Abstract
Cognitive neuroscience has witnessed increased interest in investigating the neural correlates of the mind when it drifts away from an ongoing task and the external environment. To that end, functional neuroimaging research has consistently implicated the default mode network and frontoparietal control network in mind-wandering. Yet, it remains unknown which subregions within these networks are necessary and how they facilitate mind-wandering. In this review, we synthesize evidence from lesion, transcranial direct current stimulation and intracranial EEG studies demonstrating the causal relevance of brain regions, and providing insights into the neuronal mechanism underlying mind-wandering. We propose that the integration of complementary approaches is the optimal strategy to establish a comprehensive understanding of the neural basis of mind-wandering.
Keywords: mind-wandering, lesion, transcranial direct current stimulation, intracranial EEG, default mode network, frontoparietal control network
Beyond neuroimaging correlates of mind-wandering
An exceptional feature of the human mind is its capacity to wander away from the here and now[1]. This ubiquitous experience predicts wide ranging functional outcomes in both the lab and in everyday life[2]. Regardless of how mind-wandering[3–7] is defined (see Glossary), its prevalence and impact has sparked a substantial increase in cognitive neuroscience research investigating the neural correlates of mind-wandering in the past 15 years[8]. Leveraging the superb spatial resolution of functional and structural magnetic resonance imaging (MRI) techniques, these studies revealed multiple brain structures involved in this pervasive cognitive phenomenon[9–14]. However, unanswered questions remain about the necessity of these brain regions and the neuronal processes underlying mind-wandering. By integrating evidence from lesion, transcranial direct current stimulation (tDCS) and intracranial EEG (iEEG) studies, we present a synthesis that establishes the causal relevance of brain regions, and provides insight into the neural mechanisms underlying mind-wandering.
Empirical studies investigating the neural basis of mind-wandering have primarily relied on functional MRI. They have identified a consistent set of brain regions involved in mind-wandering, providing valuable insights on where in the brain the action takes place. Given the correlational nature and limited temporal resolution of functional and structural MRI, other techniques are available to address the causality of brain regions in mind-wandering, the mechanistic relationship between these regions, and the temporal dynamics of mind-wandering. Therefore, the current review examines two important aspects of the neural basis of mind-wandering by highlighting studies involving the lesion, tDCS and intracranial EEG approaches. Box 1 describes the unique insights afforded by each of these approaches in more detail. In this review, we first address the causal relevance of brain regions in mind-wandering and then examine the neural mechanism underlying this ubiquitous experience. Given the increasingly recognized role of context, we also underline the importance of accounting for context in examining the neural basis of mind-wandering.
Box 1. The value of lesion, tDCS and intracranial EEG approaches.
The lesion, transcranial direction current stimulation (tDCS), and intracranial EEG (iEEG) approaches have uniquely advanced our understanding of the neural basis of cognitive functions (see Figure I).
For over a century, lesion studies documenting the impact of permanent damage to focal brain regions in humans have revealed important insights into their causal links to fundamental cognitive processes[88]. Changes in one cognitive function but not another in individuals with lesions compared to those with an intact brain provide compelling evidence that the damaged brain region is necessary for the disrupted cognitive function. The outcome of this permanent damage appears to be distinct from that of temporary changes elicited by brain stimulation, underscoring the unique information afforded by the lesion approach[23]. To that end, foundational and contemporary lesion studies have revealed subcomponent processes of high-level cognitive functions[24,89,90].
In contrast to the permanency of brain damage, tDCS temporarily modulates a brain region using a portable stimulator that delivers low intensity electrical currents through scalp electrodes. Importantly, changes in stimulation parameters can differentially impact the targeted cognitive function. This includes the stimulation montage (e.g. bipolar versus high-definition) which impacts the spatial precision of the stimulation[23]; polarity (e.g. anodal=excitation and cathodal=inhibition) which informs the functional role of brain regions[40,41,43,57]; and intensity (e.g. 1mA versus 2mA) which reveals dosage dependent effects[60,61]. Despite inconsistent findings in part due to methodological differences (see Box 3), the temporary effect of the stimulation enables the examination of causal relevance of brain regions in larger, more representative samples and is a potential avenue for improving cognitive functions.
Finally, recordings of iEEG are obtained directly from electrodes implanted in the brains of epilepsy patients during surgery for identifying the source of epileptic seizures. Neural activity recorded from non-epileptic sites[91] in patients resemble activity from healthy brains, emphasizing the utility of iEEG for basic research. This type of data offers millimeter anatomical precision and millisecond temporal precision useful for revealing neuronal activity unfolding over time during cognitive processes[92,93]. Moreover, iEEG data has a high signal-to-noise ratio allowing for single-trial analyses and provides access to high frequency activity which tracks neuronal firing rates and the BOLD signal[94,95]. Despite these advantages over scalp EEG, iEEG is inherently invasive and comes at the cost of low sample size and partial brain coverage. The advantages of iEEG enable us to understand not just where and when, but how, fundamental cognitive processes are implemented in the human brain[92,96].
Figure I. Lesion, transcranial direct current stimulation (tDCS) and intracranial EEG (iEEG) approaches.

A) Lesion studies have examined the default mode network (DMN), as exemplified in these MRI images. Adapted from [32]. B) tDCS studies have targeted the DMN and DLPFC in the fronto-parietal control network (FPCN). Simulation of left DLPFC stimulation using the two-electrode montage (left panels) shows that the impact spreads into medial PFC and beyond whereas the four-electrode high-definition montage (right panels) results in focalized electric field intensity restricted to the left DLPFC. Adapted from [60]. C) In studies using iEEG, grid, strip and stereotactic electrodes have been used to target regions in DMN and FPCN.
Neural regions and circuits necessary for mind-wandering
Functional MRI (fMRI) evidence has consistently implicated the interaction within and across two major large-scale networks in mind-wandering. One common finding converges on the default mode network (DMN)[10–12,15]. Parcellation of this network has revealed fine-grained differential relationships between its two subsystems and phenomenological experiences that are commonly reported during mind-wandering[9,16]. Another prominent network consistently linked to mind-wandering is the frontoparietal control network (FPCN). These networks are illustrated in Figure 1. Based on its role in goal-directed processes[17,18], co-activation of the FPCN and the DMN are linked to the occurrence of mind-wandering and related internal processes[19–21]. Box 2 further elaborates on the differential roles of the DMN and FPCN in mind-wandering, as well as other cognitive processes, highlighting a nuanced context-dependent brain-behavior functional relationship. Although additional regions beyond these networks, including the motor cortex[22], are also recruited during mind-wandering, the current review focuses on the DMN and FPCN as they have been the most extensively examined. Over a decade of fMRI research laid the foundation for our understanding of neural networks involved in mind-wandering. We now focus on two complementary approaches that build on the knowledge obtained from fMRI by establishing the causal relevance of subregions in these networks: lesion and tDCS.
Figure 1. Graphical summary of the role of DMN and FPCN subregions in mind wandering.
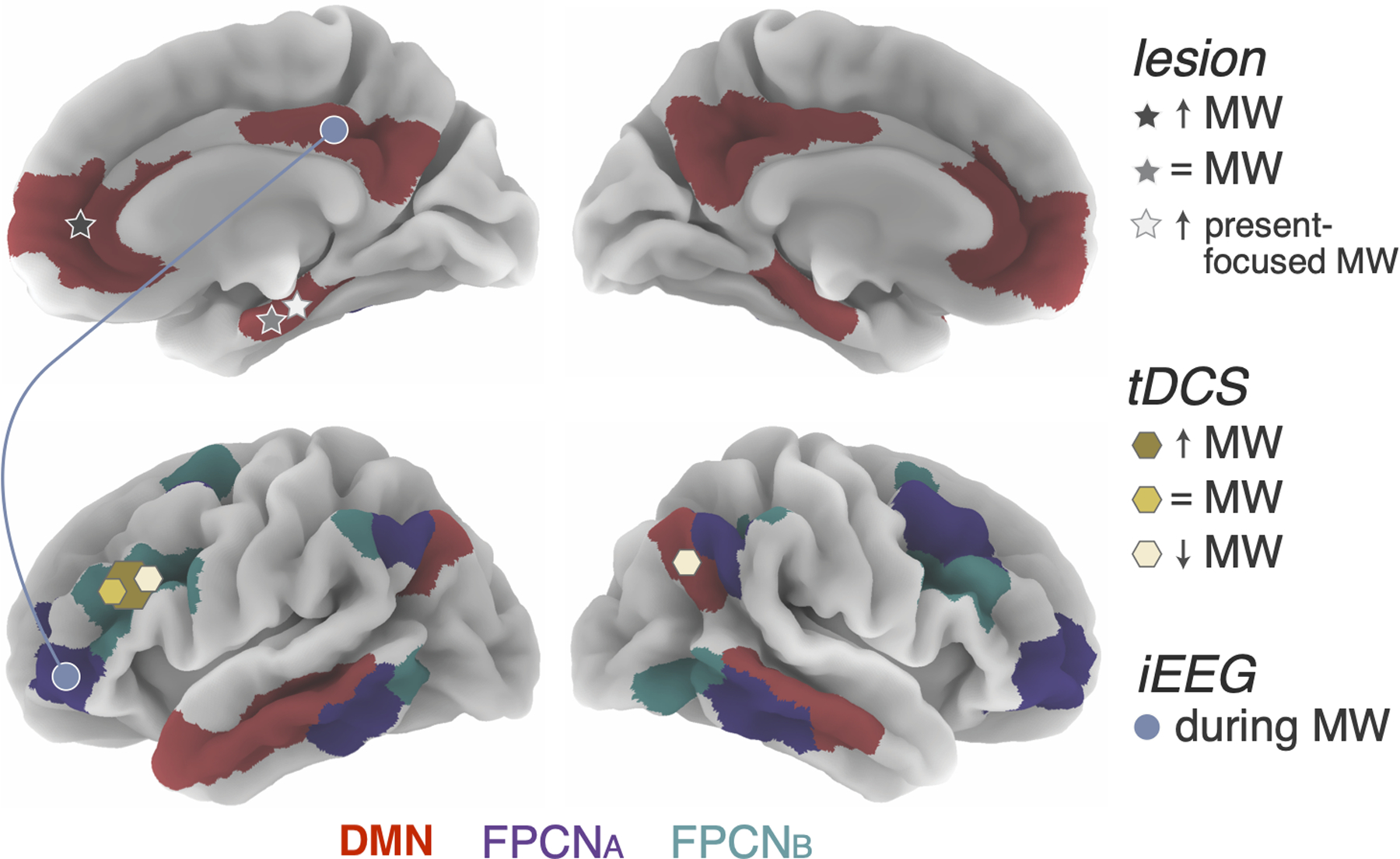
The effects of lesion on mind-wandering frequency and temporal focus of mind-wanderirng are indexed by star shapes. Darker grey indicates a lesion in that location was shown to increase mind-wandering frequency (primarily in the ventromedial PFC); the medium shade of grey indicates an absence of significant effect on mind-wandering frequency (in the hippocampus); the lightest grey indicates an increase in present-focused thoughts during MW (in the hippocampus). tDCS effects primarily focusing on mind-wandering frequency are indexed by hexagonal shapes: darker yellow indicates stimulation of that region was shown to increase mind-wandering (in the DLPFC), lighter yellow indicates a decrease in mind-wandering (in the DLPFC and right IPL), and the medium shade indicates an absence of significant effect (in the DLPFC). The size of the hexagon corresponds to the proportion of papers reporting that effect; however, note the sample size across studies is not reflected in this figure. Connectivity between DMN and FPCN subsystem A during mind-wandering as revealed by iEEG studies are shown in blue circles, which reflects involvement of the entire network in which the circle is located.
Box 2. The roles of DMN and FPCN in mind-wandering.
Neuroimaging research has long converged on the integral role of the default mode network (DMN) in mind-wandering[9–12]. Based on differential relationships between the two subsystems of the DMN and thought processes that are commonly reported during mind-wandering[9,16], these subsystems presumably contribute to the different aspects of mind-wandering. While the dorsal medial prefrontal cortex subsystem is involved in mentalizing about social situations (e.g. as in theory of mind[97]), the medial temporal lobe subsystem is active during episodic memory recall and constructive mental simulation during memory tasks[16] and during mind-wandering[30,31]. Given the robust finding of DMN’s involvement in mind-wandering, a substantial amount of research has almost exclusively examined the DMN. Subsequent work has disputed this widely accepted portrayal of a one-to-one brain-behavior mapping, with a meta-analysis demonstrating that mind-wandering reliably recruits regions outside of the DMN including the fronto-parietal control network (FPCN)[8]. Moreover, accumulating evidence suggests that the DMN is not exclusively involved in mind-wandering and its related processes[98]; rather, this network is also recruited during tasks not traditionally associated with DMN[99–103], and linked to specific aspects of task-related thoughts[22]. These findings [14,22,74,102] contest the notion of DMN’s singular role as a task-negative network, and necessitate the consideration of context to more accurately depict this nuanced relationship between DMN and ongoing thoughts.
The FPCN has also been consistently linked to mind-wandering[8]. Primarily activated during executive control processes[17,104], this network appears to serve as a gateway to conscious ongoing experience and interacts with the DMN to regulate the occurrence of internally oriented thoughts[16,21,51,105] including episodic memory[19]. Similar to the DMN, subsequent work has revealed that this network is parcellated into two subsystems that are associated with distinct functional roles[51]. The FPCNA is mainly associated with internally oriented processes whereas the FPCNB is preferentially involved in externally oriented processes.
Collectively, although neuroimaging studies provide robust evidence of the involvement of the DMN and FPCN, they also demonstrate the heterogeneity of each network and their complex relationship with mind-wandering. Their role in mind-wandering and other cognitive processes further highlights the importance of considering a more nuanced functional account of these networks in explaining our ongoing thought patterns. These separate lines of research set the foundational knowledge, upon which complementary methodological approaches can then build to establish the causal relevance and mechanistic relationship underlying subcomponents within these large-scale neural networks.
Permanent damage versus temporary modulations
The classic approach to determining the necessity of specific brain regions involves the comparison of behavioral and neural patterns of individuals with and without permanent focal damage to parts of their brain[23]. This lesion methodology was famously exemplified by the discovery of the critical role of the hippocampus in memory[24]. Informed by fMRI findings, lesion studies examining mind-wandering have primarily focused on the DMN.
Another approach to determine the causal relevance of cortical regions in mind-wandering is tDCS (see [25] for a review). In contrast to the permanent damage resulting from lesions, this type of non-invasive brain stimulation technique enables the temporary and reversible modulation of cortical excitability in targeted brain regions. Importantly, the polarity of the stimulation informs the mechanistic relationship between the stimulated brain region and the cognitive function of interest. Whereas anodal stimulation is proposed to increase excitability of the underlying cortex, cathodal stimulation is thought to inhibit activity[26]. While both the DMN and FPCN have served as targets of stimulation in neurotypical individuals, tDCS studies of mind-wandering have predominantly targeted the dorsolateral prefrontal cortex (PFC) as part of the FPCN based on its established role in the high-level executive control of attention[17,18,27].
While both permanent and temporary approaches inform the causality of a brain region in a given function, evidence suggests they provide unique information about the nature of that brain-behavior relationship (c.f.[23]). Permanent lesions reveal the functions of the damaged region following reorganization of the brain over time[28], whereas temporary modulations of brain regions reveal the effects of not only the region being perturbed in the moment but also the network to which this region belongs[29]. Therefore, the altered mind-wandering experience following temporary tDCS stimulation may mirror the acute phase of a lesion, and the disruption in mind-wandering resulting from chronic lesions in patient populations may reflect the permanency of the damage that has not recovered from neural plasticity.
Default mode network’s role in mind-wandering
Given that the DMN is reliably associated with mind-wandering, studies have investigated its role in two prominent aspects of the phenomenon: the frequency of its occurrence and temporal focus of thoughts while mind-wandering. In examining the frequency of mind-wandering, both lesion and tDCS studies have focused on the medial temporal lobe subsystem of the DMN[16]. This subsystem includes regions that are implicated in episodic memory retrieval and future thinking (hippocampus), and internal perception (ventromedial PFC and medial parietal lobe). Previous neuroimaging work have specifically implicated this subsystem in the episodic thought content that arise during mind-wandering[30,31]. In lesion studies, patients with lesions in the ventromedial PFC report less mind-wandering (conceptualized as task-unrelated thoughts) compared to neurotypical controls and patients with lesions outside of the DMN[32] (as shown in Figure 2). This was assessed via thought sampling probes across three lab-based tasks varying in cognitive demands. Notably, lesion symptom and network mapping analyses involving these ventromedial PFC patients revealed that some of the regions strongly linked to reduced trait level mind-wandering include the inferior parietal lobule, inferior frontal gyrus and the ventromedial PFC[33]. This corroborates the causal relevance of this region in mind-wandering propensity, and highlights the role of connectivity to other regions. In mapping out regions that are functionally connected to the lesioned area[34], this novel analytic approach holds promise in revealing not only the necessary regions but also the circuitry involved in different aspects of mind-wandering.
Figure 2. Specific findings on the neural structures and mechanisms underlying mind-wandering.
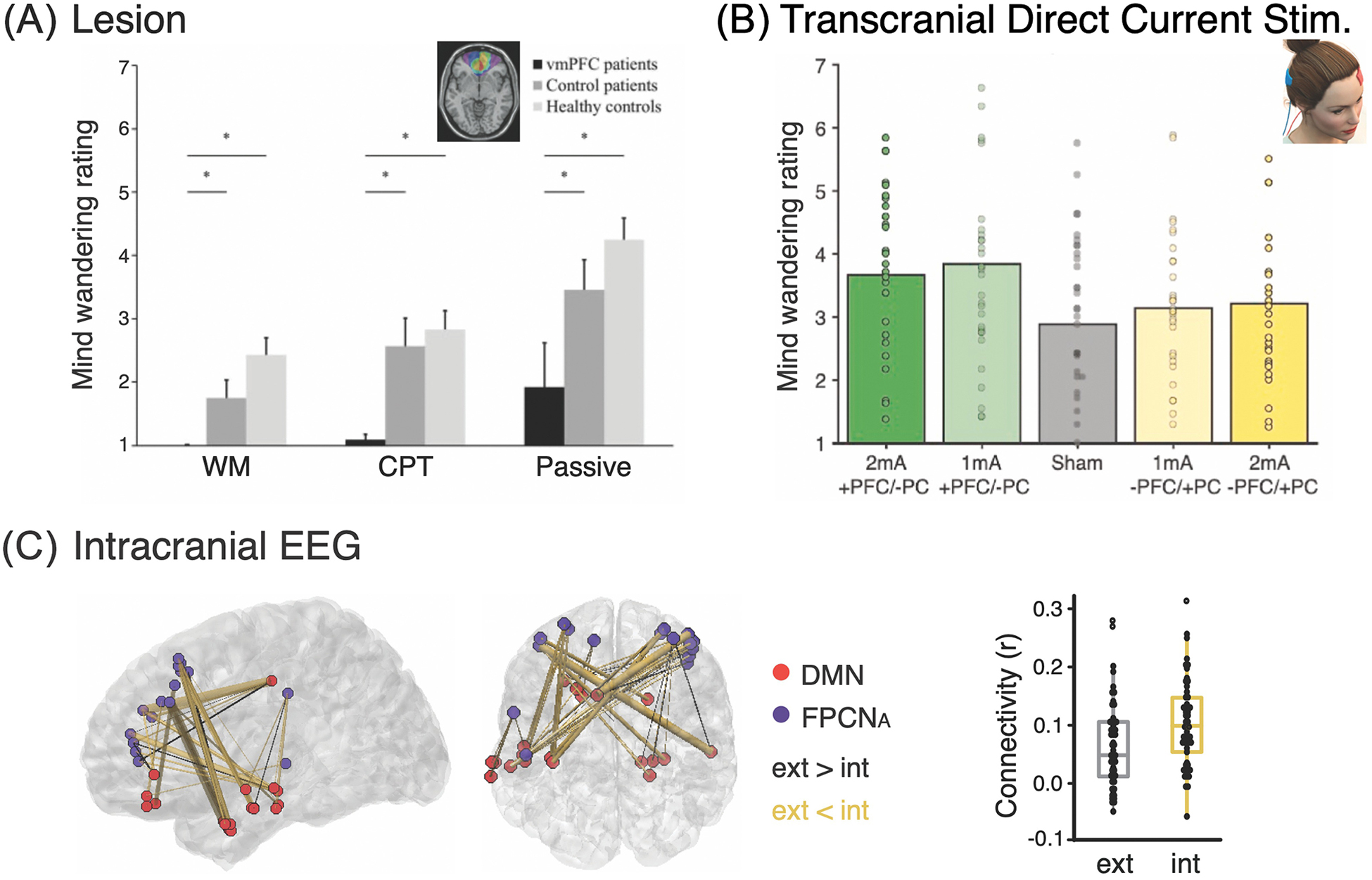
A) Patients with lesions in the ventromedial prefrontal cortex (vmPFC) reported significantly less mind-wandering episodes compared to healthy and patient controls across three tasks (i.e. WM = working memory, CPT = continuous performance task, Passive = rest). Adapted from [32]. B) tDCS anodal stimulation of the left prefrontal cortex (+PFC) and cathodal stimulation of the right inferior parietal cortex (−PC) showed increased mind-wandering frequency compared to sham stimulation, whereas the opposite montage (−PFC/+PC) did not show significant differences compared to sham, suggesting a polarity-specific effect. Adapted from [43]. C) iEEG recordings revealed increased theta connectivity between DMN and FPCN subsystem A during mind-wandering as conceptualized by internal attention (int) compared to external attention (ext). Adapted from [65].
In contrast, patients with lesions in the hippocampus, a critical node of the medial temporal lobe subsystem, report comparable levels of mind-wandering occurrence as observed in neurotypical controls[35]. This is puzzling at first glance given fMRI findings implicating the left hippocampus in the initial occurrence of spontaneous thought and mind-wandering propensity[36,37]. However, a closer examination of these authors’ conceptualization of mind-wandering and the context within which mind-wandering was assessed suggests diverging definitions and testing environments may be responsible for the observed differences. Specifically, they defined mind-wandering as stimulus-independent thought and strategically placed the thought sampling probes during moments when participants were not performing any task and were minimally engaged with the external environment[35]. Their naturalistic approach contrasts with previous studies that embedded thought probes during an experimental task in a lab setting, which captures different aspects of ongoing thoughts[38,39]. Together, this suggests the hippocampus is not necessary for perceptually decoupled thought in under-stimulated settings with minimal cognitive demands from the external environment. However, their role in task-unrelated thoughts, especially during tasks with higher cognitive demands, remains unknown.
Expanding beyond these findings, several tDCS studies have assessed the impact of DMN stimulation on mind-wandering propensity. In particular, one group found that anodal stimulation of the right inferior parietal lobule (IPL) as part of the medial temporal lobe subsystem of the DMN, and inhibitory cathodal stimulation of the left dorsolateral PFC (DLPFC), decreased mind-wandering compared to the reversed polarity montage[40]. Replicated in two subsequent studies[41,42], this stimulation effect was uniquely observed in the right but not left IPL[42]. Combining tDCS with resting state fMRI, they found that anodal stimulation of the right IPL decreased its efferent connections with the posterior cingulate cortex, a core node in the DMN, which was linked to reduced mind-wandering propensity[41]. This stimulation montage also decreased medial PFC’s efferent connections with the posterior cingulate cortex, which was linked to decreased mind-wandering. Given the facilitative role of the DLPFC in mind-wandering, these findings suggest that simultaneously inhibiting the DLPFC and increasing excitability of the right IPL reduces mind-wandering by perturbing intra-DMN connectivity. By examining functional connectivity patterns following stimulation, this combination of methodological approaches provides unique insights into the neural circuitry of the DMN and its role in mind-wandering propensity. Notably, these results should be considered along with two studies that failed to replicate the effect of reduced mind-wandering frequency when they implemented anodal stimulation of the right IPL and cathodal stimulation of the left DLPFC[43] and left cheek[44] during a less cognitively demanding task. These findings suggest the causal relevance of the right IPL as part of the DMN in mind-wandering may depend on stimulation parameters or the context in which mind-wandering was measured (see Box 3).
Box 3. Methodological challenges and suggested solutions for tDCS research.
Effects of tDCS on mind-wandering and cognitive functions are variable[106] and have been inconsistent across studies[107]. This suggests that the methodology as currently employed in many tDCS studies is of insufficient quality to provide reliable and consistent estimates of its effect on cognitive functioning. These methodological shortcomings fall into two categories: overlooking important individual factors modulating the effectiveness of tDCS, and poor study design coupled with flexible analysis methods. Regarding the first category, individual anatomy has strong effects on the distribution of the induced electric field as well as the intensity with which underlying areas of the cortex are being stimulated[81]. Another major factor shaping the electric field is the number, spatial location, material and shape of the stimulation electrodes[108]. Even small variations of these parameters can have strong impacts on the electric field. Yet, standards for these parameters are lacking from the scientific literature. Both of these challenges can be overcome by utilizing methods for individualizing both dose[109] and stimulation montages[110] based on computational models of individual structual brain scans[111], which improves standardization of the electric field across participants.
The second methodological shortcoming concerns replicability: while this issue have been raised across many scientific fields including cognitive neuroscience[112], tDCS research has been singled out as particularly vulnerable[113]. Many studies on the cognitive effects of tDCS are characterized by under-powered designs and flexible analytical methods[114], both of which have been shown to be key facilitators for non-replicable results[115]. Pre-registration (and especially pre-registered reports) has been recently identified as a powerful method to improve scientific rigor and ensure replicability[116]. By enforcing strict adherence to a pre-specified data collection and analysis plan, spurious findings can be effectively reduced. Even though several pre-registered reports have been published[57,59], adoption of pre-registration has been slow in the tDCS literature. However, this field is well-suited to benefit from the advantages of pre-registration due to the relatively low cost for collecting larger samples and the abundance of stimulation and analytical parameters.
Finally, the tDCS field has suffered from spurious results due to insufficient blinding procedures in standard protocols[117]. Brain-stimulation devices have been shown to produce powerful placebo effects[118,119], resulting in the possibility that reported findings can be attributed to effects of expectation rather than stimulation. While modern, high-definition tDCS montages have better blinding properties[120], using active control protocols can facilitate estimating the effectiveness and specificity of the primary stimulation protocols[121].
The temporal focus of thoughts is another prominent phenomenological feature of mind-wandering. In examining the content of mind-wandering thoughts, patients with lesions in both the ventromedial PFC and hippocampus report more present-focused thoughts during mind-wandering than neurotypical controls[32,35]. These findings are in line with the ventromedial PFC’s causal role in mental time travel[45] and in thoughts not bounded by external stimuli[46]. This pattern of thoughts was also observed in patients with a behavioral variant of fronto-temporal dementia, with atrophy in similar areas within the DMN and other distributed regions[37]. In addition to hippocampal patients’ preference for present-focused mind-wandering, their tendency towards semantic versus episodic thoughts and verbal versus visual thoughts[35] are also consistent with the medial temporal lobe’s role in mental simulations[9,32,47]. In other words, a damaged hippocampus appears to result in an inability to construct episodic events via visual imagery, which constitutes a major proportion of mind-wandering content[48]. It is possible that a damaged medial temporal lobe subsystem may result in the absence of mental content, causing thoughts to be more present-focused. In line with lesion findings, stimulation of bilateral IPL specifically decreased mind-wandering focused on negatively valenced past oriented thoughts compared to sham stimulation[49]. These results converge on the notion that the medial temporal lobe subsystem is causally involved in mental simulation during mind-wandering, without which our inner phenomenological experience is restricted to the present.
Collectively, notwithstanding the different conceptualizations of mind-wandering, these findings establish the spatial specificity of the medial temporal lobe subsystem of the DMN (as summarized in Figure 1). They reveal the nuanced relationship between each subregion and the frequency of mind-wandering and its phenomenological experience, suggesting they are not uniformly involved in the same aspects of mind-wandering. These patterns are consistent with neuroimaging reports of a differential relationship between the cortical thickness of two regions within the parahippocampus and varying levels of detail and task-focus of our phenomenological experience[39,50]. Moving beyond individual regions, lesion network mapping and combined tDCS and resting state fMRI converge on the mechanistic role of intra-DMN connectivity in mind-wandering occurrence. These novel approaches provide insights into the mechanistic circuitry underlying how the DMN is involved in aspects of mind-wandering beyond its propensity.
Fronto-parietal control network’s role in mind-wandering
Given the regulatory role of the FPCN in both external and internal cognition[19,51], lesion and tDCS studies have focused on the role of the DLPFC as a core node of the FPCN in mind-wandering. In our study, we compared the electrophysiological patterns observed in individuals with and without lesions in the LPFC[52]. Patients with LPFC lesions showed dysregulation of their electrophysiological response (i.e. alpha band activity) during periods when attention was focused internally (otherwise referred to as stimulus-independent thoughts). This regulatory capacity was intact in neurotypical controls and patient controls with lesions outside of the FPCN. These results suggest that the LPFC is critical for stimulus-independent thought; the impact of this lesion is manifested in the disruption of the electrophysiological activity that facilitates mind-wandering[53,54]. These findings build upon foundational work indicating the LPFC is critical for contextual processing[55].
In line with this finding, studies involving DLPFC stimulation also established the causal relevance of this region in mind-wandering, primarily designed to modulate its propensity. In an initial tDCS study, anodal stimulation of the left DLPFC increased mind-wandering compared to two control conditions involving sham stimulation and stimulation of the occipital region[56]. Subsequent studies have replicated this finding using stronger intensity stimulation that was independent of the location of cathode over right supraorbital or inferior parietal cortex[28–31] (see Figure 2). The authors also ruled out the possibility that meta-awareness as a result of stimulation contributed to the increased self-report of mind-wandering[58]. Despite the apparent robustness of this finding, we were unable to replicate the tDCS effect on mind-wandering in a well-powered, pre-registered study[59] using the identical stimulation and task parameters as those reported by the initial study[56]. By reporting Bayesian statistics, we were able to provide evidence for the absence of an effect of anodal DLPFC stimulation on mind-wandering. This finding raises important questions about the impact of methodological differences employed in the tDCS field on the variable outcomes across studies, which we address in Box 3.
In a subsequent study, we used a high-definition (HD) stimulation montage, consisting of smaller electrodes where one anode was centered over the left DLPFC and surrounded by four cathodes. Computational simulations have shown that such HD-montages provide a more focal stimulation compared to the two-electrode montage[60] used in past studies. We found that anodal stimulation of left DLPFC led to a decrease in mind-wandering using the HD-montage in a different task tailored to recruit executive function[61]. One possible explanation rests on the assumption that DLPFC stimulation enhances executive resources availability. If so, these resources may be allocated to the executive function task in this study[60], thereby decreasing mind-wandering during task performance. Since the earlier studies implemented tasks that do not require executive functions[40–43,56,57], the enhanced resources may have been allocated to mind-wandering instead, thereby increasing its occurrence. These findings are consistent with recent work indicating the same region within DLPFC flexibly connects with different networks that are relevant to its current goals, underscoring its context-dependent role in attentional allocation[62]. Future studies are needed to clarify the role of context in tDCS modulation of mind-wandering propensity.
In summary, the heterogeneity in stimulation parameters and task choice likely contributed to these opposing results of DLPFC stimulation; however, they do converge on the critical role of the DLPFC in the occurrence of mind-wandering (as summarized in Figure 1). The contrasting results raise the possibility that the specific nature of its role may depend on whether the enhanced neural resources are delegated to external or internal cognitive processes, once again underscoring the importance of considering task context in accounts of mind-wandering.
Neural mechanisms underlying mind-wandering
Investigations of neural mechanisms underlying mind-wandering have heavily relied on fMRI. Using static and dynamic functional connectivity, these studies revealed correlational relationships within and across regions of the DMN and FPCN during mind-wandering[16,19,20,51]. Nonetheless, the coarse temporal resolution of fMRI restricts its ability to capture transient, fast-acting processes of neuronal assemblies. In contrast, iEEG offers high temporal resolution and anatomical precision, rendering it an ideal tool for assessing the transient neuronal processes subserving mind-wandering. In this section, we discuss the neurophysiological mechanism underlying mind-wandering and related spontaneous processes occurring at rest as revealed by iEEG.
Neural processes during mind-wandering
In line with neuroimaging studies, iEEG research investigating mind-wandering converges on the role of the DMN and FPCN. We authored an initial iEEG study that examined mind-wandering conceptualized as stimulus-independent thought or internal attention. Our task directs subjects’ attention to be focused on the external environment (i.e. external attention) or their internal world (internal attention). Therefore, this experimental paradigm necessitates the recruitment of control regions to successfully direct attention externally or internally.
Based on the prominent role of the PFC in modulating mind-wandering as informed by the lesion, tDCS, and neuroimaging literature, we first examined the role of the PFC as part of the FPCN. Specifically, our study compared high frequency band activity (HFA; typically quantified as 70–150+ Hz) in response to auditory stimuli during external and internal attentional focus in the LPFC as well as the temporal cortex as the control region[63]. While HFA in the LPFC was greater during external compared to internal attention, there was no evidence of such differences in the temporal cortex. These findings implicate the LPFC in the top-down control of mind-wandering. Our results support the decoupling model[64], and suggests that one mechanism by which the LPFC supports mind-wandering is by withdrawing resources from the external environment as reflected in the attenuation of HFA responses to external inputs during mind-wandering.
Informed by fMRI findings of functional connectivity between DMN and FPCN, we then examined the electrophysiological mechanism underlying the relationship between these two networks during mind-wandering. Given that parcellation of the FPCN has revealed a subsystem A that is broadly implicated in internal cognition[51], we assessed the interaction between electrodes within DMN and FPCN subsystem A. Our results indicate enhanced inter-network connectivity in the theta band (4–7Hz) during internal attention relative to external attention[65] (as shown in Figures 1 and 2). This pattern was selectively observed in the theta band, consistent with studies suggesting the selectivity of theta oscillations during internal attention processes[66] within the DMN and FPCN[67,68]. Remarkably, the magnitude of the inter-network theta connectivity measure predicted attention ratings during the internal attention condition, highlighting its functional role in facilitating mind-wandering. These findings suggest that enhanced functional coupling in the theta band between the DMN and FPCN subsystem A as a potential core mechanism underlying mind-wandering.
Neural processes of mind-wandering related processes during rest
Mind-wandering is often associated with thought processes that occur at rest. Since the spontaneous processes at rest are reminiscent of the stimulus-independent thought conceptualization of mind-wandering, we reviewed iEEG studies of resting state focusing on the DMN and FPCN to further delineate the neural underpinnings of mind-wandering.
Consistent with the mind-wandering literature, evidence from iEEG studies converges on the role of the DMN during rest[69]. In particular, core hubs of the DMN have shown increased HFA during sustained periods of rest[70], the magnitude of which was shown to be specifically modulated by the theta phase in the posterior medial cortex[67]. As cross-frequency coupling is proposed to be a neuronal mechanism wherein local activity is coordinated by slower frequencies at different time scales in support of complex cognitive functions[71], these findings suggest cross-frequency coupling within the DMN may be the neurophysiological mechanism underlying spontaneous processes at rest. Implementing a more fine-grained assessment, subsequent reports have revealed unique spatial and temporal patterns of electrophysiological activity within the DMN[72] as well as between the DMN and FPCN[21] during rest. For instance, neighboring electrodes within 5–10mm of each other often display different temporal profiles[72], potentially reflecting the different stages or processes of spontaneous thoughts in which the DMN is engaged. Afforded by the granularity of iEEG, these findings underscore the heterogeneity of the spatiotemporal characteristics of the DMN during rest. Such observations corroborate lesion and tDCS findings of the nuanced relationship between subregions of the DMN and different aspects of mind-wandering, emphasizing the need to consider mind-wandering as a multi-faceted phenomenon in which the DMN plays various roles.
Taken together, iEEG has offered unique insights into the precise functional neurophysiology of mind-wandering that non-invasive neuroimaging methods are not equipped to address. Leveraging the spatiotemporal resolution of iEEG, these studies have accessed core hubs of the DMN in the medial structures of the brain with the temporal precision necessary to identify neural mechanisms and preferred frequencies. They revealed the importance of theta band in coordinating activity across large-scale neural networks during mind-wandering and in modulating HFA by way of cross-frequency coupling. They also established the neurophysiological basis for the connectivity patterns observed in the BOLD signal and provided evidence for variable temporal patterns that map onto spatial heterogeneity within the DMN and FPCN at rest.
Concluding Remarks and Future Perspectives
This review aimed to inform on the neural underpinnings of mind-wandering by synthesizing evidence from lesion, tDCS and iEEG research. These findings provide strong evidence that the medial temporal lobe subsystem of the DMN (which includes the hippocampus and ventromedial PFC) and DLPFC of the FPCN are necessary for mind-wandering, albeit for different aspects of the phenomenon. Both lesion and tDCS studies converge on the mechanistic role of intra-DMN connectivity in mind-wandering propensity, revealing that perturbation in any major node can disrupt functions of the network. The reviewed studies also demonstrated that mind-wandering relies on the coordinated functioning within and across these large-scale networks at different frequency bands, in particular the theta band and high frequency activity. We suggest that achieving an in-depth understanding of the complex neural patterns underlying mind-wandering necessitates a shift away from the predominant reliance on one method towards an integration of complementary methodological approaches. Establishing the causal and mechanistic brain-behavior relationships using these different approaches is a prerequisite to successfully pinpoint effective targets for modulating attentional focus and mind-wandering content in healthy and diseased brains. By leveraging the advantages of different methods, the field can move towards achieving the goal of creating a comprehensive understanding of the neural origins of this fundamental and pervasive cognitive phenomenon.
One essential future direction includes recognizing the panoply of thought processes and content that are often jointly characterized as mind-wandering. While behavioral and neuroimaging studies have begun to reveal the heterogeneity in processes and content during mind-wandering, studies using the methodologies described here have primarily focused on the propensity of mind-wandering or the mere presence of this phenomenon, overlooking the variety of thoughts and types of mind-wandering that occupy our everyday mental life. For example, ample evidence points to a distinction between intentional and unintentional mind-wandering, which are linked to contrasting functional correlates[73]. Another feature of mind-wandering are the dynamic characteristics of thoughts[7], which are associated with unique electrophysiological signatures[53]. Accumulating evidence from neuroimaging research have begun to unravel distinct brain profiles of diverse thought processes and content in support of conceptual distinctions[14,53,62]. The implications are two-fold. In terms of achieving a brain-behavior mapping of mind-wandering, recognizing that mind-wandering is not a unitary phenomenon will help reveal the distinct brain regions and circuitry that are differentially necessary for these various thought patterns and the neural mechanisms that may underlie them. To that end, recent work has applied principal component analysis on multi-dimensional experience sampling[74] data, which effectively quantifies the complex nature of phenomenological experience in the lab and in the real world[50,62,75]. Achieving an accurate and complete understanding of the nuanced and complex landscape of ongoing thought processes during mind-wandering is a critical first step in establishing its neural basis. Another crucial area in which the research reviewed here is relevant concerns the modulation of mind-wandering using brain stimulation: future work may benefit from focusing only on reducing the undesired types and content of mind-wandering. We discuss the practical and clinical applications in Box 4. Evidently, the diversity in our thoughts and types of mind-wandering highlight the value of using a finer grain measure of this phenomenon as an important step forward (see Outstanding Questions). A comprehensive account of the neural basis of mind-wandering will need to consider both the process by which the mind decouples from the external environment to focus on the inner milieu[76–79] as well as the multitude of phenomenological experiences that occur during mind-wandering.
Box 4. Heterogeneity of mind-wandering: real world implications.
Both theoretical and empirical work points towards the heterogeneity of the phenomenological experience during mind-wandering and the myriad ways in which we engage in this phenomenon. While the definition of mind-wandering has been under debate[6,122], we emphasize on two recent conceptualizations that have relevant practical and clinical implications. One common distinction concerns whether mind-wandering is engaged with or without intention[73]. Behavioral work on intentional and unintentional mind-wandering have revealed dissociable outcomes. Intentional mind-wandering has been associated with practical benefits, such as creativity[123] and enhanced mood. In contrast, unintentional mind-wandering has been linked to negative affect[124] in healthy individuals. Clinical work has also demonstrated increased unintentional mind-wandering but comparable intentional mind-wandering in individuals with attention deficit-hyperactivity disorder symptoms[125] compared to neurotypical controls. Given that negative outcomes uniquely associated with unintentional mind-wandering, future studies aiming to modulate mind-wandering propensity in educational or occupational settings may consider focusing on unintentional mind-wandering.
Another conceptualization of mind-wandering that is particularly relevant for clinical implications concerns the dynamic nature of thoughts[7,126,127]. Recent empirical work testing this theory revealed the ubiquitous presence of thoughts that dynamically flow from one topic to another[128,129]. Consistent with this growing theoretical recognition that our thoughts are not static, neuroimaging evidence predominantly using dynamic functional connectivity has revealed that neural activity also dynamically fluctuates over time during mind-wandering and other spontaneous thought processes[12,21,130,131], suggesting that just as our thoughts are not static, neither is the brain. These thought dynamics stand in contrast to thoughts that are constrained to one topic. For instance, our thoughts can be constrained by saliency of personal concerns, which makes it very difficult to disengage from, as commonly characterized as rumination. Thoughts can also be constrained by top-down control in service of achieving a goal, as in goal-directed processes.
The clinical implications of these various thought processes are multi-fold. As examples, future work may benefit from selectively 1) reducing undesirable constrained thoughts such as rumination characteristic of major depressive disorder, 2) increasing dynamic thought in individuals with obsessive compulsive disorder, and 3) increasing focused, goal-directed thought in individuals with attention-deficit hyperactive disorder. Although studies using the multi-dimensional experience sampling approach have reported similar thought patterns in the lab and daily life, a primary difference revealed more positive thoughts about ongoing tasks in daily life compared to the lab[39]. Future studies would benefit from considering the differential impact of performing lab-constrained versus naturalistic tasks on our phenomenological experience.
Outstanding Questions.
Lesion and tDCS studies have examined the impact of a focal brain region on mind-wandering; however, brain regions are inter-connected. How can we utilize our knowledge of large-scale brain networks to better understand its impact in the brain beyond the disrupted region, and the mechanism by which it modulates mind-wandering?
Given the heterogeneity of the phenomenological experience of mind-wandering (and distinct functional roles of subsystems in the DMN and FPCN), are there both common and idiosyncratic aspects of the causal and mechanistic relationship between brain and behavior related to mind-wandering? If this relationship is always context and content dependent, what is the extent of this variability within and across individuals?
Thus far, theta and high frequency band activity appears to play an important role in integrating activity across timescales and distant regions in facilitating mind-wandering. What are the roles of other frequency bands? How does oscillatory activity serve as a neurophysiological mechanism that underlie different types of mind-wandering? Does the nature of these mechanistic relationships change as a function of the nature of thoughts?
With accumulating evidence revealing unconstrained movement in thought patterns, referred to as freely moving thoughts, what is the timescale of these thought dynamics and how do these thought dynamics map onto neural dynamics?
What is the neurobiological basis of mind-wandering? A compelling framework suggests ascending neuromodulatory systems that promote sharp wave ripples in the hippocampus as a potential mechanism that initiates a mind-wandering episode. Another model of mind-wandering has proposed that norepinephrinergic activity in the locus coeruleus modulates the mind-wandering experience. Would empirical evidence support either or both conceptual models?
Beyond conceptual expansion, another area for development in establishing the causal relevance of brain regions in mind-wandering involves moving beyond target regions in order to capture the aforementioned diverse processes. Given neuroimaging work has reported regions beyond the DMN and FPCN implicated in mind-wandering[8], lesion studies expanding the regions of interest beyond the DMN and FPCN may reveal other brain structures necessary for different aspects of mind-wandering. To that end, one recent study recruited patients with lesions across different brain regions[33], and determined the lesion-associated networks linked to mind-wandering by implementing lesion network mapping analysis. Moving forward, this analytic approach implemented on a large sample would be particularly useful in delineating additional regions and their related circuitry that are causally involved in mind-wandering.
Stimulation of regions outside of the DLPFC will also likely prove to be informative; equally important for stimulation studies to consider is other non-invasive or invasive methods of stimulation. Thus far, tDCS is the most common approach to study mind-wandering and the majority of studies have targeted the DLPFC. Although improved stimulation protocols are being developed to enhance the anatomical precision of tDCS stimulation[60,80], modeling and simulation studies have shown that the stimulation effect of the most commonly used standard bipolar montage tends to spread well beyond the intended brain regions[60] and depends strongly on individual anatomical differences[81] (as discussed in Box 3). Notably, other non-invasive albeit less accessible approaches have been shown to provide more spatially precise stimulation. This includes transcranial magnetic stimulation[82] and transcranial focused ultrasound stimulation[83], an emerging technique in cognitive neuroscience with superior focality. Another way to enhance spatial precision of the targeted region is through intracranial electrical stimulation, which is an invasive neuromodulation technique that involves clinicians stimulating electrodes implanted into the brains of epilepsy patients to determine their corresponding behavioral effects. Although clinical risks must be carefully considered, this method offers excellent spatiotemporal precision important for tracking the transient effects of stimulation on mind-wandering manifested in behavior and neurophysiological activity, and identifying the regions and circuits causally linked to mind-wandering. A recent study summarizing these observed effects of stimulation has reported a range of behavioral responses upon stimulation of the DMN and FPCN[84].
Finally, the combination of advanced methodological and analytic techniques offers novel ways to provide mechanistic insights into the neural basis of mind-wandering. The aforementioned studies using lesion network mapping analysis[33] and tDCS combined with resting state fMRI functional connectivity analysis[41] exemplify these integrative approaches and demonstrated their effectiveness in addressing mechanistic questions. Other informative approaches include the examination of structural connections within and between networks underlying mind-wandering[85]. In addition, beyond delivering a direct electrical current in tDCS, transcranial alternating current stimulation (tACS) can modulate ongoing brain oscillations by applying oscillating electrical currents at a specific frequency band known to facilitate mind-wandering[86]. Aside from changing mind-wandering occurrence, this method potentially enables one to determine not only the necessary brain region but also the causal mechanism by which that region exerts its influence on cognition[87].
The field of cognitive neuroscience is in an unprecedented position equipped with technologically advanced tools to tackle the question of the neural underpinnings of mind-wandering. The next frontier of mind-wandering neuroscience research will likely require the integration of theory-informed, fine-grained conceptualization of mind-wandering and advanced methodological and analytical approaches to inform both basic and translational neuroscience efforts.
Highlights.
fMRI evidence has convincingly implicated the DMN and FPCN in mind-wandering. Less is known about which subregions in these networks are necessary, and how they facilitate mind-wandering.
A growing number of studies use the lesion and tDCS approaches to establish the causal link between DMN and FPCN subregions and mind-wandering. They have also identified the mechanistic role of intra-DMN connectivity in this phenomenon.
Intracranial EEG studies have revealed the neurophysiological mechanism underlying mind-wandering and its preferred frequencies in the theta and high frequency band for communication within and across large-scale neural networks.
To achieve an in-depth understanding of the where, when and how mind-wandering is implemented in the brain requires a shift away from the predominant reliance on one method toward an integration of complementary methodological approaches.
Acknowledgements
J.W.Y.K. is supported by the Natural Sciences and Engineering Research Council of Canada. R.T.K. is supported by U19 NS107609-01, NINDS R01 NS021135, and NIMH Conte Center P50 MH109429.
Glossary
- Epilepsy
Epilepsy is a neurological disorder characterized by recurrent and unpredictable seizures, during which brain activity and behavior becomes abnormal. Many individuals’ epilepsy can be treated by medication. However, if various types of medication fail, individuals who have a well-defined source may undergo surgery to determine the precise location of their seizures to be subsequently resected
- Intracranial electrical stimulation
By tracking behavioral changes resulting from the stimulation of electrodes implanted in the brains of epilepsy patients, clinicians can define the regions important for critical language and motor functions. This approach also enables researchers to infer causality by mapping brain structure with millimeter precision to behavioral, cognitive, and neural changes
- Mind-wandering
Mind-wandering is an umbrella term that encapsulates a variety of phenomenological experiences. The most widely used definition – task-unrelated thought – is narrowly characterized in contrast to an ongoing task. A broader definition is stimulus-independent thought, or internal attention, which characterize thoughts that are not focused on the external environment
- Multi-dimensional experience sampling
A form of thought sampling that asks participants to report on multiple dimensions of their ongoing phenomenological experience. In mind-wandering studies, this often includes the task-focus, detailedness, temporal focus, and valence of thoughts, among others
- Prefrontal cortex (PFC)
The PFC is situated in the frontal part of the cerebral cortex, and plays a central role in high-level cognitive functions. It can be divided into numerous subregions, associated with distinct functional roles. This review primarily discussed the ventromedial PFC as part of the DMN, and the dorsolateral PFC as part of the FPCN
- Rest
Resting state is often characterized by periods of time when participants are asked to simply do nothing and think about whatever comes to mind. Given the lack of task constraints and experimentally relevant external inputs during rest, attention is presumed and shown to be preoccupied by internally focused thoughts unrelated to their immediate environment
- Thought sampling probes
These are question prompts embedded during a task in the lab (or real life) that ask participants to describe their ongoing mental experience, which circumvents issues of retrospective bias. In mind-wandering studies, participants are typically asked whether they were paying attention to the task or not, among other phenomenological aspects of the mind-wandering experience
- Transcranial focused ultrasound stimulation
This is an emerging non-invasive brain stimulation technique that modulates neuronal activity by way of low intensity acoustic pressure waves. This neuromodulation modality is uniquely characterized by its superior focality (on the order of millimeters) and capacity to target deep brain circuits
- Transcranial magnetic stimulation
This technique involves inducing a pulsed magnetic field that creates brief electrical currents to pass in the brain to temporarily excite or inhibit a focal brain area (on the order of centimeters) below the magnetic coil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smallwood J and Schooler JW (2015) The Science of Mind Wandering : Empirically Navigating the Stream of Consciousness. Annu. Rev. Psychol 66, 487–518 [DOI] [PubMed] [Google Scholar]
- 2.Mooneyham BW and Schooler JW (2013) The costs and benefits of mind-wandering: A review. Can. J. Exp. Psychol 67, 11–18 [DOI] [PubMed] [Google Scholar]
- 3.Giambra LM (1993) The influence of aging on spontaneous shifts of attention from external stimuli to the contents of consciousness. Exp. Gerontol 28, 485–492 [DOI] [PubMed] [Google Scholar]
- 4.Antrobus JS (1968) Information theory and stimulus-independent thought. Br. J. Psychol 59, 423–430 [Google Scholar]
- 5.Stawarczyk D et al. (2011) Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychol. (Amst) 136, 370–381 [DOI] [PubMed] [Google Scholar]
- 6.Seli P et al. (2018) Mind-Wandering as a Natural Kind: A Family-Resemblances View. Trends Cogn. Sci 22, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoff K et al. (2016) Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci 17, 718–731 [DOI] [PubMed] [Google Scholar]
- 8.Fox KCR et al. (2015) The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621 [DOI] [PubMed] [Google Scholar]
- 9.Andrews-Hanna JR et al. (2010) Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 65, 550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christoff K et al. (2009) Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci 106, 8719–8724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason MF et al. (2007) Wandering minds: Stimulus-independent thought. Science (80-. ). 315, 393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucyi A and Davis KD (2014) Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 100, 471–480 [DOI] [PubMed] [Google Scholar]
- 13.Karapanagiotidis T et al. (2017) Tracking thoughts: Exploring the neural architecture of mental time travel during mind-wandering. Neuroimage 147, 272–281 [DOI] [PubMed] [Google Scholar]
- 14.Wang HT et al. (2018) Dimensions of Experience: Exploring the Heterogeneity of the Wandering Mind. Psychol. Sci 29, 56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smallwood J et al. (2013) Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage 69, 120–125 [DOI] [PubMed] [Google Scholar]
- 16.Andrews-Hanna JR et al. (2014) The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci 1316, 29–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niendam TA et al. (2012) Meta- analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci 12, 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeley W et al. (2007) Control, Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive. J. Neurosci 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spreng RN et al. (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smallwood J et al. (2012) Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 1428, 60–70 [DOI] [PubMed] [Google Scholar]
- 21.Kucyi A et al. (2020) Electrophysiological dynamics of antagonistic brain networks reflect attentional fluctuations. Nat. Commun 11, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormaz M et al. (2018) Default mode network can support the level of detail in experience during active task states. Proc. Natl. Acad. Sci 115, 9318–9323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaidya AR et al. (2019) Lesion Studies in Contemporary Neuroscience. Trends Cogn. Sci 23, 653–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scoville W and Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaieb L et al. (2019) New perspectives for the modulation of mind-wandering using transcranial electric brain stimulation. Neuroscience 409, 69–80 [DOI] [PubMed] [Google Scholar]
- 26.Filmer HL et al. (2014) Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 37, 742–753 [DOI] [PubMed] [Google Scholar]
- 27.Spreng R et al. (2013) Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci 25, doi: 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillis A (2014) Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain 137, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruff C et al. (2009) Combining TMS and fMRI: from “virtual lesions” to functional-network accounts of cognition. Cortex 45, 1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Callaghan C et al. (2015) Shaped by our thoughts - A new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn. 93, 1–10 [DOI] [PubMed] [Google Scholar]
- 31.Poerio GL et al. (2017) The role of the default mode network in component processes underlying the wandering mind. Soc. Cogn. Affect. Neurosci 12, 1047–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertossi E and Ciaramelli E (2016) Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Soc. Cogn. Affect. Neurosci 11, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philippi CL et al. (2021) Lesion network mapping demonstrates that mind-wandering is associated with the default mode network. J. Neurosci. Res 99, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boes AD et al. (2015) Network localization of neurological symptoms from focal brain lesions. Brain 138(10), 3061–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick C et al. (2018) Mind-wandering in people with hippocampal damage. J. Neurosci 38, 2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellamil M et al. (2016) Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage 136, 186–196 [DOI] [PubMed] [Google Scholar]
- 37.O’Callaghan C et al. (2019) Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl. Acad. Sci. U. S. A 116, 3316–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane MJ et al. (2017) For Whom the Mind Wanders, and When, Varies Across Laboratory and Daily-Life Settings. Psychol. Sci 28, 1271–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho NSP et al. (2019) Individual variation in patterns of task focused, and detailed, thought are uniquely associated within the architecture of the medial temporal lobe. Neuroimage 202, 116045. [DOI] [PubMed] [Google Scholar]
- 40.Kajimura S and Nomura M (2015) Decreasing propensity to mind-wander with transcranial direct current stimulation. Neuropsychologia 75, 533–537 [DOI] [PubMed] [Google Scholar]
- 41.Kajimura S et al. (2016) Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage 133, 21–30 [DOI] [PubMed] [Google Scholar]
- 42.Kajimura S et al. (2019) Challenge to unity: Relationship between hemispheric asymmetry of the default mode network and mind wandering. Cereb. Cortex 29, 2061–2071 [DOI] [PubMed] [Google Scholar]
- 43.Filmer HL et al. (2021) Stimulating task unrelated thoughts: tDCS of prefrontal and parietal cortices leads to polarity specific increases in mind wandering. Neuropsychologia 151, 107723. [DOI] [PubMed] [Google Scholar]
- 44.Coulborn S et al. (2020) Effect of tDCS Over the Right Inferior Parietal Lobule on Mind-Wandering Propensity. Front. Hum. Neurosci 14, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormick C et al. (2018) Comparing and Contrasting the Cognitive Effects of Hippocampal and Ventromedial Prefrontal Cortex Damage: A Review of Human Lesion Studies. Neuroscience 374, 295–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight R et al. (1995) Role of human prefrontal cortex in attention control. Adv. Neurol 66, 21–36 [PubMed] [Google Scholar]
- 47.Bertossi E et al. (2016) Ventromedial prefrontal cortex damage causes a pervasive impairment in episodic remembering and future thinking. Neuropsychologia 90, 12–24 [DOI] [PubMed] [Google Scholar]
- 48.Baird B et al. (2011) Back to the future: Autobiographical planning and the functionality of mind-wandering. Conscious. Cogn 20, 1604–1611 [DOI] [PubMed] [Google Scholar]
- 49.Chou T et al. (2020) Transcranial Direct Current Stimulation of Default Mode Network Parietal Nodes Decreases Negative Mind-Wandering About the Past. Cognit. Ther. Res 44, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho NSP et al. (2020) Facing up to why the wandering mind: Patterns of off-task laboratory thought are associated with stronger neural recruitment of right fusiform cortex while processing facial stimuli. Neuroimage 214, 116765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon ML et al. (2018) Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci 115, E1598–E1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kam JWY et al. (2018) Lateral prefrontal cortex lesion impairs regulation of internally and externally directed attention. Neuroimage 175, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kam JWY et al. (2021) Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. Proc. Natl. Acad. Sci 118, e2011796118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Compton RJ et al. (2019) The wandering mind oscillates: EEG alpha power is enhanced during moments of mind-wandering. Cogn. Affect. Behav. Neurosci 19, 1184–1191 [DOI] [PubMed] [Google Scholar]
- 55.Fogelson N et al. (2009) Prefrontal cortex is critical for contextual processing: Evidence from brain lesions. Brain 132, 3002–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axelrod V et al. (2015) Increasing propensity to mind-wander with transcranial direct current stimulation. Proc. Natl. Acad. Sci 112, 3314–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filmer HL et al. (2019) For a minute there, I lost myself … dosage dependent increases in mind wandering via prefrontal tDCS. Neuropsychologia 129, 379–384 [DOI] [PubMed] [Google Scholar]
- 58.Axelrod V et al. (2018) Transcranial stimulation of the frontal lobes increases propensity of mind-wandering without changing meta-awareness. Sci. Rep 8, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boayue N et al. (2019) Increasing propensity to mind-wander by transcranial direct current stimulation? A registered report. Eur. J. Neurosci 51, 755–780 [DOI] [PubMed] [Google Scholar]
- 60.Csifcsák G et al. (2018) Effects of transcranial direct current stimulation for treating depression: A modeling study. J. Affect. Disord 234, 164–173. 10.1016/j.jad.2018.02.077 [DOI] [PubMed] [Google Scholar]
- 61.Boayue NM et al. (2021) The interplay between executive control, behavioural variability and mind wandering: Insights from a high-definition transcranial direct-current stimulation study. Eur. J. Neurosci 53, 1498–1516 [DOI] [PubMed] [Google Scholar]
- 62.Turnbull A et al. (2019) Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kam JWY et al. (2021) Top–Down Attentional Modulation in Human Frontal Cortex: Differential Engagement during External and Internal Attention. Cereb. Cortex 31, 873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smallwood J (2013) Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychol. Bull 139, 519–535 [DOI] [PubMed] [Google Scholar]
- 65.Kam JWY et al. (2019) Default network and frontoparietal control network theta connectivity supports internal attention. Nat. Hum. Behav 3, [DOI] [PubMed] [Google Scholar]
- 66.Baird B et al. (2014) The decoupled mind: mind wandering disrupts cortical phase-locking to perceptual events. J. Cogn. Neurosci 26, 2596–2607 [DOI] [PubMed] [Google Scholar]
- 67.Foster BL et al. (2013) Human Retrosplenial Cortex Displays Transient Theta Phase Locking with Medial Temporal Cortex Prior to Activation during Autobiographical Memory Retrieval. J. Neurosci 33, 10439–10446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fellrath J et al. (2016) Theta-band functional connectivity in the dorsal fronto-parietal network predicts goal-directed attention. Neuropsychologia 92, 20–30 [DOI] [PubMed] [Google Scholar]
- 69.Fox KCR et al. (2018) Intracranial Electrophysiology of the Human Default Network. Trends Cogn. Sci 22, 307–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dastjerdi M et al. (2011) Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc. Natl. Acad. Sci. U. S. A 108, 3023–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canolty RT and Knight RT (2010) The functional roles of cross-frequency coupling. Trends Cogn. Sci 14, 506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daitch AL and Parvizi J (2018) Spatial and temporal heterogeneity of neural responses in human posteromedial cortex. Proc. Natl. Acad. Sci. U. S. A 115, 4785–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seli P et al. (2016) Mind-Wandering With and Without Intention. Trends Cogn. Sci 20, 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smallwood J et al. (2016) Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One 11, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKeown B et al. (2021) The impact of social isolation and changes in work patterns on ongoing thought during the first COVID-19 lockdown in the United Kingdom. Proc. Natl. Acad. Sci. U. S. A 118, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schooler JW et al. (2011) Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn. Sci 15, 319–326 [DOI] [PubMed] [Google Scholar]
- 77.Kam JWY et al. (2011) Slow fluctuations in attentional control of sensory cortex. J. Cogn. Neurosci 23, 460–70 [DOI] [PubMed] [Google Scholar]
- 78.Smallwood J et al. (2008) Going AWOL in the brain: mind wandering reduces cortical analysis of external events. J. Cogn. Neurosci 20, 458–69 [DOI] [PubMed] [Google Scholar]
- 79.Kam JWY et al. (2013) Mind wandering and the adaptive control of attentional resources. J. Cogn. Neurosci 25, [DOI] [PubMed] [Google Scholar]
- 80.De Witte S et al. (2018) Left prefrontal neuronavigated electrode localization in tDCS: 10–20 EEG system versus MRI-guided neuronavigation. Psychiatry Res Neuroimaging 274, 1–6. 10.1016/j.pscychresns.2018.02 [DOI] [PubMed] [Google Scholar]
- 81.Opitz A et al. (2015) Determinants of the electric field during transcranial direct current stimulation. Neuroimage 109, 140–150 [DOI] [PubMed] [Google Scholar]
- 82.Hallett M (2000) Transcranial magnetic stimulation and the human brain. Nature 406, 147–50. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 83.Blackmore J et al. (2019) Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol 45, 1509–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox KCR et al. (2020) Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat. Hum. Behav 4, 1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ho NSP et al. (2020) Missing the forest because of the trees: Slower alternations during binocular rivalry are associated with lower levels of visual detail during ongoing thought. Neurosci. Conscious 2020, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Helfrich R et al. (2014) Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol 24, 333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 87.Herrmann CS et al. (2013) Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margulies DS et al. (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci 113, 12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szczepanski SM and Knight RT (2014) Insights into Human Behavior from Lesions to the Prefrontal Cortex, 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirman D et al. (2015) Neural organization of spoken language revealed by lesion–symptom mapping. Nat. Commun 6, 6762 10.1038/ncomms7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossini L et al. Seizure activity per se does not induce tissue damage markers in human neocortical focal epilepsy. Ann. Neurol 82, 331–341 [DOI] [PubMed] [Google Scholar]
- 92.Parvizi J and Kastner S (2018) Human Intracranial EEG: Promises and Limitations. Nat. Neurosci 21, 474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson EL et al. (2020) Insights into human cognition from intracranial EEG: A review of audition, memory, internal cognition, and causality. J. Neural Eng 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Logothetis NK et al. (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–7 [DOI] [PubMed] [Google Scholar]
- 95.Rich EL and Wallis JD (2017) Spatiotemporal dynamics of information encoding revealed in orbitofrontal high-gamma. Nat. Commun 8, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson EL et al. (2020) Insights into human cognition from intracranial EEG: A review of audition, memory, internal cognition, and causality. J. Neural Eng 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spreng RN et al. (2009) The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- 98.Smallwood J et al. (2021) The default mode network in cognition: a topographical perspective. Nat. Rev. Neurosci 22, 503–513 [DOI] [PubMed] [Google Scholar]
- 99.Konishi M et al. (2015) Shaped by the past: The default mode network supports cognition that is independent of immediate perceptual input. PLoS One 10, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murphy C et al. (2018) Distant from input: Evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage 171, 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murphy C et al. (2019) Modes of operation: A topographic neural gradient supporting stimulus dependent and independent cognition. Neuroimage 186, 487–496 [DOI] [PubMed] [Google Scholar]
- 102.Vatansever D et al. (2015) Default Mode Dynamics for Global Functional Integration. J. Neurosci 35, 15254–15262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vatansever D et al. (2017) Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. U. S. A 114, 12821–12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dodds CM et al. (2011) Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb. Cortex 21, 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turnbull A et al. (2019) The ebb and flow of attention: Between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. Neuroimage 185, 286–299 [DOI] [PubMed] [Google Scholar]
- 106.Wiethoff S et al. (2014) Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 7, 468–475 [DOI] [PubMed] [Google Scholar]
- 107.Medina J and Cason S (2017) No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 94, 131–141 [DOI] [PubMed] [Google Scholar]
- 108.Saturnino G et al. (2015) On the importance of electrode parameters for shaping electric field patterns generated by tDCS. Neuroimage 120, 25–35 [DOI] [PubMed] [Google Scholar]
- 109.Caulfield K et al. (2020) Transcranial electrical stimulation motor threshold can estimate individualized tDCS dosage from reverse-calculation electric-field modeling. Brain Stimul. 13, 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saturnino G et al. (2019) Accessibility of cortical regions to focal TES: Dependence on spatial position, safety, and practical constraints. Neuroimage 203, 116183. [DOI] [PubMed] [Google Scholar]
- 111.Windhoff M et al. (2013) Electric field calculations in brain stimulation based on finite elements: an optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp 34, 923–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szucs D and Ioannidis J (2017) Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol. 5, e2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parkin B et al. (2015) Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron 87, 932–945 [DOI] [PubMed] [Google Scholar]
- 114.Bestmann S et al. (2015) Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cogn. Sci 19, 13–20 [DOI] [PubMed] [Google Scholar]
- 115.Button K et al. (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14, 365–376 [DOI] [PubMed] [Google Scholar]
- 116.Nosek B et al. (2018) The preregistration revolution. Proc. Natl. Acad. Sci 115, 2600–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turi Z et al. (2019) Blinding is compromised for transcranial direct current stimulation at 1 mA for 20 min in young healthy adults. Eur. J. Neurosci 50, 3261–3268 [DOI] [PubMed] [Google Scholar]
- 118.Turi Z et al. (2018) Evidence for Cognitive Placebo and Nocebo Effects in Healthy Individuals. Sci. Rep 8, 17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turi Z et al. (2017) Placebo Intervention Enhances Reward Learning in Healthy Individuals. Sci. Rep 7, 41028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reckow J et al. (2018) Tolerability and blinding of 4×1 high-definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimul. 11, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Filmer H et al. (2020) Modulating brain activity and behaviour with tDCS: Rumours of its death have been greatly exaggerated. Cortex 123, 141–151 [DOI] [PubMed] [Google Scholar]
- 122.Christoff K et al. (2018) Mind-Wandering as a Scientific Concept: Cutting through the Definitional Haze. Trends Cogn. Sci 22, 957–959 [DOI] [PubMed] [Google Scholar]
- 123.Agnoli S et al. (2018) Exploring the link between mind wandering, mindfulness, and creativity: A multidimensional approach. Creat. Res. J 30, 41–53 [Google Scholar]
- 124.Carciofo R and Jiang P (2021) Deliberate and spontaneous mind wandering in Chinese students: Associations with mindfulness, affect, personality, and life satisfaction. Pers. Individ. Dif 180, 110982 [Google Scholar]
- 125.Seli P et al. (2015) On the relation of mind wandering and ADHD symptomatology. Psychon. Bull. Rev 22, 629–636 [DOI] [PubMed] [Google Scholar]
- 126.Andrews-Hanna JR et al. (2020) Dynamic Regulation of Internal Experience,
- 127.Irving ZC (2016) Mind-wandering is unguided attention: Accounting for the “purposeful” wanderer. Philos. Stud 173, 547–571 [Google Scholar]
- 128.Mills C et al. (2018) Is an off-task mind a freely-moving mind? Examining the relationship between different dimensions of thought. Conscious. Cogn 58, 20–33 [DOI] [PubMed] [Google Scholar]
- 129.Mills C et al. (2021) How task-unrelated and freely moving thought relate to affect: Evidence for dissociable patterns in everyday life. Emotion DOI: 10.1037/emo0000849 [DOI] [PubMed] [Google Scholar]
- 130.Kucyi A (2018) Just a thought: How mind-wandering is represented in dynamic brain connectivity. Neuroimage 180, 505–512 [DOI] [PubMed] [Google Scholar]
- 131.Mooneyham B et al. (2017) States of Mind: Characterizing the Neural Bases of Focus and Mind-wandering through Dynamic Functional Connectivity. J. Cogn. Neurosci 29, 495–506 [DOI] [PubMed] [Google Scholar]


