Abstract
Objective:
Chronic inflammation contributes to the high burden of cardiovascular disease (CVD) in persons with HIV (PWH). HIV has broad effects on innate and adaptive immune cells, including innate lymphoid cells (ILCs) and CD4+ T-helper cells. At present, the relationship between CVD and plasma cytokines reflecting ILC/T-helper responses in PWH is not well defined. We investigated relationships between plasma cytokines and subclinical atherosclerosis.
Design:
Cross-sectional study.
Methods:
We recruited 70 PWH on a single antiretroviral regimen (efavirenz, tenofovir, and emtricitabine) with at least 12 months of suppressed viremia and 30 HIV-negative controls. We quantified plasma cytokines and chemokines including interferon-γ, interleukin (IL)-4, IL-13, and IL-17A, markers of macrophage activation, and endothelial activation using multiplex assays and ELISA. Cytokines were grouped using Ward’s hierarchical clustering. Brachial artery flow-mediated dilation (FMD) and carotid plaque burden were determined using ultrasound. Multivariable linear regression and negative binomial regression analyses were used to assess the relationships of plasma biomarkers and endpoints adjusted for CVD risk factors.
Results:
We identified three distinct clusters in PWH, one containing Th1/Th2/ILC1/ILC2 type cytokines, one with Th17/ILC3/macrophage-related cytokines, and a less specific third cluster. Lower FMD was associated with higher plasma IL-17A and macrophage inflammatory protein-1α. In contrast, IL-4, a Th2/ILC2 type cytokine, was associated with carotid plaque. When HIV-negative controls were added to the models clustering was more diffuse, and these associations were attenuated or absent.
Conclusions:
Th17/ILC3 and Th2/ILC2-mediated immune mechanisms may have distinct roles in endothelial dysfunction and atherosclerotic plaque formation, respectively, in PWH.
Keywords: Atherosclerosis, cardiovascular disease, endothelial dysfunction, interleukin-17, interleukin-4, HIV, T cells, Cardiovascular Disease, Atherosclerosis, Endothelial Dysfunction, plasma cytokines, Coronary Artery Disease, HIV
Introduction
Persons with HIV (PWH) have a higher incidence of cardiovascular disease (CVD) that is not accounted for by traditional risk factors. The increased CVD risk, which persists despite effective suppression of plasma viremia on antiretroviral therapy (ART), is attributed, in part, to chronic innate and adaptive immune activation which persists despite effective suppression of plasma viremia on antiretroviral therapy (ART) (1). Although inflammation is important in atherosclerosis, the roles of CD4/CD8 T cells and innate lymphoid cells (ILCs) are less well defined in PWH. The vascular endothelium regulates vessel homeostasis, interacts with cells of the bloodstream, and modulates the vascular response to mechanical damage and circulating mediators, and endothelial dysfunction predisposes to atherogenesis. Markers of endothelial activation, such as soluble vascular cell adhesion molecule 1 (sVCAM-1) and intercellular cell adhesion molecule 1 (sICAM-1), are elevated before the initiation of ART and decrease, but do not fully normalize, with suppression of plasma viremia (2). Endothelial activation or dysfunction defines a state of impaired nitric oxide signaling, a propensity for thrombosis, and enhanced recruitment of inflammatory cells including macrophages, CD4+ T cells, CD8+ T cells, neutrophils and B cells (3). These events occur early in the development of atherosclerosis, before any clinically apparent structural changes. Several studies have shown that flow-mediated dilation (FMD) is impaired in PWH compared to HIV-negative controls creating an arterial environment permissive for atherogenesis (4).
Inflammation, both vascular and non-vascular, accelerates the development of atherosclerosis in diverse conditions including obesity, infection (5), and rheumatoid arthritis (6). Atherosclerosis is characterized by arterial injury, proliferation of arterial smooth muscle cells, and increased intima-media thickness (IMT), which can be followed by atheroma formation and rupture (7). This process is driven, in part, by cells of the innate and adaptive immune system (3, 8–12). Most data on immune cells in atherosclerosis has highlighted the role of macrophages in atherosclerosis (13), but, importantly, monocyte/macrophage activity is regulated in part by other cells of the innate and adaptive immune system. CD4+ T-helper (Th) cells are one of the key mediators of inflammation. They are abundant in atherosclerotic lesions (9) and can promote or suppress vascular dysfunction and atherosclerosis depending on context and activation status (for review (14)). Different T-helper cell subsets are thought to have separate roles in this process. Th1 cells express interleukin (IL)-2 and interferon (IFN)-γ and promote atherogenesis (15). Th17 cells express IL-17A, which is elevated in the plasma of hypertensive PWH (16) and increased in mouse models of hypertension and atherosclerosis (17). Th2 cells express IL-4, IL-5, and IL-13 and may have dual roles: anti-inflammatory in some contexts (18) but proatherogenic in others (19, 20). Finally, T regulatory cells (Tregs) are important modulators of immune responses and are thought to prevent atherosclerosis and promote a stable plaque phenotype (21). Notably, a recent study showed that Tregs have different transcriptional and functional profiles in PWH on ART (22). Tregs can mediate their effects by direct contact or through cytokines including IL-10, transforming growth factor (TGF)-β (23), and IL-35 (24). In PWH, higher serum IL-10 has been associated with lower odds of prevalent coronary atherosclerotic plaque (25). Lastly, ILCs that express IFN-γ (ILC1), IL-5, IL-13 (ILC2), and IL-17, IL-22 (ILC3), may also play a role in atherosclerosis; however, their role is less clear (26).
HIV has broad effects on the innate and adaptive immune compartments and, given prior studies showing a likely role for CD4+ T helper cell cytokines in CVD pathogenesis, changes in CD4+ T helper cell polarization, ILCs, and cytokine expression may contribute to the high burden of atherosclerotic CVD in PWH. In this study, we investigated the relationship of plasma cytokines reflective of CD4+ T-helper cell, ILC, and macrophage activity with endothelial function and atherosclerotic plaque in a cohort of PWH on stable, long-term ART.
Methods
Study cohort
We recruited 70 PWH without diabetes or prior cardiovascular events (e.g., stroke or myocardial infarction) and 30 HIV-negative controls with similar age, sex, and race at Vanderbilt University Medical Center in Nashville, Tennessee (27). All PWH were on the same ART regimen of efavirenz, tenofovir, and emtricitabine for at least 6 months before enrollment with a CD4+ T cell count >350 cells/μl, and had consistent viral suppression (HIV-1 RNA viral load <50 copies/ml) for at least 12 months. Other inclusion criteria for all participants included no treatment with HMG CoA reductase inhibitors in the prior 6 months, and no known history of CVD, diabetes, or rheumatologic disease. The cohort was designed to include approximately 50% obese PWH (body mass index [BMI] >= 30kg/m2) to enrich for cardiometabolic disease risk factors, and HIV-negative controls were predominantly obese. Smoking status was documented for all participants.
Plasma biomarkers
Soluble biomarkers were measured as previously published (27). We measured T-helper effector plasma cytokines including Th1 (IFN-γ, tumor necrosis factor (TNF)-α, IL-2, IL-12p70), Th2 (IL-4, 5,13), IL-10, and Th17 (IL-17A) cytokines in duplicate using a multiple immunoassay panel (MesoScale, Rockville, MD). Additional cytokines and chemokines included markers of inflammation (IL-1β, IL-6), proteolytically cleaved and soluble forms of TNF-α receptors (sTNF-αR1 and TNF-αR2), CCL2 (also known as monocyte chemoattractant protein-1 [MCP-1]), CCL3 (also known as macrophage inflammatory protein-1α [MIP-1α]), CCL4 (also known as macrophage inflammatory protein-1β (MIP-1β]), serum amyloid A protein (SAA), sVCAM-1 and sICAM-1. Monocyte activation markers, soluble CD14 (sCD14), and soluble CD163 (sCD163) were measured by ELISA (R&D Systems, Minneapolis, MN).
Vascular Evaluations
Carotid artery plaque burden and brachial-artery FMD were measured using a Philips iE33 ultrasound with an L9-3 linear transducer as published (28). The maximum diameter of the vessel was determined and the percent change in diameter (% vasodilation) was calculated. This technique yields an intra-observer variability of 0±0.15% and inter-observer variability of 0.05±0.16% (29–33). For carotid plaque evaluation, we defined lesions greater than 1.5mm at the right and left common carotid arteries, carotid bulbs, and internal carotid arteries as carotid plaques, the sum of which was the carotid plaque burden as published (34).
Dual-energy X-ray absorptiometry (DEXA) scan
A full body DEXA scan was performed on all participants to measure regional fat mass (GE Lunar Prodigy). Visceral fat mass was estimated using the pre-programmed software algorithm.
Statistical Analyses
Clinical and demographic characteristics are expressed as median and inter-quartile range (IQR) or percentage. Differences between PWH stratified by sex and obesity were compared using Wilcoxon rank-sum test for continuous variables, and Chi-square tests for categorical variables. We performed hierarchical clustering to group inflammatory biomarkers using Ward clustering with Euclidean distances after normalizing biomarker values (subtracting mean and dividing by standard deviation). Cluster uncertainty was estimated by computing approximately unbiased probability values (AU) by multiscale bootstrapping with 10,000 bootstrap replications; AU≥95% was considered to be supported by the data. Cluster analyses were performed with the pvclust package in R statistical software (35).
Multivariable linear regression analyses – adjusted for age, BMI, fasting low-density lipoprotein (LDL), hypertension, sex, and smoking status – were used to assess the relationship between soluble biomarkers and brachial artery FMD. Skewed exposure variables with non-normal distributions were square root transformed. Beta estimates were normalized by comparing the 75th and 25th percentiles of cytokines to facilitate comparisons between cytokines with different ranges. Negative binomial regression analyses were used to assess the relationship between plasma cytokines and carotid plaque burden (counts). Similar to the linear regression analyses, we normalized beta estimates using the interquartile range. Analyses of subsets of participants defined by obesity were adjusted for age and hypertension. We controlled for false discovery using the Benjamini Hochberg procedure with a critical value for false discovery set at 0.25. Statistical analysis was performed using R version 3.6.1 and 4.0.2 (http://www.R-project.org), and GraphPad Prism version 9.0 (GraphPad Software, San Diego CA).
Images
The model figure was created with BioRender (https://biorender.com).
Study Approval
This study was approved by the Vanderbilt University Medical Center Institutional Review Board. Participants provided written informed consent. The investigators carried out studies per guidelines of the United States Department of Health and Human Services. This study is registered on clinicaltrials.gov (NCT04439448).
Results
Cohort clinical demographics
Characteristics of the cohort stratified by sex are found in Supplemental Table 1. The median age was similar in men and women (p=0.6). Women were more likely to be non-white (p=0.009). There was no difference in BMI, total cholesterol, LDL, and triglycerides by sex, but high-density lipoprotein (HDL) was higher in women (p=0.001). Men had lower brachial artery FMD (p=0.02) and more carotid plaque (p=0.03). Among the CD4 T-helper/ILC effector cytokines, there was no significant difference in plasma IFN-γ, IL-10, IL-13, and IL-17A by sex, but IL-4 (p<0.05) was higher in women while IL-5 (p=0.004) and TNF-α (p=0.004) were higher in men. Other markers including IL-6, SAA, and sCD14 were higher in women. A similar analysis of the cohort was stratified by HIV status (Supplemental Table 2). HIV-negative participants were younger and more obese (p=0.001) with no difference in race or sex. They had similar FMD and carotid plaque burden. PWH had higher plasma IL-2, IL-8, MCP1, sCD14, sVCAM-1, and sICAM-1 (p<0.05 for all). HIV-negative participants on the other hand had higher levels of plasma IL-10, IL-12, and IL-13 (p<0.05 for all).
Given the large number of plasma biomarkers measured, we used a hierarchical clustering approach to group biomarkers by plasma levels in the full cohort (Figure 1A). When limited to PWH, we observed that the plasma cytokines fell into three main clusters with AU values ≥98% (designated clusters 1, 2, and 3, Figure 1B). IFN-γ, TNF-α, IL-4, IL-10, and IL-1β grouped in cluster 1, and IL-17A, IL-5, TNF-αR1, sVCAM-1, and sICAM-1 grouped in cluster 2. Cluster 3 was the largest and included sCD14, SAA, IL-6, and hsCRP.
Figure 1. Cluster dendrogram of plasma cytokines.

Ward hierarchical clustering using the Euclidean distance matrix and pvclust function in R was used to perform hierarchical clustering with multiscale bootstrap resampling in all participants (A) and PWH only (B). The height (left axis) estimates cytokine similarity. Clusters with au≥ 95% are marked by the red rectangles. (au– approximately unbiased p value).
Plasma IL-17A is associated with lower brachial artery FMD
We first performed correlation analyses to identify CD4+ T helper/ILC effector cytokines that were associated with FMD. Of the T helper/ILC plasma cytokines grouped in cluster 2, IL-17A was inversely correlated with FMD in the full cohort (Supplemental Figure 1A). All other cluster 2 biomarkers were not significant (Supplemental Figure 1B–H). Using univariable linear regression, FMD was associated with IL-17A (β=−0.43 and p= 0.02) in all participants. This was stronger in PWH (β=−0.57 and p= 0.001). We included an interaction term between IL-17A and HIV status to determine whether the association between FMD and IL-17A differed based on HIV status. This interaction approached significance in all participants, while a significant difference by HIV status was present when the analysis was limited to the obese participants (Supplemental Figure 2). There were no interactions between HIV status and other cluster-2 cytokines (Supplemental Figure 2C–D).
Cluster-2 cytokines IL-17A and MIP-1α are negatively associated with lower brachial artery FMD in PWH
Given the higher degree of cytokine clustering when the cohort was limited to PWH, the divergent relationships between cytokines and FMD by HIV status (Supplemental Figure 2), and the much smaller number of HIV-negative controls, we limited the remainder of the analysis to PWH. We observed a negative correlations between FMD and IL-17A, IL-5, MIP-1α, and TNF-αR2 (Figure 2A–D). The other cluster-2 markers were not significant (Figure 2E–H).
Figure 2. FMD is negatively correlated with IL-17A, IL-5, CCL3 (MIP-1α) and TNF-αR2 in PWH.

Correlations of FMD and cluster 2 cytokines, IL-17A (A), IL-5 (B), CCL3 (C), TNF-αR2 (D), TNF-α R1 (E), sCD163 (F), sVCAM-1 (G) and sICAM-1 (F). Spearman’s rank correlations.
We used multivariable linear regression analysis adjusted for traditional CVD risk factors to assess the relationships between plasma cytokines and FMD (Supplemental Table 4, Figure 3). Of the CD4+ T-helper/ILC effector cytokines, higher IL-17A and MIP-1α in cluster 2 were associated with lower FMD. They both met the criteria for statistically significant when controlling the false discovery rate at 0.25. Other cytokines IL-4 and IL-1β were positively associated with FMD and met the criteria for statistically significant when controlling the false discovery rate at 0.25.
Figure 3. Multivariable linear regression analysis of the relationships between cluster 1, 2 and 3 cytokines with brachial-artery FMD among PWH.
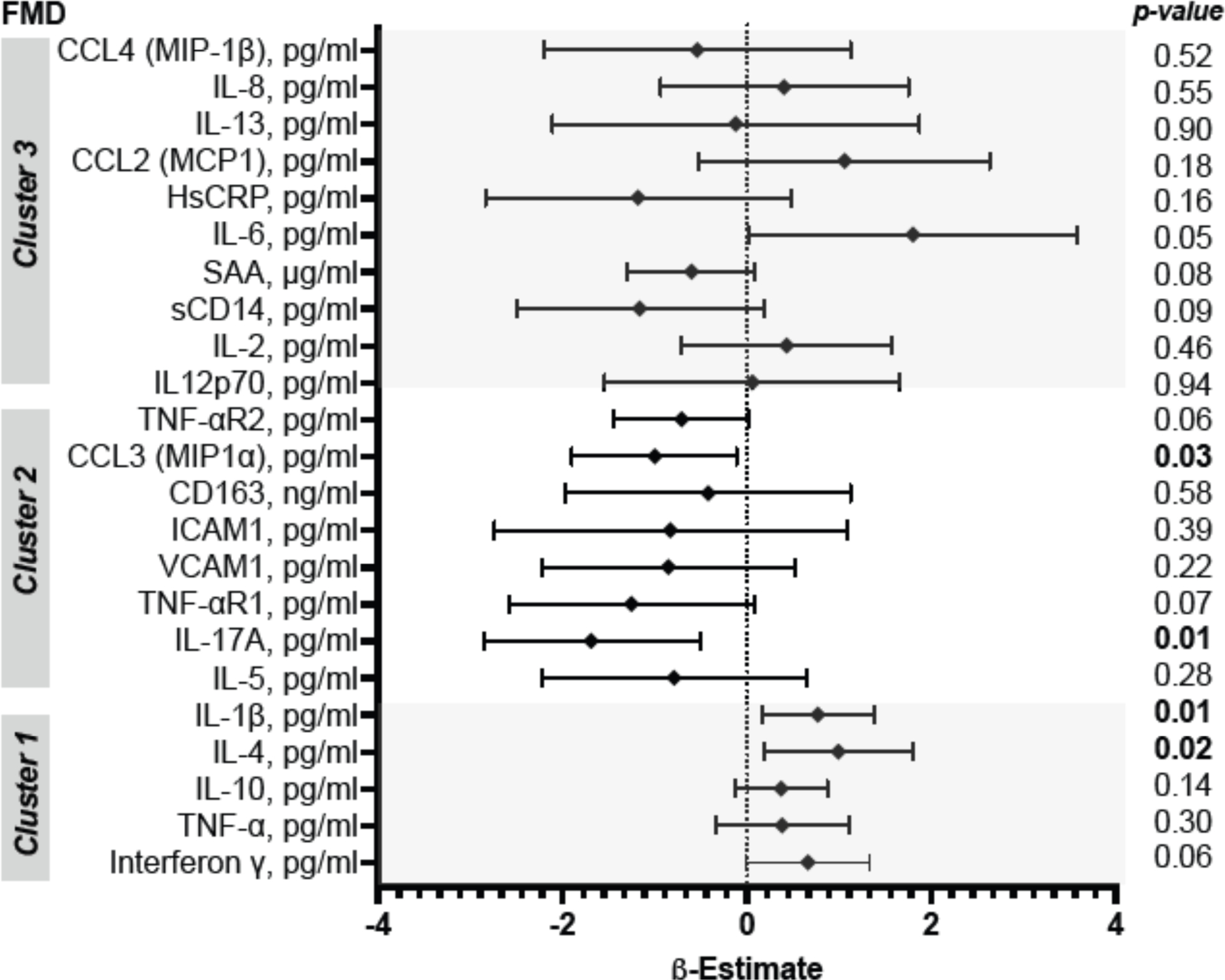
Adjusted for sex, age, body mass index, current hypertension, fasting LDL and smoking status. Beta estimates are the differences between conditional expectations of FMD comparing the 75th and 25th percentiles of the cytokines. P-values are not adjusted for multiple comparisons.
IL-4 and IL-10 were associated with carotid plaque in PWH
In our cohort, 29% of PWH had carotid plaque, comprising 24% of women and 33% of men (Supplemental Table 3). Of the T helper cytokines in clusters 1 and 3, IL-4, IL-10, and IL-13 plasma levels were similar in PWH with and without plaque (Supplemental Figure 3). We performed negative binomial regression analysis adjusted for CVD risk factors. We found that PWH with IL4 levels at the 75th percentile had an expected number of carotid plaques 44% higher than those at the 25th percentile (Supplemental Table 5, Figure 4). A similar trend was seen with IL-10 and IL-13, however only IL-4 met the criteria for statistical significance with a false discovery rate of 0.25. No significant associations were found between cluster 2 cytokines and carotid plaque.
Figure 4. Multivariable negative binomial regression analysis of the relationships between cluster 1, 2, and 3 cytokines with carotid plaque burden among PWH.

Adjusted for sex, age, body mass index, current hypertension, fasting LDL, and smoking status. Estimates are the ratios of the conditional expectations of carotid plaque burden comparing the 75th and 25th percentiles of the cytokines. P-values are not adjusted for multiple comparisons.
Visceral adiposity as a possible contributor to IL-17A and IL-5 in PWH
Obesity is associated with higher levels of circulating IL-17 in women (36), and higher proportions of visceral adipose tissue Th17 cells in patients undergoing bariatric surgery (37). We measured visceral adipose tissue mass by DEXA and assessed the relationship with plasma IL-17A in PWH. There was a positive correlation between visceral adipose tissue mass and IL-17A in all PWH (p=0.05) (Figure 5A) that was driven principally by obese PWH (p=0.006) (Figure 5B). There was no correlation between BMI and IL-17A (R=0.08, p=0.5, data not shown). When limited to women, visceral adipose tissue was higher with obesity (p=0.007) but there was no difference in IL-17A (Figure 5C–D). The correlations of visceral adipose tissue mass in obese and non-obese women had positive slopes but did not reach significance (Figure 5E). A similar analysis in the men showed higher levels of both visceral adipose tissue mass (p<0.0001) and IL-17A levels (p=0.04) with obesity (Figure 5E–F). Unlike women, the positive correlation between visceral adipose tissue mass and IL-17A was principally driven by obese men (Figure 5H). After incorporating an interaction term, we did not observe a significant difference in the relationship of visceral adipose tissue and IL-17A by sex.
Figure 5. Visceral adipose tissue mass is correlated with IL-17A in obese PWH.

Correlation plots showing the relationship between visceral adipose tissue mass and IL-17A in all PWH (A) and PWH stratified by obesity (B). Violin plots showing visceral adipose tissue mass (grams) (C) and plasma IL-17A (D) of non-obese and obese women with HIV. Correlation plots showing the relationship between visceral adipose tissue mass and IL-17A in obese and none-obese women with HIV (E). Violin plots showing visceral adipose tissue mass (grams) (F) and plasma IL-17A (G) of non-obese and obese men with HIV. Correlation plots showing the relationship between visceral adipose tissue mass and IL-17A in obese and none-obese men with HIV (H). Wilcoxon test used (C,D, F and G) and Spearman’s rank correlation used for statistical analysis.
Plasma IL-5 was another cluster 2 cytokine that grouped with IL-17. This was not different by obesity in PWH (Supplemental Figure 4A) but was higher in obese men with HIV (Supplemental Figure 4B–C). There was a positive correlation between Il-5 and visceral adipose tissue in obese PWH (Supplemental Figure 4D). Correlation plots stratified by sex showed similar trends as with IL-17A but were not significant (Supplemental Figure 4E–F). We noted a strong correlation between plasma IL-5 and IL-17A in all PWH (Supplemental Figure 4G). Using a multivariable linear regression model in all PWH, adjusted for traditional CVD risk factors, visceral adipose tissue mass as the dependent variable was associated with cluster 2 cytokines including IL-17A, TNF-αR1, and sVCAM-1 that remained significant when controlling the false discovery rate at 0.25 (Supplemental Table 6).
Discussion
In a cohort of PWH on long-term ART and HIV-negative individuals recruited from the same health system, we identified three distinct clusters of cytokines that were associated with measures of early and later stages of atherosclerosis. We found that Th17/ILC3 and Th2/ILC2-mediated immune mechanisms may have a role in endothelial dysfunction and atherosclerotic plaque formation, respectively, in PWH. The Th17/ILC3 effector cytokine IL-17A clustered with markers associated with endothelial activation (sICAM-1 and sVCAM-1), while IL-17A and MIP-1α were both associated with endothelial dysfunction as measured by FMD. In contrast, IL-4, a Th2/ILC2 type cytokine, and IL-10 were associated with carotid plaque burden. These associations were attenuated when HIV-negative controls were added to the models. Taken together, our study suggests that distinct plasma cytokines profiles, likely reflective of underlying cellular immune processes, are associated with different aspects of CVD pathogenesis in PWH.
In a prior analysis from this cohort, IL-17A expression was higher in hypertensive compared to normotensive PWH (16). This is similar to studies in HIV-negative diabetic persons which found higher levels of plasma IL-17A in hypertensive compared to non-hypertensive counterparts (17). IL-17A could exert its effects on endothelial function by several mechanisms including the direct induction of vascular smooth muscle cells to express inflammatory cytokines (17) or by stimulating the expression of inducible nitric oxide (NO) synthase (iNOS) (38). There are three distinct forms of NOS, endothelial nitric oxide synthase (eNOS), neuronal NOS (nNOS), and iNOS. The first two are constitutive and calcium-dependent while iNOS is induced upon infection, is transcriptionally regulated and not calcium-dependent, and is associated with high nitric oxide levels (39). PWH who have concomitant opportunistic infections such as cytomegalovirus have more nitric oxide (40), and nitric oxide synthesis by iNOS has been shown to enhance HIV replication in macrophages (41). Notably, nitric oxide levels drop to lower levels after ART initiation (42). Earlier studies suggested that nitric oxide may be elevated or decreased depending on ART such as protease inhibitors and duration of ART in PWH (43). However, others have reported a significant improvement in endothelial function after initiation of ART on regimens including a nucleoside reverse transcriptase inhibitors (NRTIs) + efavirenz regimen (44), as in our cohort. Future studies are warranted to explore whether there are direct effects of IL-17A on nitric oxide synthesis in PWH.
Higher IL-17A expression in human carotid artery plaques obtained during surgery was associated with vulnerable plaque and more inflammation (45). A mechanistic study in IL17A/apolipoprotein E (ApoE−/−) double deficient mice on a high-fat diet found lower leukocyte infiltration and superoxide production in their aorta compared to ApoE−/− control mice (46). Although plaque burden was not altered in this model, the plaques were more stable and with fewer aortic leukocytes and dendritic cells. This study concluded that IL-17A contributes to vascular inflammation but does not alter plaque burden, suggesting that plasma IL-17A may correlate with plaque instability and needs to be studied in the future. The potential interaction between IL-17A and HIV status and its association with FMD suggests that other indirect mechanisms might be important. One possible explanation could be a role for the Kynurenine (Kyn) pathway. PWH on efavirenz have been shown to have higher kynurenine-tryptophan (KT) ratios due to decreased Kyn pathway activity (47). Kyn pathway activity can suppress IL-17A expression and mediate arterial dilation under conditions of systemic inflammation due to infection (48). Suppression of the Kyn pathway in patients on efavirenz could therefore decrease FMD and increase IL-17A, which might explain some of the differences between PWH and HIV-negative.
Although Th1/Th17 cytokines have previously been associated with the development of atherosclerosis, there is some evidence that Th2-type cells or cytokines may also be important. IL-4 specifically can be pro-atherogenic, has been shown to induce oxidative stress, and may act synergistically with IL-1β and TNF-α to increase VCAM-1 expression (for review(19)). Similarly, we report an association between IL-4/IL-10, with carotid plaque burden in PWH. This suggests a role for Th2-type responses in plaque buildup. We propose the following model (Supplemental Figure 5), that in PWH Th17 cells known to decrease in early stages of infection, rebound with ART. Suppression of the Kyn pathway due to antiretroviral therapy may increase IL-17A expression. Both circulating and tissue-resident Th17/ILCs cells may express inflammatory cytokines such as IL-17A which may increase iNOS expression and in synergy with Th1 cytokines may directly stimulate endothelial cells to express sVCAM-1 and sICAM-1. This may increase the recruitment of CD4+ T helper cells into inflamed endothelium. Th2 cytokines such as IL-4/IL-10 expressed by Th2/ILC2 cells may stimulate B cell responses. CD4+ T cells can also in return stimulate macrophages to express IL-6 and IL1-β which are pro-atherogenic. It is unclear what other function Th2/ILC2 cells may have that could promote plaque growth. T cell activation directly or indirectly by cells other cell types such as neutrophils may also be a differentiating and important factor that may be enhanced in PWH and needs to be further investigated (12).
Study strengths and limitations
Our study has several strengths. The study group included a similar number of men and women with HIV who had no previous history of CVD. We used an unsupervised approach to group plasma cytokines and identify clusters that appear to be associated with endothelial dysfunction as measured by FMD and carotid plaque. Furthermore, all PWH were virally suppressed and on the same non-nucleoside reverse transcriptase inhibitor ART regimen. Limitations of this study include the cross-sectional design which precluded an assessment of temporal changes or causality. Plasma cytokines are also an accessible marker of T cell activity, however, they may more accurately reflect circulating cells as opposed to cells within a vessel wall or atherosclerotic plaque. Future studies should broadly look at circulating and plaque resident cell profiles in the context of CVD pathogenesis. Furthermore, other cells of the immune system including neutrophils, the most abundant leukocytes which may accelerate atherosclerosis by recruiting monocytes but are also involved in repair functions, warrant further investigation (11). Finally, our sample size may have been too small to detect associations between some cytokines and endpoints with greater biologic variability. Larger cohorts with persons on different therapies, including integrase inhibitor-based regimens, are needed.
Conclusion
As PWH survive decades on ART the burden of atherosclerotic CVD is increasing in this population, and inflammation and immune activation are likely contributors. Our study suggests that IL-17A is associated with brachial artery flow-mediated dilation while IL-4 with carotid plaque burden. Further studies are needed to assess the cellular processes driving this cytokine expression and whether addressing innate and adaptive immune cell polarization or activation could serve as a future therapeutic target to reduce the burden of CVD in the HIV population.
Supplementary Material
Financial Support:
This work was funded by NIH grants K23 100700 (JK), R01 DK112262 (JRK and CNW), HL131977 (JB), R56 DK108352 (JRK), the Vanderbilt Clinical and Translational Science Award from NCRR/NIH grant UL1 RR024975, the Vanderbilt Infection Pathogenesis and Epidemiology Research Training Program (VIPER) grant T32 AI007474, CTSA award no. KL2 TR002245 from the National Center for Advancing Translational Sciences, and the Tennessee Center for AIDS Research grant P30 AI110527 (SAM). The funding authorities had no role in study design; data collection, analysis, or interpretation; the decision to publish; or preparation of the manuscript.
Abbreviations and Acronyms
- CCL2/3/4
chemokine ligand 2/3/4
- sCD14
soluble CD14
- sCD163
soluble CD163
- hsCRP
high-sensitivity C-reactive protein
- FMD
brachial artery flow-mediated dilation
- HDL
high-density lipoprotein
- sICAM-1
soluble intercellular adhesion molecule 1
- IL-
interleukin
- LDL
low-density lipoprotein
- MCP-1
monocyte chemoattractant protein 1
- MIP1-α
macrophage inflammatory protein 1-α
- MIP1-β
macrophage inflammatory protein 1-β
- SAA
serum amyloid A
- TNF-α
tumor necrosis factor-α
- TNFαR1/2
TNF-α receptor 1 and 2
- sVCAM-1
soluble vascular cell adhesion molecule 1
Footnotes
Conflict of interest
The authors have no competing interests or financial disclosures to disclose.
REFERENCES
- 1.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol. 2019. 12;16(12):745–59. Epub 2019/06/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francisci D, Giannini S, Baldelli F, Leone M, Belfiori B, Guglielmini G, et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS. 2009. Mar;23(5):589–96. eng. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011. Mar;12(3):204–12. eng. [DOI] [PubMed] [Google Scholar]
- 4.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006. May;42(9):1325–32. Epub 2006/03/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell LA, Rosenfeld ME. Infection and Atherosclerosis Development. Arch Med Res. 2015. Jul;46(5):339–50. Epub 20150521. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol. 2015. Jul;11(7):390–400. Epub 20150331. eng. [DOI] [PubMed] [Google Scholar]
- 7.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994. May;89(5):2462–78. eng. [DOI] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009. Jul;7 Suppl 1:328–31. eng. [DOI] [PubMed] [Google Scholar]
- 9.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 1986. Mar-Apr;6(2):131–8. eng. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988. Aug;72(2–3):135–41. eng. [DOI] [PubMed] [Google Scholar]
- 11.Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020. 06;17(6):327–40. Epub 20200129. eng. [DOI] [PubMed] [Google Scholar]
- 12.Dunsmore G, Rosero EP, Shahbaz S, Santer DM, Jovel J, Lacy P, et al. Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection. PLoS Biol. 2021. 08;19(8):e3001387. Epub 20210819. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010. Apr;464(7293):1367–70. Epub 2010/03/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020. Mar. Epub 2020/03/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999. Jul;145(1):33–43. eng. [DOI] [PubMed] [Google Scholar]
- 16.Masenga SK, Elijovich F, Hamooya BM, Nzala S, Kwenda G, Heimburger DC, et al. Elevated Eosinophils as a Feature of Inflammation Associated With Hypertension in Virally Suppressed People Living With HIV. J Am Heart Assoc. 2020. 02;9(4):e011450. Epub 2020/02/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010. Feb;55(2):500–7. Epub 2009/12/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benagiano M, Azzurri A, Ciervo A, Amedei A, Tamburini C, Ferrari M, et al. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003. May;100(11):6658–63. Epub 2003/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YW, Kim PH, Lee WH, Hirani AA. Interleukin-4, Oxidative Stress, Vascular Inflammation and Atherosclerosis. Biomol Ther (Seoul). 2010. Apr;18(2):135–44. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002. Mar;22(3):456–61. eng. [DOI] [PubMed] [Google Scholar]
- 21.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999. Oct;85(8):e17–24. eng. [DOI] [PubMed] [Google Scholar]
- 22.Shahbaz S, Jovel J, Elahi S. Differential transcriptional and functional properties of regulatory T cells in HIV-infected individuals on antiretroviral therapy and long-term non-progressors. Clin Transl Immunology. 2021;10(5):e1289. Epub 20210526. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, et al. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016. Mar;13(3):167–79. Epub 2015/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 24.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010. Dec;11(12):1093–101. Epub 20101017. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fourman LT, Saylor CF, Cheru L, Fitch K, Looby S, Keller K, et al. Anti-Inflammatory Interleukin 10 Inversely Relates to Coronary Atherosclerosis in Persons With Human Immunodeficiency Virus. J Infect Dis. 2020. 02;221(4):510–5. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelbertsen D, Lichtman AH. Innate lymphoid cells in atherosclerosis. Eur J Pharmacol. 2017. Dec;816:32–6. Epub 2017/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 27.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS. 2016. Jan;30(1):83–91. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T Cell and Macrophage Activation with Arterial Vascular Health in HIV. AIDS Res Hum Retroviruses. 2017. 02;33(2):181–6. Epub 2016/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen PL, Jarolim P, Basaria S, Zuflacht JP, Milian J, Kadivar S, et al. Androgen deprivation therapy reversibly increases endothelium-dependent vasodilation in men with prostate cancer. J Am Heart Assoc. 2015. Apr;4(4). Epub 2015/04/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nohria A, Kinlay S, Buck JS, Redline W, Copeland-Halperin R, Kim S, et al. The effect of salsalate therapy on endothelial function in a broad range of subjects. J Am Heart Assoc. 2014. Jan;3(1):e000609. Epub 2014/01/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009. Nov;50(5):1063–70. Epub 2009/08/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckman JA, Goldfine AB, Dunaif A, Gerhard-Herman M, Creager MA. Endothelial function varies according to insulin resistance disease type. Diabetes Care. 2007. May;30(5):1226–32. Epub 2007/01/29. eng. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996. Dec;78(11):1210–4. eng. [DOI] [PubMed] [Google Scholar]
- 34.Wanjalla CN, Mashayekhi M, Bailin S, Gabriel CL, Meenderink LM, Temu T, et al. Anticytomegalovirus CD4. Arterioscler Thromb Vasc Biol. 2021. Feb:ATVBAHA120315786. Epub 2021/02/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006. Jun;22(12):1540–2. Epub 2006/04/04. eng. [DOI] [PubMed] [Google Scholar]
- 36.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond). 2009. Jan;33(1):151–6. Epub 2008/11/04. eng. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014. Dec;34(12):2637–43. Epub 2014/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miljkovic D, Trajkovic V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004. Feb;15(1):21–32. eng. [DOI] [PubMed] [Google Scholar]
- 39.Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc Res. 1999. Aug 15;43(3):650–7. eng. [DOI] [PubMed] [Google Scholar]
- 40.Torre D, Ferrario G, Speranza F, Orani A, Fiori GP, Zeroli C. Serum concentrations of nitrite in patients with HIV-1 infection. J Clin Pathol. 1996. Jul;49(7):574–6. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blond D, Raoul H, Le Grand R, Dormont D. Nitric oxide synthesis enhances human immunodeficiency virus replication in primary human macrophages. J Virol. 2000. Oct;74(19):8904–12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soccal RM, de Carvalho JA, Bochi GV, Moresco RN, da Silva JE. Nitric oxide levels in HIV-infected, untreated patients and HIV-infected patients receiving antiretroviral therapy. Biomed Pharmacother. 2016. Apr;79:302–7. Epub 2016/03/14. eng. [DOI] [PubMed] [Google Scholar]
- 43.Zangerle R, Fuchs D, Reibnegger G, Werner-Felmayer G, Gallati H, Wachter H, et al. Serum nitrite plus nitrate in infection with human immunodeficiency virus type-1. Immunobiology. 1995. Jun;193(1):59–70. eng. [DOI] [PubMed] [Google Scholar]
- 44.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008. Aug 12;52(7):569–76. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol. 2011. Jan;106(1):125–34. Epub 20101201. eng. [DOI] [PubMed] [Google Scholar]
- 46.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011. Jul;31(7):1565–72. Epub 2011/04/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnittman SR, Deitchman AN, Beck-Engeser G, Ahn H, York VA, Hartig H, et al. Abnormal Levels of Some Biomarkers of Immune Activation Despite Very Early Treatment of Human Immunodeficiency Virus. J Infect Dis. 2021. May 20;223(9):1621–30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Zhao J, Tan L, Huang Y, Li D, Quan S, et al. Bone Marrow Mesenchymal Stem Cells Alleviate Extracellular Kynurenine Levels, as Detected by High-Performance Liquid Chromatography. Inflammation. 2015. Aug;38(4):1450–7. eng. [DOI] [PubMed] [Google Scholar]
- 49.Soghoian DZ, Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines. 2010. Dec;9(12):1453–63. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


