Abstract
It has been well established that understanding the underlying heterogeneity of numerous complex disease process needs new strategies that present in precision medicine for prediction, prevention and personalized treatment strategies. This approach must be tailored for each individual’s unique omics that lead to personalized management of disease. The correlation between different omics data should be considered in precision medicine approach. The interaction provides a hypothesis which is called domino effect in the present minireview. Here we review the various potentials of omics data including genomics, transcriptomics, proteomics, metabolomics, pharmacogenomics. We comprehensively summarize the impact of omics data and its major role in precision medicine and provide a description about the domino effect on the pathophysiology of diseases. Each constituent of the omics data typically provides different information in associated with disease. Current research, although inadequate, clearly indicate that the information of omics data can be applicable in the concept of precision medicine. Integration of different omics data type in domino effect hypothesis can explain the causative changes of disease as it is discussed in the system biology too. While most existing studies investigate the omics data separately, data integration is needed on the horizon of precision medicine by using machine learning.
Keywords: Omics, Precision medicine, Personalized medicine
Introduction
During the last decade, the omics sciences has revolutionized translational medicine [1]. Omics (X-omics) describes high-throughput experimental technologies providing the tools for widely monitoring the disease development at a molecular level that focuses on big data. The publication of the full human genome sequence was a breakthrough in the history of omics research [2, 3].
The suffix –ome- derives from “chromosome” and includes a complete set of biological fields such as genomics, transcriptomics, proteomics, metabolomics and other omics. The “omics” approach implies a comprehensive evaluation set of molecules [4].
Traditional molecular methods are time-consuming and not adequately efficient, while omics sciences which are based on high-throughput analytical methods have proven to be accurate and more efficient, enabling scientists to better understand the genetic architecture of common diseases [5–7]. Multi-omics (X-omics) is a neologism that provides tremendous opportunity improvement for precision medicine. Precision medicine (PM) offers a way to change the clinical approaches which provide precise prevention, diagnosis, and treatment options. With the development of Next Generation Sequencing (NGS) and RNA sequencing (RNA-Seq) technologies, precision medicine is becoming attractive and practical that holds greater promise for the future of medicine [7]. The combination of metabolomics, genomics, transcriptomics, proteomics with epigenomics studies will lead to a better understanding of the disease pathophysiology. So this viewpoint considerate a key step forward toward precision medicine [8].
Multi-omics information are essentially valuable for drug development if they indicate new drug targets or a disease signatures that associate with disease outcome and/or treatment response [9].
Improvement in genetic and molecular approach in pharmacotherapy has led many progresses from the present traditional treatment. The clinical practice of genomic medicine is now a reality and most practitioners are evolving with new skills.
Genomics
Genomics is the first omics science has emerged as the systematic study of an organism’s whole genome and its functions. Genomics is divided into structural and functional genomics. Genomics is simple and comparatively fast, and works as a starting point for the other clinical omics approach. Between different types of omics approach, genomics is the most critical one [10]. Genomics studies provided a valuable infrastructure for gene mapping and identifying genetic variants contributing to both single gene and multi-factorial diseases [11]. The entire human haploid genome consists of three billion DNA base pairs (bp), encoding approximately 20,000 genes [1]. These protein-coding regions are also known as exomes, which make up 1–2% of the genome while the remaining portion holds structural significance. With the exception of genetic variations, the genome of an organism remains essentially constant over time and only less than 1% of the human genome sequence is different between individuals [12]. Genomic medicine is at the heart of precision medicine. Genome Wide Association Studies (GWAS) and Whole Exome Sequencing (WES) are new tools in the field of genomics for understanding associated variants of common multifactorial diseases. Genomic advances have the potential to revolutionize medicine and public health.
Transcriptomics
Transcription is the key regulatory step of gene expression. Transcriptomics has become an inspiring field of molecular sciences research in the post-genome era as it can reflect the potential of genome expression [13]. The term “transcriptome” was first used in the 1990s [14, 15]. Transcriptome is the complete set of Ribonucleic acid (RNA) transcripts, including the classical messenger RNAs (mRNA), ribosomal RNAs (rRNA), and transfer RNAs (tRNA) which encoded by the genome of a specific cell type or tissue. The mRNA sequence encodes proteins through ribosome machinery (including rRNA and ribosomal proteins). Additional RNAs have been known that do not encode protein which characterized as non-coding RNAs (ncRNA). These ncRNAs comprise microRNAs and long ncRNAs and have more recently been demonstrated to have regulatory functions involving gene expression and protein function. Therefore, total RNAs have important roles in gene expression and regulation in many cellular processes [16]. More than 90% of the human genome is transcribed into RNAs, among which only 2% is from the coding region of the genome [3, 17]. After the completion of the Human Genome Project, transcriptome analysis allows us to identify the expression of genome at the transcription level [3]. Transcriptome evaluation will further reveal the regulation network of biological processes and eventually give some guidance in disease prediction and prevention [10].
Proteomics
The proteome is described as a set of all proteins expressed by the genome at a specific time, in a particular condition, and in one place such as a cell, a tissue, or an organism [18]. Proteomics is a wide study protein profile on a large scale, which encompasses the study of protein composition, structure, and function as well as, the expression, post-translational changes, interactions between different proteins and those with other molecules [18].
Proteomic studies are subdivided in to three broad categories; Expression proteomics: The large-scale quantitative analysis of protein expression between different samples which is compared between diseased and healthy tissue. Therefore, the presence of a protein only within a diseased tissue is often indicative of a drug target or diagnostic marker. Structural proteomics: The structural study of protein complexes in a specific cell since proteins do not function in isolated cells, but often apply their effects in conjunction with other proteins and non-protein substances. This scientific approach aims to determine the location of proteins, the drug binding site on proteins, as well as protein- protein interactions [19]. Functional proteomics: A comprehensive analysis of protein interactions to determine the function of proteins, in which protein interactions are observed in protein signaling and biosynthesis pathway [20]. Generally, proteomics technology which characterize the complex protein combinations into their individual components have three following steps, protein separation by two-dimensional gel electrophoresis (2-DE), obtaining protein information through mass spectrometry (MS), and using databases (bioinformatics) [19, 21]. The goal of proteomics is to describe the flow of intracellular information through identifying all expressed proteins in the cell and to show their position and functions by making a comprehensive three-dimensional (3-D) structural model of the cell. This is mainly because the functions of many proteins can only be characterized by examination of their 3-D structure [22].
Proteomics is the result of interactions between genes and the environment with greater complexity than the study of genomics, because unlike the sequencing of an organism’s genome which is done irrespective of cell type, the proteome differs from cell to cell in developmental stages or environmental conditions. It is continuously changing following translation and assembly via biochemical interactions with the genome in different patterns of gene expression and protein modification [18]. For this reason, proteomics can be classified as a post-genomics science [23] which has significant clinical implications, especially in disease prevention and diagnosis [24]. Proteomics is particularly important since genomic study alone is not adequate to reveal all necessary information for characterizing protein modifications, discovering drug targets, and the genotype – phenotype correlation of the cell [19]. It is also crucial to understanding the mechanism of disease, aging and environmental effects, which cannot only be determined via genomic studies [25]. Proteomic technologies are also essential in precision medicine which require establishing a relationship between diagnosis and treatment of disease [26] through the discovery of protein biomarkers [21]. Other important applications of include designing novel drugs [27], discovering proteomic-based diagnostic mechanisms involved in cellular processes, the use of protein biochips [21]. Moreover, understanding the protein biomarkers can help in discovering new drugs and their targets [28].
Metabolomics
The profiling of metabolites in biological matrices is the most recent of ‘omic’ sciences that reveals modifications arising at all molecular levels [29, 30]. The therapeutic effects of pharmaceutical agents have been evaluated through determining the association between the standard metabolic profiles of patients and their clinical outcomes (safety and effectiveness), i.e., pharmaco-metabolomics, prediction of disease susceptibility among population in advance, patient stratification [31, 32].
Recent reports suggest that this specific field of ‘omics‘is especially important in determining the phenotypes of various diseases through discovering the changes in the pattern of metabolite expression as well as alterations in the concentration of individual metabolites and biochemical pathways [29, 30]. The susceptibility of individuals to diseases varies due to differences in genetic composition and also metabolic factors, which explains the different drug responses observed in patients who use the same pharmacological agent for the same disease (pharmaco-genomics) [33]. Metabolomics with its functional readout ability enables us to identify new diagnostic biomarkers [29, 34].
Other omics
Pharmacogenomics
Pharmacogenomics, as an extension of pharmacogenetics, is a main area in the field of PM which studies the interactions between pharmaceuticals and genes [35, 36]. It is important to distinguish between pharmacogenetics and Pharmacogenomics. As Food and Drug Administration (FDA) and European Medicines Agency (EMA) had published in their Guidance for industry (E15 Definitions) on year 2008, Pharmacogenomics is “The study of variations of genes as related to drug response and/ or adverse drug reactions” and pharmacogenetics is defined as “The study of variations in a single gene as related to drug response and/ or adverse drug reactions” [37].
Pharmacogenomics science help to improve the safety and efficacy of a treatment by giving medicines that are suitable to a person’s gene structure, expression and function [36]. It has two subcategories; personalized drug therapy with regard to germ line variations and guided cancer therapy via somatic and genetic variations and molecular-targeted approach [35, 38]. This area of science is going toward evidence building of individual’s genetic associations with drug dose, response or toxicity and patient-related outcomes [35]. The aims of pharmacogenomics are evidence-base creation for precision medicine, uncovering the effects of gene expressions and genetic variations on drug response and safety, and also discovering new targets for future medications [36, 39]. Pharmacogenomics has changed the basis of clinical trials and drug development processes by developing more effective drugs with less adverse drug reactions (ADRs) in a faster, more cost-effective manner especially in cancer area [38, 40] (Table 1).
Table 1.
The different type omics sciences in precision medicine approach
| Omics levels | Definition | Technique | Ref |
|---|---|---|---|
| Genomics | Genomics is the most mature of the omics fields. In medical research, genomics focuses on identifying genetic variants linked to disease, therapy response, and patient prognosis in the future. | DNA sequencing, genetic profiling, genetic mapping, recombinant DNA technology, structural and functional analysis of genome. | [41] |
| Transcriptomics |
Transcriptomics is the study of the transcriptome, which is the complete set of RNA transcripts produced by the genome in a specific cell under certain conditions, utilizing high-throughput technologies like microarray analysis. Comparison of transcriptomes provides the identification of genes that are differentially expressed in distinct cell populations, or in response to different treatments. Transcriptomics evaluate RNA levels genome-wide, both qualitatively and quantitatively. |
RNA sequencing, expression profiling, and transcriptional regulation. Microarrays are used to simultaneously assess the expression of thousands of genes and to generate gene expression profiles, which reflect changes in the transcriptome in response to a certain condition or therapy. | [42–44] |
| Proteomics | Proteomics involve the large-scale study of whole proteome or the sum of all proteins from an organism, tissue, cell or biofluid, their structure and physiological role or functions. | Protein identification, quantification, and post-translational modification. | [45, 46] |
| Metabolomics |
Metabolomics is comprehensive analysis of small molecules, commonly known as metabolites, within cells, biofluids, tissues or organisms. Overall, these small molecules and their interactions within a biological system are known as the metabolome. Metabolomics is a strong method because metabolites and their concentrations directly represent the underlying metabolic activity and condition of cells and tissues. As a result, metabolomics is the best representation of the molecular phenotype. |
Two main analytical techniques are currently being used in metabolomics: NMR spectroscopy and HRMS. Modern separation techniques, such as LC, GC, or CE, are often coupled with HRMS. | [47–49] |
| Pharmacogenomics | The pharmacogenetics is the study of how genetic variation affects medication treatment outcomes. While the terms pharmacogenetics and pharmacogenomics are frequently used interchangeably, pharmacogenetics refers to the effects of a particular genetic marker, and pharmacogenomics refers to the combined influence of variability across the genome to control health. Both the pharmacokinetics and pharmacodynamics of drugs may be affected by pharmacogenetics. The interindividual genetic variations (mutation and SNPs) in genes encoding of pharmacodynamic components (Receptors, Ion channels, enzymes, and Immune seystem) lead to change in pharmacokinetic (Absorbsion, distribution, metabolism, and excertion) and consequently influence the efficacy and toxicity of numerous drugs. | The common genotyping methods used in pharmacogenomics studies, including PCR-RFLP analysis, pyrosequencing, TaqMan, mass spectrometry, and DHPLC. | [50–52] |
| Microbiome | Microbiomics is a rapidly expanding science in which all of the microorganisms in a given population (called a “microbiota”) are studied collectively. This could be the microbiota from an environmental sample (such as soil or water), a specific body region (such as the gut or the mouth), or a specific organism (e.g. farm or zoo animals). | The most widely used method in microbiome analysis is 16S rRNA genes sequencing to detecting even rare species. 16S rRNA genes are highly conserved between bacterial species, but vary in a manner that allows species identification. | [53–55] |
| Stem-cellomics | Stem-cellomics aims to identify and model the biological features of stem cells and the genomic relationships | Tissue engineering and regenerative medicine method | [56, 57] |
DNA deoxyribonucleic acid, RNA ribonucleic acid, NMR nuclear magnetic resonance, HRMS spectroscopy and high-resolution mass spectrometry, LC liquid chromatography, GC gas chromatography, CE capillary electrophoresis, SNPs single nucleotide polymorphisms, PCR-RFLP polymerase chain reaction-restriction fragment length polymorphism, DHPLC denaturing high pressure liquid chromatography, rRNA ribosomal RNA
One of the barriers that is minatory to pharmacogenomics and ban its advance to clinical use is physician’s level of familiarity and comfort with pharmacogenomics data and genotype-guided pharmacotherapies [58]. This barrier could overcome by using educational programs and availability of physician-friendly resources, such as pharmacogenomics clinical practice guidelines [35, 58]. The use of pharmacogenomics can empower physicians to predict the effectiveness and safety of a treatment, prior to its application [39].
Application of prognostic genetic markers has been well recognized and established in personalized therapy in many modern clinical fields; Such as oncology, cardiovascular diseases (CVD), transplantation medicine and clinical psychiatry [59, 60]. For example, in the field of oncology, detection of tumor tissue for somatic mutations could help physicians to decide their treatment strategies (selection of transcription factor inhibitors or tyrosine kinase) [59, 61].
Microbiome
The advent of microbiome research has determined the microbiome as significant component of precision medicine and human health [62–64]. The human microbiome is the ecological community of commensal, symbiotic, and pathogenic micro-organisms [65] both within and upon us [66, 67]. It is normally located in specific body site including, skin, gastrointestinal tract, and vagina [68] the sequencing of a complete bacterial genome. Microbiome composition and function variations depend on their location, age, sex, race, and diet of their host [69]. The microbiota community specially has an important role in human health through different mechanisms. It is complicated in human normal biological function such as physiology (including immunity and development), metabolism and biosynthesis [68]. Furthermore, disequilibrium in the distribution of mirobiome population may contribute to the pathogenesis of many conditions such as liver, infectious, gastrointestinal [70], respiratory, metabolic [71], psychiatric [72], and autoimmune problems [71] which is not inherited by DNA. That may be as a consequence of interaction between the genetic, environmental factors, and changes in microbiome and dysregulated immune responses [73]. Several studies have demonstrated human microbiome is the main component of the system of biology. Any changes in the human microbiome would be one of the major reason of human diseases pathogenesis [70]. Improvement in the understanding of human microbiome and also its association with the mechanism of diseases can lead to development of next generation diagnosis and new therapeutic approaches for various diseases [67, 70]. This subject was considered generally in many studies [71, 74, 75].
Human microbiome is different between individuals and can easily be modified [76]. Microbiome is an important part of personalized or precision medicine approach to progress diagnosis, and enhance early detection and treatment as abundant of human functioning and metabolism is dependent upon microbiome. Microbiome can be targeted as a variable factor [62] by probiotic and prebiotic supplements, diet, as well as fecal microbiota transplant.
Stem-cellomics
Stem-cellolomics is an innovative term composed of “stem cell” and “omics”. Stem cells are undifferentiated foundation cells with two main features: ability for self-renewal and potency to differentiation. In human body, there are two types of stem cells: embryonic stem cells and adult stem cells. Similarly, there are some engineering types including induced pluripotent stem cells (iPSCs), which are produced in the laboratory by converting tissue-specific cells to express embryonic stem cells [77]. Several studies have introduced iPSCs as a unique tool to recapitulate disease phenotypes in vitro, that is useful to evaluate the effect of disease and individualized treatment-related polymorphism [78]. Since, these kinds of cell have an ability of differentiated into different cell types and keep their genome integrity during the large number of passage, they are the right options for study on genetic variation associated with human disease [79]. On the other hand, they are robust candidate for cell therapy that should be discussed elsewhere [80]. The suffix of “omics” refers to a field of study in biology that has explained in previous parts of this article. Hence, stem-cellolomics aims to identify and model the biological features of stem cells and the genomic relationships. This aspect of knowledge helps researchers to improve diagnosis and treatment of disease based on individuals’ cell features in bench to bedside translational studies. Previous studies have used stem cells as a modeling tool in a wide range of diseases including diabetes, cancers, neurological, cardiovascular and immune system disease [80–86]. Furthermore, there are some challenges and limitations ahead of the using cell as a modeling tool. Some more important challenges including, ethical guidelines, cost efficiency, clinical validation, regulatory issues and new demand to developing new analytical methods and supporting technologies [82]. So, these challenges are expected to be ahead of stem-cellolomics as a new concept, too.
Domino effect hypothesis in precision medicine
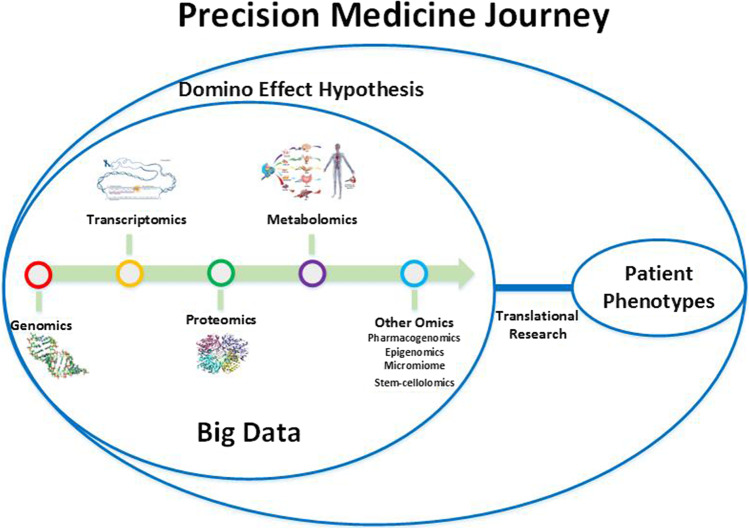
Precision medicine can profoundly improve the practice of clinical medicine by multi-omics approaches. Omics discoveries have opened new windows to precision medicine. These technologies have enabled us to better understand the micro-architecture of many diseases, especially cancer, and also are crucial in pharmaceutical research (Fig. 1) [5, 6, 87]. In this article, we propose a new point of view in the field of precision medicine about the role of omics disciplines. It indicated that the precision medicine is influenced from a phenomenon which may be called domino effect (as a hypothesis). It seems that the domino effect has an important role in pathophysiology progression of multifactorial diseases and there is a complex network between genomics, proteomics, metabolomics, and other disciplines of omics in this hypothesis. Actually, Domino effect can provide a new insight into the pathogenesis of disease. Most common diseases are multifactorial and it occurs as the result of interaction between genetic and environmental factors. In the domino effect we consider an upstream to downstream of inter-connected network that beyond this pathways epigenetic factors impact and accelerate the disease pathogenesis. The epigenetic factors may explain the variation in the phenotypic presentation of diseases. The pathway of domino effect is started by genomics. If the genomics undergoes a change such as a mutation or single nucleotide polymorphism (SNP), a huge cascade of events will happen following a tiny modification. Any problem in the genomics can target other omics this hypothesis suggested that genomics is the core part of disease pathogenesis and progression which can be a trigger for other omics. With this complicated network between omics; disease treatment and prognosis will be most challengeable because we should know the omics information of each patient.
Fig. 1.
Precision medicine approach is a journey that should pass through domino effect hypothesis. The multi-omics approaches including genomics, transcriptomics, proteomics, metobolomics, etc. by producing a large amounts of data and in combination with patient phenotypes integrate a great scientific revolution in the practice of medicine
Conclusion
Information from genomics, transcriptomics, proteomics, metabolomics, and other omics would be combined to support prediction, prevention and personalized treatment of disease and to increase our knowledge about human pathophysiology of diseases. The real challenge in the omics science is translating acquired omics big data into applicable decision in clinical practice with the aim of achieving precision medicine goals. By introducing of high-throughput omics technologies and the computational surge, we are also able to use machine learning that can target different type of molecules in order to predict, to prevent and personalized treatment of diseases in the precision medicine approach. Actually it requires supercomputing power, exclusive algorithms and artificial intelligence (AI). This is the future perspective of precision medicine which leads to make patients in the center of point-of-care.
Abbreviations
- PM
Precision medicine
- NGS
Next Generation Sequencing
- RNA-Seq
RNA sequencing
- bp
base pairs
- GWAS
Genome Wide Association Studies
- WES
Whole Exome Sequencing
- RNA
Ribonucleic acid
- 2-DE
Two-dimensional gel electrophoresis
- MS
Mass spectrometry
- 3-D
Three-dimensional
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- ADRs
Adverse drug reactions
- CVD
Cardiovascular diseases
- iPSCs
induced pluripotent stem cells
- SNP
Single nucleotide polymorphism
- AI
Artificial intelligence
Authors’ contributions
MH: edit and approve the final manuscript; NS, NA, MA, FKH: write the first draft of the manuscript (based on their specialties); SHN and SECH: provide professional advice; and HRAM: design of the study.
Declarations
Conflict of interest
None of the authors have competing interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manzoni C, Kia DA, Vandrovcova J, Hardy J, Wood NW, Lewis PA, et al. Genome, transcriptome and proteome: the rise of omics data and their integration in biomedical sciences. Brief Bioinform. 2016;19(2):286–302. doi: 10.1093/bib/bbw114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan SK, Liu RH, Jin HZ, Liu XR, Ye J, Shan L, et al. "Omics" in pharmaceutical research: overview, applications, challenges, and future perspectives. Chin J Nat Med. 2015;13(1):3–21. doi: 10.1016/S1875-5364(15)60002-4. [DOI] [PubMed] [Google Scholar]
- 3.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 4.D’Adamo GL, Widdop JT, Giles EM. The future is now? Clinical and translational aspects of “omics” technologies. Immunol Cell Biol. 2021;99(2):168–176. doi: 10.1111/imcb.12404. [DOI] [PubMed] [Google Scholar]
- 5.Bluett J, Barton A. Precision medicine in rheumatoid arthritis. Rheum Dis Clin N Am. 2017;43(3):377–387. doi: 10.1016/j.rdc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Au TH, Wang K, Stenehjem D, Garrido-Laguna I. Personalized and precision medicine: integrating genomics into treatment decisions in gastrointestinal malignancies. J Gastrointest Oncol. 2017;8(3):387–404. doi: 10.21037/jgo.2017.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Maria MR, Di Sante G, Piro G, Carbone C, Tortora G, Boldrini L, et al. Translational research in the era of precision medicine: where we are and where we will go. J Pers Med. 2021;11(3):216. doi: 10.3390/jpm11030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob M, Lopata AL, Dasouki M, Abdel Rahman AM. Metabolomics toward personalized medicine. Mass Spectrom Rev. 2019;38(3):221–238. doi: 10.1002/mas.21548. [DOI] [PubMed] [Google Scholar]
- 9.Hartl D, de Luca V, Kostikova A, Laramie J, Kennedy S, Ferrero E, et al. Translational precision medicine: an industry perspective. J Transl Med. 2021;19(1):1–14. doi: 10.1186/s12967-021-02910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodadadian A, Darzi S, Haghi-Daredeh S, Sadat Eshaghi F, Babakhanzadeh E, Mirabutalebi SH, et al. Genomics and transcriptomics: the powerful Technologies in Precision Medicine. Int J Gen Med. 2020;13:627–640. doi: 10.2147/IJGM.S249970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res 2015;43(Database issue):D1079–D1085. [DOI] [PMC free article] [PubMed]
- 13.Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405(6788):827. doi: 10.1038/35015701. [DOI] [PubMed] [Google Scholar]
- 14.Piétu G, Mariage-Samson R, Fayein N-A, Matingou C, Eveno E, Houlgatte R, et al. The Genexpress IMAGE knowledge base of the human brain transcriptome: a prototype integrated resource for functional and computational genomics. Genome Res. 1999;9(2):195–209. [PMC free article] [PubMed] [Google Scholar]
- 15.Velculescu VE, Zhang L, Zhou W, Vogelstein J, Basrai MA, Bassett DE, et al. Characterization of the yeast transcriptome. Cell. 1997;88(2):243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- 16.Eddy SR. Non–coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2(12):919. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 17.Carninci P, Yasuda J, Hayashizaki Y. Multifaceted mammalian transcriptome. Curr Opin Cell Biol. 2008;20(3):274–280. doi: 10.1016/j.ceb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Horgan RP, Kenny LC. ‘Omic’technologies: genomics, transcriptomics, proteomics and metabolomics. Obstet Gynaecol. 2011;13(3):189–195. [Google Scholar]
- 19.Graves PR, Haystead TA. Molecular biologist's guide to proteomics. Microbiol Mol Biol Rev. 2002;66(1):39–63. doi: 10.1128/MMBR.66.1.39-63.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figeys D. Functional proteomics: mapping protein-protein interactions and pathways. Curr Opin Cell Biol. 2002;4(3):210–215. [PubMed] [Google Scholar]
- 21.Beranova-Giorgianni S. Proteome analysis by two-dimensional gel electrophoresis and mass spectrometry: strengths and limitations. TrAC Trends Analyt Chem. 2003;22(5):273–281. [Google Scholar]
- 22.Barh D, Khan MS, Davies E. PlantOmics: the omics of plant science. Springer; 2016.
- 23.Holmes C, Carlson SM, McDonald F, Jones M, Graham J. Exploring the post-genomic world: differing explanatory and manipulatory functions of post-genomic sciences. New Genet Soc. 2016;35(1):49–68. doi: 10.1080/14636778.2015.1133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins IJ. The role of clinical proteomics, Lipidomics, and genomics in the diagnosis of Alzheimer’s disease. Proteomes. 2016;4(2):14. doi: 10.3390/proteomes4020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boopathi NM. Genetic mapping and marker assisted selection: basics, practice and benefits. Springer Science & Business Media; 2012.
- 26.Jain KK. Role of proteomics in the development of personalized medicine. Adv Protein Chem Struct Biol. 2016;102:41–52. doi: 10.1016/bs.apcsb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Scarano E, Fiorita A, Picciotti P, Passali G, Calo L, Cabras T, et al. Proteomics of saliva: personal experience. Acta Otorhinolaryngol Ital. 2010;30(3):125. [PMC free article] [PubMed] [Google Scholar]
- 28.Hale EJ, Gelfanova V, Ludwig RJ, Knierman MD. Application of proteomics for discovery of protein biomarkers. Brief Funct Genomics. 2003;2(3):185–193. doi: 10.1093/bfgp/2.3.185. [DOI] [PubMed] [Google Scholar]
- 29.Puchades-Carrasco L, Pineda-Lucena A. Metabolomics applications in precision medicine: an oncological perspective. Curr Top Med Chem. 2017;17(24):2740–2751. doi: 10.2174/1568026617666170707120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baraldi E, Carraro S, Giordano G, Reniero F, Perilongo G, Zacchello F. Metabolomics: moving towards personalized medicine. Ital J Pediatr. 2009;35(1):30. doi: 10.1186/1824-7288-35-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, He X, Jia W, Li H. Novel applications of metabolomics in personalized medicine: a mini-review. Molecules. 2017;22(7):1173. doi: 10.3390/molecules22071173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15(7):473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 33.Long NP, Nghi TD, Kang YP, Anh NH, Kim HM, Park SK, et al. Toward a Standardized Strategy of Clinical Metabolomics for the Advancement of Precision Medicine. Metabolites. 2020;10(2). [DOI] [PMC free article] [PubMed]
- 34.Bekri S. The role of metabolomics in precision medicine. Expert Rev Precis Med Drug Dev. 2016;1(6):517–532. [Google Scholar]
- 35.Johnson JA, Weitzel KW. Advancing pharmacogenomics as a component of precision medicine: how, where, and who? Clin Pharmacol Ther. 2016;99(2):154–156. doi: 10.1002/cpt.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlab R, Zhang L. Pharmacogenomics: historical perspective and current status. Methods Mol Biol. 2013;1015:3–22. doi: 10.1007/978-1-62703-435-7_1. [DOI] [PubMed] [Google Scholar]
- 37.FDA. International Conference on Harmonisation; Guidance on E15 Pharmacogenomics Definitions and Sample Coding; Availability. Notice. Federal register. 2008;73(68):19074–6. [PubMed]
- 38.Nakatani K, Nobori T. Pharmacogenomics. Rinsho byori The Japanese journal of clinical pathology. 2013;61(11):1018–1025. [PubMed] [Google Scholar]
- 39.Lee JW, Aminkeng F, Bhavsar AP, Shaw K, Carleton BC, Hayden MR, et al. The emerging era of pharmacogenomics: current successes, future potential, and challenges. Clin Genet. 2014;86(1):21–28. doi: 10.1111/cge.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hockings JK, Pasternak AL, Erwin AL, Mason NT, Eng C, Hicks JK. Pharmacogenomics: an evolving clinical tool for precision medicine. Clevel Clin J Med. 2020;87(2):91–99. doi: 10.3949/ccjm.87a.19073. [DOI] [PubMed] [Google Scholar]
- 41.McGuire AL, Gabriel S, Tishkoff SA, Wonkam A, Chakravarti A, Furlong EE, et al. The road ahead in genetics and genomics. Nat Rev Genet. 2020;21(10):581–96. doi: 10.1038/s41576-020-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Consortium EP An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12(2):87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khodadadian A, Darzi S, Haghi-Daredeh S, Sadat Eshaghi F, Babakhanzadeh E, Mirabutalebi SH, et al. Genomics and transcriptomics: the powerful technologies in precision medicine. Int J Gen Med. 2020;13:627–40. doi: 10.2147/IJGM.S249970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hein MY, Sharma K, Cox J, Mann M. Proteomic analysis of cellular systems. Handbook of systems biology: concepts and insights. Academic Press; 2013. p. 3–25.
- 46.Jain KK. Role of Proteomics in the Development of Personalized Medicine. Adv Protein Chem Struct Biol. 2016;102:41–52. doi: 10.1016/bs.apcsb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Shin S-Y, Fauman EB, Petersen A-K, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543–50. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gieger C, Geistlinger L, Altmaier E, Hrabé de Angelis M, Kronenberg F, Meitinger T, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4(11):e1000282. [DOI] [PMC free article] [PubMed]
- 49.Patti GJ, Yanes O, Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13(4):263–9. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinshilboum RM, Wang L. Pharmacogenomics: Precision Medicine and Drug Response. Mayo Clin Proc. 2017;92(11):1711–22. doi: 10.1016/j.mayocp.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JW, Aminkeng F, Bhavsar AP, Shaw K, Carleton BC, Hayden MR, et al. The emerging era of pharmacogenomics: current successes, future potential, and challenges. Clin Genet. 2014;86(1):21–8. doi: 10.1111/cge.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, et al. Pharmacogenomics. Lancet (London, England). 2019;394(10197):521–32. doi: 10.1016/S0140-6736(19)31276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Org E, Parks BW, Joo JWJ, Emert B, Schwartzman W, Kang EY, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015;25(10):1558–69. doi: 10.1101/gr.194118.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekri S. The role of metabolomics in precision medicine. Expert Rev Precis Med Drug Dev. 2016;1(6):517–32. [Google Scholar]
- 56.Michal K Stachowiak EST. Stem cells, from mechanisms to technologies. USA: Word Scientific; 2012.
- 57.Hamazaki T, El Rouby N, Fredette NC, Santostefano KE, Terada N. Concise review: Induced pluripotent stem cell research in the era of precision medicine. Stem Cells. 2017;35(3):545–50. doi: 10.1002/stem.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J Pharm Sci. 2017;106(9):2368–2379. doi: 10.1016/j.xphs.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 59.Cascorbi I, Tyndale R. Progress in pharmacogenomics: bridging the gap from research to practice. Clin Pharmacol Ther. 2014;95(3):231–235. doi: 10.1038/clpt.2013.235. [DOI] [PubMed] [Google Scholar]
- 60.Sanoudou D. Pharmacogenomics: achievements, challenges and prospects, for patients, pharmaceutical industries and healthcare systems. Curr Pharm Des. 2010;16(20):2182–2183. doi: 10.2174/138161210791792840. [DOI] [PubMed] [Google Scholar]
- 61.Gillis NK, Patel JN, Innocenti F. Clinical implementation of germ line Cancer Pharmacogenetic variants during the next-generation sequencing era. Clin Pharmacol Ther. 2014;95(3):269–280. doi: 10.1038/clpt.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap PC, Chia N, Nelson H, Segal E, Elinav E, editors. microbiome at the Frontier of Personalized Medicine. Mayo Clinic Proceedings; 2017: Elsevier. [DOI] [PMC free article] [PubMed]
- 63.Petrosino JF. The microbiome in precision medicine: the way forward. Genome Med. 2018;10(1):12. doi: 10.1186/s13073-018-0525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cammarota G, Ianiro G, Ahern A, Carbone C, Temko A, Claesson MJ, et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat Rev Gastroenterol Hepatol. 2020;17(10):635–648. doi: 10.1038/s41575-020-0327-3. [DOI] [PubMed] [Google Scholar]
- 65.Eloe-Fadrosh EA, Rasko DA. The human microbiome: from symbiosis to pathogenesis. Annu Rev Med. 2013;64:145–163. doi: 10.1146/annurev-med-010312-133513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgan XC, Segata N, Huttenhower C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013;29(1):51–58. doi: 10.1016/j.tig.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blum HE. The human microbiome. Adv Med Sci. 2017;62(2):414–420. doi: 10.1016/j.advms.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–1458. doi: 10.1053/j.gastro.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu Rev Pathol. 2012;7:99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering. 2017;3(1):71–82. [Google Scholar]
- 72.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 75.Lu H, Zhang C, Qian G, Hu X, Zhang H, Chen C, et al. An analysis of microbiota-targeted therapies in patients with avian influenza virus subtype H7N9 infection. BMC Infect Dis. 2014;14(1):359. doi: 10.1186/1471-2334-14-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vázquez-Baeza Y, Callewaert C, Debelius J, Hyde E, Marotz C, Morton JT, et al. Impacts of the human gut microbiome on therapeutics. Annu Rev Pharmacol Toxicol. 2018;58:253–270. doi: 10.1146/annurev-pharmtox-042017-031849. [DOI] [PubMed] [Google Scholar]
- 77.Michal K, Stachowiak EST. Stem cells, from mechanisms to technologies. Word Scientific: USA; 2012. [Google Scholar]
- 78.Hamazaki T, El Rouby N, Fredette NC, Santostefano KE, Terada N. Concise review: induced pluripotent stem cell research in the era of precision medicine. Stem Cells. 2017;35(3):545–550. doi: 10.1002/stem.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun Y, Ding Q. Genome engineering of stem cell organoids for disease modeling. Protein Cell. 2017;8(5):315–327. doi: 10.1007/s13238-016-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Sa SF, Almeida PN, Rettore JV, Maranduba CP, de Souza CM, de Souza GT, et al. Toward personalized cell therapies by using stem cells: seven relevant topics for safety and success in stem cell therapy. J Biomed Biotechnol. 2012;2012:758102. doi: 10.1155/2012/758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdelalim EM, Bonnefond A, Bennaceur-Griscelli A, Froguel P. Pluripotent stem cells as a potential tool for disease modelling and cell therapy in diabetes. Stem Cell Rev. 2014;10(3):327–337. doi: 10.1007/s12015-014-9503-6. [DOI] [PubMed] [Google Scholar]
- 82.Zhu W, Zhang XY, Marjani SL, Zhang J, Zhang W, Wu S, et al. Next-generation molecular diagnosis: single-cell sequencing from bench to bedside. Cell Mol Life Sci. 2017;74(5):869–880. doi: 10.1007/s00018-016-2368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gener P, Rafael DF, Fernandez Y, Ortega JS, Arango D, Abasolo I, et al. Cancer stem cells and personalized cancer nanomedicine. Nanomedicine (London, England). 2016;11(3):307–20. [DOI] [PubMed]
- 84.Morokoff A, Ng W, Gogos A, Kaye AH. Molecular subtypes, stem cells and heterogeneity: implications for personalised therapy in glioma. J Clin Neurosci. 2015;22(8):1219–1226. doi: 10.1016/j.jocn.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Moretti A, Laugwitz KL, Dorn T, Sinnecker D, Mummery C. Pluripotent stem cell models of human heart disease. Cold Spring Harb Perspect Med. 2013;3(11). [DOI] [PMC free article] [PubMed]
- 86.Than NN, Jeffery HC, Oo YH. Autoimmune hepatitis: Progress from global immunosuppression to personalised regulatory T cell therapy. Can J Gastroenterol Hepatol. 2016;2016:7181685. doi: 10.1155/2016/7181685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bai JPF, Melas IN, Hur J, Guo E. Advances in omics for informed pharmaceutical research and development in the era of systems medicine. Expert Opin Drug Discov. 2018;13(1):1–4. doi: 10.1080/17460441.2018.1394839. [DOI] [PubMed] [Google Scholar]