Abstract
In a nationwide sample of Black women in the U.S., we assessed preferences for HIV preexposure prophylaxis (PrEP) products, including long-acting injectable (LAI) PrEP and once-daily oral PrEP. Among 315 respondents, 32.1% were aware of PrEP and 40.6% were interested in using it; interest increased to 62.2% if PrEP were provided for free. Oral PrEP was the preferred option (51.1%), followed by LAI PrEP (25.7%), vaginal gel (16.5%), and vaginal ring (6.7%). When examining oral and LAI PrEP alone, most (62.7%) preferred oral PrEP. LAI PrEP was more likely to be preferred among respondents with concerns about healthcare costs or PrEP-related stigma, and among those who reported inconsistent condom use and multiple sexual partners. Although most Black women preferred oral PrEP, LAI PrEP may be appealing to a subset with social and structural barriers to PrEP use, such as cost and stigma, and those at increased risk of HIV infection.
Keywords: Black women, HIV prevention, Long-acting injectable (LAI), Cabotegravir, Preexposure prophylaxis (PrEP)
Introduction
After more than four decades of the HIV epidemic and two decades of highly effective treatment, over 35,000 people in the U.S. contract HIV each year [1]. Despite recent decreases in HIV incidence among men who have sex with men, HIV incidence did not decline among Black women in the United States during 2015–2019 [1]. In 2019, HIV incidence among Black women was 11 times the rate for White women and 4 times the rate for Hispanic/Latina women [1]. An estimated 93% of new infections among Black women in 2016 would not have occurred if the HIV incidence rate for Black women were as low as the rate for White women [2].
Daily oral preexposure prophylaxis (PrEP) is highly effective in reducing the risk of HIV acquisition and is a woman-controlled HIV-prevention method that may be especially beneficial for women who face challenges with accessing and negotiating condoms with male sexual partners. However, women—and particularly Black women—have been underserved by PrEP implementation initiatives to date. Overall, 5.6% of PrEP users are women, of whom only 26% are Black [3], despite Black women accounting for 57% of new HIV diagnoses among women [4]. Barriers to oral PrEP use among Black women include low PrEP awareness, misinformation about PrEP eligibility, low perceived risk of HIV infection, medical mistrust, and stigma [5–13]. Black women have also reported pill-related barriers to oral PrEP uptake, adherence, and persistence, including the added burden of taking another pill in addition to daily contraception and other medications, as well as competing lifestyle and caregiving demands that may contribute to missed PrEP doses [14–18]. As a result, among Black women who initiate oral PrEP, persistence is low [19].
Long-acting injectable (LAI) cabotegravir PrEP administered every 8 weeks is an alternative PrEP agent that was recently shown to have superior efficacy compared with daily oral PrEP in multiple priority populations, including cisgender women [20]. The U.S. Food and Drug Administration approved LAI PrEP for use in adults and adolescents in December 2021 [21], and the Centers for Disease Control and Prevention (CDC) has issued guidelines for LAI PrEP prescribing [22]. In studies of U.S. women overall, reasons that women preferred LAI PrEP over oral PrEP included high perceived efficacy, convenience, confidentiality, and eliminated pill burden [16, 23–25]. By addressing barriers to oral PrEP initiation, adherence, and persistence, the availability of LAI PrEP has the potential to increase PrEP uptake and reduce HIV incidence among Black women. In addition to LAI PrEP and oral PrEP, other PrEP products, including a vaginal ring and vaginal gels, are at various phases of development and clinical trials [26, 27]. The dapivirine vaginal ring has been approved for use by European regulators and is under review by the FDA [28], while the timing of availability for vaginal gels is unknown. Preferences for these emerging PrEP modalities have been explored in subpopulations of women in Sub-Saharan Africa [29–31]. However, few studies have evaluated PrEP product preferences among Black women in the U.S., which are needed to inform PrEP implementation strategies that can overcome historical inequities in use.
To inform a public health approach that will counteract existing systems that undermine PrEP access, we conducted an online survey of adult Black women in the U.S. to assess PrEP product preferences, including PrEP delivered as a daily oral pill, LAI, vaginal gel, or vaginal ring. Given that LAI PrEP has demonstrated high acceptability and greater efficacy than oral PrEP, we also assessed the prevalence and correlates of preference for LAI PrEP over oral PrEP among Black women. Because barriers and facilitators of PrEP use in Black women are multifactorial [10, 32, 33], we explored individual (e.g., sexual behaviors), social (e.g., PrEP stigma), and structural factors (e.g., health care access) as potential correlates of PrEP product preferences.
Methods
Study Design
We conducted a cross-sectional survey using the Qualtrics online platform (Qualtrics, Provo, UT). Respondents were recruited through the Qualtrics panel research service to obtain a non-representative, nationwide sample of adult Black women. The Qualtrics research panel consists of individuals recruited from various sources who have agreed to respond to Qualtrics online surveys in exchange for compensation. Panel aggregation is increasingly recognized as an acceptable online data source for HIV research [34] and has been successfully used for HIV and PrEP-related studies among Black Americans [8, 9, 35–37].
Adults who met the following eligibility criteria were invited to participate: (1) self-identified as a cisgender woman, (2) self-identified as Black/African American, (3) aged 18 to 44, (4) could complete a survey in English, (5) no prior HIV diagnosis, and (6) engaged in sexual intercourse with a male partner in the 12 months prior to survey administration. Upon providing electronic informed consent, respondents were asked to complete the online survey. Surveys were self-administered and anonymous, and the average time to survey completion was 17 min. IP addresses were not collected and cookies were not used, thereby protecting the respondent’s privacy and preventing further data collection mechanisms. Respondents who completed the survey received a $16 online gift card. Only completed surveys were included in the final study sample. The Institutional Review Board at Washington University in St. Louis approved this study.
Survey Development
Before finalizing and administering the survey, we sought input from an established community advisory board comprising adult Black women who resided in St. Louis, Missouri. We requested open-ended feedback on the survey, including question comprehension, cultural applicability, and potential response bias due to sensitive questions, and then modified the survey content and format accordingly.
Measures
The survey included self-reported sociodemographic information, including age, relationship status, education level, household income, health insurance status, and employment status. Health care engagement was assessed by asking respondents if they visited a doctor/health care provider in the past 12 months, and if they did not receive health care in the past 12 months due to cost. Sexual history was assessed by asking respondents about number of sexual partners and frequency of condom use in the past 6 months; if they had ever exchanged sex for money, drugs, housing, or any other commodity; if they had received an HIV test in the last 12 months; and if they had ever been treated for a sexually transmitted infection (STI) in their lifetime. A single item assessed worry about acquiring HIV infection: “Do you ever worry that you could get HIV?”
PrEP stigma was assessed using the PrEP Anticipated Stigma Scale, which has been validated with HIV-negative, PrEP-inexperienced, heterosexually active women [12]. The scale includes two subscales: PrEP-User Stereotypes subscale (5 items), which measures perceived cultural associations with PrEP (e.g., “People would assume I slept around if they knew I took PrEP”), and the PrEP Disapproval by Others subscale (3 items), which measures expected judgements from others for using PrEP [e.g., “My sexual partner(s) would approve of me taking PrEP”]. Participants responded using 4-point scales ranging from strongly disagree to strongly agree. Items were reverse-coded as needed so that higher values indicated higher stigma. We treated them as separate subscales to measure two distinct dimensions of stigma for this study, PrEP disapproval (Cronbach’s alpha = 0.85) and PrEP stereotypes (Cronbach’s alpha = 0.81).
After providing education about PrEP, which included a brief statement defining PrEP and an infographic describing the FDA approval and efficacy of oral PrEP, we assessed PrEP awareness and interest. Respondents were asked: “Before today have you heard of PrEP?” Respondents were then asked: “Are you interested in using PrEP to prevent HIV infection?” and “If provided for free, would you be interested in taking PrEP for HIV infection?” Background information was only provided for oral PrEP, and respondents were asked about their interest and preferences for other PrEP modalities following the oral PrEP section of the survey.
To measure PrEP product preferences, respondents were asked to respond to the following questions, “Of the four ways of taking PrEP, please tell me which you prefer most and which the least?” Response options included: (1) “Oral pill or tablet you would take once a day (like ‘the pill’),” (2) “injection/shot every 3 months (like ‘the shot’),” (3) “a ring that you insert into your vagina once per month (like ‘the ring’),” or (4) “a gel you would insert into your vagina prior to sexual intercourse (like a lubricant).” Respondents were asked to rank the PrEP products according to their preferences. Respondents who ranked LAI PrEP higher than oral PrEP were classified as preferring LAI PrEP over oral PrEP.
Statistical Analysis
We analyzed data in SAS 9.5 (Cary, NC). We used descriptive statistics to characterize the overall sample of respondents. We then used log-binomial regression to compute unadjusted prevalence ratios (PRs) and corresponding 95% confidence intervals (CIs) for each independent variable in relation to preference for LAI PrEP over oral PrEP. Our goal was to describe PrEP product preferences rather than assess a causal relationship; thus, we did not conduct multivariable analyses to adjust for confounding [38].
Results
Sample Characteristics
The survey was distributed from April through May 2019, and approximately 1249 Black women met eligibility criteria and received the study invitation. Of the initial 523 respondents, 23.7% did not complete the survey (n = 124) and 16.1% (n = 84) were excluded because they did not provide valid data on key variables, leaving the final sample of 315 Black women. Descriptive characteristics are presented in Table 1. The mean age was 29 years (range 22–36 years). The geographic distribution of respondents was similar to the distribution of Black Americans across the U.S.; most respondents (55.2%) resided in the South at the time of survey administration. Most respondents had at least some college education (50.5%), were employed (68.3%), and had health insurance (88.6%), and 44.6% had an annual income of $40,000 or higher. In the past 12 months, 88.3% had visited a health care provider and 26.3% did not receive health care because of concerns about cost. More than half of respondents (52.6%) were in a relationship. In the past 6 months, most (66.7%) reported inconsistent or no condom use, 22.2% had 2 or more sexual partners, and 12.4% reported both inconsistent or no condom use and multiple sexual partnerships. Few respondents (7.6%) had exchanged sex for money or goods, and nearly half (41.0%) had received treatment for an STI in their lifetime. Overall, more than half (57.8%) reported being worried about acquiring HIV infection; of those who reported both inconsistent or no condom use and multiple sexual partners in the past 6 months, 15.4% reported being worried about acquiring HIV infection.
Table 1.
Characteristics of respondents (N = 315)
| N (%) | |
|---|---|
| Age M (SD) | 29 (7.46) |
| Age groups | |
| 24 years or younger | 105 (33.3) |
| 25–29 years | 69 (21.9) |
| 30–39 years | 104 (33.0) |
| 40–49 years | 37 (11.7) |
| Relationship status | |
| Single | 149 (47.3) |
| In relationship | 166 (52.7) |
| Education | |
| Less than high school degree | 6 (1.9) |
| High school/GED | 150 (47.6) |
| Technical/associate degree | 70 (22.2) |
| Bachelors or higher | 89 (28.3) |
| Employment | |
| Unemployed | 69 (21.9) |
| Student | 31 (9.8) |
| Part-time employment | 68 (21.6) |
| Full-time employment | 147 (46.7) |
| Household income (USD) | |
| Less than 20,000 | 104 (33.0) |
| 20,000–40,000 | 102 (32.4) |
| 40,001–60,000 | 74 (23.5) |
| 60,001–80,000 | 35 (11.1) |
| Geographic region | |
| Midwest | 72 (22.8) |
| Northeast | 42 (13.3) |
| South | 174 (55.2) |
| West | 27 (8.7) |
| Health insurance status | |
| Uninsured | 36 (11.4) |
| Private health plan | 112 (35.6) |
| Medicaid | 122 (38.7) |
| Other government plan | 35 (11.1) |
| Other | 10 (3.2) |
| Visited a health care provider past 12 months | 278 (88.3) |
| Didn’t receive healthcare due to cost in past 12 months | 83 (26.3) |
| Ever treated for STI in lifetime | 129 (41.0) |
| Received test for HIV in the past 12 months | 165 (52.4) |
| Ever had sex with someone to receive money, food, housing | 24 (7.6) |
| Number of sexual partners in the past 6 months | |
| 0–1 Sex partner | 245 (77.8) |
| 2–3 Sexual partners | 57 (18.1) |
| 4 Or more sexual partners | 13 (4.1) |
| Inconsistent or no condom use in the past 6 months | 210 (66.7) |
| Inconsistent or no condom use and multiple partners past 6 months | 39 (12.4) |
| Worry about HIV Infection | 182 (57.8) |
| Aware of PrEP | 116 (36.8) |
| Interested in PrEP for HIV prevention | 128 (40.6) |
| Would use PrEP if provided for free | 196 (62.2) |
| Comfortable speaking to provider about PrEP | 249 (79.0) |
| PrEP disapproval by others subscale score M (SD) | 6.40 (2.33) |
| My friends would approve of me taking PrEP | 241 (76.5) |
| My family would approve of me taking PrEP | 234 (74.3) |
| My sexual partner(s) would approve of me taking PrEP | 214 (67.9) |
| PrEP-user stereotypes subscale score M (SD) | 10.9 (3.66) |
| I would feel ashamed to tell other people that I was taking PrEP | 121 (38.4) |
| People would assume I slept around if they knew I took PrEP | 152 (48.2) |
| People would assume I am HIV positive if they knew I took PrEP | 134 (42.5) |
| People would assume I am a bad person if they knew I took PrEP | 80 (24.5) |
| People would assume I am gay if they knew I took PrEP | 74 (23.5) |
PrEP disapproval by others subscale scores range from 3 to 12, with higher scores indicating greater anticipated disapproval. PrEP-user stereotypes subscale scores range from 5 to 20, with higher scores indicating greater anticipated stereotyping
PrEP preexposure prophylaxis, HIV human immunodeficiency virus, STI sexually transmitted infection
Most respondents (67.9%) were unaware of PrEP prior to the survey. When made aware of oral PrEP, many (41.6%) were interested in using oral PrEP for HIV prevention when cost was not specified, and almost two-thirds (62.2%) were interested in PrEP if it were provided for free. Most respondents (79.0%) felt comfortable speaking with their health care provider about PrEP, and most felt their friends (76.5%), family (74.3%), and sexual partners (67.9%) would approve of their using PrEP. Less than half of respondents anticipated being stereotyped for their PrEP use, including feeling ashamed to tell other people that they were taking PrEP (38.4%) or endorsing that people would assume that they slept around (48.2%), were HIV-positive (42.5%), were a bad person (24.5%), or were gay (23.5%) if they knew that the respondents took PrEP.
PrEP Product Preferences
Oral PrEP was the most preferred form of PrEP (51.1%), followed by LAI PrEP (25.7%), vaginal gel (16.5%), and vaginal ring (6.7%) (Fig. 1). When examining preferences for oral and LAI PrEP alone, 37.8% of respondents preferred LAI PrEP over oral PrEP (Table 2). Preference for LAI PrEP over oral PrEP was more common among respondents who were not able to receive health care due to costs (PR = 1.36; 95% CI = 1.02 to 1.82), among those with 4 or more sexual partners in the past 6 months compared with those who had one or no sex partners (PR = 1.9; 95% CI = 1.28 to 2.81), and among those who reported both inconsistent/no condom use and multiple partners in the past 6 months compared with those who had no partners or had one sex partner and used condoms consistently (PR = 1.43; 95% CI = 1.01 to 2.01). Preference for LAI PrEP over oral PrEP was also more common among those with higher PrEP-user stereotypes subscale scores (PR = 1.04; 95% CI = 1.00 to 1.08), including those who endorsed that they would feel ashamed if people knew that they took PrEP (PR = 1.63; 95% CI = 1.23 to 2.15), that people would assume they were gay if they took PrEP (PR = 1.41; 95% CI 1.05 to 1.89), and that people would assume they were HIV-positive if they took PrEP (PR = 1.32; 95% CI = 1.01 to 1.76).
Fig. 1.
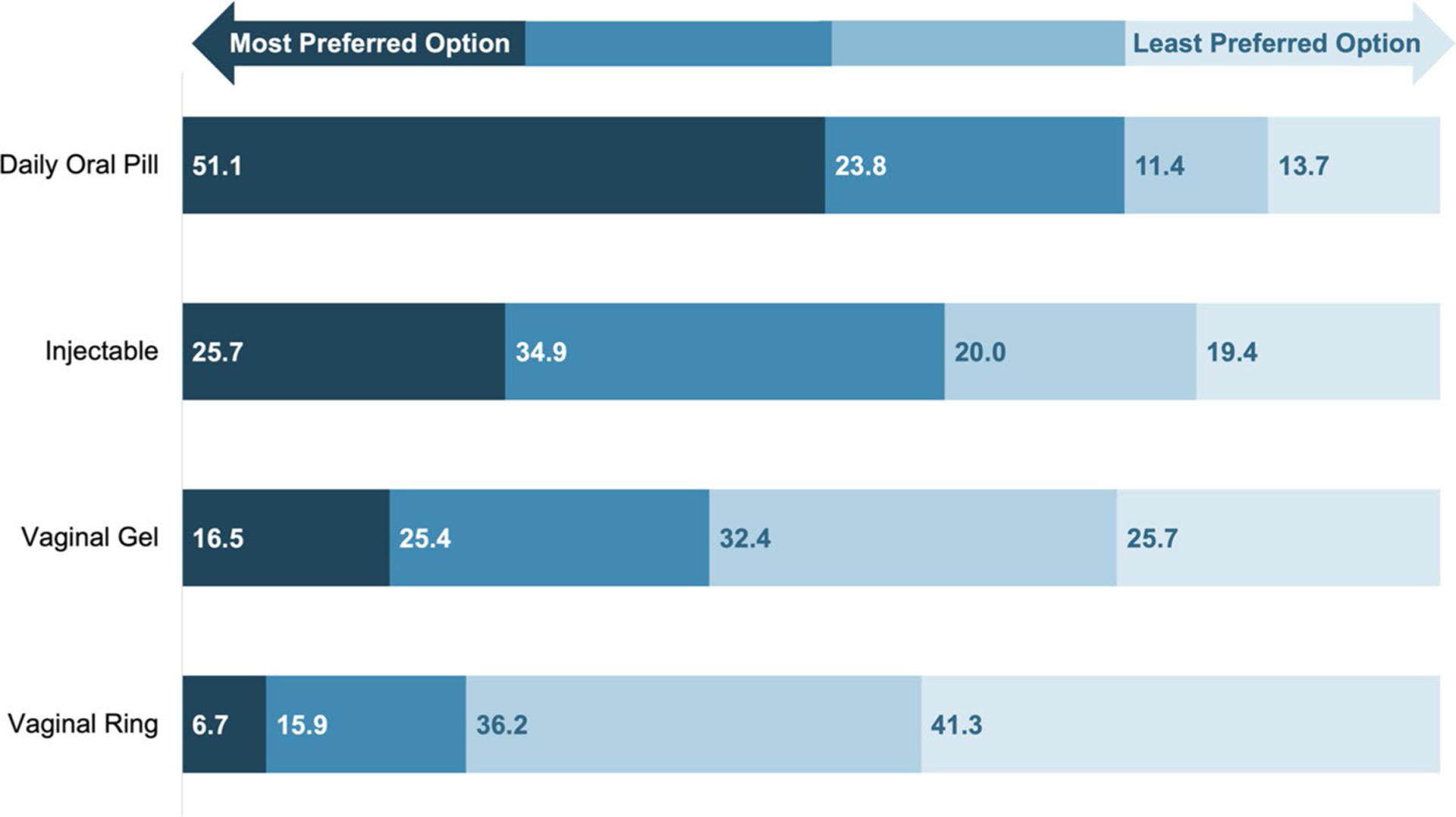
Percentage of respondents who ranked each PrEP product from most to least preferred (N = 315). Figure depicts the percentage of respondents who ranked each PrEP product from most preferred to least preferred (1 to 4). Darkest shade of color represents most preferred, next shade represents second preferred, lighter shade represents third preferred, and the lightest shade of color represents least preferred
Table 2.
Factors associated with preference for injectable over oral PrEP among Black women (N = 315)
| Variable | Prefer injectable over oral PrEP (N = 119) | |||
|---|---|---|---|---|
| % | PR | (95% CI) | P | |
| Age | ||||
| ≤ 30 | 39.1 | 1.08 | (0.76, 1.34) | 0.95 |
| Older than 30 years (ref) | 36.2 | |||
| Relationship status | ||||
| In a relationship | 38.0 | 1.01 | (0.76, 1.34) | 0.95 |
| Single (ref) | 37.6 | |||
| Employment status | ||||
| Employed | 38.6 | 1.11 | (0.77, 1.59) | 0.57 |
| Unemployed (ref) | 34.8 | |||
| Income | ||||
| 40,000+ | 39.4 | 1.07 | (0.79, 1.43) | 0.65 |
| Less than $40,000 (ref) | 36.9 | |||
| Insurance status | ||||
| Insured | 36.6 | 0.77 | (0.53, 1.13) | 0.18 |
| Uninsured (ref) | 47.2 | |||
| Visit health provider past 12 months | ||||
| Yes | 37.1 | 0.85 | (0.57, 1.27) | 0.44 |
| No (ref) | 43.2 | |||
| Did not receive healthcare due to cost in past 12 months | ||||
| Yes | 47.0 | 1.36 | (1.02, 1.82) | 0.03 |
| No (ref) | 34.5 | |||
| Ever diagnosed with STI | ||||
| Yes | 38.0 | 1.00 | 0.75, 1.34) | 0.95 |
| No (ref) | 37.6 | |||
| HIV test past 12 months | ||||
| Yes | 39.4 | 1.09 | (0.82, 1.45) | 0.53 |
| No (ref) | 36.0 | |||
| Ever exchange sex | ||||
| Yes | 45.8 | 1.23 | (0.77, 1.95) | 0.36 |
| No (ref) | 37.1 | |||
| Inconsistent condom or no use past 6 months | ||||
| Yes | 41.0 | 1.30 | (0.94, 1.80) | 0.11 |
| No (ref) | 31.4 | |||
| Number of sexual partners past 6 months | ||||
| 4+ Sexual partners | 69.2 | 1.90 | (1.28, 2.81) | 0.0013 |
| 2–3 Sexual partners | 38.6 | 1.02 | (0.71, 1.47) | 0.88 |
| 0–1 Sex partner (ref) | 35.9 | |||
| Inconsistent or no condom use and multiple partners past 6 months | ||||
| Yes | 51.3 | 1.43 | (1.01, 2.01) | 0.04 |
| No (ref) | 35.9 | |||
| Ever worry about HIV | ||||
| Yes | 37.4 | 0.94 | (0.73, 1.29) | 0.86 |
| No (ref) | 38.3 | |||
| Comfort discussing PrEP with provider | ||||
| Yes | 37.3 | 0.95 | (0.67, 1.33) | 0.76 |
| No (ref) | 39.4 | |||
| PrEP disapproval by others subscale score | 0.96 | (0.90, 1.03) | 0.28 | |
| My family would approve of me taking PrEP | ||||
| Agree | 43.2 | 0.83 | (0.61, 1.25) | 0.23 |
| Disagree (ref) | 35.9 | |||
| My friends would approve of me taking PrEP | ||||
| Agree | 36.8 | 0.91 | (0.66, 1.25) | 0.57 |
| Disagree (ref) | 40.5 | |||
| My sexual partner(s) would approve of me taking PrEP | ||||
| Agree | 40.2 | 1.23 | (0.88, 1.70) | 0.21 |
| Disagree (ref) | 32.7 | |||
| PrEP-user stereotypes subscale score | 1.04 | (1.00, 1.08) | 0.03 | |
| I would feel ashamed to tell other people that I was taking PrEP | ||||
| Agree | 49.6 | 1.63 | (1.23, 2.15) | < 0.001 |
| Disagree (ref) | 30.4 | |||
| People would assume I slept around if they knew I took PrEP | ||||
| Agree | 37.5 | 0.98 | (0.74, 1.31) | 0.92 |
| Disagree (ref) | 38.0 | |||
| People would assume I am gay if they knew I took PrEP | ||||
| Agree | 48.6 | 1.41 | (1.05, 1.89) | 0.02 |
| Disagree (ref) | 34.4 | |||
| People would assume I am a bad person if they knew I took PrEP | ||||
| Agree | 42.5 | 1.17 | (0.86, 1.59) | 0.30 |
| Disagree (ref) | 36.2 | |||
| People would assume I am HIV-positive if they knew I took PrEP | ||||
| Agree | 44.0 | 1.32 | (1.01, 1.76) | 0.048 |
| Disagree (ref) | 33.1 | |||
PrEP, preexposure prophylaxis; HIV, human immunodeficiency virus; STI, sexually transmitted infection; PR, prevalence ratio; CI, confidence interval
Age, relationship status, employment status, income, insurance status, STI history, HIV testing, sex exchange, worry about HIV infection, comfort discussing PrEP with provider, and the PrEP disapproval subscale and scale items were not associated with preference for LAI PrEP over oral PrEP.
Discussion
Despite stark racial inequities in PrEP use and HIV incidence, Black women in the U.S. have been neglected in PrEP research and implementation initiatives to date. Using a national sample, this study contributes some of the first data on Black cisgender women’s preferences for PrEP products. Although oral PrEP was the most preferred product in this nationwide sample of Black women, with a vaginal ring and vaginal gel being the least preferred PrEP products, we found that a third of respondents preferred LAI PrEP over oral PrEP. LAI PrEP was more likely to be preferred over oral PrEP among Black women with health care cost concerns, those with concerns that they may be stigmatized if others knew they were taking PrEP, and those who had inconsistent condom use and multiple sexual partners. Our findings suggest that LAI PrEP may be an important addition to the prevention toolkit, with potential to engage Black women who are at increased risk of HIV infection because of sexual behaviors, perceived or actual cost barriers, and anticipated stigma.
PrEP product preferences among Black women in our study were consistent with prior studies of U.S. women that found that most women preferred oral PrEP, but that a significant minority preferred LAI PrEP, while a vaginal ring and gel were preferred by fewer women [5, 25, 39]. The diversity of preferences provides evidence that expanding options will be critical for greater reach of PrEP among Black women. Women’s preference for and adherence to oral PrEP may be attributed in part to their prior experience with contraceptive pills [19, 25]. However, Black women have reported that they would still like to have nondaily options, such as a weekly or monthly PrEP product, partly because nondaily PrEP products offer greater discretion than a daily pill [19]. Black women have also expressed a lack of motivation to take PrEP every day because their sexual behaviors are often unplanned or intermittent [19]. Event-driven or 2–1–1 PrEP with tenofovir disoproxil fumarate/emtricitabine has been shown to be efficacious for men who have sex with men [40, 41]. In contrast, event-driven PrEP has not been evaluated or recommended for women, in part because PrEP is highly efficacious for women when 6–7 pills per week are taken [42]. LAI PrEP offers extended HIV protection that can help women overcome the challenges of adhering to daily PrEP in the context of unpredictable sexual behaviors and dynamic HIV risk. Despite evidence to date that women generally prefer oral or LAI PrEP, and that PrEP as a vaginal ring or vaginal gel may be less effective, the vaginal ring and gel are acceptable PrEP options for some women in the U.S. [43–45], including subsets of Black women in our sample. Our study suggests that Black women’s product preferences are heterogeneous, and that emerging PrEP modalities, such as LAI PrEP, will increase the population impact of all available PrEP products (i.e., mosaic effectiveness [46]) by appealing to a substantial fraction of women who would have declined to take oral PrEP. Diversifying PrEP products can also empower Black women by providing options that align with their sexual health lifestyle, experience, and preferences.
The 2021 CDC PrEP guidelines recommend that all sexually active adolescents and adults be informed about PrEP, and that people who report inconsistent condom use with multiple sexual partners be offered a PrEP prescription [22]. We found that Black women who reported multiple sexual partners in general, and the combination of multiple sexual partners and inconsistent or no condom use, tended to prefer LAI PrEP over oral PrEP. Our results suggest that LAI PrEP may be a preferred option for Black women who are at increased risk of contracting HIV, highlighting the potential for real-world impact. With the impending availability of LAI PrEP in the U.S., there is a need to prepare for broad and equitable implementation of this new strategy, including by increasing awareness of LAI PrEP among sexually active Black women and their health care providers. We also found that most women reported being worried about acquiring HIV, but HIV worry was much lower among women who engaged in sexual behaviors associated with HIV risk. Self-perceived risk of HIV is a critical determinant of PrEP use among women [47–50], but HIV worry is only one dimension of perceived risk—i.e., affective perceived risk [47]. More robust qualitative research and measurement of HIV risk perception is needed to explore the potential role of HIV risk perception in PrEP product preferences and uptake in Black women.
We found that Black women who experienced cost as a barrier to receiving health care were more likely than other women to prefer LAI PrEP over oral PrEP. Concerns about the costs of PrEP care have been associated with greater interest in nondaily PrEP regimens (e.g., 2–1–1) among men who have sex with men [51], and prior studies have found that cost is a concern for Black women who may benefit from PrEP [6, 10, 16, 52]. At $3700 per dose, LAI PrEP is 50 to 60 times more expensive than generic oral formulations of PrEP, but some Black women may perceive infrequent injections as more affordable than a daily pill. Most women in our study were not initially interested in using any form of PrEP for HIV prevention; however, most women indicated that they would use PrEP if provided for free, highlighting the need to address perceived and actual costs as barriers to PrEP uptake for Black women. Black women who use PrEP and receive resources related to PrEP discount and coverage programs do not express concerns about cost [19]. Ensuring that Black women are informed of and connected to health insurance plans and PrEP assistance programs, and that those plans and programs cover LAI PrEP in addition to oral PrEP, is critical to mitigating cost barriers to PrEP uptake and ensuring that Black women can access the full range of PrEP options. LAI PrEP should also be included in the U.S. Preventive Services Task Force Grade A recommendation for PrEP, which would require coverage without patient cost-sharing under the Affordable Care Act.
We found that anticipated PrEP stigma was associated with a preference for LAI PrEP over oral PrEP, suggesting that the discretion of LAI PrEP may be particularly appealing to Black women who do not want to be seen taking PrEP pills. In addition to overall PrEP stigma scores, preference for LAI PrEP was associated with the endorsement of beliefs that if people knew that the respondents took PrEP there would be assumptions about their sexual orientation and HIV status, and that they would feel ashamed if others knew they were taking PrEP. Consistent with our findings, Philbin et al., found that women preferred LAI PrEP over oral PrEP because they feared that others would see that they were taking a pill or carrying a bottle of medication and make assumptions about their HIV status or sexual behaviors [23]. In that study, women felt that receiving LAI PrEP in the privacy of a health care facility would mitigate those concerns. Although LAI PrEP may reduce the fear and shame associated with taking pills by facilitating confidentiality, stigma will likely remain a significant barrier to PrEP uptake in Black communities [53–56]. Interventions that target both individual- and community-level stigma, including faith-based strategies [36, 57–61] and interventions based in barbershops and beauty salons [62–64], have been found to be effective in Black communities. Integrating PrEP education and components that specifically target PrEP stigma into such programs may facilitate PrEP uptake among Black women [62].
Our study has several limitations. First, the recruitment strategy involved convenience sampling, thus limiting generalizability to the broader population of Black women in the U.S. Second, survey responses may have been influenced by social desirability bias, although we minimized this potential bias through anonymous survey administration and by limiting the collection of any identifying information. We also leveraged our partnership with a community advisory board throughout the development of the survey and incorporated their feedback on survey questions, including potential response bias, prior to survey administration. Third, LAI PrEP was described to respondents as an injection “every 3 months,” which is longer than the 8-week window for injections that was efficacious in clinical trials [20]. It is possible that fewer women would have expressed interest in LAI PrEP if presented with a shorter interval between injections. Fourth, we provided more background information about oral PrEP than the other modalities, and participants were informed that only oral PrEP had been approved by the FDA at the time of survey administration. This imbalance in the information we provided to participants may have contributed to oral PrEP being the most preferred modality. Finally, we explored theoretical preferences for the various PrEP modalities and did not provide information about their relative costs or efficacy. Thus, our findings may not translate into actual PrEP uptake as cost and efficacy will vary across modalities.
Conclusions
This study fills a gap in the literature on PrEP product preferences among Black women, who are disproportionately affected by HIV and have been underserved by PrEP implementation initiatives to date. Our findings on factors associated with preference for LAI PrEP over oral PrEP are timely, as LAI cabotegravir PrEP was approved by the FDA in December 2021 and the dapivirine vaginal ring is pending regulatory approval in the U.S. The diversification of PrEP products may increase uptake for women, just as it has for contraception [65], and discreet and long-acting PrEP modalities have the potential to further strengthen PrEP as woman-controlled HIV-prevention options. However, product diversification alone will be insufficient to optimize PrEP use among Black women if social and systemic barriers remain unaddressed. Our study suggests that anticipated stigma and concerns about costs are potential determinants of not only PrEP interest but also PrEP product preferences among Black women. As disproportionately high HIV incidence rates persist in Black women, further insight is needed into the unique barriers and facilitators of engagement in biomedical HIV prevention in this high-priority population. To ensure successful scale-up of PrEP in the U.S., implementation efforts must identify and meet the needs and preferences of Black women.
Funding
This publication was made possible by Grant Numbers T32 AI007433 from the National Institute of Allergy and Infectious Diseases, T32 DA15035 from the National Institute on Drug Abuse, R25 MH08362 from the National Institute on Mental Health, and the National Association for Social Workers (NASW) Foundation’s Jane B Aron Dissertation Award to Whitney Irie. Elvin Geng is supported by Grant Number K24 AI134413 from the National Institute of Allergy and Infectious Diseases. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the NASW.
Conflict of interest
Dr. Calabrese received partial support from Gilead for conference attendance in 2019 unrelated to this work. Dr. Marcus previously consulted for Kaiser Permanente Northern California on a research grant from Gilead Sciences unrelated to the submitted work. Dr. Mayer has received unrestricted educational grants from Gilead Sciences and served on a scientific advisory board for them unrelated to this work. Dr. Geng has received an educational grant from Viiv Healthcare. All other authors declare no conflicts.
Footnotes
Ethical Approval All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board at Washington University in St. Louis. (IRB201902109) and with the 1964 Helsinki Declaration and its later amendments.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Data Availability
The data that support the findings of this study are available from the corresponding author, Whitney Irie, upon reasonable request.
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019, vol. 26, HIV Surveillance Supplemental Report 2021. CDC; 2021. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 8 July 2021. [Google Scholar]
- 2.Bradley ELP, Williams AM, Green S, Lima AC. Disparities in Incidence of human immunodeficiency virus infection among Black and White women—United States, 2010–2016. Morb Mortal Wkly Rep. 2019;68(18):416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y-LA, Zhu W, Smith DK, Harris N, Hoover KW. HIV preexposure prophylaxis, by race and ethnicity—United States, 2014–2016. Morb Mortal Wkly Rep. 2018;67:1147–50. https://www.iqvia.com/locations/united-states/commercial-operations/. Accessed 8 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). HIV and women: HIV diagnoses. HIV Surveillance Report. CDC; 2021. https://www.cdc.gov/hiv/group/gender/women/diagnoses.html. Accessed 8 July 2021. [Google Scholar]
- 5.Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29(2):102–10. 10.1089/apc.2014.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flash CA, Stone VE, Mitty JA, Mimiaga MJ, Hall KT, Krakower D, et al. Perspectives on HIV prevention among urban Black women: a potential role for HIV pre-exposure prophylaxis. AIDS Patient Care STDS. 2014;28(12):635–42. 10.1089/apc.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goparaju L, Praschan NC, Jeanpiere LW, Experton LS, Young MA, Kassaye S. Stigma, partners, providers and costs: potential barriers to PrEP uptake among US women. J AIDS Clin Res. 2017;8(9):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojikutu BO, Bogart LM, Higgins-Biddle M, Dale SK, Allen W, Dominique T, et al. Facilitators and barriers to pre-exposure prophylaxis (PrEP) use among Black individuals in the United States: results from the National Survey on HIV in the Black Community (NSHBC). AIDS Behav. 2018;22(11):3576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogart LM, Ransome Y, Allen W, Higgins-Biddle M, Ojikutu BO. HIV-related medical mistrust, HIV testing, and HIV risk in the National Survey on HIV in the Black Community. Behav Med. 2019;45(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschhorn LR, Brown RN, Friedman EE, Greene GJ, Bender A, Christeller C, et al. Black cisgender women’s PrEP knowledge, attitudes, preferences, and experience in Chicago. J Acquir Immune Defic Syndr. 2020;84(5):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koren DE, Nichols JS, Simoncini GM. HIV Pre-exposure prophylaxis and women: survey of the knowledge, attitudes, and beliefs in an urban obstetrics/gynecology clinic. AIDS Patient Care STDS. 2018;32(12):490–4. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese SK, Dovidio JF, Tekeste M, Taggart T, Galvao RW, Safon CB, et al. HIV pre-exposure prophylaxis stigma as a multidimensional barrier to uptake among women who attend planned parenthood. J Acquir Immune Defic Syndr. 2018;79(1):46–53. http://insights.ovid.com/crossref?an=00126334-201809010-00007. Accessed 12 Apr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert CC, Marrazzo J, Amico RK, Mugavero MJ, Elopre L. PrEParing women to prevent HIV: an integrated theoretical framework to PrEP Black women in the United States. J Assoc Nurses AIDS Care. 2018;29(6):835–48. http://journals.lww.com/00001782-201811000-00005. Accessed 12 Apr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DK, Toledo L, Smith DJ, Adams MA, Rothenberg R. Attitudes and program preference of African-American urban young adults about pre-exposure prophylaxis (PrEP). AIDS Educ Prev. 2012;24(5):408–21. [DOI] [PubMed] [Google Scholar]
- 15.Collier KL, Colarossi LG, Sanders K. Raising awareness of pre-exposure prophylaxis (PrEP) among women in New York City: community and provider perspectives. J Health Commun. 2017;22(3):1–7. 10.1080/10810730.2016.1261969. [DOI] [PubMed] [Google Scholar]
- 16.Chandler R, Hull S, Ross H, Guillaume D, Paul S, Dera N, et al. The pre-exposure prophylaxis (PrEP) consciousness of Black college women and the perceived hesitancy of public health institutions to curtail HIV in Black women. BMC Public Health. 2020;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nydegger LA, Dickson-Gomez J, Ko KT. A longitudinal, qualitative exploration of perceived HIV risk, healthcare experiences, and social support as facilitators and barriers to PrEP adoption among Black women. AIDS Behav. 2021;25(2):582–91. 10.1007/s10461-020-03015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond KT, Gunn AJ. Perceived advantages and disadvantages of using pre-exposure prophylaxis (PrEP) among sexually active Black women: an exploratory study. J Black Sex Relatsh. 2016;3(1):1–24. https://muse.jhu.edu/article/647629. Accessed 12 Apr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyra M, Johnson AK, Devlin S, Uvin AZ, Irby S, Stewart E, et al. HIV pre-exposure prophylaxis use and persistence among Black ciswomen: “Women Need to Protect Themselves, Period.” J Racial Ethn Health Disparities. 2021. 10.1007/s40615-021-01020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HIV Prevention Trials Network. HPTN 084 Study demonstrates superiority of CAB LA to oral FTC/TDF for the prevention of HIV. HIV Prevention Trials Network; 2020. https://www.hptn.org/news-and-events/press-releases/hptn-084-study-demonstrates-superiority-of-cab-la-to-oral-ftctdf-for. Accessed 25 June 2021 [Google Scholar]
- 21.FDA U.S. Food and Drug Administration. FDA approves first injectable treatment for HIV pre-exposure prevention. FDA News Release. FDA; 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention. Accessed 20 Dec 2021 [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update clinical practice guideline. CDC; 2021. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. Accessed 8 Dec 2021. [Google Scholar]
- 23.Philbin MM, Parish C, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, et al. Interest in long-acting injectable pre-exposure prophylaxis (LAI PrEP) among women in the Women’s Interagency HIV Study (WIHS): a qualitative study across six cities in the United States. AIDS Behav. 2021;25(3):667–78. 10.1007/s10461-020-03023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolley EE, Li S, Zangeneh SZ, Atujuna M, Musara P, Justman J, et al. Acceptability of a long-acting injectable HIV prevention product among US and African women: findings from a phase 2 clinical Trial (HPTN 076). J Int AIDS Soc. 2019;22(10):1–9. 10.1002/jia2.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese SK, Galvao RW, Dovidio JF, Willie TC, Safon CB, Kaplan C, et al. Contraception as a potential gateway to pre-exposure prophylaxis: US women’s pre-exposure prophylaxis modality preferences align with their birth control practices. AIDS Patient Care STDS. 2020;34(3):132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coelho LE, Torres TS, Veloso VG, Landovitz RJ, Grinsztejn B. Pre-exposure prophylaxis 2.0: new drugs and technologies in the pipeline. Lancet HIV. 2019;6(11):e788–99. [DOI] [PubMed] [Google Scholar]
- 27.Beymer MR, Holloway IW, Pulsipher C, Landovitz RJ. Current and future PrEP medications and modalities: on-demand, injectables, and topicals. Curr HIV/AIDS Rep. 2019;16(4):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Partnership for Microbicides. IPM’s new drug application for dapivirine vaginal ring to reduce HIV risk in women accepted for filing by US Food and Drug Administration. International Partnership for Microbicides; 2021. https://www.ipmglobal.org/content/ipm’s-new-drug-application-dapivirine-vaginal-ring-reduce-hiv-risk-women-accepted-filing-us. Accessed 4 Mar 2021. [Google Scholar]
- 29.van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, et al. The tablets, ring, injections as options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. 2018;21(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minnis AM, Browne EN, Boeri M, Agot K, van der Straten A, Ahmed K, et al. Young womenʼs stated preferences for biomedical HIV prevention. J Acquir Immune Defic Syndr. 2019;80(4):394–403. http://insights.ovid.com/crossref?an=00126334-201904010-00004. Accessed 8 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browne EN, Montgomery ET, Mansfield C, Boeri M, Mange B, Beksinska M, et al. Efficacy is not everything: eliciting women’s preferences for a vaginal HIV prevention product using a discrete-choice experiment. AIDS Behav. 2019. 10.1007/s10461-019-02715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nydegger LA, Dickson-Gomez J, Ko TK. Structural and syndemic barriers to PrEP adoption among Black women at high risk for HIV: a qualitative exploration. Cult Health Sex. 2021;23(5):659–73. 10.1080/13691058.2020.1720297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sales JM, Steiner RJ, Brown JL, Swartzendruber A, Patel AS, Sheth AN. PrEP eligibility and interest among clinic- and community-recruited young Black women in Atlanta, Georgia, USA. Curr HIV Res. 2018;16(3):250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beymer MR, Holloway IW, Grov C. Comparing self-reported demographic and sexual behavioral factors among men who have sex with men recruited through Mechanical Turk, Qualtrics, and a HIV/STI clinic-based sample: implications for researchers and providers. Arch Sex Behav. 2018;47(1):133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojikutu BO, Bogart LM, Mayer KH, Stopka TJ, Sullivan PS, Ransome Y. Spatial access and willingness to use pre-exposure prophylaxis among Black/African American individuals in the United States: cross-sectional survey. JMIR Public Health Surveill. 2019;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ransome Y, Bogart LM, Nunn AS, Mayer KH, Sadler KR, Ojikutu BO. Faith leaders’ messaging is essential to enhance HIV prevention among Black Americans: results from the 2016 National Survey on HIV in the Black Community (NSHBC). BMC Public Health. 2018;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ransome Y, Bogart LM, Kawachi I, Kaplan A, Mayer KH, Ojikutu B. Area-level HIV risk and socioeconomic factors associated with willingness to use PrEP among Black people in the U.S. South. Ann Epidemiol. 2020;42:33–41. 10.1016/j.annepidem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernán MA, Robins JM. Causal inference: what if. Boca Raton: Chapman Hill/CRC; 2020. [Google Scholar]
- 39.Flash CA, Stone VE, Mitty JA, Mimiaga MJ, Hall KT, Krakower D, et al. Perspectives on HIV prevention among urban Black women: A potential role for HIV pre-exposure prophylaxis. AIDS Patient Care STDS. 2014;28(12):635–42. http://www.ncbi.nlm.nih.gov/pubmed/25295393%5Cn, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4250961/pdf/apc.2014.0003.pdf. Accessed 12 Apr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–46. [DOI] [PubMed] [Google Scholar]
- 41.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–10. [DOI] [PubMed] [Google Scholar]
- 42.Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frew P, Parker K, Horton T, Hixson B, Flowers L, Priddy F, et al. Assessment of a microbicide candidate among a diverse cohort of urban Southern US women and their male sexual partners. J AIDS Clin Res. 2012. 10.4172/2155-6113.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montgomery ET, Noguchi LM, Dai JY, Pan J, Biggio J, Hendrix C, et al. Acceptability of and adherence to an antiretroviral-based vaginal microbicide among pregnant women in the United States. AIDS Behav. 2018;22(2):402–11. 10.1007/s10461-017-1811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craddock JB, Mangum LC, Aidoo-Frimpong G, Whitfield DL. The associations of HIV pre-exposure prophylaxis interest and sexual risk behaviors among young Black women. AIDS Patient Care STDS. 2021;35(7):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glidden DV, Mehrotra ML, Dunn DT, Geng EH. Mosaic effectiveness: Measuring the impact of novel PrEP methods. Lancet HIV. 2019;6(11):e800–6. https://linkinghub.elsevier.com/retrieve/pii/S2352301819302279. Accessed 8 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sales JM, Sheth AN. Associations among perceived HIV risk, behavioral risk and interest in PrEP among Black women in the southern US. AIDS Behav. 2018. 10.1007/s10461-018-2333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garfinkel DB, Alexander KA, McDonald-Mosley R, Willie TC, Decker MR. Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care. 2017;29(6):751–8. https://linkinghub.elsevier.com/retrieve/pii/S0031938416312148. Accessed 12 Apr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackstock OJ, Patel VV, Felsen U, Park C, Jain S. Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care. 2017;29(7):866–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sewell WC, Patel RR, Blankenship S, Marcus JL, Krakower DS, Chan PA, et al. Associations among HIV risk perception, sexual health efficacy, and intent to use PrEP among women: an application of the risk perception attitude framework. AIDS Educ Prev. 2020;32(5):392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sewell WC, Powell VE, Mayer KH, Ochoa A, Krakower DS, Marcus JL. Nondaily use of HIV preexposure prophylaxis in a large online survey of primarily men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2020;84(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson AK, Fletcher FE, Ott E, Wishart M, Friedman EE, Terlikowski J, et al. Awareness and intent to use pre-exposure prophylaxis (PrEP) among African American women in a family planning clinic. J Racial Ethn Health Disparities. 2020;7(3):550–4. [DOI] [PubMed] [Google Scholar]
- 53.Ojikutu BO, Nnaji C, Sithole J, Schneider KL, Higgins-Biddle M, Cranston K, et al. All Black people are not alike: differences in HIV testing patterns, knowledge, and experience of stigma between U.S.-Born and non-U.S.-Born Blacks in Massachusetts. AIDS Patient Care STDS. 2013;27(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutchler MG, McDavitt B, Ghani MA, Nogg K, Winder TJA, Soto JK. Getting PrEPared for HIV prevention navigation: young Black gay men talk about HIV prevention in the biomedical era. AIDS Patient Care STDS. 2015;29(9):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eaton LA, Kalichman SC, Price D, Finneran S, Allen A, Maksut J. Stigma and conspiracy beliefs related to pre-exposure prophylaxis (PrEP) and interest in using PrEP among Black and White men and transgender women who have sex with men. AIDS Behav. 2017;21(5):1236–46. 10.1007/s10461-017-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks RA, Nieto O, Landrian A, Fehrenbacher A, Cabral A. Experiences of pre-exposure prophylaxis (PrEP)-related stigma among Black MSM PrEP users in Los Angeles. J Urban Health. 2020;97(5):679–91. 10.1007/s11524-019-00371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aholou TM, Cooks E, Murray A, Sutton MY, Gaul Z, Gaskins S, et al. “Wake Up! HIV is at Your Door”: African American faith leaders in the Rural South and HIV perceptions: a qualitative analysis. J Relig Health. 2016;55(6):1968–79. [DOI] [PubMed] [Google Scholar]
- 58.Derose KP, Griffin BA, Kanouse DE, Bogart LM, Williams MV, Haas AC, et al. Effects of a pilot church-based intervention to reduce HIV stigma and promote HIV testing among African Americans and Latinos. AIDS Behav. 2016;20(8):1692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muturi N, An S. HIV/AIDS stigma and religiosity among African American women. J Health Commun. 2010;15(4):388–401. http://www.ncbi.nlm.nih.gov/pubmed/20574877. Accessed 8 July 2021. [DOI] [PubMed] [Google Scholar]
- 60.Payne-Foster P, Bradley ELP, Aduloju-Ajijola N, Yang X, Gaul Z, Parton J, et al. Testing our FAITHH: HIV stigma and knowledge after a faith-based HIV stigma reduction intervention in the Rural South. AIDS Care. 2018;30(2):232–9. 10.1080/09540121.2017.1371664. [DOI] [PubMed] [Google Scholar]
- 61.Duthely LM, Sanchez-Covarrubias AP, Brown MR, Thomas TE, Montgomerie EK, Dale S, et al. Pills, PrEP, and Pals: adherence, stigma, resilience, faith and the need to connect among minority women with HIV/AIDS in a US HIV epicenter. Front Public Health. 2021;9(June):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Randolph SD, Johnson R, Meyers D, Washington D, Saint-Hillaire L. Leveraging social networks of Black women in beauty salons to improve uptake of pre-exposure prophylaxis. Health Educ J. 2021;80(1):95–105. [Google Scholar]
- 63.Wilson TE, Fraser-White M, Williams KM, Pinto A, Agbetor F, Camilien B, et al. Barbershop talk with brothers: Using community-based participatory research to develop and pilot test a program to reduce HIV risk among Black heterosexual men. AIDS Educ Prev. 2014;26(5):383–97. http://libproxy.wustl.edu/login?url=, http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=25299804&site=ehost-live&scope=site. Accessed 8 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker JL, Brawner B, Cederbaum JA, White S, Davis ZM, Brawner W, et al. Barbershops as venues to assess and intervene in HIV/STI risk among young, heterosexual African American men. Am J Mens Health. 2012;6(5):368–82. [DOI] [PubMed] [Google Scholar]
- 65.Ross J, Stover J. Use of modern contraception increases when more methods become available: analysis of evidence from 1982–2009. Glob Health Sci Pract. 2013;1(2):203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Whitney Irie, upon reasonable request.


