Abstract
Objective
Glucomannan is a dietary fiber that slows the absorption of carbohydrates and suppresses appetite, thereby reducing blood glucose. This meta-analysis sought to examine the effect of glucomannan supplementation on Fasting Blood Glucose (FBG) and Postprandial Glucose (PPG) in adults.
Method
We searched PubMed, and SCOPUS databases, and Google Scholar from inception to May 2020, using relevant keywords. All randomized controlled clinical trials (RCTs) that examined the effect of glucomannan supplementation on FBG and PPG in adults were included. Weighted mean differences (WMD) and their 95% confidence interval (CI) were calculated using Stata. Subgroup analysis was used to discern possible sources of heterogeneity.
Results
Overall, 6 trials were included, consisting of 124 participants. We found that glucomannan supplementation significantly reduced FBG (WMD): -0.60 mmol/L, 95% CI: -1.16, -0.05; P=0.03, but not PPG (WMD: -2.07mmol/L ; 95% CI: -5.09, 0.95; P=0.18), compared with controls group. We conducted subgroup analysis based on dosage and duration of intervention and health status of the population. Findings from subgroup analysis revealed a significant effect of glucomannan supplementation on FBG in diabetic patients (WMD: -1.28 mmol/L, 95% CI: -2.54, -0.02; P=0.04).
Conclusion
Glucomannan supplementation can elicit significant reductions in FBG, but has no significant impact on PPG, in adults. More RCTs may find the exact effect of glucomannan on FBG and PPG.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-00993-6.
Keywords: Glucomannan, Blood glucose, Diabetes mellitus, Postprandial glucose
Introduction
According to the International Diabetes Federation) IDF(, about 463 million people worldwide are afflicted by diabetes, and it is estimated to increase up to 700 million people by the year 2045 [1]. About 90% of people with diabetes have Type 2 Diabetes Mellitus (T2DM) [2, 3]. Diabetes mellitus (DM) is defined by elevated blood glucose levels as a result of impaired insulin secretion or dysfunctional hormone response [4–6]. Genetics, obesity, low levels of physical activity, low socioeconomic status, and psychological stress are common risk factors for DM [7–11]. Prolonged hyperglycemia can lead to a wide variety of macro and microvascular complications in people with diabetes, including coronary heart disease, stroke, dialysis, retinopathy, and neuropathy, in addition to Lower-Extremity Amputations (LEA) [4, 12–14]. Medical Nutrition Therapy (MNT) and physical activity are considered vital in the prevention and treatment of DM and its associated complications and comorbidities [3, 15].
One of the most prevalent dietary recommendations is the consumption of dietary fibers [16]. Glucomannan is a fermentable water-soluble dietary fiber, that is emanate from konjac root (Amorphophallus konjac) [17]. It is used as an additive in traditional Asian cooking and also as a fiber supplement in the purified form [18]. The possible effects of glucomannan have been investigated in many studies, where it has been shown to reduce Total Cholesterol (TC) and Low-Density Lipoprotein Cholesterol (LDL-C) [19–21], induce body weight loss [22], and effectively reduce blood glucose level [18, 21, 23]. The underlying mechanism of glycemic parameter improvement is posited to be the slowed absorption of carbohydrates and suppression of appetite [24–26]. The high viscosity of glucomannan increases the gastrointestinal content viscosity as well; in fact, by extending the time for gastric emptying, absorption of nutrients in the small intestine decreases. Thus, the lowering rate of glucose absorption leads to an improved glycemic response [18, 27, 28].
However, several clinical studies dispute the advantages of glucomannan. One study displayed that insulin level was not different between glucomannan and placebo-treated groups [18]. Wood et al. concluded that adding Konjac-Mannan (KM) to a carbohydrate-restricted diet would not alter insulin level [29]; whilst Vuksan et al. reported that glucose and insulin level were not significantly changed by glucomannan in T2DM patients [25]. In 2008, Sood et al. conducted a systematic review and meta-analysis on the impact of glucomannan on plasma lipids, Fasting Blood Glucose (FBG), body weight, and blood pressure [26]. As reported by that meta-analysis, glucomannan favorably affected FBG, but this effect was not observe in people with diabetes. Moreover, a clinical study in 2013 reported that glucomannan did not significantly alter glucose parameters [30]. Therefore, given the inconsistent data, we conducted a systematic review and meta-analysis to evaluate the existing evidence for the effect of glucomannan supplementation on glycemic responses in people with and without diabetes.
Methods
The present study was conducted in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) [31]. We registered the protocol of this study in PROSPRO and the registration code is ID=CRD42021291876. We removed this sentences from methods section and insert that we restricted the search until May 2020 and also excluded if not published in English
Eligibility criteria
We included studies that met the following inclusion criteria: 1) RCTs 2) study participants were adults (≥18 years old) 3) Oral glucomannan supplements or studies that used glucomannan in conjunction with another diet if consumed in both treatment and control groups 4) FBG or/and PPG were outcome measures. Studies were excluded if they were literature reviews, observational studies, case reports, republished data, animal studies, and we searched grey literature for relevant studies.
Study selection
Titles and abstracts of all reached articles in the initial search were evaluated independently by 2 investigators (MR-A and R-Z). With utilizing a screen form with a hierarchical approach, based on study design, population or exposure, and outcome, articles that did not meet the eligibility criteria were excluded. Then, full-texts of eligible articles were retrieved and subjected to a second evaluation by the same investigators. Each discordances were argued and resolved by consensus.
Data extraction
Two independent investigators (R-Z and A-M) separately extracted data from the included publications using a standard data extraction form. The following data were collected from each study: first author’s name, study location, publication year, study sample size, number of participants in each group, health status of the population, study design (parallel/cross-over), gender of participants, mean or range of age, mean body mass index (BMI), study duration, type of diet, type and dose of glucomannan supplements, and mean and Standard Deviation (SD) of FBG and PPG at baseline and end of the study (Tables 1 and 2).
Table 1.
General characteristics of included studies
| Author (year) | Subjects and gender | Age range (y) | Design | Medication dosage (g/day) | Duration (week) | Outcomes | Outcome | Any other intervention | Notes about subjects | type of treatment of control group | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Mean ±SD | Control Mean ± SD | ||||||||||
| Wood et al. [29] | 30 Men20-69 | 20-69 | parallel | 3 | 12 | FBS | Before mmol/L): 5.09 ±0.83 | Before (mmol/L): 5.15±0.63 | carbohydraterestricted diet | 30 overweight and obese men | carbohydraterestricted diet |
| After (mmol/L):4.8 ±0.64 | After (mmol/L): 5.01±0.56 | ||||||||||
| Vuksan et al. [23] | 11men/women | 45–65 | crossover8 | 8-13 | 8 | FBS | Before (mmol/L):6.8±1.65 | Before (mmol/L):6.6± 0.99 | - | 11 subjects with impaired glucose tolerance | wheat bran |
| After (mmol/L):5.9±0.99 | After (mmol/L):5.9±1.32 | ||||||||||
| Chen et al. [21] | 22men/women | 52–77 | crossover | 3.6 | 8 | FBS | (FBS) | (FBS) | - | 22 subjects with hyperlipide mia and type 2 diabetes | Placebo |
| PPG | |||||||||||
| Before(mmol/L):9.4±2.3 | Before(mmol/L):8.7±1.8 | ||||||||||
| After (mmol/L): 8±1.8 | After (mmol/L):9.6±2.4 | ||||||||||
| (PPG) Before (mmol/L):13.8±4,6 | (PPG) Before (mmol/L):13±3.7 | ||||||||||
| After (mmol/L):11.5±3.2 | After (mmol/L):14.3±4.3 | ||||||||||
| Vuksan et al. [25] | 11 men/women | 45–65 | crossover | 15 | 8 | FBS | Before (mmol/L): 9.63±2.95 | Before (mmol/L):9.29±2.45 | - | 11 subjects with hyperlipidemia and type 2 diabetes | Wheat bran |
| After (mmol/L):8.62±3.15 | After (mmol/L): 8.99±2.58 | ||||||||||
| Chearskul et al. [18] | 20 Men/women | 30-70 | crossover | 3 | 10 | FBS | (FBS) | 8.8±0.26 | - | ||
| PPG | Before (mmol/L):9.1±3.3 | After (mmol/L):9.05±2.41 (PPG) | |||||||||
| After (mmol/L):8.83±3.08PPG) | Before (mmol/L): 16.89±3.89 | ||||||||||
| Before(mmol/L):17.36±4.02 | After (mmol/L):17.18±4.29 | ||||||||||
| After (mmol/L):17.13±4.42 | |||||||||||
| Zhang et al [34] | 30 Men/women | 19-65 | crossover | 6 | 4 | FBS | (FBS) Before (mmol/L): 5.1±0.79 | (FBS) Before (mmol/L):5.08±0.81 | - | 30 subjects with schizophre nia and dyslipidemia | Placebo |
| After (mmol/L):5.11±1.14 | After (mmol/L):5.27±0.87 | ||||||||||
Abbreviations: FBS, fasting blood glucose; PPG, post parandial glucose; SD, standard deviation
Table 2.
Subgroup analysis of included RCTs in meta-analysis of the effect of glucomannan supplementation on FBG
| Subgroup | WMD (95% Cl)'a | P value'* | P-heterogeneity | I2 b (%) | P for between subgroup heterogeneity | studies for each subgroup |
|---|---|---|---|---|---|---|
| Glucomannan dosage | <0.001 | |||||
| 3 g | -017 (-0.49, 0.14) | 0.28 | 0.592 | 0.0 | 2 | |
| >3 g | -.82 (-1.83, 0.19) | 0.11 | <0.0001 | 86.5 | 4 | |
| Duration (week) | <0.001 | |||||
| >8 | -0.17 (-0.49, 0.14) | 0.28 | 0.592 | 0.0 | 3 | |
| 8 | -.82 (-1.83, 0.19) | 0.11 | <0.0001 | 86.5 | 3 | |
| Health status | <0.001 | |||||
| Obese or overweight | -0.17 (-1.16, -0.05) | 0.12 | 0.98 | 0.0 | 2 | |
| Diabetic | -1.28 (-.2.54, -0.02) | 0.04 | 0.36 | 70.0 | 4 |
Quality assessment of studies
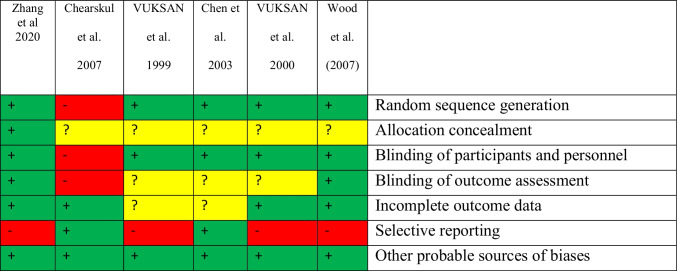
Two investigators (A-M and R-Z) assessed the quality of each selected study using the Cochrane risk of bias tools for RCTs. The risk of bias for the contained studies was assessed utilizing the Cochrane quality assessment tool for RCTs. The quality of studies was assessed using the following seven criteria: 1) random sequence generation 2) allocation concealment 3) blinding of participants and personnel 4) blinding of outcome assessment 5) incomplete outcome data 6) selective reporting and 7) other probable sources of biases. To evaluate the quality of studies, each study was judged as low risk, high risk, or unclear risk of bias. The quality assessment results of the articles are shown in Table 3.
Table 3.
Riskof bias summary for included studies
Statistical analysis
To analyze the effect size of FBG and PPG the mean change and its SD for both the intervention and control groups were extracted.
The random-effects model (DerSimonian and Laird method) was used to find the relationships [32]. we calculated any within-group changes that were not reported by subtracting the baseline mean from the final mean value in each group. The SDs for mean differences were computed utilizing the following formula [33]:
For trials which only reported the Standard Error of the Mean (SEM), SD was calculated using the following formula: SD =, where “n” is the number of participants in each group. Serum concentrations of glucose were converted to the units that most frequently used (mmol/L). Heterogeneity between studies was assessed by Cochrane's Q test (significance point at P<0.1) and I2 index.
Subgroup analysis based on the dose of supplementation, duration of the study, and health status of participants was performed to explore the potential sources of heterogeneity. Between subgroup heterogeneity was assessed using a fixed-effect model. Sensitivity analysis was conducted by removing each study, one by one, and recalculating the pooled evaluations. Begg's rank correlation test and Egger's regression asymmetry test were performed for detecting potential publication bias. Statistical analysis was conducted using STATA, Version 11.2 (Stata Corp, College Station, TX). The threshold for statistical significance value was, a priori, defined as P< 0.05.
Results
Study selection
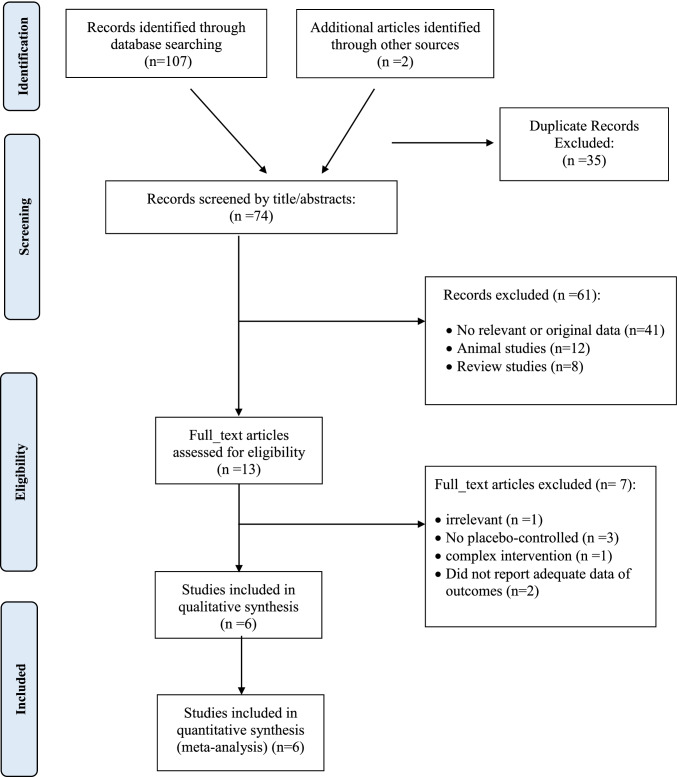
Of the 109 relevant publications that were initially identified in our search, 35 duplicate publications were removed. The remaining 74 records were screened, of which 61 irrelevant articles were omitted based on title and abstract screening. Then, 13 publications were selected for further evaluation of full texts. An additional 7 records were excluded for the following reasons: irrelevant studies (n = 1), studies without a suitable control group (n = 3), did not report adequate data of outcomes (n = 2), and complex intervention (n=1). Finally, a total of 6 eligible clinical trials were included in the meta-analysis. Out of 6 studies, 6 investigated the effect of glucomannan on FBG [18, 21, 23, 25, 29, 34], and 2 provided data on PPG [18, 21]. The flow diagram of the study selection process is presented in Fig. 1.
Fig. 1.
Flow chart of the number of studies identified and selected into the meta-analysis
Study characteristics
Overall, 6 studies were included in the current meta-analysis. The general characteristics of the 6 eligible RCTs are shown in Table 1. All studies were published between 1999 and 2020 and conducted in Thailand [18], Taiwan [21], Canada [23, 25], the USA [29], and China [34]. Sample sizes varied from 11 to 30 people, totaling 124 subjects enrolled in the studies. The age of participants ranged from 19 to 77 years, and mean baseline BMIs varied from 25 to 30 kg/m2 across studies. All trials were performed in men and women, except for the study by Wood et al. [29] which was just performed in men. Glucomannan was used as the intervention in the included studies, the dosage of glucomannan supplements ranged from 3 to 15 g/day, and the duration of glucomannan administration was from 4 to 12 weeks. Three trials [23, 25, 29] recommended a special diet concomitant to the glucomannan supplementation. Five trials employed a crossover design [18, 21, 23, 25, 34], while Wood et al. [29] was a parallel design. Two studies included overweight or obese people [23, 29] and three studies included people with T2DM [18, 21, 25] and one study included people with schizophrenia and dyslipidemia [34]. All included studies measured serum concentrations of FBG as the outcome and two trials also considered PPG as the outcome [18, 21].
Effects of glucomannan on FBG
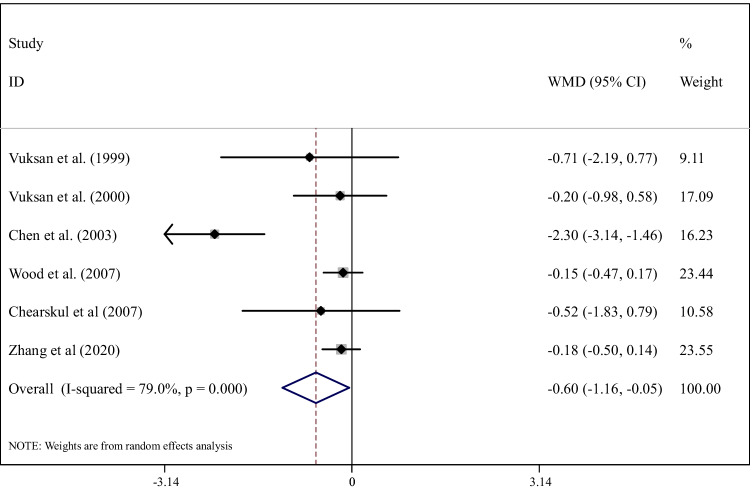
The effect of glucomannan supplementation on FBG and PPG was assessed in 6 studies, including 124 participants. We observed that glucomannan supplementation significantly reduced FBG (weighted mean differences (WMD): -0.60 mmol/L, 95% CI: -1.16, -0.05; P= 0.03) compared with controls (Fig. 2). Because of a high between-study heterogeneity (I2 =79.0%, P <0.0001), we conducted subgroup analysis based on the dosage of intervention (3g/d and >3g/d), duration of the intervention (8 weeks and >8 weeks), and health status of the population (overweight or obese people and person with type 2 diabetes), which considerably removed the heterogeneity (Table 2). These analyses indicated that glucomannan supplementation significantly reduced FBG in people with type 2 diabetes (WMD: -1.28 mmol/L, 95% CI: -2.54, -0.02; P=0.04). No other subgroup yielded significant changes.
Fig. 2.
Forest plot detailing weighted mean difference and 95% confidence intervals for the effect of glucomannan supplementation on FBG
Effects of glucomannan on PPG
The effect of glucomannan supplementation on PPG was assessed in 2 studies, including 42 participants. We observed that glucomannan supplementation significantly reduced PPG (weighted mean differences (WMD): -3.60 mmol/L, 95% CI: -5.20, -2.00) compared with controls in study of chen et al. However We did not observe that glucomannan supplementation significantly reduced PPG (weighted mean differences (WMD): -0.52 mmol/L, 95% CI: -5.09, 0.95) compared with controls in study of chearskul et al.
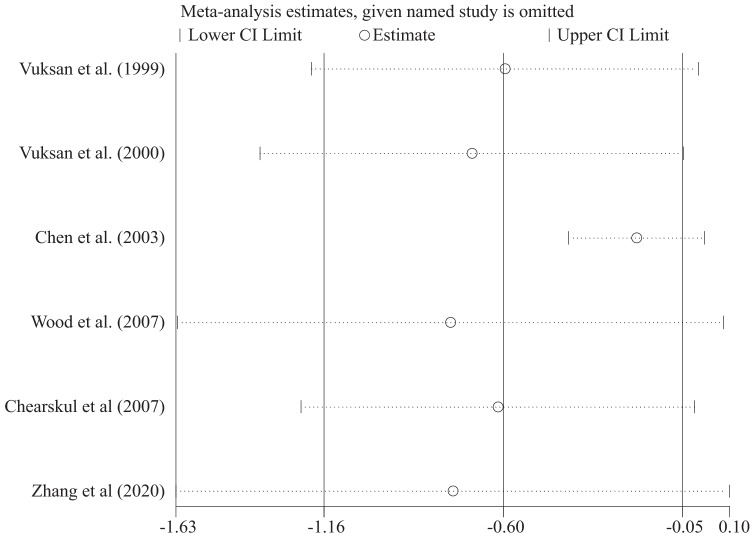
Sensitivity analysis
To identify the influence of every trial on the total effect size, we excluded each study and repeated the analysis of the effect sizes for the influence of glucomannan on FBG (Fig. 3).
Fig. 3.
Sensitivity analysis for the effect of glucomannan supplementation on FBG
Publication bias
Begg's test and Egger’s linear regression were implemented for the assessment of publication bias in the meta-analyses of glucomannan supplementation effects on FBG and PPG. These tests did not provide any evidence of publication bias; Begg's test (P= 0.091) and Egger’s linear regression (P=0.274). Moreover, visual inspection of the funnel plots approved the result of Egger’s linear regression.
Discussion
The systematic review and meta-analysis of current available RCTs, that investigated the effects of glucomannan supplementation on blood glucose, indicated a significant reduction in FBG, but not in PPG, in response to glucomannan supplementation in adults. In addition, subgroup analysis showed glucomannan supplementation significantly decreased FBG in people with diabetes.
Concordant with our results, several previous clinical trials indicated no significant effects of glucomannan on blood glucose [17, 23, 29, 30, 34, 35]. However, a previous clinical trial reported that glucomannan supplementation significantly reduced FBG in people with diabetes [21], and in an animal study, the administration of Konjac Glucomannan (KGM) significantly decreased the levels of FBG in T2DM rats, compared with the control group [36]. Moreover, Devaraj et al., in a critical review, reported that KGM supplementation could reduce blood glucose in people with diabetes who ingested a KGM-rich diet daily (0.7 g KGM/100 kcal intake) [28].
However, in some previous clinical trials, supplementation with glucomannan did not elicit any significant effects on FBG in people with diabetes [18, 25, 26] and reported that glucomannan significantly reduced PPG in people with diabetes [18] and healthy adults [37], respectively. In Boers et al., the effect of high fiber flour flatbreads containing 2/4 g KM on PPG in normal-weight adults was assessed. Accordingly, flatbreads with 4 g KM decreased PPG ≥30 % (p < 0.01), while consumption of 2 g KM did not significantly alter in PPG vs. the control group [37]. In this present review, the effect of glucomannan on PPG was only assessed in doses <4 g/d, in addition to the limited number of studies (2 RCTs), which are plausible reasons for no significant effects of glucomannan supplementation on PPG being found. Further, one previous meta-analysis of RCTs showed a significant reduction of FBG in adults, but this effect was not indicated in people with diabetes [26].
T2DM is a chronic metabolic disease that is typically caused by Insulin Resistance (IR), leading to increases in FBG and PPG. Genetic factors, weight gain caused by overeating, and reduced physical activity are common causes of, or contributors to, diabetes. Overeating leads to unnecessary insulin secretion, which negatively affects the capacity of insulin secretory pancreatic β-cells, leading to obesity and IR [28]. Unfortunately, most medication used in the treatment of diabetes concurrently increase body weight, contributing to dysfunctional glycemic control [18]. Glucomannan is a soluble dietary fiber that absorbs high amounts of water and increases the volume of content in the stomach, accordingly, this can lead to delays in stomach emptying, increases in satiety, and prevention of overeating [26, 28]. Glucomannan is a low-calorie fiber that decelerates the absorption of dietary sugar and it may reduce hepatic glucose production [28, 29]. Indeed, in vitro analysis has indicated that glucomannan can improve glycemic control and can be a beneficial dietary supplement for people with diabetes. Other health benefits previously described for glucomannan include lowering of triglycerides, cholesterol, blood pressure, and body weight, in addition to promoting intestinal activity and preventing constipation. On the basis of the available data, glucomannan appears to be well tolerated, although it has been associated with various gastrointestinal complaints, including abdominal discomfort, diarrhea, loose stools, and flatulence [26, 28].
Strengths and limitations
In the present review, we assessed the effect of glucomannan supplementation on FBG and PPG in people with and without diabetes, which is novel. In addition, a previous meta-analysis only assessed the effects of glucomannan on FBG, but not PPG, while in this meta-analysis, we evaluated the effects of glucomannan supplementation on both FBG and PPG. However, despite the novelty of the present study, some limitations should be considered, including the limited number of RCTs. In addition, between included studies dosage of glucomannan, duration of studies, and types of intervention were different, however, we tried to consider them in the subgroup analysis. Based on the present study, it is clear that further RCTs, of varying duration and glucomannan dose, be conducted, particularly in relation to PPG.
Conclusion
The current study revealed that glucomannan supplementation can elicit a significant reduction in FBG in adults, but not in PPG. Moreover, subgroup analysis indicated that glucomannan supplementation may be particularly useful in people with diabetes.
Supplementary Information
(PDF 82 kb)
Abbreviations
- CI
Confidence Intervals
- DM
Diabetes mellitus
- FBG
Fasting Blood Glucose
- IDF
International Diabetes Federation
- IR
Insulin Resistance
- KGM
konjac Glucomannan
- KM
Konjac-Mannan
- LDL-C
Low-Density Lipoprotein Cholesterol
- LEA
Lower-Extremity Amputations
- MeSH
Medical Subject Headings
- MNT
Medical Nutrition Therapy
- PPG
Postprandial Glucose
- PRISMA-P
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
- RCTs
Randomized Controlled Trials
- SEM
Standard Error of the Mean
- TC
Total Cholesterol
- T2DM
Type 2 Diabetes Mellitus
- WMD
Weighted Mean Differences
Author contributions
AM and RZ contributed to the study concept and design; MRA and RZ designed search strategy and screened Papers; SM performed statistical analysis; AM, RZ, NR, SP and FA wrote the first draft of the manuscript; KhM was supervisor of the article; all authors read and approved the final manuscript.
Funding
This study was supported by the Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no competing interest in this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hassanein M, Amod A, Khunti K, Lee MK, Mohan V. Introduction: Real-World Evidence in Type 2 Diabetes. Diabetes Ther. 2020;11(2):29–32. doi: 10.1007/s13300-020-00832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahed K, Sasangohar F, Mehta R, Erraguntla M, Qaraqe K: Diabetes management experience and the state of hypoglycemia: A national online survey. J MIR Diabetes 2020. [DOI] [PMC free article] [PubMed]
- 3.Khazrai YM, Defeudis G, Pozzilli P. Effect of diet on type 2 diabetes mellitus: a review. Diabetes Metab Res Rev. 2014;30(Suppl 1):24–33. doi: 10.1002/dmrr.2515. [DOI] [PubMed] [Google Scholar]
- 4.Kaura Parbhakar K, Rosella LC, Singhal S, Quinonez CR. Acute and chronic diabetes complications associated with self-reported oral health: a retrospective cohort study. BMC Oral Health. 2020;20:66. doi: 10.1186/s12903-020-1054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly F, Dan W, Wei C, Wu A. Vascular cognitive impairment and dementia in type 2 diabetes mellitus: An overview. Life Sci. 2020;254:117771. doi: 10.1016/j.lfs.2020.117771. [DOI] [PubMed] [Google Scholar]
- 6.Moscovici K, Wainstock T, Sheiner E, Pariente G. The association between family history of diabetes mellitus and offspring long-term neurological hospitalisation. Acta Paediatr. 2020;109:1236–1242. doi: 10.1111/apa.15078. [DOI] [PubMed] [Google Scholar]
- 7.Bitton S, Wainstock T, Sheiner E, Landau D, Avigan L, Pariente G. Is there an association between family history of diabetes mellitus and long-term cardiovascular hospitalizations of offspring? Prim Care Diabetes. 2019;13:529–534. doi: 10.1016/j.pcd.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 9.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 10.Irene R. Diabetes Mellitus and Stroke - A cross Sectional Study of 2.5 Million Adults in the United States. Maedica (Buchar) 2020;15:24–31. doi: 10.26574/maedica.2020.15.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 13.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 14.Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z: Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev 2015:Cd004714. [DOI] [PMC free article] [PubMed]
- 15.Birkeland E, Gharagozlian S, Birkeland KI, Valeur J, Mage I, Rud I, Aas AM. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr. 2020;59(7):3325–3338. doi: 10.1007/s00394-020-02282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.5. Lifestyle Management Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S46–s60. doi: 10.2337/dc19-S005. [DOI] [PubMed] [Google Scholar]
- 17.Suwannaporn P, Tester RF, Al-Ghazzewi FH, Artitdit P. Effect of short term administration of konjac glucomannan hydrolysates on adult blood lipid parameters and glucose concentrations. Nutr Food Sci. 2015;45(4):616–624. doi: 10.1108/NFS-02-2015-0012. [DOI] [Google Scholar]
- 18.Chearskul S, Sangurai S, Nitiyanant W, Kriengsinyos W, Kooptiwut S, Harindhanavudhi T. Glycemic and lipid responses to glucomannan in Thais with type 2 diabetes mellitus. Med J Med Assoc Thailand. 2007;90:2150. [PubMed] [Google Scholar]
- 19.Martino F, Martino E, Morrone F, Carnevali E, Forcone R, Niglio T. Effect of dietary supplementation with glucomannan on plasma total cholesterol and low density lipoprotein cholesterol in hypercholesterolemic children. Nutr Metab Cardiovasc Dis. 2005;15:174–180. doi: 10.1016/j.numecd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Vanstone CA, Parsons WD, Zawistowski J, Jones PJ. Effect of plant sterols and glucomannan on lipids in individuals with and without type II diabetes. Eur J Clin Nutr. 2006;60:529–537. doi: 10.1038/sj.ejcn.1602347. [DOI] [PubMed] [Google Scholar]
- 21.Chen HL, Sheu WH, Tai TS, Liaw YP, Chen YC. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects--a randomized double-blind trial. J Am Coll Nutr. 2003;22:36–42. doi: 10.1080/07315724.2003.10719273. [DOI] [PubMed] [Google Scholar]
- 22.Birketvedt GS, Shimshi M, Erling T, Florholmen J. Experiences with three different fiber supplements in weight reduction. Med Sci Monit. 2005;11:Pi5–Pi8. [PubMed] [Google Scholar]
- 23.Vuksan V, Sievenpiper JL, Owen R, Swilley JA, Spadafora P, Jenkins DJ, Vidgen E, Brighenti F, Josse RG, Leiter LA, et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23:9–14. doi: 10.2337/diacare.23.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Jenkins AL, Wolever TM, Vuksan V, Rao AV, Thompson LU, Josse RG. Low glycemic index: lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr. 1994;59:706s–709s. doi: 10.1093/ajcn/59.3.706S. [DOI] [PubMed] [Google Scholar]
- 25.Vuksan V, Jenkins DJ, Spadafora P, Sievenpiper JL, Owen R, Vidgen E, Brighenti F, Josse R, Leiter LA, Bruce-Thompson C. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care. 1999;22:913–919. doi: 10.2337/diacare.22.6.913. [DOI] [PubMed] [Google Scholar]
- 26.Sood N, Baker WL, Coleman CI. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: systematic review and meta-analysis. Am J Clin Nutr. 2008;88:1167–1175. doi: 10.1093/ajcn/88.4.1167. [DOI] [PubMed] [Google Scholar]
- 27.Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:790–801. doi: 10.1111/nure.12076. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj RD, Reddy CK, Xu B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int J Biol Macromol. 2019;126:273–281. doi: 10.1016/j.ijbiomac.2018.12.203. [DOI] [PubMed] [Google Scholar]
- 29.Wood RJ, Fernandez ML, Sharman MJ, Silvestre R, Greene CM, Zern TL, Shrestha S, Judelson DA, Gomez AL, Kraemer WJ. Effects of a carbohydrate-restricted diet with and without supplemental soluble fiber on plasma low-density lipoprotein cholesterol and other clinical markers of cardiovascular risk. Metabolism. 2007;56:58–67. doi: 10.1016/j.metabol.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Keithley JK, Swanson B, Mikolaitis SL, DeMeo M, Zeller JM, Fogg L, Adamji J. Safety and efficacy of glucomannan for weight loss in overweight and moderately obese adults. J Obes. 2013;2013:610908. doi: 10.1155/2013/610908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Han Y, Zhao Z, Liu X, Xu Y, Cui G, Zhang X, Zhang R. Beneficial effects of konjac powder on lipid profile in schizophrenia with dyslipidemia: A randomized controlled trial. Asia Pac J Clin Nutr. 2020;29:505–512. doi: 10.6133/apjcn.202009_29(3).0009. [DOI] [PubMed] [Google Scholar]
- 35.Morgan L, Tredger J, Wright J, Marks V. The effect of soluble-and insoluble-fibre supplementation on post-prandial glucose tolerance, insulin and gastric inhibitory polypeptide secretion in healthy subjects. Br J Nutr. 1990;64:103–110. doi: 10.1079/BJN19900013. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Nie Q, Hu J, Huang X, Zhang K, Pan S, Nie S. Hypoglycemic and hypolipidemic effects of glucomannan extracted from konjac on type 2 diabetic rats. J Agric Food Chem. 2019;67:5278–5288. doi: 10.1021/acs.jafc.9b01192. [DOI] [PubMed] [Google Scholar]
- 37.Boers HM, MacAulay K, Murray P, Ten Hoorn JS, Hoogenraad A-R, Peters HP, Vente-Spreeuwenberg MA, Mela DJ. Efficacy of different fibres and flour mixes in South-Asian flatbreads for reducing post-prandial glucose responses in healthy adults. Eur J Nutr. 2017;56:2049–2060. doi: 10.1007/s00394-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 82 kb)
Data Availability Statement
Not applicable.