Abstract
Background
Diabetic mellitus (DM) and cardiovascular diseases (CVD) cause significant healthcare burden globally and often co-exists. Current approaches often fail to identify many people with co-occurrence of DM and CVD, leading to delay in healthcare seeking, increased complications and morbidity. In this paper, we aimed to develop and evaluate a two-stage machine learning (ML) model to predict the co-occurrence of DM and CVD.
Methods
We used the diabetes complications screening research initiative (DiScRi) dataset containing >200 variables from >2000 participants. In the first stage, we used two ML models (logistic regression and Evimp functions) implemented in multivariate adaptive regression splines model to infer the significant common risk factors for DM and CVD and applied the correlation matrix to reduce redundancy. In the second stage, we used classification and regression algorithm to develop our model. We evaluated the prediction models using prediction accuracy, sensitivity and specificity as performance metrics.
Results
Common risk factors for DM and CVD co-occurrence was family history of the diseases, gender, deep breathing heart rate change, lying to standing blood pressure change, HbA1c, HDL and TC\HDL ratio. The predictive model showed that the participants with HbA1c >6.45 and TC\HDL ratio > 5.5 were at risk of developing both diseases (97.9% probability). In contrast, participants with HbA1c >6.45 and TC\HDL ratio ≤ 5.5 were more likely to have only DM (84.5% probability) and those with HbA1c ≤5.45 and HDL >1.45 were likely to be healthy (82.4%. probability). Further, participants with HbA1c ≤5.45 and HDL <1.45 were at risk of only CVD (100% probability). The predictive accuracy of the ML model to detect co-occurrence of DM and CVD is 94.09%, sensitivity 93.5%, and specificity 95.8%.
Conclusions
Our ML model can significantly predict with high accuracy the co-occurrence of DM and CVD in people attending a screening program. This might help in early detection of patients with DM and CVD who could benefit from preventive treatment and reduce future healthcare burden.
Keywords: Diabetes mellitus, Cardiovascular disease, Multi-diseases prediction, Classification and regression, Co-morbidity
Introduction
The prevalence and burden of chronic diseases including diabetes mellitus (DM), cardiovascular diseases (CVD), chronic respiratory diseases and cancers have been increasing over the past three decades in many countries worldwide [1, 2]. Globally, there are 415 million individuals with DM (8.8% of the total world’s population) and the International Diabetes Federation predicts that the number of people with DM will increase to 642 million by 2040 [3]. Similarly, CVD is the leading cause of disease burden in the world [4] and attributes to 17.7 million deaths annually [5]. The prevalence of CVD nearly doubled from 271 million in 1990 to 523 million in 2019 [4]. During the same period the number of CVD deaths also increased from 12.1 million to 18.6 million [4].
Comorbidity is a common problem in many people with chronic diseases such as individuals with DM commonly present with obesity, hypertension, dyslipidaemia and CVD. There are ample evidences of the association between DM and CVD [6, 7]. Both CVD and DM share similar cardiometabolic, behavioral, environmental, and social risk factors. For example, most of the CVD risk factors such as hypertension, obesity, and dyslipidaemia are common in people with DM [7–9]. In contrast, DM is a primary risk factor for CVD [10]. The abnormalities in physiological factors of CVD or DM often result in more than one disease at the same time [11]. People with diabetes have shown to have poor quality of life, increased healthcare expenditure and suffer from more depressive symptoms compared to those without diabetes [12–15]. The comorbid presence of DM and CVD significantly contributes to the increased complications and death [16, 17]. People with DM and CVD are 1.7 times more likely to die compared to those suffering from CVD only [18]. Moreover, both CVD and DM are directly associated with cardiovascular autonomic neuropathy which can increase complications and deaths [19, 20].
DM and CVD often remains undetected in the early phases of the disease and therefore untreated, leading to complications and premature deaths. Therefore, it is essential to provide a practical and viable model to predict these diseases early together in order to reduce the future morbidities and premature deaths. A number of studies have used ML approaches to predict DM and CVD in different populations and using different methods [21–25]. However, evidence on ML approaches for predicting co-occurrence of DM and CVD is lacking. Perceiving the common risk factors and developing a predictive model for co-occurrence of DM and CVD is more important for prevention and management of these diseases than targeting individual diseases. Therefore, in this study we aimed to identify the common risk factors for DM and CVD, develop a multi-diseases predictive model capable of predicting DM and CVD simultaneously and evaluate the performance of the prediction model.
Materials and methods
Design
We conducted secondary analysis from a retrospective cohort study. We developed a two-stage approach to predict the occurrence of DM and CVD comorbidities based on their common risk factors. In the first stage, logistic regression (LR) and the Evimp functions (EVF) were implemented in Multivariate Adaptive Regression Splines (MARS) model to infer the significant common risk factors based on voting criteria. Afterward, the correlation matrix is applied to reduce the redundancy of common risk factors. In the second stage, Classification and Regression (CART) algorithm is employed in constructing a predictive model of DM and CVD.
Participants, location and data collection
We used the diabetes complications screening research initiative (DiScRi) datasets which contains data from 2000 participants on more than 200 variables collected from Charles Stuart University in New South Wales, Australia from 2004 [26]. Patients were recruited through a public media campaign, including newspaper, radio, local television, and advertisements posted in general practice and community health centres. People were requested to contact the university if they wished to undergo a health check, and an appointment was made to attend the clinic. All participations older than 40 years were eligible to participate [27]. Participants with existing cardiovascular, respiratory and renal disease as well as depression, schizophrenia and Parkinson’s disease were excluded. The data collection procedure involved the following steps: (1) all participants were required to stop smoking or to consume drinks like alcohol and coffee 24 h before being tested. They were required to fast, beginning from midnight prior to the testing day. The tests were conducted from 9:00 am to 12:00 pm.
Ethics
Written informed consent was obtained from all participants before data collection. The protocol for the DiScRi study was approved by the Ethics in Human Research Committee of the Charles Sturt University (Protocol # 03/164).
Variables and measurements
The DiScRi dataset contain data on participants sociodemographic information, diseases history, measurements of blood pressure (BP), heart rate, electrocardiograms, blood biochemistry tests and Ewing battery tests. In this study we used the following variables: family history (FH) of disease, gender, age, body mass index (BMI) measured as height in cm divided by weight in kg2 waist circumference, hypertension status (yes or no), glycated hemoglobin (HbA1c), blood lipid profile including triglyceride (TG), Total cholesterol (TC), High-density lipoproteins (HDL), Low-density lipoproteins (LDL) and ratio of total cholesterol to high-density lipoprotein (TC/HDL ratio). Blood glucose estimations included fasting glucose test (FGT) and Glucose Tolerance Test (GTT) measured in (mmol/L).
Finally, we recorded the DM and CVD status of the participants and conducted Ewing’s Test including: 1. Lying to standing heart rate (LSHR) change expressed by 30:15 ratio. Such test indicates to the ratio of longest R-R interval (ranged from 20 to 40 beat) to the shortest R-R interval (ranged from 5 to 25 beat) produced by a change in position (from a horizontal position to vertical position); 2. Deep breathing heart rate (DBHR) change, which refers to the evaluation of beat-to-beat Heart Rate variation (R-R variation) based on deep breathing; 3. Valsalva maneuver heart rate (VAHR) change measuring the response of heart rate during and after increasing the intra-abdominal and intrathoracic pressure; 4. Handgrip blood pressure (HGBP) change measuring the change in diastolic BP after using a handgrip dynamometer; and 5. Lying to standing blood pressure (LSBP) change measuring the difference in the baroreflex-mediated BP after a change in the position.
ML models: DM and CVD comorbidity predictive model
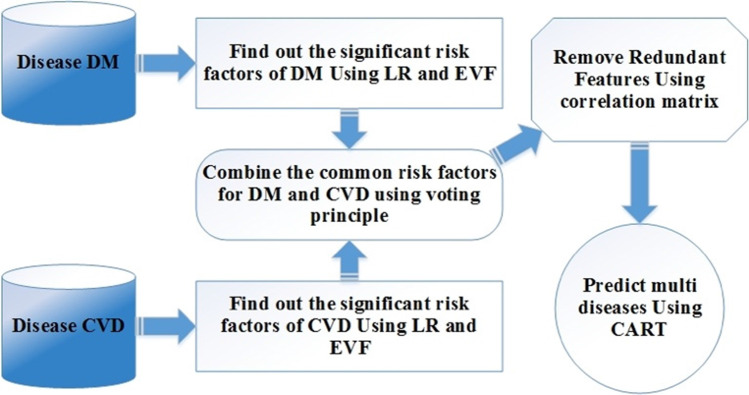
Figure 1 illustrates the proposed model, which consists of two main stages. The first stage focuses on the extraction of the common risk factors from the dataset. The output of the first stage becomes the input of the second stage, which deals with predicting the co-occurrence of the two diseases. In the following subsections, the two stages are explained in detail.
Fig. 1.
The Framework of the Predictive Model
Data analysis
Extracting common risk factors
The common risk factors refer to all factors that show a significant association in both diseases. We used two feature selection methods to extract the common risk factors of DM and CVD: logistic regression (LR) [28], and the Evimp function (EVF) implemented in multivariate adaptive regression splines model (MARS) [29]. These methods were chosen due to their efficiency in determining the association between the independent variables and the outcome [30–32].
Logistic regression
Logistic Regression (LR) model computes the probability of occurrence of dependent variable based on the predictability of independent variables. In general, the LR can be expressed as follows:
| 1 |
where π represents the probability of occurrence of an outcome (dependent variable) based on the selected independent variables. β indicates the regression coefficients of each independent variables. In this paper, we use logistic regression with forward stepwise method to select the significant risk factors with (P < 0.05).
Estimate variable importance implemented in MARS
The Evimp function (EVF) is a method implemented in multivariate adaptive regression splines model (MARS) [29]. The EVF returns a matrix presenting the relative significance of the features in the model and uses three criteria in estimating the importance of features as follows:
The (nsub-set) criterion calculates the number of sub-sets that involves the feature. Features that are involved in more sub-sets are considered most important.
The raw residual sum-of-squares (RSS) criterion contains two steps. Firstly, it computes the reduction in the RSS for each sub-set and compares the reduction with the value of the previous subset. After, for each involved feature, RSS aggregates these reductions for all sub-sets that involve the feature. In the end, the total aggregation of reductions is interpreted. Features that cause a massive reduction in RSS are most important.
The generalised cross-validation (GCV) criterion is similar to RSS criterion; however, it uses GCV instead of RSS. GCV evaluates the performance features in sub-sets and selects the most significant sub-set (lower values of GCV are useful).
Voting method
After identifying the risk factors (statistically significant) for each disease by LR and EVF, a voting method was applied to determine the common risk factors of DM and CVD. The correlation matrix method was then performed to remove the redundant common risk factors and avoid the problem of multicollinearity using cut-off = 0.5.
Comorbidity predictive model
In the second stage, classification and regression (CART) algorithm [33] was used to construct the predictive model of DM and CVD comorbidity based on the extracted common risk factors from the first stage. CART was used due to its several advantages. For example, in comparison with other ML algorithms, CART outcomes are easy to interpret visually using If-then condition, which is like a human decision. It does not require specifying the association between independent variables and the outcome. CART can handle both classification and regression problems. Furthermore, CART is capable of dealing with the continuous and discrete dependent variables. It automatically amends the constructed tree to reduce the impacts of the measured impurities and identifies the efficiency of the node for a final decision. In general, CART algorithm uses Gini index to build the decision trees. Gini index measures the impurity or purity of the features. The general formula Gini index used by CART is as follows:
| 2 |
In fact, the initially constructed tree in Eq. (2) does not represent the optimal result. Therefore, CART algorithm prunes the created tree using node error rate as follows:
| 3 |
where Xei gives the number of misclassified instances in the node, and provides the total number of instances in the node. The process of pruning tree starts from bottom to top based on the total error rate condition. If the rate of total error is higher than the stated threshold, then it stops the process of pruning.
Evaluation of the predictive model
We evaluated the multi-stage comorbidity prediction model using various measures such as accuracy, sensitivity, specificity and confusion matrix measurements. The evaluation measures used are as follows:
Sensitivity: defines the number of participants that are correctly predicted with the positive disease.
| 4 |
Specificity: refers to the number of participants that are correctly predicted with the negative disease.
| 5 |
Accuracy: exposes the total number of participants that are correctly predicted with the positive and negative diseases.
| 6 |
The 10-fold cross-validation (CV) approach was applied to obtain a balanced evaluation of the generalisation error. Cross-validation is an approach used to evaluate the performance of predictive models by partitioning the entire dataset into k number of sub-sets. It uses 10-fold cross-validation to randomly divide the entire dataset into ten sub-sets; 9 sub-sets are used for training stage (90%), and the remaining sub-set is used for the testing stage (10%) with replacement in the sub-sets. The hardware used are: Intel Core i9 10850K 3.6Ghz Comet Lake 10 Core 20 Thread LGA1200, GeForce RTX 3070 GAMING Z TRIO 8G LHR GRAPHIC CARD and MPG Z590 Gaming Edge WiFi LGA1200 ATX Desktop Motherboard. Data were analyzed using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA) and R language.
Results
A total of 812 participants were included in this study (244 with CVD, 237 with DM, 139 with CVD and DM simultaneously, and 192 healthy disease-free participants).
Common risk factors of DM and CVD
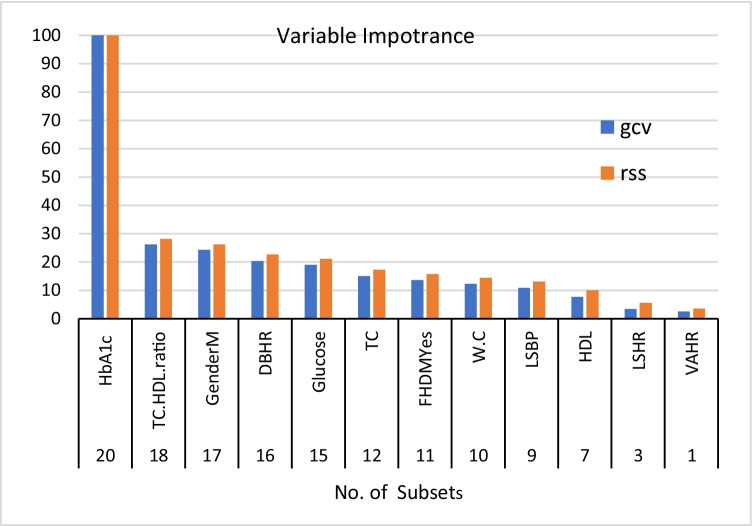
Table 1 shows the risk factors of DM selected by LR and the Evimp function (EVF). In the LR model family history of diabetes, gender, age, lying to standing heart rate change, deep breathing heart rate change, lying to standing BP change, HbA1c, FGT, TC, HDL and TC\HDL ratio were significantly associated with DM (P < 0.05). The outcomes of applying an Evimp function (EVF) with DM demonstrates that family history of diabetes, gender, waist circumference, lying to standing heart rate change, deep breathing heart rate change, Valsalva maneuver heart rate change, lying to standing BP change, HbA1c, FGT, TC, HDL and TC\HDL ratio were the most important features. Figure 2 illustrates the features’ importance of DM by Evimp function (EVF). Risk factors that were common in both LR and EVM models are shown in Table 1.
Table 1.
Summarizes DM risk factors under LR and EVF
| Covariates | LR | (EVF) | Risk factors of DM |
|---|---|---|---|
| FH | * | * | * |
| Gender | * | * | * |
| Age | * | ||
| W.C | * | ||
| BMI | |||
| LSHR | * | * | * |
| DBHR | * | * | * |
| VAHR | * | ||
| HGBP | |||
| LSBP | * | * | * |
| HbA1c | * | * | * |
| Triglyceride | |||
| Glucose | * | * | * |
| TG | |||
| HT Status | |||
| TC | * | * | * |
| HDL | * | * | * |
| TC\HDL ratio | * | * | * |
| LDL |
LR Logistic Regression, EVF Evimp functions, FH Family History, W.C Waist Circumference, BMI Body Mass Index measured in kg/m2, LSHR Lying to standing heart rate, DBHR Deep breathing heart rate, VAHR Valsalva maneuver heart rate, HGBP Handgrip blood pressure, LSBP Lying to standing blood pressure, HbA1c glycated hemoglobin, TG triglyceride, HT Hypertension, TC Total cholesterol, HDL High-density lipoproteins, LDL Low-density lipoproteins
Fig. 2.
Essential Features of DM by EVF. Note: HbA1c glycated hemoglobin, TC Total cholesterol, HDL High-density lipoproteins, LDL Low-density lipoproteins, DBHR Deep breathing heart rate, FHDM Family History of DM, W.C Waist Circumference, LSBP Lying to standing blood pressure, VAHR Valsalva maneuver heart rate
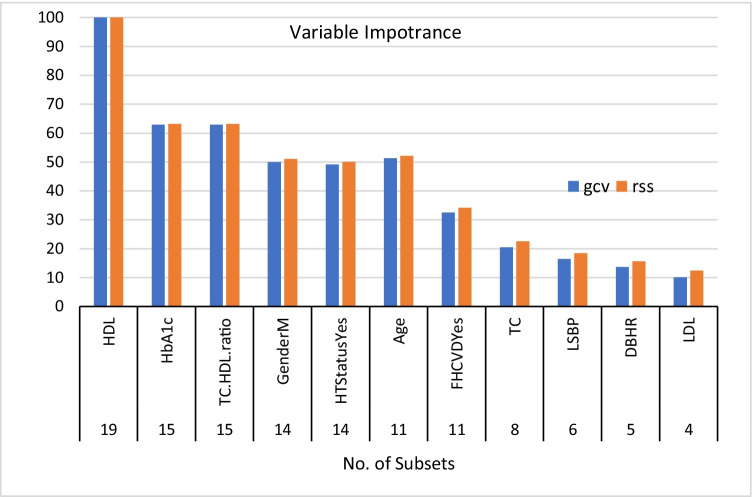
Similarly, the risk factors of CVD as selected by logistic regression (LR) and the Evimp function (EVF) are presented in Table 2. Family history of CVD, gender, age, deep breathing heart rate change, lying to standing BP change, HbA1c, hypertension status, TC, HDL and TC\HDL ratio were significantly associated with CVD (P < 0.05). The EVF result shows that family history of CVD, gender, age, deep breathing heart rate change, lying to standing BP change, HbA1c, hypertension status, TC, HDL, LDL and TC\HDL ratio were significantly correlated with CVD. Figure 3 clarifies the feature significance of CVD by using EVF. As shown in Table 3, the common risk factors for both diseases are family history of the disease, gender, deep breathing heart rate change, lying to standing BP change, HbA1c, TC, HDL and TC\HDL ratio. Later, the remove redundant features method was applied and showed there was inversely related correlation between TC feature and TC\HDL ratio with r = − 0.8. Therefore, the TC feature is removed from the common risk factor set. Thus, the final set of common risk factors was FH of the disease, gender, DBHR, LSBP, HbA1c, HDL and TC\HDL ratio.
Table 2.
Summary of CVD risk factors by LR and EVF
| Covariates | LR | (EVF) | Risk factors of CVD |
|---|---|---|---|
| FH | * | * | * |
| Gender | * | * | * |
| Age | * | * | * |
| W.C | |||
| BMI | |||
| LSHR | |||
| DBHR | * | * | * |
| VAHR | |||
| HGBP | |||
| LSBP | * | * | * |
| HbA1c | * | * | * |
| Triglyceride | |||
| Glucose | |||
| TG | |||
| HT Status | * | * | * |
| TC | * | * | * |
| HDL | * | * | * |
| TC\HDL ratio | * | * | * |
| LDL | * |
LR Logistic Regression, EVF Evimp functions, FH Family History, W.C Waist Circumference, BMI Body Mass Index measured in kg/m2, LSHR Lying to standing heart rate, DBHR Deep breathing heart rate, VAHR Valsalva maneuver heart rate, HGBP Handgrip blood pressure, LSBP Lying to standing blood pressure, HbA1c glycated hemoglobin, TG triglyceride, HT Hypertension, TC Total cholesterol, HDL High-density lipoproteins, LDL Low-density lipoproteins
Fig. 3.
Essential Features of CVD by EVF. Note: HDL High-density lipoproteins, HbA1c glycated hemoglobin, TC Total cholesterol, HDL High-density lipoproteins, HT Hypertension, FHCVD Family History of CVD, LSBP Lying to standing blood pressure, DBHR Deep breathing heart rate, LDL Low-density lipoproteins
Table 3.
Common risk factors of DM and CVD
| Covariates | Risk Factors of DM | Risk Factors of CVD | The common risk factors |
|---|---|---|---|
| FH | * | * | * |
| gender | * | * | * |
| Age | * | ||
| W.C | |||
| BMI | |||
| LSHR | * | ||
| DBHR | * | * | * |
| VAHR | |||
| HGBP | |||
| LSBP | * | * | * |
| HbA1c | * | * | * |
| Triglyceride | |||
| Glucose | * | ||
| SG | |||
| HT Status | * | ||
| TC | * | * | * |
| HDL | * | * | * |
| TC\HDL ratio | * | * | * |
| LDL |
LR Logistic Regression, EVF Evimp functions, FH Family History, W.C Waist Circumference, BMI Body Mass Index measured in kg/m2, LSHR Lying to standing heart rate, DBHR Deep breathing heart rate, VAHR Valsalva maneuver heart rate, HGBP Handgrip blood pressure, LSBP Lying to standing blood pressure, HbA1c glycated hemoglobin, TG triglyceride, HT Hypertension, TC Total cholesterol, HDL High-density lipoproteins, LDL Low-density lipoproteins
Performance of the proposed model
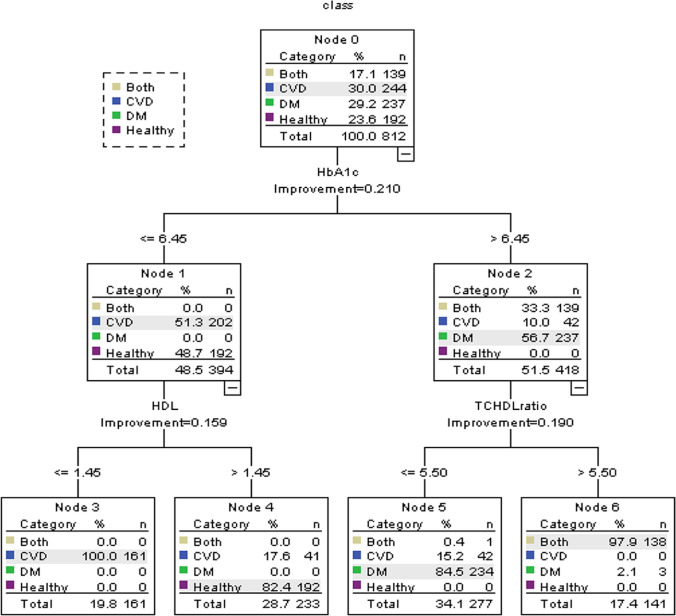
Figure 4 depicts the obtained tree of the evaluation of participants with the final set of common risk factors of multi-diseases using the predictive model. As shown in Fig. 4, HbA1c, HDL, and TC\HDL ratio risk factors played significant roles in creating the rules of the model. The predictive model showed that the participants with HbA1c >6.45 and TC\HDL ratio > 5.5 are at risk of developing both diseases with probability 97.9%. In contrast, the participants with HbA1c >6.45 and TC\HDL ratio ≤ 5.5 are more likely to gain only DM with probability of 84.5%. Participants with HbA1c ≤5.45 and HDL > 1.45 are likely to fall under the healthy group with probability of 82.4%. The predictive model also showed participants with HbA1c ≤5.45 and HDL <1.45 are at risk of only CVD with probability (100%).
Fig. 4.
The tree constructed of the proposed model
Table 4 summarizes the rules and the diagnosis outcomes of the created tree of multi-diseases. The created rules are straightforward to interpret, and thus physicians can use them to make proper decisions. The multi-diseases prediction outcomes of the validation sample based on the most common risk factors are presented in Table 5 (confusion matrix).
Table 4.
Induction rules of the proposed model
| Node | Rule | Class |
|---|---|---|
| 6 | If HbA1c is >6.45 and TC\HDL ratio > 5.5 Then | Both |
| 5 | If HbA1c is >6.45 and TC\HDL ratio ≤ 5.5 Then | DM |
| 4 | If HbA1c ≤5.45 and HDL > 1.45 Then | Healthy |
| 3 | If HbA1c ≤5.45 and HDL < 1.45 Then | CVD |
HbA1c glycated hemoglobin, TC Total cholesterol, HDL High-density lipoproteins
Table 5.
Confusion matrix of multi-diseases prediction model
| Predictive Class | Actual Class | |||
|---|---|---|---|---|
| A | B | C | D | |
| 218 | 12 | 0 | 14 | A = CVD |
| 9 | 225 | 3 | 0 | B=DM |
| 0 | 2 | 137 | 0 | C = Both |
| 8 | 0 | 0 | 184 | D = Healthy |
As evident from the confusion matrix, the performance of the predictive model was very good with an accuracy of 94.09%. Meanwhile, the sensitivity and the specificity of the predictive model were 93.5% and 95.8% respectively. As presented in the confusion matrix, out of 244 there were 26 CVD participants incorrectly predicted. As for DM, the number of incorrectly predicted participants were 12 out of 237. In the same context, out of 192 healthy participants, the model incorrectly predicted eight participants. As for participants with both diseases two out of 139 participants were incorrectly predicted. The outstanding performance of this model is due to all the included tests were significantly associated with both diseases (P < 0.05).
Discussion
In this study, we developed a two-stage ML model to predict the co-occurrence of DM and CVD based on their common risk factors and evaluated its prediction accuracy, sensitivity and specificity. Our results suggest that a ML model can significantly predict with high accuracy the co-occurrence of DM and CVD in people attending a screening program. Thus, increasing early detection of patients who could benefit from preventive treatment and reduce future healthcare burden. In recent years, several studies have developed predictive models for DM [34–37] and CVD [38–42]. However, the existing models can predict only a single disease (CVD or DM) at a time. Since patients may suffer from multiple related diseases at the same time, these models are inadequate for predicting the co-occurrence of DM and CVD simultaneously.
A number of studies have developed models for predicting several diseases and comorbidities [9, 43–48]. Chun and colleagues [49] have introduced a comorbidity prediction method using filtering technique to predict likely comorbid conditions for individuals and a trajectory prediction graph model to reveal progression paths of the conditions. A recent work [50] used social network patient data as evidence-based knowledge to support decision making in disease progression for comorbidities. The proposed model was based on statistical modelling of the constructed knowledge base. However, the work calculated the similarity between a patient’s record and other patients’ records and derived the risk of a certain medical condition using patients self-reported data. Another research [51] proposed a cascade data mining approach for frequent pattern mining enriched with context information, including a new algorithm MIxCO for maximal frequent patterns mining. The work explicated some population specific comorbidities such as schizophrenia, hyperprolactinemia and Type 2 DM. Other studies have identified potential comorbidities based on mutant genes, enzymes and protein-protein interactions [52–55]. A study [56] proposed a two-phase predictive model to simultaneously predict hypertension and hyperlipidaemia based on their common risk factors.
Both DM and CVD share common risk factors. DM is a complex disease influenced by multiple factors like genetics, lifestyle and environmental conditions [57]. Blood tests for HbA1c and glucose are reliable tests for diagnosing diabetes as recommended by the American Diabetes Association and the World Health Organization [58, 59]. High lipid profile including TC, HDL and TC\HDL ratio are significant predictors of DM [60, 61]. As for Ewing’s tests such as LSHR, DBHR, LSBP are the gold standard tests for cardiac autonomic neuropathy which is directly associated with DM. Further, family history of CVD, gender, age, and hypertension status are common risk factors for CVD and used in the Framingham Risk Score [62, 63]. Dyslipidaemia is also a main risk factors for CVD in people with DM [64, 65]. Increase in HbA1c showed progressively increasing risks of CVD [66]. Ewing tests has shown to an independent prognostic indicator of sudden arrhythmic death risk [67]. Abnormalities in HbA1c was associated with both DM and CVD [59, 68, 69]. Compared to LDL cholesterol, the HDL cholesterol level is a robust risk factor for coronary heart disease and DM [70]. The ratio of total cholesterol to HDL is also a risk factor for cardiovascular events [65, 71, 72]. Presence of family history was found to be significantly correlated with the prevalence of both diseases [73, 74].
We found common risk factors for DM and CVD co-occurrence were family history of the diseases, gender, deep breathing heart rate change, lying to standing BP change, HbA1c, HDL and TC\HDL ratio. Our predictive model showed that the participants with HbA1c >6.45 and TC\HDL ratio > 5.5 were at risk of developing both diseases (97.9% probability). In contrast, participants with HbA1c >6.45 and TC\HDL ratio ≤ 5.5 were more likely to have only DM (84.5% probability) and those with HbA1c ≤5.45 and HDL >1.45 were likely to be healthy (82.4% probability). Further, participants with HbA1c ≤5.45 and HDL <1.45 were at risk of only CVD (100% probability). Our results indicate that Ewing’s tests (DBHR and LSBP) could be used in the prediction of DM and CVD co-occurrence.
A major strength of this study in the use of two robust ML models to detect DM and CVD co-occurrence which can be a medical revolutionary. This study has potential limitations that should be considered for interpreting the results. First, data were collected from a small number of participants attending a screening program in a rural health centre in Australia. Therefore, the model might not produce the same results in another setting. Second, data on other important markers of diabetes and CVD for example, heart rate variability, retinal scans, peripheral nerve function, and various parameters derived from electrocardiogram recordings were not available. Third, we did not record the run time for the machine learning modlels which might be useful for its clinical application. Finally, our ML model has not been tested in a clinical population. Future research involving representative participants with larger sample size in multiple clinics with long-term follow-up are needed. Future machine learning studies should attempt to compare the algorithms developed in similar other datasets for better comparisons. Also, there is a need to explore other important cardiovascular and metabolic markers in the model which could improve the prediction power and accuracy.
The analysis and evaluation of the proposed model shows that it is very efficient and seamless to employ and use. The prediction accuracy of existing computational approach also requires to be improved as well as computationally complex. Evaluation of the model in clinics and training healthcare providers to use the ML models will improve the success of DM and CVD screening. Previous research have shown that digital health approaches could be useful for prevention and management of DM and CVD [75, 76]. Evidence suggest that using simple mobile phone services such as text messaging are effective and cost-effective approaches for controlling DM and CVD [77–80]. Our ML models could easily be employed as a tool for web-based and mobile phone application, thus increasing its reach among people with DM, CVD and healthcare providers.
Conclusion
Our ML model provides high accuracy, sensitivity and specificity, making it potential for utilization by primary healthcare providers in the clinics. Early detection of both DM and CVD will facilitate the planning of timely intervention and creates greater awareness of the risk of the diseases.
Acknowledgements
We are grateful to Herbert F. Jelinek from School of Community Health, Charles Sturt University, Albury, Australia for providing us access to the datasets. SMSI is supported by a Fellowship from NHMRC and National Heart Foundation of Australia.
Author contributions
Concept-ASA, JA; Data collection and project management-HFJ; Data analysis and interpretation-ASA, JA, TA, HFJ, SMSI, Drafting- ASA, JA, SMSI. All authors have reviewed the final manuscript and agreed to submission.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflict of interest
Authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahmad Shaker Abdalrada, Email: aabdalra@uowasit.edu.iq.
Jemal Abawajy, Email: jemal.abawajy@deakin.edu.au.
Tahsien Al-Quraishi, Email: tahsien.alquraishi@uowasit.edu.iq.
Sheikh Mohammed Shariful Islam, Email: shariful.islam@deakin.edu.au.
References
- 1.Islam SMS, et al. Non-communicable diseases (NCDs) in developing countries: a symposium report. Glob Health. 2014;10(1):1–8. doi: 10.1186/s12992-014-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . IDF Diabetes Atlas. 7. Brussels, Belgium: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 4.Roth GA, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(WHO), W.H.O. Cardiovascular diseases (CVDs). 2017 [cited 2018; Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 6.Al-Zubayer MA, Ahammed B, Sarder MA, Kundu S, Majumder UK, Islam SMS. Double and triple burden of non-communicable diseases and its determinants among adults in Bangladesh: Evidence from a recent demographic and health survey. Int J Clin Pract. 2021;7575:e14613. 10.1111/ijcp.14613 [DOI] [PubMed]
- 7.Islam SMS, et al. Prevalence of risk factors for hypertension: a cross-sectional study in an urban area of Bangladesh. Global cardiology science and practice. 2015;2015(4):43. doi: 10.5339/gcsp.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam SMS, et al. Cardiovascular diseases risk prediction in patients with diabetes: Posthoc analysis from a matched case-control study in Bangladesh. J Diabetes Metabol Disord. 2021;20(1):417–425. doi: 10.1007/s40200-021-00761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaboration ERF. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Institute of Health and Welfare. Evidence for chronic disease risk factors. Apr 19 , 2016 [cited 2018; Available from: https://www.aihw.gov.au/reports/chronic-disease/evidence-for-chronic-disease-risk-factors/contents/behavioural-and-biomedical-risk-factors.
- 12.Islam, S. M. S., Ferrari, U., Seissler, J., Niessen, L., & Lechner, A. Association between depression and diabetes amongst adults in Bangladesh: a hospital based case–control study. Journal of Global Health. 2015;5(2). [DOI] [PMC free article] [PubMed]
- 13.Islam SMS, Rawal LB, Niessen LW. Prevalence of depression and its associated factors in patients with type 2 diabetes: a cross-sectional study in Dhaka, Bangladesh. Asian J Psychiatry. 2015;17:36–41. doi: 10.1016/j.ajp.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Safita N, et al. The impact of type 2 diabetes on health related quality of life in Bangladesh: results from a matched study comparing treated cases with non-diabetic controls. Health Qual Life Outcomes. 2016;14(1):1–9. doi: 10.1186/s12955-016-0530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam SMS, et al. Healthcare use and expenditure for diabetes in Bangladesh. BMJ Glob Health. 2017;2(1):e000033. doi: 10.1136/bmjgh-2016-000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Angelantonio E, et al. Association of cardiometabolic multimorbidity with mortality. Jama. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matheus, A. S. D. M., Tannus, L. R. M., Cobas, R. A., Palma, C. C. S., Negrato, C. A., & Gomes, M. D. B. Impact of diabetes on cardiovascular disease: an update. International Journal of Hypertension. 2013; 2013. [DOI] [PMC free article] [PubMed]
- 18.Centers for Disease Control and Prevention . National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. p. 2014. [Google Scholar]
- 19.Cha S-A, et al. Diabetic cardiovascular autonomic neuropathy predicts recurrent cardiovascular diseases in patients with type 2 diabetes. PLoS One. 2016;11(10):e0164807. doi: 10.1371/journal.pone.0164807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pop-Busui, R., Braffett, B. H., Zinman, B., Martin, C., White, N. H., Herman, W. H., ... & DCCT/EDIC Research Group. Cardiovascular autonomic neuropathy and cardiovascular outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes care. 2017;40(1):94–100. [DOI] [PMC free article] [PubMed]
- 21.Alaa AM, et al. Cardiovascular disease risk prediction using automated machine learning: a prospective study of 423,604 UK Biobank participants. PLoS One. 2019;14(5):e0213653. doi: 10.1371/journal.pone.0213653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas H et al. Predicting diabetes in healthy population through machine learning. in 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS). 2019. IEEE.
- 23.Hossain ME, Uddin S, Khan A. Network analytics and machine learning for predictive risk modelling of cardiovascular disease in patients with type 2 diabetes. Expert Syst Appl. 2021;164:113918. [Google Scholar]
- 24.Garcia-Carretero R, et al. Pulse wave velocity and machine learning to predict cardiovascular outcomes in prediabetic and diabetic populations. J Med Syst. 2020;44(1):1–10. doi: 10.1007/s10916-019-1479-y. [DOI] [PubMed] [Google Scholar]
- 25.Weng SF, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone.0174944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelinek HF, Wilding C, Tinely P. An innovative multi-disciplinary diabetes complications screening program in a rural community: a description and preliminary results of the screening. Australian J Prim Health. 2006;12(1):14–20. [Google Scholar]
- 27.White F, Wang L, Jelinek HF. Management of hypertension in patients with diabetes mellitus. Exp Clin Cardiol. 2010;15(1):5–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Walker SH, Duncan DB. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54(1–2):167–179. [PubMed] [Google Scholar]
- 29.Friedman, J. H. Multivariate adaptive regression splines. The Annals of Statistics, 1991;1-67.
- 30.Hosmer David W, Lemeshow S, Sturdivant Rodney X. Applied logistic regression. New York: Wiley; 2000. [Google Scholar]
- 31.Zhang W, Goh AT, Zhang Y. Multivariate adaptive regression splines application for multivariate geotechnical problems with big data. Geotech Geol Eng. 2016;34(1):193–204. [Google Scholar]
- 32.Park S, et al. Evaluation of logistic regression and multivariate adaptive regression spline models for groundwater potential mapping using R and GIS. Sustainability. 2017;9(7):1157. [Google Scholar]
- 33.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Routledge; 2017. [Google Scholar]
- 34.Alghamdi M, et al. Predicting diabetes mellitus using SMOTE and ensemble machine learning approach: the Henry ford ExercIse testing (FIT) project. PLoS One. 2017;12(7):e0179805. doi: 10.1371/journal.pone.0179805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samant P, Agarwal R. Machine learning techniques for medical diagnosis of diabetes using iris images. Comput Methods Prog Biomed. 2018. [DOI] [PubMed]
- 36.Barakat N, Bradley AP, Barakat MNH. Intelligible support vector machines for diagnosis of diabetes mellitus. IEEE Trans Inf Technol Biomed. 2010;14(4):1114–1120. doi: 10.1109/TITB.2009.2039485. [DOI] [PubMed] [Google Scholar]
- 37.Wu H, et al. Type 2 diabetes mellitus prediction model based on data mining. Inform Med Unlocked. 2018;10:100–107. [Google Scholar]
- 38.Das R, Turkoglu I, Sengur A. Effective diagnosis of heart disease through neural networks ensembles. Expert Syst Appl. 2009;36(4):7675–7680. doi: 10.1016/j.cmpb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Amiri AM, Armano G. Early diagnosis of heart disease using classification and regression trees. in Neural Networks (IJCNN), The 2013 International Joint Conference on. 2013. IEEE.
- 40.Uyar K, İlhan A. Diagnosis of heart disease using genetic algorithm based trained recurrent fuzzy neural networks. Proc Comp Sci. 2017;120:588–593. [Google Scholar]
- 41.Masetic Z, Subasi A. Congestive heart failure detection using random forest classifier. Comput Methods Prog Biomed. 2016;130:54–64. doi: 10.1016/j.cmpb.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Yu S-N, Lee M-Y. Conditional mutual information-based feature selection for congestive heart failure recognition using heart rate variability. Comput Methods Prog Biomed. 2012;108(1):299–309. doi: 10.1016/j.cmpb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Aktar S, et al. Machine learning approach to predicting COVID-19 disease severity based on clinical blood test data: statistical analysis and model development. JMIR Med Inform. 2021;9(4):e25884. doi: 10.2196/25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khozeimeh F, et al. Combining a convolutional neural network with autoencoders to predict the survival chance of COVID-19 patients. Sci Rep. 2021;11(1):1–18. doi: 10.1038/s41598-021-93543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moni MA, et al. Network-based computational approach to identify delineating common cell pathways influencing type 2 diabetes and diseases of bone and joints. IEEE Access. 2019;8:1486–1497. [Google Scholar]
- 46.Rashed-Al-Mahfuz M, et al. Deep convolutional neural networks based ECG beats classification to diagnose cardiovascular conditions. Biomed Eng Lett. 2021;11(2):147–162. doi: 10.1007/s13534-021-00185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satu M, et al. Short-term prediction of COVID-19 cases using machine learning models. Appl Sci. 2021;11(9):4266. [Google Scholar]
- 48.Dinh A, et al. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Dec Making. 2019;19(1):1–15. doi: 10.1186/s12911-019-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji X, Chun SA, Geller J. Predicting comorbid conditions and trajectories using social health records. IEEE Transact Nanobiosci. 2016;15(4):371–379. doi: 10.1109/TNB.2016.2564299. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamurthy M et al. Representing Social Network Patient Data as Evidence-Based Knowledge to Support Decision Making in Disease Progression for Comorbidities. IEEE Access. 2018.
- 51.Boytcheva S, et al. Mining comorbidity patterns using retrospective analysis of big collection of outpatient records. Health Inform Sci Syst. 2017;5(1):3. doi: 10.1007/s13755-017-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He F, et al. Pcid: a novel approach for predicting disease comorbidity by integrating multi-scale data. IEEE/ACM Transact Comput Biol Bioinform (TCBB) 2017;14(3):678–686. doi: 10.1109/TCBB.2016.2550443. [DOI] [PubMed] [Google Scholar]
- 53.Park J, et al. The impact of cellular networks on disease comorbidity. Mol Syst Biol. 2009;5(1):262. doi: 10.1038/msb.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng C-H, et al. Tumor clustering using nonnegative matrix factorization with gene selection. IEEE Trans Inf Technol Biomed. 2009;13(4):599–607. doi: 10.1109/TITB.2009.2018115. [DOI] [PubMed] [Google Scholar]
- 55.Xia J-F, Zhao X-M, Huang D-S. Predicting protein–protein interactions from protein sequences using meta predictor. Amino Acids. 2010;39(5):1595–1599. doi: 10.1007/s00726-010-0588-1. [DOI] [PubMed] [Google Scholar]
- 56.Chang C-D, Wang C-C, Jiang BC. Using data mining techniques for multi-diseases prediction modeling of hypertension and hyperlipidemia by common risk factors. Expert Syst Appl. 2011;38(5):5507–5513. [Google Scholar]
- 57.Prasad RB, Groop L. Genetics of type 2 diabetes—pitfalls and possibilities. Genes. 2015;6(1):87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Association AD. Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Organization WH. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011. [PubMed]
- 60.Rhee E-J, et al. Increased risk for diabetes development in subjects with large variation in total cholesterol levels in 2,827,950 Koreans: a nationwide population-based study. PLoS One. 2017;12(5):e0176615. doi: 10.1371/journal.pone.0176615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wada M, et al. Effect of serum cholesterol on insulin secretory capacity: Shimane CoHRE study. PLoS One. 2016;11(2):e0149452. doi: 10.1371/journal.pone.0149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachmann JM et al. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center longitudinal study. Circulation. 2012: CIRCULATIONAHA. 111.065490. [DOI] [PMC free article] [PubMed]
- 63.Pandey AK, et al. Family history of coronary heart disease and markers of subclinical cardiovascular disease: where do we stand? Atherosclerosis. 2013;228(2):285–294. doi: 10.1016/j.atherosclerosis.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 64.Dixit AK, et al. The prevalence of dyslipidemia in patients with diabetes mellitus of ayurveda hospital. J Diabetes Metabol Disord. 2014;13(1):58. doi: 10.1186/2251-6581-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gimeno-Orna J, Faure-Nogueras E, Sancho-Serrano M. Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidaemia. Diabet Med. 2005;22(1):26–31. doi: 10.1111/j.1464-5491.2004.01341.x. [DOI] [PubMed] [Google Scholar]
- 66.Eeg-Olofsson K, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268(5):471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 67.Metelka R, Cibičková L, Gajdová J, Krystyník O. Heart rate variability evaluation in the assessment of cardiac autonomic neuropathy in patients with type 2 diabetes. Cor et Vasa. 2018;60(4):e335–e344. [Google Scholar]
- 68.Stranieri A, et al. Data-analytically derived flexible HbA1c thresholds for type 2 diabetes mellitus diagnostic. Artif Intell Res. 2015;5(1):111. [Google Scholar]
- 69.Sherwani SI et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016. 11: BMI. S38440. [DOI] [PMC free article] [PubMed]
- 70.Schmidt MI, et al. Identifying individuals at high risk for diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28(8):2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 71.Barter P, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 72.American Heart Association. Cholesterol Abnormalities and Diabetes. Jan 29,2018 [cited 2018; Available from: http://www.heart.org/HEARTORG/Conditions/More/Diabetes/WhyDiabetesMatters/Cholesterol-Abnormalities-Diabetes_UCM_313868_Article.jsp#.WrSe_4huaUl.
- 73.Vornanen M, et al. Family history and perceived risk of diabetes, cardiovascular disease, cancer, and depression. Prev Med. 2016;90:177–183. doi: 10.1016/j.ypmed.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, et al. Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS One. 2015;10(2):e0117044. doi: 10.1371/journal.pone.0117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Islam, S. M. S., & Maddison, R. Digital health approaches for cardiovascular diseases prevention and management: Lessons from preliminary studies. Mhealth. 2021;7. [DOI] [PMC free article] [PubMed]
- 76.Moses JC et al. Application of Smartphone Technologies in Disease Monitoring: A Systematic Review. in Healthcare. 2021. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed]
- 77.Islam SMS, Chow CK, Redfern J, Kok C, Rådholm K, Stepien S, Hackett ML. Effect of text messaging on depression in patients with coronary heart disease: a substudy analysis from the TEXT ME randomised controlled trial. BMJ open. 2019;9(2):e022637. [DOI] [PMC free article] [PubMed]
- 78.Islam SMS, et al. Mobile phone text-messaging interventions aimed to prevent cardiovascular diseases (Text2PreventCVD): systematic review and individual patient data meta-analysis. Open Heart. 2019;6(2):e001017. doi: 10.1136/openhrt-2019-001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Islam SMS, et al. Effects of Mobile phone SMS to improve glycemic control among patients with type 2 diabetes in Bangladesh: a prospective, parallel-group, randomized controlled trial. Diabetes Care. 2015;2015(38):112–113. doi: 10.2337/dc15-0505. [DOI] [PubMed] [Google Scholar]
- 80.Chow CK, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. Jama. 2015;314(12):1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]