Abstract
Background:
Buprenorphine is a life-saving medication for people with opioid use disorder (OUD). U.S. federal law allows advanced practice clinicians (APCs), such as nurse practitioners (NPs) and physician assistants (PAs), to obtain a federal waiver to prescribe buprenorphine in office-based practices. However, states regulate APCs’ scope of practice (SOP) variously, including requirements for physician supervision. States may also have laws entirely banning NP/PA buprenorphine prescribing or requiring that supervising physicians have a federal waiver to prescribe buprenorphine. We sought to identify prevalence of state laws other than SOP laws that either 1) prohibit NP/PA buprenorphine prescribing entirely, or 2) require supervision by a federally waivered physician.
Methods:
We searched for state statutes and regulations in all 50 states and Washington D.C. regulating prescribing of buprenorphine for OUD by APCs during summer 2021. We excluded general scope of practice laws, laws only applicable to Medicaid-funded clinicians, laws not applicable to substance use disorder (SUD) treatment, and laws only applicable to NPs/PAs serving licensed SUD treatment facilities. We then conducted content analysis.
Results:
One state prohibits all APCs from prescribing buprenorphine for OUD, even though the state’s general SOP laws permit APC buprenorphine prescribing. Five states require PA supervision by a federally waivered physician. Three states require NP supervision by a federally waivered physician.
Conclusions:
Aside from general scope of practice laws, several states have created laws explicitly regulating buprenorphine prescribing by APCs outside of licensed state SUD facilities.
Keywords: Nurse practitioners, Advanced care practitioners, Physician assistants, State law, Buprenorphine, Waiver, Collaboration, Supervision, Scope of practice, Opioid use disorder, Medications for opioid use disorder
1. Introduction
Medications for opioid use disorder (MOUD)—including formulations of methadone, buprenorphine, and naltrexone—decrease the risk of all-cause mortality among people with opioid use disorder (OUD) by as much as 50% (Santo et al., 2021). In the United States, the federal and state governments tightly regulate clinicians’ ability to prescribe or dispense MOUD, particularly methadone and buprenorphine (Frank et al., 2018; Saloner et al., 2021). For example, clinicians can only dispense methadone for OUD in licensed opioid treatment programs (OTPs). Additionally, while clinicians can prescribe formulations of buprenorphine for OUD outside of OTPs, clinicians are bound by federal and state laws, which may restrict and/or regulate prescribing.
Federally, the Drug Addiction Treatment Act of 2000 (DATA-2000), the Comprehensive Addiction and Recovery Act (CARA) (Comprehensive Addiction and Recovery Act of 2016, 2016), and the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities (SUPPORT) Act (“Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, U.S.C. 823(g)(2)(G)(iii),” 2018) enable and regulate prescribing of buprenorphine for OUD outside of OTPs. For example, CARA and subsequent regulations enable certain advanced practice clinicians (APCs)—including physician assistants (PAs) and nurse practitioners (NPs)—to prescribe buprenorphine following a 24-hour training (“Registration of…” 2018; Comprehensive Addiction and Recovery Act of 2016, 2016) and submission of a Notice of Intent to the U.S. Drug Enforcement Agency for an “x-waiver” DEA license. A federal guideline change in 2021 enables both physicians and APCs to obtain an X-waiver for prescribing buprenorphine for OUD to 30 or fewer patients without specialized training in buprenorphine prescribing (Practice Guidelines…, 2021). Federal law requires physicians prescribing buprenorphine to more than 30 patients to obtain eight hours of specialized training and APCs prescribing buprenorphine to more than 30 patients to obtain twenty-four hours of specialized training (Comprehensive Addiction and Recovery Act of 2016, 2016). Furthermore, federal law sets limits on the number of patients to whom waivered practitioners can prescribe buprenorphine for OUD (“Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, U.S.C. 823(g)(2)(G)(iii),” 2018).
Expanding the number of clinicians who prescribe buprenorphine for OUD in the United States is a national priority (Frank et al., 2018; Saloner et al., 2021). NPs/PAs have demonstrated willingness to prescribe buprenorphine and have improved access to buprenorphine (Cotton & Ferszt, 2018; Leahy, 2017; Lee & McNeely, 2019; Roose et al., 2008; Tierney et al., 2015; Wen et al., 2019). Since being granted permission to prescribe buprenorphine through CARA, NPs/PAs have obtained X-waivers at a greater rate than physicians. NPs/PAs may fill a critical niche in buprenorphine provision in rural areas where they may be the sole prescriber (Andrilla & Patterson, 2021; Andrilla et al., 2018; Barnett et al., 2019; Fornili & Fogger, 2017; Klein et al., 2020). However, individual states regulate NPs’/PAs’ scope of practice (SOP)—including the degree of independent practice, prescriptive authority, and ability to prescribe controlled substances, including buprenorphine (Nguyen et al., 2021; Spetz et al., 2019; Strobbe & Hobbins, 2012). Thus, while federal law, notably CARA, allows for the provision of buprenorphine by APCs, state laws may limit NPs’/PAs’ prescribing buprenorphine. For example, state SOP laws may require NPs/PAs to have a supervising/collaborating physician for Schedule III prescribing—the controlled substance schedule to which buprenorphine belongs. In addition to general SOP laws, states can require that a supervising/collaborating physician have an X-waiver, or states can ban buprenorphine prescribing by NPs/PAs entirely. Therefore, we sought to identify the prevalence of state laws other than general SOP laws that either 1) prohibit NP/PA buprenorphine prescribing entirely, or 2) require supervision by a federally waivered physician.
2. Material and methods
Two research team members searched the Westlaw legal database in May 2021 for state regulations and statutes using terms related to NPs, PAs, and MOUD for all U.S. states plus Washington D.C. We focused our search on NPs and PAs specifically given previous scholarship, suggesting these APCs’ importance in expanding buprenorphine access in underserved, particularly rural, areas (Andrilla et al., 2020). See Appendix for detailed search terms. We then focused on laws regulating buprenorphine prescribing by NPs/PAs outside of licensed facilities. We excluded definitions, controlled substance schedules, and laws not relevant to OUD treatment (e.g., rules related to pain management.) Following a review by a licensed attorney and MOUD subject matter expert, we subsequently excluded the following types of laws from analysis: a) MOUD prescribing for withdrawal or detoxification rather than maintenance treatment; b) pilot programs not applicable to the whole state; c) laws applicable only to state licensed SUD facilities (i.e., as opposed to non-licensed office-based practices); d) rules not specific to MOUD (e.g., rules applicable to prescribers of all controlled substances); e) general SOP rules without specific mention of MOUD; f) pharmacy dispensing rules; g) rules only applicable to treating Medicaid patients or only applicable to treating Workers Compensation patients; and h) rules about how to provide MOUD (e.g., required urine drug screenings; prescription drug monitoring program checks) rather than whether buprenorphine treatment could be provided at all or who must serve as collaborator/supervisor (if needed). In the rare cases where exclusion decisions were unclear, the team held discussions with a larger group of MOUD subject matter experts until everyone reached consensus. For example, in West Virginia office-based buprenorphine prescribing can only occur in state-licensed office-based practices (Taylor, 2016), and after team discussion we chose to exclude NP/PA buprenorphine prescribing laws in West Virginia due to the state’s unique requirements regarding settings in which buprenorphine may be prescribed.
We created a codebook using the final dataset, assessing whether the law: (1) allowed an NP to prescribe buprenorphine, (2) required a collaborating or supervising physician, and (3) required the collaborating or supervising physician to have an X-waiver. We created an identical set of codes for PAs. Two research team members subsequently independently coded the final dataset, discussing differences in coding to reach consensus. We coded laws specifying that buprenorphine must be part of the supervising/collaborating physician’s regular practice as requiring a waiver for the physician. The entire research team reviewed final codes, along with the relevant legal text and citations, and discussed interpretations of the legal text to achieve consensus. Last, we compared our dataset to the general state SOP laws for NPs and PAs for schedule III substances using datasets of SOP laws from McMichael and Markowitz (2020) (McMichael & Markowitz, 2020) and the National Conference of State Legislatures (National Conference of State Legislature, 2021).
3. Results
General SOP laws for Schedule III controlled substances:
In May 2021, all states permitted NP and PA prescribing of Schedule III controlled substances within their SOP laws. All states also required that PAs have physician collaboration or supervision for Schedule III controlled substance prescribing. Twenty-nine states allowed NPs to prescribe Schedule III controlled substances without formal physician oversight; and Virginia allowed NPs to practice independently after five years of oversight.
Buprenorphine-specific prescribing laws (separate from SOP laws):
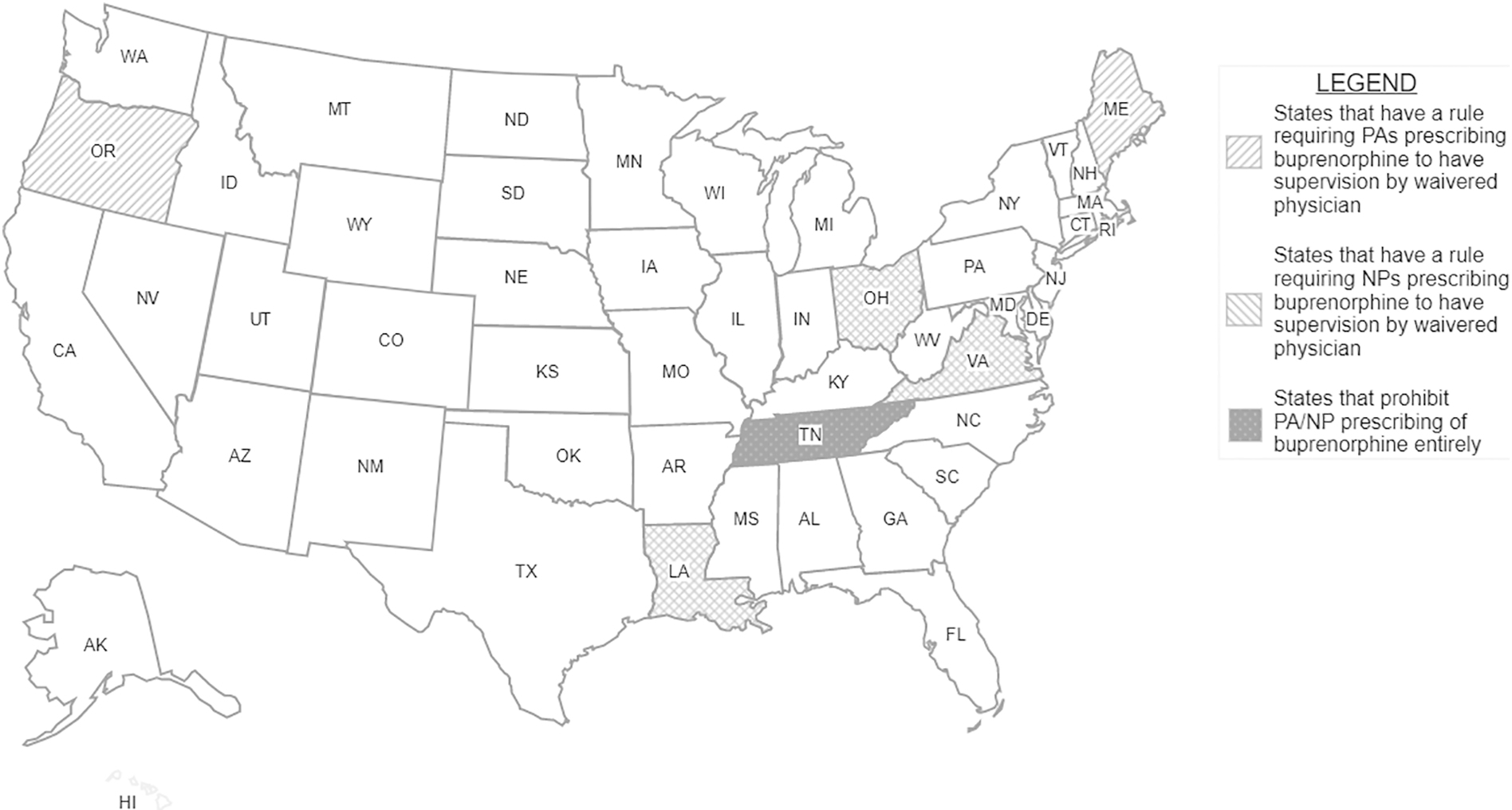
We found one state, Tennessee, that explicitly prohibited buprenorphine prescribing by APCs in a law separate from its SOP law. We found five states (Louisiana, Maine, Ohio, Oregon, and Virginia) that not only required a supervising/collaborating physician for PAs in the general SOP law, but also had a law requiring supervising/collaborating physicians to have an X-waiver. Similarly, we found three states (Louisiana, Ohio, and Virginia) that required a supervising/collaborating physician for NPs to have an X-waiver. See Figure 1 for a map of NP/PA laws specific to buprenorphine prescribing for OUD. See Table 1 for relevant quotes and effective dates from state laws and Table 2 for a comparison of relevant laws across all jurisdictions. Among the states that we identified with laws, Tennessee’s law is the oldest, becoming effective in 2015. The laws in all the other states became effective after January 2017, with Ohio’s law regulating NP prescribing of buprenorphine being the most recent (effective as of February 2021.)
Fig. 1:
NP PA paper.
Table 1:
Relevant legal excerpts and citations (italics added for emphasis)
| State | Citation | Legal text | Practitioner to whom law applies | Earliest version containing excerpt | Current version |
|---|---|---|---|---|---|
| LA | LA § 913. | (c) Advanced practice registered nursing may include the provision of medication-assisted treatment (MAT), as authorized by the United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration and in accordance with rules promulgated by the board. At a minimum, rules promulgated by the board shall include a requirement that in order for the APRN to provide MAT, his collaborating physician shall also be authorized and in compliance with all federal and state laws and rules authorizing the provision of MAT. For purposes of this Subparagraph, “MAT” means the use of medications with counseling and behavioral therapies to treat substance use disorders and prevent opioid overdose. | NP | Effective: August 1, 2019 | Effective: August 1, 2019 |
| LA § 1360.31. | (4) A physician assistant may provide medication-assisted treatment (MAT), as authorized by the United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration and in accordance with rules promulgated by the board. At a minimum, rules promulgated by the board shall include a requirement that in order for the PA to provide MAT, his supervising physician shall also be authorized and in compliance with all federal and state laws and rules authorizing the provision of MAT. For purposes of this Subparagraph, “MAT” means the use of medications with counseling and behavioral therapies to treat substance use disorders and prevent opioid overdose. | PA | Effective: August 1, 2019 | Effective: August 1, 2019 | |
| ME | ME § 3 | 1. Clinicians who wish to provide Approved Medications for OUD in an OBOT must: A. Hold a current license issued by the Board; B. Hold a current controlled substance registration issued by the DEA; C. Obtain a DATA 2000 Waiver and complete buprenorphine training; D. In the case of a physician assistant, be delegated the authority to provide OBOT pursuant to a written plan of supervision by a supervising physician who also meets the criteria for providing OBOT; and E. Comply with this joint rule. 2. Patient limits. Clinicians must be aware of and comply with limits established by the DEA regarding the number of patients that can be treated with Approved Medications in OBOT. |
PA | Effective: April 29, 2020 | Effective: April 29, 2020 |
| OH | OH 4723-913 |
(B) An advanced practice registered nurse with a current valid license issued by the board and designated as a clinical nurse specialist, certified nurse midwife or certified nurse practitioner may provide medication-assisted treatment, including prescribing controlled substances in schedule III, IV or V, if the advanced practice registered nurse: (1) Complies with section 3719.064 of the Revised Code, and all federal and state laws and regulations governing the prescribing of the medication, including but not limited to incorporating into the advanced practice registered nurse’s practice knowledge of Chapter 4729. of the Revised Code, and Chapter 4731. of the Revised Code and rules adopted under that Chapter that govern the practice of the advanced practice registered nurse’s collaborating physician; (2) Completes at least 8 h of continuing nursing education in each renewal period related to substance abuse and addiction. Courses completed in compliance with this requirement shall be accepted toward meeting the continuing education requirements for biennial renewal of the advanced practice registered nurse license; and (3) Only provides medication-assisted treatment if the treatment is within the collaborating physician’s normal course of practice and expertise. |
NP | Effective: February 1, 2021 | Effective: February 1, 2021 |
| OH 4730-403 |
(A) A physician assistant who provides OBOT shall comply with the following requirements: (1) Before initiating OBOT, the physician assistant shall comply with section 3719.064 of the Revised Code. (2) Comply with all federal and state laws and regulations governing the prescribing of the medication; (3) Complete at least eight hours of “Category 1” continuing medical education relating to substance abuse and addiction every two years. Courses completed in compliance with this requirement shall be accepted toward meeting the continuing medical education requirement for biennial renewal of the physician assistant’s license; and (4) Only provide OBOT if the provision of OBOT is within the supervising physician’s normal course of practice and expertise. |
PA | Effective: April 30, 2019 | Effective: April 30, 2019 | |
| OR | OR 847-050-0041 |
(4) A physician assistant may prescribe and dispense buprenorphine for medication-assisted treatment for opioid dependency if the requirements in (1) or (2) are fulfilled and the following conditions are met: (a) The physician assistant has obtained a buprenorphine waiver from the Drug Enforcement Administration; (b) The physician assistant has been granted dispensing authority if the physician assistant will dispense buprenorphine; (c) The scope of practice of the physician assistant’s supervising physician includes use of buprenorphine for medication-assisted treatment for opioid dependency; (d) The physician assistant’s practice agreement includes use of buprenorphine for medication-assisted treatment for opioid dependency as a delegated medical service; and (e) The physician assistant complies with all federal and state requirements for recordkeeping specific to buprenorphine treatment. |
PA | Effective: July 14, 2017 | Effective: January 5, 2018 |
| TN | TN § 53-11-311 | (c)(1) Notwithstanding any other provision of this title, and except as otherwise provided in subdivision (c)(2), a physician licensed under title 63, chapter 6 or 9, is the only healthcare provider authorized to prescribe any buprenorphine product for any federal food and drug administration approved use in recovery or medication-assisted treatment. | NP; PA | Effective: July 1, 2015 | Effective: August 1, 2020 |
| VA | VA 85-21-130 | C. Physician assistants and nurse practitioners who have obtained a SAMHSA waiver shall only prescribe buprenorphine for opioid addiction pursuant to a practice agreement with a waivered doctor of medicine or doctor of osteopathic medicine. | PA | Effective: August 8, 2018. | Effective: August 8, 2018. |
| VA 90-40-250 | C. Nurse practitioners who have obtained a SAMHSA waiver shall only prescribe buprenorphine for opioid addiction pursuant to a practice agreement with a SAMHSA-waivered doctor of medicine or doctor of osteopathic medicine unless the nurse practitioner has been authorized by the boards for autonomous practice. | NP | Effective: July 10, 2019. | Effective: July 10, 2019. |
Table 2:
Summary of laws
| SOP law permits PA prescribing of schedule III? | Other state law prohibits PA bupren orphine prescribing? | SOP law requires PA physician supervision/collaboration? | PA’s collaborating/supervising physician needs x-waiver? | SOP law permits NP prescribing of schedule III? | Other state law prohibits NP bupren orphine prescribing? | SOP law requires NP physician supervision/collaboration? | NP’s collaborating/supervising physician needs x-waiver? | |
|---|---|---|---|---|---|---|---|---|
| AL | yes | no | yes | no | yes | no | Yes | no |
| AK | yes | no | yes | no | yes | no | No | no |
| AZ | yes | no | yes | no | yes | no | no | no |
| AR | yes | no | yes | no | yes | no | yes | no |
| CA | yes | no | yes | no | yes | no | yes | no |
| CO | yes | no | yes | no | yes | no | no | no |
| CT | yes | no | yes | no | yes | no | no | no |
| DE | yes | no | yes | no | yes | no | no | no |
| FL | yes | no | yes | no | yes | no | no | no |
| GA | yes | no | yes | no | yes | no | yes | no |
| HI | yes | no | yes | no | yes | no | no | no |
| ID | yes | no | yes | no | yes | no | no | no |
| IL | yes | no | yes | no | yes | no | no | no |
| IN | yes | no | yes | no | yes | no | yes | no |
| IA | yes | no | yes | no | yes | no | no | no |
| KS | yes | no | yes | no | yes | no | yes | no |
| KY | yes | no | yes | no | yes | no | yes | no |
| LA | yes | no | yes | yes | yes | no | yes | yes |
| MA | yes | no | yes | no | yes | no | no | no |
| MD | yes | no | yes | no | yes | no | no | no |
| ME | yes | no | yes | yes | yes | no | no | no |
| MI | yes | no | yes | no | yes | no | yes | no |
| MN | yes | no | yes | no | yes | no | no | no |
| MS | yes | no | yes | no | yes | no | yes | no |
| MO | yes | no | yes | no | yes | no | yes | no |
| MT | yes | no | yes | no | yes | no | no | no |
| NE | yes | no | yes | no | yes | no | no | no |
| NV | yes | no | yes | no | yes | no | no | no |
| NH | yes | no | yes | no | yes | no | no | no |
| NJ | yes | no | yes | no | yes | no | yes | no |
| NM | yes | no | yes | no | yes | no | no | no |
| NY | yes | no | yes | no | yes | no | no | no |
| NC | yes | no | yes | no | yes | no | yes | no |
| ND | yes | no | yes | no | yes | no | no | no |
| OH | yes | no | yes | yes | yes | no | yes | yes |
| OK | yes | no | yes | no | yes | no | yes | no |
| OR | yes | no | yes | yes | yes | no | no | no |
| PA | yes | no | yes | no | yes | no | yes | no |
| RI | yes | no | yes | no | yes | no | no | no |
| SC | yes | no | yes | no | yes | no | yes | no |
| SD | yes | no | yes | no | yes | no | no | no |
| TN | yes | yes | yes | n/a | yes | yes | yes | n/a |
| TX | yes | no | yes | no | yes | no | yes | no |
| UT | yes | no | yes | no | yes | no | no | no |
| VT | yes | no | yes | no | yes | no | no | no |
| VA | yes | no | yes | yes | yes | no | no | yes |
| WA | yes | no | yes | no | yes | no | no | no |
| WV | yes | no | yes | no | yes | no | no | no |
| WI | yes | no | yes | no | yes | no | yes | no |
| WY | yes | no | yes | no | yes | no | no | no |
Dark grey indicates requirement for APC supervision by waivered physician when prescribing buprenorphine for OUD. Light grey indicates prohibition of buprenorphine prescribing for OUD by APCs.
4. Discussion
Buprenorphine is an underutilized life-saving treatment for OUD (National Academies of Science Engineering & Medicine, 2019; Wakeman et al., 2020; Wu et al., 2016). Since the passage of CARA, scholars have described NPs and PAs as a key resource in mitigating underprescribing of buprenorphine for OUD in the United States (Andrilla et al., 2018; Barnett et al., 2019; Wen et al., 2019). Nevertheless, we found that six states either entirely prohibit APC buprenorphine prescribing or have specific APC supervision policies that may limit these clinicians from providing effective treatment for OUD outside of licensed SUD facilities (e.g., in office-based practices).
CARA requires that APCs in states with SOP collaboration/supervision requirements work with a physician “qualified” to obtain a waiver (Comprehensive Addiction and Recovery Act of 2016, 2016). A physician being “qualified” to obtain a waiver is not the same, however, as a physician possessing a waiver. Nevertheless, we found five states that go beyond the federal law and require that the supervising/collaborating physician have an X-waiver. Whether legal requirements for a physician to possess an X-waiver as opposed to merely “qualifying” for a waiver would have measurably different effects on APC prescribing rates is unclear. If so, then it is particularly concerning that Ohio and Maine require NPs and/or PAs to collaborate with or be supervised by waivered physicians, as both Ohio and Maine were among the top ten states in terms of opioid-involved death rates in 2019 (Kaiser Family Foundation, 2019). Furthermore, among all U.S. states, Maine has the highest proportion of rural residents (61.3%) (United States Census Bureau, 2012); and NPs/PAs are critical for addressing buprenorphine underutilization in rural areas with few waivered physicians (Andrilla & Patterson, 2021). Importantly, scholars, activists, practitioners, and policy-makers have called for legislation eliminating the federal X-waiver requirement (Marino et al., 2019; Saloner et al., 2021), which, if passed, would render waiver-related concerns moot.
Surprisingly, despite CARA and the state’s own general SOP laws, Tennessee prohibits APC prescribing of buprenorphine for OUD. This policy likely decreases access to buprenorphine for Tennessee residents, despite buprenorphine’s relative safety among opioids, its lifesaving potential for people with OUD (Santo et al., 2021), and Tennessee having one of the nation’s highest drug overdose mortality rates. Tennessee’s policies are especially likely to constrain treatment access, as 93% of the state is rural (Tennessee Department of Health, 2021). Rural areas are least likely to have waivered physicians and thus APCs, such as NPs/PAs, could make a substantial difference in providing treatment access in these locations (Andrilla & Patterson, 2021).
Our study has several important limitations. We excluded several laws that could impact NP/PA buprenorphine prescribing and should be examined in future studies: Medicaid rules, Workers Compensation rules, and laws applicable only to APCs working in state-licensed SUD treatment facilities, including West Virginia’s laws requiring office-based practices that provide buprenorphine treatment to have state licenses. Also, through other regulatory mechanisms, states may have other buprenorphine treatment requirements not captured in our study, which could further constrain buprenorphine treatment. For example, states may require NPs/PAs to obtain additional continuing medical education credits when prescribing buprenorphine, limit the days’ supply of controlled substances an NP/PA can prescribe, require NPs/PAs to provide or refer patients to counseling, and require NPs/PAs to conduct routine urine drugs screens. Therefore, our study likely underreports the extent to which state laws influence NP/PA buprenorphine prescribing. Additionally, we did not examine laws regulating buprenorphine prescribing by other types of APCs, such as clinical nurse specialist, certified registered nurse anesthetists, and certified nurse midwives. Future work, including qualitative studies of NPs’/PAs’ experiences, should disentangle the role of different types of laws on NPs’/PAs’ buprenorphine prescribing behaviors. Finally, we know little about how NPs/PAs find physicians with X-waivers with whom to collaborate, nor to what extent finding such physicians and establishing a collaborative relationship acts as a barrier to APC buprenorphine prescribing.
5. Conclusion
Our study found that several states have created laws regulating APC supervision when prescribing buprenorphine in ways that go above and beyond oversight requirements in the states’ general SOP laws, such as by requiring supervising physicians to have an X-waiver. Our results highlight the need for empirical studies of state APC supervision policies to examine the effects of laws beyond general SOP laws.
The opioid epidemic continues to be a significant public health crisis, with more than 90,000 fatal overdose deaths in 2020, more than ever before (Ahmad et al., 2021). Expanding access to the most effective treatments, such as buprenorphine, is critical to an effective response to this crisis. Since the passage of CARA, NPs and PAs have played an increasingly important role in increasing access to buprenorphine (Andrilla et al., 2018). Yet states with among the highest opioid-related overdose rates either entirely prohibit APC buprenorphine prescribing or have specific APC supervision policies that may limit these clinicians’ provision of effective treatment for OUD outside of licensed SUD facilities (e.g., in office-based practices).
Supplementary Material
HIGHLIGHTS.
One state prohibits all advanced practice clinicians from prescribing buprenorphine
Five states require a waivered supervising physician for PAs prescribing buprenorphine
Three states require a waivered supervising physician for NPs prescribing buprenorphine
Studies of state NP/PA supervision should include non-scope of practice laws
Acknowledgments
FUNDING
This work was supported by NIH National Institute on Drug Abuse Awards R01DA045800 and P50DA046351. AG and CS were further supported by NIH/NIDA [1UG1DA049444] and VA [PII 19-321 (HX003009)]. JS was supported by NIH/NIDA [1R01DA047379]. Supporting organizations had no further role in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the paper for publication. The authors are solely responsible for the content of this article. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Federal Government, including the Department of Veterans Affairs, Veterans Health Administration and the National Institute of Health, National Institute on Drug Abuse or any of the authors’ academic affiliates.
Footnotes
CONFLICTS OF INTEREST:
Authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Ahmad F, Rossen L, Spencer M, Warner M, & Sutton P (2021). Provisional drug overdose death counts. National Center for Health Statistics. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm#citation [Google Scholar]
- Andrilla CHA, Jones KC, & Patterson DG (2020). Prescribing Practices of Nurse Practitioners and Physician Assistants Waivered to Prescribe Buprenorphine and the Barriers They Experience Prescribing Buprenorphine. The Journal of Rural Health, 36(2), 187–195. 10.1111/jrh.12404 [DOI] [PubMed] [Google Scholar]
- Andrilla CHA, & Patterson DG (2021). Tracking the geographic distribution and growth of clinicians with a DEA waiver to prescribe buprenorphine to treat opioid use disorder. The Journal of Rural Health. 10.1111/jrh.12569 [DOI] [PubMed] [Google Scholar]
- Andrilla CHA, Patterson DG, Moore TE, Coulthard C, & Larson EH (2018). Projected Contributions of Nurse Practitioners and Physicians Assistants to Buprenorphine Treatment Services for Opioid Use Disorder in Rural Areas. Medical Care Research and Review, 77(2), 2018–2216. 10.1177/1077558718793070 [DOI] [PubMed] [Google Scholar]
- Barnett ML, Lee D, & Frank RG (2019). In Rural Areas, Buprenorphine Waiver Adoption Since 2017 Driven By Nurse Practitioners And Physician Assistants. Health Affairs, 38(12), 2048–2056. 10.1377/hlthaff.2019.00859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comprehensive Addiction and Recovery Act of 2016, Pub. L. No. 114–198 (2016).
- Cotton BP, & Ferszt GG (2018). The role of NPs in medication-assisted treatment for opioid use disorder. The Nurse Practitioner, 43(5), 8. 10.1097/01.NPR.0000531074.42053.50 [DOI] [PubMed] [Google Scholar]
- Fornili KS, & Fogger SA (2017). Nurse Practitioner Prescriptive Authority for Buprenorphine: From DATA 2000 to CARA 2016. Journal of Addictions Nursing, 28(1), 43–48. 10.1097/JAN.0000000000000160 [DOI] [PubMed] [Google Scholar]
- Frank JW, Wakeman SE, & Gordon AJ (2018). No end to the crisis without an end to the waiver. Substance Abuse, 39(3), 263–265. 10.1080/08897077.2018.1543382 [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. (2019). Opioid Overdose Death Rates and All Drug Overdose Death Rates per 100,000 Population (Age-Adjusted). Retrieved November 1 from https://www.kff.org/other/state-indicator/opioid-overdose-death-rates/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Opioid%20Overdose%20Death%20Rate%20(Age-Adjusted)%22,%22sort%22:%22desc%22%7D
- Klein TA, Geddes J, & Hartung D (2020). The Geographic Impact of Buprenorphine Expansion to Nurse Practitioner Prescribers in Oregon. The Journal of Rural Health. 10.1111/jrh.12538 [DOI] [PubMed] [Google Scholar]
- Leahy LG (2017). The Opioid Epidemic: What Does it Mean for Nurses? Journal of Psychosocial Nursing and Mental Health Services, 55(1), 18–23. 10.3928/02793695-20170119-03 [DOI] [PubMed] [Google Scholar]
- Lee JD, & McNeely J (2019). Commentary on Jones & McCance-Katze (2019): Buprenorphine and the glass half full-why can’t we prescribe more of it, and will nurse practitioners and physician assistants fulfill a chronic unmet need? Addiction, 114(3), 483–484. 10.1111/add.14545 [DOI] [PubMed] [Google Scholar]
- Marino R, Perrone J, Nelson LS, Wiegand TJ, Schwarz ES, Wax PM, & Stolbach AI (2019). ACMT Position Statement: Remove the Waiver Requirement for Prescribing Buprenorphine for Opioid Use Disorder. Journal of Medical Toxicology, 15(4), 307–309. 10.1007/s13181-019-00728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael BJ, & Markowitz S (2020). Toward a Uniform Classification of Nurse Practitioner Scope of Practice Laws. National Bureau of Economic Research. 10.3386/w28192 [DOI] [PubMed] [Google Scholar]
- National Academies of Science Engineering & Medicine (2019). Medications for Opioid Use Disorder Save Lives (Press NA, Ed.). The National Academies Press. 10.17226/25310 [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislatures (2021). National Conference of State Legislatures: Physician Assistants. Retrieved May 23, 2021 from https://scopeofpracticepolicy.org/practitioners/physician-assistants/ [Google Scholar]
- Nguyen T, Muench U, Andraka-Christou B, Simon K, Bradford WD, & Spetz J (2021). The Association Between Scope of Practice Regulations and Nurse Practitioner Prescribing of Buprenorphine After the 2016 Opioid Bill. Medical Care Research and Review. 10.1177/10775587211004311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder, 86 Fed. Reg. No. 80 22439–22440 (April 28, 2021) https://www.govinfo.gov/content/pkg/FR-2021-04-28/pdf/2021-08961.pdf
- Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances, 21 C.F.R. § 1301 (2018).
- Roose RJ, Kunins HV, Sohler NL, Elam RT, & Cunningham CO (2008). Nurse practitioner and physician assistant interest in prescribing buprenorphine. Journal of Substance Abuse Treatment, 34(4), 456–459. 10.1016/j.jsat.2007.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Andraka Christou B, Gordon AJ, & Stein BD (2021). It will end in tiers: A strategy to include “dabblers” in the buprenorphine workforce after the X-waiver. Substance Abuse, 42(2), 153–157. 10.1080/08897077.2021.1903659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo T Jr., Clark B, Hickman M, Grebely J, Campbell G, Sordo L, Chen A, Bharat C, Padmanathan P, Cousins G, Dupouy J, Kelty E, Muga R, Nosyk B, Min J, Pavarin R, Farrell M, & Degenhardt L (2021). Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry. 10.1001/jamapsychiatry.2021.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetz J, Toretsky C, Chapman S, Phoenix B, & Tierney M (2019). Nurse Practitioner and Physician Assistant Waivers to Prescribe Buprenorphine and State Scope of Practice Restrictions. JAMA, 321(14), 1407–1408. 10.1001/jama.2019.0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe S, & Hobbins D (2012). The prescribing of buprenorphine by advanced practice addictions nurses. Journal of Addictions Nursing, 23(1), 82–83. 10.3109/10884602.2011.649026 [DOI] [PubMed] [Google Scholar]
- Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, U.S.C. 823(g)(2)(G)(iii), Pub. L. No. 115–271, (2018).
- Taylor P (2016). Re: 69 CSR 12 Medication-Assisted Treatment - Office-Based Medication-Assisted Treatment. https://www.asam.org/docs/default-source/advocacy/wv-obmat-comment-letter-8-5-16.pdf?sfvrsn=0
- Tennessee Department of Health (2021). Rural Areas. https://www.tn.gov/health/cedep/environmental/healthy-places/healthy-places/land-use/lu/rural-areas.html#:~:text=Most%20of%20the%20land%20area,square%20miles%20of%20rural%20Tennessee.
- Tierney M, Finnell DS, Naegle MA, LaBelle C, & Gordon AJ (2015). Advanced Practice Nurses: Increasing Access to Opioid Treatment by Expanding the Pool of Qualified Buprenorphine Prescribers. Substance Abuse, 36(4), 389–392. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. (2012). Growth in Urban Population Outpaces Rest of Nation, Census Bureau Reports. https://www.census.gov/newsroom/releases/archives/2010_census/cb12-50.html [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, & Sanghavi DM (2020). Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Network Open, 3(2). 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Borders TF, & Cummings JR (2019). Trends In Buprenorphine Prescribing By Physician Specialty. Health Affairs, 38(1), 24–28. 10.1377/hlthaff.2018.05145 [DOI] [PubMed] [Google Scholar]
- Wu L-T, Zhu H, & Swartz MS (2016). Treatment utilization among persons with opioid use disorder in the United States. Drug and alcohol dependence, 169, 117–127. 10.1016/J.DRUGALCDEP.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


