Abstract
Background:
Bacterial sepsis is a relatively common, life-threatening condition with a high case fatality rate. The current primary diagnostic tools for detecting bacterial infection in fluids are bacterial culture and fluid cytology. While culture is the gold standard, it can take up to several days for results to be made available to clinicians, which can delay recognition of bacterial sepsis and negatively impact patient outcomes.
Objectives:
The aim of this study was to evaluate the diagnostic accuracy of cytology for detecting bacterial infection in body fluids.
Methods:
We retrospectively reviewed 10 years of medical records at the Ohio State University’s Veterinary Medical Center for mammalian patients with both cytology and bacterial culture of fluid samples, including body cavity fluids (abdominal and thoracic effusion), blood, joint fluid, and CSF. The overall sensitivity and specificity of cytology relative to the reference method of bacterial culture was recorded, as well as among the subcategories of fluid type.
Results:
The overall sensitivity and specificity of cytology for the diagnosis of sepsis were 42.6% and 93.0%, respectively. Individual sensitivities and specificities were also calculated for each fluid type. Thoracic fluid cytology had relatively high sensitivity and low specificity, in contrast to the other fluid types analyzed.
Conclusions:
Overall, cytology is poorly sensitive but highly specific for the detection of bacterial infection in fluid samples. The results from this study will allow a better comparison between the diagnostic accuracy of cytology and emerging diagnostic tests for the detection of bacterial sepsis in mammalian patients.
Keywords: canine, culture, equine, sensitivity, sepsis, specificity
Introduction
Bacterial sepsis is a complex, critical condition seen in both human and veterinary medicine. Although commonly seen in veterinary patients, there is less information in the literature about bacterial sepsis in animals than in people. Bacterial sepsis is currently defined in human medicine as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 The reported mortality rate for sepsis ranges from 30%−70%; possible complications from sepsis include disseminated intravascular coagulation (DIC) and multiple organ dysfunction syndrome (MODS).2 Sepsis is seen in many veterinary species, including small animals such as dogs, cats, and large animals, such as cattle and horses. Gram-negative bacteria are the most common cause of bacterial sepsis in dogs and cats, with Escherichia coli as the most common isolate.3 Some of the most common anatomic locations of bacterial infections leading to sepsis in the clinic are the joints, abdominal (peritoneal), and thoracic (pleural) cavities.2
The current primary diagnostic tools for detecting bacterial infection in body fluids from these sites in veterinary patients are fluid cytology and bacterial culture, the latter of which is considered the reference method (gold standard). A major disadvantage of bacterial culture is the length of time it takes for bacterial growth to be identified, which is approximately 48–72 hours.4 This prolonged period of time before the clinician receives the culture results can have a negative impact on the survival of a septic patient. Indeed, sepsis is an emergent condition in which rapid diagnosis and instigation of treatment are critical for patient survival.3 Cytology offers a rapid and cost-effective alternative to bacterial culture by allowing the clinician to institute appropriate therapy within an acceptable period of time, thereby minimizing patient morbidity and mortality.5 The goal of this study was to evaluate the diagnostic accuracy of cytology for detecting bacterial infection in body fluid samples from the sites most commonly associated with clinical sepsis in veterinary patients. We hypothesized that the sensitivity of cytology is low but that the specificity is high for the detection of bacterial infection in fluid samples.
Materials and Methods
Medical records from a ten-year period from 2010–2019 were reviewed using the Vetstar and VADDS (Advanced Technology Corp.) system database at the Ohio State University’s Veterinary Medical Center. Mammalian cases that included a diagnosis of bacterial infection in body cavity fluids (abdominal and thoracic effusions), blood, joint fluid, or cerebrospinal fluid (CSF) were included. To be included in the study, bacterial culture and cytology (or blood smear evaluation, in the case of bacteremia) must have been performed on the same sample. Specific keywords were entered in the “diagnosis” search field of archived discharge summaries using File Maker Pro Advanced (Claris International Inc.), and medical records that fell within the ten-year period were considered. These keyword search terms included: “sepsis,” “bacterial sepsis,” “septicemia,” “endotoxemia,” “septic shock,” “bacterial infection in effusion,” “septic peritonitis,” “septic abdomen,” “pyothorax,” “septic thorax,” “septic arthritis,” “septic joint fluid,” “septic CSF,” and “bacterial meningitis.” The medical records that met the inclusion criteria were then correlated with the respective cytologic and culture reports in VADDS. Culture reports before 2015 were not available electronically, so the paper medical records were reviewed instead.
Cases with duplicate samples, non-mammalian species, or an inconclusive diagnosis by bacterial culture were excluded from the study. Information extracted from the medical record included medical record number, signalment (species, age, sex, and neuter status), and type of fluid (abdominal fluid, thoracic fluid, joint fluid, blood, or CSF). The infection type was identified from the diagnostic section of the medical record, rather than the Laboratory Information System (LIS), due to the limited search capabilities of the LIS program. From the cytologic report, data regarding the fluid type, cell count, protein content, percent neutrophils, and final cytologic interpretation were collected. From the bacterial culture report, data on the genus and species of the bacterial growth, if any, the type of growth (very light, light, medium, heavy), as well as the susceptibility and resistance pattern were collected. Antibiotic use at the time of sampling was also evaluated, and the type of antibiotic used was recorded when indicated in the medical record. The ultimate clinical diagnosis and status at discharge (alive, dead, dead/necropsied, euthanized, euthanized/necropsied) were also extracted from the medical records.
Based on the results of the cytology report with bacterial culture as the gold standard, cases were categorized as either true positive, false positive, true negative, or false negative. An overall sensitivity and specificity were calculated using the standard formulae, as well as the sensitivity and specificity among the subcategories of fluid type.
Results
Two hundred forty-four cases met inclusion criteria. The most frequent species were horses (54.5%), followed by dogs (34.8%) (Figure 1). Cases fell into one of the following categories for fluid type: blood, abdominal fluid, thoracic fluid, joint fluid, or CSF, and each fluid category varied in species distribution. For example, bacteremia cases were primarily horses (86.5%, n=90), while septic abdomen cases were primarily dogs (51.3%, n=20). Cases with septic thorax consisted primarily of either cats or dogs (89.5%, n=17), with only two equine cases which met search and inclusion criteria.
Figure 1:
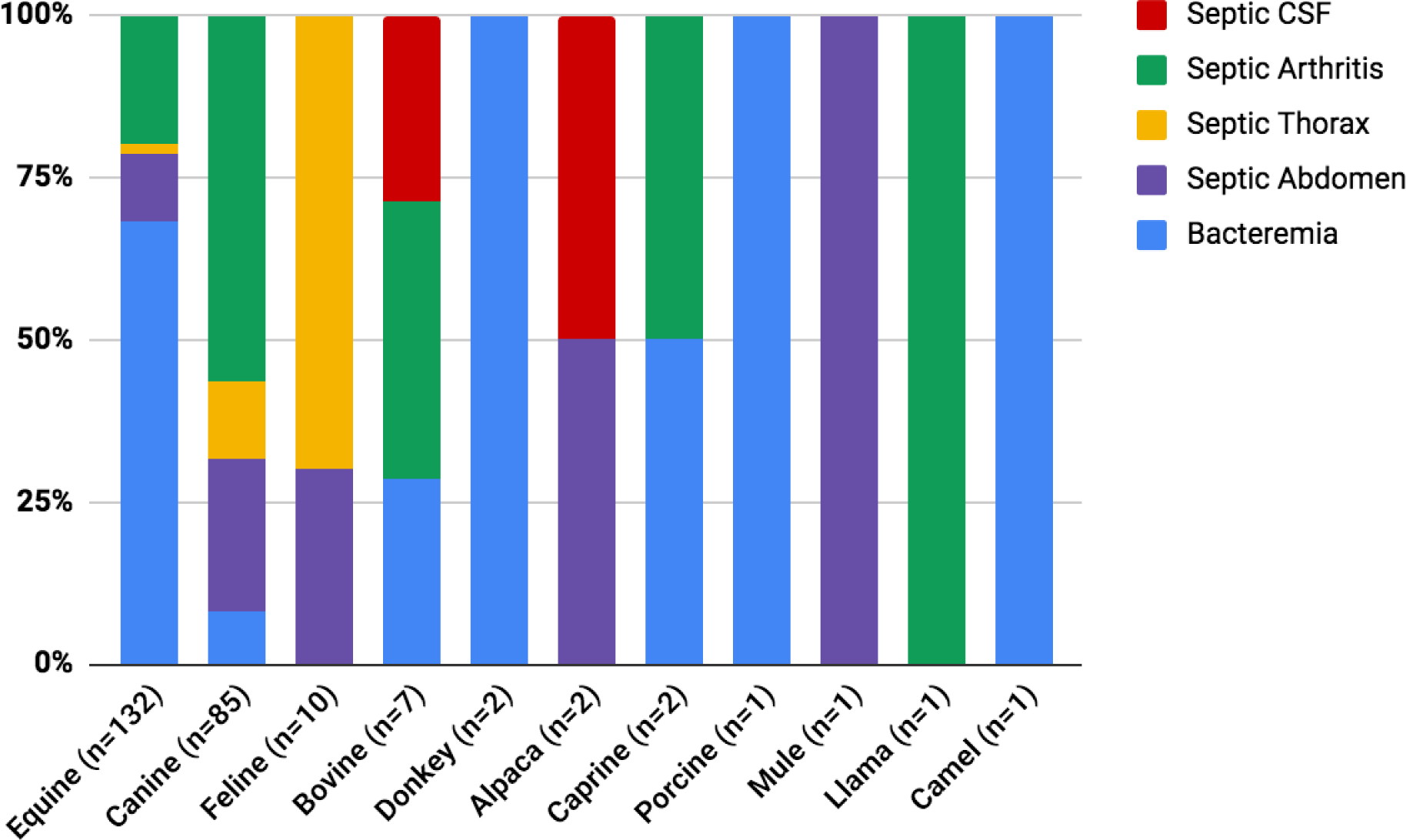
A 100% stacked column chart shows the relative percentages of different infection types across various species included in this study.
The average age and sex distribution for each fluid type are listed in Table 1. Infection type was documented based on the fluid sample type submitted for evaluation (blood, abdominal fluid, thoracic fluid, joint fluid, or CSF), and discharge status was recorded for each infection type. For this study, “alive” was defined as currently living at the time of discharge, though it should be noted that there were a few cases in which the clinician recommended euthanasia, but the client opted to go back to their regular veterinarian for euthanasia or continue home monitoring for longer.
Table 1:
Diagnostic accuracy of cytology for detecting bacterial infection in various fluid types
| All Fluid Types | Blood | Abdominal Fluid | Thoracic Fluid | Joint Fluid | CSF | |
|---|---|---|---|---|---|---|
|
| ||||||
| Total Cases (N) | 244 | 104 | 39 | 19 | 79 | 3 |
| Sensitivity (%) | 42.6 | 16.1 | 82.8 | 87.5 | 38.2 | 100.0 |
| Specificity (%) | 93.0 | 97.9 | 100.0 | 63.6 | 93.4 | 100.0 |
| Average Age (years) | 3.72 | 0.34 | 8.40 | 6.15 | 4.99 | 2.43 |
| F, N (%) | 80 (32.8%) | 45 (43.3%) | 13 (33.3%) | 3 (15.8%) | 19 (24.1%) | 0 (0%) |
| FS, N (%) | 26 (10.7%) | 2 (1.9%) | 7 (17.9%) | 5 (26.3%) | 12 (15.1%) | 0 (0%) |
| M, N (%) | 86 (35.2%) | 53 (51.0%) | 7 (17.9%) | 4 (21.1%) | 19 (24.1%) | 3 (100%) |
| MC, N (%) | 52 (21.3%) | 4 (3.8%) | 12 (30.8%) | 7 (36.8%) | 29 (36.7%) | 0 (0%) |
| Alive, N (%) | 187 (76.6%) | 74 (71.2%) | 22 (56.4%) | 14 (73.7%) | 75 (94.9%) | 2 (66.7%) |
| Died (no necropsy), N (%) | 9 (3.7%) | 4 (3.8%) | 2 (5.1%) | 2 (10.5%) | 1 (1.3%) | 0 (0%) |
| Died/Necropsy, N (%) | 14 (5.7%) | 13 (12.5%) | 0 (0%) | 0 (0%) | 1 (1.3%) | 0 (0%) |
| Euthanized (no necropsy), N (%) | 13 (5.3%) | 4 (3.8%) | 7 (17.9%) | 1 (5.3%) | 1 (1.3%) | 0 (0%) |
| Euthanized/Necropsy, N (%) | 21 (8.6%) | 9 (8.7%) | 8 (20.5%) | 2 (10.5%) | 1 (1.3%) | 1 (33.3%) |
F, female; FS, female spayed; M, male; MC, male castrated; N, number
For this study, overall sensitivity and specificity were calculated, as well as an individual sensitivity and specificity for each fluid type (Table 1). We found an overall sensitivity of 42.6% and an overall specificity of 93.0% for the diagnosis of bacterial infection in body fluids. Excluding blood samples, the overall sensitivity and specificity of cytology for the detection of bacterial infection in fluid samples (n=140) were 63.0% and 89.6%, respectively. An additional analysis was performed to compare the sensitivity and specificity in all cases that received antibiotic therapy at the time of sampling (n=82) versus those that did not (n=162). The sensitivity and specificity for the group that received antibiotics were 43.9% and 97.6%, respectively. For the 162 cases without a history of previous antibiotic use, a sensitivity of 41.6% and a specificity of 91.8% were obtained. Furthermore, a similar analysis was performed on body (thoracic and abdominal) fluids alone. Those cases with a history of previous antibiotic use (n=50) at the time of sampling showed a sensitivity of 61.5% and a specificity of 95.8%. In contrast, those with no prior antibiotic use (n=90) demonstrated a sensitivity of 63.8% and a specificity of 88.4%.
Cases categorized as false negative (i.e., positive bacterial culture but negative cytology) that had a record of previous antibiotic use at the time of sampling were documented. In order of frequency, the following antibiotics were reported in these cases: gentamicin, metronidazole, ceftiofur, enrofloxacin, clindamycin, tulathromycin, penicillin, trimethoprim/sulfamethoxazole, cephalexin, amikacin, and ciprofloxacin.
For this study, microscopic blood smear evaluation (as part of the CBC) was used as the ‘cytologic’ testing for cases with bacteremia, and cell count, serum protein concentrations, and neutrophil percentages were recorded using the correlating CBC. Cytologic interpretations included whether or not bacterial infection (ie, intracellular organisms) was seen and the morphology of the organism if noted. Most cases noted that the blood smear was negative for organisms, but the few cases that did note bacteria were also reviewed by the microbiology laboratory for confirmation. In these cases, Gram-negative rods and occasional Gram-positive cocci were noted on the microbiology report. The cytologic findings and most common clinical diagnosis for each fluid type were recorded (Table 2). Some cases were excluded from the average cell count because the laboratory reported an insufficient quantity to perform a cell count, the sample was characterized as “too flocculant,” or the laboratory did not report a cell count for unknown reasons. It should be noted that for calculations, cases with total protein concentrations reported as “<2.5 g/dL” were not included in the average protein concentration but were considered separately instead. We separated out the cases with the low protein concentrations because the cytologic reports did not list a numerical value, and to arbitrarily assign these cases a number to calculate a mean would be inaccurate and misleading.
Table 2:
Cytologic findings in fluids with and without positive bacterial culture results.
| Blood | Abdominal Fluid | Thoracic Fluid | Joint Fluid | CSF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Bacterial Culture | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) |
| Number of Cases (N) | 56 | 48 | 29 | 10 | 8 | 11 | 34 | 46 | 2 | 1 |
| Average Cell Count (cells/uL) | 6,204 | 7,846 | 91,516 | 141,334 | 195,784 | 125,594 | 148,749 | 64,218 | 11,700 | 1 |
| Percent Neutrophils (%) | 63.9 | 68.2 | 89.4 | 86.4 | 84.6 | 80.5 | 93.4 | 83.9 | 68.0 | 0 |
| Average Protein Content (g/dL) | 5.76 | 5.36 | 4.34 | 4.27 | 4.76 | 4.59 | 5.22 | 4.92 | 0.53 | 0.022 |
| Cases with protein content <2.5 uL (N) | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Bacterial species | Actinobacillus sp. Enterobacter sp. Escherichia coli Bacillus sp. Enterococus sp. |
N/A | Klebsiella sp. Escherichia coli Streptococcus sp. Enterococus sp. |
N/A | Bacteroides sp. Escherichia coli |
N/A | Streprococus sp. Escherichia coli |
N/A | Enterococus sp. Streptococus sp. |
N/A |
| Clinical Diagnosis | Failure of passive transfer, neonatal adjustment syndrome | Endocarditis, aspiration pneumonia, hemorrhagic enterocolitis | GI perforation, pancreatitis, hepatitis | Neoplasia, GI impaction, abscess, intusussception | Pyothorax, aspiration pneumonia | Gastroenteritis, FIP, cholangiohepatitis | Septic arthritis, osteoarthritis | IMPA, septic arthritis | Meningitis | Meningitis |
It is worth noting that the culture results on two separate septic arthritis cases included comments, “that the culture was positive on enrichment broth only, and that positive culture could represent a possible contaminant.” Very light growth of Pasturella dagmatis was isolated from the first case, and the genus and species of the bacterial growth for the second case were not recorded. The final clinical diagnosis for the first case was a swollen right stifle, multiple abscesses, and possible septicemia. The second case was diagnosed as septic arthritis of the right carpal joint. These cases were categorized as false negatives since cytology did not detect the etiologic agents present.
A total of 359 cases were excluded from this study for a variety of reasons: no culture report could be located, the culture was canceled by the clinicians, the culture was of tissue rather than the relevant fluid sample, and/or there was no corresponding cytologic report.
Discussion
Bacterial sepsis is a critical condition that carries a high mortality rate, and early detection is paramount for survival. Bacterial culture is considered the gold standard method for the diagnosis of bacterial infection in fluids, but cytology can provide clinicians and owners with more rapid results. In the current study, we evaluated the diagnostic accuracy of cytology as a tool for the diagnosis of bacterial infection in fluid samples. Overall, we found that cytology is poorly sensitive (42.6%) but quite specific (93.0%) for the detection of bacterial infection. Excluding blood samples and focusing on fluid cytology only, the sensitivity was more moderate (63.0%), but the specificity was slightly worse (89.6%).
Cytology had very low sensitivity but very high specificity for the detection of bacteremia. This was expected because we used blood smears for our cytologic sample, and bacteria are not commonly noted in the microscopic evaluation of blood. Based on the 97.9% specificity, a positive result by cytology can be considered confirmatory. A blood smear review can be easily performed in-clinic in a reasonable amount of time while waiting for blood culture results on a suspected septicemic patient.
The calculated specificity for abdominal fluid was very high (100%), which indicates that cytology of abdominal fluid is very useful to rule-in bacterial infection. However, limited abdominal fluid samples were included in this study (n=39). Out of the five false-negative cases in abdominal fluid, only one was noted to have concurrent antibiotic use (ciprofloxacin) at the time of sampling. Obtaining a specimen prior to administration of antimicrobial agents is important to ensure the isolation and identification of the pathogen because previous antibiotic use may make identifying intracellular bacteria difficult, thus resulting in a false-negative cytology result.6
In contrast to other fluid types, the sensitivity of cytology for the detection of bacterial infection in thoracic effusion cases was higher than the specificity. Thus, cytology is better at ruling out septic thorax than ruling it in, though our calculated sensitivity (87.5%) was still only moderate. We speculate that thoracic fluid has a different sensitivity and specificity compared with the other fluid types due to the unique types of bacterial organisms typically infecting this location and the way these cases typically present. For example, thoracic effusion cases are often caused by a penetrating injury (eg, bite wound), migrating foreign bodies, or extension of pneumonia, whereas abdominal effusion is most often caused by a ruptured gastrointestinal (GI) tract or other loss of GI wall integrity.6 These factors may affect the species, morphology, and number of bacteria present in a fluid sample. Other fluid characteristics (eg, cell number and distribution, neutrophil degeneration, protein content) or patient characteristics (eg, clinical history and index of suspicion for sepsis) may affect the amount of time a pathologist spends searching for bacterial organisms. Additionally, anaerobes, such as filamentous Actinomyces and Nocardia spp., are more commonly seen in septic thorax cases; therefore, it is possible that the thorax favors bacteria that are more difficult to culture.2 This may help explain the lower specificity (higher rate of “false positives”) observed in thoracic fluids. Our sample included only 19 cases of thoracic fluids, so these results should be interpreted with some caution.
The sensitivity of cytology for joint fluid was low (38.2%), and the specificity was high (93.4%) in this study, indicating that cytology can be used as a confirmatory diagnostic tool for bacterial infection of the joint. Identification of bacteria in synovial fluid is known to be problematic, which makes determining the accuracy of cytology for septic arthritis challenging.7 A cytology of joint fluid should be performed, despite a low sensitivity for identification of bacteria because if bacteria are present, the results can guide antimicrobial selection while culture results are pending.8 It should be noted that joint culture suffers from low sensitivity, so if no organisms are seen, septic arthritis cannot be ruled out. The most common joint affected in this study was the stifle joint, followed by the elbow. Septic arthritis is a common condition seen in veterinary medicine, especially in large animal patients; 41.5% of cases in our study were large animals. The prognosis of foals with septic arthritis is dependent on several factors, including the prolonged duration (>24 hours) of signs before the initiation of treatment, but short-term survival is considered good, with studies reporting 71% to 81% survival to discharge.8 Thus, early detection of bacteria by cytology could improve the prognosis for these animals.
It is suspected that bacterial meningitis/encephalitis is very rare in our patient population and that clinicians rarely order CSF culture and cytology at the same time, which is partially why our search yielded so few cases. Only 3 cases of septic CSF were returned by our search criteria, and cytology appropriately diagnosed bacterial infection in all three. Due to this limitation, relatively little can be concluded about the sensitivity and specificity of cytology for bacterial infection in CSF using this data.
An additional analysis of cases that had received antibiotics at the time of sampling was performed. Surprisingly, our study did not find that antibiotic use decreased sensitivity. Fluid cytology showed an overall sensitivity of 42.6%, and cases that included antibiotic therapy at sampling time had a sensitivity of 43.9%. It is worth noting that bacterial culture may be negative with antibiotic use, thus impacting the number of false positives and true positives when comparing cytology to bacterial culture. Therefore, strict reliance on culture as our reference method is a limitation to this study.
Here, we evaluated the accuracy of cytology for the diagnosis of bacterial infection in body fluids among veterinary mammalian patients. As expected, we found cytology has an overall low sensitivity but high specificity, with some variations based on fluid type. The general trend, excluding thoracic fluid, was that cytology is better at ruling in than ruling out bacterial infection. This retrospective study was not without limitations. The small sample size for some individual fluid types made calculating the true accuracy of cytology difficult for those subsets. Due to the limited search capabilities of the medical record database, the retrospective nature of this study, and the use of specific search keywords, the cases gathered were likely significantly skewed towards sepsis-positive patients. We very likely missed out on cases with paired cytology and bacterial culture that were non-septic, and even some that were septic, due to these limitations. A further potential limitation is the strict reliance on fluid culture as our reference method (“gold standard”). For example, joint fluid culture is known to be insensitive.7 As a result of this biased prevalence of sepsis, the positive and negative predictive values (PPV and NPV) could not be calculated using these data. Future studies could evaluate the accuracy of cytology for the detection of bacterial organisms in a prospective manner to avoid some of these limitations.
There are currently various methods used to diagnose early bacterial sepsis in veterinary medicine, one of which is the sepsis scoring system. This system is commonly used to diagnose sepsis in foals, and it uses subjective clinical criteria and objective clinicopathologic data to assign a number for each criterion.4 The points are then added and compared to a cut point. Some variables used in this scoring system include the median l-lactate concentration, blood glucose, and lymphocyte count.4 A recent study found that the sensitivity and specificity of a modified sepsis score were 62.0% and 64.0%, respectively.4 Based on the overall performance of the sepsis score, it was recommended that clinicians use this score with caution and in conjunction with clinical assessments and judgments and blood culture results.4 Due to the low accuracy and the lack of use in patients other than foals, the sepsis scoring system alone does not appear to be an accurate early diagnostic tool for sepsis in mammalian patients. Cytology offers a high specificity (93.0% overall) for diagnosing bacterial infection, and it can be performed in conjunction with bacterial culture. Although not directly comparable to the sepsis scoring system, a cytologic diagnosis of bacterial infection in a patient with other clinicopathologic findings consistent with sepsis is highly supportive of sepsis. The addition of cytologic evidence of bacterial infection may be a valuable parameter to add to the sepsis scoring system, and an investigation into the accuracy of the sepsis scoring system with and without a cytologic parameter is an appealing avenue for future research.
Another method that can be used to diagnose bacterial infection is assessing the differences between abdominal and blood glucose and lactate concentrations. This diagnostic test is used for suspected septic abdomen cases because a higher lactate concentration in abdominal fluid than in blood and a lower glucose concentration in abdominal fluid than in blood is suggestive of a septic abdomen.9 Abdominal glucose is decreased due to its utilization by bacteria, and abdominal lactate is increased because of increased production from the bacteria. The reported sensitivity and specificity for the difference between blood and abdominal fluid glucose concentrations vary by method, cutoff (eg, > 20 mg/dL), species, and study. Reported sensitivities range from 41.2 to 100%, and reported specificity ranges from 77.8 to 100%.9,10 One study reported that < −2.0 mmol/L difference between blood and abdominal fluid lactate concentrations was 100% sensitive and specific for septic abdomen, though this sample included only seven patients.9 A more recent study of 37 dogs showed that an effusion lactate concentration of > 4.2 mmol/L was 72.2% sensitive and 84.2% specific for the diagnosis of septic abdomen.11 Our study found that cytology is 82.8% sensitive and 100% specific for the detection of septic abdomen (out of 39 cases). Thus, cytology offers similar or better diagnostic accuracy relative to the biochemical tests described here.
Cytology has the major benefit of a reduced output time for results, giving a potentially septic patient a much better chance at survival. Cytology can also be performed in-house and suggest or confirm an underlying etiology. However, as shown here, cytology is poorly sensitive for the detection of bacterial infection, particularly in peripheral blood. Thus, there is currently an unmet need for a more sensitive but still rapid diagnostic technique to fill this niche. Flow cytometry, PCR, biochemical-based test strips (eg, RapidBac12), and various biomarkers (eg, procalcitonin and acute-phase proteins) are all possible avenues of further exploration. We hope to use the results from this retrospective study on the accuracy of cytology to inform future studies aimed at developing novel diagnostic tests for the rapid detection of bacterial sepsis in mammalian patients.
Acknowledgments
A stipend for BAA was funded by the NIH T35 Training Grant (#10977-12).
References
- 1.Singer M Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315 (8):801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etienne C Clinical Veterinary Advisor: Dogs and Cats, 3rd ed. St. Louis, MO: Elsevier; 2011:929–931. [Google Scholar]
- 3.DeClue AE.Chapter 132: Sepsis and the Systemic Inflammatory Response Syndrome. In: Junaid J, Textbook of Veterinary Internal Medicine, 8th edition. St. Louis, Missouri: Elsevier; 2017:1492–1502. [Google Scholar]
- 4.Wong DM, Ruby RE, Dembek KA, et al. Evaluation of updated sepsis scoring systems and systemic inflammatory response syndrome criteria and their association with sepsis in equine neonates. J Vet Intern Med. 2018;32(3):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connally HE. Cytology and fluid analysis of the acute abdomen. Clin Tech Small Animal Practice. 2003;18(1):39–44. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey SM, Ewing PJ. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J Am Anim Hosp Assoc. 2011;47(1):1–11. [DOI] [PubMed] [Google Scholar]
- 7.Scharf VF, Lewis ST, Wellehan JF, et al. Retrospective evaluation of the efficacy of isolating bacteria from synovial fluid in dogs with suspected septic arthritis. Australian Veterinary Journal. 2015;93(6)200–203. [DOI] [PubMed] [Google Scholar]
- 8.Hardy J Chapter 38: Diseases of the Bones, Joints, and Connective Tissues. In: Smith BP, Large Animal Internal Medicine, 5th edition. St. Louis, Missouri: Elsevier; 2015:1094–1097. [Google Scholar]
- 9.Bonczynskii JJ, Ludwig LL, Barton LJ, Loar A, Peterson ME. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg. 2003;32(2):161–166. [DOI] [PubMed] [Google Scholar]
- 10.Koenig A, Verlander LL. Usefulness of whole blood, plasma, peritoneal fluid, and peritoneal fluid supernatant glucose concentrations obtained by a veterinary point-of-care glucometer to identify septic peritonitis in dogs with peritoneal effusion. J Am Vet Med Assoc. 2015;247(9):1027–1032. [DOI] [PubMed] [Google Scholar]
- 11.Martiny P, Goggs R. Biomarker Guided Diagnosis of Septic Peritonitis in Dogs. Front Vet Sci. 2019. Jun 27;6:208.Erratum in: Front Vet Sci. 2020 Mar 19;7:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stapleton AE, Cox ME, DiNello RK, et al. Performance of a New Rapid Immunoassay Test Kit for Point-of-Care Diagnosis of Significant Bacteriuria. J Clin Microbiol. 2015. Sep;53(9):2805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


