Abstract
Objectives:
Tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) is used as a biomarker of ART adherence. Recent treatment studies have shown that TFV-DP predicts future viremia in persons with HIV (PWH) but there are few data from high-burden settings. We investigated whether TFV-DP in DBS predicts future viral breakthrough in South African PWH.
Design:
Prospective observational cohort
Methods
We enrolled 250 adults receiving tenofovir-containing regimens, currently virally suppressed (<50 copies/mL), but at risk of future viral breakthrough, from four primary health clinics in Cape Town. Paired viral load (VL) and DBS for TFV-DP were collected monthly for 12 months. Viral breakthrough was the first confirmed VL >400 copies/mL. Logistic regression estimated the odds ratio (OR) and 95% confidence intervals for future viral breakthrough at the next visit.
Results:
Participants provided 2,944 paired DBS and VL samples. Median (IQR) age was 34 (27 – 42) years; median duration on ART at study entry was 11 (4–12) months; 78% were women. Twenty-one (8%) participants developed viral breakthrough. Participants with TFV-DP ≤400 fmol/punch had an adjusted OR of 16.1 (95% CI: 3.9–67.4; p<0.001) for developing viral breakthrough one month later compared to participants with TFV-DP >800 fmol/punch.
Conclusions:
TFV-DP in DBS strongly predicted future viral breakthrough in a clinical cohort of South African PWH. A biomarker able to identify PWH at risk for future viral breakthrough has the potential to improve health outcomes through timely intervention. Future studies exploring the clinical use of TFV-DP in DBS in conjunction with viral load in ART monitoring are warranted.
Keywords: HIV, antiretroviral therapy, tenofovir diphosphate, adherence, dried blood spots
Introduction:
Antiretroviral therapy (ART) non-adherence is a critical public health problem in HIV clinical care, particularly in low-resource settings. Low ART adherence can negatively influence treatment outcomes and propagate onward HIV transmission.[1,2] South Africa has the largest population of persons with HIV (PWH; 7.6 million) and the largest ART program in the world, with 70% of PWH in South Africa receiving ART.[3] However, UNAIDS estimates that only 64% of South Africans on ART are virally suppressed to <1000 copies/mL.[3] Although durable high adherence to ART is necessary to achieve and maintain viral suppression, objectively quantifying ART adherence through clinically actionable measures remains a challenge.[4,5]
In South Africa, tenofovir disoproxyl fumarate (TDF) forms the backbone of first-line regimens and some second-line regimens. Its widespread use makes it an ideal drug to target in adherence monitoring. Tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) is a biomarker of cumulative ART adherence that is informative about average adherence in the preceding 6–8 weeks due to its long intracellular half-life of 17 days in red blood cells, which are abundant in DBS.[6,7] To date, most studies using TFV-DP in DBS have involved persons at high risk for HIV using Pre-Exposure Prophylaxis (PrEP). In PWH, TFV-DP in DBS has been shown to correlate with other adherence measures such as pharmacy refills[8] and electronic adherence monitoring.[9] Most recently, other studies demonstrated that TFV-DP in DBS is strongly associated with viral suppression in PWH, and that this association strengthened with increasing TFV-DP concentrations.[10,11] However, only one of those studies was in South Africa and exclusively among post-partum women.
While the association of a pharmacologic measure with viral suppression is informative, a measure of ART adherence that can predict viral breakthrough before it happens, and even identify PWH at risk for developing ART resistance, could provide unique opportunities for intervention.[12] This would be of particular interest in countries like South Africa, where ongoing clinical monitoring and treatment options are limited.[13] For example, in South Africa, due to limited healthcare resources, PWH on ART only receive adherence-enhancing interventions after viral breakthrough has been identified; and viral loads are only drawn at 4 months and 12 months post-ART initiation and annually thereafter.[14] Thus, intervening before viral breakthrough to maintain viral suppression would promote better health outcomes, and help reduce transmission.[12] Furthermore, an intervention prior to viral breakthrough could reduce the risk for patients developing antiretroviral drug resistance, and thus conserve the use of first-line single-tablet treatment regimens. To date, this predictive value has been established for TFV-DP and emtricitabine triphosphate (FTC-TP) in DBS in PWH in the US[15] and in post-partum women in South Africa,[16] but has not been assessed in a larger cohort of PWH in this setting. To address this, we present data from a longitudinal cohort of South African PWH on ART that assessed TFV-DP as a predictor of time to first viral breakthrough. We also describe the ranges of TFV-DP in DBS that are associated with sustained viral suppression and detectable viremia in this cohort.
Methods:
Design, participants and setting
Study participants were recruited from four public sector ART clinics (Gugulethu Community Health Center, Vuyani Clinic, Nyanga Community Health Center, and Gugulethu Clinic) in Cape Town, South Africa between November 2016 and November 2018. We recruited adult PWH who met the following inclusion criteria: virally suppressed (<50 copies/ml), on first-line (TDF-based) treatment, and at increased risk of viral breakthrough either due to being in their first year of treatment[17,18] or by having documented previous adherence challenges. These challenges were determined by either routine pharmacy refill data, pill counts, electronic adherence monitoring data or previous viral breakthrough. No resistance data were available, as this is not routinely collected in the South African ART program.
Study procedures
Individuals who met the inclusion criteria on folder review were approached by the study team and offered study participation. All participants provided written informed consent in their preferred language (English or Xhosa). Participants were required to attend a baseline visit followed by 12 monthly visits, approximately 30 days apart. Demographics and medical history were collected through questionnaires and medical chart review. Several months after study initiation a protocol amendment allowed for the collection of height and weight for body mass index (BMI) at each study visit.
Whole blood for DBS was collected at each study visit by venipuncture. To prepare the DBS, 25 microliters of whole blood were spotted five times onto a Whatman 903 ProteinSaver card and dried for a minimum of 3 hours and up to overnight before being individually packaged in a plastic bag with dessicant and a humidity indicator. The cards were stored frozen at −80˚C prior to shipment. DBS samples were batched and shipped to the Colorado Antiviral Pharmacology Laboratory (CAVP) to be processed using a validated assay for TFV-DP.[6,19] Hematocrit was assessed at baseline and every other visit thereafter.
Anticoagulated whole blood for PCR HIV-1 RNA (viral load) was collected at every visit and transported to the National Health Laboratory Service (NHLS) to be assayed using the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 Test. Plasma viral load results >400 copies/ml were confirmed by running a repeat assay from the original blood sample. A confirmed viral breakthrough event was defined as two assay results >400 copies/ml from the same sample.
Statistical Analysis
Descriptive statistics were calculated and reported for the full sample. For those who remained virally suppressed throughout the study, descriptive statistics were calculated for TFV-DP in DBS separately by BMI category by kg/m2 (<18.5 = underweight; 18.5–24.9 = normal; 25–29.9 = overweight; ≥30 = obese).
We used logistic regression to estimate the odds ratio (OR), adjusted OR (aOR) and 95% confidence intervals (CI) for the association between first viral breakthrough and TFV-DP concentrations from 1 month or 2 months prior. Adjusted models controlled for age and hematocrit at enrollment, gender, and first available BMI. These adjustment variables were selected based on the known pharmacology of tenofovir in plasma[20] and TFV-DP in DBS.[7,21] For those who remained virally suppressed throughout the study, their median TFV-DP concentration from all available study visits was used. We categorised TFV-DP concentrations into three groups: 1) ≤400 fmol/punch, 2) >400–800 fmol/punch, and 3) >800 fmol/punch. The concentration dividing the lower categories was based on the Receiver Operating Characteristic (ROC) analysis (400 fmol/punch) and the concentration dividing the upper categories was based on the lower end of the interquartile range (IQR) of PWH who were suppressed throughout the study (800 fmol/punch). In our primary analysis, we examined the association between TFV-DP concentration and the first viral breakthrough (defined as viral load of >400 copies/ml) one month later. We also conducted sensitivity analyses where we explored different definitions for viral breakthrough (viral load >50, viral load >1000 copies/mL) at different timepoints (one month later, two months later). As illustrated in Figure 1, TFV-DP concentrations measured one month before viral load reflect adherence behavior up to two months prior. Participants with viral breakthrough who did not have TFV-DP collected at the selected time point were excluded from analysis.
Figure 1:
The relationship between the adherence lookback period captured by tenofovir disphosphate (TFV-DP) concentrations in Dried Blood Spots (DBS) and the viral load taken at each study visit. A TFV-DP in DBS taken at e.g. Month 3 will reflect the adherence of the previous 6–8 weeks i.e. the period between Month 1 and Month 3.
Ethics
The study was approved by the National Institute of Allergy and Infectious Diseases Division of AIDS Clinical Science Review Committee, the University of Cape Town Human Research Ethics Committee, and the New York State Psychiatric Institute Institutional Review Board.
Results:
Study population
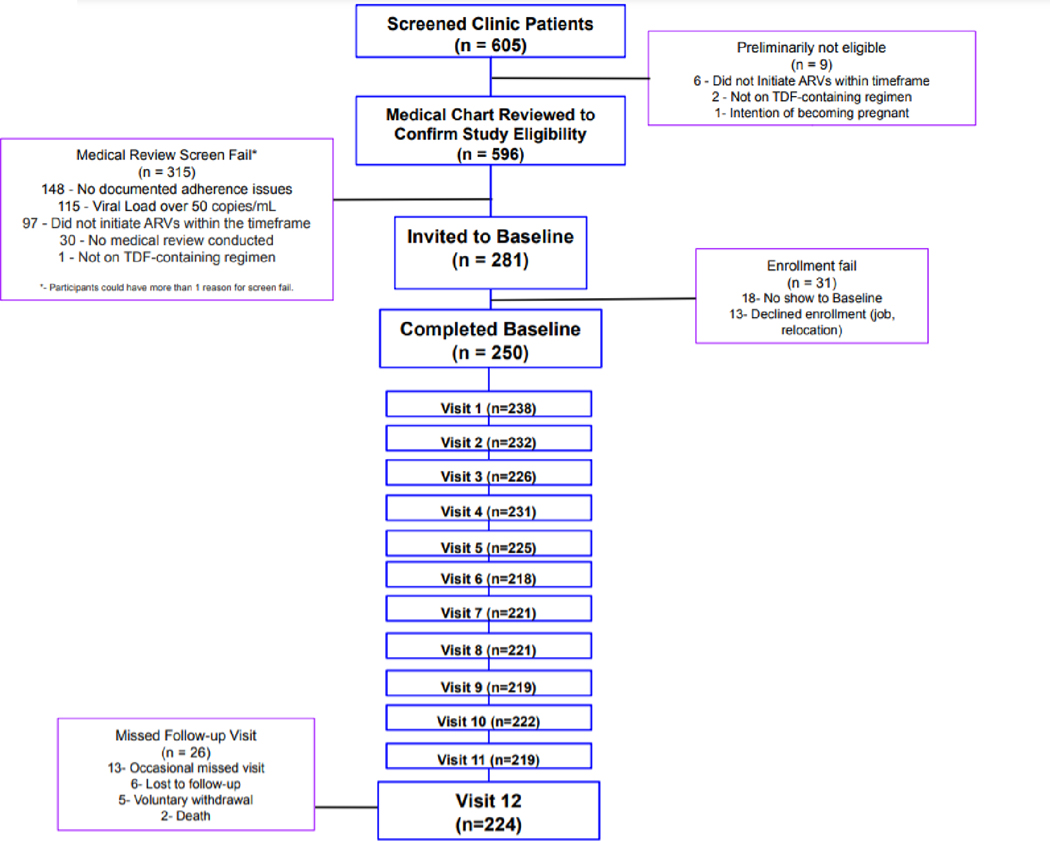
We enrolled 250 PWH, who provided a total of 2,944 paired DBS and HIV viral load samples. Overall 224 participants completed all 13 study visits (Figure 2). The demographics of the cohort are presented in Table 1. The majority of the participants were female (n=195, 78%), with a median (IQR) age of 34 (27–42) years. Median (IQR) duration on ART at study entry was 11 (5–13) months. All participants were receiving a TDF-based regimen in the form of single-tablet tenofovir/emtricitabine and efavirenz (TDF/FTC/EFV).
Figure 2:
CONSORT diagram of participant recruitment and follow-up during each phase of the study from screening to completion of the final study visit (visit 12). Reasons for screen failure, enrollment failure and missed visits are shown.
Table 1:
Baseline demographics and clinical details of the study population
| Total (N=250) | |
|---|---|
|
| |
| Female: N (%) | 195 (78%) |
| Age (years): Median (IQR) | 34 (27–42) |
| Body Mass Index (kg/m2)*: Median (IQR) | 27.1 (23.0–32.3) |
| <18.5: N (%) | 5 (2.5) |
| 18.5–24.9: N (%) | 68 (34.2) |
| 25–29.9: N (%) | 61 (30.7) |
| >30: N (%) | 65 (32.7) |
| Haematocrit (%): Median (IQR) | 37 (34–40) |
| Males: Mean (SD) | 40 (38–43) |
| Females: Mean (SD) | 36 (34–39) |
| Months on ART at study start: Median (IQR) | 11 (5–13) |
First BMI if not measured at enrollment
Concentrations of TFV-DP in DBS
Median (IQR) TFV-DP concentration across all study visits was 1,041 (727–1,355) fmol/punch. Twenty-one participants (8%) developed viral breakthrough, with a median (IQR) viral load of 9,505 (1,430–45,481) copies/mL, and a median (IQR) TFV-DP concentration of 325 (79–652) fmol/punch at the time of first breakthrough (Figure 3). For the 229 participants who did not experience viral breakthrough, their median (IQR) TFV-DP concentration across all study visits was 1,057 (768–1365) fmol/punch. BMI was available for 199 participants. Median (IQR) TFV-DP in DBS concentration was 1,433 (847–1,588) for BMI <18.5 kg/m2, 1,146 (857–1354) for BMI 18.5–24.9 kg/m2, 1,100 (759–1,295) for BMI 25–29.9 kg/m2, and 1,038 (724–1,322) for BMI ≥30 kg/m2.
Figure 3:
Median (IQR) tenofovir diphosphate (TFV-DP) concentration (fmol/punch) in dried blood spots (DBS) by viral breakthrough status. Each color represents a tenofovir dosing category base on Directly Observed Therapy (DOT) studies in HIV negative populations.[7] Dark green represents daily dosing, light green represents 4–6 doses/week, yellow represents 2–3 doses/week and red represents <2 doses/week. The horizontal lines in the figure represent the interquartile range with the dot representing the median TFV-DP concentration for each group of participants.
Predictive value of TFV-DP in DBS on viral outcomes
A threshold TFV-DP concentration in DBS of 400 fmol/punch maximized sensitivity and specificity (using the Youden Index) to detect future viral breakthrough (viral load ≥400 copies/ml). Participants with TFV-DP ≤400 fmol/punch had an aOR of 16.1 (95% CI: 3.9–67.4 p<0.001) of developing viral breakthrough one month later when compared to participants with TFV-DP >800 fmol/punch (Table 2). When assessing the TFV-DP concentration from two months prior to breakthrough, the aOR increased slightly to 21.3 (95% CI: 4.4–101.8; p<0.001).
Table 2:
Odds Ratio and 95% Confidence Intervals for the association between tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) and viral load >400 copies/ml one month later
| TFV-DP in DBS (fmol/punch) | N (%) | OR (95% CI) | P Value | aOR (95% CI)* | P Value |
|---|---|---|---|---|---|
|
| |||||
| ≤400 | 14 (5.8) | 15.6 (4.4–55.7) | <0.001 | 16.1 (3.9–67.4) | <0.001 |
| >400–800 | 54 (22.3) | 1.2 (0.3–4.8) | 0.8 | 0.9 (0.2–4.9) | 0.9 |
| >800 | 174 (71.9) | 1 | 1 | ||
Abbreviations: aOR, adjusted odds ratio; BLQ. Below level of quantification; CI, confidence interval; OR, odds ratio; TFV-DP, tenofovir diphosphate.
Adjusted for age, gender, body mass index and haematocrit
Sensitivity analyses compared different definitions for viral breakthrough (>50 copies/mL, >1000 copies/mL) one and two months later. Drug concentration was consistently positively associated with future viral breakthrough (with one expection: the adjusted model where breakthrough was defined as viral load >50 copies/mL). This association was generally strongest when viral breakthrough was defined as >1000 copies/mL and examined two months later, and weakest (and not statistically significant) when viral breakthrough was defined as >50 copies/mL and examined one month later (Supplementary Table 1). Across all analyses, TFV-DP concentrations of >400–800 fmol/punch were not significantly associated with future viral breakthrough.
Discussion:
In this study we established the range for TFV-DP concentrations in DBS among a South African population on TDF-based ART associated with sustained viral suppression, and identified a threshold concentration strongly predictive of future viral breakthrough. While the median TFV-DP concentration in DBS (1,041 fmol/punch) was higher than has been found in other South African studies,[9,11,16,22] it was still lower in this population than in US PWH (median 1,874 fmol/punch).[8] This difference could be due to biologic differences in study populations, timing of sampling in relation to ART start, use of generic TDF, use of and/or unboosted ART regimens. As seen in other populations,[21] TFV-DP concentrations were inversely related to BMI, with lower median TFV-DP concentrations noted as BMI increased. While other studies have established the strength of association of TFV-DP concentrations of DBS for future viremia,[15,16] this study is the first to explore the association at different time-points. We showed that while both were highly predictive, a TFV-DP concentration in DBS of below 400 fmol/punch measured two months prior to viral load draw had higher odds of future viral breakthough (aOR 21.3) than a drug concentration obtained one month prior to the viral load draw (aOR 16.1). With the current turn-around time for results of TFV-DP in DBS at 2–3 weeks, this finding has significant clinical benefits as it would allow a clinical team sufficient time to both receive results and initiate an intervention before viral breakthrough occurs.
Compared to a previous study in the US, where TFV-DP in DBS was predictive of future viremia at all categories,[15] our study did not show a significant association between TFV-DP concentrations in DBS between >400–800 fmol/punch and future viral breakthrough. This result may reflect the increased efficacy of newer NNRTI-based regimens, which have been shown to require lower levels of adherence to maintain viral suppression[23,24]. This group was also more diverse and contained both individuals who had maintained viral suppression and those who had not. This demonstrates that relying on viral load alone to assess adherence could be inadequate, as it has been previously shown that mid-range adherence is more strongly associated with the development of ART resistance.[25] It has also been shown that TFV-DP in DBS, in combination with viral load, can be used to identify PWH with suspected ART resistance.[22,25]
Interestingly, the observed association between TFV-DP in DBS and viral load breakthrough increased with higher breakthrough thresholds. There was no association between TFV-DP in DBS and future viral load >50 copies/ml. As per the protocol, viral loads between 50 and 400 copies/ml (only viral load >400 copies/ml) were not repeated so analysis of this group would have included PWH experiencing “blips” (single viral loads of 50–200 copies/ml), which have been shown to have no association with drug adherence.[26,27]
Our findings have multiple clinical implications. Concentrations of TFV-DP in DBS can reliably provide HIV care providers with an early signal of future viral breakthrough. This signal in turn allows providers to initiate supportive interventions during a time of lower adherence, and thus prevent viremia and subsequent clinical consequences.[12] The values of the DBS drug concentrations can also give HIV care providers more nuanced insight into their patients’ clinical management as they review more continuous, real-time patterns of adherence than is provided by viral load. Furthermore, when used in conjunction with viral load, high drug concentrations in DBS in the presence of viremia may help identify patients with ART drug resistance.[12,22,25] These findings create opportunities for future research in exploring the most impactful ways to use long-acting drug metabolite testing in conjunction with traditional viral load testing and how best to provide drug concentration-based feedback to participants and providers.
The strengths of our study include the generalizability of this cohort, which is representative of patients accessing ART services in SA. In addition, the large numbers of DBS and viral load pairs established a “suppressive range” for this population. A result in the suppressive range would provide reassurance and positive feedback on a patient’s adherence, whereas a result below the threshold may provide an opportunity for a clinic level intervention to improve adherence through education and support, prior to the patient experiencing virologic failure.[12] Last, DBS are easy to collect and transport in a clinic setting and, furthermore, there is a current study evaluating home collection of DBS to quantify TFV-DP[28]. However, the current cost of the TFV-DP assay and the time taken to receive results would prohibit its routine use in resource-poor settings. In response to this, a new point-of-care TFV-DP assay is currently under development[29,30]. Future studies exploring simple, low-cost strategies for providing timely adherence feedback to both providers and PWH using this adherence biomarker are warranted.
A limitation to these findings is that there were relatively few episodes of viral breakthrough during the study; however, these were still sufficient to establish a threshold concentration. While monthly study visits and electronic monitoring may increase initial adherence[31] and reduce the total number of participants who experienced viral breakthroughs, we do not believe this would impact the determination of a suppressive range for TFV-DP or the association seen in those who did experience breakthrough, as these participants were shown to have poor adherence despite monthly visits (Figure 3). In addition, only viral loads of >400 copies/ml were confirmed by a second assay. Thus some of the viral loads between 50–400 copies/ml included in the models may have been less precise for analysis than those confirmed to be >400 copies/ml. Last, while we amended the study to include BMI, we were unable to capture creatinine clearance in our study participants. However, since all study participants were taking tenofovir, and creatinine monitoring is part of routine care in South Africa, we can assume that few participants had clinically relevant reduction in creatinine clearance.
In summary, our study established a threshold concentration of TFV-DP in DBS that can predict future virologic breakthrough in South Africans taking TDF-based ART. We also established a range of TFV-DP values in DBS at which virologic breakthrough is unlikely. These findings provide new insights into the use of this biomarker as an adherence measure that can be used in conjunction with viral load to monitor ART. Further research into its application in a real-world clinical setting is needed.
Supplementary Material
Acknowledgements
The authors wish to thank the people who participated in this study as well as the study team in Gugulethu, including Colleen Herman, Alicia James and Sphamandla Mpisane as the clinical site coordinators for the study.
Conflict of interest and source of funding
Author CO has received honoraria from ViiV and MSD. For the remaining authors none were declared. This work was supported by the National Institutes of Health [R01-AI122300].
References:
- 1.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007; 146:564–573. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS 2006; 20:223–231. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS data 2020. | UNAIDS. https://www.unaids.org/en/resources/documents/2020/unaids-data (accessed 8 Oct2021).
- 4.Thompson MA, Mugavero MJ, Rivet Amico K, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an international association of physicians in AIDS care panel. Ann. Intern. Med. 2012; 156:817–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Updated recommendations on service delivery for the treatment and care of people living with HIV. https://www.who.int/publications/i/item/9789240023581 (accessed 20 Jul2021). [PubMed]
- 6.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson PL, Liu AY, Castillo-mancilla JR, Gardner EM, Seifert SM, Mchugh C, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Searls K, Caraway P, Zheng JH, Gardner EM, Predhomme J, et al. Short communication: Tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses 2015; 31:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warne P, Robbins R, Anderson P, Gouse H, Joska J, C-S L. Utility of Dried Blood Spot-Derived ARV Biomarkers as an Objective Measure of Treatment Adherence in South Africa. In: 10th International Conference on HIV Treatment and Prevention Adherence, Miami, FL.; 2015. [Google Scholar]
- 10.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng JH, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019; 68:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips TK, Sinxadi P, Abrams EJ, Zerbe A, Orrell C, Hu N-C, et al. A comparison of plasma efavirenz and tenofovir, dried blood spot tenofovir-diphosphate, and self-reported adherence to predict virologic suppression among South African women. J Acquir Immune Defic Syndr 2019; 81:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristofich M, Anderson PL, Castillo-Mancilla JR. Beyond HIV viral load: application of pharmacologic measures to identify ART adherence mismatch: Ther Adv Infect Dis 2021; 8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Republic of South Africa National Department of Health. 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy, Adolescents, children, Infants and Neonates. 2019.https://www.nicd.ac.za/wp-content/uploads/2019/11/2019-ART-Clinical-Guidelines-25-Nov.pdf (accessed 8 Oct2021).
- 14.Western Cape Department of Health. The Western Cape Consolidated Guidelines for HIV Treatment: Prevention of Mother-to-Child Transmission of HIV (PMTCT), Children, Adolescents and Adults. 2020. [Google Scholar]
- 15.Morrow M, MaWhinney S, Coyle RP, Coleman SS, Gardner EM, Zheng J-H, et al. Predictive Value of Tenofovir Diphosphate in Dried Blood Spots for Future Viremia in Persons Living With HIV. J Infect Dis 2019; 220:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odayar J, Orrell C, Phillips TK, Hu N-C, Kabanda S, Malaba TR, et al. Use of tenofovir diphosphate levels to predict viremia during the postpartum period in women living with HIV: a nested case-control study. Clin Infect Dis Published Online First: 3 January 2022. doi: 10.1093/CID/CIAB1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orrell C, Kaplan R, Wood R, Bekker L-G. Virological Breakthrough: A Risk Factor for Loss to Followup in a Large Community-Based Cohort on Antiretroviral Therapy. AIDS Res Treat 2011; 2011. doi: 10.1155/2011/469127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker LG, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther 2007; 12:83–88. [PubMed] [Google Scholar]
- 19.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxi SM, Greenblatt RM, Bacchetti P, Scherzer R, Minkoff H, Huang Y, et al. Common clinical conditions-age, low BMI, ritonavir use, mild renal impairment-affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS 2014; 28:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyle RP, Morrow M, Coleman SS, Gardner EM, Zheng J-H, Ellison L, et al. Factors associated with tenofovir diphosphate concentrations in dried blood spots in persons living with HIV. J Antimicrob Chemother 2020; 75:1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo-Mancilla JR, Zhao Y, Brijkumar J, Johnson B, Edwards A, Selvan P, et al. Tenofovir Diphosphate in Dried Blood Spots Predicts Virologic Failure and Resistance. In: CROI.; 2020. [Google Scholar]
- 23.Viswanathan S, Detels R, Mehta SH, Macatangay BJC, Kirk GD, Jacobson LP. Level of Adherence and HIV RNA Suppression in the Current Era of Highly Active Antiretroviral Therapy (HAART). AIDS Behav 2015; 19:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann. Pharmacother. 2011; 45:372–379. [DOI] [PubMed] [Google Scholar]
- 25.Yager JL, Coyle RP, Coleman SS, Ellison L, Zheng JH, Bushman L, et al. Moderately High Tenofovir Diphosphate in Dried Blood Spots Indicates Drug Resistance in Viremic Persons Living with HIV. J Int Assoc Provid AIDS Care 2019; 18:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer A, Wang X, Ganesan A, Deiss RG, Agan BK, O’Bryan TA, et al. Factors associated with HIV viral load “blips” and the relationship between self-reported adherence and efavirenz blood levels on blip occurrence: a case–control study. AIDS Res Ther 2016 131 2016; 13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller LG, Golin CE, Liu H, Hays RD, Hua J, Wenger NS, et al. No evidence of an association between transient HIV viremia (“blips”) and lower adherence to the antiretroviral medication regimen. J Infect Dis 2004; 189:1487–1496. [DOI] [PubMed] [Google Scholar]
- 28.HIV Outpatient Monitoring Evaluation Through Self-collection of Dried Blood Spots - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04979728?term=yager&draw=2&rank=1 (accessed 6 Jan2022). [Google Scholar]
- 29.Pu F, Pandey S, Bushman LR, Anderson PL, Ouyang Z, Cooks RG. Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem 2020; 412:1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olanrewaju AO, Sullivan BP, Bardon AR, Lo TJ, Cressey TR, Posner JD, et al. Pilot evaluation of an enzymatic assay for rapid measurement of antiretroviral drug concentrations. Virol J 2021; 18. doi: 10.1186/S12985-021-01543-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschamps AE, Wijngaerden E Van, Denhaerynck K, Vandamme AM, Geest S De. Use of electronic monitoring induces a 40-day intervention effect in HIV patients [2]. J Acquir Immune Defic Syndr 2006; 43:247–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.