Abstract
This narrative review paper is aimed to critically evaluate recent studies of the associations between air pollution and the outcomes in the COVID-19 pandemic. The main air pollutants we have considered are carbon monoxide (CO), nitrogen dioxide (NO2), ground-level ozone (O3), particulate matter (PM2.5 and PM10), and sulfur dioxide (SO2). We, specifically, evaluated the influences of these pollutants, both individually and collaboratively, across various geographic areas and exposure windows. We further reviewed the proposed biological mechanisms underlying the association between air pollution and COVID-19. Ultimately, we aim to inform policy and public health practice regarding the implications of COVID-19 and air pollution.
Keywords: Air pollution, Biological complications, COVID-19, Pollutants
1. Introduction
The global scale of the ongoing pandemic of coronavirus disease 2019 (COVID-19) is unmatched in our lifetime. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the type of coronavirus that causes COVID-19 disease. (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020, Grant et al., 2020) Common symptoms include headache, loss of smell and taste, nasal congestion and rhinorrhea, cough, muscle pain, sore throat, fever, diarrhea, and breathing difficulties. (Clinical characteristics of COVID-19 2022) COVID-19 transmission occurs via person-to-person contact through respiratory droplets and airborne aerosol transmission. (Transmission of COVID-19 2022)
Now in the third year of the COVID-19 pandemic, our knowledgebase regarding risk factors for increased transmission and disease severity has greatly improved. A growing body of evidence has highlighted the important role of environmental factors, including air pollution. Here, we first reviewed the epidemiological and statistical evidence regarding the impact of air pollution on COVID-19 outcomes. We paid specific attention to the geographic variation in these associations, the different types of air pollutants evaluated and the variation in air pollutant exposure window (long-term versus short-term exposure). Second, we evaluated proposed plausible biological mechanisms of interaction between air pollutants and this novel coronavirus.
2. Air pollutants
Air pollution is a leading environmental cause of disease and premature death globally. (GBD 2017 Risk Factor Collaborators 2017) The Environmental Protection Agency has identified six criteria pollutants, namely; carbon monoxide (CO), particulate matter (PM2.5 and PM10), ground-level ozone (O3), nitrogen oxides (NO2), sulfur oxides (SO2), and lead. (Air Pollutants | Air | CDC. Published 2021)
CO is the most dangerous product of incomplete fossil fuel combustion. The hemoglobin affinity for CO is even greater than that of oxygen (O2). Thus, when inhaled by humans, CO can result in decreased peripheral and cerebral oxygenation causing symptoms ranging from headache, confusion, chest pain and dyspnea to loss of consciousness or death. (Manisalidis et al., 2020)
Particulate matter refers to the solid and liquid particles suspended in the air. Fine particulate matter is produced as a byproduct of fossil fuel combustion and is characterized by the diameter of the particle. PM2.5 comprises inhalable particles with diameters 2.5 micrometers or smaller, while PM10 particles are 10 micrometers or smaller. Inhalation of PM2.5 and PM10 can cause mechanical trauma to human airways. (Manisalidis et al., 2020)
Ground level ozone forms as a secondary pollutant from the reaction between nitrogen oxides (NOx) and volatile organic compounds (VOCs). When inhaled, O3, can penetrate deep into the lungs causing inflammation. (Manisalidis et al., 2020)
Similarly, NO2, increases bronchial sensitivity. (Hesterberg et al., 2009) Primarily a traffic-related air pollutant, high atmospheric concentrations are associated with pulmonary edema and immune suppression. Long-term exposure can result in chronic lung disease. (Manisalidis et al., 2020)
Sulfur dioxide is primarily emitted from fossil fuel consumption and industrial activities, as well as natural sources such as fires and phytoplankton. SO2 exposure increases the risk of respiratory tract infections, causing increased airway resistance. SO2 can also interfere with airway mucus production. (Hesterberg et al., 2009, Chen et al., 2007)
Lastly, lead is emitted by industrial plants, petrol engines and some aircraft. Accumulating in bones, blood, and soft tissues, lead can have developmental and neurotoxic effects. Notably, little data exists addressing the role of lead exposure in COVID. (Manisalidis et al., 2020)
Researchers delineate the unique effects of each pollutant on human health, but, in reality, communities and individuals are exposed to sources (e.g. air pollution, heavy industry) that emit a mixture of all these pollutants. Although we will discuss research specifically addressing each criteria pollutant in turn, each pollutant should be conceptualized as more of a marker of a complex set of exposures, rather than an individual exposure itself.
3. Search methodology
We conducted a literature search of the National Library of Medicine's PubMed database (National Institutes of Health et al.). The search date was on November 23, 2021 and searched for the last one year. The “Air pollution” and “COVID-19” were used as the keywords for the searches, along with their synonyms, including “PM2.5,” “NO2,” “ozone,” “O3,” etc., for air pollution, and “coronavirus,” “SARS-CoV-2,” etc., for COVID-19. Other key words included were "biological", "epidemiological", "Full text", "Classical Article", "Clinical Study", "Meta-Analysis", "Randomized Controlled Trial", "Review", "Systematic Review", and "English". We updated the search using the same strategy on April 1, 2022.
4. Epidemiological evidence on the impact of air pollution on COVID-19 mortality and infectivity
In this section, we surveyed epidemiological evidence on the impact of various air pollutants on COVID-19 mortality and infectivity. We further characterized the results by exposure window and geographic variation.
We identified literature evaluating the impact of all criteria pollutants on COVID outcomes except lead (Table 1 ). PM2.5, NO2 and O3 were all frequently identified as culprits implicated in exacerbations of COVID-19 incidence and mortality. At the population level, positive associations between high air pollution levels and worse COVID-19 outcomes were identified despite significant variation in study design. Study approaches varied in methodologies of air pollution measurement, COVID-19 data collection (documented new cases, Emergency Department [ED] visits, deaths, etc.) and modeling strategy. Some authors evaluated bivariate correlations while others accounted for potential environmental and regional confounders. Despite this variation, the sum of existing evidence suggests that PM2.5 and NO2 likely exacerbate COVID-19. (Pansini and Fornacca, 2020, Lavigne et al., 2022, Sarkodie and Owusu, 2021, Zoran et al., 2020, Filippini et al., 2021) Using data extrapolated from the US and China, one group found the effect of PM2.5 to be so significant as to contribute 15% to the overall COVID mortality burden. (Pozzer et al., 2020) Although a handful of studies failed to detect an effect for NO2 or PM2.5, (Liang et al., 2020, Xu et al., 2021) no studies found a protective effect from either.
Table 1.
Epidemiologic Studies on COVID-19 and Air Pollution.
| Title | Authors | Year | Journal | Geography | Pollutant(s) | Exposure Duration | Direction of Outcome | Outcome |
|---|---|---|---|---|---|---|---|---|
| Role of the chronic air pollution levels in the COVID-19 outbreak risk in Italy | Fattorini D & Regoli F | 2020 | Environ Pollution | Italy | NO2 O3 PM2.5 PM10 |
Long-term | Exacerbated COVID incidence | Pearson correlations across 71 Italian provinces were significant for the following exposure measures and outcomes; - Incidence of Covid-19 cases vs levels of A) NO2, B) PM2.5 and C) PM10 (four years means) - Incidence of Covid-19 cases vs number of days per year exceeding regulatory limits of D) O3 and E) PM10 (three years means) |
| COVID-19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources | Hendryx M & Luo J | 2020 | Environ Pollution | United States | O3 PM2.5 |
Long-term | No effect on COVID prevalence or mortality | When accounting for diesel particulate matter (DPM), neither O3 nor PM2.5 were found to have a significant association with COVID-19 prevalence or mortality in mixed linear multiple regression models. |
| Urban Air Pollution May Enhance COVID-19 Case-Fatality and Mortality Rates in the United States | Liang D, Shi L, Zhao J, Liu P, Sarnat JA, Gao S, Schwartz J, Liu Y, Ebelt ST, Scovronick N, Chang HH | 2020 | Innova-tion | United States | NO2 O3 PM2.5 |
Long-Term | NO2 exacerbated COVID case-fatality and mortality rate O3 & PM2.5 had no effect |
County-level average NO2 concentrations were positively associated with both COVID-19 case-fatality rate and mortality rate in single-, bi-, and tri-pollutant models (p-values<0.05). Concluded that long-term exposure to NO2, which largely arises from urban combustion sources such as traffic, may enhance susceptibility to severe COVID-19 outcomes, independent of long-term PM2.5 and O3 exposure. |
| Regional and global contributions of air pollution to risk of death from COVID-19 | Pozzer A, Dominici F, Haines A, Witt C, Münzel T, Lelieveld J | 2020 | Cardio Res | US China (estimate worldwide effects) |
PM2.5 | Long-term | Exacerbated COVID mortality | Applied the global atmospheric chemistry general circulation model (EMAC) to estimate that particulate air pollution contributed 15% to mortality (95% confidence interval 7–33%) to COVID-19 mortality worldwide (27% (13 – 46%) in East Asia, 19% (8–41%) in Europe, and 17% (6–39%) in North America). |
| Exposure to Air Pollution and COVID-19 Mortality in the United States | Wu X, Nethery RC, Sabath MB, Braun D, Dominici F | 2020 | Science Advance | United States | PM2.5 | Long-term | Exacerbated COVID mortality | Developed a binomial mixed model and found that the association between long term PM2.5 exposure and the COVID mortality rate ratio (MRR) which was 1.08 (1.02-1.15)for a 1mcg/m3 increase in PM2.5 |
| Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China | Zhu Y, Xie J, Huang F, Cao L | 2020 | Total Environ | China | CO NO2 O3 PM2.5 PM10 SO2 |
Short-term | All exacerbated COVID incidence, except SO2 reduced COVID incidence | Used a generalized additive model to determine the Spearman correlation coefficients with new confirmed COVID-19 cases, incorporating lag effects. Across multiple lags (0-21 days), PM2.5, PM10, NO2, O3 and CO were all associated with increased daily counts of COVID-19 cases. SO2 was negatively associated with COVID-19 confirmed cases across lag 0-14 days. |
| Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy | Zoran MA, Savastru RS, Savastru DM, Tautan MN | 2020 | Sci Total Environ | Italy | PM2.5 PM10 |
Short-term | Both exacerbated COVID incidence | Identified a significant positive association between confirmed daily new cases and air pollution, PM10 & PM2.5. |
| Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide | Filippini T, Rothman KJ, Cocchio S, Narne E, Mantoan D, Saia M, Goffi A, Ferrari F, Maffeis G, Orsini N, Baldo V, Vinceti M | 2021 | Sci Total Environ | Italy | NO2 | Short-term | Exacerbated COVID mortality | Ecological study utilizing negative binomial regression model identified positive, non-linear associations between high NO2 trophospheric levels shortly before COVID 2020 lockdowns in several Italian provinces and mortality lagged at 14, 28 and 42 days. |
| Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries | Pansini R & Fornacca D | 2021 | Atmosphere | China United States Italy Iran France Spain Germany United Kingdom |
PM2.5 PM10 NO2 CO O3 |
Long-Term | China - CO, NO2, PM2.5 & PM10 exacerbated COVID mortality & case-fatality; O3 had no effect United States - CO, NO2, PM2.5 & PM10 exacerbated COVID incidence, mortality & case-fatality rates Italy - NO2, PM2.5 & PM10 exacerbated COVID incidence & case-fatality rate Iran - NO2 exacerbated COVID incidence; PM2.5 had no effect France - NO2 & PM2.5 exacerbated COVID case-fatality Spain - NO2 & PM2.5 had no effect Germany - PM2.5 reduced COVID incidence but exacerbated case-fatality rate; NO2 had no effect United Kingdom - PM2.5 & NO2 had no effect on COVID incidence but exacerbated case-fatality rate |
Assessed bivariable Kendall correlations for aggregated long-term air quality estimates with COVID incidence rates (per 100,000 inhabitants), COVID mortality rates (per 100,000 inhabitants) and case-fatality rates (deaths per infections). |
| Global effect of city-to-city air pollution, health conditions, climatic & socio-economic factors on COVID-19 pandemic | Sarkodie SA & Owusu PA | 2021 | Sci Total Environment | 615 cities globally | NO2 O3 PM2.5 |
Long-Term | All exacerbated COVID incidence | Aggregated city-level COVID data and aligned it with World Air Quality Index project data to develop ln-transformed linear models estimating the effect of air pollutants on incident COVID cases. |
| Weather, air pollution, and SARS-CoV-2 transmission: a global analysis | Xu R, Rahmandad H, Gupta M, DiGennaro C, Ghaffarzadegan N, Amini H, Jalali MS | 2021 | Lancet Planetary Health | 211 countries globally | NO2 O3 PM2.5 SO2 |
Short-term | O3 & SO2 exacerbated COVID reproduction number PM2.5 & NO2 had no effect |
Developed a linear model predicting the reproduction number utilizing daily documented infections and previously defined methods for estimating viral reproduction. |
| Short-term exposure to ambient air pollution and individual emergency department visits for COVID-19: a case-crossover study in Canada | Lavigne E, Ryti N, Gasparrini A, Sera F, Weichenthal S, Chen H, To T, Evans GJ, Sun L, Dheri A, Lemogo L, Kotchi SO, Stieb D | 2022 | Thorax | Canada | NO2 O3 PM2.5 |
Short-Term | PM2.5 & NO2 exacerbated COVID-19 ED visits O3 had no effect |
Applied conditional logistic regression to a case-crossover study of 78,000 emergency department (ED) visits in two Canadian provinces finding that ED visits were highest 3 days following elevated NO2 and PM2.5 ambient concentrations. |
On the other hand, evidence regarding ground-level ozone was mixed. Studies drawing on data directly from the US, China and Canada found no effect from O3 on mortality ED visits. (Sarkodie and Owusu, 2021, Hendryx and Luo, 2020, Fattorini and Regoli, 2020, Zhu et al., 2020) Yet, several global studies and regional work in Italy and China identified a positive association between O3 and COVID incidence. (Sarkodie and Owusu, 2021, Xu et al., 2021, Fattorini and Regoli, 2020, Zhu et al., 2020) Overall, such data suggest O3 may increase vulnerability to COVID transmission without exacerbating the severity of disease.
Only one study identified any protective effects associated with air pollution exposure. Specifically, Zhu et al (2020) found SO2 exposure within the preceding two weeks to be protective against COVID-19 incidence. (Zhu et al., 2020) The authors noted that these findings conflicted with work in other respiratory disease processes, where SO2 exposure has been associated with worse outcomes. They proposed that SO2 may have virucidal properties but emphasize the need for further investigation.
4.1. Geographic variation
Both air pollution and COVID-19 exposure vary significantly by topography and community. The studies we reviewed incorporated data from across the globe. However, Italy, the US and China were relatively over-represented compared to other nations. Such emphasis likely reflects the national capacity for tracking both COVID-19 and air pollution.
Overall, high-income countries were disproportionately represented in the literature we reviewed. China and Iran were the only middle-income countries evaluated, although some global studies incorporated data from cities of a wide variety of income levels around the world. However, even these more globally focused studies note the underrepresentation from low-income countries and the notable effect of this lack of data on confidence in results for these countries. (Pozzer et al., 2020) These differences likely greatly impact the amount and types of air pollution to which communities are exposed and the national public health infrastructure available to respond to the pandemic.
Studies in the US mostly found positive correlations to COVID-19 measures. One study that examined only PM2.5 found a positive correlation with death rate. (21) Liang et al used county-level long-term pollutant exposure data in the US and showed mixed results; NO2 correlated with COVID-19 case-fatality and mortality rates, while PM2.5 and O3 did not. (Liang et al., 2020) In Pansini and Formacca's multi-country study, the US data suggested that CO, NO2, PM10, PM2.5 all were associated with exacerbated infection rates, death rates, and mortality rates. (Pansini and Fornacca, 2020)
For China, Zhu et al used a generalized additive model to find short-term PM2.5, PM10, CO, NO2, and O3 all were positively correlated with daily confirmed COVID-19 cases and SO2 was negatively correlated. (Zhu et al., 2020) Pansini and Formacca reported CO, NO2, PM2.5, and PM10 all were positively associated with death rate and morality versus O3 had no correlation. (Pansini and Fornacca, 2020)
Positive associations between air pollutant exposure and exacerbation of the COVID-19 pandemic were most consistently demonstrated in Italy. These positive correlations included short-term PM2.5 and PM10 to number of new cases daily; (Zoran et al., 2020) long-term NO2, O3, PM2.5, and PM10 and confirmed cases; (Fattorini and Regoli, 2020) short-term NO2 and mortality; (Filippini et al., 2021) long-term NO2, PM2.5, PM10 with death and infection rates. (Pansini and Fornacca, 2020)
Rather than focusing on a specific region, some authors aggregated data on air pollution and COVID globally. Inherent in this study approach is the assumption that geography does not modify the effect of pollution exposure on COVID. While this assumption may be debated, these studies do provide broader guidance for global policymaking and pandemic readiness. In these studies, most findings were positive correlations. Sarkodie and Owusu investigated the effects of long-term exposure to PM2.5, O3, and NO2 on aggregated city-level COVID-19 incidence and found a positive association. (Sarkodie and Owusu, 2021) Xu et al noted positive correlations for O3 and SO2 but no effect for PM2.5 and NO2. (Xu et al., 2021) Although Xu's study relied on many modeling assumptions and imputations for missing data, the model was robust to multiple iterations of simulated epidemiologic conditions. (Xu et al., 2021) Finally, Pozzer et al. extrapolated data from the USA and China to estimate that particulate air pollution contributed approximately 15% to COVID-19 mortality worldwide. (Pozzer et al., 2020)
Lastly, Pansini and Formacca's evaluated air pollution and COVID in 8 countries without aggregating the underlying data, enabling practical comparisons across national borders. (Pansini and Fornacca, 2020) In both Italy and Iran, air pollution independent of population density correlated with the distribution pattern of the virus for each country. PM2.5 had no effect in Iran, the country whose initial COVID-19 data was most limited. No correlations between pollutants and COVID-19 were found in Spain, and this was attributed to fairly even distribution of high air quality levels nationally with minimal differences between provinces. Germany's mixed findings, alternatively, were attributed to the fairly even spread of high pollution levels throughout the country's districts. In the United Kingdom (UK), PM2.5 and NO2 had no effect on infection rates but positively correlated to death and mortality rates, a country noted to enforce containment measures much later compared to other countries. For China and the US, pollutants were found to correlate positively with infection, death, and morality rates except for no correlation of O3 in China. (Pansini and Fornacca, 2020)
Consistently across these studies, population density was highlighted as a potential geographic confounder of the relationship between air pollution and COVID-19 disease burden. Dense population could drive both disease transmission and anthropogenic pollution. Both Pansini et al. and Sarkodie et al. specifically highlight this potential limitation. (Pansini and Fornacca, 2020, Sarkodie and Owusu, 2021) More specifically, in Liang's US study the authors could not exclude the possibility that NO2, a traffic-related air pollutant, might simply be a proxy of urbanicity. (Liang et al., 2020)
4.2. Exposure windows
We classified studies into short-term and long-term pollution exposure groups. Short-term exposure studies evaluated the impact of near-simultaneous air pollutant exposure at the time of COVID-19 transmission. Short-term air pollution levels could theoretically be impacted by acute changes in human activity, such as reduced productivity as witnessed during pandemic lockdowns. The long-term exposure window was measured in years and reflected the chronic level of air pollution in a given region, less affected by day-to-day changes in human activity.
In general, short-term air pollution exposure exacerbated COVID-19 outcomes. In one study, PM2.5, PM10, SO2, CO, NO2, and O3 levels in China were correlated to daily COVID-19 confirmed cases. Only SO2 had a negative correlation with daily confirmed COVID-19 cases, whereas all other pollution measures were positively correlated with increasing COVID-19 daily cases. (Zhu et al., 2020) Two other short-term studies, one in Canada and one in 211 countries worldwide, showed mixed effects of short-term pollutants on COVID measures. The Canadian showed that PM2.5 and NO2 were correlated with more COVID-19 emergency room visits while O3 had no effect. (Lavigne et al., 2022) Xu et al estimated COVID-19 reproduction numbers for 211 countries worldwide and determined higher O3 and SO2 exposure related to greater COVID-19 reproduction number, while PM2.5 and NO2 were found to have no relation to COVID-19 reproduction number. (Xu et al., 2021) Lastly, a study in Italy determined that short-term PM2.5 and PM10 levels correlated to greater transmission of disease represented by daily COVID-19 case number. (Zoran et al., 2020)
The majority of studies examining long-term pollution measures accounting for chronic exposures found that pollution was positively correlated with COVID transmission and mortality. In Sarkodie and Owusu's study, they used aggregated city-level COVID-data for 615 cities globally and World Air Quality Index data to determine that PM2.5, O3, NO2 were associated with exacerbated COVID-19 incidence. (Sarkodie and Owusu, 2021) Another multi-country paper correlated various aggregated air quality estimates with COVID-19 incidence rates, death rates, and mortality rates and found a mix of mostly positive and no correlations, except for in Germany where they found that PM2.5 was associated with improved COVID-19 infection rates but a worse death rate. (Pansini and Fornacca, 2020) Conversely in Germany, NO2 had no association with COVID-19. (Pansini and Fornacca, 2020) Liang's cross-sectional US study on NO2, PM2.5, O3 long-term exposures and correlation to COVID-19 case-fatality and mortality rates also showed mixed effects: NO2 positive associations to both, PM2.5 marginal positive for mortality rate, and O3 no association to either. (Liang et al., 2020) Also in the US and then more globally, two studies investigating the long-term exposure to PM2.5 on COVID-19 reported positive correlations. (Pozzer et al., 2020, 21) Fattorini's study focused on long-term NO2, O3, PM2.5, and PM10 exposure in Italy found all positive correlations to COVID-19. (Fattorini and Regoli, 2020)
Overall, both long- and short-term exposure window studies demonstrated mostly positive associations to COVID-19 transmission and outcomes. The lack of consistent findings between the studies for which measures had positive, negative, or null correlations to COVID-19 measures, highlights the complex nature of the relationship between pollution measures, population density, and COVID-19 transmission versus outcome measures. However, when comparing the short- and long-term exposure window studies, there was fairly consistent variation between both groups suggesting that both short- and long-term exposure to pollution may be useful and valid measures for future studies.
5. Biological mechanisms: air pollution and COVID-19
Beyond identifying epidemiologic trends in associations between air pollutants and COVID-19 outcomes, we sought to better elucidate the proposed mechanisms by which these pollutants exert health effects in the COVID-19 disease pathway. We reviewed literature highlighting the role of air pollutants in COVID lung infection, clotting complications and neurologic symptoms (Table 2 ). While a wide variety of air pollutants were evaluated for epidemiologic associations with COVID-19, mechanistic proposals focused on NO2 and PM2.5.
Table 2.
Studies of Biological Mechanisms of Air Pollution Exacerbating COVID-19.
| Lead Author | Year | Mechanism | Findings |
|---|---|---|---|
| Ni | 2020 | Lung | ACE-2 serves as the transmembrane protein that facilitates SARS-CoV-2 cellular entry |
| Frontera | 2020 | Lung | Correlated PM2.5 & NO2 with COVID 19 cases and ICU admissions in Italy Proposed ACE-2 as the receptor responsible for SARS-CoV-2 cellular entry Suggested PM2.5 & NO2 must up-regulate ACE-2 |
| Borro | 2020 | Lung | Correlated PM2.5 & COVID rates in Italy Identified consensus motifs for the transcription factor AhR in the regulative region of the ACE-2 gene Highlighted that AhR is stimulated by air pollutants, including PM |
| Watzky | 2021 | Lung | Investigated chemicals that modify the expression of ACE-2, TMPRSS2, FURIN, and CATHEPSINs (all previously linked to COVID cell entry) |
| Liu | 2020 | Lung | Compared heart and lung expression of other proteins potentially involved in SARS-CoV-2 cell entry including TMPRSS2, FURIN, CTSL, S protein Found proteins more over-expressed in lung tissue |
| Li | 2021 | Lung | Elucidated the pathway that PM further up-regulates ACE2 and TMPRSS2 via IL-8 Used murine models with bleomycin-induced pulmonary fibrosis |
| Zhang | 2020 | Thrombosis | Identified SARS-CoV-2 viral particles within platelets Identified ACE-2 expression on murine and human platelets |
| Hottz | 2020 | Thrombosis | Found high circulating levels of CRP and fibrinogen in COVID-19 patients correlated with markers of platelet activation/aggregation Monocyte TF was over-expressed in COVID-19 Platelet aggregation was mediated by platelet P-selectin and integrin AII/b3 |
| Zaid | 2020 | Thrombosis | Identified SARS-CoV-2 viral particles within platelets |
| Manne | 2020 | Thrombosis | Identified SARS-CoV-2 viral particles within platelets |
| Reyes | 2020 | Neurologic | Reviewed two potential mechanisms for SARS-CoV-19 to access the brain: 1. Via neuronal spread across the olfactory membrane 2. Via hematologic spread across the blood-brain barrier |
| Nalleballe | 2020 | Neurologic | Epidemiologic review of neurologic symptoms in COVID-19 patients globally in the TriNetX database |
| Guerrero | 2021 | Neurologic | Identified SARS-CoV-2 in the CSF of a minority of COVID-19 patients with neurologic symptoms |
| Solomon | 2020 | Neurologic | Found no immunohistochemical evidence of SARS-CoV-19 in brain tissue at 18 serial autopsies All brains demonstrated hypoxic changes |
| Liu | 2021 | Neurologic | Reviewed two potential mechanisms for SARS-CoV-19 to access the brain: 1. Via neuronal spread across the olfactory membrane 2. Via hematologic spread across the blood-brain barrier |
| Heusinkveld | 2016 | Neurologic | Proposed that PM could enter brain tissue via intranasal canal neuronal passage via the olfactory bulb or through the cerebrovascular circulation |
| Calderon-Garciduenas | 2020 | Neurologic | Described accelerated neurodegenerative change in young COVID-19 patients in a highly polluted community (Mexico City) who experienced accelerated neurodegeneration in Alzheimer's and Parkinson's disease |
| Borisova | 2021 | Neurologic | Proposed that the viral lipid membrane of SARS-CoV-2 could bind to water-suspended PM PM would act as a carrier for the virus, both trans-membrane in neuronal cells and via environmental distribution |
5.1. Air pollution and COVID lung infection
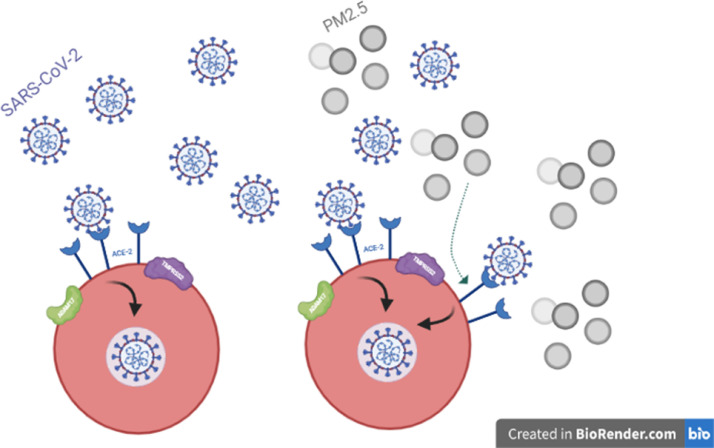
Angiotensin-converting enzyme-2 (ACE-2) is a transmembrane protein expressed in most human tissues, but highly expressed in the lungs. The spike glycoprotein on the SARS-CoV-2 viral envelope binds to one of the sub-domains on ACE-2, enabling cellular entry (Fig. 1 ). (Ni et al., 2020)
Fig. 1.
Air pollution and COVID-19 cellular entry.
Chronic exposure to PM2.5 and NO2 has been associated with overexpression of ACE-2in the lung. (Frontera et al., 2020) In a bioinformatic analysis, Borro et al (2020) defined consensus motifs for the aryl hydrocarbon receptor (AhR) in the regulative region of ACE-2. (Borro et al., 2020) Airway exposure to PM2.5, among other toxicants, promotes an inflammatory milieu, which promotes up-regulation of ACE-2 via AhR stimulation. (Borro et al., 2020, Watzky et al., 2021) It is through this over-expression of ACE-2 that air pollution is believed to exacerbate COVID-19 lung infection.
However, ACE-2 expression is higher in heart than lung tissue. The higher prevalence in the lungs of other transmembrane proteins that support viral uptake are believed to explain the predominantly respiratory complications of COVID-19. Transmembrane protease serine 2 (TMPRSS2) primes the viral spike protein for uptake while a disintegrin metallopeptidase domain 17 (ADAM17) cleaves ectodomains of ACE-2 further promote cellular entry. Both the greater prevalence of these associated proteases in lung tissue and the primary method of COVID transmission via airborne particles likely account for the respiratory predominance of the disease process. (Liu et al., 2020, Zhang et al., 2020, Li et al., 2021)
5.2. Air pollution and COVID thrombotic complications
Both epidemiologic and biologic evidence for an association between air pollutant exposure and COVID-associated thrombosis is sparse relative to respiratory manifestations. However, two previously proposed pathways via which COVID-19 exerts effects on platelet function could be vulnerable to air pollution.
First, the pro-inflammatory state promoted by SARS-CoV-2 infection has been associated with platelet hyperactivity and aggregation. (Zhang et al., 2020, Manne et al., 2020, Hottz et al., 2020) High circulating plasma levels of CRP and fibrinogen have been correlated with platelet activation, while No consensus exists on the exact pathway by which COVID-19 promotes these effects, but several pathways have been suggested. Generally, COVID-19 patients have high circulating plasma levels of CRP and fibrinogen, which correlate with platelet activation. (Hottz et al., 2020) Further, SARS-CoV-2 may promote over-expression of monocyte TF, precipitating increased monocyte-platelet aggregation mediated by platelet P-selectin and integrin aIIb/b3. (Hottz et al., 2020) Air pollution is known to induce similar inflammatory states and could therefore further promote the same pro-thrombotic pathways during COVID-19 infection.
Second, a handful of studies have identified SARS-CoV-2 within platelets. (Zhang et al., 2020, Manne et al., 2020, Zaid et al., 2020) However, only Zhang et al (2020) definitively identified ACE-2 expression on platelets in both mice and humans. (Zhang et al., 2020) These discordant findings highlight the need for further analysis. But if at least some proportion of the global population have platelet ACE-2 expression, upregulation of this receptor with air pollutant exposure could theoretically exacerbate thrombotic outcomes.
5.3. Air pollution & neurologic COVID-19 complications
Recent work suggests exposure to particulate matter, such as PM2.5 and PM10 could influence and augment the neurological impacts of COVID-19. (Reyes and Medina, 2020) Neurological symptoms caused by COVID-19 in patients, range from mild headaches and dizziness to seizures and encephalopathy. (Nalleballe et al., 2020) These symptoms may be a result of a neuroinflammatory response of the Central Nervous System (CNS) to a SARS-CoV-2 infection. (Reyes and Medina, 2020) Because sampling brain tissue is challenging, consensus has not been reached on whether SAS-Cov-2 regularly penetrates brain tissue. One systematic review detected viral particles in the cerebrospinal fluid (CSF) of a minority of COVID-19 patients with neurologic symptoms. (Guerrero et al., 2021) Another histopathologic analysis of 18 consecutive patients who died of COVID-19 identified hypoxic changes to the brain tissue but could not definitively identify the virus in brain tissue via immunohistochemical analysis at autopsy. (Solomon et al., 2020)
If SARS-CoV-2 does reach the CNS, two main mechanisms of transport have been proposed; either through the cerebrovascular circulation by crossing through the capillary endothelium, or by passage from the intranasal canal through the olfactory bulb via a trans-synaptic neuronal route. The latter pathway could explain the anosmia associated with COVID-19. (Reyes and Medina, 2020) Prior work suggests particulate matter accesses the brain by both of these proposed mechanisms. (Heusinkveld et al., 2016) The presence of both viral particles and particulate matter may synergistically promote intra-cerebral inflammation. Clinicians in Mexico City have suggested that this synergy could explain the acceleration of neurodegenerative diseases such as Alzheimer's and Parkinson's Diseases they witnessed in young COVID-19. (Calderón-Garcidueñas et al., 2020)
Further, SARS-CoV-2 is an enveloped virus with a lipid membrane, which supports its passage through a host's cellular membrane. PM can also interact with the lipid components of cell membranes to facilitate entry. Therefore, Borisova and Komisarenko (2021) hypothesized that PM dissolved in airborne water particles could serve as a carrier for SARS-Cov-2, both in the environment and across the olfactory membrane. (Borisova and Komisarenko, 2021)
6. Discussion
Our review suggests that threat of the SARS-CoV-2 virus increases when paired with air pollutant exposure. In different countries around the world, associations and correlations have been drawn between increased concentrations of PM2.5, PM10, and NO2 and other types of pollutants and increased mortality rates and infectivity of COVID-19. Analyzing the overall significance of air pollutants is important in modelling and forecasting future mortality and infection rates, as policymakers plan for future waves of infection. While researchers specifically evaluated associations between COVID outcomes and unique criteria pollutants, policymakers should consider these individual findings as markers of a more complex amalgamated group of air pollutant exposures when considering the comprehensive effects of air pollution in this pandemic. Further, understanding the underlying biological pathways by which air pollution exacerbates COVID-19 infectivity and severity can inform both policy initiatives and clinical management of disease.
In sum, our review suggests air pollution, in both the long- and short-term, is an important and modifiable environmental risk factor for COVID-19. Any public health response seeking to reduce air pollution would result in additional benefits for COVID incidence and severity. Prior work has highlighted the disparate impact of air pollution on poor communities, which further supports an imperative to reduce air pollution as a component of COVID management to reduce socioeconomic health disparities.
This study has some limitations. As with any review, our evaluation of the current literature is affected by publication bias, which may inflate the perceived strength and reproducibility of the associations between air pollutants and COVID-19. The relationship between air pollution and COVID-19 is also likely more nuanced than is captured with our search strategy. Governments often imposed lockdowns in areas where COVID rates were especially high, which would likely reduce the local short-term burden of air pollution. Although the reduction of air pollution during the COVID pandemic is beyond the scope of this review, work is currently ongoing to better understand this dichotomy. Finally, regarding the biologic mechanism studies, the rapidly evolving nature of the virus, may render current bioinformatic studies and their results inaccurate in the near future.
Conclusion
We collected state-of-the-science evidence when assessing the influence of air pollutants on mortality and infectivity. Indications of an association between increased air pollutants and COVID-19 mortality are largely consistent in studies around the world, providing promising but informative insights into how reduction of air pollution can result in lowering mortality rates worldwide.
Author contributions statement
E.Y., K.Z. and T.D. devised the project, the main conceptual ideas, conceived of the presented idea. E.Y., K.Z., A.N., C. F., and T.D. designed the idea. E.Y., K.Z., A.N., and C. F. took the lead in the writing of the manuscript. All authors discussed the results and commented on the manuscript. E.Y., X.W and T.D. supervised the project and were in charge of overall direction and planning.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical characteristics of COVID-19 . 2022. European Centre for Disease Prevention and Control.https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical Accessed May 8. [Google Scholar]
- Transmission of COVID-19 . 2022. European Centre for Disease Prevention and Control.https://www.ecdc.europa.eu/en/covid-19/latest-evidence/transmission Accessed May 8. [Google Scholar]
- GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study. Lancet Lond. Engl. 2017;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Air Pollutants | Air | CDC. Published July 20, 2021. Accessed May 8, 2022. https://www.cdc.gov/air/pollutants.htm.
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front. Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit. Rev. Toxicol. 2009;39(9):743–781. doi: 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- Chen TM, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 2007;333(4):249–256. doi: 10.1097/MAJ.0b013e31803b900f. [DOI] [PubMed] [Google Scholar]
- Pansini R, Fornacca D. 2020. COVID-19 Higher Induced Mortality in Chinese Regions with Lower Air Quality. Published online April 162020.04.04.20053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Ryti N, Gasparrini A, et al. Short-term exposure to ambient air pollution and individual emergency department visits for COVID-19: a case-crossover study in Canada. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-217602. Published online March 31thoraxjnl-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkodie SA, Owusu PA. Global effect of city-to-city air pollution, health conditions, climatic & socio-economic factors on COVID-19 pandemic. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran MA, Savastru RS, Savastru DM, Tautan MN. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T, Rothman KJ, Cocchio S, et al. Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A, Dominici F, Haines A, Witt C, Münzel T, Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116(14):2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Shi L, Zhao J, et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innov. N Y N. 2020;1(3) doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Rahmandad H, Gupta M, et al. Weather, air pollution, and SARS-CoV-2 transmission: a global analysis. Lancet Planet Health. 2021;5(10):e671–e680. doi: 10.1016/S2542-5196(21)00202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx M, Luo J. COVID-19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources. Environ. Pollut. Barking Essex 1987. 2020;265(Pt A) doi: 10.1016/j.envpol.2020.115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2022;6(45):eabd4049. doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A, Cianfanelli L, Vlachos K, Landoni G, Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J. Infect. 2020;81(2):255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borro M, Di Girolamo P, Gentile G, et al. Evidence-based considerations exploring relations between SARS-CoV-2 pandemic and air pollution: involvement of PM2.5-mediated up-regulation of the viral receptor ACE-2. Int. J. Environ. Res. Public Health. 2020;17(15):E5573. doi: 10.3390/ijerph17155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzky M, de Dieuleveult M, Letessier A, Saint-Ruf C, Miotto B. Assessing the consequences of environmental exposures on the expression of the human receptor and proteases involved in SARS-CoV-2 cell-entry. Environ. Res. 2021;195 doi: 10.1016/j.envres.2020.110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Gai S, Wang X, et al. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc. Res. 2020;116(10):1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. OncolJ Hematol. Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HH, Liu CC, Hsu TW, et al. Upregulation of ACE2 and TMPRSS2 by particulate matter and idiopathic pulmonary fibrosis: a potential role in severe COVID-19. Part. Fibre Toxicol. 2021;18(1):11. doi: 10.1186/s12989-021-00404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottz ED, Azevedo-Quintanilha IG, Palhinha L, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;17 doi: 10.1161/CIRCRESAHA.120.317703. Published online September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes MSS, Medina PMB. Environmental pollutant exposure can exacerbate COVID-19 neurologic symptoms. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalleballe K, Reddy Onteddu S, Sharma R, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero JI, Barragán LA, Martínez JD, et al. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect. Dis. 2021;21(1):515. doi: 10.1186/s12879-021-06185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinkveld HJ, Wahle T, Campbell A, et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. doi: 10.1016/j.neuro.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Torres-Jardón R, Franco-Lira M, et al. Environmental nanoparticles, SARS-CoV-2 brain involvement, and potential acceleration of Alzheimer's and Parkinson's diseases in young urbanites exposed to air pollution. J. Alzheimers Dis. JAD. 2020;78(2):479–503. doi: 10.3233/JAD-200891. [DOI] [PubMed] [Google Scholar]
- Borisova T, Komisarenko S. Air pollution particulate matter as a potential carrier of SARS-CoV-2 to the nervous system and/or neurological symptom enhancer: arguments in favor. Environ. Sci. Pollut. Res. Int. 2021;28(30):40371–40377. doi: 10.1007/s11356-020-11183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]