Abstract
DNA adducts are central in the mechanism of carcinogenesis by genotoxic agents. We compared levels of a DNA adduct of acrolein, a genotoxic carcinogen found in e-cigarette vapor, in oral cell DNA of e-cigarette users and non-users of any tobacco or nicotine product. e-Cigarette users and non-users visited our clinic once monthly for 6 months, and oral brushings and urine samples were collected. For this study, we analyzed oral cell DNA adducts from three monthly visits in e-cigarette users and non-users as confirmed by urinary cyanoethyl mercapturic acid and total nicotine equivalents. DNA was isolated from the oral brushings and analyzed by a validated liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry method for the acrolein DNA adduct 8R/S-3-(2’-deoxyribos-1’-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10-(3H)-one (γ-OH-Acr-dGuo). The median value of this DNA adduct in the e-cigarette users was 179 fmol/µmol dGuo (range 5.0 - 793 fmol/µmol dGuo) while that for non-users was 21.0 fmol/µmol dGuo (range 5.0 - 539 fmol/µmol dGuo), P = 0.001. These results demonstrate for the first time that e-cigarette users have elevated levels of a carcinogen–DNA adduct in their oral cells.
DNA adducts of acrolein were significantly greater in oral cells of 20 e-cigarette users sampled monthly for 3 months than in 20 non-users. This is the first identification of a carcinogen–DNA adduct in any tissue of an e-cigarette user.
Graphical Abstract
Introduction
e-Cigarettes continue to grow in popularity. It has been estimated that there were 68 million adult e-cigarette users in the world in 2020 (1). In the United States in 2021, 2.06 million middle and high school students used e-cigarettes in the past 30 days, while in 2019, 4.5–4.8% of adults were current e-cigarette users (2–4). The public health consequences of e-cigarette use are still unclear (5). e-Cigarettes lack tobacco leaf and combustion, resulting in a toxicity profile which is less hazardous than that of combustible tobacco products. However, e-cigarette vapor does contain some toxicants as well as nicotine, a highly addictive substance that could lead to continued use or transition to cigarette smoking. The study reported here focuses on acrolein, a toxicant and carcinogen present in e-cigarette vapor.
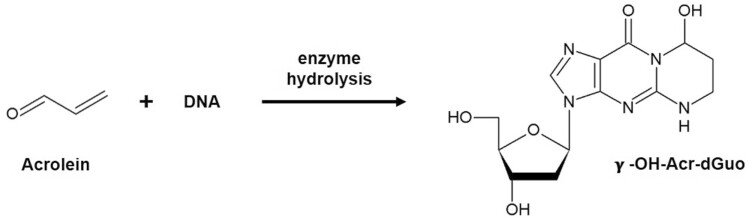
Acrolein is considered ‘probably carcinogenic to humans’ (Group 2A) by the International Agency for Research on Cancer (6). Exposure to acrolein by inhalation significantly increased the incidence of malignant lymphoma in female mice and caused rare rhabdomyoma and squamous cell carcinoma of the nasal cavity in female rats (6). Acrolein readily reacts with DNA to produce well characterized adducts among which (8R/S)-3-(2ʹ-deoxyribos-1ʹ-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)-one (γ-OH-Acr-dGuo, Figure 1) is the most prevalent and extensively studied.
Figure 1.
Reaction of acrolein with DNA to produce the major adduct γ-OH-Acr-dGuo.
γ-OH-Acr-dGuo has some mutagenic properties and has been detected in previous studies in oral cells and tissue, with levels higher in smokers than in nonsmokers (7–10). Our recent study using a validated liquid chromatography–nanoelectrospray ionization-high resolution tandem mass spectrometry (LC–NSI-HRMS/MS) method demonstrated that levels of γ-OH-Acr-dGuo were 27 times higher in oral cells of cigarette smokers than nonsmokers (11). Thus, γ-OH-Acr-dGuo in oral cell DNA has emerged as a highly specific biomarker of acrolein exposure and DNA damage. We applied this methodology to quantify levels of this DNA adduct in oral cells of e-cigarette users and non-users of any nicotine-containing product.
There is no doubt that acrolein is present in most e-cigarette vapor, and multiple recent studies have evaluated its levels. Uchiyama et al. reported levels of acrolein up to 150 µg/10 puffs in nine brands of Japanese e-cigarettes (12). Ogunwale et al. reported levels of acrolein from 0.02 to 5.8 µg/10 puffs from different e-cigarette devices and liquids (13). Farsalinos and Voudris found emitted levels of acrolein ranging from 0.9 to 19.4 µg/g e-liquids with different atomizers and power settings (14) and also reported acrolein emission levels of 8.6 and 11.7 µg/g e-liquid at 9.0 and 13.5W power settings (15). Bitzer et al. did not detect acrolein in aerosols generated from a standardized research e-cigarette as well as other closed-system, breath activated, commercially available e-cigarettes (16). Urena et al. quantified vapor levels of acrolein ranging from 2 to 7 µg/g of liquid consumed from four differently flavored e-cigarette liquids (17). Gillman et al. found mean levels of acrolein ranging from 0.81 to 1.22 µg/g e-liquid consumed (18). Zelinkova and Wenzl confirmed the frequently reported observation that acrolein levels depend on the applied power, and that exceeding the recommended power range resulted in large increases in acrolein production (19). Uchiyama et al. also observed exponential increases in acrolein production at high power levels (20). Belushkin et al. reported acrolein levels in a broad range of e-cigarette device emissions under a wide variety of conditions (21). Li et al. studied the impact of e-liquid composition, coil temperature, and puff topography on the aerosol chemistry of e-cigarettes and demonstrated that vegetable glycerin is a major precursor to acrolein (22). Zhou et al. reported increased levels of acrolein with increasing voltage and the effects of voltage and e-liquid on the deposition of nicotine in the oral region of a human oral-trachea cast model (23). Collectively, these results demonstrate that use of e-cigarettes exposes the oral cavity to acrolein (24).
Several biomarker studies have evaluated levels of 3-hydroxypropyl mercapturic acid (3-HPMA), an established biomarker of acrolein exposure, in the urine of e-cigarette users versus non-users of any tobacco or nicotine-containing product. In general, mixed results have been obtained with some studies showing increases in 3-HPMA in e-cigarette users versus non-users while others found no difference, as recently reviewed by Hiler et al. (25). Some specific results were as follows. Shahab et al. reported similar levels of 3-HPMA in e-cigarette-only users and nicotine replacement therapy-only users, both significantly lower than in cigarette smokers (26). Lorkiewicz et al. did not detect a difference in 3-HPMA levels between e-cigarette users and non-users (27). Dawkins et al. examined the effects of different power settings and nicotine concentrations on 3-HPMA excretion in e-cigarette users and found no significant effects (28). Rubinstein et al. found significantly higher levels of 3-HPMA in the urine of adolescent e-cigarette users versus non-user controls (29). Keith et al. and De Jesus et al. reported significantly higher levels of 3-HPMA in e-cigarette users versus non-users of tobacco (30,31). The mixed results of these studies with respect to 3-HPMA levels probably result from the multiple sources of acrolein exposure including use of cooking oils at high temperatures, exposure to vehicle exhaust and other sources of polluted air, intake from the diet, and endogenous processes, which may not have been fully controlled (6). On the other hand, multiple studies, as exemplified by the Population Assessment of Tobacco and Health (PATH) and National Health and Nutrition Examination Survey (NHANES), clearly and consistently demonstrate that 3-HPMA levels in the urine of cigarette smokers are 3–4 times higher than in nonsmokers (32,33). Cigarette smoking entails a far greater exposure to acrolein than does e-cigarette use.
Our data demonstrating much higher levels of γ-OH-Acr-dGuo in oral cell DNA of smokers compared to nonsmokers suggested that this DNA adduct could be a highly sensitive and selective biomarker of acrolein exposure with possible applicability to e-cigarette users in whom acrolein exposure would be considerably lower than in cigarette smokers (11). Therefore, we have performed a clinical study in which samples were collected from e-cigarette users, non-users of e-cigarettes or any tobacco product, and cigarette smokers at monthly intervals.
Materials and methods
Chemicals
Standards and internal standards for the analysis of γ-OH-Acr-dGuo in oral cell DNA were obtained as described, as were other reagents and chemicals for the DNA adduct analysis (11).
Clinical study design and sample collection
This study was approved by the University of Minnesota Institutional Review Board. Buccal cell brushings and urine samples were collected and analyzed from 8 smokers, 20 e-cigarette users, and 20 nonsmokers. All participants were recruited through the Tobacco Research Programs, University of Minnesota. Inclusion criteria were as follows: age >18, in stable and good physical and mental health, excellent oral health, no current infection as determined by medical history and investigator assessment, and no current use of medicinal nicotine products or any tobacco products other than those required for the specified groups. Subjects avoided the use of marijuana for at least 2 days before study visits and had no current use of antibiotics or anti-inflammatory agents. They had a body mass index ≤40 kg/m2, consumed less than 21 alcoholic drinks per week, and were not pregnant, nursing or planning on becoming pregnant while enrolled in the study. e-Cigarette users were required to have exclusively used e-cigarettes for at least 3 months and at least 4 days per week and have exhaled CO <6 ppm. Smokers were required to have a stable smoking pattern of at least five cigarettes per day for a minimum of four days per week for the past year and have exhaled CO >8 ppm. Neither e-cigarette users nor smokers intended to quit using these products in the next 6 months. Nonsmokers were required to have smoked no more than 100 cigarettes in their lifetime and have exhaled CO <6 ppm, and not to have used any other tobacco product including e-cigarettes. Average urinary total nicotine equivalents (TNE, the molar sum of nicotine, cotinine, and 3’-hydroxycotinine and their glucuronides) and cyanoethyl mercapturic acid (CEMA) were quantified to confirm e-cigarette use (TNE > 3 nmol/ml, range 1.5–388 nmol/ml and CEMA < 27 pmol/ml) or nonsmoking, non-user status (TNE < 0.1 nmol/ml and CEMA < 27 pmol/ml) (33,34). One e-cigarette user had average TNE 0.18 nmol/ml for the monthly visits and another had CEMA 206 pmol/ml at one visit; exclusion of these subjects from the statistical analysis presented below had no effect on the conclusions. Cigarette smokers had TNE >3 nmol/ml and CEMA >27 pmol/ml (Supplementary Table 1).
Participants visited the clinic once monthly for 6 consecutive months. For this study, we analyzed γ-OH-Acr-dGuo in oral cells from 3 monthly visits because of the high cost of the LC–NSI-HRMS/MS methodology employed. The participants were asked to visit in the morning before eating breakfast or after at least a 4-h fast, and not to have ingested alcohol, or used cigarettes or any other tobacco product or e-cigarettes immediately before the clinic visit. At each visit, participants provided a spot urine sample and buccal brushings. The urine sample was collected in a 100 ml sterile specimen cup, aliquoted, and immediately frozen at −20°C. For the oral samples collection, the subjects were asked to brush their teeth 15 min before giving a buccal cell sample, which was collected by brushing the oral mucosa inside one cheek with a clean ‘Cytobrush’ and swirling the brush for 20 s in a sterile polypropylene centrifuge tube prefilled with 5 ml of saline to transfer the collected buccal cells from the brush into the liquid. The process was then repeated on the other cheek with a new brush and a new tube. Buccal cells rather than mouthwash samples were collected in this study because they potentially provide a cleaner source of exposed tissue DNA. After the collection, the samples were centrifuged at 2500g for 15 min at 4°C to pellet cells; the pellets were washed twice with 1 ml of phosphate buffered saline (pH 7.4), pipetted into 2 ml DNA Lo-bind tubes, and stored at −20°C until DNA isolation and analysis. Buccal cell samples were collected at the clinic visit both prior to e-cigarette or cigarette use and immediately after having vaped or smoked using their own e-cigarette devices and e-liquids at the clinic visit. Specifically, at each clinic visit, e-cigarette users and cigarette smokers were invited to use their products in the smoking lab. The participants were instructed to take a puff of their cigarette or e-cigarette every 30 s, for a total of 10 puffs. Upon completion of the 10th puff, another oral brushing sample was immediately collected.
Samples were selected for this study based on availability, e.g. not having been used in other studies of the larger project. Fourteen of the 20 subject samples were collected on consecutive months, e.g. months 2, 3 and 4 of the study, while the other 6 were not collected on consecutive months, e.g. months 2, 3 and 5 of the study. There was no significant difference in the results between these two groups. Samples collected from the right and left cheek were combined for DNA extraction for nonsmokers, smokers and for the analysis of e-cigarette users when comparing levels before and after e-cigarette exposure. The remaining samples from e-cigarette users were analyzed by combining the samples from the right cheek collected before and after the exposure.
DNA isolation from buccal cells, hydrolysis and sample purification, and analysis of γ-OH-Acr-dGuo by LC-NSI-HRMS/MS
These were carried out as described previously, using [13C1015N5]Acr-dGuo as internal standard (11). Samples were dissolved in 20 μl of H2O for LC-NSI-HRMS/MS analysis. The analysis was carried out on an Orbitrap Fusion Lumos instrument (Thermo Scientific, San Jose, CA) interfaced with a UPLC system (Ultimate 3000 RSLCnano UPLC, Thermo Scientific, Waltham, MA) using nanoelectrospray ionization (NSI). The UPLC was equipped with a 5 μl loop and the separation was performed using a capillary column (75 μm ID, 20 cm length, 15 μm orifice) prepared by hand packing a commercially available fused-silica emitter (New Objective, Woburn MA) with Luna C18 5µ bonded separation media (Phenomenex, Torrance, CA). The mobile phase consisted of 2 mM NH4OAc and CH3CN. The gradient started at 1% CH3CN for 6 min at a flow rate of 0.9 μl/min, increased to 13% CH3CN in 20 min at a flow rate of 0.3 μl/min and then to 30% CH3CN in 1 min, holding at this composition for 4 min. The gradient was then returned to 1% CH3CN in 1 min and the system was re-equilibrated at this mobile phase composition for 1 min at a flow rate of 0.9 μl/min before the next injection. The source temperature was set at 300°C and the spray voltage was static at 2200 V. The maximum injection time was 300 ms and the normalized automatic gain control (AGC) was set at 1000%. The precursor ions were isolated by the quadrupole with an isolation width of m/z 1.5 and fragmented by higher energy collisional dissociation (HCD) at 20% and the fragment ions were detected by the Orbitrap detector at a resolution of 60 000. The ion transitions monitored with accurate mass extracted were: m/z 324.1 → m/z 208.0829 ([M+H]+ → [BH]+) and m/z 324.1 → m/z 164.0542 for γ-OH-Acr-dGuo, and m/z 339.1 → m/z 218.0849 and m/z 339.1 → m/z 174.0560 for [13C1015N5]γ-OH-Acr-dGuo. To monitor the possible artifactual formation of the Acr-dGuo adducts in the samples, [15N]DNA was added to the samples before the enzymatic hydrolysis, and the following ion transitions were included in the method: m/z 329.1 → m/z 213.0681 for [15N5]γ-OH-Acr-dGuo. Calibration curves were prepared using standard solutions of the adduct in increasing concentrations (5–500 amol/μl) added to a constant concentration of the isotopically labeled internal standard (200 amol/μl).
Quantitation of dGuo
Quantitation of dGuo was similar as that reported previously for the abasic site determination in oral cells (35), and was performed using a TSQ Vantage triple-quadrupole mass spectrometer (Thermo Scientific, Waltham, MA) interfaced with a Dionex Ultimate 3000 UHPLC system (NCS-3500RS pump and WPS-3000PL autosampler). Analysis was performed on a Luna C18 column (5 μm, 150 × 0.5 mm, Phenomenex) at a flow rate of 10 µl/min at room temperature. Sample injection volume was 4 μl. The mobile phase consisted of 5 mM NH4OAc and CH3CN with a linear gradient from 3 to 40% CH3CN over 20 min, followed by ramping to 90% CH3CN within 1 min and holding at this composition for 4 min. The gradient was then returned to 3% CH3CN in 1 min followed by 10 min re-equilibration. The ESI source was operated in positive ion mode, monitoring m/z 268.1 [M+H]+ → m/z 152.1 [C5H6N5O]+ for dGuo and the corresponding transition m/z 283.1 → 162.1 for [13C1015N5]dGuo. The collision gas was Ar at 1 mTorr with a collision energy of 15 eV. The S-lense was set at 85. The quadrupoles Q1 and Q2 were both operated at a resolution of 0.7 Da.
Statistical analysis
For the samples from the first visit, in which oral cells were collected before and after using an e-cigarette, the values for γ-OH-Acr-dGuo were averaged since there was no statistical difference between them based on the non-parametric Wilcoxon signed rank test for paired samples. Then the amounts of γ-OH-Acr-dGuo for each subject at the 3 time points were averaged, with not detected (ND) values being replaced by 5 fmol/ml, which was half the detection limit. The medians were calculated, and the non-parametric Wilcoxon rank sum test was used for statistical comparison of γ-OH-Acr-dGuo in e-cigarette users versus non-users of e-cigarettes or any tobacco product. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and P-values <0.05 were considered statistically significant.
Results
Analysis of γ-OH-Acr-dGuo in oral cell DNA
Our LC-NSI-HRMS/MS method can quantify γ-OH-Acr-dGuo as well as 1,N6-etheno-dAdo and 3,N4-etheno-dCyd (11). However, the latter two DNA adducts and α-OH-Acr-dGuo were not detected in most samples. In the first round of analyses of DNA adducts in buccal cells of 20 e-cigarette users, we collected cells both before and immediately after e-cigarette use in the clinic. As shown in Table 1, there was no significant difference between the levels of γ-OH-Acr-dGuo in buccal cell DNA collected prior to and after e-cigarette use in the clinic (P = 0.09). Therefore in the two subsequent rounds of analyses of monthly buccal cell samples collected from these 20 subjects, we combined the “prior and post use” buccal cell samples, thus providing more DNA for a more sensitive analysis (an average of 400 ng of DNA were used for each sample analysis). Characteristics of the subjects and their e-cigarette brands are presented in Supplementary Table 1.
Table 1.
Levels of γ-OH-Acr-dGuo in the DNA of oral cells collected from e-cigarette users who made 3 monthly visits to the clinic
| Participant number | Visit 1 | Visit 2 | Visit 3 | |
|---|---|---|---|---|
| Pre-exposure | Post-exposure | Pre-post exposure samples combined | Pre-post exposure samples combined | |
| γ-OH-Acr-dGuo (fmol/µmol dGuo) |
γ-OH-Acr-dGuo
(fmol/µmol dGuo) |
γ-OH-Acr-dGuo (fmol/µmol dGuo) | γ-OH-Acr-dGuo (fmol/µmol dGuo) | |
| 1 | 336 | 454 | 529 | 165 |
| 2 | 223 | 205 | 132 | 320 |
| 3 | 85 | 1851 | 162 | 226 |
| 4 | 52 | 91 | 471 | 281 |
| 5 | ND | 409 | ND | 172 |
| 6 | ND | 403 | ND | 380 |
| 7 | 137 | 1256 | 105 | 127 |
| 8 | 95 | 197 | 224 | 173 |
| 9 | 284 | 575 | 158 | 375 |
| 10 | ND | 72 | 144 | 348 |
| 11 | ND | ND | ND | ND |
| 12 | ND | ND | ND | ND |
| 13 | 67 | 42 | ND | 261 |
| 14 | 50 | ND | ND | 149 |
| 15 | ND | 224 | 86 | 654 |
| 16 | 41 | 104 | 83 | 274 |
| 17 | 67 | 48 | ND | 317 |
| 18 | 839 | ND | 527 | 1430 |
| 19 | ND | 56 | ND | 150 |
| 20 | 211 | ND | ND | 246 |
| Median (range) | 59.5 (5/839) | 97.5 (5/1851) | 84.5 (5/529) | 254 (5/1430) |
At Visit 1, samples were collected before and after e-cigarette use, as described in Materials and methods. At subsequent visits, only a single sample was collected because there was no significant difference in DNA adduct levels between samples collected before and after product use. Samples collected from the right and left cheek were combined for DNA extraction for nonsmokers and for the analysis of e-cigarette users when comparing levels before and after e-cigarette exposure. The remaining samples from e-cigarette users were analyzed by combining the samples from the right cheek collected before and after the exposure. Further subject characteristics are presented in Supplementary Table 1.
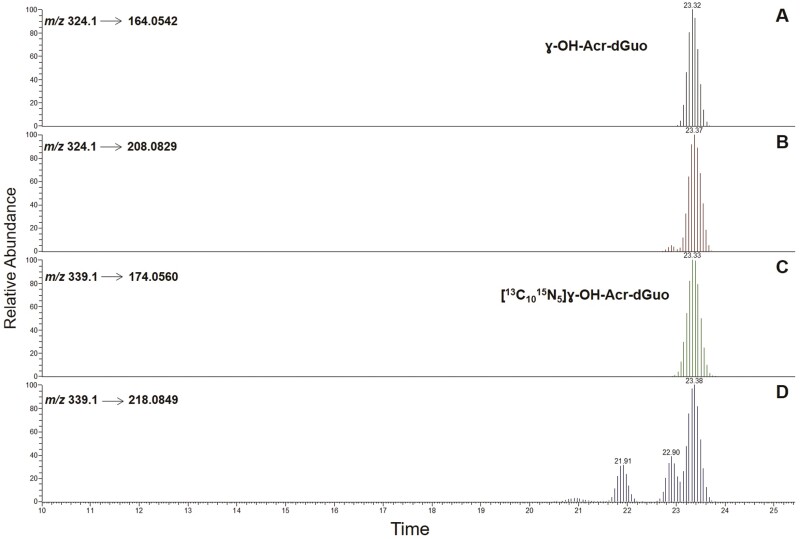
A typical LC-NSI-HRMS/MS chromatogram obtained upon analysis of buccal cell DNA from an e-cigarette user is illustrated in Figure 2. The results of the analyses are summarized in Table 1 (e-cigarette users) and Table 2 (non-users of e-cigarettes or any tobacco product).
Figure 2.
LC–NSI-HRMS/MS chromatograms obtained upon analysis of human oral cell DNA obtained from an e-cigarette user. SRM was carried out monitoring the transitions: m/z 324.1 → 164.0542 (A) and m/z 324.1 → 208.0829 (B) for γ-OH-Acr-dGuo, and m/z 339.1 → 174.0560 (C) and m/z 339.1 → 218.0849 (D) for [13C1015N5]γ-OH-Acr-dGuo. Panel D includes the 2 peaks corresponding to [13C1015N5]α-OH-Acr-dGuo, that were included in the isotopically labeled OH-Acr-dGuo internal standard mix.
Table 2.
Levels of γ-OH-Acr-dGuo in the DNA of oral cells from non-users of e-cigarettes or any tobacco products who made 3 monthly visits to the clinic
| Participant number | Visit 1 | Visit 2 | Visit 3 |
|---|---|---|---|
| γ-OH-Acr-dGuo (fmol/µmol) | γ-OH-Acr-dGuo (fmol/µmol) | γ-OH-Acr-dGuo (fmol/µmol) | |
| 1 | 65 | ND | ND |
| 2 | 33 | 124 | ND |
| 3 | ND | ND | ND |
| 4 | 72 | ND | 226 |
| 5 | 47 | ND | ND |
| 6 | 567 | 128 | 128 |
| 7 | ND | ND | ND |
| 8 | ND | ND | ND |
| 9 | 40 | ND | 1571 |
| 10 | 24 | 50 | 37 |
| 11 | ND | ND | ND |
| 12 | 259 | 38 | 68 |
| 13 | ND | ND | ND |
| 14 | ND | ND | ND |
| 15 | 55 | ND | ND |
| 16 | 1006 | 85 | 123 |
| 17 | 124 | ND | ND |
| 18 | 51 | ND | ND |
| 19 | ND | ND | ND |
| 20 | ND | ND | ND |
| Median(range) | 36.5 (5/1006) | 5.0 (5-128) | 5.0 (5/1571) |
Product use was confirmed by urinary TNE and CEMA as described in Materials and methods. CEMA and TNE data are presented in Supplementary Table 1.
The average median value and range of γ-OH-Acr-dGuo for e-cigarette users was 178.8 fmol/µmol dGuo (range 5.0–793.1) while that for non-users was 21.0 fmol/µmol dGuo (range 5.0–538.7), a significant 9-fold difference (P = 0.001). There was no significant difference between median levels of samples collected from subjects in consecutive months (N = 14 subjects) versus those collected in nonconsecutive months (N = 6), P = 0.364. We also determined γ-OH-Acr-dGuo levels in buccal cells of 8 cigarette smokers participating in this study, who provided samples during the first visit. The median value was 446 fmol/µmol dGuo (range 158–5830), significantly higher than that in e-cigarette users (P < 0.001) and essentially consistent with the results of our previous study in which we observed a mean value ± S.D. of 259 ± 540 adducts/109 nucleotides in buccal cell brushings from 19 cigarette smokers (11). Therefore, cigarette smokers’ DNA samples were not further analyzed in this study.
We also investigated the relationship of gender and increasing age to levels of γ-OH-Acr-dGuo in DNA. Gender had no significant effect but we did observe a correlation of adduct levels with age in both e-cigarette users (non-parametric Spearman correlation coefficient, 0.66 (P = 0.002), and non-users, 0.47 (P = 0.036). There was no significant interaction between age and study group (P = 0.234).
Analysis of bacterial DNA
Oral cells could contain bacterial DNA. Using a primer set reported by Maeda et al. (36) which has been used to detect bacteria in dental plaque via qPCR, we analyzed total bacterial DNA by amplification of 16S rDNA in Escherichia coli, genome position 1048–1194, using qPCR for buccal cell samples from five e-cigarette users and five non-users. By assuming that the 16s rDNA gene copy is the same across all bacteria, the target region of a variety of which was shown to be very conservative (37), we estimated the range of contributions of bacterial DNA to the analyzed DNA was 0.002–2.7% for e-cigarette users and 0.006–4.4% for non-users. The bacterial content of oral cells in our recently published paper also fell into this range (35).
Discussion
This is the first study to demonstrate DNA adduct formation in any tissue of e-cigarette users. The use of a validated LC-NSI-HRMS/MS method for its analysis leaves little doubt regarding the amounts or identity of γ-OH-Acr-dGuo in buccal brushing DNA of e-cigarette users. Our results show that its levels were 9 times greater in e-cigarette users than in non-users of e-cigarettes or any tobacco product. DNA adducts are central to the carcinogenic process because they can cause miscoding in DNA resulting in activation of oncogenes and inactivation of tumor suppressor genes, processes that are well established in the etiology of cancers occurring in cigarette smokers and nonsmokers (38,39). Site-specific mutagenesis studies of γ-OH-Acr-dGuo in E. coli demonstrate that it can cause low frequencies of G–T transversion mutations (10). While the potential consequences of γ-OH-Acr-dGuo with respect to oral pathologies in e-cigarette users remain to be determined, our results present a warning signal. Increased DNA adduct formation from acrolein in the oral cavity could suggest possible elevated cancer risk. We note that two cases of oral cancer in chronic exclusive e-cigarette users with no other apparent risk factors have been reported (40).
Exposure to acrolein from cigarette smoking is greater than from e-cigarette use, but it is difficult to compare them because of the many different conditions used in e-cigarette studies. In one recent study, levels of acrolein in mainstream cigarette smoke under ISO conditions ranged from 30.8 to 82.6 µg per cigarette (41). Farsalinos and Gillman estimated that acrolein levels from consuming 5 g of e-liquid with a puffing regime of 50 ml volume, 5 s duration, and 30 s inter-puff interval, would be 94–99% lower than from smoking 20 cigarettes per day (24). Our data in this study, although limited for cigarette smokers, indicate that levels of γ-OH-Acr-dGuo were 2.5 times higher in buccal cell DNA of cigarette smokers compared to e-cigarette users.
The recent IARC monograph concluded that ‘there is consistent and coherent evidence that acrolein exhibits key characteristics of carcinogens’ (6). It is a strongly electrophilic enal that easily reacts with DNA and protein in vivo and in vitro (6,10,42). They note that the adduct detected here, γ-OH-Acr-dGuo, has also been detected in various human DNA samples including those isolated from lung, liver, brain, urothelial mucosa, and saliva (6). Published data demonstrate that, in acrolein-treated human lung cells, its DNA adducts were formed at hotspots of TP53 mutations in lung cancer (43). Acrolein is genotoxic and induced DNA strand breaks and DNA protein crosslinks in human primary cells. It also induced mutations and micronucleus formation in cultured human cell lines (6). Acrolein is mutagenic in Salmonella strains without metabolic activation, inducing both base pair substitution and frameshift mutations (6). Acrolein alters multiple DNA repair pathways and causes genomic instability (44). It decreases glutathione concentration in studies of cultured cells, both human and rodent, and causes oxidative stress. It is also immunosuppressive, induces chronic inflammation, and alters cell proliferation and cell death. It may also induce epigenetic changes (6).
The oral mucosa is the first site of exposure of e-cigarette users to acrolein. Thus, acrolein-DNA adducts in oral cells, in addition to their potential biological effects, also serve as an excellent biomarker of acrolein exposure. Formation of these DNA adducts does not require metabolism and involves the direct reaction of acrolein with dGuo. Thus, the DNA adduct biomarker is distinguished from the urinary biomarker 3-HPMA, which requires reaction with glutathione, most likely in the liver, followed by metabolic processing of the initially formed adduct and excretion in the urine. There are endogenous processes such as lipid peroxidation that generate acrolein and also contribute to urinary 3-HPMA (45). This can partially explain why oral cell DNA adducts of acrolein are 27 times higher in cigarette smokers than nonsmokers, while 3-HPMA is only 3–4 times higher.
While our data raise a warning flag regarding the potential health risk of e-cigarette use, it should be emphasized that cigarette smoking is far worse. Cigarette smoking entails not only higher exposure to acrolein, but also to multiple other carcinogens such as tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons that are generally not present in e-cigarette vapor. The NASEM report concluded that the net public health effect of e-cigarettes depends not only on their intrinsic toxicity, which is the topic of this study, but also on their effects on youth initiation and adult cessation of combustible tobacco products, which could outweigh any effects of intrinsic e-cigarette toxicity (5).
Conclusion
This study clearly demonstrates exposure of e-cigarette users to the toxic and carcinogenic compound acrolein by analysis of the corresponding oral cell DNA adduct levels, which were significantly elevated compared to non-users of these products. This is the first study to demonstrate DNA adduct formation in e-cigarette users, and indicates the need for further research on the potential toxic and carcinogenic effects of e-cigarette use.
Supplementary Material
Acknowledgements
We thank Nicole Thomson of the Sharon E. Murphy laboratory for analysis of total nicotine equivalents, Romel Dator and Alex Strom for assistance with the extraction of bacterial [15N] DNA and Qiyuan Han of the Natalia Tretyakova laboratory for help with bacterial content determination in the oral cells. We also would like to thank Dr Peter Villalta Director of the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, for assistance with mass spectrometry analysis.
Glossary
Abbreviations
- CEMA
cyanoethyl mercapturic acid
- HCD
higher energy collisional dissociation
- HPMA
3-hydroxypropyl mercapturic acid
- LC-NSI-HRMS/MS
liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry
- ND
not detected
- PATH
Population Assessment of Tobacco and Health
Contributor Information
Guang Cheng, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Jiehong Guo, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Steven G Carmella, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Bruce Lindgren, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Joshua Ikuemonisan, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Brittany Niesen, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Joni Jensen, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Dorothy K Hatsukami, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Silvia Balbo, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Stephen S Hecht, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Funding
This study was supported by grant number CA-203851 from the U.S. National Cancer Institute and the Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the Food and Drug Administration. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, supported in part by Cancer Center Support Grant CA-077598. Salary support for Dr Villalta was provided by the National Cancer Institute (R50-CA211256).
References
- 1. Jerzyński, T., et al. (2021) Estimation of the global number of e-cigarette users in 2020. Harm. Reduct. J., 18, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park-Lee, E., et al. (2021) E-Cigarette use among middle and high school students – National Youth Tobacco Survey, United States, 2021. MMWR Morb Mortal Wkly Rep., 70, 1387–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornelius, M.E., et al. (2020) Tobacco product use among adults – United States, 2019. Morb. Mortal. Wkly. Rep., 69, 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandi, P., et al. (2021) Trends in E-cigarette use by age group and combustible cigarette smoking histories, U.S. adults, 2014–2018. Am. J. Prev. Med., 60, 151–158. [DOI] [PubMed] [Google Scholar]
- 5. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division. (2018) Public Health Consequences of E-Cigarettes. National Academies Press, Washington, DC. [Google Scholar]
- 6. International Agency for Research on Cancer. (2021) Acrolein, Crotonaldehyde, and Arecoline. Vol. 128. IARC, Lyon, France, pp. 1–133. [PubMed] [Google Scholar]
- 7. Weng, M.W., et al. (2018) Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. U.S.A., 115, E6152–E6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nath, R.G., et al. (1998) 1,N2-propanodeoxyguanosine adducts: potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res., 58, 581–584. [PubMed] [Google Scholar]
- 9. Bessette, E.E., et al. (2009) Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal. Chem., 81, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minko, I.G., et al. (2009) Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem. Res. Toxicol., 22, 759–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paiano, V., et al. (2020) Quantitative liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of acrolein-DNA adducts and etheno-DNA adducts in oral cells from cigarette smokers and nonsmokers. Chem. Res. Toxicol., 33, 2197–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uchiyama, S., et al. (2016) Determination of chemical compounds generated from second-generation E-cigarettes using a sorbent cartridge followed by a two-step elution method. Anal. Sci., 32, 549–555. [DOI] [PubMed] [Google Scholar]
- 13. Ogunwale, M.A., et al. (2017) Aldehyde detection in electronic cigarette aerosols. ACS Omega, 2, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farsalinos, K.E., et al. (2018) Do flavouring compounds contribute to aldehyde emissions in e-cigarettes?. Food Chem. Toxicol., 115, 212–217. [DOI] [PubMed] [Google Scholar]
- 15. Farsalinos, K.E., et al. (2018) Aldehyde levels in e-cigarette aerosol: findings from a replication study and from use of a new-generation device. Food Chem. Toxicol., 111, 64–70. [DOI] [PubMed] [Google Scholar]
- 16. Bitzer, Z.T., et al. (2019) Emissions of free radicals, carbonyls, and nicotine from the NIDA standardized research electronic cigarette and comparison to similar commercial devices. Chem. Res. Toxicol., 32, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ureña, J.F., et al. (2020) Impact of atomizer age and flavor on in vitro toxicity of aerosols from a third-generation electronic cigarette against human oral cells. Chem. Res. Toxicol., 33, 2527–2537. [DOI] [PubMed] [Google Scholar]
- 18. Gillman, I.G., et al. (2020) Determining the impact of flavored e-liquids on aldehyde production during vaping. Regul. Toxicol. Pharmacol., 112, 104588. [DOI] [PubMed] [Google Scholar]
- 19. Zelinkova, Z., et al. (2020) Influence of battery power setting on carbonyl emissions from electronic cigarettes. Tob. Induc. Dis., 18, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchiyama, S., et al. (2020) Determination of thermal decomposition products generated from E-cigarettes. Chem. Res. Toxicol., 33, 576–583. [DOI] [PubMed] [Google Scholar]
- 21. Belushkin, M., et al. (2020) Selected harmful and potentially harmful constituents levels in commercial e-cigarettes. Chem. Res. Toxicol., 33, 657–668. [DOI] [PubMed] [Google Scholar]
- 22. Li, Y., et al. (2021) Impact of e-liquid composition, coil temperature, and puff topography on the aerosol chemistry of electronic cigarettes. Chem. Res. Toxicol., 34, 1640–1654. [DOI] [PubMed] [Google Scholar]
- 23. Zhou, Y., et al. (2021) Voltage and e-liquid composition affect nicotine deposition within the oral cavity and carbonyl formation. Tob. Control, 30, 485–491. [DOI] [PubMed] [Google Scholar]
- 24. Farsalinos, K.E., et al. (2017) Carbonyl emissions in E-cigarette aerosol: a systematic review and methodological considerations. Front. Physiol., 8, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiler, M., et al. (2021) Systemic biomarkers of exposure associated with ENDS use: a scoping review. Tob. Control. doi:10.1136/tobaccocontrol-2021-056896. [DOI] [PubMed] [Google Scholar]
- 26. Shahab, L., et al. (2017) Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann. Intern. Med., 166, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorkiewicz, P., et al. (2019) Comparison of urinary biomarkers of exposure in humans using electronic cigarettes, combustible cigarettes, and smokeless tobacco. Nicotine Tob. Res., 21, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawkins, L., et al. (2018) ‘Real-world’ compensatory behaviour with low nicotine concentration e-liquid: subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction, 113, 1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubinstein, M.L., et al. (2018) Adolescent exposure to toxic volatile organic chemicals from e-cigarettes. Pediatrics, 141, e20173557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keith, R.J., et al. (2020) Characterization of volatile organic compound metabolites in cigarette smokers, electronic nicotine device users, dual users, and nonusers of tobacco. Nicotine Tob. Res., 22, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Jesus, V.R., et al. (2020) Urinary biomarkers of exposure to volatile organic compounds from the Population Assessment of Tobacco and Health study wave 1 (2013-2014). Int. J. Environ. Res. Public Health, 17, 5408; doi:10.3390/ijerph17155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alwis, K.U., et al. (2015) Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005-2006. Environ. Health Perspect., 123, 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goniewicz, M.L., et al. (2018) Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw. Open, 1, e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo, X., et al. (2020) Urinary cyanoethyl mercapturic acid, a biomarker of the smoke toxicant acrylonitrile, clearly distinguishes smokers from nonsmokers. Nicotine Tob. Res., 22, 1744–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo, J., et al. (2021) Liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of apurinic/apyrimidinic sites in oral cell DNA of cigarette smokers, e-cigarette users, and nonsmokers. Chem. Res. Toxicol., 34, 2540–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maeda, H., et al. (2003) Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol., 39, 81–86. [DOI] [PubMed] [Google Scholar]
- 37. Horz, H.P., et al. (2005) Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: general implications and practical use in endodontic antimicrobial therapy. J. Clin. Microbiol., 43, 5332–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. U.S. Department of Health and Human Services. (2010) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [PubMed] [Google Scholar]
- 39. Bond, J.A., et al. (2019) Electrophilic agents. In Tumour Site Concordance and Mechanisms of Carcinogenesis. International Agency for Research on Cancer, Lyon, France, pp. 1–21. [PubMed] [Google Scholar]
- 40. Nguyen, H., et al. (2017) Oral carcinoma associated with chronic use of electronic cigarettes. Otolaryngol (Sunnyvale), 7, 2. [Google Scholar]
- 41. Cecil, T.L., et al. (2017) Acrolein yields in mainstream smoke from commercial cigarette and little cigar tobacco products. Nicotine Tob. Res., 19, 865–870. [DOI] [PubMed] [Google Scholar]
- 42. Chung, F.L., et al. (1984) Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res., 44, 990–995. [PubMed] [Google Scholar]
- 43. Feng, Z., et al. (2006) Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. U.S.A., 103, 15404–15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang, H.T., et al. (2012) Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J. Biol. Chem., 287, 12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stevens, J.F., et al. (2008) Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res., 52, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.