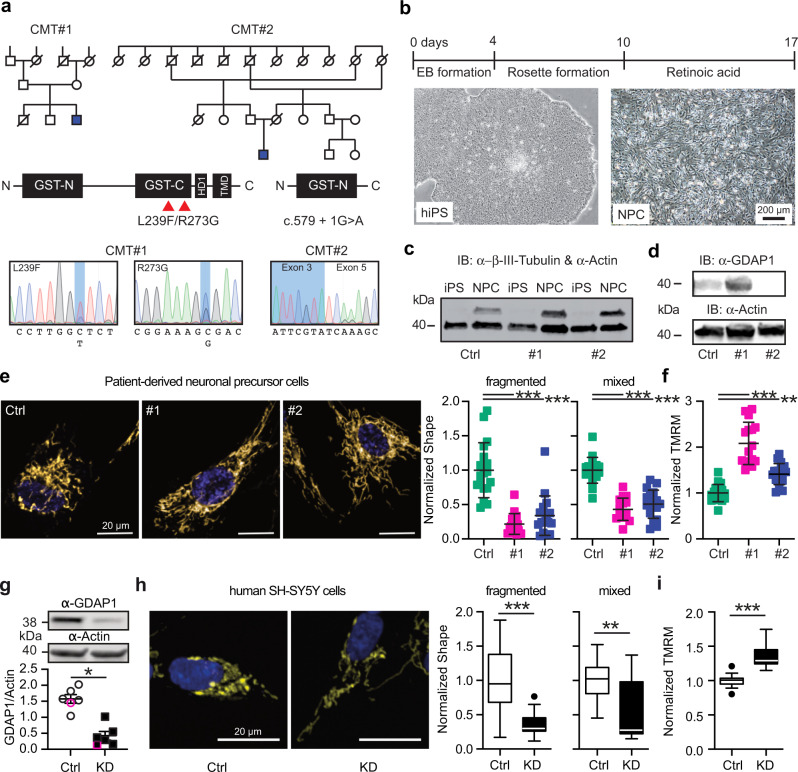
Fig. 1. More tubular mitochondria and an increased mitochondrial membrane potential in CMT4A patient-derived neuronal cells and GDAP1 knockdown cells.
a Pedigree and GDAP1 DNA sequences from two patients suffering from autosomal-recessive CMT4A disease. b Differentiation protocol to obtain neuronal precursor cells from induced-pluripotent stem cells (iPSCs). c Immunoblot demonstrating expression of the neuronal marker β-tubulin III in the NPCs but not in iPSCs. d Control and CMT#1 but not CMT#2 neuronal cells express GDAP1 shown by immunoblotting. Size is indicated, Actin served as loading control. e, h Representative images of automated high-content confocal microscopy analysis of mitochondrial shape (MitoTracker) in patient-derived (e) and GDAP1 KD (h) cells demonstrating elongated mitochondria in GDAP1 loss-of-function cells. f, i Increased mitochondrial membrane potential (TMRM) in patient-derived (f) and GDAP1 KD (i) cells. The values obtained in control cells were set as 1. g GDAP1 immunoblot and quantification demonstrating successful knockdown. Size is indicated, Actin served as loading control. Data in e and f are from 3 independent experiments with 4–8 replicates per experiment with a range of 74 to 1908 cells per well. Data in (h) and (i) are from 4 independent experiments with 4–8 replicates with a range of 343 to 4977 cells per well. Statistical variation is shown as scatter plot (e–g) or Tukey boxplot (h, i) and significance calculated using one-way ANOVA (e, f) or Mann–Whitney (g–i) tests, *p < 0.05, **p < 0.001, ***p < 0.0001.
We generated induced-pluripotent stem cells from fibroblast cell lines26 from these patients using non-integrative expression of the Yamanaka factors and differentiated them to neuronal precursor cells (NPCs) (Fig. 1b) that express the neuronal marker protein β-III tubulin (Fig. 1c). In contrast to fibroblasts26, control and CMT#1 NPCs express GDAP1 detectable by immunoblotting (Fig. 1d). NPCs from CMT#2 lacked GDAP1 expression (Fig. 1d). Because the polyclonal antiserum targets an antigen which should still be present in the patient, this is probably due to nonsense-mediated mRNA decay or degradation of the truncated protein.
We quantified mitochondrial shape and membrane potential (Δψm) using automated high-content confocal microscopy analysis of cells simultaneously stained with the fluorescent dyes mitotracker and tetramethylrhodamine methyl ester (TMRM). Patient-derived neuronal cells contained significantly more tubular mitochondria (Fig. 1e) with a more negative mitochondrial membrane potential (Fig. 1f). Applying the same methodology to GDAP1 KD cells (Fig. 1g), we found similar changes; more elongated mitochondria (Fig. 1h) and a significantly more negative membrane potential (Fig. 1i). The consistency between the phenotypes of patient-derived cells and GDAP1 KD cells suggests that GDAP1 KD cells are a suitable model to study CMT4A disease.
GDAP1 knockdown uncouples mitochondrial respiration from ATP generation
Mitochondria produce ATP by consuming oxygen and the energetic electron donors NADH and FADH2 in a process called oxidative phosphorylation. NADH is provided by the TCA, PDH and β-oxidation. To assess the effect of GDAP1 KD on this all-important process, we measured mitochondrial oxygen consumption using high-resolution respirometry and found a higher mitochondrial routine respiration in GDAP1 KD cells (Fig. 2a). The maximal capacity of the electron transfer system (ETS), determined by titrating in the uncoupler FCCP, was however similar in both cell lines (Fig. 2a). Normalization of the respiratory states to the maximum ETS capacity, the so-called flux control ratio, revealed that the GDAP1 KD cells use a higher fraction of their maximal capacity for routine, leak and phosphorylating respiration (Fig. 2a′). An increased ratio between non-phosphorylating leak respiration (electron flow coupled to proton pumping to compensate for proton leaks) and ETS capacity suggests intrinsic uncoupling or dysfunction in GDAP1 KD cells. Quantification of ATP content using the ratiometric reporters BTeam targeted either to the cytosol or to the mitochondrial matrix27 also demonstrated a reduced mitochondrial ATP generation in GDAP1 KD cells despite the increased Δψm and routine respiration (Fig. 2b). Taken together, these data suggest that GDAP1 KD results in the uncoupling of mitochondrial respiration and ATP generation via oxidative phosphorylation.
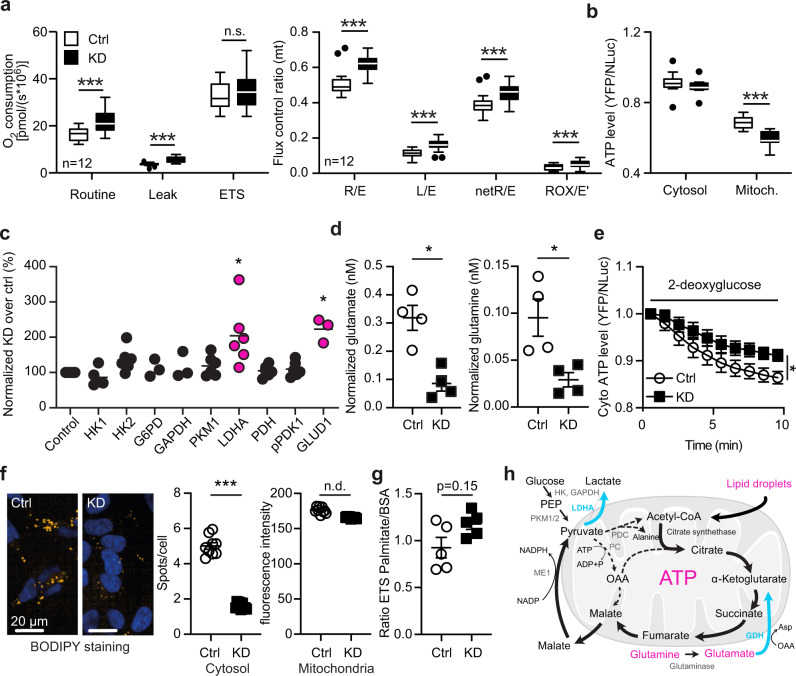
Fig. 2. GDAP1 knockdown uncouples mitochondrial respiration from ATP generation and shifts metabolism towards glutaminolysis.
a Mitochondrial oxygen consumption of intact cells in regular growth medium measured by high-resolution respirometry and corrected for ROX. ETS, electron transfer system; L, leak respiration; R, routine respiration; E, ETS capacity; ROX, residual oxygen consumption; netR, oxygen consumption minus ROX. Calculation of flux control ratios. b Comparison of cytosolic and mitochondrial ATP content using the genetically encoded ATP sensor BTeam. Calculation of BTeam YFP/NLuc emission ratios under basal conditions revealed reduced mitochondrial ATP levels. c Immunoblot quantification of proteins involved in glycolysis in Ctrl and KD cells shows an increase in lactate dehydrogenase A (LDHA) and glutamate dehydrogenase 1 (GLUD1) levels. HK, Hexokinase; G6PD, glucose-6-phosphate dehydrogenase; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; PKM1, pyruvate kinase M1; PDH, pyruvate dehydrogenase; pPDK1, phospho-pyruvate dehydrogenase kinase. Actin expression served as loading control. d Diminished glutamate and glutamine levels determined by a luminescence-based assay. e Comparison of cytosolic ATP content using the genetically encoded ATP sensor BTeam. BTeam YFP/NLuc emission ratios after treatment with 25 µM 2-deoxyglucose reveals increased non-glucose dependent ATP generation capacity in KD cells. f Automated high-content confocal microscopy analysis of BODIPY-stained fatty acids demonstrating less lipid droplets in KD cells identified by Höchst staining of nuclei. Lipid droplets close to mitochondria were identified by MitoTracker staining. g ETS capacity in the presence of palmitate or BSA as substrates measured by high-resolution respirometry. h Schematic illustration of metabolic changes observed in GDAP1 KD cells. Upregulation is shown in blue and bold lines; downregulation in magenta and dashed lines. ME, malic enzyme 1; PDC, pyruvate dehydrogenase complex; PKM1/2, pyruvate kinase M1/2; OAA, oxaloacetate. Data in (a) and (a′) are from 12 independent experiments performed in duplicate. Data in (b) are from 4 independent experiments performed in triplicates. Data in (e) are from 6 independent experiments performed in triplicates. Data in (f) were from >10,000 cells in total and were analyzed in 3 independent experiments performed in triplicates. Statistical variation is shown as Tukey’s boxplots in (a) and (b), XY graph in (c), scatter plots in (d) and (f), mean ± SEM in (e). Significance was calculated using the student’s t test in (a), 2-way ANOVA in b, the non-parametric Kruskal–Wallis test in (c), the Mann–Whitney test in (d–g), *p < 0.05, **p < 0.001, ***p < 0.0001.
GDAP1 knockdown shifts cellular metabolism towards glutaminolysis
To identify metabolic pathways in GDAP1 KD cells that compensate for the diminished capacity for oxidative phosphorylation, we quantified the expression levels of various metabolic enzymes in control and KD cells by immunoblotting. This revealed a significant higher protein levels of lactate dehydrogenase (LDHA) and glutamate dehydrogenase 1 (GLUD1) in GDAP1 KD cells (Fig. 2c, Supplementary Fig. 1). LDHA catalyzes the interconversion of pyruvate to lactate. GLUD1, in contrast, converts glutamate to α-ketoglutarate, the precursor of succinyl-CoA in the TCA. In line with the increased expression of GLUD1, GDAP1 KD cells consume significantly more glutamate, the substrate of GLUD1, and more glutamine which can be converted to glutamate by glutaminase (Fig. 2d). We also observed an attenuated decline in cytosolic ATP content in KD cells after treatment with the glucose antimetabolite 2-deoxyglucose (Fig. 2e) in line with a reduced use of glucose as the major fuel for the TCA cycle. The TCA metabolite upstream of α-ketoglutarate is citrate, which is produced by the transfer of acetyl-CoA to oxaloacetate. Acetyl-CoA is the product of the PDC in the mitochondrial matrix or of mitochondrial β-oxidation. We suspected a dysfunction of the TCA cycle in GDAP1 KD cells at the level of the PDC and therefore quantified the amount of lipid droplets as alternative sources for acetyl-CoA via β-oxidation in GDAP1 KD cells. We quantified the amount of lipid droplets by BODIPY C12 staining and indeed found decreased cytosolic lipid droplet levels in GDAP1 KD cells, whereas lipid droplets associated with mitochondria were unchanged (Fig. 2f). Maximum oxygen consumption of GDAP1 KD cells indeed tended to be higher in the presence of the fatty acid oxidation substrate palmitate (Fig. 2g). Together, these results are in line with a high lipid catabolism that serves to replenish fatty acids and consequently acetyl-CoA levels in the TCA cycle. We posit that the increased demand of glutamine and fatty acids in GDAP1 KD probably serve as compensatory mechanisms, clearly pointing towards a dysfunction of the TCA cycle in GDAP1 KD cells at the level of the PDC (summarized in Fig. 2h).
Hyperphosphorylated pyruvate dehydrogenase and reduced mitochondrial Ca2+ levels in GDAP1 KD cells
Increased mitochondrial Ca2+ levels activate the PDC by stimulating PDH phosphatase28. Ca2+ also activates isocitrate dehydrogenase which is upstream of α-ketoglutarate, the product of GLUD1, and α-ketoglutarate dehydrogenase29. Based on the reported attenuated mitochondrial respiration upon SOCE in GDAP1 KD cells22, we suspected altered PDH phosphorylation levels driven by changes in mitochondrial Ca2+ content as the reason for PDC inhibition (see scheme in Fig. 3a). To test this idea, we probed phosphorylation of serine 293 of the E1 PDH subunit, which has been directly linked to PDC activity30. Immunoblotting showed a significantly increased phosphorylation of PDH E1 serine 293 in GDAP1 KD cells as compared to total E1 PDH (Fig. 3b). This is in line with a decreased activity of the PDC. We then measured mitochondrial Ca2+ levels by imaging live cells stained with Rhod2-AM or expressing the genetically encoded mitochondrial Ca2+ sensor mito-CEPIA31 normalized to mito-FarRed. The Ca2+ sensor GEM-CEPIA1er targeted to the ER served as a control. Both methods revealed a reduction in steady-state mitochondrial Ca2+ levels in GDAP1 KD cells, whereas ER calcium levels were not affected (Fig. 3c). We conclude that the PDC malfunction in GDAP1 KD cells is likely caused by a reduction in mitochondrial Ca2+ levels resulting in an increased phosphorylation of PDH.
Fig. 3. Hyperphosphorylated pyruvate dehydrogenase and reduced mitochondrial Ca2+ levels in GDAP1 KD cells.
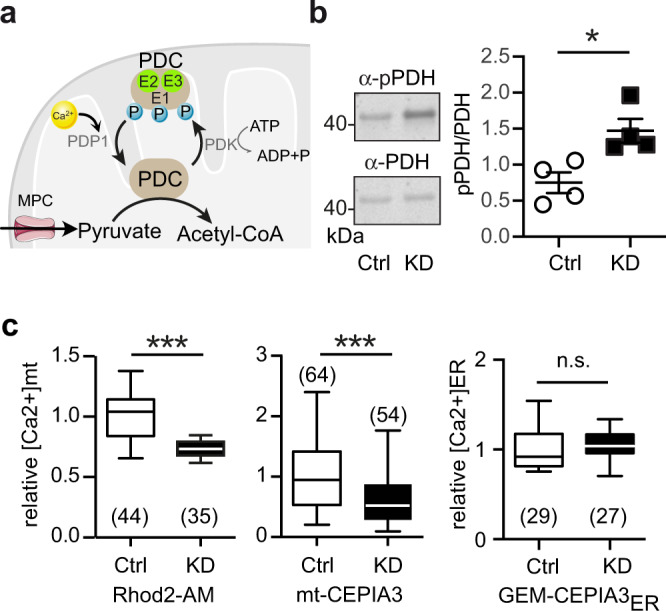
a Scheme showing the regulation of the pyruvate dehydrogenase complex (PDC). The PDC E1 subunit can be phosphorylated by the catalytic activity of the PDH kinase (PDK). The PDH phosphatase subunit 1 (PDP1) in turn dephosphorylates the serine residues upon activation by Ca2+. MPC, mitochondrial pyruvate carrier;PDK1, pyruvate dehydrogenase kinase 1. b Immunoblots from whole cell lysates for quantification of PDH E1 phosphorylation (serine 293), normalized to total PDH E1 levels revealed increased phosphorylation of PDH in GDAP1 KD cells. c Mitochondrial Ca2+ measured with the fluorescent dye Rhod2-AM or mito-CEPIA normalized to mito-FarRed indicated reduced mt[Ca2+] levels. ER[Ca2+] levels measured with the genetically encoded Ca2+ sensor GEM-CEPIA3ER did not show any variation between the cell lines. Data in (c) were from 3 independent experiments with the indicated number of cells. Data for GEM-CEPIA3ER were from 2 independent experiments. Statistical variation is shown as scatter plots in (b) and Tukey boxplots in (c). Significance was calculated using the non-parametric Mann–Whitney in (b) and Student’s t test in (c), *p < 0.05, **p < 0.001, ***p < 0.0001.
Patient-derived cells are similarly characterized by increased glutaminolysis, reduced lipid droplets and reduced mitochondrial Ca2+ levels
We next tested whether the findings of reduced mitochondrial Ca2+ levels and increased glutaminolysis also apply to patient-derived cells. We set out to specifically test this in motoneurons, a cell type affected by CMT4A. Motoneurons were differentiated from NPCs following established protocols (Fig. 4a) and motoneuronal identity was confirmed by immunostaining against the dendrite marker MAP2 and the motoneuronal neurofilament H marker antibody Smi32 (Fig. 4b). Control and patient-derived motoneurons stained similarly for these markers as shown by high-content imaging (Supplementary Fig. 2). These motoneurons express GDAP1 similar to NPCs with cells from patient CMT#2 lacking GDAP1 expression (Fig. 4c). High-content microscopy of MitoTracker-stained cells revealed an increased area occupied by mitochondria (Fig. 4d). Immunoblotting reproduced the increased expression of GLUD1 seen upon GDAP1 KD (Figs. 4e, 2c). Motoneurons differentiated from patient-derived cells also increased the consumption of the GLUD1-substrate glutamate (Figs. 4f, 2d) and its precursor glutamine. Furthermore, motoneurons showed a decrease in lipid droplets normalized to the nuclear area (Fig. 4g), indicative of increased fatty acid consumption. Together, these data further highlight the similarity between patient-derived cells and GDAP1 KD cells.
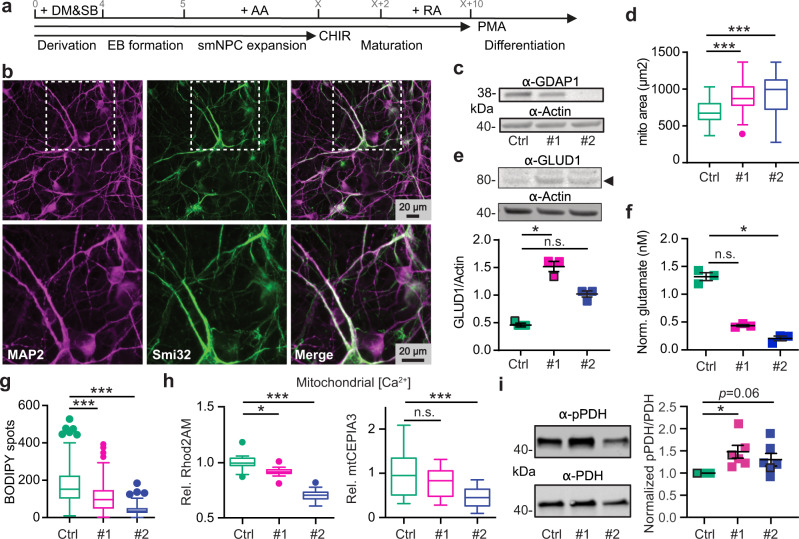
Fig. 4. Patient-derived cells are similarly characterized by an anaplerotic state and reduced mitochondrial Ca2+ levels.
a Differentiation protocol to obtain motoneurons (MN) from induced-pluripotent stem cells (iPSCs) and (b) confirmatory immunocytochemistry by staining against dendrite marker MAP2 and motoneuronal neurofilament Smi32. c Immunoblot of total MN cell lysates for validation of GDAP1 expression, actin served as loading control, size is indicated. d Automated high-content microscopy analysis of MitoTracker-stained mitochondria revealed an increased total mitochondrial mass per well. e Immunoblot of total MN cell lysates demonstrated increased glutamate dehydrogenase (GLUD1, arrowhead) in patient-derived MNs, actin served as loading control. f Reduced glutamate levels in CMT4A patient-derived MN determined by a luminescence-based assay indicating increased glutamine consumption compared to Ctrl. g Automated high-content microscopy analysis of BODIPY-positive spots normalized to the nuclei area in MN revealed a reduced number of lipid droplets in CMT4A patient-derived cells. h Reduced relative mt[Ca2+] levels of NPCs determined by confocal microscopy with Rhod2-AM and mito-CEPIA3. i Immunoblots and quantification demonstrating increased phosphorylation of PDH E1 serine 293, normalized to total PDH E1 levels and control cells. Data in (d–g) were from 3 independent differentiations and data in (d, g) were obtained from n > 40,000 cells in quadruplicate, experiments in (f) were performed in duplicate. Data in (h) were from 20–22 Rhod2-AM-stained cells and 22–33 cells mitoCEPIA3 transfected from three independent experiments. Data in (i) were from 6 different passages. Data points corresponding to the example blots are highlighted. Statistical variation is shown as Tukey boxplots in (d, e) or scatter plots in (i) with mean ± SEM indicated. Significance was calculated using the non-parametric Kruskal–Wallis test, *p < 0.05, **p < 0.001, ***p < 0.0001.
Because motoneuronal cells showed a low transfection efficiency which made it difficult to identify single neurons in the cultures, we reverted to the patient-derived NPCs instead of fully differentiated motoneurons to assess the mitochondrial Ca2+ phenotype. We measured lower resting Ca2+ levels with Rhod2-AM and mito-CEPIA (Fig. 4h) in NPCs, similar to KD cells. Patient-derived NPCs also had increased levels of pPDH as compared to total PDH levels (Fig. 4i). We conclude that neuronal cells from patients with GDAP1 mutation not only have the same changes in mitochondrial shape and membrane potential as GDAP1 KD cells (Fig. 1) but also feature the same metabolic changes and alterations of Ca2+ levels. Together this suggests that GDAP1-mediated CMT4A is caused by inhibition of PDH activity resulting in an anaplerotic state.
GDAP1 knockdown reduces the number of contact sites between mitochondria and the endoplasmic reticulum
We suspected that changes in MERCS might underlie the changes in mitochondrial Ca2+ levels especially as a reduced colocalization of ER and mitochondrial markers hint to changes in MERCS, as shown previously19. MERCS are hot spots of interactions between the ER and mitochondria defined by a distance of mitochondrial and ER membranes between ~10 and ~50 nm32 and are important signaling hubs (reviewed in ref. 33). Using transmission electron microscopy, we indeed found (i) a decrease in MERCS in GDAP1 KD cells defined as the percentage of mitochondrial perimeter covered by the ER, (ii) a decreased number of MERCS per mitochondrion, (iii) a decreased length of MERCS (Fig. 5a) and (iv) an increased width between the ER and mitochondria (Fig. 5a, b). As an additional readout to quantify the distance between mitochondria and the ER, we used a well-established assay based on Förster resonance emission transfer (FRET) between two fluorescent proteins targeted to the surface of mitochondria and the ER, both facing the cytosol34,35. The lower FRET ratio (Fig. 5c) confirmed the data obtained by the morphometric analysis with an increased width between the organelles in GDAP1 KD cells. Comparing the amount of ER protein present in mitochondrial cell fractions using immunoblotting of the membrane ER protein Sec62 and the mitochondrial protein Cox4 also confirmed the reduction in contact sites (Fig. 5d). Such a reduction and increased distance should result in decreased Ca2+ transfer from the ER to the mitochondrial matrix. We studied this by triggering ER Ca2+ release with the cholinergic agent carbachol, which activates inositol trisphosphate receptors (IP3Rs) leading to Ca2+ release into the cytosol, generating Ca2+ microdomains at MERCS, which sustain rapid Ca2+ uptake by mitochondria34,36. Surprisingly, we did not observe a difference in mitochondrial Ca2+ uptake measured by mitochondrially-targeted aequorin upon treatment with carbachol (Fig. 5e). The direct reducing effect of carbachol on PDH E1 phosphorylation was also similar in both cell lines while the baseline levels were significantly increased in GDAP1 KD cells (Fig. 5f). Apparently, upregulated MCU levels (Fig. 5g) and hyperpolarization (see Fig. 1I) can compensate for the increased distance between the organelles under conditions of high Ca2+ influx. The other components of the ER-mitochondrial Ca2+ uptake complex37, the IP3R ER Ca2+ release channels, the molecular chaperone glucose-regulated protein 75 (Grp75, official name Heat Shock Protein Family A (Hsp70) Member (HSPA)), and the mitochondrial voltage-dependent anion channel 1 (VDAC1) at the outer mitochondrial membrane were however similarly expressed in GDAP1 KD and control cells (Fig. 5h). Taken together our findings support altered MERCS with an increased distance between the ER and mitochondria in GDAP1 KD cells.
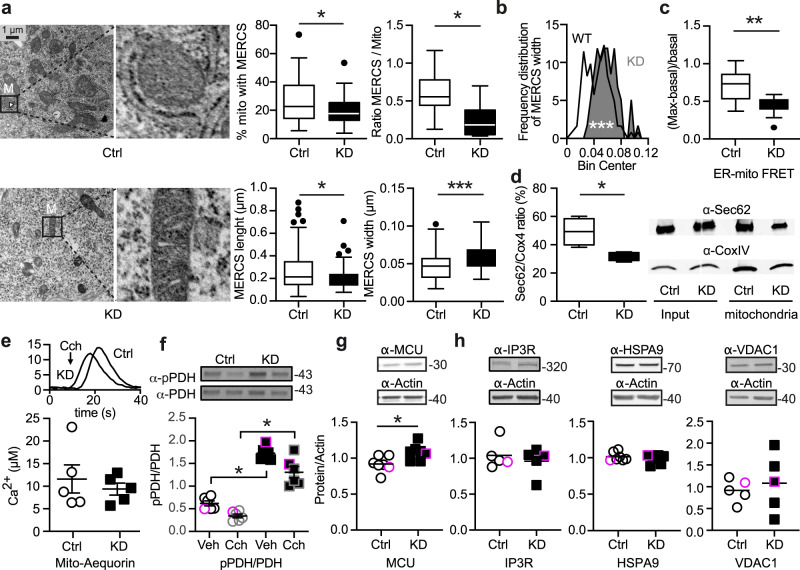
Fig. 5. GDAP1 knockdown reduces the number of contact sites between mitochondria and the endoplasmic reticulum.
a Transmission electron microscopy of knockdown (KD) and control (Ctrl) cells. The indicated parameters were quantified using ImageJ by a blinded investigator. M, mitochondrion. b Histogram showing the distribution of MERCS’ widths, and an increased distance in KD cells between the ER and mitochondria. c Proximity of ER and mitochondria was measured using a FRET-FEMP sensor comprising ER CFP-Sac1 and mitochondrial YFP-Akap1. Proximity leads to high intensity of YFP-FRET-emission (410-430/520-560 ex/em). 100 nM rapamycine was added to achieve the closest possible distance, and the measurement of FRET ratio was calculated as (FRETmax-FRETbasal)/FRETbasal. d Quantification of ER membrane (Sec62) and mitochondrial (Cox4) proteins in mitochondrial fractions. e Representative curve for the aequorin Ca2+ measurement and quantification of mitochondrial Ca2+ levels after addition of 200 µM carbachol (CCH), which releases Ca2+ from the ER by activation of a G-protein-coupled receptor. f) Immunoblot of Ctrl and KD cells lysed 10 min after 200 µM CCH addition to quantify the PDH phosphorylation (pPDH/(PDHtotal) after Ca2+ release into the MERCS and mitochondria. g, h Immunoblot of total cell lysates of Ctrl and KD cells showing increased expression levels of MCU but not pan-IP3R, HSPA9 or VDAC1, actin served as loading control, size is indicated. Data in (a) were obtained from 11 (Ctrl) and 9 (KD) cells, in (b) from 4 independent experiments in triplicate or quintuplicate with a range of 162–818 cells per experiment, and in (d) from 4 independent experiments. Data in (e) from 5 independent experiments performed in triplicates. Data points corresponding to the example blots are highlighted. Statistical variation is shown as Tukey boxplots or scatter plots with the indication of mean ± SEM and significance was calculated using the non-parametric Mann–Whitney test, *p < 0.05, **p < 0.001, ***p < 0.0001.
Increased levels of proteins involved in β-oxidation in GDAP1 KD cells
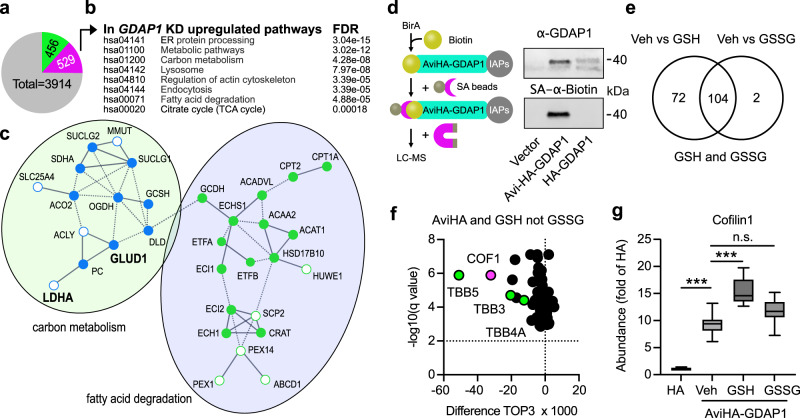
To clarify how GDAP1 KD affects MERCS and mitochondrial Ca2+ levels, we next set out to identify proteins that are dysregulated in KD cells by label-free quantitative liquid chromatography coupled to mass spectrometry (LC-MS)38. Comparing the proteome of control and GDAP1 KD cells identified 985 significantly dysregulated proteins out of 3914 proteins—456 proteins were found to be less abundant and 529 to be more abundant (Fig. 6a). Then, we submitted the list of upregulated proteins to STRING39 and observed that the following KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways were over-represented with an false-detection rate below 2 × 10−4: protein processing in the ER, lysosome, regulation of the actin cytoskeleton, endocytosis, fatty acid degradation and citrate cycle (Fig. 6b). The analysis corroborated the increased levels of LDHA and GLUD1 observed by immunoblotting (Fig. 2c). It also demonstrated increased levels of proteins involved in β-oxidation (Fig. 6c) like carnitine palmitoyltransferase 1 (CPT1A) which catalyzes the transfer of the acyl group of long-chain fatty acid-CoA conjugates onto carnitine to transport the resulting acylcarnitine long-chain fatty acids inside the mitochondria. These results suggest that the decrease in lipid droplets observed in GDAP1 KD cells (Fig. 2f) indeed reflects a high lipid catabolism that serves to provide the TCA cycle with acetyl-CoA.
Fig. 6. LC-MS proteomics identifies proteins involved in β-oxidation as being more abundant in GDAP1 KD cells and hints to alterations of the actin cytoskeleton mediated by redox-dependent interactions with GDAP1.
a Number of proteins dysregulated in GDAP1 KD cells identified by label-free proteomics from three independent experiments, measured in quadruplicates, green less abundant, magenta more abundant. Statistical significance was determined by Student’s t test with Bonferroni correction. b KEGG pathways significantly altered in proteins more abundant in GDAP1 KD cells. c Section of a STRING analysis of proteins more abundant in GDAP1 KD cells corroborating altered carbon metabolism (blue) and fatty acid degradation (green) in these cells. Open dots correspond to more abundant interacting proteins that do not fall directly into these categories. Proteins in bold were found to be more abundant independently using immunoblotting. d Schematic illustration showing the biotinylation of the AviTag fused to GDAP1 protein and subsequent pulldown and enrichment of biotinylated GDAP1 protein via streptavidin-labeled magnetic beads. Immunoblot of cell lysates prior to pulldown of biotinylated GDAP1 stained against GDAP1 and Biotin with an infrared-labeled streptavidin (SA) dye shows an overlap of biotinylation and GDAP1 protein. Streptavidin pulldown was performed with primary neuronal cultures from BirA-expressing E16 mouse embryos. e Results from label-free quantitative proteomics performed with GSH or GSSG added to the preparation, (f) shows the 72 proteins with an altered expression after addition of GSH but not GSSG. g TOP3 quantification of Cofilin-1 abundance and increased Cofilin-1 abundance upon GSH, but not GSSG addition indicated an interaction with GDAP1 in a redox-dependent manner. Statistical significance in g was determined by one-way ANOVA and the Tukey test, ***p < 0.0001.
GDAP1 interacts with proteins of the actin cytoskeleton in a redox-dependent manner
We then used label-free proteomics to identify GDAP1-interacting proteins to get a more complete picture of how GDAP1 loss of function affects cellular metabolism. We transduced primary cortical cultures from mice expressing the biotin ligase BirA40 with adeno-associated viruses expressing GDAP1 tagged with an Avi-tag, a specific substrate for BirA41. Only Avi-HA-tagged GDAP1 but not the HA-tagged control was biotinylated (Fig. 6d). By treating the mitochondrial preparations with GSH or GSSG as described42, we further aimed to identify proteins whose interaction is affected by their specific redox state. This was driven by the fact that GDAP1 can bind potential GST substrates9,10 and the assumption that such an interaction might depend on the local redox environment. A total of 268 proteins were pulled down from Avi-HA but not or less from HA-GDAP1-transduced cultures. 176 of these proteins showed a statistically different abundance in preparations treated with GSH compared to vehicle and 106 in preparations treated with GSSG. For 72 proteins the interaction with GDAP1 was altered only by GSH and not by GSSG treatment (Fig. 6e). Interestingly, the most strongly and most significantly regulated proteins can be linked to the cytoskeleton like tubulins (Fig. 6f, green), crucial components of the cytoskeleton that can serve as a scaffold for mitochondrial transport43. This is consistent with regulation of the actin cytoskeleton being one of the top hits in the KEGG analysis of proteins upregulated in GDAP1 KD cells (Fig. 6b, Supplementary fig. 3). In addition, an interaction between GDAP1 and β-tubulin TUBB has been reported in a yeast-two-hybrid experiment44. This led us to further concentrate our subsequent analysis on a potential misregulation in actin dynamics in CMT4A-disease models.
GDAP1 knockdown reduces Cofilin-1 abundance at mitochondria
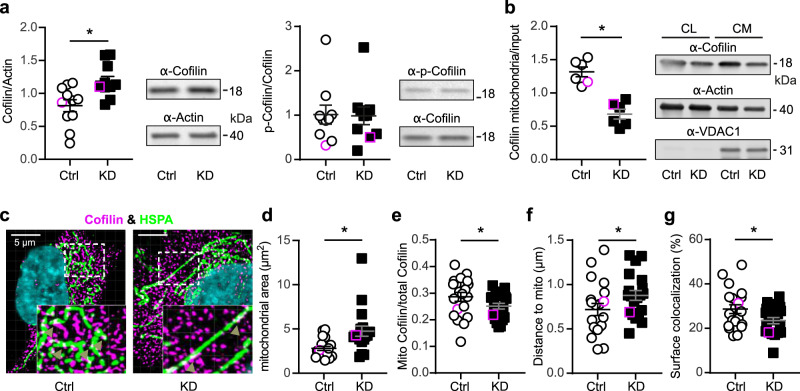
The actin-regulatory protein Cofilin-1 (Fig. 6g) specifically caught our attention because it affects mitochondrial shape by inducing depolymerization of actin filaments in mitochondrial proximity which restricts access of DRP1 to mitochondria21. Its antagonist is the ER-anchored INF2, which also causes CMT disease when mutated20. We further explored the identified interaction between GDAP1 and Cofilin-1, and hypothesized that GDAP1 alters actin abundance, polymerization or both at mitochondrial sites by interacting with Cofilin-1. The actin-binding ability of Cofilin-1 is inhibited by phosphorylation of serine 3 which is controlled by the presence of intramolecular disulfide bridges45,46 or protein glutathionylation47 and could thus be a target of GDAP1’s GST activity. Alternatively, GDAP1 could just recruit Cofilin-1 to the mitochondrial surface in a redox-dependent manner. We studied Cofilin-1 abundance and intracellular localization and phosphorylation in GDAP1 KD and control cells. GDAP1 KD increased the total abundance of Cofilin-1 but did not affect phosphorylation (Fig. 7a). However, when we compared the protein levels in mitochondrial fractions, we observed the opposite, a significant reduction in GDAP1 KD cells (Fig. 7b). To further test for the amount and localization of Cofilin-1 close to mitochondria, we used HSPA9, a mitochondrial matrix protein, as a marker as it was not regulated in GDAP1 KD cells (Fig. 5h) and plays a role in ER-mitochondrial Ca2+ transfer37. Confocal microscopy demonstrated an increase in the area covered by mitochondria (Fig. 7c and d) in line with the more tubular network in GDAP1 KD cells (Fig. 1h) and a reduction of mitochondrial Cofilin-1 compared to total Cofilin-1 in GDAP1 KD cells (Fig. 7e), in line with the fractionation assays. In addition, the distance of Cofilin-1 spots from mitochondria was significantly increased (Fig. 7f) and the colocalization of the surfaces of Cofilin-1 and HSPA9-expressing structures was reduced (Fig. 7g). These results imply that GDAP1 controls the presence of Cofilin-1 in proximity to the mitochondrial surface, possibly at MERCS.
Fig. 7. Reduced presence of Cofilin-1 at mitochondria.
a Immunoblot of whole cell lysates and quantification of Cofilin-1 and p-Cofilin-1 (S3) protein levels. Actin served as loading control. b Analysis of Cofilin-1 abundance at the mitochondria after fractionation of crude mitochondria (CM) normalized to Cofilin-1 levels in whole cell lysates (CL). c Immunostaining of Ctrl and GDAP1 KD cells for Cofilin-1 and HSPA9 as mitochondrial protein. Nuclei were counterstained with DAPI. Imaris analysis confirmed (d) the increased mitochondrial area in KD cells and (e) a reduction of mitochondrially-located Cofilin-1 (white, arrowheads) concomitant with (f) an increased distance between mitochondria and cofilin-1. Conversion of the structures into surfaces using contact XTension demonstrated (g) that the proportion of the mitochondrial surface area in contact with Cofilin-1 was significantly reduced. d, f Data are from a total of n = 20 cells per cell line from three independent experiments. Data points corresponding to the example are highlighted. Statistical variation is shown as scatter plots with the indication of mean ± SEM and significance calculated using the non-parametric Mann–Whitney test, *p < 0.05.
GDAP1 KD reduces F-actin fibers at mitochondrial surfaces and restricts access of DRP1 to the mitochondria resulting in less DRP1 and MFF
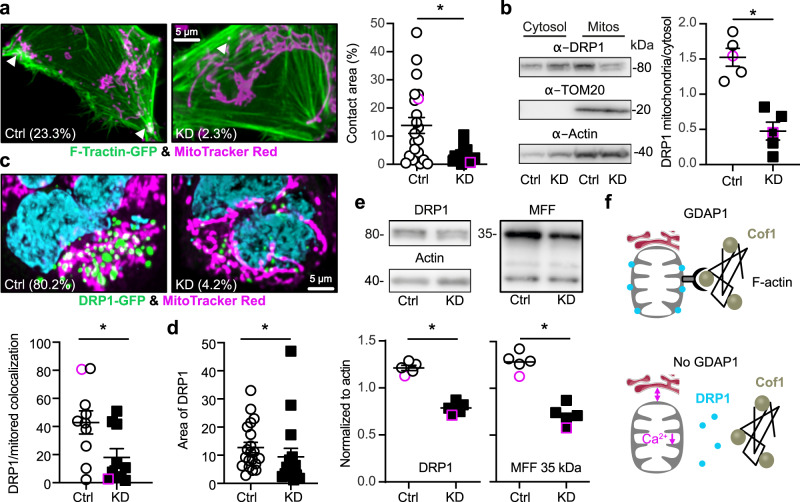
Because Cofilin-1 is an actin-binding protein, we suspected less actin in the proximity of mitochondria in GDAP1 KD cells. We quantitated F-actin levels and its colocalization with mitochondria in living cells by transiently transfecting control and GDAP1 KD cells with GFP-tagged F-tractin and labeling of mitochondria with mitotracker red. F-tractin does not perturb actin rearrangement48–50 and was previously used to study the effects of INF2 perturbation on mitochondrial dynamics18,51. Our data revealed a significant reduction of surface colocalization between F-actin and mitochondria in GDAP1 KD cells (Fig. 8a) in line with our hypothesis. As expected from previous work17, this severely reduced DRP1 localization in mitochondrial fractions (Fig. 8b). The decreased colocalization of DRP1 with mitochondria was also evident when we overexpressed GFP-tagged DRP1 in control and GDAP1 KD cells (Fig. 8c). Interestingly, DRP1 also covered less space in GDAP1 KD cells (Fig. 8d). To rule out that this is caused by a reduced transfection efficiency, we quantified total endogenous DRP1. This also revealed a reduced abundance of DRP1 and its receptor at the mitochondrial surface, mitochondrial fission factor (MFF) (Fig. 8e). In summary, these findings imply that in the absence of GDAP1, F-actin fibers are less present at the mitochondrial surface which restricts the access of ER tubules and DRP1 to sites of mitochondrial constriction and decreased levels of DRP1 and its receptor MFF resulting in a dysfunction of mitochondrial dynamics (Fig. 8f).
Fig. 8. GDAP1 KD reduces F-actin fibers at mitochondrial surfaces and restricts access of DRP1 to the mitochondria resulting in less DRP1 and MFF.
a Live cell imaging of GFP-F-tractin transfected cells. Mitochondria were stained with MitoTracker and images analyzed for contact area using Imaris. b Immunoblot of DRP1 in cytosolic and mitochondrial fractions demonstrating a reduced DRP1 abundance in mitochondrial fractions. TOM20 and actin served as loading controls, size is indicated. c, d Live cell imaging of GFP-DRP1 transfected cells. Mitochondria were stained with MitoTracker and images analyzed for (c) colocalization and (d) area. e Immunoblot of total DRP1 and MFF demonstrating a reduced abundance of both proteins. Actin served as loading control, size is indicated. f Scheme depicting our findings. Cof1, Cofilin-1. In the absence of GDAP1 mitochondrial F-actin is reduced resulting in an increased distance between ER and mitochondria, reduced mitochondrial Ca2+ levels, and a reduced presence of DRP1 at mitochondrial constriction sites. Data from (a) are from n = 19 cells per cell line from three independent experiments performed in triplicates. Data from (c) are from n = 10 and (d) from n = 16 cells per cell line from three independent experiments. Data points corresponding to the examples are highlighted. Statistical variation is shown as scatter plots with the indication of mean ± SEM and significance calculated using the non-parametric Mann–Whitney test, *p < 0.05.
Discussion
In this work, we used neuronal GDAP1 KD in human SH-SY5Y neuroblastoma cells and patient-derived cells to study the pathophysiology of CMT4A. We found that GDAP1 interacts with the actin-interacting protein Cofilin-1 and that loss of GDAP1 results in a reduction of F-actin fibers in mitochondrial proximity. This limits the access of ER tubules to mitochondria which connects two processes: it impedes DRP1 recruitment to mitochondrial constriction sites resulting in more tubular mitochondria and it disrupts mitochondria-ER contact sites causing reduced mitochondrial Ca2+ levels. The reduced mitochondrial Ca2+ levels inhibit the PDC and result in a rewired cellular metabolism characterized by dependence on glutamine and fatty acids to compensate for an impaired TCA cycle. We therefore conclude that the reduction in F-actin presence at mitochondria caused by GDAP1 loss of function represents the probable cause of autosomal-recessive CMT4A.
The reduced mitochondrial Ca2+ levels in GDAP1 KD and GDAP1-loss-of-function NPCs are difficult to understand because over-expression of the MCU was shown previously to increase the matrix Ca2+ concentration52. Other, unchanged components of the ER-mitochondrial complex that regulate Ca2+ transfer, such as the ER release channels, the mitochondrial VDAC1 channels and the linking protein HSPA9 (Grp75)37 (Fig. 5h) might account for this. In addition, the amount and stoichiometry of the MCU-interacting proteins MICU1 and MICU1, which are regulatory subunits of the large protein complex which mediates mitochondrial Ca2+ uptake, also affect the matrix Ca2+ concentration52 and were not further studied here as it is difficult to fathom how the outer mitochondrial membrane protein GDAP1 could affect or modify these proteins. Despite the reduction in MERCs in GDAP1 KD cells, agonist-induced increases in mitochondrial Ca2+ levels were unchanged. Such Ca2+ transients are believed to instigate the increase in PDC activity28,29. We therefore have to conclude that the reported blunting of the Ca2+-dependent increase of mitochondrial respiration upon SOCE22 also affects PDC activity. In line with this, it was recently shown that in mouse Gdap1−/− motoneurons, glutamate treatment results in a decreased decay of the Ca2+ signal and a reduction in respiration53. The authors attributed this to defects in mitochondrial movement resulting in a lack of correct positioning of mitochondria at sites where an intense stimulation of ATP production by oxidative phosphorylation is required like the plasma membrane. Interestingly, the actin cytoskeleton is essential for short-distance mitochondrial movements and for immobilization of mitochondria at the actin cortex, a specialized layer of cytoplasmic proteins on the inner face of the cell membrane (reviewed in54) suggesting that the changes described here could also underlie these defects in mitochondrial positioning. Alternatively, other yet unknown mechanisms exist that link the actin cytoskeleton to steady-state mitochondrial Ca2+ levels.
We found that GDAP1 interacts with Cofilin-1 in a redox-dependent manner and restricts its presence at the mitochondrial surface. This was shown by confocal microscopy using HSPA9 as a marker protein. HSPA9 (Grp75) is recognized to be enriched in MERCS. An enrichment of Cofilin-1 in MERCs has not been reported yet and we reproduced the reduced abundance of Cofilin-1 in GDAP1 KD cells in fractions containing crude mitochondria (Fig. 7b). We therefore conclude that the reduction of Cofilin-1 at mitochondrial surfaces is not restricted to MERCS. Very recently, Cofilin-1 was shown to affect mitochondrial shape and function55. Theoretically, GDAP1 could affect Cofilin-1 function through its still unresolved potential GST-like enzymatic activity6,8–10 because Cofilin-1 contains four potential GST target cysteine residues at the positions 39, 80, 139 and 147. Cysteines 39 and 80 are buried inside the protein while cysteines 139 and 147 are located on the surface of the protein56. Redox-mediated modifications of these cysteine residues clearly affect the function of Cofilin-1. Treatment with hydrogen peroxide leads to the formation of an intramolecular disulfide bond resulting in a conformational change that prevents phosphorylation and thereby actin polymerization45. Moreover, intermolecular disulfides and oligomeric forms of Cofilin-1 have been described. Monomeric Cofilin-1 possesses severing activity, whereas the dimeric and oligomeric forms have actin-bundling activity57. Interestingly, only monomeric Cofilin-1 is phosphorylated58 which represents the most-studied post-translational modification of Cofilin-1 to date. Cofilin-1 can be post-translationally modified by phosphorylation of serine 3. Increased phosphorylation inhibits the actin-binding ability of Cofilin-159; dephosphorylated Cofilin-1 preferentially localizes to mitochondria, whereas a mutation mimicking the phosphorylated protein prevents translocation from the cytosol to mitochondria60. We found no changes in Cofilin-1 phosphorylation in whole cell lysates and no phosphorylated Cofilin-1 in mitochondrial fractions. Upon oxidation of all four cysteine residues and dephosphorylation at serine 3, Cofilin-1 loses its affinity for actin, translocates from the cytosol to mitochondria and induces apoptosis60,61. Oxidation of methionine 115 apparently also prevents its actin depolymerization activity and induces forced mitochondrial translocation and apoptosis62. In addition, Cofilin-1 can be glutathionylated, which represents the presumed enzymatic activity of GDAP1. Glutathionylation of Cofilin-1 was demonstrated in lymphocytes treated with the thiol-oxidizing reagent diamide63 and in cells of the rat nucleus accumbens during cued cocaine seeking in the absence of cocaine47. Interestingly, glutathionylation reduces cofilin-1-dependent depolymerization of F-actin, implying regulatory functions in cell signaling47. It remains to be clarified whether GDAP1 affects the redox state and function of Cofilin-1 by specific glutathionylation.
Mutations in other proteins that target the same pathway also cause CMT disease. Mutated PDK3 also inhibits the PDC by hyperphosphorylation of the PDH E1α subunit similar to GDAP1 loss of function. This causes CMTX624. Loss of function of the actin-polymerizing protein INF2 alters mitochondrial shape by changing DRP1 access to mitochondria similar to GDAP1 KD17. INF2 is also implicated in the abundance of MERCS and changes in mitochondrial Ca2+ dynamics18 which we also observed in GDAP1 loss-of-function cells. Mutations in INF2 also cause CMT disease, CMTDIE20. Mutations in Dynamin-2 (DNM2), a ubiquitously expressed large GTPase that interacts tightly with the actin and microtubule network64,65 also cause CMT disease, dominant intermediate CMT (CMTDIB)66 and autosomal-dominant CMT2M67. Similar to GDAP1, DNM2 works in concert with DRP1 to orchestrate mitochondrial constriction events that result in division68. DNM2 therefore directly interferes with the same processes that we found to be affected by GDAP1 loss of function. Finally mutations in BSCL2 (Bernardinelli-Seip Congenital Lipodystrophy Type 2) also known as seipin cause a distal hereditary motor neuronopathy (HMN5C) which can present with features of axonal CMT269. BSCL2 is an ER protein crucial in the formation of lipid droplets70,71. BSCL2 also interacts with the adaptor protein YWHAB (14-3-3-beta) which then recruits cofilin-1 to remodel the actin cytoskeleton for adipocyte differentiation72.
In summary, our results shed light on the pathophysiology of CMT4A and highlight the importance of GDAP1 and actin for mitochondrial function. The fact that mutations in diverse proteins that all interact with the overarching mechanisms described here—metabolic and mitochondrial remodeling caused by changes in the the interaction of the actin cytoskeleton with mitochondria—cause CMT disease strengthens the importance of this mechanism for the health of the peripheral nervous system.
Methods
Generation of iPSCs
iPSC lines from two CMT4A patients and one healthy donor were generated with Sendai virus (CytoTune, DNAVEC Corporation) coding for POU5F1, SOX2, KLF4 and MYC. All subjects gave written informed consent prior and the study was approved by the local ethics committee at the Universities of Warsaw (patient #1), Düsseldorf (patient #2) and Bonn (healthy donor). In brief, Sendai virus-infected primary fibroblasts were immediately centrifuged for 45 min at 32 °C with 1500 g (spinfection) and cultivated in Advanced DMEM containing 5% fetal calf serum (FCS) and 1% GlutaMAX™ (all from Life Technologies). On the following day the virus-containing medium was replaced with fresh culture medium. Five days post infection (d5), transduced fibroblasts were trypsinized and seeded onto mouse feeder-coated dishes in DMEM/F-12 containing 10% KnockOut™ Serum Replacement, 1% nonessential amino acids (NEAA), 1% GlutaMAX™, 1% pyruvate, 0.1 mM β-mercaptoethanol and 10 ng/ml basic fibroblast growth factor (bFGF) (all from Life Technologies). Medium was changed every other day until clonal iPSC colonies were manually picked and adapted to feeder-free culture conditions. Several clonal lines were subjected to SNP genotyping in order to identify iPSC clones with normal karyotype.
SNP analysis of iPSC lines
Genomic DNA was prepared using the DNeasy Blood & Tissue Kit (Qiagen). Whole-genome single nucleotide polymorphism (SNP) genotyping was performed at the Institute of Human Genetics at the University of Bonn. Genomic DNA at a concentration of 60 ng/µL was used for whole-genome amplification. Afterwards, the amplified DNA was fragmented and hybridized to sequence-specific oligomers bound to beads on an Illumina HumanOmniExpress-12v1.0 chip. Data were analyzed using Illumina Bead Studio.
Differentiation of hiPSCs into neural cells
Induced-pluripotent stem cells were grown using mTeSR™1 medium (Stemcell Tech., 05850) on matrigel (Corning, 354277). Human iPS cells were detached from matrigel using ReLeSRTM (Stemcell Tech., 05872), resuspended in medium consisting of Dulbecco’s modified Eagle’s medium/ F12 supplemented with 10% knockout serum (Invitrogen, 10828-028), 1% N2 supplement (Gibco, 17502-048), 0.05% B27 (Gibco, 17504-044), 20 ng/ml epidermal growth factor (Sigma, E9644) and 10 ng/ml basic fibroblast growth factor (Gibco, PHG0024), and plated within a low-attachment petri dish to induce embryoid body (EB) formation for 4 days. The EBs were plated on polyornithine (Sigma, P3655)/laminin (Sigma, L2020)-coated dishes for additional 6 days to induce the formation of neural rosettes. Neural rosettes were then manually removed, dissociated with accutase (Stemcell Tech., 07920), plated on poly-L-ornithine/laminin-coated dishes, and then treated with 3 µM retinoic acid for 7 days. The medium was changed daily and cultures were passaged weekly by accutase and plated on matrigel-coated dishes in the above-mentioned neural medium.
Small molecule differentiation of hiPSCs into motoneurons
iPSCs differentiation was performed by the addition of small molecules adapted from a previously described protocol73. Briefly, for neuronal induction, iPSCs were seeded as colonies resuspended in E8 medium 2 days prior differentiation. To start the differentiation, medium was changed to N2B27 medium (50% Neurobasal medium/ 50% DMEM-F12 medium with 1:200 N2 supplement, 1:100 B27 supplement without vitamin A and 1% penicillin, streptomycin and L-glutamine respectively) supplemented with the small molecules 10 µM SB-431542 (SB), 1 µM dorsomorphin (DM), 3 µM CHIR 99021 and 0.5 µM PMA for four days. Subsequently, SB and DM were replaced by 150 µM Ascorbic Acid (AA), and cells were fed daily until epithelium-like structures emerged. Neural epithelial structures were picked, dissociated mechanically and plated on 12-well plates, which were coated with Matrigel (1:100 in DMEM/F12) overnight. After around five passages the smNPC cells reached a high purity and were further kept in culture on Matrigel-coated plates and N2B27 medium supplemented with CHIR, PMS and AA. Detaching of the cells for passaging was performed with accutase. Starting from smNPC passage 13, differentiation to MN could be initiated. N2B27 medium with 1 µM PMA was added 3 days after passaging. After two more days, 1 µM retinoic acid (RA) and 1 µM PMA were supplemented to the medium for the following 8 days, until culturing in maturation medium began, consisting of N2B27 medium with BDNF, GDNF and dbcAMP for two more weeks.
Immunoblotting
Denatured total cellular protein samples were separated on SDS polyacrylamide gels 4-15% Mini-PROTEAN® TGX Stain-Free™ gels (Bio-Rad) and transferred onto a nitrocellulose membrane using the Trans-Blot® Turbo™ Transfer System (Bio-Rad). Membranes were blocked with 3% (w/v) milk powder in PBS-T or TBS-T (1x PBS or TBS, 0.05% (v/v) Tween 20) for 1 h at room temperature (RT). Chameleon Duo Pre-stained Protein Ladder (Li-Cor Biosciences) was used as a molecular weight standard. Primary antibodies were anti-actin mAB (clone C4, 1:4000; Millipore MAB1501), anti-Cofilin-1 mAB (clone D3F9, 1:1000, Cell Signaling, 5175), anti-p-Cofilin-1 mAB (Ser3, clone 77G2, 1:1000, Cell Signaling 3313), anti-DRP1 mAB (clone 4E11B11, 1:1000, Cell Signaling 14647), anti-GDAP1 (1:750, Sigma HPA014266), anti-G6PD mAB (clone D5D2, 1:1000, Cell Signaling 12263), anti-GAPDH mAB (clone 14C10, 1:2000, Cell Signaling 2118), anti-GLUD1 mAB (clone D9F7P, 1:1000, Cell Signaling 12793), anti-HK1 mAB (clone C35C4, 1:1000; Cell Signaling 2024), anti-HK2 mAB (clone C64G5, 1:1000, Cell Signaling 2687), anti-HSPA9 (clone N52A/42, 1:1000, UC Davis/NIH NeuroMab Facility, Davis, USA, 73-127), anti-LDHA mAb (clone C4B5, 1:1000, Cell Signaling 3582), anti-MCU (1:1000, Sigma HPA016480), anti-MFF (1:1000, Proteintech 17090-1-AP), anti-MFN2 mAB (clone M03, 1:500; Abnova H00009927-M03), anti-Nestin mAB (clone rat-401, 1:1000, Merck chemicals MAB353), anti-PDH mAb (E1α, clone C54G1, 1:1000, Cell Signaling 3205), anti-PDH mAB (E2,E3bp, clone 13G2AE2BH5, 1:1000, Abcam, ab110333) anti-p-PDH (Ser293, 1:1000, Cell Signaling 31866), anti-PKM1/2 mAb (clone C103A3, 1:1000, Cell Signaling 3582), anti-TOM20 (1:1000, Sigma HPA011562), anti-βIII-Tubulin mAB (clone TuJ-1, 1:1000, R&D Systems MAB1195), anti-VDAC1 (clone 20B12AF2, 1:1000, Abcam ab14734). The membranes were incubated with the primary antibodies overnight at 4 °C. For visualization, membranes were incubated with an infrared fluorescence IRDye® 680RD Streptavidin for biotinylation staining or IRDye 800-conjugated anti-mouse, 800-conjugated anti-rabbit, or 680-conjugated anti-mouse IgG secondary antibody (1:15,000; Licor), for 1 h at RT and detected with the Odyssey Infrared Imaging System (Licor). Western Blots were analyzed with the Image Studio Lite Software (Li-Cor Biosciences).
Immunocytochemistry
Neuronal cells were grown on matrigel-coated µ-Slide 8 Well, ibiTreat (Ibidi, 80826), fixed with 4% paraformaldehyde (PFA) (CarlROTH, 3105.2) and permeabilized by 0.25% (v/v) Triton X-100 in PBS. Unspecific binding of antibodies was blocked with 1X Roti®-Block (CarlROTH, A151.4) for 30 min. Primary antibodies anti-Nestin (1:100, Ebioscience, 14-9843-82), anti-MAP2 (1:500, Synaptic Systems, 188004), anti-Smi32 (Clone SMI32, 1:1000, Biolegend 801702), anti-beta-III tubulin (1:1000, R&D Systems, MAB1195) were treated in 1X Roti®-Block at 4 °C overnight. The cells were washed three times with PBS and incubated with the fluorescent conjugated secondary antibody (Molecular Probes, A-11001) in 1X Roti®-Block for 1 h at RT. Subsequently, three PBS washing steps were done. Nuclei were stained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI). Pictures were taken with a Leica TCS SP5 inverted confocal microscope with a 63x/NA1.4 oil immersion objective, a BX51 Fluorescence microscope (Olympus) with a 20x objective or an Opera PhenixTM spinning disc high-content screening microscope (Perkin Elmer, USA) equipped with two 16-bit sCMOS cameras and a 40x, 1.1 NA water immersion objective. For Cofilin-1 evaluations, SH-SY5Y cells were grown on 12 mm glass cover slides. Primary antibodies were anti-Cofilin-1 mAB (clone D3F9, 1:100, Cell Signaling, 5175), anti-GRP75 mAB (clone N52A/42, 1:20, Neuromab 75-127). Pictures were taken with a Leica TCS SP8 inverted confocal microscope with a 63x/1.4 NA oil immersion objective in z-stacks. Images were analyzed in Imaris 9.5.1 (Bitplane). For motoneurons analysis, images were imported into marcopolo columbus database and underwent batch analysis that determined the fluorescence intensity of each marker. The signal intensities of Smi32 and MAP2 were normalized to β-tubulinIII each.
Confocal microscopy and image analysis
High-content light microscopy analysis was conducted with the Opera PhenixTM spinning disc microscope. Fluorescence (Ex/Em) for Hoechst, MitoTrackerTM Green FM and tetramethylrhodamine methyl ester perchlorate (TMRM) was measured at 405/435-480, 488/500-550 and 561/570-630 nm ex/em, respectively. BODIPY™ 558/568 C12 was added to the cells in parallel to MitoTracker in a final concentration of 1 µM for 15 min and subsequently, cells were incubated with normal growth medium. The images were analyzed with the software packages Harmony (version 4.5) and Columbus (version 2.7.1) containing the PhenoLOGICTM machine learning plugin (Perkin Elmer). Cells were selected via nuclear staining with HOECHST, but the cytosol region was defined using the mitochondrial stain. Within this selection, mitochondria and LDs were defined as isolated populations and analyzed respectively for their area and intensity. For mitochondria-associated LDs, the intensity of BODIPYTM 558/568 C12 staining was determined within the mitochondrial area. The complete image analysis sequences are available upon request. Live-cell imaging of actin filament analysis was conducted two days after transfection of GFP-F-tractin (gift of Henry N. Higgs, Dartmouth University) and together with staining of mitochondria with 100 nM MitoTracker™ Red CMXRos. Cells were imaged using the Leica TCS SP8 inverted confocal microscope with a 63x/1.4 NA oil immersion objective in z-stacks. Cells were captured at 1024×1024 with sequential image capturing. Step size was set at 0.25 µm. Images from confocal microscopy for Cofilin-1, HSPA9 and actin filament quantification were analyzed in Imaris 9.5.1 (Bitplane). To improve the signal-to-noise ratio, iterative deconvolution was performed (Huygens Essential 20.10). The software then created 3D surfaces of mitochondria and Cofilin-1 spots to measure the surface area and to determine the shortest distance from the Cofilin-1 surface to the mitochondrial surface. Colocalizing Cofilin-1/actin with a distance ≤ 0 µm was duplicated to a new surface and taken for the Imaris surface-surface contact XTension to quantify the contact area, defined as the percentage of mitochondrial surface contacting Cofilin-1. Similarly, 3D surfaces of Actin and the mitochondria were created. The software computed the contact area between the two surfaces as well as the shortest distance between the actin and mitochondria surfaces. Live-cell imaging of DRP1 surface analysis was conducted two days after transfection of pcDNA3-GFP-DRP1 (gift of Prof. Dr. Culmsee, Philipps-Universität Marburg) and together with staining of mitochondria with 100 nM MitoTracker™ RedCMXRos. Images were captured in a similar manner using the Leica TCS SP8 inverted confocal microscope. Images were then imported to and analyzed in Imaris 9.7.2 (Bitplane) where 3D surfaces of Actin and the mitochondria were created. The software computed the contact area between the two surfaces as well as colocalization XTension to quantify the colocalized voxels between the Drp1 surface and the mitochondrial surface.
Cell culture and generation of stable cell lines
SH-SY5Y cell lines were grown in DMEM/F12 medium (Gibco) supplemented with 10% (v/v) fetal calf serum (FCS; Thermo Scientific), 2 mM L-glutamine (Gibco), 1 x MEM non-essential amino acids, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco) in a humidified incubator at 37 °C, 5% CO2 and 95% air. The SH-SY5Y cell lines pLKO-NT (control) and G4 (knockdown) were a kind gift of David Pla-Martin and Francesc Palau19 and were grown in growth medium containing 2 µg/ml puromycin (InvivoGen).
Measurement of mitochondrial oxygen consumption
Mitochondrial respiration and oxygen consumption were analyzed using the Oxygraph-2k (Oroboros Instruments). Cells in suspension at a density of 1.5–2.0 × 106 cells/ml were analyzed under continuous stirring at 750 rpm and 37 °C. All chemicals with the exception of palmitate-BSA were purchased from Sigma. Palmitate-BSA was purchased from Biomol. In a phosphorylation-control-protocol, the routine respiration of cells in their general growth medium was measured following the addition of 2 µg/ml oligomycin to inhibit the ATP synthase and measure leak respiration. By titration of the protonophore carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone in 0.5 µM steps the respiration was stimulated up to a maximum oxygen flow and the electron transfer system capacity was determined. By the addition of 0.5 µM rotenone and 2.5 µM antimycin A the respiration was inhibited and the non-mitochondrial residual oxygen consumption was measured. In 2 s intervals, the oxygen concentration and the oxygen flow per cells were recorded using the DatLab software 5.1 (Oroboros Instruments). All measurements were performed after daily calibration of the polarographic oxygen sensors and using instrumental background correction. The measured respiratory states were analyzed after correction with ROX to compare only mitochondrial oxygen consumption.
ATP measurements
Cytosolic and mitochondrial ATP levels were quantified as described27. Cells were transiently transfected with plasmids carrying the bioluminescence energy transfer (BRET)-based ATP biosensor BTeam without targeting signal sequence (for cyto-ATP determination) or targeted to mitochondria (for mito-ATP determination) using TurboFectin reagent (OriGene). The plasmids were a kind gift of Hiroshi Imamura, University of Kyoto. 48 h later, the cells were incubated for 30 min in phenol red-free medium supplemented with 30 μM NanoLuciferase (NLuc) inhibitor to avoid disturbance from the BTeam released from dead cells. Afterwards, NLuc substrate (Promega) was added to the medium and the plate incubated for 20 min. Subsequently, luminescent emissions from the cells were measured at 37 °C at 520/560 nm (Yellow Fluorescent Protein (YFP) emission) and at 430/470 nm (NLuc emission) using a Tecan Spark® Multimode Microplate Reader.
Glutamine and glutamate measurements
The glutamine-glutamate-glo assay (Promega, # J8021) was performed according to manufacturer’s instructions to determine intracellular glutamine and glutamate concentrations. Briefly, 20,000 cells of the SH-SY5Y cell line and 100,000 motoneurons were plated in triplicates per experiment on white 96-well plates (Greiner, # 655083) two or seven days prior to the experiment, respectively. On the day of the experiment, cells were washed twice with PBS and 30 µl of PBS as well as 15 µl of 0.3 HCl solution was added to the cells and mixed for 5 min. 15 µl of 450 mM Tris solution, pH 8.0 was added and incubated for further 60 sec. From each lysate, 25 µl was transferred into a new white 96-well plate for I) glutamine plus glutamate and II) glutamate only measurement. Glutaminase was only added to the first set of wells and incubated for 30 min at RT. The detection reagent composed of Luciferin detection solution, reductase, reductase substrate, GLUD1 and NAD was added to all wells and incubated for 60 min at RT before luminescence detection in a Tecan Infinite 200 pro plate reader. Concentrations were calculated with glutamine and glutamate standard curves (0.78 to 50 µM) and a blank was included to remove any assay background signal. Glutamate levels were calculated by subtracting the glutamate-only signal from the signal containing glutamate and glutamine levels together.
Electron microscopy and analysis
Cells were pelleted by centrifugation at 1300 rpm for 3 min and fixed in 3% glutaraldehyde overnight. Following several rinses in 0.2 M sodium cacodylate buffer (pH 7.3), the samples were postfixed in 1% osmium tetroxide in cacodylate buffer for 2 h, dehydrated through an ascending series of ethanol concentrations and embedded in resin with propylene oxide as an intermediary. Semi-thin (0.65 µm) sections for light microscopy and ultrathin (50 nm) sections for electron microscopy were cut on a Leica Ultracut UCT ultramicrotome. Semi-thin sections were stained with methylene blue. Ultrathin sections were stained with an alcoholic solution of 1% uranyl acetate and lead citrate in sodium hydroxide and examined with a Zeiss EM-910 transmission electron microscope. For morphometric and quantitative analysis, representative cells were photographed at a magnification of 10,000 and 18,000. Analyses were done with ImageJ. MERCs were defined by a distance of mitochondrial and ER membranes between ~10 and ~50 nm. The frequency distribution of the MERCs width was calculated using GraphPad.
Biochemical analysis of mitochondria-ER contact sites
Cells were detached using trypsin/ETDA and resuspended in 1–2 ml of a buffer containing 0.32 M sucrose, 10 mM Tris-HCl, 1 mM EDTA and protease (Roche Diagnostics, 04693124001) and phosphatase (Roche Diagnostics, 04906845001) inhibitors. Cells were disrupted by a nitrogen decompression instrument (Parr Instrument Company, 4639) and centrifuged (2000 g, 10 min). The supernatant was transferred to a new microtube, centrifuged at 10,000 g for 10 min and the cell pellet resuspended in 1 ml of the same buffer containing the same components as above except 0.5 M sucrose. Finally, mitochondria were sedimented by centrifugation at 10,000 g for 10 min and resolved in an appropriate amount of RIPA buffer for the subsequent experiments.
FRET-based FEMP probe to quantify mitochondria-ER contact sites
MERCS were quantified with a FRET-based sensor indicating the proximity between the ER and mitochondria. The plasmid encodes for a YFP-linked outer mitochondrial membrane protein Akap1 and a CFP-conjugated ER-protein Sac1, as well as a fused FKBP and FRB domain respectively and is available from Marta Giacomello, University of Padova. These domains can form heterodimers upon rapamycin treatment. The specific localization of these proteins is ensured by the introduction of a self-cleavable Tav2A sequence34,35. The FEMP plasmid (FRET-based ER-mitochondria probe) was transfected with GenJet. 48 h later, images were taken with the Perkin Elmer Operetta High-Content Imaging System acquiring the CFP- (410-430/460-500 ex/em), YFP- (490-510/520-560 ex/em) and YFPFRET-emission (410-430/520-560 ex/em) with a ×40 water objective for determination of the basal distances between the organelles. Subsequently, the cells were treated with 100 nM rapamycine for 15 min for FKBP-FRB dimerization induction and to reach a maximum of YFPFRET signal. Cells were fixed for another 20 min with 1% PFA and imaging was performed again with equal microscopy settings. For the analysis, the Harmony software was used. First, the cells were identified using the YFP channel. Within each cell and region of interest (ROI), the intensities of the three acquired channels were calculated, including background subtraction. FRET basal and FRET max were calculated as: (FYFP-FRETcell-FYFP-FRETbackground)/ (FCFPcell-FCFPbackground); FRET Ratio was calculated as (FRETmax–FRETbasal)/FRETbasal.
Mitochondrial Ca2+ measurement
Dye: Cells were plated (20,000 cells/cm2) in 8-well µ-slides ibiTreat (ibidi, 80826) and treated with 5 µM of Rhod2-AM (Molecular Probes, R1245MP) in culture medium without FBS for 60 min at 37 °C the next day. Rhod2-AM fluorescent signals were analyzed at (Ex/Em) 549/578 nm wavelengths using a Leica SP5 confocal microscope and analyzed by ImageJ. Genetically encoded reporter: Cells were transfected with the genetically encoded reporters and analyzed two days later in an inverted TCS-SP5 confocal microscope (Leica) with appropriate excitation/emission wavelengths as reported for CEPIA3mt (Addgene, 58219) and GEM-CEPIA1er (Addgene, 58217)31. The CEPIA3mt construct was co-transfected and normalized to mito-TurboFarRed.
Aequorin Ca2+ measurements
For Ca2+ measurements, the biosensor aequorin (AEQ) was used, which is a 22 kDa calcium-binding photoprotein isolated from jellyfish Aequorea Victoria. The plasmid is available from Marta Giacomello, University of Padova. SH-SY5Y cells were grown on 12 mm glass coverslips to a confluence of 40–50% and transfected with cytosolic or mitochondria-targeted AEQ (cytAEQ/mtAEQ) using GenJet. On the day of the experiment, the cells were treated with 5 µM coelenterazine-N-AM (Santa Cruz Biotechnology sc-205904) in basic saline solution (BS, 135 mM NaCl2, 5 mM KCl, 0.4 mM KH2PO4, 1 mM MgSO4 x 7 H2O, 20 mM HEPES, 0.1% (w/v) glucose, pH 7.4 adjustment at 37 °C with NaOH 10 N) containing 1 mM CaCl2 for 2 h at 37 °C and 5% CO2. Coelenterazine served as a substrate for AEQ. Cover slides were then placed in the luminometer with constant buffer perfusion with BS supplemented with I) 1 mM Ca2+ (30 s) II) 200 µM EGTA (30 s) III) 200 µM Carbachol (CCH) + 200 µM EGTA (120 s) IV) 100 µM Digitonin + 5 mM CaCl2 (220 s).
Precipitation of biotinylated Avi-GDAP1
Primary cortical neuron cultures were prepared from embryos (E16) from the transgenic mouse line Gt(ROSA)26Sortm1(birA)Mejr (ROSA26-BirA) of a C57BL/6 N background. In this mouse strain, the biotin ligase BirA was inserted into the gene locus of the ROSA26 promoter40. Cortical neurons were cultured on poly-d-lysine coated plates (0.05 mg/ml) in Neurobasal medium (NBM, Life Technologies, 21103049) supplemented with 2% (v/v) B-27 supplement (Life Technologies, 17504-044), 1% (v/v) L-glutamine (Sigma-Aldrich, G1251) and 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, P0781). Medium change was performed at day 1 and day 4 after isolation. For transduction of the primary neurons, viruses were added in a volume that equaled 6 × 107−8 × 107 copies/µl per well of a 6 well plate containing 4 ml of NBM and neurons on day 4 after isolation. Neurons were cultured for further 7 days. Cells were harvested, washed with PBS and lysed in RIPA buffer supplemented with protease inhibitors. The samples were centrifuged at 21,000 g for 30 min at 4 °C, the proteins concentration was determined via BC-Assay and 20 µg protein lysate was removed as input-control. Protein lysates were either directly incubated with Dynabeads™ MyOne™ Streptavidin T1 according to the manufacturer’s protocol or taken for GSH or GSSG incubation (5 mM GSH or GSSG in 10 mM HEPES pH 7.4, 35 mM sucrose, 40 mM KCl, 0.25 mM EGTA, 2 mM Mg(CH3COO)2, 0.5 mM GTP, 1 mM ATP (K+), 5 mM Na-succinate, 0.08 mM ADP, 2 mM K2HPO4 for 30 min at 37 °C). Dynabeads™ MyOne™ Streptavidin T1 were incubated for 45 min at RT on a shaker. Dynabeads were washed three times with RIPA buffer and once with PBS and stored at −80 °C until further processing for LC-MS.
Protein elution, lysis and digestion
Bound proteins were eluted from Dynabeads in 10 mM Tris pH 8.0, 2% SDS, 1 mM Biotin at 80 °C. Whole cells were lysed in 5 µl of 10% SDS at 95 °C for 5 min. Protein samples were digested using the SP3 (“Single-Pot Solid-Phase-Enhanced Sample Preparation”) protocol74 with modifications75. Proteins were reduced by adding 5 µl of 200 mM Dithiothreitol (DTT) per 100 µl lysate (45 °C, 30 min). Free cysteines were subsequently alkylated by adding 10 µl 100 mM Iodoacetamide (IAA) per 100 µl lysate (RT, 30 min, in the dark). Subsequently, remaining IAA was quenched by adding 10 µl 200 mM DTT per 100 µl lysate. Magnetic carboxylate modified particles Beads (SpeedBeads, Sigma) were used for protein clean-up and acetonitrile (ACN), in a final concentration of 70%, was added to the samples to induce the binding of the proteins to the beads by hydrophilic interactions (18 min RT). By incubating the bead-protein mixture on a magnetic stand for 2 min, the sample was bound to the magnet and the supernatant removed, followed by two washing steps with 70% ethanol (EtOH), addition of 180 µl ACN, incubation for 15 s and removal of the solvent. Finally, 5 µl digest buffer (50 mM ammonium bicarbonate, 1:25 w/w trypsin:protein ratio) was added to the air-dried bead-protein mixtures and incubated overnight at 37 °C. To purify peptides after digestion, ACN was added to a final concentration of 95%. After another washing step. the beads were resuspended in 10 µl 2% DMSO (in water), put into an ultrasonic bath for 1 min and then shortly centrifuged. 10 µl of the resulting supernatant was mixed with 5 µl 100 fmol/µl Enolase digest (Waters Corporation) and acidified with 5 µl 1% formic acid (FA).
LC-MS analysis
Liquid chromatography (LC) of tryptic peptides was performed on a NanoAQUITY UPLC system (Waters Corporation) equipped with 75 µM × 250 mm HSS-T3 C18 column (Waters corporation). Mobile phase A was 0.1% (v/v) formic acid (FA) and 3% (v/v) DMSO in water. Mobile phase B was 0.1% (v/v) FA and 3% (v/v) DMSO in ACN. Peptides were separated running a gradient from 5 to 40% (v/v) mobile phase B at a flow rate of 300 nL/ min over 90 min. The column was heated to 55 °C. MS analysis of eluting peptides was performed by ion-mobility enhanced data-independent acquisition (UDMSE). Precursor ion information was collected in low-energy MS mode at a constant collision energy of 4 eV. Fragment ion information was obtained in the elevated energy scan applying drift-time specific collision energies. The spectral acquisition time in each mode was 0.7 s with a 0.05 s interscan delay resulting in an overall cycle time of 1.5 s for the acquisition of one cycle of low and elevated energy data. [Glu1]-fibrinopeptide was used as lock mass at 100 fmol/μL and sampled every 30 s into the mass spectrometer via the reference sprayer of the NanoLockSpray source. All samples were analyzed in three technical replicates.
Data processing and label-free protein quantification
UDMSE data processing and database search was performed using ProteinLynx Global Server (PLGS, ver. 3.0.2, Waters Corporation). The resulting proteins were searched against the UniProt proteome database (species: Mus musculus, UniProtK-Swissprot release 2019_05, 17.051 entries; Homo sapiens, UniProtK-Swissprot release 2019_10, 20367 entries) supplemented with a list of common contaminants. The database search was specified by trypsin as enzyme for digestion and peptides with up to two missed cleavages were included. Carbamidomethyl cysteine was set as fixed modification and oxidized methionine as variable modification. False discovery rate assessment for peptide and protein identification was done using the target-decoy strategy by searching a reverse database and was set to 0.01 for database search in PLGS. Retention time alignment, exact mass retention time (EMRT), as well as normalization and filtering were performed in ISOQuant ver.1.8. By using TOP3 quantification, absolute in-sample amounts of proteins were calculated.
Statistics and reproducibility
Normal distribution was tested using the D’Agostino-Pearson omnibus normality test. Statistical significance was then verified using appropriate parametric (Student’s t test or ANOVA) or non-parametric tests (Mann–Whitney and Kruskal–Wallis tests) followed by multiple comparison tests as indicated. The Wilcoxon signed rank test was used when normalization to 100% was necessary as indicated. Statistical analysis of mass spectrometry data was performed using two-tailed, paired t-tests and subsequent Bonferroni correction. Here, a corrected p < 0.01 was considered significant for the biotinylated Avi-GDAP1 pulldown experiment and p < 0.05 for whole cell lysates. In all other data a p < 0.05 was considered to be statistically significant. Pathway over-representation analysis was performed using the STRING database with default parameters39. KEGG pathway visualization was performed using the R package clusterProfiler76.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The excellent support by the IMB Core Facility Microscopy is gratefully acknowledged. This work was supported by grants of the Deutsche Forschungsgemeinschaft to A.M. (CRC1080 project A10) and to A.M. and E.M.H. and S.T. (DFG ME1922 16-1; Ha 8334/2-2; TE599/6-1) within the priority program 1710. This work was supported by an Excellence Grant of the Luxembourg National Research Fund (FNR) within the PEARL program (FNR; FNR/P13/6682797 to R.K.). Work of R.K. is further supported by a grant from the FNR within the National Centre of Excellence in Research on Parkinson’s disease (NCER-PD). We thank the Disease Modelling and Screening Platform of the Luxembourg Institute of Systems Biomedicine and the Luxembourg Institute of Health for their support on high-content imaging of motoneurons. We thank Marion Silies for excellent and exhaustive proofreading.
Author contributions
C.W., A.P., S.B., AnnP, D.B., P.M., F.d.B., C.V., I.B., D.G., P.M. carried out experiments; K.D., M.P., and O.B. generated the iPS cells; J.S. and S.R. helped establishing high-content microscopy; S.A., D.G., and S.T. analyzed quantitative proteomic analyses; M.G. supervised and analyzed the FEMP and aequorin measurements; R.K. supervised and analyzed the experiments with human motoneurons; C.W., M.S., and E.M.H. analyzed data and helped writing the manuscript; A.M. devised experiments, analyzed data and wrote the manuscript.
Peer review
Peer review information
This manuscript has been previously reviewed at another Nature Portfolio journal. The manuscript was considered suitable for publication without further review at Communications Biology. Primary Handling Editors: Eve Rogers.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository77 with the data set identifiers: <PXD024555> for the biotinylated Avi-GDAP1 coprecipitation experiment; and <PXD028460> for the GDAP1 KD label-free quantification experiment. Plasmids used are mentioned in the Methods section and can be requested from those that generated them upon reasonable request. Supplementary Data 1 contains all raw data and uncropped immunoblots.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03487-6.
References
- 1.Baxter RV, et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat. Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 2.Cuesta A, et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat. Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 3.Senderek J, et al. Mutations in the ganglioside-induced differentiation-associated protein-1 (GDAP1) gene in intermediate type autosomal recessive Charcot-Marie-Tooth neuropathy. Brain. 2003;126:642–649. doi: 10.1093/brain/awg068. [DOI] [PubMed] [Google Scholar]
- 4.Chung KW, et al. A novel GDAP1 Q218E mutation in autosomal dominant Charcot-Marie-Tooth disease. J. Hum. Genet. 2008;53:360–364. doi: 10.1007/s10038-008-0249-3. [DOI] [PubMed] [Google Scholar]
- 5.Marco A, Cuesta A, Pedrola L, Palau F, Marín I. Evolutionary and structural analyses of GDAP1, involved in Charcot-Marie-Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol. Biol. Evol. 2004;21:176–187. doi: 10.1093/molbev/msh013. [DOI] [PubMed] [Google Scholar]
- 6.Shield AJ, Murray TP, Board PG. Functional characterisation of ganglioside-induced differentiation-associated protein 1 as a glutathione transferase. Biochem. Biophys. Res. Commun. 2006;347:859–866. doi: 10.1016/j.bbrc.2006.06.189. [DOI] [PubMed] [Google Scholar]
- 7.Wagner KM, Rüegg M, Niemann A, Suter U. Targeting and function of the mitochondrial fission factor GDAP1 are dependent on its tail-anchor. PLoS ONE. 2009;4:e5160. doi: 10.1371/journal.pone.0005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedrola L, et al. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum. Mol. Genet. 2005;14:1087–1094. doi: 10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- 9.Huber N, et al. Glutathione-conjugating and membrane-remodeling activity of GDAP1 relies on amphipathic C-terminal domain. Sci. Rep. 2016;6:36930. doi: 10.1038/srep36930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Googins MR, et al. Structural and functional divergence of GDAP1 from the glutathione S-transferase superfamily. FASEB J. 2020;34:7192–7207. doi: 10.1096/fj.202000110R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber N, Guimaraes S, Schrader M, Suter U, Niemann A. Charcot-Marie-Tooth disease-associated mutants of GDAP1 dissociate its roles in peroxisomal and mitochondrial fission. EMBO Rep. 2013;14:545–552. doi: 10.1038/embor.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemann A, Wagner KM, Ruegg M, Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol. Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Ingerman E, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 17.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti R, et al. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J. Cell Biol. 2018;217:251–268. doi: 10.1083/jcb.201709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pla-Martín D, et al. Silencing of the Charcot-Marie-Tooth disease-associated gene GDAP1 induces abnormal mitochondrial distribution and affects Ca2+ homeostasis by reducing store-operated Ca2+ entry. Neurobiol. Dis. 2013;55:140–151. doi: 10.1016/j.nbd.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Boyer O, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 2011;365:2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- 21.Rehklau K, et al. Cofilin1-dependent actin dynamics control DRP1-mediated mitochondrial fission. Cell Death Dis. 2017;8:e3063. doi: 10.1038/cddis.2017.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Sánchez P, et al. CMT-linked loss-of-function mutations in GDAP1 impair store-operated Ca2+ entry-stimulated respiration. Sci. Rep. 2017;7:42993. doi: 10.1038/srep42993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chance B. The energy-linked reaction of calcium with mitochondria. J. Biol. Chem. 1965;240:2729–2748. doi: 10.1016/S0021-9258(18)97387-4. [DOI] [PubMed] [Google Scholar]
- 24.Kennerson ML, et al. A new locus for X-linked dominant Charcot-Marie-Tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene. Hum. Mol. Genet. 2013;22:1404–1416. doi: 10.1093/hmg/dds557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pla-Martín D, et al. Junctophilin-1 is a modifier gene of GDAP1-related Charcot-Marie-Tooth disease. Hum. Mol. Genet. 2015;24:213–229. doi: 10.1093/hmg/ddu440. [DOI] [PubMed] [Google Scholar]
- 26.Noack R, et al. Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum. Mol. Genet. 2012;21:150–162. doi: 10.1093/hmg/ddr450. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Kakizuka A, Imamura H. BTeam, a novel BRET-based biosensor for the accurate quantification of ATP concentration within living cells. Sci. Rep. 2016;6:39618. doi: 10.1038/srep39618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem. J. 1972;128:161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem. J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, et al. Site-directed mutagenesis of phosphorylation sites of the branched chain alpha-ketoacid dehydrogenase complex. J. Biol. Chem. 1994;269:18583–18587. doi: 10.1016/S0021-9258(17)32349-9. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki J, et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giacomello M, Pellegrini L. The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ. 2016;23:1417–1427. doi: 10.1038/cdd.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim. Biophys. Acta. 2014;1837:461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Csordás G, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naon D, et al. Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc. Natl Acad. Sci. USA. 2016;113:11249–11254. doi: 10.1073/pnas.1606786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacomello M, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenzer S, et al. Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J. Proteome Res. 2013;12:2869–2884. doi: 10.1021/pr400193j. [DOI] [PubMed] [Google Scholar]
- 39.Szklarczyk D, et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2020;49:D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driegen S, et al. A generic tool for biotinylation of tagged proteins in transgenic mice. Transgenic Res. 2005;14:477–482. doi: 10.1007/s11248-005-7220-2. [DOI] [PubMed] [Google Scholar]
- 41.Cull MG, Schatz PJ. Biotinylation of proteins in vivo and in vitro using small peptide tags. Methods Enzymol. 2000;326:430–440. doi: 10.1016/S0076-6879(00)26068-0. [DOI] [PubMed] [Google Scholar]
- 42.Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012;13:909–915. doi: 10.1038/embor.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bittermann E, et al. Differential requirements of tubulin genes in mammalian forebrain development. PLoS Genet. 2019;15:e1008243. doi: 10.1371/journal.pgen.1008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estela A, Pla-Martín D, Sánchez-Piris M, Sesaki H, Palau F. Charcot-Marie-Tooth-related gene GDAP1 complements cell cycle delay at G2/M phase in Saccharomyces cerevisiae fis1 gene-defective cells. J. Biol. Chem. 2011;286:36777–36786. doi: 10.1074/jbc.M111.260042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klemke M, et al. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Gellert M, Hanschmann E-M, Lepka K, Berndt C, Lillig CH. Redox regulation of cytoskeletal dynamics during differentiation and de-differentiation. Biochim. Biophys. Acta. 2015;1850:1575–1587. doi: 10.1016/j.bbagen.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Kruyer A, Ball LE, Townsend DM, Kalivas PW, Uys JD. Post-translational S-glutathionylation of cofilin increases actin cycling during cocaine seeking. PLoS ONE. 2019;14:e0223037. doi: 10.1371/journal.pone.0223037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melak M, Plessner M, Grosse R. Actin visualization at a glance. J. Cell Sci. 2017;130:525–530. doi: 10.1242/jcs.204487. [DOI] [PubMed] [Google Scholar]
- 49.Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell. 2011;22:4047–4058. doi: 10.1091/mbc.e11-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji W-K, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patron M, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Civera-Tregón, A. et al. Mitochondria and calcium defects correlate with axonal dysfunction in GDAP1-related Charcot-Marie-Tooth mouse model. Neurobiol. Dis. 152, 105300 (2021). [DOI] [PubMed]
- 54.Boldogh IR, Pon LA. Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophys. Acta. 2006;1763:450–462. doi: 10.1016/j.bbamcr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann L, et al. Cofilin1 oxidation links oxidative distress to mitochondrial demise and neuronal cell death. Cell Death Dis. 2021;12:953. doi: 10.1038/s41419-021-04242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pope BJ, Zierler-Gould KM, Kühne R, Weeds AG, Ball LJ. Solution structure of human cofilin: actin binding, pH sensitivity, and relationship to actin-depolymerizing factor. J. Biol. Chem. 2004;279:4840–4848. doi: 10.1074/jbc.M310148200. [DOI] [PubMed] [Google Scholar]
- 57.Pfannstiel J, et al. Human cofilin forms oligomers exhibiting actin bundling activity. J. Biol. Chem. 2001;276:49476–49484. doi: 10.1074/jbc.M104760200. [DOI] [PubMed] [Google Scholar]
- 58.Goyal P, et al. Cofilin oligomer formation occurs in vivo and is regulated by cofilin phosphorylation. PLoS ONE. 2013;8:e71769. doi: 10.1371/journal.pone.0071769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siudeja K, Grzeschik NA, Rana A, de Jong J, Sibon OCM. Cofilin/Twinstar phosphorylation levels increase in response to impaired coenzyme a metabolism. PLoS ONE. 2012;7:e43145. doi: 10.1371/journal.pone.0043145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua BT, et al. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat. Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 61.Klamt F, et al. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat. Cell Biol. 2009;11:1241–1246. doi: 10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo S, Uehara H, Shacter E. Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation. Free Radic. Biol. Med. 2014;75:84–94. doi: 10.1016/j.freeradbiomed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Fratelli M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl Acad. Sci. USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl Acad. Sci. USA. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee E, De Camilli P. Dynamin at actin tails. Proc. Natl Acad. Sci. USA. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Züchner S, et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
- 67.Fabrizi GM, et al. Two novel mutations in dynamin-2 cause axonal Charcot-Marie-Tooth disease. Neurology. 2007;69:291–295. doi: 10.1212/01.wnl.0000265820.51075.61. [DOI] [PubMed] [Google Scholar]
- 68.Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luigetti M, et al. Seipin S90L mutation in an Italian family with CMT2/dHMN and pyramidal signs. Muscle Nerve. 2010;42:448–451. doi: 10.1002/mus.21734. [DOI] [PubMed] [Google Scholar]
- 70.Boutet E, et al. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. doi: 10.1016/j.biochi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Tian Y, et al. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011;7:e1001364. doi: 10.1371/journal.pgen.1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang W, et al. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum. Mol. Genet. 2014;23:502–513. doi: 10.1093/hmg/ddt444. [DOI] [PubMed] [Google Scholar]
- 73.Reinhardt P, et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS ONE. 2013;8:e59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hughes CS, et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014;10:757. doi: 10.15252/msb.20145625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sielaff M, et al. Evaluation of FASP, SP3, and iST Protocols for Proteomic Sample Preparation in the Low Microgram Range. J. Proteome Res. 2017;16:4060–4072. doi: 10.1021/acs.jproteome.7b00433. [DOI] [PubMed] [Google Scholar]
- 76.Wu T, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. J. 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vizcaíno JA, et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013;41:D1063–D1069. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository77 with the data set identifiers: <PXD024555> for the biotinylated Avi-GDAP1 coprecipitation experiment; and <PXD028460> for the GDAP1 KD label-free quantification experiment. Plasmids used are mentioned in the Methods section and can be requested from those that generated them upon reasonable request. Supplementary Data 1 contains all raw data and uncropped immunoblots.