Abstract
Active wound dressing with physicochemical and biological characteristics is more effective in healing diabetic foot ulcer (DFU). In this study, a 3-layer electrospun nanofiber wound dressings was fabricated, while its outer, middle and inner layers of the scaffold were made of PCL, PCL/collagen and collagen nanofibers, respectively. Various amounts of Melilotus officinalis extract were also loaded in the collagen nanofibers as a biologically active compound. The diameter and morphology of the obtained nanofibers were investigated by scanning electron microscopy (SEM) and FT-IR spectroscopy to analyse the composition of prepared dressings. The efficacy of the fabricated dressings as wound healing agent was assessed in streptozotocin-induced diabetic rats. The results demonstrated that the mean diameter of nanofibers are 373 ± 179 nm, 266 ± 108 nm, 160 ± 52 nm, and 393 ± 131 nm for PCL, PCL/collagen, pure collagen, and collagen nanofibers containing 0.08 g extract, respectively. The histo-pathology and histomorphometry assessments demonstrate the herbal extract-loaded electrospun dressings (especially containing 0.08 g of the extract) are promising in improving the diabetic ulcer healing. Our results indicated that the combination of drug did not compromise the physicochemical characteristics of wound dressing, while improving its biological activities.
Keywords: PCL, Collagen, Melilotus officinalis extract, Wound dressing
Introduction
Diabetes is considered as one the most life threatening diseases with estimated 592 million patients around 2035[1]. Some patients suffer from diabetic wounds (diabetic foot ulcer with around 15–25% of cases) that may result in amputation in chronic conditions [2, 3]. The healing complexity arises from highly inflammatory wound environment, microbial infections, overexpression of enzymes (such as MMP-9) and diseases of peripheral vascular system [4, 5]. In this respect, providing wound healing procedures are in demand. Until now, various kinds of dressings have been proposed for wound healing[6–8], however, electrospun nanofiber dressings having prominent physico-chemical properties illustrated special characteristics against diabetic ulcers [9, 10]. Indeed, nanofiber structures mimicking the native extracellular matrix (ECM) would provide biophysical and biochemical cues for skin cell conduction, proliferation and differentiation. Furthermore, due to their excellent porous morphology and designable 3D geometry, these dressings would help proper oxygen permeability and exudate absorption [10, 11]. Meanwhile, these structures could be loaded with different drugs to improve the healing process [12]. A variety of different polymers such as polyurethane (PU) [13], poly-L-lactic acid (PLLA) [14], polyvinylalcohol (PVA) [15] and polyvinylpyrolydone (PVP) [16] have been utilized to prepare electrospun nanofibrous wound dressings. Polycaprolactone (PCL) is one of the most commonly used synthetic polymers in different biomedical fields [17]. Being biocompatible and biodegradable, PCL demonstrate unique properties to meet the requirements for appropriate wound dressing production [17]. Pure electrospun PCL nanofibers have shown to support different wound healing processes, however, its hydrophobic nature and slow degradation resulted in the idea of developing biopolymer-blended PCL nanofibrous scaffolds. In this regard, electrospun PCL nanofibers blended with biopolymers such as chitosan [18, 19] and gelatin [20, 21] have been reported. Also, another biopolymer, collagen, is utilized to improve biofunctionality of PCL polymer scaffolds [22–24]. Collagen as the most abundant ECM protein provides a bio-structural scaffold to support cellular binding, proliferation, differentiation and migration [25]. Nevertheless, pure collagen nanofiber scaffolds show weak mechanical properties that requires blending with synthetic polymers such as PCL for wound healing applications. To render additional bio-activity to the electrospun wound dressings, different herbal extracts have been added to nanofibers [26, 27]. Previously, Melilotus officinalis extract has been formulated in ANGIPARS™ and expected to enhance angiogenesis for dermal regeneration and wound healing [28, 29]. Accordingly, the Melilotus officinalis extract has been incorporated inside nanofibrous dressings to investigate their wound healing capabilities [30, 31]. In fact, electrospun nanofibrous offer a membrane for the direct incorporation and sustained release of drugs and other bioactive compounds for a long period. Furthermore, the unique characteristics of these types of nanofibrous such as high porosity, large surface area to volume ratio, high drug loading and encapsulation efficiency, ability to modulate release and also its cost effectiveness makes them suitable in various drug delivery applications.
In the present study, 3-layered electrospun nanofibrous wound dressings comprised of PCL, collagen and Melilotus officinalis extract was fabricated. The dressing layers were made of nanofibrous PCL, PCL-collagen and collagen-extract for outer, middle and inner layers, respectively. In such 3-layered structure, collagen-extract layer would promote angiogenesis along with providing a substrate for skin cell attachment and functions. Also, it has a proper gelation capability that helps absorption of wound exudates. The middle layer (PCL-Collagen) and outer layer PCL nanofibers would robust mechanical properties of the collagen layer. Also, PCL nanofibers form a physical barrier against any contaminations. The morphology and diameter of obtained nanofibers for each layer were analyzed by SEM. Also, composition of the resultant scaffolds was investigated by FT-IR spectroscopy. Thereafter, cyto-compatibility of the electrospun dressings on L929 cells was evaluated by MTT assay. Finally, in vivo studies were performed on rat models of diabetic ulcer in 6 groups. The results illustrated the special wound healing capabilities of 3-layered electrospun dressings containing Melilotus officinalis extract.
Materials and methods
Materials
Poly (Ɛ-caprolactone) polymer with molecular weight of 70 kDa was received from Hangzhou Ruijiang Chemical Co., Ltd, China. Collagen type I was extracted from rat tail according to our previous study [32]. Glacial acetic acid, chloroform and methanol were obtained from Merck Co., Germany. Melilotus officinalis extract was kindly donated by NanoBioPharma Ltd, Tehran, Iran. All of the materials were utilized without further purification. Lab-scale electrospinning set was purchased from Fanavaran Nano-Meghyas Co. Ltd, Tehran, Iran.
Preparation of electrospun scaffolds
The present electrospun wound dressing was designed based on 3-layered structure. Accordingly, different solutions were prepared. The outer layer of the dressing was made of PCL polymer in a mixture of chloroform/methanol [4/1, v/v] at a concentration of 10% (wt.). For the second or middle layer, PCL polymer was dissolved in glacial acetic acid to prepare a solution of 11% (wt.) and collagen type I was prepared in 90% acetic acid to obtain a solution of 11% (wt.). Then, a blend solution of PCL and collagen was provided with a weight ratio of 50/50, v/v. For electrospinning of the third or inner layer of the wound dressing, collagen 10% (wt.) containing different amounts of the Melilotus officinalis extract was prepared. In this regard, three weight amounts of 0.02, 0.04 and 0.08 g extract solution (20% wt. in DMSO) were added to the 1 g of collagen solution and stirred thoroughly. Thereafter, for electrospinning of each of the mentioned-layers, as-prepared solutions were loaded into 5 ml syringes ended to a 23 G needle. Then, before starting the electrospinning, an aluminium foil was wrapped around a rotating drum to collect the nanofibers. The rotation rate was set at 100 rpm and electrospinning was performed at a high voltage of 15 kV, a feeding rate of 1 ml/h, spinneret to collector distance of 10 cm and at about 30 ℃. The resultant nanofibrous scaffolds were preserved for further investigations.
Characterization
Scanning electron microscopy (SEM)
To observe the surface morphology and diameter of the obtained nanofibers, SEM (XL 30, Philips, USA) was used at an accelerating voltage of 25.0 kV. Prior to observation, a piece of each sample including each of the electrospun layers of the dressing was sputter-coated with gold and then, micrographs were recorded. Thereafter, specimen images were analysed by image analysis software (imagj.nih.gov/ij) to calculate the average size of about 100 nanofibers.
ATR-FTIR analyses
For compositional evaluation of the prepared samples including PCL, collagen, collagen-extract and pure extract, ATR-FTIR spectrometry of specimens was carried out using an Equinox 55, Bruker instrument (Germany). The spectra were obtained at a resolution of 4 cm−1 in the range of 4000–500 cm−1.
Viability of cells on electrospun scaffolds
The effect of the prepared 3-layered electrospun wound dressing materials on the viability and proliferation rate of L929 mouse skin fibroblast cells was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. First, circular specimens of 3-layered electrospun scaffolds (n = 5) having different amounts of the extract (0.02, 0.04 and 0.08 g per 1 g of collagen solution) or lack of the extract were sterilised with UV-irradiation (20 min) and gentamycin. Then, the samples were put into 96-well plates and 1 × 104 L929 cells were seeded on the collagen side of the 3-layered scaffolds in DMEM supplemented with 10% FBS and 1% pen/strep. Finally, the plates were kept in an incubator at 37 °C in humidified atmosphere with 5% CO2. At pre-set times including day 1, 3 and 5, the media on the seeded cells was aspirated and then, MTT solution (100µL) was added to the cell culture wells. Thereafter, the MTT solution was removed and 100 µL of DMSO was replaced to dissolve the formazan crystals. Finally, the absorbance of the specimens was read at 570 nm by a plate reader.
In vivo studies of wound healing
Diabetes induction in rats
The in vivo studies were performed according to the approval of Ethics committee of Tehran University of Medical Sciences, Tehran, Iran. Male Wistar rats (5 weeks old) were divided into 6 groups of 6 ones in the negative control (without any treatment with wound dressings or extract), positive control (treated with Angipars drug), and 4 other test groups of electrospun wound dressings lack of or having different amounts of the extract (0.02, 0.04 and 0.08 g). The induction of diabetes was done using intraperitoneal injection of streptozotocin drug (STZ, 55 mg/kg of the rat body weight). After 48 h, the level of blood glucose was measured in rats and the animals with glucose level higher than 280 mg/dL were considered to be diabetic. Also, the diabetic animals showed increased urination and a strong smell of the urine that stably continued until 18 days of the investigation. The animals were randomly divided into 6 groups of the experiment.
Rat diabetic wound dressing
According to the above-mentioned animal groups, rats were anesthetized using ketamine/xylazine (4:1, v/v) and then, dorsal part of animal bodies were completely shaved and sterilized. Subsequently, one full-thickness excision (2 × 2cm2) was made on each of the shaved rats. Finally, animal wounds were treated with a thin layer of commercial ANGIPARS™ 3% gel (positive control group), 3-layered electrospun dressing lack of the extract or each of the extract-loaded 3-layered wound dressings (0.02, 0.04 and 0.08; test groups). For the negative control group, no treatment with extract or electrospun dressings was done and it was only performed with conventional dressings.
Histopathology study
Diabetic rats of the mentioned groups were euthanized 7, 14 and 18 days after treatment and the tissues of their skin were harvested and then, fixed in formalin (10%, pH: 7.26) for 48 h. Thereafter, the fixed specimens were paraffin embedded and 5 µm-thick sections were prepared. Then, staining of the obtained sections was done using haematoxylin and eosin (H&E) and also, Masson’s trichrome (MT). Finally, resultant tissue slides were analysed utilizing light microscopy (Olympus BX51; Olympus, Tokyo, Japan). Accordingly, some parameters such as epithelialization, infiltration of inflammatory cells and formation of granulation tissue have been evaluated.
Histo-morphometry analysis
For evaluation of the rate of epithelialization, a semi-quantitative 5 point scale including 0 (0% of epithelialization), 1 (25%), 2 (50%), 3 (75%), and 4 (100%) was utilized and a comparative analysis was done. Furthermore, the density of deposited collagen was evaluated by Image-Pro Plus® V.6 (Media Cybernetics, Inc., Silver Spring, USA) to analyse histomorphometry in samples.
Statistical analysis
Kruskal Wallis analyses and Dunn’s test post-hoc were done to compare the results with considering P values of less than 0.05 as statistically significant results (SPSS software, USA).
Results and discussion
Morphology of electrospun nanofibers
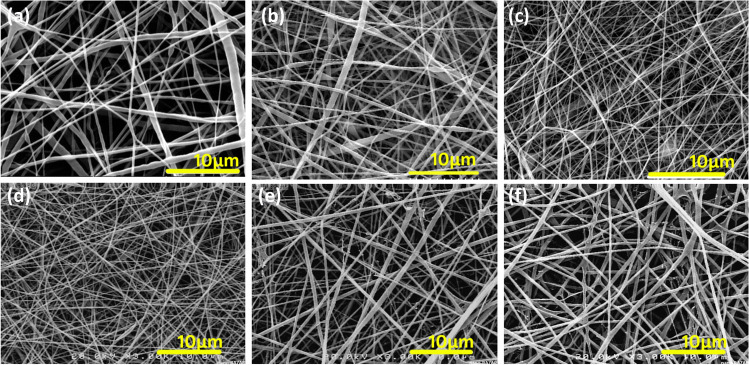
The presented wound dressing was designed as a 3-layered electrospun structure. The outer layer was made of PCL nanofibers using chloroform/methanol (4/1, v/v). It is expected that this 3-layered structure would mimic the structure of natural skin. The outer PCL layer is hydrophobic and might provide a protection against environmental contaminations, meanwhile, improving the handling of the collagen-based dressings. The inner layer was comprised of collagen type I that is the main component of skin ECM. This layer was loaded with different amounts of Melilotus officinalis extract. As the collagen is hydrophilic and could not directly interact with hydrophobic PCL, a middle layer of PCL/Collagen was added. According to the Fig. 1, PCL, PCL/collagen, pure collagen and extract-loaded collagen nanofibers show smooth and bead-less morphologies. It is clear that incorporation of herbal extract had no adverse effects on the nanofibers. The mean diameter of the fibers are 373 ± 179 nm, 266 ± 108 nm, 160 ± 52 nm, 153 ± 31 nm, 225 ± 72 nm and 393 ± 131 nm for PCL, PCL/collagen, pure collagen, collagen nanofibers containing 0.02 g, 0.04 g and 0.08 g extract, respectively. For electrospinning of blend PCL/collagen solutions, acetic acid solvent was utilized. As Fig. 1a-b shows, addition of collagen to PCL solution for the middle layer led to the smaller fibers. Electrospinning of acetic acid PCL solutions result in finer fibers compared to chloroform/methanol solutions. It could be attributed to the higher conductivity of acid solvent in comparison with the chloroform/methanol. As the solvent for PCL and collagen is glacial acetic acid and 90% acetic acid, respectively, it was resulted in lowering the diameter of nanofibers, in spite of the increased polymer concentrations. Also, Fig. 1c-f illustrates the changes in size of electrospun collagen nanofiber layer at its pure or plant extract added form. It is obvious that increasing the amount of extract led to the formation of thicker fibers. SEM micrographs of 3-layered electrospun wound dressings are shown in Fig. 2.
Fig. 1.
SEM images of electrospun nanofibers of PCL (a), PCL/collagen (b), pure collagen (c), and collagen nanofibers containing 0.02 g (d), 0.04 g (e) and 0.08 g extract (f)
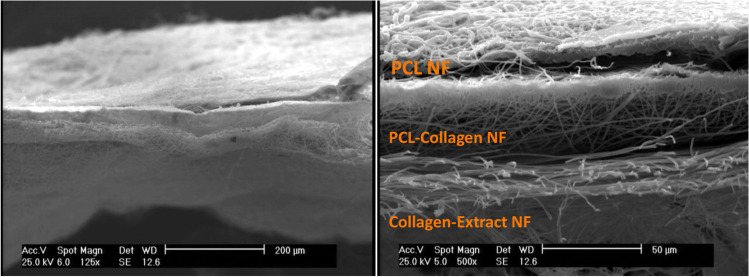
Fig. 2.
SEM micrograph of 3-layered structure of the electrospun wound dressing containing PCL NF, PCL/Collagen and Collagen-Extract NF layers (Scale bars: 200 & 50 µm)
FT-IR analyses
Figure 3 shows the FT-IR spectra of different compositions in the 3-layered electrospun wound dressing. As mentioned before, the outer and middle layers of the dressings are made of PCL and PCL/collagen, respectively.
Fig. 3.
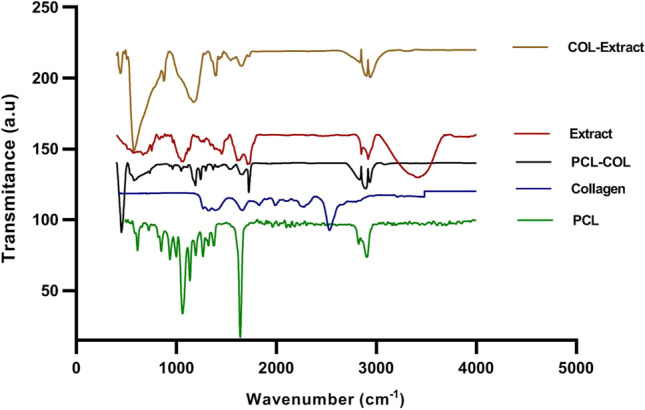
ATR-FTIR spectra of PCL, collagen, Melilotus officinalis Extract and collagen-extract samples
According to the ATR-FTIR spectrum of PCL nanofibers, the characteristic peaks at 2950 cm−1 and 2870 cm−1 could be attributed to the asymmetric and symmetric stretching of CH2, respectively. The peak at 1725 cm−1 is assigned to stretching of carbonyl. Also, other peaks at 1171 cm−1 and 1240 cm−1 could be ascribed to the symmetric and asymmetric stretching of C–O–C, respectively [33, 34]. The collagen spectrum illustrates characteristic bands at 3265 cm−1 (stretching of O–H or N–H), 1638 cm−1 (amide I, C = O stretching), 1526 cm−1 (amide II, N–H bending), 1231 cm−1 (amide III) and 1438 cm−1 (-COO-) [35, 36]. The spectrum of the plant extract shows a broad band at 3450 cm−1 (stretching of O–H or N–H) and also, 2922 cm−1 (symmetrical and asymmetrical stretching of C-H) and 1638 cm−1 (amide I, C = O stretching) [36]. The characteristic bending vibration bonds of O–H or N–H (3450 cm−1), C = O (1638 cm−1), amide II, N–H (1526 cm−1), -COO- (1438 cm−1), amide III (1231 cm−1), and C-H (2922 cm−1) were observed collagen-extract nanofibers. Furthermore, the spectra of collagen-extract show the presence of characteristic absorption bonds of extract, which proved the successful incorporation of extract into collagen-extract nanofibers [37, 38].
Cell study and MTT assay
The biocompatibility of PCL and PCL/collagen scaffolds for tissue engineering applications has been previously demonstrated [32, 39]. Figure 4 illustrates the viability percentage of the L929 fibroblast cells on different electrospun nanofibrous wound dressings. As it is shown, all dressings including extract-free nanofibers or specimens containing 0.02 g, 0.04 g or 0.08 g extract demonstrated appropriate cell viability in 5 days of investigation. However, significant differences were observed mainly between the extract-free dressings and extract-loaded ones (*p value < 0.05, **p value < 0.01). Totally, the results of cell study support the safe application of the prepared electrospun dressings composed of PCL and collagen nanofibers loaded with Melilotus officinalis extract.
Fig. 4.
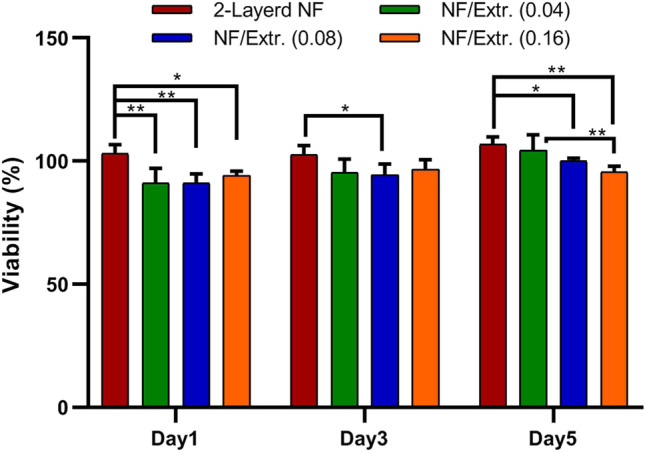
MTT assay of L929 fibroblasts on different electrospun nanofibrous dressings including extract free 3-layered nanofibers or dressings containing 0.02 g, 0.04 g or 0.08 g extract
Histological analysis of the skin wounds
Figures 5, 6 and 7 depict H&E stained diabetic skin specimens. Accordingly, in the control group, the results illustrated infiltration of polymorphonuclear inflammatory cells and also, formation of granulation tissue. However, the epidermal layer is not formed and a crusty scab could be observed on the wound (Fig. 5). The positive control (ANGIPARS-treated group) at day 7 exhibited severe inflammatory cells infiltration into the defect area. A thick layer of epithelial cells could be detected at day 14, and the inflammation in defect area is significantly reduced in comparison with the negative control. Finally, the re-epithelialization process is completed at 18-day and also, skin-appendage rejuvenation is evidently occurred (Figs. 5, 6 and 7). Investigations on group NF demonstrated existence of a thick crusty scab on the wound at days 7 and 14 post treatments. Also, proliferation of the epidermis is started at day 18, although, being incomplete. In addition, the inflammation is significantly reduced compared to the specimens of days 7 and 14. The test group 1 (NF + 0.02) showed a close similarity to the negative control so that a crusty scab on the wound area without the epidermal formation could be seen at days 7, 14 and 18. Also, the presence of inflammation in wound area was evident (Fig. 6). At day 18, formation of epidermis and dermis is started and inflammatory responses are notably reduced (Fig. 7). Histological analyses of group 2 (NF + 0.04) illustrated epidermal proliferation and an increase in the epidermal layer 18 days post-treatment. It is observed that granulation tissue and inflammatory responses were gradually reduced in 18 days of treatment (Fig. 7).
Fig. 5.
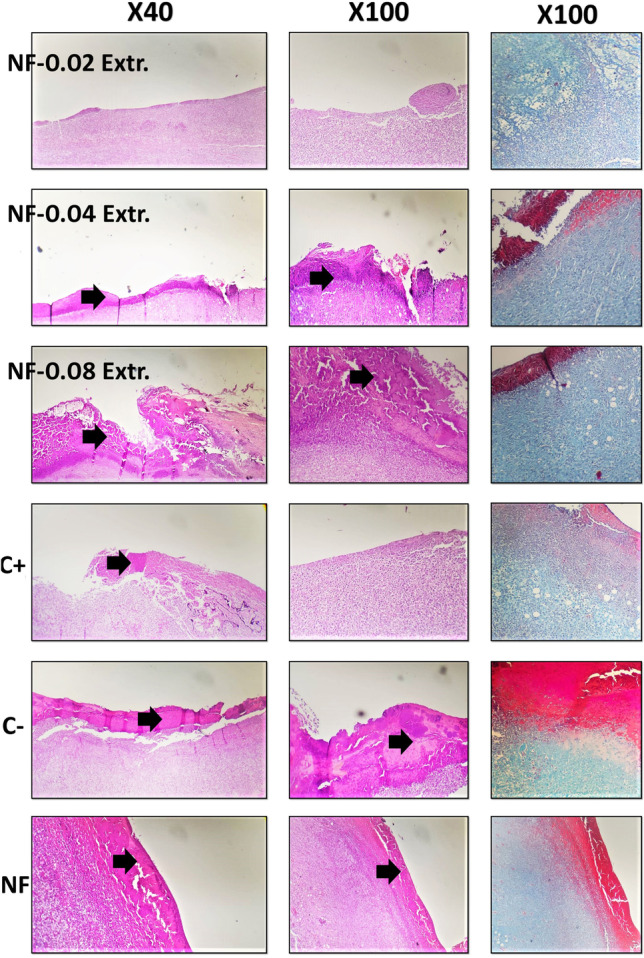
H&E and MT stained microscopic sections of the skin samples of the wounds after 7 days of treatment. Black arrows: crusty scab
Fig. 6.
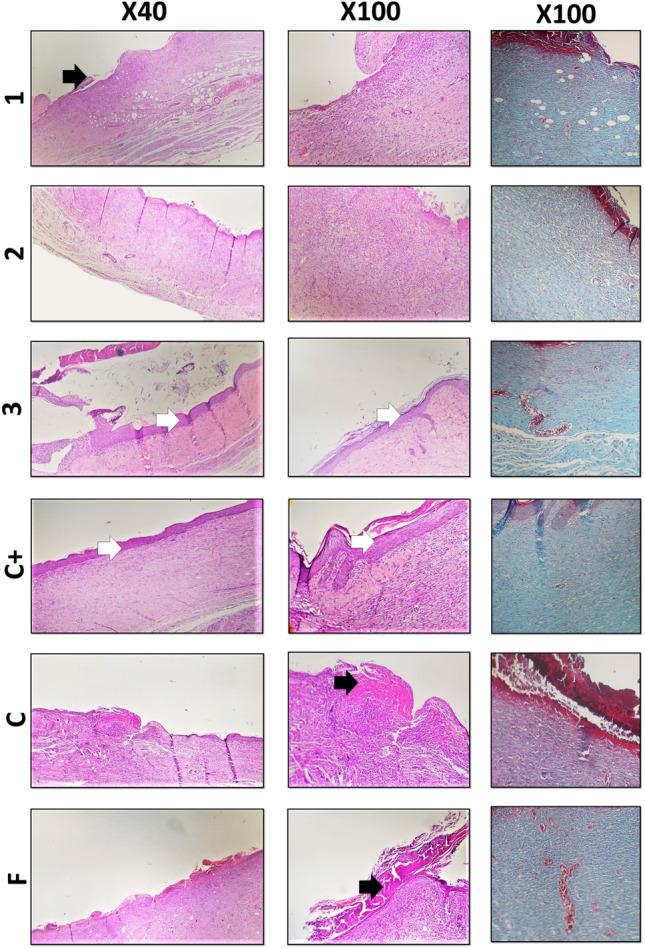
H&E and MT stained microscopic sections of the skin samples of the wounds after 14 days of treatment. Black arrows: crusty scab, white arrows: epithelial layer
Fig. 7.
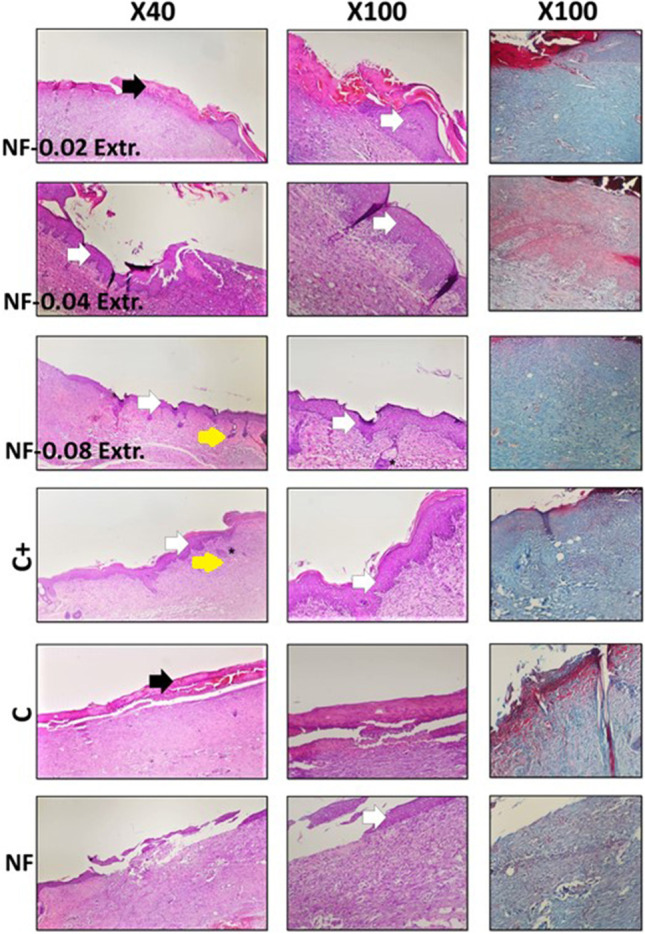
H&E and MT stained microscopic sections of the skin samples of the wounds after 18 days of treatment. Black arrows: crusty scab, white arrows: epithelial layer, Yellow arrow: hair follicle, and asteroids: Sebaceous gland
The evaluation of specimens in group 3 (NF + 0.08) revealed a notable decrease in inflammation at day 14 compared with the others. This group illustrated more similarities to a normal skin structure, with a thin epidermis and the presence of normal rete ridges and skin-appendage rejuvenation. It could be concluded that this group illustrates the best results in comparison to the negative control and other test groups.
Histomorphometric analysis
The histo-morphometric analyses were performed 18 days post treatment and the results are presented in the Table 1. Amongst all groups, re-epithelialization in the extract-free scaffolds and negative control groups was least and it was mainly filled with immature granulation tissue (P < 0.05). The best re-epithelialization was observed in the test group 3 (NF + 0.08). Overall, the healing capability of the scaffold dressings of test group 3 was more similar to that of the positive control at day 18 and even much better, which has the best cosmetic appearance; indeed, this nanofibrous dressing showed formation of the epidermal layer with normal thickness and rejuvenation of the hair follicles and skin appendages (Sebaceous gland).
Table1.
Histomorphometric analysis of different experimental groups
| Group | Epitheliogenesis Score |
|---|---|
| NF (lack of the Extract) |
0,0,0,0 (7 d) 1,1,2,0 (14 d) 2,3,3,1 (18 d) * |
| NF-0.02 g Extract |
0,0,0,0 (7 d) 1,0,0,1 (14 d) 2,1,0,2 (18 d) |
| NF-0.04 g Extract |
0,0,0,0 (7d) 1,1,1,1 (14 d) 3,2,2,3 (18 d) * |
| NF-0.08 g Extract |
0,0,0,0 (7 d) 2,1,2,2 (14 d) * 4,3,4,4 (18 d) ** |
| C + |
1,0,0,0 (7 d) 2,3,2,1 (14 d) ** 4,4,3,4 (18 d) ** |
| C− |
0,0,0,0 (7 d) 0,0,0,0 (14 d) 1,1,0,0 (18 d) |
*, **, ***: values indicate treatment group versus control group; * P < 0.05, ** P < 0.01, ***P < 0.001
As the wound healing procedure is dependent to the amount of collagen synthesis and deposition, therefore, sections of animal skin tissues were evaluated by the Masson’s Trichrome (MT) staining to further investigate the effect of different nanofibrous dressings on wound healing. The results demonstrated that among the experimental groups, group 3 (NF + 0.08) had the greatest collagen synthesis. In contrast, the amount of collagen synthesis and deposition in the wounds of negative control group was lowest. According to the Table 2, increasing the amount of the extract in the electrospun dressing resulted in the improvement in the percentage of collagen production and deposition. In this regard, collagen density (%) was investigated morphologically in the stained wound sections. Through MT staining, collagen fibers become green. Five randomly-selected high-power fields (× 400) were taken for any sample and then, percentage of the deposited collagen was analysed (Image Pro-Plus software, version 6, Media Cybernetics, USA).
Table 2.
The collagen density in different experimental groups
| Group | Collagen density (%) |
|---|---|
| NF (lack of the Extract) |
31.7 ± 5.3(7 d) 62.2 ± 4.7(14 d) *** 55.0 ± 5.7(18 d) |
| NF-0.02 Extract |
41.5 ± 3.1(7 d) ** 61.5 ± 4.2(14 d) *** 57.0 ± 2.9(18 d) * |
| NF-0.04 g Extract |
48.5 ± 5.5(7 d) *** 60.5 ± 4.9(14 d) *** 73.2 ± 3.8(18 d) *** |
| NF-0.08 g Extract |
45.5 ± 3.6(7 d) *** 58.5 ± 5.5(14 d) *** 82.0 ± 5.4(18 d) *** |
| C + (treated with Angipars) |
48.2 ± 4.5(7 d) *** 72.2 ± 4.0(14 d) *** 82.5 ± 4.4(18 d) *** |
| C− |
28.7 ± 3.3(7 d) 37.7 ± 5.7(14 d) 46.2 ± 6.1(18 d) |
*, **: values indicate treatment group versus control groups (empty control); * P < 0.05, ** P < 0.01
Conclusion
Diabetic wounds and specially foot ulcers are among the most problematic medical issues that may lead to lower limb amputations. Therefore, electrospun nanofibrous wound dressings have been introduced to enhance the ulcer healing processes. In the present study, 3-layered electrospun nanofibrous dressings containing Melilotus officinalis extract were fabricated out of PCL, PCL-collagen and extract-loaded collagen layers. It is previously shown that Melilotus officinalis extract could successfully regenerate tissues of the skin at the diabetic ulcer. The mechanism of action is proposed to be done by stimulating the angiogenesis. SEM analyses illustrated the smooth and defect-free structure of the nanofibers in all three layers. The in vitro cell studies demonstrated appropriate viability of fibroblast cells seeded on the collagen layer of the 3-layered dressings. Also, through the 18 days of in vivo studies, 0.08 g extract-loaded dressings demonstrated proper re-epithelialization of the diabetic wounds and collagen production and deposition in the newly formed skin. The results are comparable with the ANGIPARS™ formulation, however, a 3-layered nanofibrous wound dressing would provide a better handling for application on the wound surface along with its capacity to be loaded with other drugs. In this regard, antimicrobial molecules or growth factors could be sequestered inside the nanofibers to prevent infections and even more accelerate wound healing process. Therefore, according to the findings, the prepared electrospun nanofibrous dressing containing Melilotus officinalis extract would be promising in diabetic ulcer healing.
Acknowledgements
Research reported in this publication was supported by a grant from the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences in Iran.
Declarations
Ethical approval
The procedures were performed in accordance with the ethical approval of Tehran University of Medical Sciences and with the 1964 Helsinki Declaration and subsequent amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Ali Derakhshan, Email: ma_derakhshan@sums.ac.ir.
Kobra Omidfar, Email: omidfar@tums.ac.ir.
References
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine. 2010;38(11):602–606. doi: 10.1016/j.mpmed.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui AR, Bernstein JM. Chronic wound infection: Facts and controversies. Clin Dermatol. 2010;28(5):519–526. doi: 10.1016/j.clindermatol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 5.Dinh T, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong L, et al. Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(36):30103–30114. doi: 10.1021/acsami.8b09191. [DOI] [PubMed] [Google Scholar]
- 7.Griffin DR, et al. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14(7):737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano. 2019;13(10):11686–11697. doi: 10.1021/acsnano.9b05608. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, et al. Asymmetric wettable composite wound dressing prepared by electrospinning with bioinspired micropatterning enhances diabetic wound healing. ACS Appl Bio Mater. 2020;3(8):5383–5394. doi: 10.1021/acsabm.0c00695. [DOI] [PubMed] [Google Scholar]
- 10.Memic A, et al. Latest progress in electrospun nanofibers for wound healing applications. ACS Appl Bio Mater. 2019;2(3):952–969. doi: 10.1021/acsabm.8b00637. [DOI] [PubMed] [Google Scholar]
- 11.Stapelfeldt K, et al. Controlling the multiscale structure of nanofibrous fibrinogen scaffolds for wound healing. Nano Lett. 2019;19(9):6554–6563. doi: 10.1021/acs.nanolett.9b02798. [DOI] [PubMed] [Google Scholar]
- 12.dr M et al. Electrospun nanofiber-based drug delivery platform: advances in diabetic foot ulcer management. Expert Opin Drug Del. 2020; 1–18. [DOI] [PubMed]
- 13.Sofi HS, et al. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int J Pharm. 2019;569:118590. doi: 10.1016/j.ijpharm.2019.118590. [DOI] [PubMed] [Google Scholar]
- 14.Bi H, et al. In Vitro and in vivo comparison study of electrospun PLA and PLA/PVA/SA fiber membranes for wound healing. Polymers. 2020;12(4):839. doi: 10.3390/polym12040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norouzi MA, et al. Flower buds like PVA/ZnO composite nanofibers assembly: Antibacterial, in vivo wound healing, cytotoxicity and histological studies. Polym Test. 2021;93:106914. doi: 10.1016/j.polymertesting.2020.106914. [DOI] [Google Scholar]
- 16.Rosa RM, et al. Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater Lett. 2017;207:145–148. doi: 10.1016/j.matlet.2017.07.046. [DOI] [Google Scholar]
- 17.Mochane MJ, et al. Morphology and properties of electrospun PCL and its composites for medical applications: a mini review. Appl Sci. 2019;9(11):2205. doi: 10.3390/app9112205. [DOI] [Google Scholar]
- 18.Fahimirad S, et al. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. 2021;259:117640. doi: 10.1016/j.carbpol.2021.117640. [DOI] [PubMed] [Google Scholar]
- 19.Chanda A, et al. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int J Biol Macromol. 2018;116:774–785. doi: 10.1016/j.ijbiomac.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 20.Rather HA, et al. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioactive Materials. 2018;3(2):201–211. doi: 10.1016/j.bioactmat.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unalan I, et al. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics. 2019;11(11):570. doi: 10.3390/pharmaceutics11110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JI, Kim CS. Harnessing nanotopography of PCL/collagen nanocomposite membrane and changes in cell morphology coordinated with wound healing activity. Mater Sci Eng, C. 2018;91:824–837. doi: 10.1016/j.msec.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Mohamadi F, et al. Electrospun nerve guide scaffold of poly (ε-caprolactone)/collagen/nanobioglass: an in vitro study in peripheral nerve tissue engineering. J Biomed Mater Res A. 2017;105(7):1960–1972. doi: 10.1002/jbm.a.36068. [DOI] [PubMed] [Google Scholar]
- 24.Park S, et al. Fabrication of strong, bioactive vascular grafts with PCL/collagen and PCL/silica bilayers for small-diameter vascular applications. Mater Des. 2019;181:108079. doi: 10.1016/j.matdes.2019.108079. [DOI] [Google Scholar]
- 25.Dong C, Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8(2):42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Ronca S, Mele E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials. 2017;7(2):42. doi: 10.3390/nano7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilchez A, et al. Applications of electrospun nanofibers with antioxidant properties: a review. Nanomaterials. 2020;10(1):175. doi: 10.3390/nano10010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamimi NK, et al. Topical application of Semelil (ANGIPARS™) in treatment of pressure ulcers: a randomized clinical trial. 2008.
- 29.Bahrami A, et al. Clinical application of oral form of ANGIPARS™ and in combination with topical form as a new treatment for diabetic foot ulcers: a randomized clinical trial. Daru. 2008;16(SUPPL. 1):41–48. [Google Scholar]
- 30.Shahrousvand M, Haddadi-Asl V, Shahrousvand M. Step-by-step design of poly (ε-caprolactone) /chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int J Biol Macromol. 2021;180:36–50. doi: 10.1016/j.ijbiomac.2021.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Mirzaei E, et al. Herbal extract loaded chitosan-based nanofibers as a potential wound-dressing. J Adv Med Sci Appl Technol. 2016;2(1):141–150. doi: 10.18869/nrip.jamsat.2.1.141. [DOI] [Google Scholar]
- 32.Sharifi-Aghdam M, et al. Preparation of collagen/polyurethane/knitted silk as a composite scaffold for tendon tissue engineering. Proc Inst Mech Eng H J Eng Med. 2017; 0954411917697751. [DOI] [PubMed]
- 33.Gautam S, Dinda AK, Mishra NC. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater Sci Eng C. 2013;33(3):1228–1235. doi: 10.1016/j.msec.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Eskandarinia A, et al. A novel bilayer wound dressing composed of a dense polyurethane/propolis membrane and a biodegradable polycaprolactone/gelatin nanofibrous scaffold. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-59931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman MS, et al. Fabrication of biocompatible porous scaffolds based on hydroxyapatite/collagen/chitosan composite for restoration of defected maxillofacial mandible bone. Prog Biomater. 2019;8(3):137–154. doi: 10.1007/s40204-019-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghorbani M, Nezhad-Mokhtari P, Ramazani S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int J Biol Macromol. 2020;153:921–930. doi: 10.1016/j.ijbiomac.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 37.Soofi M, et al. Preparation of nanobiocomposite film based on lemon waste containing cellulose nanofiber and savory essential oil: a new biodegradable active packaging system. Int J Biol Macromol. 2021;169:352–361. doi: 10.1016/j.ijbiomac.2020.12.114. [DOI] [PubMed] [Google Scholar]
- 38.Nisar T, et al. Characterization of citrus pectin films integrated with clove bud essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Int J Biol Macromol. 2018;106:670–680. doi: 10.1016/j.ijbiomac.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 39.Hou J, et al. Sustained release of N-acetylcysteine by sandwich structured polycaprolactone/collagen scaffolds for wound healing. J Biomed Mater Res A. 2019;107(7):1414–1424. doi: 10.1002/jbm.a.36656. [DOI] [PubMed] [Google Scholar]