Abstract
Due to its low invasiveness and controllability, chemogenetic approaches offer a highly attractive option to modulate neuronal activity in basic research and in future clinical applications. Chemogenetics have revolutionized neuroscience research by facilitating manipulations of selective brain circuits. To date, however, the large majority of these studies have been conducted in rodent models while the wide application of chemogenetics in non-human primates (NHPs) is yet to occur. Still, important progress has been achieved in the use of chemogenetics in NHP studies in the last few years. Here we review the studies that have been published using chemogenetics in NHPs and discuss the current limitations of the technique to its more widespread use in NHPs, and possible ways to overcome them.
Introduction
The chemogenetic techniques are a genetic based approach that can achieve modulation of specific cell types, based on the expression of artificial proteins designed to be inert to endogenous ligands, but activated by systemic administration of compounds that exclusively target these receptors. [1]
Chemogenetic control of neuronal activity presents several advantages over traditional pharmacological methods. The expression of the mutated proteins can be targeted to selective cell populations (using transgenic animals or viral vectors driven by cell-specific promoters). The compounds to activate the receptors can be administered systemically, largely minimizing the invasiveness of the technique, and the desired level of control of the neuronal modulation can be achieved by dosing the compound and/or the level of expression of the mutated receptors. Furthermore, as opposed to permanent lesions or ablations, the chemogenetic effects can be reversible. These properties make chemogenetic tools particularly attractive for eventual application in clinic.
Chemogenetic tools based on G protein-coupled receptors (GPCRs) and ligand-gated ion channels (LGICs) are the most commonly used in systems neuroscience research. [2,3] Among these, the GPCR chemogenetic receptors known as DREADDs (Designer receptor exclusively activated by designer drugs) have become the most popular [3,4]. The DREADDs hM3Dq and hM4Di, based on human muscarinic acetylcholine receptors, are coupled respectively to Gq and Gi proteins [3,4]. hM3Dq and hM4Di display minimal sensitivity to the endogenous neurotransmitter acetylcholine but are, instead, activated by clozapine-N-oxide (CNO), clozapine or other exogenous compounds as described below. Once activated, DREADDs will facilitate or attenuate neuronal firing, after activation of Gq/s or Gi proteins, respectively. One experimental consideration is that both hM3Dq and hM4Di are activated by the same ligand(s), and thus it is not possible to both increase and decrease the activity of the same group of neurons. An alternative is the use of kappa-opioid receptor based DREADD (KORD), a Gi protein-coupled receptor, which is activated by the otherwise inert compound salvinorin B [5]. Since KORD is an inhibitory DREADD, it can be combined with the excitatory hM3Dq receptor to achieve bidirectional control of neuronal activity.
In contrast to GPCR-based chemogenetic methods, which relay on the pharmacological control of intracellular signaling pathways, chemogenetic LGICs allow direct control of neuronal electrical activity. A novel example of this type of chemogenetic tools are the Pharmacologically Selective Actuator Modules (PSAMs). PSAMs are chimeric LGICs comprising the α7 nicotinic acetylcholine receptor ligand binding domain, combined with the ion pore domain of either the excitatory cation-selective serotonin receptor 3 (5HT3), or the inhibitory chloride-selective glycine receptor. PSAMs can be activated by specifically designed PSAM agonists (ultrapotent Pharmacologically Selective Effector Molecules (uPSEMs, [6,7]), or by low doses of clinically approved drugs (e.g, the smoking cessation drug varenicline).
Besides GPCRs and LGICs, several chemogenetic proteins have been developed [2,3]. Relevant to this review, is a technique based on the expression of tetanus neurotoxin (TeNT) [8]. When activated, TeNT cleaves the synaptic vesicle protein synaptobrevin, interfering with neurotransmitter release. TeNT is genetically encoded in a viral vector and expressed under control of a tetracycline-responsive element. Thus, the TeNT is only transcribed in the presence of a tetracycline analog such as doxycycline (Dox), a compound that can be administered systemically. The TeNT-based chemogenetic system can only be used to silence, but not to activate, neuronal activity.
Chemogenetic methods have been extensively used in research, from molecular to systems neurosciences, to manipulate the activity of neuronal and non-neuronal cells. Studies include manipulations in normal animals as well as in animal models of neurological and neuropsychiatric diseases. Several recent reviews have described the applications of DREADDs and other chemogenetic platforms in neuroscience [2,3,9]. Suffice to say here that chemogenetics have revolutionized neuroscience research, facilitating manipulations of selective brain circuits related to learning and memory, movement, sleep, attention, appetite, anxiety, and pain. Although the large majority of publications reporting use of chemogenetics have used rodent models, a growing number of studies has accomplished chemogenetic neuronal manipulation in non-human primates (NHPs).
Given the similarities of NHPs to humans, including development, social behavior and brain structure [10,11], application of chemogenetics in these animals is an essential step to translate chemogenetic approaches to treat human neurological and neuropsychiatric conditions. Here we review the studies that have been published using chemogenetics in NHPs. We also discuss the current limitations of the technique to its more widespread use in NHPs and possible ways to overcome them.
Advances in the use of chemogenetics in NHPs
Prior to genetic based approaches, the manipulation of neuronal activity in NHPs has largely been limited to studies utilizing permanent lesions, transient pharmacological inactivation, or electrical stimulation. While these techniques have provided profound insight into behavioral neuroscience, they also have limitations. For example, neural plasticity after permanent lesions can lead to reorganization and compensation from other brain structures and intracerebral drug infusions require surgical placement of skull-mounted chambers, which are not suitable for developmental neuroscience studies [12]. Therefore, chemogenetic tools provide an opportunity to manipulate neuronal activity while avoiding the pitfalls of permanent lesions or chambers. Yet, for nearly two decades chemogenetics have been widely used in rodent preclinical models of neuropsychiatric disorders, neurodegenerative diseases, and addiction [3,13], whereas the application of chemogenetics in NHP models has only just emerged over the last few years. While genetically modified strains of rodents (e.g. Cre recombinase expressing) provide a unique opportunity for probing neural pathway specific questions, transgenic NHP models are not available. To date, most NHP chemogenetic studies have used inhibitory DREADDs (hM4Di) or TeNT to investigate reward value, learning and memory, socioemotional behavior, and sensory-motor functions. The primary reason for the focus on inhibitory chemogenetic tools originates from the vast wealth of knowledge of how permanent lesions or chemical inactivation of brain areas impact behavior. The comparison between lesion and chemogenetic studies in NHPs provide an essential step toward translating these tools for clinical use in humans. Table 1 provides a summary of published studies using chemogenetic techniques in NHPs.
Table 1.
Summary of studies in non-human primates utilizing chemogenetic modulation of brain circuitry and behavior
| Study | NHP Species (n, sex, age) | Viral vector(s) | Injection location(s)* | Chemogenetic actuator(dose) |
|---|---|---|---|---|
| Kinoshita, et al., 2012 | Japanese and Rhesus macaque (5, age not reported) | (1) HiRet-TRE-EGFP.eTeNT (2) AAV2-CMV-rtTAV16 |
(1) Spinal cord segments C6-T1 (2) Spinal cord segments C2-C5 |
Dox (5–15 mg/kg, p.o.) |
| Aguilar, et al., 2015 * | Rhesus macaque (1, male, 1 female, age not reported) | AAV5-hSyn-HA.hM4Di-IRES-mCitrine | Substania nigra (unilateral) | CNO (2–10mg/kg, i.m.) C21 (5mg/kg, i.m.) Perlapine (5mg/kg, i.m.) |
| Eldridge, et al., 2016 | Rhesus macaque (2 female & male, 9–14 years old) | Lenti-hSyn-hM4Di.CFP | Orbital frontal cortex areas 11 & 13 (unilateral with contralateral lesion to rhinal cortex) | CNO (10mg/kg, i.m.) |
| Grayson, et al., 2016 | Rhesus macaque (4, male, ~5.25 years old) | AAV5-hSyn-hM4D-mCherry | Amygdala (bilateral) | CNO (10mg/kg, i.v.) |
| Nagai, et al., 2016 | Rhesus macaque (4 males, 5–14 years old) | AAV2-CMV-hM4Di or AAV2-CMV-HA-hM4Di | Rostromedial caudate (bilateral) | CNO (3mg/kg, i.v.) |
| Tohyama et al., 2017 | Macaques (6, not reported) | (1) HiRet/FuG-E/NeuRet-TRE-EGFP.eTeNT (2) AAV2/DJ-CMV-rtTAV16 |
(1) Spinal cord segments C6-T1 (2) Spinal cord segments C2-C4 |
Dox (15 mg/kg, p.o.) |
| Upright, et al., 2018 | Rhesus macaque (5 males, 4–6 years old) | AAV5-hSyn-hM4Di. mCherry | Dorsolateral prefrontal cortex (bilateral) | CNO (20mg/kg, i.m.) |
| Magnus, et al., 2019 | Rhesus macaque (1, 5 years old) | AAV8-hSyn-PSAM4GlyR-IRES-EGFP | Internal Globus Pallidus (unilateral) | Varenicline (0.1mg/kg, s.c.) |
| Raper, et al., 2019 | Rhesus macaque (2, female and male, 9 months old) | AAV5-hSyn-HA.hM4Di-IRES-mCitrine | Basolateral amygdala (bilateral) | CNO (10mg/kg, s.c.) Clozapine (0.1mg/kg, s.c.) |
| Kinoshita, et al., 2019 | Rhesus and Japanese macaques (1 each, males) | (1) HiRetTRE-eGFP.eTeNT (2) AAV1-CMV-rtTAV |
(1) Ventrolateral pulvinar (2) Superior colliculus |
Dox (15–25 mg/kg, p.o.) |
| Hayashi, et al. 2020 | Japanese macaque (5, males and females, 4–10 years old) | HiRet-hM4Di-WPRE | Medial prefrontal cortex (bilateral) | CNO (3mg/kg, i.v.) |
| Nagai, et al., 2020 | Rhesus and Japanese macaque (2, female & male, 5–7 years old) | AAV1-hSyn-hM4Di-IRES-AcGFP | Dorsolateral prefrontal cortex (bilateral) | DCZ (100 μg/kg, i.m.) |
| Ninomiya et al. 2020 | Rhesus macaques (2, males, 6 years old) | (1) AAV-DJ-TRE-EGFP-eTeNT (2) AAV2-retro-CMV-rTAV16 |
(1) Medial prefrontal cortex (2) Premotor cortex |
Dox (25–30 mg/kg, p.o.) |
| Vancraeyenes t et al. 2020 | Rhesus macaque (2 female, 6 males, 4–7 years old) | (1) HiRet -TRE-EGFP.eTeNT-EGFP/mCherry or NeuRet-TRE-EGFP.eTeNT-EGFP/mCherry (2) AAV2-CMV-rtTAV16 |
(1) Nucleus accumbens (2) Ventral tegmental area |
Dox (15 mg/kg, p.o.) |
| Allen, et al., 2021 | Rhesus macaque (7, male, 5–6 years old) | AAV1-hSyn-hM4Di.mCherry | Nucleus accumbens (bilateral) | CNO hydrochloride salt (1.7–5.6 mg/kg, i.m.) |
| Hirabayashi, et al., 2021 | Japanese macaque (3, female and male, 5–10 years old) | AAV1-hSyn-hM4Di-IRES2-AcGFP or AAV2-CMV-hM4Di | Hand region of somatosensory cortex (unilateral) | DCZ (0.1 mg/kg, i.v.) |
| Mimura, et al., 2021 | Marmoset (3, female & male, 2.5–4 years old) | AAV1-hSyn-hM3Dq-IRES-AcGFP or AAV1-THFLAG-hM3Dq | Substania nigra (unilateral) | DCZ (100 μg/kg, p.o. or 3 μg/kg, i.p.) |
| Oguchi, et al., 2021 | Japanese macaque (2 males, 7–8 years old) | (1) FuGE-Cre-2A-GFP (2) AAV5-hSyn-DIO-hM4Di.mCherry |
(1) Caudate (2) Dorsolateral prefrontal cortex |
CNO (5mg/kg, i.v.) |
| Oyama, et al., 2021 | Rhesus and Japanese macaque (2, female & male, 5–6 years old) | AAV1-hSyn-hM4Di-IRES-AcGFP | Dorsolateral prefrontal cortex | DCZ (100 μg/kg, i.m.) DCZ (2–3 ul, 100 nM, localized microinfusions in the caudate or the mediodorsal thalamus) |
| Roseboom, et al., 2021 | Rhesus macaque (5, female & male, ~2.19 years old) | AAV5-hSyn-HA.hM4Di | Central amygdala (bilateral) | Clozapine (0.030.1mg/kg, i.m.) |
| Upright et al, 2021 * | Rhesus macaque (2 males, 8 years old) | (1) CAV2-Cre (2) AAV5-hSyn-DIO-hM3Dq.mCherry |
(1) Nucleus basalis of Meynert (2) Dorsolateral prefrontal cortex |
DCZ (0.1mg/kg, i.m.) |
The numbers indicate which of vectors were injected in each region
Indicates studies published only as conference proceedings
Abbreviations: DCZ, deschloroclozapine, Dox, doxycycline, CNO, clozapine
Notes: HiRet is also known as FuG-B2, NeuRet is also known as FuG-C
Motor Systems:
The earliest report of the use of chemogenetics in primates was in a study questioning if propiospinal neurons (PNs) contributed to dexterous hand movement in primates[8]. The study used a sophisticated approach to selectively and reversibly silence the activity of PNs. A retrograde vector (HiRet) encoding TeNT was injected in spinal cord segments C6 to T1 in which the terminals of PNs are located, while a second vector, an AAV carrying a tetracycline transactivator, was injected in more rostral segments where the PN somata are located. Thus, only these PNs expressed both the TeNT and the transactivator. After Dox administration, TeNT disrupted neurotransmission between PNs and their postsynaptic motoneurons, while other inputs to these neurons remain functional. Such disruption caused a marked impairment of reach and grasp movements in the monkeys, which was fully reversed once the Dox treatment stopped. The study demonstrates the use of a combination of viral vectors to functionally dissect a specific pathway and constitutes the first demonstration of a genetic based approach to modulate neuronal activity that result in disruption of behavior in NHPs. It also exemplifies how these methods can be used to study questions specific to primate species, such as the connectivity and functions of the cortico-motoneuronal system, which is unique to dexterous primates. In a follow-up study, using similar methods, TeNT-mediated silencing of PNs was used to demonstrated that PNs contribute to recovery of dexterous hand movement after lesions of the corticospinal tract[14].
More recent studies have used DREADD-based approaches to study extrapyramidal motor systems in primates. Both studies involved modulation of the substantia nigra (SN). The SN pars compacta (SNc) contains dopaminergic neurons that project to the striatum, while the GABergic pars reticulata (SNr) is one of the output nucleus of the basal ganglia, projecting to the superior colliculus, motor thalamus and other targets. One study in macaques used inhibitory DREADDs to reversible silence the SNr, producing rotations contralateral to the treated side [15], similar to results obtained in earlier studies with pharmacological inhibition of the nucleus. In a more recent study, the expression of excitatory hM3Dq DREADDs was targeted to the SNc neurons, using a tyrosine hydroxylase (catecholaminergic neuron selective) promoter to drive the expression of the DREADDs [16]. In agreement with previous studies using electrical or pharmacological stimulation of the nigrostriatal pathway, activation of SNc neurons resulted in contralateral rotations [16].
Other basal ganglia structures, the primate internal and external segment of the globus pallidus (GPe and GPi respectively, both part of the basal ganglia), are characterized by spontaneous high frequency neuronal firing, making these structures particularly attractive to conduct electrophysiological experiments which are essential to verify the effects of chemogenetic manipulation on cell firing. Local CNO applications resulted, predominantly, in decreased neuronal activity of hM4Di-expressing GPe in anesthetized monkeys[17]. A LGIC-based chemogenetic method was used to manipulate the activity of GPi neurons[6]. In this study, extracellular recordings were conducted in awake monkeys to verify that systemic administration of varenicline decreased the firing rate of GPi neurons expressing the inhibitory PSAM-GlyR. Although neither of these studies included assessment of motor behavior, they provided electrophysiological evidence of the effectiveness of chemogenetic agents to modulate neuronal firing in primates.
Sensory systems.
After lesions to the primary visual cortex (V1), humans and NHPs may exhibit residual vision (“blindsight”). To study the mechanisms of blindsight, which have been controversial for many years, Kinoshita et al. (2019) silenced the synaptic transmission from superior colliculus (SC) to the ventrolateral pulvinar (vlPul), using the dual-vector and Dox-inducible TeNT methodology [8]. Dox-induced blockade of the SC-vlPul pathway diminished residual vision in macaques with V1 lesions [18], demonstrating the contribution of this pathway to blindsight. The results of this study suggested that previous discrepant result may be a consequence of non-selective and non-reversible degeneration methods.
Chemogenetic methods have also been applied to investigate somatosensory networks. Using a functional MRI-guided approach, Hirabayashi and colleagues[19] injected inhibitory DREADDs (unilaterally) into the hand regions of the somatosensory cortex in macaques. On a grasping task, somatosensory inhibition via DREADD-activation impacted manual fine motor dexterity for the contralateral hand, sparing dexterity in the ipsilateral hand. Interestingly, despite the lack of DREADD expression in the downstream somatosensory foot sole region, there was significant and consistent increase in fMRI BOLD signal and hypersensitivity of the foot with DREADD-activation. These results suggest a bidirectional effect of DREADD silencing on the operations of the somatosensory network.
Reward and Learning:
The brain reward pathway involves areas that are also involved in learning and memory. Two studies have investigated reward circuity using inhibitory DREADDs and a reward-discrimination task. The first one (which was also the first publication using DREADDs in NHPs) focused on disconnection of the reward pathway using unilateral lesions of the rhinal cortex and DREADD inhibition of the contralateral orbital frontal cortex (OFC) in two macaques [20]. The results revealed that the interaction between the rhinal and orbital frontal cortices were critical for making judgments of reward size [20]. The second study used inhibitory DREADDs bilaterally in the rostromedial caudate (rmCD), which receives strong projections from the OFC [21]. Bilateral silencing of the rmCD in macaques impaired reward estimation [21].
The roles of the nucleus accumbens (NAcc) in reward-based learning have also been examined with chemogenetic tools. The ventral tegmental area (VTA) sends mostly dopaminergic input to the NAcc. This connection was examined using a combination of pathway specific targeting and TeNT based inactivation. [22] During VTA-NAcc pathway silencing (induced by Dox administration) there was an increased functional network connectivity involving cortical regions, as assessed by MRI. Surprisingly, however, the chemogenetic silencing of this pathway did not affect reinforcement-based learning, but, instead, decreased motivation to perform in a decision-making task[22].
More recently, the rewarding effects of alcohol have been examined using inhibitory DREADDs. In an ethanol drug discrimination task, macaques received inhibitory DREADDs bilaterally into the NAcc [23]. Decreased NAcc output was expected to create a leftward shift in the ethanol dose discrimination curve (indicating a reduced preference for ethanol), however, the behavioral shift was proportional to the expression of hM4Di in the NAcc, highlighting that individual variability in expression levels can be an important factor in NHP chemogenetic studies (see also [24]).
Cognitive Functions:
The dorsolateral prefrontal cortex (dlPFC) is important for working memory and executive functioning, and dysfunction of the dlPFC has been implicated in cognitive deficits characteristic of disorders such as schizophrenia and dementia. Macaque monkeys were tested on a spatial delayed response task after bilateral transduction of inhibitory DREADDs in the dlPFC [24]. There was considerable variability in the behavioral results, and an extensive stereology study demonstrated a monotonic relationship between extent of receptor transduction and behavioral effect. Interestingly, only 3% of dlPFC neurons were transduced with DREADD receptors in two macaques that exhibited the largest behavioral deficit [24]. This study underscores that proper interpretation of behavioral results depends on thorough histological characterization of receptor expression, as further discussed below.
The group of Minamimoto has used DREADDs in several studies of cognitive functions in NHPs. They demonstrated that DREADD inhibition of the dlPFC could reliably and transiently impair spatial working memory for up to 2 hours, with recovered memory function 24 hours after DREADD activation [25]. In other study, DREADD inhibition was used to probe the prefronto-subcortical pathways important for spatial working memory or decision making [26]. Bilateral inhibition of the dlPFC by systemic administration of DREADD ligands or microinfusions directly targeting DREADD expressing axon terminals in the mediodorsal (MD) thalamus resulted in impaired spatial working memory. In contrast, inhibition of the dlPFC-caudate resulted in impaired decision making [26]. Lastly, using an ocular delayed response task, Oguchi and colleagues [27] selectively suppressed lateral PFC neurons projecting to the CD, demonstrating that suppression of this pathway resulted in dysfunction of inhibitory control of impulsive behavior [27]. This study is one of the few in which pathway-selective chemogenetic modulation has been used in monkeys (see Table 1), although the technique is widely used in rodent models.
A recent study demonstrates the use of chemogenetic manipulation to explore cognitive abilities unique to primates. Theory of mind, the ability to infer the mental states of others, has been attributed to the brain network that includes mPFC, superior temporal sulcus, and temporo-parietal junction. Yet, whether neural activation in these areas causally linked to theory of mind abilities are unknown. To probe the role of the mPFC in theory of mind, Hayashi et al. [28] trained monkeys in an anticipatory looking ‘false belief’ paradigm. DREADD-inhibition of the mPFC abolished the implicit gaze bias toward ‘false belief’ targets, suggesting its key role in one’s ability to infer the mental states of others.
The studies cited demonstrate that chemogenetics can be used to probe cognitive systems and related brain pathways, and to broaden our understanding of the contributions of specific brain structures or neural pathways to cognitive functions that are unique to primate species.
Socioemotional Behavior:
Primates are social creatures, and in large part their behavior is regulated around social exchanges. To demonstrate the role of cortico-cortical pathways in monitoring social actions, Ninomiya et al [29] used a double viral vector approach and the TeNT-based chemogenetic method to selectively block the synaptic transmission from the premotor to the mPFC. After Dox administration, to block neurotransmission in this pathway, monkeys showed deficits in processing observed actions executed by social partners [29].
Among subcortical structures, the amygdala plays an important role in social behavior and emotional responses. To date, three studies have utilized inhibitory DREADDs in relation to amygdala functions and connectivity [12,30,31]. Activation of inhibitory DREADDs with CNO not only decreased amygdala functional connectivity (as expected), but it also led to large differential changes in neural network dynamics as compared to saline administration [30]. Neural network analyses found that resting state functional connectivity of the limbic and default mode networks were fragmented by DREADD-inhibition of the amygdala. The study by Grayson et al. [30] demonstrates that chemogenetics can provide a powerful tool to examine functional circuits in vivo. Two studies utilized an acute social stress task (human intruder paradigm) to examine changes in emotional reactivity after DREADD-induced inhibition of the basolateral [12] or central amygdala [31], in infant and adolescent macaques, respectively. Despite targeting different areas of the amygdala for DREADD-mediated inhibition, both studies found decreased freezing (with no impact on vocalization or hostile behavior expressions [12]) during the task, indicating reduced anxiety-related behaviors. In the study conducted in infant monkeys, during a social attention eye tracking task, the animals spent more time looking toward the mouth of conspecifics, as well as increased time looking at neutral and aversive objects when the basolateral amygdala was chemogenetically inhibited [12]. These results provide additional evidence that amygdala silencing shifts attention for socially salient cues. Results from these studies are largely consistent with previous findings from chemical inactivation or permanent lesions of the amygdala, which supports the use of chemogenetic manipulation of socio-emotional systems in NHPs.
To date, the majority of NHP chemogenetic studies appear to have been focused on comparing inhibitory or excitatory DREADDs to permanent lesions or transient pharmacological neuromodulation (e.g. muscimol or bicuculline) to demonstrate the effectiveness of the tools in NHPs. In some cases, studies have demonstrated that transient DREADD inhibition produced fewer behavioral differences compared to permanent lesions [12]. Considering that the above studies have established the proof of principle that chemogenetic tools work in NHP neurons, new studies are beginning to test the potential for chemogenetic neuromodulation to be used as a neurotherapeutic method for treating anxiety disorder [31] or for circuit-specific treatment of cognitive impairments seen in aging and disease [32]. Establishing a reliable and reproducible effects of chemogenetic tools in NHPs is an essential step toward translating these tools for clinical use in humans.
Challenges and possible solutions
The studies described above and in Table 1 demonstrate not only that the application of chemogenetic techniques is feasible in NHPs, but also that it can be used to gather new knowledge about primate brain pathways and functions. However, several challenges remain to further expand the use of chemogenetics in NHP basic and translational research, which we have grouped as follows: (1) difficulty to target specific cell subtypes, (2) inconsistent level and pattern of transgene expression after viral injections, (3) immune responses to viral vectors or transgenes and (4) lack of efficacy or specificity of actuators. Except for the last point, these issues are common to other genetic based approaches that depend on the use of viral vectors. Although these obstacles are no unique to studies in NHPs, for various reasons that we describe below they are more difficult to address in these species than in rodent (or other) experimental models.
Targeting specific cell populations
The main advantage of genetic-based approaches is the possibility of targeting specific cell types. However, in NHP studies a major challenge is the scarcity of tools to selectively direct the expression of the chemogenetic receptor to neuronal subpopulations. Most studies using chemogenetic tools in mice take advantage of the numerous easily accessible genetic lines. Since transgenic NHPs are not yet widely available, genetic based approaches in these species depend on the use of viral vectors. The selectivity of a viral vector for a cell type derives from its tropism (i.e., the preference of the viral vector to transduce one cell type over others) [33–36] and the regulatory sequences (promoter or enhancer) that drive the expression of the transgene to genetically-defined cell types.
The known cell tropisms of most commonly used viral vectors and serotypes seem to be consistent across species[37]. However, few studies have compared, side by side, transduction achieved with the same viral vectors in the CNS of NHPs and other species. These studies have, indeed, found that cell-type specific transduction may vary across species depending on the administration route [38], brain pathway targeted [39], or the combination of the AAV capsid and promoter [40].
There is a small (but growing) number of promoter or enhancer sequences that have been demonstrated to target selective cell types in NHPs after viral vector administrations (Table 2). The promoters and enhancers listed in table 2 were not originally developed based on primate genetic sequences. Still, they have been shown to be effective to target specific cell types in NHPs. Efforts are underway to characterize transcriptional patterns and gene expression in the brain of primates (including human), that will help to design primate-specific regulatory sequences [41,42].
Table 2:
Regulatory genetic sequences used in non-human primate (NHP) studies to drive transgene expression using viral vectors (only studies in which the use of the sequence was verified in NHPs are included)
| Regulatory sequence | Cell-type specificity | Transgene expressed | References |
|---|---|---|---|
| Human Synapsin1 (SYN) | Neurons | Opsins, chemogenetic receptors, fluorescent proteins | Numerous, see e.g. Table 1 |
| CaMKIIα | Mainly excitatory projection neurons, but also in some interneurons | Opsins, fluorescent proteins | [81–83] |
| Tyrosine hydroxylase (TH) | Catecholaminergic neurons | Opsins, chemogenetic receptors, fluorescent proteins | [16,35,44] |
| Dlx5/6 | Telencephalic GABAergic neurons | GFP Opsins |
[84,85] |
| Parvalbumin (PV) | PV-expressing GABAergic neurons | Opsins | [85] |
| E2 | PV-expressing GABAergic neurons | Opsins | [43] |
| GFAP | Astrocytes | GFP | [86] |
| L7 | Purkinje cells | Opsins | [87] |
| ChAT | Cholinergic interneurons | GFP | [88] |
The most commonly used viral vectors in NHP chemogenetic experiments, lentivirus and AAVs, have a relatively small vector capacity [37], limiting the length of the regulatory sequences that can be efficiently incorporated in the plasmid. Short regulatory elements [43] and mini-promoters [41], developed based on recent advances in transcriptomic and epigenetic analysis at the single cell level, promise an expanded variety of neuronal and non-neuronal cell types. In addition, a dual viral vector approach could circumvent the need of a short promoter, by making the expression of the transgene of interest Cre-dependent [44].
An alternative (or complementary) method to selectively express a transgene in a cell subtype is by leveraging the anatomical connections of the neurons of interest [45]. Pathway-selective expression can be achieved, for example, by using viral vectors with retrograde properties (injecting the viral vector at the terminal fields of the neurons of interest). As mentioned above, the HiRet and NeuRet retrograde vectors have been used several times to target specific brain pathways and achieve chemogenetic modulation in NHPs [8,14,18,22,27,29].
Pseudotyped rabies virus permit the delivery of chemogenetic receptors to manipulate neurons presynaptically connected to targeted cells (reviewed in [37]. Although this is a powerful technique that can be used to manipulate neurons that synapse onto targeted cell types, the high toxicity of these vectors limits the survival time after viral injection, thus making them unsuitable for long term NHP studies. Nontoxic versions of the rabies viral vectors are available (e.g, [46]), but their use in NHPs have not yet been demonstrated.
Inconsistent level and pattern of transgene expression
An important challenge faced by users of chemogenetic techniques is inconsistent expression of the chemogenetic receptors after intracerebral injections of viral vectors. Due to limited diffusion of the virus solution in the tissue, the injections can result in receptor expression restricted to a small volume of brain tissue, which may be large enough for rodent models, but too small for practical use in primates. Injections of the virus solutions in CSF compartments could help achieve a more widespread distribution of the virus throughout the whole brain (e.g., [47]). One step further would be systemic delivery of the virus solution using vectors that can penetrate the blood brain barrier (BBB) [48]. While such vectors are rapidly developing, and are already available for studies in rodents, further optimization may be needed before application in primate species.
Besides the tissue volume reached by the virus, several other intersecting factors affect the virus transduction, including the type of virus used, the volume of virus solution, the injection method and interactions between the viral capsid and the promoter [34,35,40]. There are examples in the literature showing inconsistency among transduction efficiency, tropism, and direction of axonal transport among brain regions, both in rodents and NHP studies (e.g., [39,49]).
In addition, mistargeting of virus injections can be a severe obstacle and may account for a high number of failures in NHP studies. Although these issues could be resolved by using larger numbers of animals, selecting only the subjects with strong and on-target expression of the receptors, for practical and ethical reasons this is not possible in NHP studies. Thus, methods should be employed to refine placement of viral vector solutions in the brain. For example, the injection target can be significantly improved using real time guidance during the virus injection, with electrophysiological recordings [6] or MRI [50].
Similarly, detection of receptor expression in vivo is critical to make corrections to the experimental approach during the study. Imaging approaches provide an excellent opportunity to non-invasively verify transgene expression [51], and chemogenetic studies in NHPs have started to take advantage of these tools. Positron emission tomography (PET) imaging has been used to monitor in a non-invasive manner the extent and temporal course of the artificial receptor expression using radioactive versions of the chemogenetic ligands ([11C]-clozapine, [11C]- deschloroclozapine, and [18F]JHU37107 for DREADDs [21,25,52] or [18F]-ASEM for PSAMs [6].
While the continued use of imaging methods should be a consistent component of chemogenetic (and other genetic based approaches), to increase the reliability and reproducibility of NHP studies, postmortem histological examination remains the definitive method to confirm the level and pattern of expression of chemogenetic receptors. The level of expression, which can be measured histologically as the proportion of transgene-positive neurons, directly correlates with behavioral effects. As discussed above, in chemogenetic experiments this proportion could be relatively small (around 3% of neurons [24]). However, this may vary depending on the brain circuit explored and the chemogenetic receptor used.
The localization pattern of the receptors is equally important. To be activated by the systemically injected ligand, the receptors should be localized at the plasma membrane. We have found that the tag protein can interfere with the trafficking of DREADDs to the membrane. In our study, fusion of the inhibitory DREADD hM4Di to the fluorescent protein mCherry resulted in sparse expression at the membrane in monkey neurons (Fig. 1). In contrast, in mouse neurons most of the mCherry labeling was observed at the plasma membrane [53]. These discrepant expression patterns indicate a species difference in the trafficking and membrane insertion of exogenous proteins, although the mechanisms behind these differences remain to be determined. We found, however, that when we traded the mCherry for the haemagglutinin (HA) tag resulted in strong neuronal plasma membrane expression (Fig. 1). These results emphasize that the choice of tag protein is relevant to successful chemogenetic receptor expression. While the use of fluorescent proteins may not always hinder the receptor expression, the use of smaller tags (such as HA) or the use of IRES/P2A systems could help improve the chemogenetic expression in NHP studies (the use of smaller tags could also benefit rodent studies, as recently demonstrated [54]).
Figure 1.
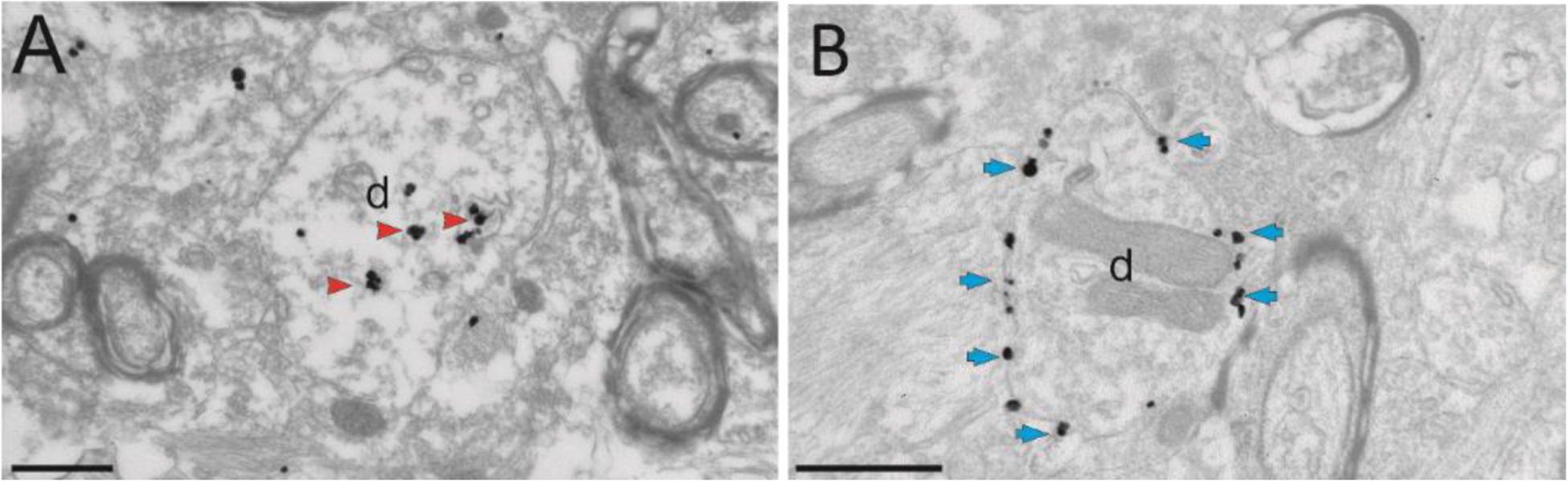
Ultrastructural localization of hM4Di in monkeys, using the pre-embedding immunogold method to reveal the tag proteins mCherry (in A) or hemagglutinin (HA, B). A and B show electron micrographs of dendrites (d) in the amygdala of two monkeys, that received intra-amygdala injections of AAV5-hSyn-hM4Di-mCherry (A) or AAV5-hSyn-HA-hM4Di (B). The large majority of immunogold particles labeling hM4Di-mCherry are localized in the intracellular compartment (red arrowheads), while the gold particles labeling HA-hM4Di are bound to the plasma membrane (blue arrows). Scale bars: 0.6 μm (from Galvan et al 2019)
Immune responses to viral vectors or transgenes
Innate immune responses to the viral vectors is a well-known challenge in the gene therapy field [55]. Although this issue has been reported in murine species, (e.g., [56]), it has not been known to be a major roadblock in rodent studies. However, it poses a significant problem to the use of chemogenetics in NHPs. Neutralizing antibodies (nAbs) against AAVs, which can prevent the viral vectors from reaching the target cells, are prevalent in many primate species [55]. Although circulating antibodies are usually not a concern in the CNS, most chemogenetic experiments in NHPs involve the use of craniotomies and/or intracerebral injections, which compromise the BBB and could lead to a possible infiltration of nAbs to the brain parenchyma. The strategy adopted by many NHP researchers is to screen animals for pre-existing nAbs, including in the studies only animals with negligible titers of nAbs against the serotype of interest. But even in seronegative subjects, an initial experimental administration of AAVs can result in strong production of nAbs [57], limiting the possibilities of conducting sequential AAV administrations. Neuroscience researchers using NHP models can benefit from advances in gene therapy to evade the immune responses, including engineering approaches to mutate the AAV capsid [55] and the use of IgG degrading enzymes to reduce circulating nAbs [58].
Lack of efficacy or specificity of actuators
Central to the effectiveness of chemogenetic receptors is understanding the pharmacokinetics and BBB permeability of systemically administered ligands. Although CNO has been the most widely used ligand for DREADD activation, studies have demonstrated several problems with using CNO. Firstly, CNO has poor brain penetrance because it is a substrate for P-glycoprotein (P-gp) efflux pump, which limits its permeability to cross the BBB [59,60]. Secondly, CNO is subject to reductive metabolism to clozapine, an atypical antipsychotic drug that can bind to both DREADD receptors and endogenous receptors (e.g. serotonin and dopamine) [59–61]. Clozapine has a high affinity for the DREADDs, so that low-dose administration of clozapine effectively activates these receptors [4,12,31,59]. Still, even low-dose clozapine can have off-target side effects, as shown by decreased cortisol secretions in the recent study from Roseboom and colleagues [31]. Reductive metabolism of CNO into clozapine is not only an issue for NHP studies, but has been demonstrated in rodents [62,63] and it is this conversion of CNO to clozapine that is responsible for DREADD occupancy [59]. CNO administration can impact locomotor and anxiety behavior of DREADD naïve rodents [39,59,62,64]. Given these complications with the use of CNO or low-dose clozapine to activate DREADDs, other ligands with better brain penetrance and no active metabolites are needed. Compound 21 (C21) was the first DREADD ligand developed to address the issue of CNO’s reverse metabolism to clozapine [65]. C21, however, was shown to have low brain penetrance in both rodents and NHPs [59]. Chronic administration of C21 in DREADD naïve rodents can impact exploration behavior. [39]. The development of high affinity DREADD ligands is essential to advance the translational potential of DREADD technology. Along this line, novel DREADD ligands have been developed including JHU compounds (JHU37152 and 60) [52] and deschloroclozapine (DCZ) [25]. Both JHU compounds and DCZ have high BBB permeability and have been shown to be potent, selective, and fast-acting DREADD ligands [25,52]. Unlike clozapine, low dose DCZ had no off-target effects in DREADD naïve monkeys performing a working memory task [66]. Last, but not least, as discussed above, JHU and DCZ are available as radioligands for quantifying DREADD receptor expression in vivo using PET [25,52,67].
For LGIC-based chemogenetic approaches, activation of PSAMs can be achieved with low doses of the anti-smoking drug varenicline [6]. Alternatively, PSAMs can be activated by uPSEMs, selective and highly potent PSAMs agonists derived from varenicline [6]. To expand the use of uPSEMs in NHP basic research, however, the pharmacokinetic properties of uPSEMs should be thoroughly characterized in these species.
Dox has been used as tetracycline analog in TeNT-based chemogenetic studies. In contrast to DREADD and PSAM-ligands, tetracycline derivatives (including Dox) were not specifically selected or designed to exclusively activate an artificial receptor. Dox, a commonly used antibiotic [68], is used as tetracycline activator due to its BBB-crossing ability. However, long term use of Dox could lead to gastrointestinal and/or toxic effects. Also, the slow release kinetics of Dox can make it unreliable to predict experimental responses [68]. Finally, the use of Dox would void the use of other tetracycline- based approaches to modulate expression of other transgenes (for example, calcium sensors) in the same animal.
Other considerations
The studies described above constitute, to the best of our knowledge, published reports of the use of chemogenetic methods in NHPs. The large majority of these reports have used DREADDs based on muscarinic acetylcholine receptors. However, the chemogenetic toolbox includes other receptor types and proteins [3], which have not yet been tested in NHPs, but could expand the experimental landscape. For example, as mentioned above, KORDs could be used to multiplex chemogenetic control of neurons [5]. Salvinorin B, the effector for KORDs, is known to rapidly enter the brain after systemic administration in macaques [69]. However, its short half-life and its limited solubility [5], encumbers its use in NHP studies.
Some reports in rodents have indicated that the use of inhibitory PSAMs or DREADDs can lead to unexpected effects. In one case, activation of the GlyR conductance of PSAM-GlyR induced depolarization in in vitro recordings of striatal projection neurons [70], while other study showed that activation of hM4Di enhanced evoked potentials in dentate gyrus neurons [71]. It was also demonstrated that hM3Dq-mediated activation of interneurons in the hippocampus reduced the firing of pyramidal neurons, as predicted, but unexpectedly, the activity of many of the interneurons decreased as well, and there were short and long-term reorganization in the neuronal circuits involved [72]. These examples highlight the importance of conducting direct electrophysiologic verification that chemogenetic receptor activation leads to the intended inhibitory or excitatory effect in the neurons of interest, but such validation experiments are rarely conducted (even in rodent experimentation). Exceptionally, two reports in NHPs have provided evidence that activation of hM4Di and PSAM-GlyRs reduce neuronal firing in pallidal neurons [6,17].
The potential adverse effects of chronic expression of chemogenetic receptors in neurons, or repeated exposure to chemogenetic ligands has yet to be thoroughly described. Although this consideration is not specific to NHPs, it may be of particular importance in NHP studies that can last months or years. In rodents, transduction of hM4Di with a high titer AAV (10e13 vg/ml) resulted in marked loss of hippocampal neurons, accompanied by neuroinflammatory reactions [71]. It remains to be determined that such toxic effects of chemogenetic receptor expression occur in NHP neurons, but studies have shown that AAV-mediated expression of exogenous proteins can induce immune responses and neuroinflammation in NHPs [73]. Therefore, we strongly recommend a careful characterization of the expression of the chemogenetic receptors in neurons and the long-term effect on the neuron’s health, in NHP brains. Because of their larger brain size and longer axonal projections, primate neurons have greater metabolic demands than rodent neurons [74], thus, over-expressed foreign proteins, such as viral-vector transduced chemogenetic receptors, may represent extra metabolic demands on neurons.
The effects of repeated administration of chemogenetic ligands deserve careful consideration. Few studies have used chronic (i.e., more than four weeks) administration of these ligands (but see studies using Dox, Table 1). Some reports indicate that systemic administration of CNO over the course of many weeks does not have off-target effects on behaviors of naïve animals.[63,75] However, it has also been shown that the effects of short- and long-term activation of DREADDs can differ. For instance, when expressed on serotonergic neurons, acute activation of DREADDs with CNO results in anxiogenic and antidepressant effects, but after longer-term activation the anxiogenic effects are blunted, while the antidepressant effects persist [76]. Long-term exposure to chemogenetic ligands could also affect non-CNS systems, such as the gut microbiota, as demonstrated in mice [77]. Due to the long-term duration of many NHP studies and given the potential of clinical applicability of chemogenetics (which would require chronic dosing), detailed studies of the effects of repeated administration of chemogenetic ligands in NHP are needed.
Closing remarks
Capitalizing on advances from the fields of gene therapy, imaging and molecular biology, NHP researchers are making steady progress to expand the genetic-based approaches in NHP research. The available evidence shows that chemogenetics can be successfully used to manipulate neuronal activity and behavior in NHP neuroscience research. Even more, studies using chemogenetic approaches have started to bring unprecedent knowledge about functions of specific brain circuits. The expanding availability of novel promoters and enhancer sequences, combined with the use of dual virus approaches, will increase the ability to target specific cell types.
As far as we can tell, there is no consensus in the field regarding which chemogenetic system is preferable in neuroscience research and, more to the point, in NHP studies. The selection of the chemogenetic tool to use would depend on the goals of the study and the previous data available on the transgene use and expression. Clearly, DREADDs have been used more extensively, thus their advantages and limitations have been better described (for the most part, in rodent studies). A shortcoming of DREADDs is the fact that these receptors work by activating endogenous G-proteins and second-messenger pathways, and the cell’s activity could be modulated by multiple mechanisms, which may be poorly characterized for many cell types. [2]. Thus, we emphasize the importance of validating the physiological effect of DREADD activation in each neuronal system. On the other hand, DREADDs have shown their immense utility to neuroscience research and the abundant DREADD studies in rodents provide a wealth of information that has been used by NHP researchers as starting point. Furthermore, as mentioned earlier, by combining DREADDs with KORDs, one could achieve multiplexed chemogenetic modulation, although this approach remains to be demonstrated in NHPs.
In contrast to DREADDs, LGIC-based chemogenetic methods offer the advantage of direct modulation of the neuron’s electrical activity. Even though PSAM were recently developed and optimized, [6] their use has been expanding in rodent neuroscience studies (e.g., [43,78]).
Both the DREADD and PSAM chemogenetic platforms share aspects that are important in translational NHP research. Each of these receptor types can be activated by low doses of clinically approved drugs (clozapine and varenicline, respectively), which could help advance potential chemogenetic-based treatments for eventual use in human disease. However, the occurrence of side effects should be carefully characterized in naïve animals, even when using very low doses of these compounds. Furthermore, to allow visualization of expression receptor in vivo radioligands tracers are available for both PSAMs and DREADDs.
The Dox-inducible TeNT method to silence neurotransmission has been used successfully in various studies in NHPs. Some disadvantages of this technology are the inability to increase neuronal activity and its slow and variable time course. The time needed for the emergence of the TeNT effects after Dox administration may range from one to 10 days [18]. This variability may depend on the neuronal circuit targeted and may need to be defined on a case by case basis. Such prolonged time course may not be acceptable in certain studies.
Given the translational importance of NHPs, along with the effort and expenses associated with NHP research, constant and open communication among researchers is key to maximize progress, avoid repetition of unfruitful experiments and consolidate redundant pilot testing. For example, accessibility to open databases [79,80] and collaborative efforts will help overcome the challenges stated above.
Funding
The authors’ research cited in this review was funded by the National Institutes of Health (NINDS 1R21NS106346 (AG), and in part by the National Institutes of Mental Health (NIMH) Autism Center of Excellence Center Grant P50 MH100029and the Office of Research Infrastructure Programs P51 OD011132 (base grant to the Yerkes Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors report no conflict of interest
REFERENCES CITED
- 1.Sternson SM, Roth BL: Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 2014, 37:387–407. [DOI] [PubMed] [Google Scholar]
- 2.Atasoy D, Sternson SM: Chemogenetic Tools for Causal Cellular and Neuronal Biology. Physiol Rev 2018, 98:391–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth Bryan L: DREADDs for Neuroscientists. Neuron 2016, 89:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL: Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 2007, 104:5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, et al. : A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, et al. : Ultrapotent chemogenetics for research and potential clinical applications. Science 2019, 364:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM: Chemical and genetic engineering of selective ion channel-ligand interactions. Science 2011, 333:1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, et al. : Genetic dissection of the circuit for hand dexterity in primates. Nature 2012, 487:235–238. [DOI] [PubMed] [Google Scholar]
- 9.Campbell EJ, Marchant NJ: The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br J Pharmacol 2018, 175:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffalo EA, Movshon JA, Wurtz RH: From basic brain research to treating human brain disorders. Proc Natl Acad Sci U S A 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, et al. : Why primate models matter. Am J Primatol 2014, 76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raper J, Murphy L, Richardson R, Romm Z, Kovacs-Balint Z, Payne C, Galvan A: Chemogenetic Inhibition of the Amygdala Modulates Emotional Behavior Expression in Infant Rhesus Monkeys. eNeuro 2019, 6:ENEURO.0360–0319.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KS, Bucci DJ, Luikart BW, Mahler SV: DREADDS: Use and application in behavioral neuroscience. Behav Neurosci 2016, 130:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohyama T, Kinoshita M, Kobayashi K, Isa K, Watanabe D, Kobayashi K, Liu M, Isa T: Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proc Natl Acad Sci U S A 2017, 114:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar BL, Elorette C, Huizenga MN, Forcelli PA, Malkova L: Chemogenetic control of motor behavior in the nonhuman primate: DREADD-mediated silencing of the substantia nigra pars reticulata. In Society for Neuroscience. Edited by. Chicago; 2015:802.810. [Google Scholar]
- 16.Mimura K, Nagai Y, Inoue KI, Matsumoto J, Hori Y, Sato C, Kimura K, Okauchi T, Hirabayashi T, Nishijo H, et al. : Chemogenetic activation of nigrostriatal dopamine neurons in freely moving common marmosets. iScience 2021, 24:103066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deffains M, Nguyen TH, Orignac H, Biendon N, Dovero S, Bezard E, Boraud T: In vivo electrophysiological validation of DREADD-based modulation of pallidal neurons in the non-human primate. Eur J Neurosci 2020. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita M, Kato R, Isa K, Kobayashi K, Kobayashi K, Onoe H, Isa T: Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nat Commun 2019, 10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi T, Nagai Y, Hori Y, Inoue K-i, Aoki I, Takada M, Suhara T, Higuchi M, Minamimoto T: Chemogenetic sensory fMRI reveals behaviorally relevant bidirectional changes in primate somatosensory network. Neuron 2021, 109:3312–3322.e3315. [DOI] [PubMed] [Google Scholar]
- 20.Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, Ji B, Higuchi M, Minamimoto T, Richmond BJ: Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci 2016, 19:37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai Y, Kikuchi E, Lerchner W, Inoue KI, Ji B, Eldridge MA, Kaneko H, Kimura Y, Oh-Nishi A, Hori Y, et al. : PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun 2016, 7:13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vancraeyenest P, Arsenault JT, Li X, Zhu Q, Kobayashi K, Isa K, Isa T, Vanduffel W: Selective Mesoaccumbal Pathway Inactivation Affects Motivation but Not Reinforcement-Based Learning in Macaques. Neuron 2020, 108:568–581 e566. [DOI] [PubMed] [Google Scholar]
- 23.Allen DC, Jimenez VA, Carlson TL, Walter NA, Grant KA, Cuzon Carlson VC: Characterization of DREADD receptor expression and function in rhesus macaques trained to discriminate ethanol. Neuropsychopharmacology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upright NA, Brookshire SW, Schnebelen W, Damatac CG, Hof PR, Browning PGF, Croxson PL, Rudebeck PH, Baxter MG: Behavioral Effect of Chemogenetic Inhibition Is Directly Related to Receptor Transduction Levels in Rhesus Monkeys. J Neurosci 2018, 38:7969–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, Takahashi M, Huang XP, Slocum ST, DiBerto JF, et al. : Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 2020, 23:1157–1167. [DOI] [PubMed] [Google Scholar]
- 26.Oyama K, Hori Y, Nagai Y, Miyakawa N, Mimura K, Hirabayashi T, Inoue KI, Suhara T, Takada M, Higuchi M, et al. : Chemogenetic dissection of the primate prefronto-subcortical pathways for working memory and decision-making. Sci Adv 2021, 7:eabg4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oguchi M, Tanaka S, Pan X, Kikusui T, Moriya-Ito K, Kato S, Kobayashi K, Sakagami M: Chemogenetic inactivation reveals the inhibitory control function of the prefronto-striatal pathway in the macaque brain. Commun Biol 2021, 4:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T, Akikawa R, Kawasaki K, Egawa J, Minamimoto T, Kobayashi K, Kato S, Hori Y, Nagai Y, Iijima A, et al. : Macaques Exhibit Implicit Gaze Bias Anticipating Others’ False-Belief-Driven Actions via Medial Prefrontal Cortex. Cell Rep 2020, 30:4433–4444 e4435. [DOI] [PubMed] [Google Scholar]
- 29.Ninomiya T, Noritake A, Kobayashi K, Isoda M: A causal role for frontal cortico-cortical coordination in social action monitoring. Nat Commun 2020, 11:5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA, Amaral DG: The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron 2016, 91:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roseboom PH, Mueller SAL, Oler JA, Fox AS, Riedel MK, Elam VR, Olsen ME, Gomez JL, Boehm MA, DiFilippo AH, et al. : Evidence in primates supporting the use of chemogenetics for the treatment of human refractory neuropsychiatric disorders. Mol Ther 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Upright NA, Elorette C, Fujimoto A, Croxson PL, Russ BE, Rudebeck PH, Baxter MG: Pathway-specific chemogenetic neuromodulation enhances working memory in rhesus monkeys. In Society for Neuroscience. Edited by. Online; 2021:P315.302. 2021. [Google Scholar]
- 33.An H, Cho DW, Lee SE, Yang YS, Han SC, Lee CJ: Differential Cellular Tropism of Lentivirus and Adeno-Associated Virus in the Brain of Cynomolgus Monkey. Exp Neurobiol 2016, 25:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerits A, Vancraeyenest P, Vreysen S, Laramee ME, Michiels A, Gijsbers R, Van den Haute C, Moons L, Debyser Z, Baekelandt V, et al. : Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics 2015, 2:031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerchner W, Corgiat B, Der Minassian V, Saunders RC, Richmond BJ: Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain. Gene Ther 2014, 21:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, Elsworth JD, Roth RH, Samulski RJ, Redmond DE Jr.: Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol Ther 2010, 18:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Holmes TC, Luo MH, Beier KT, Horwitz GD, Zhao F, Zeng W, Hui M, Semler BL, Sandri-Goldin RM: Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron 2020, 107:1029–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liguore WA, Domire JS, Button D, Wang Y, Dufour BD, Srinivasan S, McBride JL: AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-human Primates Compared with Mice. Mol Ther 2019, 27:2018–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albaugh DL, Smith Y, Galvan A: Comparative analyses of transgene expression patterns after intra-striatal injections of rAAV2-retro in rats and rhesus monkeys: A light and electron microscopic study. Eur J Neurosci 2020, 52:4824–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohlen MO, McCown TJ, Powell SK, El-Nahal HG, Daw T, Basso MA, Sommer MA, Samulski RJ: Adeno-Associated Virus Capsid-Promoter Interactions in the Brain Translate from Rat to the Nonhuman Primate. Hum Gene Ther 2020, 31:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portales-Casamar E, Swanson DJ, Liu L, de Leeuw CN, Banks KG, Ho Sui SJ, Fulton DL, Ali J, Amirabbasi M, Arenillas DJ, et al. : A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proc Natl Acad Sci U S A 2010, 107:16589–16594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He J, Kleyman M, Chen J, Alikaya A, Rothenhoefer KM, Ozturk BE, Wirthlin M, Bostan AC, Fish K, Byrne LC, et al. : Transcriptional and anatomical diversity of medium spiny neurons in the primate striatum. Curr Biol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vormstein-Schneider D, Lin JD, Pelkey KA, Chittajallu R, Guo B, Arias-Garcia MA, Allaway K, Sakopoulos S, Schneider G, Stevenson O, et al. : Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans. Nat Neurosci 2020, 23:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W: Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell 2016, 166:1564–1571 e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sjulson L, Cassataro D, DasGupta S, Miesenbock G: Cell-Specific Targeting of Genetically Encoded Tools for Neuroscience. Annu Rev Genet 2016, 50:571–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee S, Sullivan HA, MacLennan BJ, Xu R, Hou Y, Lavin TK, Lea NE, Michalski JE, Babcock KR, Dietrich S, et al. : Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat Neurosci 2018, 21:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galvan A, Petkau TL, Hill AM, Korecki AJ, Lu G, Choi D, Rahman K, Simpson EM, Leavitt BR, Smith Y: Intracerebroventricular Administration of AAV9-PHP.B SYN1-EmGFP Induces Widespread Transgene Expression in the Mouse and Monkey Central Nervous System. Hum Gene Ther 2021, 32:599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, et al. : Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017, 20:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aschauer DF, Kreuz S, Rumpel S: Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 2013, 8:e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yazdan-Shahmorad A, Tian N, Kharazia V, Samaranch L, Kells A, Bringas J, He J, Bankiewicz K, Sabes PN: Widespread optogenetic expression in macaque cortex obtained with MR-guided, convection enhanced delivery (CED) of AAV vector to the thalamus. J Neurosci Methods 2018, 293:347–358. [DOI] [PubMed] [Google Scholar]
- 51.Boehm MA, Bonaventura J, Gomez JL, Solis O, Stein EA, Bradberry CW, Michaelides M: Translational PET applications for brain circuit mapping with transgenic neuromodulation tools. Pharmacol Biochem Behav 2021, 204:173147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonaventura J, Eldridge MAG, Hu F, Gomez JL, Sanchez-Soto M, Abramyan AM, Lam S, Boehm MA, Ruiz C, Farrell MR, et al. : High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nature Communications 2019, 10:4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvan A, Raper J, Hu X, Pare JF, Bonaventura J, Richie CT, Michaelides M, Mueller SAL, Roseboom PH, Oler JA, et al. : Ultrastructural localization of DREADDs in monkeys. Eur J Neurosci 2019, 50:2801–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desloovere J, Boon P, Larsen LE, Goossens MG, Delbeke J, Carrette E, Wadman W, Vonck K, Raedt R: Chemogenetic Seizure Control with Clozapine and the Novel Ligand JHU37160 Outperforms the Effects of Levetiracetam in the Intrahippocampal Kainic Acid Mouse Model. Neurotherapeutics 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Samulski RJ: Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 2020, 21:255–272. [DOI] [PubMed] [Google Scholar]
- 56.Janelidze S, Nordstrom U, Kugler S, Brundin P: Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson’s disease. J Gene Med 2014, 16:300–308. [DOI] [PubMed] [Google Scholar]
- 57.Mendoza SD, El-Shamayleh Y, Horwitz GD: AAV-mediated delivery of optogenetic constructs to the macaque brain triggers humoral immune responses. J Neurophysiol 2017, 117:2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmore ZC, Oh DK, Simon KE, Fanous MM, Asokan A: Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight 2020, 5:e139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, et al. : Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A: Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chem Neurosci 2017, 8:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen DC, Carlson TL, Xiong Y, Jin J, Grant KA, Cuzon Carlson VC: A comparative study of the pharmacokinetics of clozapine N-oxide and clozapine N-oxide hydrochloride salt in rhesus macaques. J Pharmacol Exp Ther 2018, 368:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D: The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 2018, 8:3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Katzel D, Nissen W, Pekcec A: Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 2019, 9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD: Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro 2016, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J: The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci 2015, 6:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Upright NA, Baxter MG: Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology 2020, 45:1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan X, Telu S, Dick RM, Liow JS, Zanotti-Fregonara P, Morse CL, Manly LS, Gladding RL, Shrestha S, Lerchner W, et al. : [(11)C]deschloroclozapine is an improved PET radioligand for quantifying a human muscarinic DREADD expressed in monkey brain. J Cereb Blood Flow Metab 2021:271678X211007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krueger C, Pfleiderer K, Hillen W, Berens C: Tetracycline derivatives: alternative effectors for Tet transregulators. BioTechniques 2018, 37. [DOI] [PubMed] [Google Scholar]
- 69.Hooker JM, Munro TA, Beguin C, Alexoff D, Shea C, Xu Y, Cohen BM: Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology 2009, 57:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gantz SC, Ortiz MM, Belilos AJ, Moussawi K: Excitation of medium spiny neurons by ‘inhibitory’ ultrapotent chemogenetics via shifts in chloride reversal potential. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goossens MG, Larsen LE, Vergaelen M, Wadman W, Van den Haute C, Brackx W, Proesmans S, Desloovere J, Christiaen E, Craey E, et al. : Level of hM4D(Gi) DREADD Expression Determines Inhibitory and Neurotoxic Effects in the Hippocampus. eNeuro 2021, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers S, Rozman PA, Valero M, Doyle WK, Buzsaki G: Mechanisms and plasticity of chemogenically induced interneuronal suppression of principal cells. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samaranch L, San Sebastian W, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, DeArmond S, Forsayeth J, Bankiewicz KS: AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther 2014, 22:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isler K, van Schaik CP: Metabolic costs of brain size evolution. Biol Lett 2006, 2:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tran FH, Spears SL, Ahn KJ, Eisch AJ, Yun S: Does chronic systemic injection of the DREADD agonists clozapine-N-oxide or Compound 21 change behavior relevant to locomotion, exploration, anxiety, and depression in male non-DREADD-expressing mice? Neurosci Lett 2020, 739:135432. [DOI] [PubMed] [Google Scholar]
- 76.Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, Aryal DK, Farrell MS, Lowery-Gionta E, Olsen RH, et al. : Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacology 2016, 41:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo W, Wan X, Ma L, Zhang J, Hashimoto K: Abnormalities in the composition of the gut microbiota in mice after repeated administration of DREADD ligands. Brain Research Bulletin 2021. [DOI] [PubMed] [Google Scholar]
- 78.Koga K, Shiraishi Y, Yamagata R, Tozaki-Saitoh H, Shiratori-Hayashi M, Tsuda M: Intrinsic braking role of descending locus coeruleus noradrenergic neurons in acute and chronic itch in mice. Mol Brain 2020, 13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tremblay S, Acker L, Afraz A, Albaugh DL, Amita H, Andrei AR, Angelucci A, Aschner A, Balan PF, Basso MA, et al. : An Open Resource for Non-human Primate Optogenetics. Neuron 2020, 108:1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Messinger A, Sirmpilatze N, Heuer K, Loh KK, Mars RB, Sein J, Xu T, Glen D, Jung B, Seidlitz J, et al. : A collaborative resource platform for non-human primate neuroimaging. Neuroimage 2021, 226:117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein C, Evrard HC, Shapcott KA, Haverkamp S, Logothetis NK, Schmid MC: Cell-Targeted Optogenetics and Electrical Microstimulation Reveal the Primate Koniocellular Projection to Supra-granular Visual Cortex. Neuron 2016, 90:143–151. [DOI] [PubMed] [Google Scholar]
- 82.Galvan A, Caiola MJ, Albaugh DL: Advances in optogenetic and chemogenetic methods to study brain circuits in non-human primates. J Neural Transm (Vienna) 2018, 125:547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, Ozawa K, Isa T, Yamamori T: Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res 2014. [DOI] [PubMed] [Google Scholar]
- 84.Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, Xu Q, Liu R, Lu C, Chu J, et al. : A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci 2016, 19:1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Shamayleh Y, Horwitz GD: Primate optogenetics: Progress and prognosis. Proc Natl Acad Sci U S A 2019, 116:26195–26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinohara Y, Konno A, Takahashi N, Matsuzaki Y, Kishi S, Hirai H: Viral Vector-Based Dissection of Marmoset GFAP Promoter in Mouse and Marmoset Brains. PLoS One 2016, 11:e0162023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El-Shamayleh Y, Kojima Y, Soetedjo R, Horwitz GD: Selective Optogenetic Control of Purkinje Cells in Monkey Cerebellum. Neuron 2017, 95:51–62 e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martel AC, Elseedy H, Lavigne M, Scapula J, Ghestem A, Kremer EJ, Esclapez M, Apicella P: Targeted Transgene Expression in Cholinergic Interneurons in the Monkey Striatum Using Canine Adenovirus Serotype 2 Vectors. Front Mol Neurosci 2020, 13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]


