Abstract
Purpose
Non-alcoholic fatty liver disease (NAFLD) is caused by the increase of fat in the liver. The present study aimed to study the association between different dietary patterns and NAFLD in adults.
Methods
This study included 121 adult patients with NAFLD and 119 non-NAFLD. Dietary intake was calculated by a 168-item food frequency questionnaire. Biochemical markers were measured. Dietary patterns were determined by factor analysis. The association between dietary patterns and NAFLD was evaluated using multiple logistic regression analysis.
Results
Two dietary patterns (healthy, western) were recognized in participants. Western dietary pattern was related with 72 percent increase in the odds of NAFLD (OR: 1.72; 95% CI: 1.32,2.14), after adjustment for covariates. Healthy dietary pattern was associated with 38 percent lower odds of NAFLD (OR: 0.38; 95% CI: 0.11, 0.65). Adherence to the western diet was related to 0.486 greater amounts of ALT, 3.248 mg/dl higher levels of FBS, and 3.989 mg/dl greater amounts of TG and 2.354 mg/dl greater amounts of MDA after adjusting for confounding factors (p > 0.001, p = 0.042, p > 0.001, p = 0.036 respectively). The healthy dietary pattern score was negatively associated with FBS and Cholesterol and TG levels (p = 0.035, p = 0.048, and p = 0.025), respectively. Moreover, it was associated with 3.211 mg/dl higher levels of TAC (p = 0.049).
Conclusions
There is a significant relationship between dietary patterns and non-alcoholic fatty liver disease. Adherence to a western dietary pattern is related to an increase in non-alcoholic fatty liver disease.
Keywords: Non-alcoholic fatty liver disease, Diet, Western diet, Healthy diet, Dietary patterns
Introduction
Fatty liver disease is one of the most common liver disorders and is known to be the main reason of liver damage in developed countries [1]. The increasing prevalence of NAFLD has led to a significant increase in health care and the economic burden. The 10-year NAFLD forecast is projected to increase to $1.005 trillion in the United States and €334 billion in Europe [2]. NAFLD has been suggested as a major cause of cirrhosis and liver carcinoma in recent years. NAFLD also increases the risk of all-cause mortality and cardiovascular disease [3]. Previous studies showed that 34% of people in the United States who had this disease were within the ages of 30–65 [4]. According to a previous study, the non-alcoholic fatty liver disease (NAFLD) prevalence in the general population of Iran is 10–35% [5]. Its prevalence increased by age [6] and it was higher in men [7]. Many factors are considered to be involved in the pathology of this disorder like genetic, metabolic, microbial, and environmental [1]. NAFLD along with fat accumulation in hepatocytes and it usually occurs in people with obesity and increased insulin resistance [4].
Dietary composition is an environmental factor that may influence the risk of developing NAFLD [8]. Overeating or diets rich in high-glycemic carbohydrates, saturated fats of fats, and cholesterol are associated with risk of NAFLD in adults [9]. In NAFLD patients, high consumption of red meat, fat and sweets was seen, while consumption of whole grains, fruits and vegetables was low [10]. High consumption of red meat and sugary drinks in the western diet causes oxidative stress throughout the body and increases body fat, which can lead to NAFLD [11]. Many biochemical processes such as oxidative stress, mitochondrial dysfunction, and increased expression of pro-inflammatory markers are responsible for initiating NAFLD [12]. It is evident that higher fruit and vegetable consumption is associated with increased total antioxidant capacity(TAC) and glutathione peroxidase activity(GSH-PX) [12]. Studies have shown that insufficient consumption of antioxidants increase oxidative stress [13]. According to another study, NAFLD patients reported lower intake of antioxidants, including vitamin C, vitamin A, vitamin E and selenium, compared to healthy individuals [14].
There are some articles about the association between dietary intake and NAFLD, concentrating on the effects of individual foods or nutrients rather than whole dietary patterns [15, 16]. However, diets are made of a diverse range of nutritional components, which may have independent as well as synergistic properties that are beyond the effect of individual nutrients in isolation [17]. Therefore, we aimed to investigate the relationship between dietary patterns and NAFLD. In addition, the association between some biochemical markers including white blood cells (WBC), Hemoglobin (HB), aspartate aminotransferase (AST), Serum alanine aminotransferase (ALT), creatinine (CR), fasting blood sugar (FBS), cholesterol (CHOL), triglycerides (TG), serum total antioxidant capacity (TAC) and Serum Malondialdehyde (MDA) with NAFLD in adults was examined.
Methods
Subjects
This case–control study was conducted at Isfahan from September 2019 to February 2020. This investigation was permitted by the Ethics Committee of Isfahan University of Medical Sciences. (Ethics code: IR.MUI.REC. 1398.279 and study project code: 398,293). Written informed consent was obtained from all subjects prior to participation and were entitled to withdraw from the study at any time. The sample size for this study (n = 240) was powered to detect a statistically significant effect using an alpha of 0.05 and a power of 90% as a significant result measure (odds ratio (OR)) of 1.45 [18].
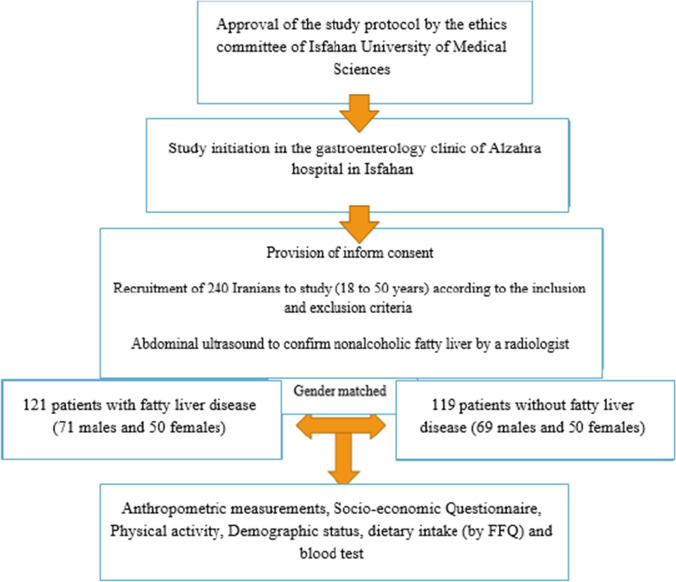
To begin with, 254 participants at the age of 18–50 years were involved in this study. They were all from Isfahan provinces. We excluded all participants with chronic liver disease, including 1 participant had cirrhosis, 8 participants who were positive for the hepatitis B virus and 2 participants who were positive for the hepatitis C virus. The remaining participants (n = 240), who were included in the study, were divided into two separate groups (121 cases and 119 controls). Individuals who were diagnosed with NAFLD by a specialist physician based on the liver sonography (presence of steatosis) were included in the case group. The control group were selected randomly from the general population during the same time period and frequency-matched for sex to the case group. People in the control group did not have fatty liver disease. The recruitment procedure for eligible participants are represented in Fig. 1. These subjects were recruited consecutively.
Fig. 1.
Chart for selection and registration of study subjects (cases and controls). NAFLD: Nonalcoholic fatty liver disease; FFQ: Food Frequency Questionnaire
Inclusion criteria were the following items: A) Confirmation of fatty liver disease by abdominal ultrasonography (cases only); B) willingness to participate in the study; C) age of 18 to 50 years; D) absence of concomitant liver injury at the time of recruitment (viral, autoimmune, genetic or drug induced); E) not following a specific diet from the time of diagnosis. Individuals with any of the following criteria were excluded: A) subjects without demographic or anthropometric data; B) individuals who report daily calorie intake > 5000 or < 800 kilocalories C) weight loss of more than 10% in the last three months [19] D) Menopausal women.
Dietary assessment
Dietary intake of all participants were evaluated by a validated semi-quantitative food frequency questionnaires, which included 168 food items [20]. The participants reported the frequency of consuming each food item on a daily, weekly, monthly or yearly basis. Questions were asked via face-to-face interviews and recorded by a trained nutritionist. To assess the energy and nutrient intakes, the household measures and the USDA food composition database which modified for Iranian foods [21, 22], were used to convert the consumed food portion sizes to grams. The calculations were also performed by a modified version of NUTRITIONIST IV software for Iranian foods [23] (version 7.0; N-Squared Computing, Salem, OR, USA).
Other assessments
In this study, socioeconomic status and demographic characteristics were taken using a questionnaire. Information like age, sex, occupation, education, marital status, and current smoking status were assembled.
Weight was measured with light clothing and without shoes, by using a digital weighing scale (Seca725 GmbH & Co. Hamburg, Germany) to the nearest 100 g and the height was measured while standing and keeping the shoulders and hips against the wall without shoes, using a stadiometer (Seca, Germany) with an accuracy of 0.1 cm. Body mass index (BMI) was calculated as weight divided by height squared (expressed as kg/m2). The waist and hip circumference were measured with measuring tape, with an approximate of 0.5 cm from under the rib cage and above the iliac crest.
Physical activity was computed using the validated International Physical Activity Questionnaires (IPAQ) questionnaire [24]. Participants were requested to state their daily activities like jogging, sports, and hours dedicated to watching TV, reading, or eating. Total activity was stated for 24 h and metabolic equivalents (MET) were computed.
Biochemical assessment
All biochemical valuations were done at the hospital laboratory by standard laboratory processes. Blood samples were assembled in the morning after 12 h overnight fast. Totally, 11 mL of blood was taken from all participants. Two-mL venous blood samples were used to measure the white blood cells (WBC) and Hemoglobin (Hb) by Sysmex® Hematology instrument. Remainder samples were immediately centrifuged at 3000 rpm for 10 min to separate serum and kept at − 80C. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total cholesterol (TC), creatinine (CR), and fasting blood sugar (FBS) were determined by a photometric method using commercially available kits. Serum Malondialdehyde (MDA) level was determined by colorimetric assay using thiobarbituric acid reagent. MDA reacts with thiobarbituric acid in an acidic environment to form a pink compound which is read at 532 nm [25]. Moreover, the serum total antioxidant capacity (TAC) was determined by measuring the ability of the serum to recover ferric ions. In this method, the antioxidants in the serum ferric ion are converted to ferrous ions at low PH, which in combination with tripyridyltriazine, create a color complex the intensity of which was measured at 593 nm in this study [26].
Statistical analysis
Factor analysis was used to determine the food patterns. In this analysis, the Varimax rotation method was used to create a simple and differentiating matrix. Factor loading values greater than 0.3 were considered to determine the items of each food pattern [27]. Diet patterns were named based on the food characteristics of each pattern. Dietary patterns were divided into quartiles, that quartile 1 show low adherence and quartile 4 show high adherence to the dietary pattern. The statistical package SPSS for windows 22 (SPSS Inc., Chicago, IL) was applied for the analysis. Chi-square test was used to compare the frequency of qualitative variables between the study groups and the independent t-test used to compare the mean of quantitative variables between the study groups. Values are expressed as mean ± SD for quantitative variables, and frequency (%) for qualitative variables. Relationship between each dietary pattern and fatty liver was examined with logistic regression models. Linear regression models used to detect relations between the dietary patterns’ z-scores and NAFLD-related biochemical markers. The significance level was determined at p < 0.05.
Results
Table 1 Anthropometric and biochemical markers among case (non – alcoholic fatty liver patients) and control groups are demonstrated in Table 1. In case group, the mean ± SD of participants’ age was 38.04(6.7) year for men and 35.6(10.2) year for women. Moreover, 50(41.3%) of case were women, and BMI was 28.6 (4.03279) for men and 23.28 (4.14167) for women. The results of the independent t- test showed that the case group were older (p = 0.037), had a higher body weight (p < 0.001), higher waist circumference (p < 0.001), higher hip circumference (p < 0.001), higher BMI (p < 0.001) and lower physical activity levels (p = 0.047) than the control group. Moreover, NAFLD patients had higher levels of AST (p < 0.001), ALT(p < 0.001), FBS(p < 0.001), TG (p < 0.001) and CHOL (< 0.001) compared to controls. Demographic factors were largely the same except for job (p < 0.001) and education (p = 0.002). The level of education was higher in healthy group (Master and PHD) while in the fatty liver group, the percentage of people with a diploma was higher. The number and percentage frequency in healthy subjects in terms of Master and PhD group were 36(30.3%) while in the patient group were 19(15.7%). In patients' group, employee 36(29.7%) and in the control group, students 35(29.4%) made up high percentage of participants.
Table 1.
Characteristics of NAFLD cases and controls
| Baseline characteristics | Case (N = 121) | Control (N = 119) | *P_value |
|---|---|---|---|
| Gender | 0.91 | ||
| Men | 71(58.7%) | 69(58%) | |
| Women | 50(41.3%) | 50(42%) | |
| Smoking | 0.15 | ||
| Yes | 12(9.9%) | 6(5%) | |
| No | 109(90.1%) | 113(95%) | |
| Alcohol | 0.71 | ||
| Yes | 4 (3.3%) | 3 (2.5%) | |
| No | 117(96.7%) | 116(97.5%) | |
| Education | 0.002 | ||
| School education | 20(16.5%) | 15(12.6%) | |
| Diploma | 43(35.5%) | 27(22.7%) | |
| Bachelor | 39(32.2%) | 41(34.5%) | |
| Master and PhD | 19(15.7%) | 36(30.3%) | |
| Job | < 0.001 | ||
| Employ | 36(29.7%) | 17(14.3%) | |
| Private | 6(5%) | 10(8.4%) | |
| Student | 2(1.7%) | 35(29.4%) | |
| Housewife | 36(29.85) | 21(17.6%) | |
| self-employ | 32(26.4%) | 22(18.5%) | |
| Unemployed | 9(7.4%) | 14(11.8%) | |
| SES | 0.114 | ||
| Poor | 10 (8.3) | 10 (8.4) | |
| Middle | 78 (64.5) | 90 (75.6) | |
| Excellent | 33 (27.3) | 19 (16.0) | |
| Supplement | 0.561 | ||
| NO | 97(81.5%) | 95(78.5%) | |
| Yes | 22(18.5%) | 26(21.5%) | |
| Age (year) | 38.04(6.7) | 35.6(10.2) | 0.037 |
| Height(cm) | 170.5(11.1) | 168.06(9.4) | 0.062 |
| Weight(cm) | 82.9(11.4) | 65.7(12.4) | < 0.001 |
| Waist circumference(cm) | 102.5(10.7) | 81.7(10.3) | < 0.001 |
| Hip circumference(cm) | 102.2(10.5) | 91.9(10.5) | < 0.001 |
| BMI(kg/m2) | 28.6045(4.03279) | 23.2884(4.14167) | < 0.001 |
| Physical activity (Mets × hours/week) | 9.90(3.51) | 10.62(5.9) | 0.047 |
| Energy Intake (kcal/d) | 3220 (31.9) | 2126 (40.2) | < 0.001 |
| WBC (cells/µL) | 3185.8 ± 186.39 | 3173.86 ± 175.86 | 0.55 |
| HB (g/dl) | 14.22 ± 1.64 | 13.99 ± 1.34 | 0.12 |
| AST (IU/L) | 33.3 ± 10.35 | 21.35 ± 7.84 | < 0.001 |
| ALT (IU/L) | 44.5 ± 13.64 | 22.1 ± 6.68 | < 0.001 |
| CR (mg/dL) | 1.10 ± 0.22 | 1.10 ± 0.19 | 0.65 |
| FBS (mg/dL) | 114.23 ± 20.25 | 97.83 ± 12.61 | < 0.001 |
| CHOL (mg/dL) | 197.1 ± 44.9 | 172.05 ± 26.47 | < 0.001 |
| TG (mg/dL) | 160.32 ± 31.96 | 118.85 ± 28.18 | < 0.001 |
| TAC (nmol/mg) | 13.88 (9.54) | 17.70 (8.56) | < 0.001 |
| MDA (nmol/mg) | 2.64 (1.00) | 1.33 (0.58) | < 0.001 |
Due to the normality of the data and the homogeneity of the variance, the independent t-test was used to compare the variables in two groups. * Chi-square test was used to compare the qualitative variables in study groups. Values of P < 0.05 indicate the significance of the test. Note: Values are expressed as mean ± SD for quantitative variables, and frequency (%) for qualitative variables. SES; Socioeconomic Status, MET; metabolic equivalent of task, WBC; white blood cell, HB; hemoglobin FBS; fasting blood sugar AST; Aspartate transaminase; ALT: Alanine transaminase; TG: Triglycerides; CHOL; cholesterol CR; creatinine; TAC: Total antioxidant capacity; MDA: Malondialdehyde
Factor analysis showed two dietary patterns (healthy, western), and the main factor loadings of each pattern for participants are shown in Table2. These two dietary patterns explained 24.85% of the total variance of food intake. The healthy dietary pattern contains pumpkin, celery, melon, watermelon, pear, apple, orange, persimmon, tangerine, pomegranate, peach, grape, sweet lemon, black pepper, greengage, nectarine, kiwi, fish, nuts, lettuce, spinach, beans, dough, tea, low fat dairy, coffee, and refined grains. The western dietary pattern contains sausage, tongue, brain, Kalla Pacha, pizza, lamb, hamburger, and lunch meat.
Table 2.
Rotated factor loading matrix for the two dietary patterns identified from the food frequency questionnaires
| Food groups | The first pattern (healthy pattern) |
The second pattern (western pattern) |
|---|---|---|
| Fish | 0.59 | |
| Fruit | 0.47 | |
| Vegetables | 0.68 | |
| Nuts | 0.53 | |
| Lettuce and spinach | 0.56 | |
| Beans | 0.47 | |
| Dough | 0.52 | |
| Tea | 0.56 | |
| Low fat dairy | 0.35 | |
| Coffee | 0.68 | |
| Refined grains | -0.12 | 0.75 |
| Butter | 0.52 | |
| mayonnaise sauce | 0.92 | |
| Soft drinks | 0.49 | |
| Meat products | 0.33 | |
| Snack | 0.55 | |
| Liquid oil | 0.39 | |
| Red Meat | 0.27 | |
| Sweets and desserts | 0.49 | |
| Total explained variance (%) | 10.93 | 13.92 |
Absolute factor loading values < 0.30 for both patterns were excluded for simplicity. These two dietary patterns explained 24.85% of the total variance in food intake
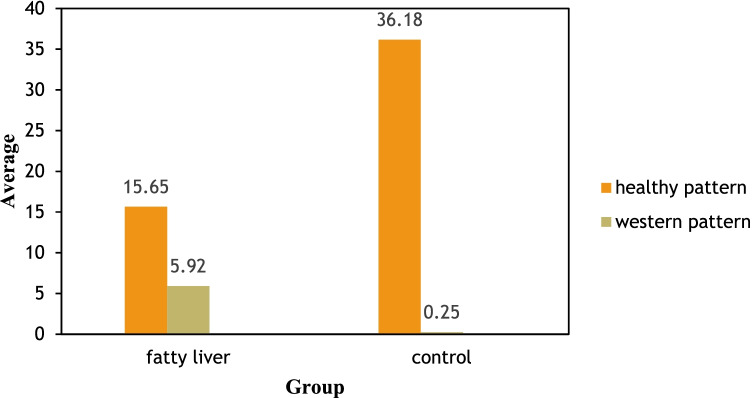
Mean scores for all dietary patterns in the case and control groups are shown in Fig. 2. Control group had a higher compliance for healthy dietary pattern (p > 0.05).
Fig. 2.
Comparison of dominant nutritional patterns in case and control group
The relationship between the dietary patterns and the odds of NAFLD is shown in Table 3. Those in the fourth quartile of western dietary pattern had a 72 percent increase in the odds of NAFLD (OR: 1.72; 95% CI; 1.32, 2.14), after adjustment for covariates in model 3. Furthermore, in the highest quartile of healthy dietary pattern we observed a 38 percent reduction in odds of NAFLD (OR: 0.38; 95% CI: 0.11–0.65).
Table 3.
Association between two dietary patterns and risk of non-alcoholic fatty liver disease (N = 240)
| Model 1* Odds ratio (95% confidence interval) |
Model 2* Odds ratio (95% confidence interval) |
Model 3* Odds ratio (95% confidence interval) |
|
|---|---|---|---|
| Healthy dietary pattern | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.75(0.38- 0.1.07) | 0.69 (0.52–0.82) | 0.62 (0.37—0.84) |
| Q3 | 0.41(0.26- 0.55) | 0.45 (0.25–0.64) | 0.49 (0.31–0.68) |
| Q4 | 0.54(0.39–0.64) | 0.42 (0.17, 0.50) | 0.38 (0.11–0.65) |
| P trendc | 0.994 | 0.16 | 0.045 |
| Western dietary pattern | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.35 (0.45–1.62) | 0.61 (0.36, 0.85) | 1.18 (0.54, 1.82) |
| Q3 | 1.06 (0.48–1.79) | 1.01 (0.84, 1.19) | 1.36 (0.75, 1.98) |
| Q4 | 1.87 (0.64–3.11) | 1.52 (1.36,1.68) | 1.72 (1.32, 2.14) |
| P trendc | 0.085 | 0.056 | 0.015 |
• Model 1*: crude
• Model 2*: adjusted for age, energy intake, weight, hip circumference, education and BMI
• Model 3*: adjusted for age, energy intake, weight, hip circumference, education, BMI and PAL
• P trendc Test for trend through quartiles from logistic regression model
Model 2: adjusted for age, energy intake, weight, hip circumference and BMI. Model 3: adjusted for age, energy intake, weight, hip circumference, education, BMI and PAL.
Table 4 shows a 1-unit rise in the “western diet” score was related to 0.486 greater amounts of ALT, 3.248 mg/dl higher levels of FBS, and 3.989 mg/dl greater amounts of TG and 2.354 mg/dl greater amounts of MDA after adjusting for confounding factors (beta = 0.486 p > 0.001, beta = 3.248 p = 0.042, beta = 3.989 p > 0.001, beta = 2.354 p = 0.036), respectively. The healthy dietary pattern score was negatively associated with FBS and Cholesterol and TG levels (beta = -0.051, p = 0.035 and beta = -0.075, p = 0.048, and beta = -4.835, p = 0.025), respectively. Moreover, it was associated with 3.211 mg/dl higher levels of TAC (beta = 3.211, p = 0.049).
Table 4.
Association of z-scores of dietary patterns with NAFLD-related biomarkers; results of multivariate linear regressions
| Healthy pattern | Western pattern | ||||||
|---|---|---|---|---|---|---|---|
| Beta | SE | P | Beta | SE | p | ||
| WBC | Model 1 | 0.675 | 0.054 | 0.963 | 0.968 | 0.039 | 0.856 |
| Model 2 | 0.571 | 0.041 | 0.961 | 0.785 | 0.019 | 0.745 | |
| Model 3 | 0.223 | 0.071 | 0.892 | 0.746 | 0.084 | 0.881 | |
| HB | Model 1 | 0.254 | 0.351 | 0.417 | 0.605 | 0.125 | 0.521 |
| Model 2 | 0.368 | 0.288 | 0.635 | 0.504 | 0.198 | 0.735 | |
| Model 3 | 0.497 | 0.340 | 0.482 | 0.565 | 0.335 | 0.956 | |
| AST | Model 1 | -0.403 | 0.067 | 0.096 | 1.325 | 0.745 | 0.372 |
| Model 2 | -0.308 | 0.049 | 0.085 | 1.028 | 0.682 | 0.245 | |
| Model 3 | -0.111 | 0.026 | 0.088 | 1.098 | 0.566 | 0.115 | |
| ALT | Model 1 | -0.356 | 0.802 | 0.159 | 0.415 | 0.036 | 0.068 |
| Model 2 | -0.223 | 0.719 | 0.194 | 0.357 | 0.032 | 0.058 | |
| Model 3 | -0.048 | 0.746 | 0.231 | 0.486 | 0.097 | < 0.001 | |
| CR | Model 1 | -0.671 | 0.073 | 0.938 | 0.799 | 0.399 | 0.284 |
| Model 2 | -0.543 | 0.085 | 0.936 | 0.756 | 0.319 | 0.111 | |
| Model 3 | -0.445 | 0.097 | 0.927 | 0.727 | 0.215 | 0.126 | |
| FBS | Model 1 | -0.096 | 0.139 | 0.045 | 1.771 | 1.673 | 0.094 |
| Model 2 | -0.062 | 0.021 | 0.059 | 1.585 | 1.269 | 0.089 | |
| Model 3 | -0.051 | 0.085 | 0.035 | 3.248 | 2.575 | 0.042 | |
| CHOL | Model 1 | -0.099 | 0.074 | 0.124 | 0.449 | 0.675 | 0.219 |
| Model 2 | -0.078 | 0.065 | 0.098 | 0.405 | 0.466 | 0.191 | |
| Model 3 | -0.075 | 0.089 | 0.048 | 0.415 | 0.501 | 0.122 | |
| TG | Model 1 | -3.985 | 4.322 | 0.360 | 4.931 | 3.563 | 0.245 |
| Model 2 | -3.564 | 3.365 | 0.126 | 4.585 | 2.592 | 0.098 | |
| Model 3 | -4.835 | 3.484 | 0.025 | 3.989 | 2.162 | < 0.001 | |
| TAC | Model 1 | 1.665 | 3.959 | 0.238 | -4.895 | 1.365 | 0.334 |
| Model 2 | 2.259 | 2.766 | 0.192 | -3.644 | 1.572 | 0.289 | |
| Model 3 | 3.211 | 3.686 | 0.049 | -4.018 | 2.261 | 0.173 | |
| MDA | Model 1 | -5.545 | 5.352 | 0.245 | 3.922 | 1.095 | 0.055 |
| Model 2 | -5.342 | 4.345 | 0.143 | 2.354 | 1.112 | 0.036 | |
| Model 3 | -4.233 | 4.762 | 0.169 | 3.352 | 2.368 | 0.995 | |
• Model 1: Crude
• Model 2: adjusted for age, energy intake, weight, hip circumference, education, BMI and presence of NAFLD
• Model 3: adjusted for age, energy intake, weight, hip circumference, education, BMI and presence of NAFLD, PAL, and the other 3 dietary patterns
• SE: Standard error; p: multivariate binary logistic regression p-value. WBC; white blood cell, HB; hemoglobin FBS; fasting blood sugar AST; Aspartate transaminase; ALT: Alanine transaminase; TG: Triglycerides; CHOL; cholesterol CR; creatinine; TAC: Total antioxidant capacity; MDA: Malondialdehyde
Discussion
According to the present study, subjects with a greater adherence to a western dietary pattern were more likely to have NAFLD while participants who had a greater adherence to healthy dietary patterns were less likely to develop NAFLD.
The Western dietary pattern contains higher intakes of red and processed meats, refined grain products and sweets [28]. The western dietary pattern identified in this study was high in refined grains, sweets and desserts, mayonnaise sauce, and snacks (high sodium and high fat).
The results of our study are similar in many aspects to other previous studies. For instance, Oddy. et al., showed that western dietary pattern is associated with NAFLD. That characterized by a high intake of take away foods, confectionary, red meat, refined grains, processed meats, chips, sauces, full-fat dairy products, and soft drinks [29]. Shim et al. reported that the odds of developing NAFLD was definitely related to the intake of red and processed meats [30]. In another study among Korean adults, a healthy dietary pattern including fruits, eggs, dairy products, root vegetables, and yellow vegetables, and nuts was associated with a reduced risk of NAFLD while an unhealthy food patterns includes meat, sausages, and processed foods was related to an increased chance of developing NAFLD [31]. In the present study, the relationship between dietary patterns rich in red meat, mayonnaise sauce, and snack with NAFLD risk can be attributed to the presence of saturated and trans fatty acids in these foods.
Consumption of red meat has always been known as one of the causes of chronic diseases such as diabetes and cardiovascular diseases [32], non-alcoholic fatty liver and increasing insulin resistance [33, 34]. Red meat contains a significant amount of saturated and trans fatty acids [35]. In addition, unhealthy cooking method, such as grilling, increases heterocyclic amines which can increase insulin resistance [33]. As previously mentioned, insulin resistance is one of the predisposing factors for NAFLD [36].
A new study has shown that saturated fatty acids have harmful outcomes on lipid and glucose homeostasis, leading to the development of the metabolic syndrome and NAFLD [37]. Furthermore, in patients' group, the consumption of snacks containing saturated fat, salt and refined sugar was high. Jia et al. showed an association between the regional snack pattern and NAFLD. These snacks contain high carbohydrates and high glycemic load [17]. The higher consumption of saturated fat and trans fatty acids may also increase levels of lipid peroxidation and lower plasma antioxidant levels [38–40]. Additionally, former studies have shown that fast food consumption, obesity, and insulin resistance are the major risk factors for NAFLD [41, 42]. On the other hand, a study in Jordan showed that fast food consumption was not significantly associated with increased levels of liver enzymes ALT and AST and But it can increase calorie intake and cause obesity and metabolic syndrome. [43].
We also observed the association between healthy dietary pattern and reduced risk of NAFLD. A healthy diet usually includes plant-based foods, fresh fruits and vegetables, antioxidants, soy, nuts, sources of omega-3 fatty acids, unsaturated fats and also low amount of trans fats, animal-derived proteins, and added sugars [44]. The healthy dietary pattern identified in this study was related to high consumption of coffee, vegetables, fish, lettuce and spinach and tea. In the study of Fakhoury-Sayegh et al. a traditional Lebanese diet, including legumes, peas, corn, peas, and vegetables such as cauliflower, peppers, and lettuce, was inversely related to NAFLD [45]. Adriano et al. showed that a vegetable and dairy pattern is inversely related to non-alcoholic fatty liver disease. In that study, the healthy eating pattern included: vegetables, whole grains, legume and nuts, and dairy products [46]. Additionally, a traditional Chinese diet (nuts, fruit, egg, fish, shrimp, milk, and tea) revealed no relationship with the risk of NAFLD. However, animal food dietary pattern was positively associated the risk of NAFLD [47]. In a healthy dietary pattern, eating enough vegetables and fruits is likely to provide many antioxidant vitamins (e.g. vitamin A, C, E). These vitamins play a protective role against oxidative stress and inflammation caused by fibrosis and fatty liver [48]. Dietary fiber intake is also inversely related to insulin resistance, which is a risk factor for NAFLD [49]. In addition, foods with a low glycemic index, such as fruits, vegetables, legumes have a beneficial effect on blood sugar control and metabolic syndrome [50]. In our study, healthy people ate more fruits and vegetables. On the other hand, Xia exhibits the consumption of oranges is positively associated with the prevalence of NAFLD [51]. But Tajima Claimed the Japanese do not need to limit fruit consumption to limit the consumption of fructose as a means of preventing NAFLD [52].
In the present study, the intake of nuts was associated with a reduced risk of NAFLD. In our study, the term "nuts" included walnuts, pistachios, almonds, hazelnuts, and peanuts. In fact, these foods are rich in fiber, monounsaturated and polyunsaturated fatty acids (MUFAs and PUFAs), antioxidants and phenolic compounds [53]. The association between nuts consumption and reduced risk of nonalcoholic fatty liver disease has been shown in other studies [54–56]. But other studies have found no link between the nut consumption and liver fat [57, 58].
Other foods with high loading in the healthy diet in our study were coffee and tea. Both of them contain large amounts of antioxidants such as hydrocinnamic acid and polyphenols [59], epicatechin [60] and chlorogenic acid [61]. A systematic review study by Shen et al. Showed that regular coffee consumption can reduce liver fibrosis in patients with NAFLD [62]. Another meta-analyses by Hayat et al. demonstrated coffee intake can reduce risk of NAFLD [63]. High concentrations of antioxidants in coffee have been shown to play an important role in modulating glucose intolerance, reducing fibrosis in liver cells, and improving fatty liver disease in animal models [64]. On the other hand, some studies have shown that there is no significant difference in coffee consumption between patients without liver fibrosis and with liver fibrosis [65, 66].
We also examined the relationship of the two dietary patterns and NAFLD-related biomarkers. Higher adherence to a western dietary pattern was independently related to elevated ALT, FBS and cholesterol levels, while a higher adherence to a healthy dietary pattern was independently related with lowered FBS, cholesterol and TG amounts. Patients with NAFLD are often recognized by the asymptomatic rise of liver enzymes, most frequently of serum alanine aminotransferase (ALT), which has been used as a marker for NAFLD [67]. Elevated blood TG are one of the major cause of NAFLD [68]. The western dietary pattern is full of SFA and sugar, which have been associated with more inflammation and increased risk of NAFLD [29]. Sugar-sweetened drinks are high in fructose and fast-food meals are low in fiber and high in animal protein, which may explain the increased risk of obesity, insulin resistance, and elevated fasting blood sugar [69]. In contrast, a healthy dietary pattern has a beneficial impact on TG levels, potentially due to its anti-inflammatory components [70].
This study has some strengths. First, we used a 168-Items FFQ modified for the Iranian population in a face-to-face interview, which resulted in a better demonstration of the participants’ dietary intake. Second, we measured the antioxidant indicators such as TAC and MDA concerned with NAFLD.
Our study also had some limitations. First, due to the nature of the case–control design of the study, we cannot infer that dietary pattern are causal or defending for the disease. Second, the food frequency questionnaire is still considered the most appropriate tool for collecting food data in extensive epidemiological studies. However, some errors are inevitable. Third, in all observational studies, memory-based (or recall bias) data might cause measurement errors.
In conclusion, the current study showed that a greater adherence to a western dietary pattern significantly increases the odds of NAFLD, ALT, FBS, and TG levels. Moreover, adherence to a healthy dietary pattern may have a protective role against NAFLD. Nevertheless, high quality clinical trials and longitudinal studies are required to confirm these preliminary outcomes.
Acknowledgements
This study was extracted from MSc dissertation, which was approved by School of Nutrition & Food Science, Isfahan University of Medical Sciences. We would like to express our appreciation for all those participating in this study for their sincere cooperation.
Funding
This work was supported by the Isfahan University of Medical Sciences [grant numbers IR.MUI.REC. 1398.279].
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fateme Moradi, Email: Fateme.mrdi@nutr.mui.ac.ir.
Seyedeh Parisa Moosavian, Email: p_moosavian@yahoo.com.
Farhang Djafari, Email: farhangj74@gmail.com.
Azam Teimori, Email: drateimouri@gmail.com.
Zahra Faghih Imani, Email: Dr.faghihimani@yahoo.com.
Amirmansour Alavi Naeini, Email: am.alavi@nutr.mui.ac.ir.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Priyadarshi RN, Anand U. Non-alcoholic fatty liver disease: growing burden, adverse outcomes and associations. J Clin Transl Hepatol. 2020;8(1):76. doi: 10.14218/JCTH.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J Gastroenterol. 2017;23(23):4146. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr. 2017;8(2):240–252. doi: 10.3945/an.116.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26:153–162. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]
- 7.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos MB, Colvin R, Belt P, Molleston JP, Murray KF, Rosenthal P, et al. Correlation of vitamin E, uric acid and diet composition with histologic features of pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2012;54(1):90. doi: 10.1097/MPG.0b013e318229da1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheah MC, McCullough AJ, Goh GB. Dietary manipulations for nonalcoholic fatty liver disease (NAFLD) Bioactive food as dietary interventions for diabetes: Elsevier; 2019. pp. 69–88. [Google Scholar]
- 10.Mirmiran P, Amirhamidi Z, Ejtahed H-S, Bahadoran Z, Azizi F. Relationship between diet and non-alcoholic fatty liver disease: a review article. Iran J Public Health. 2017;46(8):1007. [PMC free article] [PubMed] [Google Scholar]
- 11.Heinonen I, Rinne P, Ruohonen S, Ruohonen S, Ahotupa M, Savontaus E. The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiol. 2014;211(3):515–527. doi: 10.1111/apha.12253. [DOI] [PubMed] [Google Scholar]
- 12.Lampret BR, Murko S, Tanšek MŽ, Podkrajšek KT, Debeljak M, Šmon A, et al. Selective Screening for Metabolic Disorders in the Slovenian Pediatric Population/Selektivni Skrining Metaboličkih Poremećaja Kod Dečije Populacije U Sloveniji. Journal of medical biochemistry. 2014;34(1):58–63. doi: 10.2478/jomb-2014-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan BL, Norhaizan ME, Liew W-P-P, Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Frontiers in pharmacology. 2018;9:1162. [DOI] [PMC free article] [PubMed]
- 14.Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Dietary quality indices and biochemical parameters among patients with non alcoholic fatty liver disease (NAFLD). Hepatitis monthly. 2013;13(7). [DOI] [PMC free article] [PubMed]
- 15.Di Minno MND, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol: WJG. 2012;18(41):5839. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Deng F. Iron overload is associated with non-alcoholic fatty liver disease (NAFLD): results from The NHANES III survey. European Journal of BioMedical Research. 2017;3(1):10–15. [Google Scholar]
- 17.Jia Q, Xia Y, Zhang Q, Wu H, Du H, Liu L, et al. Dietary patterns are associated with prevalence of fatty liver disease in adults. Eur J Clin Nutr. 2015;69(8):914–921. doi: 10.1038/ejcn.2014.297. [DOI] [PubMed] [Google Scholar]
- 18.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47(5):711–717. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Kim H-Y, Lee J, Kim J. Association between dietary inflammatory index and metabolic syndrome in the general Korean population. Nutrients. 2018;10(5):648. doi: 10.3390/nu10050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–662. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 21.Azar M, Sarkisian E. Food composition table of Iran: National Nutrition and food research institute. Tehran: Shaheed Beheshti University; 1980. [Google Scholar]
- 22.US Department of Agriculture ARS. USDA national nutrient database for standard reference, release 28. Nutrient data laboratory home page. 2011.
- 23.Ghafarpour M, Houshiar-Rad A, Kianfar H, Ghaffarpour M. The manual for household measures, cooking yields factors and edible portion of food. Tehran: Keshavarzi Press; 1999. [Google Scholar]
- 24.Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196–1202. doi: 10.1249/01.MSS.0000074446.02192.14. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Samuels P. Advice on exploratory factor analysis. 2017.
- 28.Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups, and dietary patterns: a systematic review. Nutr Rev. 2012;70(8):423–435. doi: 10.1111/j.1753-4887.2012.00490.x. [DOI] [PubMed] [Google Scholar]
- 29.Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'sullivan TA, Ayonrinde OT, et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108(5):778–785. doi: 10.1038/ajg.2013.95. [DOI] [PubMed] [Google Scholar]
- 30.Shim P, Choi D, Park Y. Association of blood fatty acid composition and dietary pattern with the risk of non-alcoholic fatty liver disease in patients who underwent cholecystectomy. Ann Nutr Metab. 2017;70(4):303–311. doi: 10.1159/000475605. [DOI] [PubMed] [Google Scholar]
- 31.Chung GE, Youn J, Kim YS, Lee JE, Yang SY, Lim JH, et al. Dietary patterns are associated with the prevalence of nonalcoholic fatty liver disease in Korean adults. Nutrition. 2019;62:32–38. doi: 10.1016/j.nut.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelber-Sagi S, Ivancovsky-Wajcman D, Isakov NF, Webb M, Orenstein D, Shibolet O, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68(6):1239–1246. doi: 10.1016/j.jhep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Hashemian M, Merat S, Poustchi H, Jafari E, Radmard A-R, Kamangar F, et al. Red meat consumption and risk of nonalcoholic fatty liver disease in a population with low meat consumption: the golestan cohort study. Am J Gastroenterol. 2021;116(8):1667–1675. doi: 10.14309/ajg.0000000000001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng H, Xie X, Pan X, Zheng J, Zeng Y, Cai X, et al. Association of meat consumption with NAFLD risk and liver-related biochemical indexes in older Chinese: a cross-sectional study. BMC Gastroenterol. 2021;21(1):1–11. doi: 10.1186/s12876-021-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Park SH. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. J Affect Disord. 2021;278:259–263. doi: 10.1016/j.jad.2020.09.073. [DOI] [PubMed] [Google Scholar]
- 37.Chao H-W, Chao S-W, Lin H, Ku H-C, Cheng C-F. Homeostasis of glucose and lipid in non-alcoholic fatty liver disease. Int J Mol Sci. 2019;20(2):298. doi: 10.3390/ijms20020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhibi M, Brahmi F, Mnari A, Houas Z, Chargui I, Bchir L, et al. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr Metab. 2011;8(1):65. doi: 10.1186/1743-7075-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Y, Burrington CM, Graff EC, Zhang J, Judd RL, Suksaranjit P, et al. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. American Journal of Physiology-Endocrinology and Metabolism. 2016;310(6):E418–E439. doi: 10.1152/ajpendo.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295(5):G987-G95. [DOI] [PMC free article] [PubMed]
- 41.Doost Mohammadi F, Vazirinejad R, Rezaeian M, Vazirinejad E, Bastam D, Ahmadinia H, et al. Fast food consumption and the risk of non-alcoholic fatty liver in adults: A community-based case-control study. Journal of Occupational Health and Epidemiology. 2019;8(4):176–184. [Google Scholar]
- 42.Scapaticci S, D’Adamo E, Mohn A, Chiarelli F, Giannini C. Non-alcoholic fatty liver disease in obese youth with insulin resistance and type 2 diabetes. Frontiers in Endocrinology. 2021;12. [DOI] [PMC free article] [PubMed]
- 43.Khatatbeh M, Momani W, Altaani Z, Al Saad R, Al Bourah AR. Fast food consumption, liver functions, and change in body weight among university students: A cross-sectional study. International Journal of Preventive Medicine. 2021;12. [DOI] [PMC free article] [PubMed]
- 44.Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol Res. 2018;131:32–43. doi: 10.1016/j.phrs.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Fakhoury-Sayegh N, Younes H, Heraoui GN, Sayegh R. Nutritional profile and dietary patterns of lebanese non-alcoholic fatty liver disease patients: a case-control study. Nutrients. 2017;9(11):1245. doi: 10.3390/nu9111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adriano LS, de Carvalho Sampaio HA, Arruda SPM, de Melo Portela CL, de Melo MLP, Carioca AAF, et al. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br J Nutr. 2016;115(12):2189–2195. doi: 10.1017/S0007114516001410. [DOI] [PubMed] [Google Scholar]
- 47.Yang C-Q, Shu L, Wang S, Wang J-J, Zhou Y, Xuan Y-J, et al. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in Chinese adults. Nutrients. 2015;7(6):4778–4791. doi: 10.3390/nu7064778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arendt BM, Allard JP. Effect of atorvastatin, vitamin E and C on nonalcoholic fatty liver disease: is the combination required? Am J Gastroenterol. 2011;106(1):78–80. doi: 10.1038/ajg.2010.310. [DOI] [PubMed] [Google Scholar]
- 49.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37(4):909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 50.Geetha K, Yankanchi GM, Hulamani S, Hiremath N. Glycemic index of millet based food mix and its effect on pre diabetic subjects. Journal of Food Science and Technology. 2020:1–7. [DOI] [PMC free article] [PubMed]
- 51.Xia Y, Lu Z, Lu M, Liu M, Liu L, Meng G, et al. Raw orange intake is associated with higher prevalence of non-alcoholic fatty liver disease in an adult population. Nutrition. 2019;60:252–260. doi: 10.1016/j.nut.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 52.Tajima R, Kimura T, Enomoto A, Saito A, Kobayashi S, Masuda K, et al. No association between fruits or vegetables and non-alcoholic fatty liver disease in middle-aged men and women. Nutrition. 2019;61:119–124. doi: 10.1016/j.nut.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Venkatachalam M, Sathe SK. Chemical composition of selected edible nut seeds. J Agric Food Chem. 2006;54(13):4705–4714. doi: 10.1021/jf0606959. [DOI] [PubMed] [Google Scholar]
- 54.bing Chen B, Han Y, Pan X, Yan J, Liu W, Li Y, et al. Association between nut intake and non-alcoholic fatty liver disease risk: a retrospective case-control study in a sample of Chinese Han adults. BMJ open. 2019;9(9):e028961. [DOI] [PMC free article] [PubMed]
- 55.Zhang S, Fu J, Zhang Q, Liu L, Meng G, Yao Z, et al. Association between nut consumption and non-alcoholic fatty liver disease in adults. Liver Int. 2019;39(9):1732–1741. doi: 10.1111/liv.14164. [DOI] [PubMed] [Google Scholar]
- 56.Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Giannini EG. Nuts and Non-Alcoholic Fatty Liver Disease: Are Nuts Safe for Patients with Fatty Liver Disease? Nutrients. 2020;12(11):3363. doi: 10.3390/nu12113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowen J, Luscombe-Marsh ND, Stonehouse W, Tran C, Rogers GB, Johnson N, et al. Effects of almond consumption on metabolic function and liver fat in overweight and obese adults with elevated fasting blood glucose: A randomised controlled trial. Clinical nutrition ESPEN. 2019;30:10–18. doi: 10.1016/j.clnesp.2018.12.088. [DOI] [PubMed] [Google Scholar]
- 58.Agebratt C, Ström E, Romu T, Dahlqvist-Leinhard O, Borga M, Leandersson P, et al. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PloS one. 2016;11(1):e0147149. [DOI] [PMC free article] [PubMed]
- 59.Sirota R, Gorelik S, Harris R, Kohen R, Kanner J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol Nutr Food Res. 2013;57(5):916–919. doi: 10.1002/mnfr.201200557. [DOI] [PubMed] [Google Scholar]
- 60.D Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Annali-Istituto Superiore di Sanita. 2007;43(4):348. [PubMed]
- 61.Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol Res. 2007;55(3):187–198. doi: 10.1016/j.phrs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Shen H, Rodriguez AC, Shiani A, Lipka S, Shahzad G, Kumar A, et al. Association between caffeine consumption and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Ther Adv Gastroenterol. 2016;9(1):113–120. doi: 10.1177/1756283X15593700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Annals of Hepatology. 2021;20:100254. [DOI] [PubMed]
- 64.Panchal SK, Poudyal H, Waanders J, Brown L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J Nutr. 2012;142(4):690–697. doi: 10.3945/jn.111.153577. [DOI] [PubMed] [Google Scholar]
- 65.Bambha K, Wilson LA, Unalp A, Loomba R, Neuschwander-Tetri BA, Brunt EM, et al. Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014;34(8):1250–1258. doi: 10.1111/liv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alferink LJ, Fittipaldi J, Kiefte-de Jong JC, Taimr P, Hansen BE, Metselaar HJ, et al. Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: The Rotterdam study. J Hepatol. 2017;67(2):339–348. doi: 10.1016/j.jhep.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Zou Y, Zhong L, Hu C, Sheng G. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: a population-based longitudinal study. Lipids Health Dis. 2020;19(1):1–10. doi: 10.1186/s12944-020-01419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Romero-Polvo A, Denova-Gutiérrez E, Rivera-Paredez B, Castañón S, Gallegos-Carrillo K, Halley-Castillo E, et al. Association between dietary patterns and insulin resistance in Mexican children and adolescents. Ann Nutr Metab. 2012;61(2):142–150. doi: 10.1159/000341493. [DOI] [PubMed] [Google Scholar]
- 70.De Biase SG, Fernandes SFC, Gianini RJ, Duarte JLG. Vegetarian diet and cholesterol and triglycerides levels. Arq Bras Cardiol. 2007;88(1):35. doi: 10.1590/s0066-782x2007000100006. [DOI] [PubMed] [Google Scholar]