Abstract
Long-term patient engagement and retention in HIV care is an ongoing challenge in South Africa’s strained health system. However, some patients thought to be “lost to follow-up” (LTFU) may have “transferred” clinics to receive care elsewhere. Through semi-structured interviews, we explored the relationship between clinic transfer and long-term patient engagement among 19 treatment-experienced people living with HIV (PLWH) who self-identified as having engaged in a clinic transfer at least once since starting antiretroviral therapy (ART) in Gugulethu, Cape Town. Our findings suggest that patient engagement is often fluid, as PLWH cycle in and out of care multiple times during their lifetime. The linear nature of the HIV care cascade model poorly describes the lived realities of PLWH on established treatment. Further research is needed to explore strategies for reducing unplanned clinic transfers and offer more supportive care to new and returning patients.
Keywords: Engagement in HIV care, clinic transfer, South Africa, loss to follow-up, Qualitative research
Introduction
Retaining people living with HIV (PLWH) on antiretroviral therapy (ART) in continuous clinical care represents a significant and growing challenge for health care systems around the world (1). Continued engagement in HIV care and adherence to ART ensures viral suppression, improves treatment outcomes, and reduces onward transmission of HIV, thus helping to curb the HIV epidemic (1, 2). PLWH are therefore expected to make a lifelong commitment to engagement in care. However, in low- and middle-income countries, disengagement is common as patients often face significant structural and individual barriers to care that prevent ongoing engagement (3–5). In South Africa in 2020, where the HIV treatment program is the largest in the world only 72% [95% CI: 48 – 93] of PLWH were on treatment, and only 66% [95% CI: 43 – 84] of PLWH were virally suppressed, illustrating a notable challenge in the context of a maturing epidemic (6).
The HIV care cascade has been used to conceptualize and measure patient engagement in care, including peoples’ interactions and movements within the HIV healthcare system (7–9). The care cascade framework, adopted by global bodies like UNAIDS, depicts a sequential stepwise process through which patients “progress” (7–10). The dominant paradigm of a successfully engaged patient is to follow this ideal treatment course, moving seamlessly through each stage of the care cascade until viral suppression is achieved (11). However, efforts to measure a patients’ engagement experience are limited by what can practically be measured, which is usually whether or not the patient attends a clinical appointment or their viral load. As a consequence, we have come to think of engagement as a binary outcome: successful navigation of the cascade on one hand or disengaged on the other. Researchers have tracked outcomes of patient’s loss to follow-up (LTFU) after they have disengaged (12–16). A systematic review estimated that on average of 18.6% of patients who have been classified as LTFU actually transferred clinics to receive care and treatment at another clinic (13). Another recent study in Cape Town, South Africa found that 31% of participants transferred at least once over a median of 32 months of follow-up on ART (17).
Clinic transferring refers to when a patient receiving care at one clinic subsequently moves to a different clinic. In South Africa, clinic transfers can be initiated in two ways: either through formal channels when the patient requests official transfer documentation, or “silently,” when a patient independently moves to a new facility without notifying either the old or new clinic of the move (16, 18). Studies suggest that the number of clinic transfers has grown with the continued expansion of ART coverage and decentralization of clinics, giving patients more options to choose where they want to receive care (13). Most healthcare systems are not equipped to adequately track patient movements from one facility to another, making it difficult to determine if a patient has transferred clinics or is truly out of care (15). Clinic transferring is thus problematic as health systems may over-report both the number of patients who have initiated ART and those that are LTFU (12). In addition clinic transferring can potentially disrupt continuity in care, leading to impaired health outcomes, particularly when breaks in care are lengthy (19).
Despite the growing concern and compelling problems of clinic transfers, there have been limited qualitative studies conducted to examine the phenomenon of clinic transferring and its relationship to patient engagement as a singular focus. Therefore, we conducted a qualitative study that sought to explore the relationship between clinic transferring and long-term patient engagement and motivations for clinic transfers in treatment-experienced PLWH in Gugulethu, Cape Town, South Africa.
Methods
Study Setting:
This study was conducted in Gugulethu, a peri-urban township located 15km from Cape Town; the majority of residents are isiXhosa speaking and of low socioeconomic status (20). The HIV prevalence in Gugulethu is approximately 27% (21), with most PLWH seeking health services and ART at fee-free local public health services. However, many of these clinics are overburdened and low-resourced, making it difficult to track patients who are lost to follow up (22).
Data collection was conducted in partnership with a community organization, Movement for Change and Social Justice (MCSJ). Established in 2016, MCSJ is primarily focused on health issues, including access to and the quality of healthcare services and issues around the high rates of HIV, TB, and chronic diseases in the area (23). Members of MCSJ are considered community activists and are well known and respected throughout Gugulethu, surrounding areas and in HIV care clinics. Three MCSJ members volunteered to assist with study recruitment and act as translators when needed during interviews.
Population and Recruitment:
A total of 19 PLWH who self-identified as clinic transferring since starting ART were interviewed for this study. Inclusion criteria for these qualitative interviews were: 1) age 18 or older, 2) reported previously being linked to HIV care services, which we defined as attending the first clinic attendance date after a positive diagnose within a time period of 3 months after diagnosis (25), 3) had previously at any point started ART, and 4) had clinic transferred either formally or silently at least once since starting ART. Inclusion criteria was ascertained by asking potential participants and then again by having them complete a brief questionnaire before the interview began. We used purposive sampling and a snowball technique to identify and recruit participants (25, 26). MCSJ staff members presented an overview of the study at a monthly community meeting. Interested community members were encouraged to contact an MCSJ staff member for initial eligibility screening and to schedule an interview. In addition, known PLWH who met the inclusion criteria were approached by an MCSJ staff member and asked if they would be interested in participating in the research study. At the end of all interviews, each participant was asked if they knew anyone else who met eligibility requirements who would be interested in the study. MCSJ staff would then contact potential participants to assess interest and eligibility for the study. Of all potential PLWH who were approached to be interviewed, no one declined to participate. We sought to interview enough participants in which thematic saturation could be achieved. Concepts and topics began to repeat in the last few of our 19 interviews, suggesting thematic saturation occurred (27).
Data collection:
In-depth, semi-structured interviews were conducted in order to elicit in-depth responses and to identify unexpected areas that can potentially lead to practical solutions and explain the participants’ experiences. The interview guide was developed using a modified funnel approach to help to build rapport with the participants and to limit social desirability bias (28). We piloted the draft interview guide with a person who met all inclusion criteria and modified the protocol accordingly to include additional questions about healthcare worker relationships with patients. The interview guide focused on five domains: 1) overall patient experience receiving HIV care, 2) motivation and reasoning for switching clinics, 3) the steps to switching clinics, 4) the process of choosing a new clinic, and 5) the experience at the new clinic.
Interviews were conducted by the first author and assisted by one of the three MCSJ staff members who were trained in qualitative interviewing procedures and translation methods. Participants were interviewed in English or in isiXhosa, the local language. When isiXhosa was chosen, the verbatim translation to English was offered by the MCSJ staff member. The interviews were audio-recorded after permission was obtained from participants (one participant declined to be audiotape-recorded; however, interview notes were included in the analysis). Each interview session was conducted in a private room at the MCSJ office or in a convenient place of the participant’s choosing. The interviews lasted a median time of 50 minutes [IQR 41, 63].
Data Analysis:
All 19 interviews were transcribed and checked for accuracy by the first author. Transcription files were uploaded into NVivo software for qualitative data management and analysis. The transcripts were analyzed using inductive and deductive principles of thematic analysis to identify and explore patterns of meaning across the interviews (29). The research team developed an initial thematic coding framework based on the structured questions from the interview guide. Using the initial coding structures, the first author coded the transcripts, and modified the scheme until no new codes were identified. The team met periodically to assess the consistency of the coding and discuss and resolve all coding discrepancies (30). Next, applying principles of grounded theory, themes were identified by further refining codes and reduced to thematic categories by noting emerging patterns within and throughout the interviews (31). Through a series of meetings, all the authors reviewed the codes and thematic results, which resulted in additional queries, discussion, and further refinement of thematic results to make sure all findings were valid and complete. An audit trail was utilized to show and keep track of analytical steps and the process of decision making (32). It was also used to note the first author’s reflexivity and positionality to the data (32).
Ethics:
This study is part of a larger study which seeks to understand the HIV care cascade among people in Gugulethu, South Africa. This study was approved by the Human Research Ethics Committee (HREC) at the University of Cape Town and the IRB at Brown University. For this analysis, written and verbal informed consent, administered in either English or in isiXhosa, was obtained from all participants before beginning data collection.
Results
Of the 19 participants who were enrolled in the study, ten were female, and nine were male (Table 1). Participants had a median age of 41 years [IQR 32–48] and had been diagnosed with HIV for a median of 15 years [IQR 8–17]. Approximately a third (31.6%) of participants were currently disengaged from care, which we defined as not receiving any clinical, laboratory or pharmacy services for at least six months. Seventeen (89.5%) of the participants had transferred clinics more than once, with a median of four clinic transfers [IQR 3–6] per participant. Ten (10/19) of the participants had transferred clinics officially once. Of those who transferred officially, three (3/10) participants were ordered by the clinic for specific medical care at another facility, six (6/10) participants requested a transfer for personal reasons and one (1/10) participant requested an official transfer due to moving home locations. In total, only two (2/19) of the participants had exclusively experienced an official transfer, while seventeen (17/19) of the participants had attempted to transfer silently at least once. However, of those who attempted to transfer silently, four (4/17) were unsuccessful and were identified as needing to return to their original clinic to obtain officially transfer documentation before changing clinics. Of the four participants who were unable to transfer silently, all were currently disengaged from care at the time of the interview.
Table 1:
Characteristics of Study Population
| Characteristics | n (%) or median (IQR) |
|---|---|
| Gender | |
| Male | 9 (47.4%) |
| Female | 10 (52.6%) |
| Age (years) | 41 [IQR 32–48] |
| Ethnicity | |
| Xhosa | 19 (100%) |
| Marriage Status | |
| Single | 11 (57.9%) |
| Married | 8 (42.1%) |
| Employment Status | |
| Formal Employment | 4 (21.1%) |
| In-Formal Employment (e.g., street vendor) | 7 (36.8%) |
| Unemployed | 8 (42.1%) |
| Number of Years Since Diagnoses | 15 [IQR 8–17] |
| Current Clinical Engagement Status | |
| Engaged and Retained in care | 13 (68.4%) |
| Disengaged from care | 6 (31.6%) |
| Clinic Switched More Than Once | 17 (89.5%) |
| Number of Clinic Switches | 4 [IQR 3–6] |
| Participants Who Transferred Officially | 10 (58.8%) |
| Participants Who Attempted to Silent Transfer at Least Once | 17 (89.5%) |
Our results indicated that participants reported cyclical engagement in HIV care, using clinic transferring as a mean to re-enter into the health care system after less than six months out of care or a period of disengagement. We identified five themes that represent various time points in a patient’s cycle of engagement (Figure 1). These themes include persistent challenges that participants faced to remain in care, followed by the internal and external stigma participants faced when they fail to live up to the ideal patient role, which often leads to their disengagement or some time out of care. We then explored the pull factors that brought participants back into formal care and discuss how clinic transferring is used to re-enter into the formal health system. We conclude with reasons participants reported for remaining in care and at their current clinic. Overall, there were no significant differences between demographic subgroups of patients and their clinic transferring experience.
Figure One:
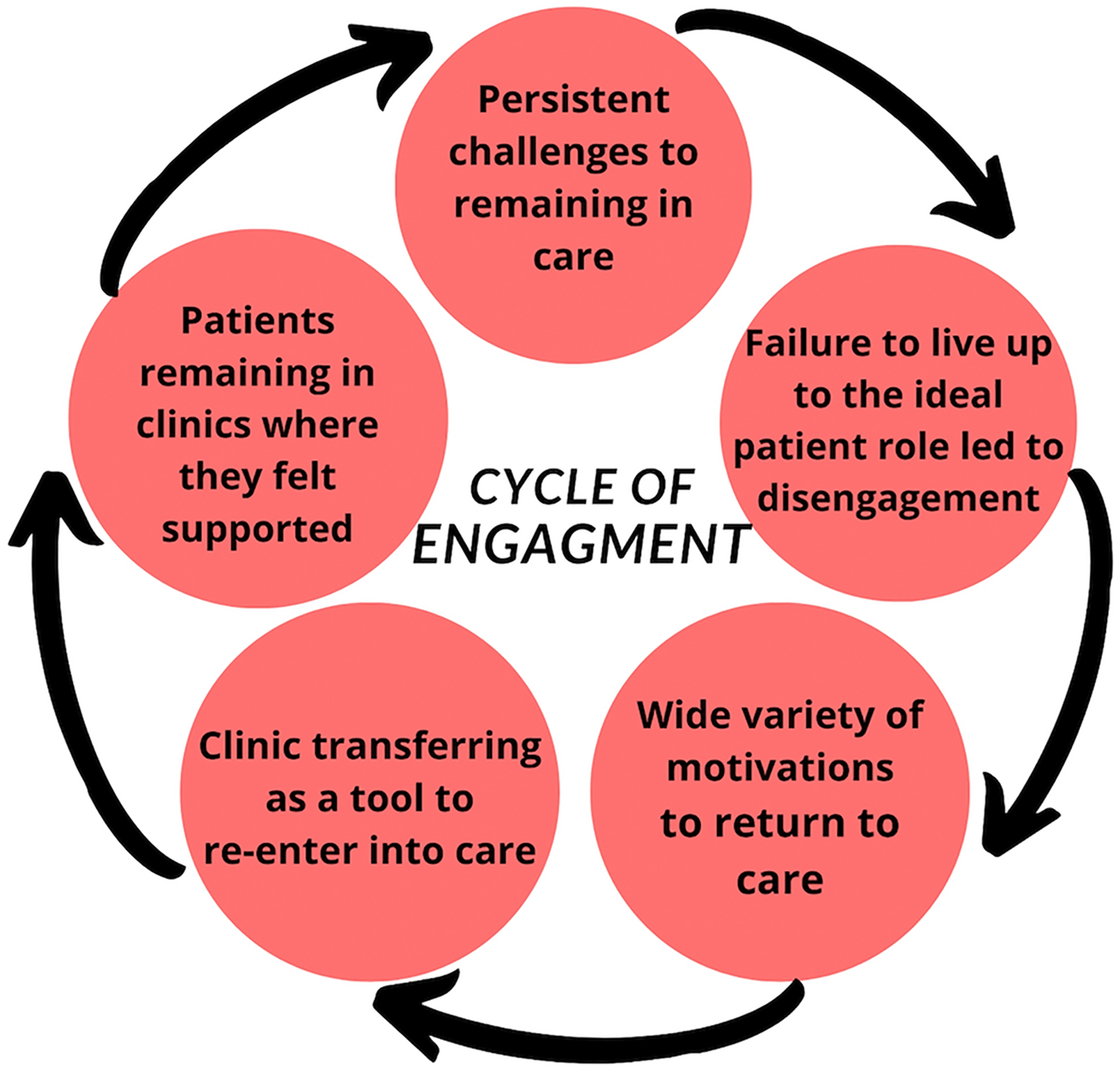
Hypothesized Cyclical process of engagement and re-engagement in HIV care
In our findings, we focus on describing the lived experiences of participants who had attempted to transfer silently, most often this occurred after a time out of care (less than six months) or a period of disengagement. While we want to acknowledge there is a plurality of pathways to clinic transfers, almost all the participants (17/19) had reported cyclical engagement in HIV care at least once in their lifetime.
Theme 1: Participants faced persistent challenges to remain in care
Among our treatment-experienced population, all participants discussed external barriers that interfered with maintaining their HIV care and engagement. Participants reported being well-adjusted to their treatment at one-point but then experiencing unforeseen challenges that endangered their engagement. For example, fundamental needs, such as the need to earn income, often interfered with HIV care engagement. One participant explained his need to search for informal work to support himself instead of going to his appointment:
“Sometimes you haven’t got support even in your homes, sometimes you haven’t got support even in your family, and you don’t work, there’s no jobs, and you can’t eat tablet (ART) without food, there’s nothing that you can do about that but go get a job… not go to your date (clinical appointment).”
(Male)
Other participants added that working as migrant laborers or changes in family responsibility also made regular clinic attendance difficult. Most participants noted not being able to attend clinic appointments at some point due to lack of money for transport to and from the clinic. Others seemed to weigh the decision whether or not to save their money that would be used for transportation for other important things such as food. As one participant explained,
“I needed more money for transportation because I was still taking the medication that side in Khayelitsha (neighborhood near Gugulethu) … sometimes I don’t have (money for) transport…. (when I would find) transport fare, then when I goes there (to the clinic), they said, “No, your date has passed.” Maybe, the date was last week, so they gave me another date. So you see now, that experience is very frustrating, because there’s no doctor. Now the 20 Rand that I had, I rather save it for food maybe or save it for something for my child, rather than to just go and know that I might get medication, or I might not get it.”
(Male)
As adherence to HIV treatment is a lifelong commitment, PLWH over their lifetime felt the expectation to continue their engagement even when unforeseen challenges arise.
Theme 2: Failure to live up to the ideal patient role often led to disengagement
Frequently, the barriers described in theme one could lead to participants missing appointment dates or not adhering to medications according to directions. Participants described their desire to live up to the ideals of the “perfect patient” for themselves and as a means to meet nurses’ expectations. Participants reported feeling like they failed at being a “perfect patient” when they missed appointments or forgot to take their medication, which led to a perception by staff they were unmotivated. Repeatedly, participants cited feelings of “shame”, “embarrassment”, or “guilt” when they were unable to keep their clinic appointments.
“After I defaulted, I was afraid to go back there because I know all the nurses and the people, that staff who’s there. They know I defaulted, so I didn’t go back there because I was ashamed.”
(Male)
Additionally, participants conveyed how their engagement experience has been more challenging compared to others who attend the same clinic. There was widespread agreement that the typical patient was someone who is well engaged, adherent, comes to all clinical appointments, is educated about HIV and does not struggle taking their medication.
After missing a clinical appointment, participants were met by “unwelcoming” healthcare staff. All participants reported instances of being shouted at or punished, both implicitly and explicitly, by healthcare workers.
“So when you miss a date, those doctors here in our location, they start to swearing you…shouting, swearing at you in front of other people. That is nothing. That thing make your dignity like this (foot stomps the ground and smooshes the floor).”
(Female)
Several participants reported that healthcare workers would publicly disclose confidential information such as a patient’s HIV status, STI status, or non-adherence in spaces like the clinic waiting area. This often culminated in an already struggling patient disengaging and leaving the health system entirely as described by this male participant:
“…That’s why I’m not taking my ARVs, because now I had to pull myself out from the system because of the way they (healthcare workers) treat me, I don’t feel like a man, I’m nothing.”
(Male)
Participants also reported being punished by healthcare workers in other ways. Common punishments included making participants wait long hours, sometimes to be told to return the next day, removal from adherence clubs and “misplacement” of patient folders which led to further delays in care. This female participant described her previous clinic experience after she returned to her clinic after ten months of not attending.
“I would spend the whole day at the clinic and wake early. I would go and wait and wait and wait and I got nothing. I’ll sit the whole day only to be shouted at to come back tomorrow. I come back tomorrow and same thing. They wouldn’t give me my treatment. No man it was too much for me. I spent the whole week going up and down to the clinic…. until they said they lost my folder. So, I decided I am just going to sit down (default).”
(Female)
Not attending a clinic as a result of poor treatment by healthcare workers is not just a problem of avoidant behavior but instead the result of internalized stigma experienced by PLWH.
Theme 3: Motivations to return to care
Participants reported varied lengths of time spent out of care. Some participants reported being out of care for a couple of weeks while others fully disengaged for over 6 months to some waiting years to return to a clinic. Among these participants, there were three distinct factors that motivated a participant’s readiness to return to care: illness, social support or pressure, and the internal sense of responsibility for one’s health.
While most tried to take extras measures to maintain their health when not attending a clinic such as eating healthy or exercising, some became ill with tuberculosis (TB) or noticed a deterioration in their health, leading them back into the health system.
“I go to the mirror to look myself, now I see the change. I see the change, I’m growing thin, all of those things. I’ve got a lot of things, breathing maybe heavy… I knew it was time (to return to care).”
(Male)
Others relied on or were convinced to return to the clinic by family, social, or community support. Support systems that influenced participants to return to care included daily check-ins or reminders from friends and family and sometimes transportation to and from the clinic:
“…(my friend will say) ‘Are you fine? Is the medication right for you? Did you take it? Should I remind you by phone? Or what?’ … That will give you courage to go back (to the clinic) and continue.”
(Female)
Participants viewed their support system as extremely helpful, giving them a safe space to talk about their struggles and successes with treatment. This man spoke about confiding in his sister about his plan to not return to the clinic. However, she was able to convince him to return to care for her sake so that he could continue to be there for their family:
I did tell my sister, I did tell her I don’t want to go (back) to (clinic name), I’d rather die. But my sister said no… That I need to keep going.”
(Male)
Nearly all participants recognized the importance of taking responsibility for managing one’s health. Regularly, participants shared the perception that if no one else is going to look after their health, they must be the ones to do so. Generally, in these statements, participants showed qualities of agency and self-efficacy that led to the perceived capability of returning and remaining in care.
“So I told myself it is the right time for me now. To stop playing hide and seek. To stop hiding in the bush. Because it’s my life. It’s my life. I have to go (to the clinic), and I have to be responsible for my life.”
(Female)
“You see? That is my medication. I must eat my medication because if I don’t eat that, when I don’t treat myself, who is going to treat myself? I treat myself so that I can be healthy.”
(Male)
Overall, there was not one specific motivating factor that brought participants back into care; instead, there was more likely to be a culmination of events and multiple factors that led participants to return to formal care.
Theme 4: Clinic transferring was used as a tool to re-enter into care
When deliberating the best way to return to care, many participants thought about how to escape the discrimination of being labeled by healthcare workers as a “difficult patient” and agreed that clinic transferring was the best way to do this. In order to be seen as a “new patient” in a clinic, multiple participants used informal silent transferring methods to attend a new clinic. Participants used a variety of tactics to return to care, sometimes using a fake name, a fake address or retesting to “find out” about their HIV status:
“I went and retested at a new clinic and didn’t tell them of my status. I pretended that I was new and needed help. I don’t want to lie. I did give them a fake address that was in Gugulethu because I was scared, they would send me back to (previous clinic name).”
(Female)
“I have changed my name when I went to the clinic, they asked me for my ID but I told them “I don’t have an ID.” And the clinic say, “Okay. We’ll assist you, but please, go and apply for ID.” And then when I arrive, there’s no more (participant’s real name). I was now (participant’s fake name).”
(Male)
Only about half of participants were aware that their official transferring documents were required for a clinic switch. Among those who did not know about the transfer letter requirement or did not request one, four were “caught” by a new computer system that used a patient’s names, date of birth, and ID number to record a patient’s history. Participants were then instructed to go back to their original clinic to retrieve the documentation in order to register to attend the new clinic. While some participants were able to successfully navigate this task, there were a few left in limbo, feeling unable to receive care at any clinic.
“I went to (new clinic name), she said to me, “No sis, I can’t help you. Because on the system, it’s written, you belong to (original clinic name). So, you have to go back to (original clinic name).” I stayed for six months without my treatment…I was running out of ideas so I thought I would try to go to (new clinic name) again, but the same nurse saw me. “Sis, how many times must we tell you that you mustn’t come back here. Sis, just go back to the (original clinic name). Please. Just go. I’m busy. I’m very busy.” You know, it’s like that. Then I go, then I went out. Because I don’t have a choice.”
(Female)
Barriers to returning to their previous clinic to retrieve a transfer letter include fear of repercussions from previous clinic staff and not being able to afford transport money back to their previous clinic. The woman quoted below was working as a migrant farmer when she began taking her medication. She currently cannot afford the transport money back to retrieve a proper transfer letter but claims no clinic in Cape Town will treat her without it. She has tested at seven different health and mobile clinics trying to gain access back into treatment.
“You know, it is painful and sad in our communities, because when it comes to people that has default, they take it as if they don’t care about themselves. Now in the case of the me I am willing to be on medication, but they are blocking me…. Where must I go? What must I do? Because (clinic name) doesn’t want to take me. (Clinic name) also. So, what must I do? I really don’t know… because I’m lost. I’m really lost. What must I do?”
(Female)
Participants also mentioned alternative tactics that they have employed to navigate re-engagement in care. One strategy by participants who knew about the need for a transfer letter policy was to lie to healthcare workers about the reason for leaving. Six participants said it was easier to acquire a transfer letter if you lied and told a healthcare worker that you were leaving Cape Town and moving to another province.
“So I decide to ask the transfer to go to another clinic. They asked me, “Where are you going now?” I said, “I’m going to Eastern Cape. I didn’t tell them I am going to Khayelitsha (a community nearby) … They would shout at me if they knew the truth”
(Female)
Three more participants also noted the best way of procuring a transfer letter was going straight to the doctors, who were reported as more understanding and helpful than nurses.
“So doctors they don’t have a problem. There is only sisters(nurses) they have a problem. If you want to transfer, you must go to the doctor new to the system because if you go to the sister, they said you must wait… But if you go to the doctor, I need the transfer I’m going now… They want you to take your tablets.”
(Female)
Participants frequently discussed how returning to care at a new clinic was a way of “starting fresh” or having a “do-over” in their engagement. As many participants performed tactical tricks to restart as a new patient, such as giving a fake name, clinic transferring was also a psychological journey of recreating their identity as a patient.
“I was (re)-tested in (clinic name). As a new patient. As a new person…. (I gave) them another address and another name.”
(Female)
In this study, clinic transferring was a tool used by participants to gain access to a new clinic. Not only was it a way to potentially avoid judgment from healthcare workers, but it was also expressed by participants as a fresh start to their engagement.
Theme 5: Participants reported remaining at a clinic where they felt they belonged
In total, seven participants said they were very happy with their current clinical care and engagement and had no future plans to change clinics. In general, participants were more content in a clinic where they felt they were receiving better care than in previous clinics and were supported by the healthcare workers.
“There is a lot of care here. They are professionals.”
(Male)
Participants regularly equated good care with positive relationships between patients and healthcare workers, mentioning provider-patient communication, trust, confidentiality, respect and understanding as key symbols of the strength of this relationship.
“They know how to talk to each other; they know how to talk to me. They don’t shout. They believe what I say.”
(Female)
Overall, participants reported that they approach their care one day at a time, often relaying the importance of understanding that their HIV care engagement is a lifelong process that will continue to evolve over time. Even participants who had been at their clinic for years and reported being virally suppressed stated there was always potential they could transfer clinics again.
Discussion
This study examined the experiences and motivations of PLWH who transferred clinics after initiating ART. The findings demonstrate how, for this patient population, participants’ descriptions of their engagement and retention in HIV care do not map neatly onto the traditional HIV care cascade. Instead, we found participants’ engagement in care to be more fluid and dynamic than predicted by the care cascade model in which participants used clinic transferring as a tool in creating a cyclical process of engagement and re-engagement in HIV care over time in order to navigate the health system (33–35).
Traditionally, the HIV care cascade has represented the health system’s perspective on HIV care, but it is limited in describing the actual lived realities of PLWH. While the care cascade may need to be an oversimplification in order to monitor HIV treatment programs at a population level, the cascade framework has expanded its utility from a monitoring tool to a policymaking framework. The logic of the care cascade has thus provided a way to conceptualize, design, and implement HIV-related service delivery (36). Yet, our findings have highlighted several limitations of the cascade, including its failure to acknowledge the unpredictable nature of longitudinal adherence, its linear and unidirectional movement of steps, and opaque details of how to re-enter into care following any period of disengagement. When PLWH could not meet the expectation of the care cascade, our study participants navigated a new path by using clinic transferring as a way to restart their engagement.
ART has continued to improve life expectancy for maturing PLWH, who, in some cases, have been in care for over ten years (37). Despite this, long-term patient engagement and adherence remain a significant challenge for health systems (38). Our study participants were, on average, diagnosed with HIV 14 years ago, of which all had reported previously been linked to care and adherent to treatment, yet reported fluctuations in their engagement since initiating ART. This finding is similar to other studies that have demonstrated the complexities of long-term HIV engagement and its ongoing challenge to remaining in care (39). While the care cascade includes viral suppression as the final step, it is conceptualized as adherence, and it’s limited to capturing a lifelong process cross-sectionally, even though patients will spend the rest of their lives in HIV care. Furthermore, the HIV care cascade assumes a linear and unidirectional movement of steps, meaning that one can only progress in one direction, one step at a time (10). Therefore, the paradigm fails to adequately capture or describe people whose engagement changes over time: individuals who may have previously progressed through the cascade but then began to struggle, needed to backtrack, or restart their treatment. As such, pathways into and out of each step are also not clearly expressed, making the cascade opaque and insufficient to clarify crucial details of its actual utilization of health services (11).
Yet, many participants described the care cascade as the “typical” pathway for PLWH and reported feelings of “shame” and “embarrassment” when this ideal could not be met for their own engagement. Our findings are similar to that of other studies in which participants attach a moral framework to engagement, creating a dichotomy between what is a “good” patient and a “bad” patient (40). When the expectation of engagement from healthcare workers or oneself could not be met, participant decisions to engage or not in HIV care were guided by an “all or nothing” approach: that as a patient, you either perform the role of the good patient and go to every clinical appointment, or you stop going to the clinic altogether.
Similar to Ware and colleagues, we found our participants showed a reluctance to return to care at their original clinic after a period of disengagement due to the failure to live up to engagement expectations and, as a result, experienced this parallel of self-disappointment and anticipated negative healthcare worker attitudes or behaviors (4). For these participants, the health system was difficult and challenging to return to after a period of disengagement. When participants were ready to return to care, they frequently used clinic transferring as a tool to re-enter into a new clinic. Not only was it used as a tactical method to gain access to care at a new clinic, but it also was experienced as an evolution of oneself as a patient, a chance at a fresh start.
Recommendations:
Participants in this study were creative and resilient, navigating a health system that was not necessarily designed to meet all of their needs. As HIV care is shifting to a chronic disease model, there is an urgent need to focus more heavily on long-term patient engagement and re-engagement. Future policies and interventions need to allow for more flexible models of care that could enhance patient and healthcare worker relationships, as well as normalize challenges to engagement. Historically, health systems have placed an emphasis on linkage to care and treatment initiation but have not been designed to “welcome back” patients who are re-engaging. There is potential to shift how clinics and healthcare workers respond to re-engaging patients by addressing barriers that were associated with their interruption from care in ways that are more supportive and less punitive. Interventions should continue to address how to best educate and change healthcare workers’ communication techniques, body language, and understanding of patients by giving healthcare workers new skills such as motivational interviewing (41).
We also recommend refining policies to ensure that patients can quickly and smoothly access care in multiple locations within and beyond provincial boundaries. This can be accomplished by integrating health information systems more effectively allowing for patient information to be accessed across clinical sites. “Health passports” could enable patients to receive care across facilities. At a minimum, official transfer letters should be redesigned to make the process more accessible and easily understood by patients, so that these transfer documentations become a facilitator, not a barrier to re-engagement in care.
Limitations:
This study offers important patient perspectives about long-term engagement in care. However, it also has limitations. Most notably, healthcare workers’ perspectives are not captured by the focus of this study. Future research should aim to include the perspectives of healthcare workers examining the knowledge, attitudes, and behaviors towards patients who have transferred clinics and those who have disengaged from care. Additionally, participants represented a convenience sample of which some who were recruited were either involved with or were closely associated with MCSJ, a health justice NGO. As such, these participants may differ in some important ways compared to others’ HIV positive clinic transferring experiences.
Conclusion
In exploring methods and motivations of clinic transfer among South Africans who have been living with HIV for more than a decade, we described a more comprehensive and explanatory model of HIV patient engagement. We identified five themes that relate to patients’ cycle of engagement: 1) persistent challenges to remaining in care, 2) failure to live up to the ideal patient role, 3) wide variety of motivations to return to care, 4) clinic transferring as a tool to re-enter care, and 5) patients remaining in clinics where they felt supported. This study found that patient engagement is often fluid as PLWH cycle in and out of care multiple times during their lifetimes using clinic transferring to maneuver and navigate a complicated and sometimes punitive health care system. In all, participants expressed that unlike the linear care cascade suggests, there is no final step when you are living with HIV. Even when you reach viral suppression, the challenge of living with HIV does not dissipate; instead, it’s a lifelong journey that embodies daily obstacles and onerous decisions to stay in care or not. Further research is needed to explore strategies for reducing unplanned clinic transfers and offer more supportive care to new and returning patients.
Acknowledgments:
The authors would like to thank all of the participants who were interviewed and told their story for this study. The authors are also grateful to the staff members of MCSJ: Mandla Majola, Nduiso Khosified Mzazi, Ncedisa Qabazi, and Zukie Madulini for their support and contribution to this study.
Source of financial support:
This work was supported by grants awarded jointly by the National Institute of Mental Health and the South African Medical Research Council (grant number R01 MH106600), the National Institute of Mental Health (R00MH112413), and the Fogarty International Center and the National Institute of Mental Health (D43 TW011308). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or the South African Medical Research Council. This work was also partially supported by Brown University Framework in Global Health Scholarship and the Brown University travel fund.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of interest: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the IRBs at the University of Cape Town and Brown University (IRB0001938, Co-PIs: Colvin and Lurie).
Consent to participate: Written and verbal informed consent, administered in either English or in isiXhosa, was obtained from all participants before beginning data collection. All data were anonymized.
Consent for publication: Consent to publish has been granted by all authors.
Availability of data and material:
The data generated and used during the current study is available from the lead author on reasonable request.
References:
- 1.Hannaford A, Moll AP, Madondo T, Khoza B, Shenoi SV. Mobility and structural barriers in rural South Africa contribute to loss to follow up from HIV care. AIDS Care. 2020. Aug 28;1–9. Available from: 10.1080/09540121.2020.1808567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nosyk B, Lourenço L, Min JE, Shopin D, Lima VD, Montaner JSG. Characterizing retention in HAART as a recurrent event process: insights into “cascade churn”. AIDS. 2015. Aug;29(13):1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frijters EM, Hermans LE, Wensing AMJ, Devillé WLJM, Tempelman HA, De Wit JBF. Risk factors for loss to follow-up from antiretroviral therapy programmes in low-income and middle-income countries. AIDS [Internet]. 2020;34(9). [DOI] [PubMed] [Google Scholar]
- 4.Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1): e1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eshun-Wilson I, Rohwer A, Hendricks L, Oliver S, Garner P. Being HIV positive and staying on antiretroviral therapy in Africa: A qualitative systematic review and theoretical model. PLoS One. 2019;14(1):e0210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. South Africa Country Factsheet: 2020. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica
- 7.Paparini S, Rhodes T. The biopolitics of engagement and the HIV cascade of care: a synthesis of the literature on patient citizenship and antiretroviral therapy. Critical Public Health. 2016;26(5):501–17. [Google Scholar]
- 8.Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: Updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2013. Oct;57(8):1164–71. [DOI] [PubMed] [Google Scholar]
- 10.Powers KA, Miller WC. Critical Review: Building on the HIV Cascade: A Complementary “HIV States and Transitions” Framework for Describing HIV Diagnosis, Care, and Treatment at the Population Level. J Acquir Immune Defic Syndr. 2015. Jul;69(3):341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallett TB, Eaton JW. A Side Door Into Care Cascade for HIV-Infected Patients? JAIDS J Acquir Immune Defic Syndr. 2013;63. [DOI] [PubMed] [Google Scholar]
- 12.Fox MP, Bor J, Brennan AT, MacLeod WB, Maskew M, Stevens WS, et al. Estimating retention in HIV care accounting for patient transfers: A national laboratory cohort study in South Africa. PLoS Med. 2018;15(6):e1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):365–79 [DOI] [PubMed] [Google Scholar]
- 14.Dalal RP, MacPhail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47(1):101–7. [DOI] [PubMed] [Google Scholar]
- 15.Lurie MN, Kirwa K, Callaway J, Cornell M, Boulle A, Bengtson AM, et al. Quantifying the HIV treatment cascade in a South African health sub-district by gender: retrospective cohort study. Trop Med Int Health. 2020;25(2):186–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2016;62(7):935–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengtson AM, Espinosa Dice AL, Kirwa K, Cornell M, Colvin CJ, & Lurie MN (2021). Patient Transfers and Their Impact on Gaps in Clinical Care: Differences by Gender in a Large Cohort of Adults Living with HIV on Antiretroviral Therapy in South Africa. AIDS and Behavior. 10.1007/s10461-021-03191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey MD, Omollo D, Salmen CR, Mattah B, Blat C, Ouma GB, et al. Movement between facilities for HIV care among a mobile population in Kenya: transfer, loss to follow-up, and reengagement. AIDS Care [Internet]. 2016/05/04. 2016. Nov;28(11):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tweya H, Feldacker C, Estill J, Jahn A, Ng’ambi W, Ben-Smith A, et al. Are They Really Lost? “True” Status and Reasons for Treatment Discontinuation among HIV Infected Patients on Antiretroviral Therapy Considered Lost to Follow Up in Urban Malawi. PLoS One. 2013. Sep 26;8(9):e75761. Available from: 10.1371/journal.pone.0075761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strategic Development Information and GIS Department (SDI&GIS). City of Cape Town – 2011 Census Suburb Gugulethu. 2013. Available from: https://resource.capetown.gov.za/documentcentre/Documents/Maps%20and%20statistics/2011_Census_CT_Suburb_Gugulethu_Profile.pdf
- 21.Busgeeth K, Rivett U. The use of a spatial information system in the management of HIV/AIDS in South Africa. Int J Health Geogr. 2004;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan SR, Oosthuizen C, Stinson K, Little F, Euvrard J, Schomaker M, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study. PLoS Med. 2017;14(11):e1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colvin CJ, van Pinxteren M, Majola M, Leon N, Swartz A, Mbokazi N, et al. Fostering a healthy public for men and HIV: a case study of the Movement for Change and Social Justice (MCSJ). Palgrave Communications. 2020;6(1). [Google Scholar]
- 24.Magnolini R, Senkoro E, Vanobberghen F, Weisser M. “Linkage to care” among people living with HIV - definition in the era of “universal test and treat” in a sub-Sahara African setting. Swiss Med Wkly. 2021;151(December 2020):w20535. [DOI] [PubMed] [Google Scholar]
- 25.Tongco MDC. Purposive sampling as a tool for informant selection. Ethnobotany research and applications. Ethnobot Res Appl. 2007;5:147–58. Available from: www.ethnobotanyjournal.org/vol5/i1547-3465-05-147.pdf [Google Scholar]
- 26.Noy C. Sampling knowledge: The hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327–44. [Google Scholar]
- 27.Guest G, Bunce A, Johnson L. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field methods. 2006;18(1):59–82. [Google Scholar]
- 28.Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: Developing best practices based on science and experience. Qual Life Res. 2009;18(9):1263–78. [DOI] [PubMed] [Google Scholar]
- 29.Vaismoradi M, Jones J, Turunen H, Snelgrove S. Theme development in qualitative content analysis and thematic analysis. JNEP. 2016;6(5):100–10 [Google Scholar]
- 30.MacPhail C, Khoza N, Abler L, Ranganathan M. Process guidelines for establishing Intercoder Reliability in qualitative studies. Qual Res. 2016;16(2):198–212. [Google Scholar]
- 31.Fereday J, Muir-Cochrane E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. Int J Qual Methods [Internet]. 2006. Mar 1;5(1):80–92. Available from: 10.1177/160940690600500107 [DOI] [Google Scholar]
- 32.Carcary M. The Research Audit Trial--Enhancing Trustworthiness in Qualitative Inquiry. Electron J Bus Res Methods. 2009;7(1). [Google Scholar]
- 33.Lee H, Wu XK, Genberg BL, Mugavero MJ, Cole SR, Lau B, et al. Beyond binary retention in HIV care: predictors of the dynamic processes of patient engagement, disengagement, and re-entry into care in a US clinical cohort. AIDS. 2018. Sep;32(15):2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajabiun S, Mallinson RK, McCoy K, Coleman S, Drainoni ML, Rebholz C, et al. “Getting me back on track”: The role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21(SUPPL. 1):20–9. [DOI] [PubMed] [Google Scholar]
- 35.Horn T, Sherwood J, Remien RH, Nash D, Auerbach JD, Group for the TAG and F for ARHIVPCW. Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc [Internet]. 2016. Jan 1;19(1):21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seckinelgin Hakan. “People Don’t Live on the Care Cascade: The Life of the HIV Care Cascade as an International AIDS Policy and Its Implications.” Global Public Health, vol. 15, no. 3, Mar. 2020, pp. 321–33, doi: 10.1080/17441692.2019.1673784. [DOI] [PubMed] [Google Scholar]
- 37.Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genberg BL, Shangani S, Sabatino K, Rachlis B, Wachira J, Braitstein P, et al. Improving Engagement in the HIV Care Cascade: A Systematic Review of Interventions Involving People Living with HIV/AIDS as Peers. AIDS Behav. 2016;20(10):2452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrickson Cheryl J., et al. “‘My Future Is Bright…I Won’t Die with the Cause of AIDS’: Ten-Year Patient ART Outcomes and Experiences in South Africa.” Journal of the International AIDS Society, vol. 21, no. 10, 2018, pp. 1–14, doi: 10.1002/jia2.25184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koester KA, Johnson MO, Wood T, Fredericksen R, Neilands TB, Sauceda J, et al. The influence of the ‘good’ patient ideal on engagement in HIV care. PLoS One. 2019;14(3):e0214636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bofill L, Weiss SM, Lucas M, Bordato A, Dorigo A, Fernandez-Cabanillas G, et al. Motivational Interviewing among HIV Health Care Providers. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2015;14(6):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and used during the current study is available from the lead author on reasonable request.


