Abstract
Background
Diabetic retinopathy, which is a common complication of diabetes, is one of the most common reasons of blindness in adults. There are several potential risk factors for diabetic retinopathy such as hypertension (HTN), hyperlipidemia (HLP), high fasting blood sugar (FBS), and high Hemoglobin A1c (HbA1c). Yet, ethnicity is another factor which may contribute to diabetic retinopathy regardless of the potential risk factors mentioned. The aim of this study, therefore, is to find the risk factors associated with diabetic retinopathy in the north of Iran.
Methods
This was a retrospective cohort study including a total of 1,125 patients divided into three groups as follows: (i) patients with no diabetic retinopathy (NDR group; n = 398); (ii) patients with non-proliferative diabetic retinopathy (non-PDR group; n = 408); (iii) patients with proliferative diabetic retinopathy (PDR group; n = 319). The laboratory data were collected from patients for analysis.
Results
Diabetic patients with retinopathy had significantly higher levels of FBS compared with those without retinopathy (p = 0.001). Patients with PDR or non-PDR had higher levels of HbA1c compared with patients without retinopathy (p = 0.001). In contrast, no association was observed between HTN or HLP and diabetic retinopathy. On the other hand, duration of diabetes was another important factor affecting diabetic retinopathy.
Conclusions
Higher levels of FBS and HbA1c were observed in patients with diabetic retinopathy. Monitoring and controlling of FBS and HbA1c of diabetic patients could prevent the occurrence of diabetic retinopathy.
Keywords: Diabetes, Diabetic retinopathy, Risk factors, Proliferative diabetic retinopathy, PDR
Introduction
Diabetic retinopathy is a common microvascular complication of diabetes and the most common reason for visual impairment. There is evidence that neurodegeneration is early characteristic of patients with diabetic retinopathy, and thereby neurovascular complication is considered as a specific characteristic of this condition [1–3].
The number of patients with diabetes is increasing over time. It is estimated that the number of patients with diabetes will rise to 366 million by 2030 [4]. The increase in the number of patients with diabetes means the increased number of patients with diabetic retinopathy. Approximately one-third of patients with diabetes have diabetic retinopathy, and one-third of patients with diabetic retinopathy have vision-threatening retinopathy [5, 6]. It worth mentioning that micro-angiopathy induced by hyperglycemia in patients with diabetes mellitus could cause to vascular leakage, diabetic macular edema, and capillary occlusion progression. On the other hand, capillary occlusion contributes to neovascularization and the proliferative stage of diabetic retinopathy through inducing retinal ischemia and increasing levels of vascular endothelial growth factor (VEGF) [7]. It is well known that controlling of blood sugar levels in diabetic patients by prescribing insulin (diabetes type 1) and metformin and glibenclamide as oral medicine and insulin (diabetes type 2) could significantly reduce the risk of retinopathy progression [8]. Diabetic retinopathy is generally categorized into proliferative diabetic retinopathy (PDR) and non-proliferative diabetic retinopathy (non-PDR) [9].
Diabetic retinopathy is usually asymptomatic at the early stages, and patients with diabetes need to be regularly monitored for timely diagnosis of diabetic retinopathy. Electroretinography (ERG), retinal blood flow and retinal blood vessel calibre are used for initial diagnosis of diabetic retinopathy. On the other hand, laser therapy and vitrectomy surgery are used in the patients with advanced diabetic retinopathy [6, 9]. Diagnosis of patients who are more susceptible to progression to diabetic retinopathy may help physicians to better manage these patients. Although several factors including hypertension (HTP), hyperlipidemia (HLP), and high blood glucose are considered as risk factors for diabetic retinopathy, these are not reported consistently in previous studies [1, 6]. Moreover, differences in ethnicity play an important role in the progression to diabetic retinopathy, as attested by the evidence that there is a higher prevalence of diabetic retinopathy among African Americans compared with white people [6].
Since ethnicity is an important factor in progression to diabetic retinopathy, the common risk factors should be interpreted with regard to this factor. Thus, in this study we set out to assess the association of several common risk factors including HLP, HTN, fasting blood sugar (FBS), hemoglobin A1c (HbA1c), and duration of diabetes with diabetic retinopathy in patients with diabetes. Moreover, using the blood creatinine and blood urea nitrogen (BUN) of our subjects, the status of their kidneys was evaluated.
Methods
Study subjects
A total of 1,125 diabetic patients were enrolled in this retrospective cohort study. Of these, 262 were male and 863 were female, with a mean (SD) age of 56 [10] years. According to different types of diabetic retinopathy, patients were divided into three groups as follows: (i) patients with no diabetic retinopathy (NDR group; n = 398); (ii) patients with non-proliferative diabetic retinopathy (non-PDR group; n = 408); (iii) patients with proliferative diabetic retinopathy (PDR group; n = 319). Written informed consent was obtained from all patients referring to the Ruhani Hospital of Babol, Iran. The study was approved by the Institutional Research Ethics Committee, Ruhani Hospital, Babol University of Medical Sciences.
The present study was done under the ethical principles and the national norms and standards for conducting medical research in Iran (IR.MUBABOL.REC.1399.354).
Ophthalmologic evaluation
Based on fundus photographs, patients with intra-retinal hemorrhages, microvascular abnormalities, or microaneurysms were considered as having diabetic retinopathy and those with retinal neovascularization were considered as having PDR. The PDR group is considered as sight-threatening diabetic retinopathy (STDR) and the non-PDR group is considered as non-STDR.
Data collection
Patients referred to the laboratory where peripheral blood was collected from them after at least 8 h fasting. Then, the variables including creatinine, BUN, cholesterol, triglyceride (TG), FBS, Hb, HbA1c, HLP, and HTN were collected from the subjects. Moreover, age, gender, and duration of diabetes of the patients were recorded. The demographic characteristics and laboratory information of patients are shown in Table 1.
Table 1.
Demographic and clinic features of patients
| Continues variables* | Number | Patients (1,562) |
|---|---|---|
| Age | 1,562 | 56 (10.6) |
| Duration of diabetes | 1,562 | 11 (7.5) |
| Creatinine | 1,055 | 1 (0.5) |
| BUN | 989 | 26 (17.1) |
| Cholesterol | 1,444 | 203 (57.4) |
| TG | 1,456 | 206 (127.2) |
| FBS | 1,531 | 194 (73.6) |
| Hb | 1,088 | 13 (1.6) |
| HbA1c | 988 | 10 (2.0) |
| Categorical variables | Number | Patients (1,562) |
| Gender | 1,562 |
Male (357) Female (1,205) |
| HLP | 1,562 |
Yes (1,086) No (476) |
| HTN | 1,562 |
Yes (660) No (902) |
Note: BUN blood urea nitrogen, TG Triglyceride, FBS fasting blood sugar, Hb hemoglobin, HbA1c hemoglobin A1c, HLP hyperlipidemia, HTN hypertension
Statistical analysis
Data were analyzed using SPSS ver. 24 (IBM, Armonk, NY, USA). The one-way analysis of variance (ANOVA) test was used for continues data, and the categorical data were analyzed by the chi square test or chi square for trend. P-values < 0.05 were considered significant in all statistical tests. Data are presented as mean ± SD.
Results
Demographic and laboratory findings of patients
Demographic and clinical features of patients are shown in Table 1. The percentage of patients in the NDR, non-PDR, and PDR groups was 35.38%, 36.27%, and 28.36%, respectively.
Association between HLP and diabetic retinopathy
Among the 1,125 patients with diabetes, 727 were diagnosed with retinopathy and 398 were identified without retinopathy. The association between HLP and different types of retinopathy was significant (p = 0.001). However, we did not observe any linear association between HLP and increased chance of diabetic retinopathy. There was no significant association between HLP and chance of diabetic retinopathy (OR 1.23; CI [0.90, 1.67]).
Association between HTN and diabetic retinopathy
The association between HTN and different types of retinopathy was not significant (p = 0.95). There was no association between HTN and chance of non-PDR (OR 1.06; CI [0.80, 1.40]) or PDR (OR 1.00; CI [0.74, 1.35]).
Association of cataract with diabetic retinopathy
There was a significant difference between lens status and diabetic retinopathy (p < 0.001). Diabetic patients with incomplete cataract had 98% increased chance of diabetic retinopathy compared with those without cataract (OR 1.98 CI [1.52, 2.59]). As far as CI was concerned, this result was conclusive. Moreover, diabetic patients with complete cataract had a 5.11-fold increase in the chance of diabetic retinopathy compared with those without cataract (OR 5.11 CI [2.47, 10.58]). Both of the results were significant.
Association of duration of diabetes and diabetic retinopathy
There was a significant difference between duration of diabetes and diabetic retinopathy (p < 0.001). Diabetic patients with a 10-year history of diabetes had a 3.56-fold increase in the chance of diabetic retinopathy compared with those with ≤ 5 years of diabetes (OR 3.56 CI [2.52, 5.02]). Regarding its CI, this result was conclusive. Moreover, diabetic patients with > 10 years of diabetes had a 12.05-fold increase in the chance of diabetic retinopathy compared with those with ≤ 5 years of diabetes (OR 12.05 CI [8.06, 18.02]). Both of these results were statistically significant.
In addition, there was a significant difference between duration of diabetic disease and two types of retinopathy. Non-PDR and PDR patients had a longer duration of diabetes compared with patients without retinopathy (p < 0.001) (Fig. 1).
Fig. 1.
Patients with retinopathy had higher duration of diabetescompared with those without retinopathy
FBS
Diabetic patients with retinopathy had significantly higher levels of FBS compared with those without retinopathy. Patients with PDR and non-PDR had higher levels of FBS compared with those without retinopathy (p = 0.001) (Fig. 2).
Fig. 2.
Patients with retinopathy had higher serum levels of FBScompared with those without retinopathy
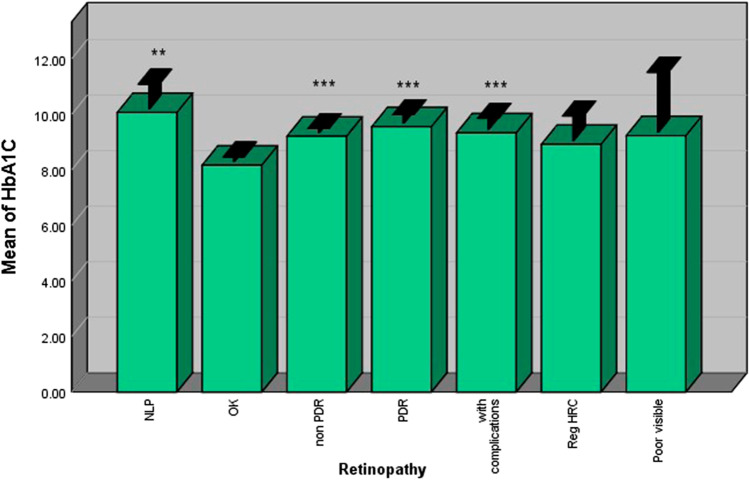
HbA1c
Diabetic patients with retinopathy had significantly higher levels of HbA1c compared with those without retinopathy. PDR and non-PDR patients had higher levels of HbA1c compared with patients without retinopathy (p = 0.001) (Fig. 3).
Fig. 3.
Patients with retinopathy had higher HbA1C levels compared withthose without retinopathy
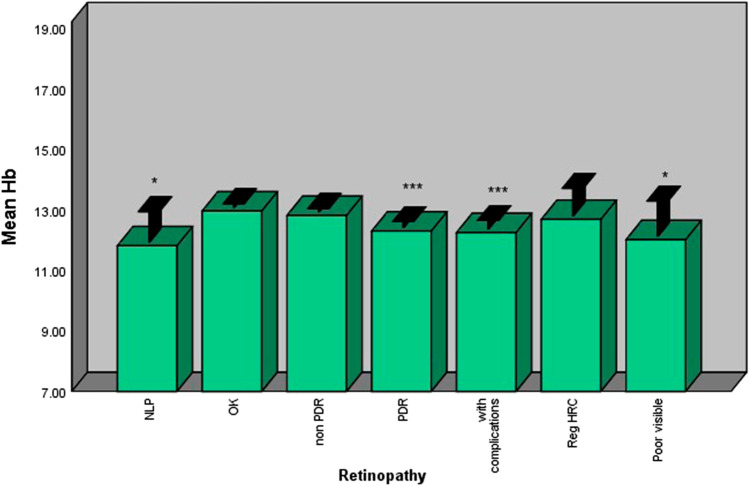
Hb
Diabetic patients with retinopathy had significantly lower levels of Hb compared with those without retinopathy. Patients with PDR had lower levels of Hb compared with those without retinopathy (p < 0.001). However, no difference was observed between non-PDR patients and patients without retinopathy in terms of their Hb levels (Fig. 4).
Fig. 4.
Patients with PDR had lower Hb levels compared with patientswith nonPDR and those without retinopathy
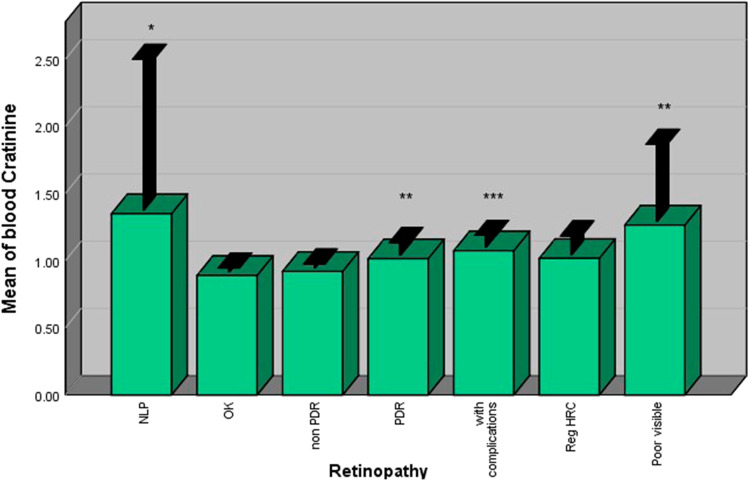
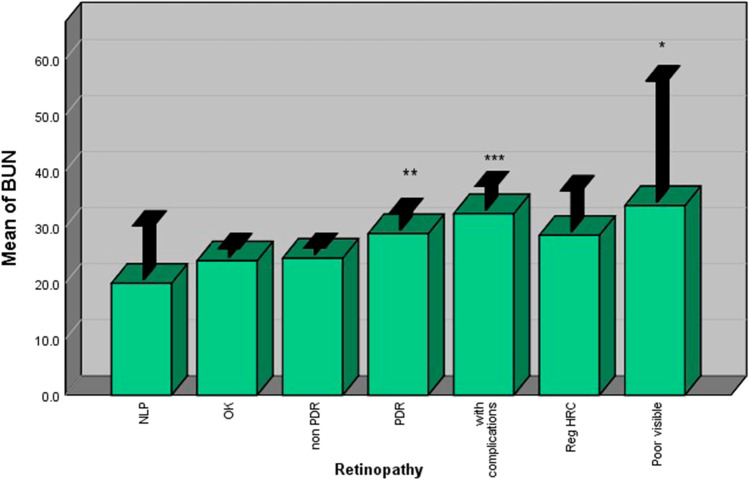
Blood creatinine and BUN
Diabetic patients with retinopathy had significantly higher levels of blood creatinine compared with those without retinopathy. Patients with PDR had higher levels of blood creatinine compared with patients without retinopathy (p < 0.001). However, no significant difference was observed between non-PDR patients and patients without retinopathy (Fig. 5). The same results were also observed for BUN (Fig. 6).
Fig. 5.
Patients with PDR had higher blood creatinine levels comparedwith patients with nonPDR and those without retinopathy
Fig. 6.
Patients with PDR had higher BUN levels compared with patientswith nonPDR and those without retinopathy
Cholesterol and TG
Cholesterol and TG did not have any significant differences between patients without retinopathy and those with non-PDR or PDR (Figs. 7 and 8).
Fig. 7.
Cholesterol levels did not show significant difference betweenstudy subjects
Fig. 8.
TG levels did not show significant difference between studysubjects
Discussion
Diabetic retinopathy, as a consequence of diabetes, is the most common reason for visual impairment and blindness. Several factors such as high blood glucose, HTN, and HLP may contribute to diabetic retinopathy. However, differences ethnicity can also contribute to retinopathy progression, independent of retinopathy risk factors. Controlling diabetic retinopathy will improve the quality of life of patients and reduce the burden of disease and the associated economic problems [4, 6, 9, 11].
We studied 1,125 patients with diabetes or diabetic retinopathy from the north of Iran and assessed the association between potential risk factors and diabetic retinopathy. In general, we did not find any significant association between HLP and diabetic retinopathy. In line with this study, Liu et al. [10] did not report any association between HLP and diabetic retinopathy. Moreover, some other studies showed that patients without diabetic retinopathy and those suffering from this condition had the same TG [12–14]. In contrast, a study reported that patients with diabetic retinopathy had significantly higher TG compared with those without diabetic retinopathy [15]. A global case-control study by Sacks et al. showed that a slightly higher risk of DR in patients with higher levels of TG was insignificant after adjustment for hypertension and HbA1c, highlighting the effect of confounders such as hypertension and HbA1c in the TG levels [16].
When we separately assessed the association between different types of retinopathy and HLP, only an inverse association between HLP and patients with PDR was observed. In other words, patients with HLP are less susceptible to progress to PDR. In contrast, other studies showed that patients with PDR had higher TG compared with non-PDR patients, but the difference was not significant [13, 17]. In general, it can be concluded that there is no association between HLP and diabetic retinopathy. However, more studies are needed to clarify if there is any association between lower lipids and PDR.
Hypertension was another potential factor that was assessed in the study subjects. We showed that hypertension was not a risk factor for developing both types of diabetic retinopathy (non-PDR and PDR). The results of other studies also showed that there is no association between hypertension and risk of diabetic retinopathy [12, 18].
In contrast to HLP and HTN which did not increase the risk of diabetic retinopathy, incomplete and complete cataract were two major reasons for developing retinopathy. Diabetic patients who had incomplete or complete cataract were more susceptible to developing diabetic retinopathy. Thus, patients with cataract should be controlled well to prevent them from developing diabetic retinopathy.
In line with some studies [12, 15, 18], we showed that there are increased levels of FBS and HbA1c in diabetic retinopathy patients compared with patients without diabetic retinopathy. Moreover, the duration of diabetes was an important factor in occurrence of diabetic retinopathy. In our study, patients without diabetic retinopathy had a diabetes history <10 years, and those with diabetic retinopathy had a diabetes history >10 years. We showed that duration of diabetes was the major reason for diabetic retinopathy progression. Moreover, FBS and HbA1c levels gradually and over time increased in the patients, making significant the difference between patients with diabetic retinopathy and those without it. Thus, controlling the FBS of patients without diabetic retinopathy could be helpful and reduce the risk of retinopathy.
Diabetic retinopathy may have a negative effect on the status of patients’ kidneys. In our study, patients with diabetic retinopathy had a significantly higher amount of BUN and blood creatinine compared with patients without diabetic retinopathy. In line with our study, some other studies have shown that patients with diabetic retinopathy have a significantly lower rate of estimated glomerular filtration rate (eGFR) compared with patients without diabetic retinopathy [19, 20].
Conclusions
In general, cataract, duration of diabetes, FBS could be considered as risk factors for diabetic retinopathy. In contrast, we did not find any significant association between HLP or HTN and diabetic retinopathy. On the other hand, patients with diabetic retinopathy are more susceptible to kidney disease.
Declarations
Conflict of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forga L, Goñi MJ, Ibáñez B, Cambra K, García-Mouriz M, Iriarte A. Influence of age at diagnosis and time-dependent risk factors on the development of diabetic retinopathy in patients with type 1 diabetes. J Diabetes Res. 2016. [DOI] [PMC free article] [PubMed]
- 2.Heng L, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640–50. doi: 10.1111/dme.12089. [DOI] [PubMed] [Google Scholar]
- 3.Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62(4):206–12. doi: 10.1159/000499541. [DOI] [PubMed] [Google Scholar]
- 4.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–77. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 5.Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J. 2016;22(6):589–99. doi: 10.12809/hkmj164869. [DOI] [PubMed] [Google Scholar]
- 6.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 7.Nentwich MM, Ulbig MW. Diabetic retinopathy-ocular complications of diabetes mellitus. World J Diabetes. 2015;6(3):489. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Control D, Group CTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision research. 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Yang J, Tao L, Lv H, Jiang X, Zhang M, Li X. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7(9):e016280. 10.1136/bmjopen-2017-016280. [DOI] [PMC free article] [PubMed]
- 11.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O’Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabetic Stud. 2015;12(1–2):159–95. doi: 10.1900/RDS.2015.12.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foo V, Quah J, Cheung G, Tan NC, Ma Zar KL, Chan CM, et al. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics: 亚洲 2 型糖尿病患者的 HbA1c 以及收缩压的变异性与糖尿病视网膜病变之间的关系. J Diabetes. 2017;9(2):200–7. doi: 10.1111/1753-0407.12403. [DOI] [PubMed] [Google Scholar]
- 13.Laiginhas R, Madeira C, Lopes M, Neves JS, Barbosa M, Rosas V, et al. Risk factors for prevalent diabetic retinopathy and proliferative diabetic retinopathy in type 1 diabetes. Endocrine. 2019;66(2):201–9. doi: 10.1007/s12020-019-02047-z. [DOI] [PubMed] [Google Scholar]
- 14.Penman A, Hancock H, Papavasileiou E, James M, Idowu O, Riche DM, et al. Risk factors for proliferative diabetic retinopathy in African Americans with type 2 diabetes. Ophthalmic Epidemiol. 2016;23(2):88–93. doi: 10.3109/09286586.2015.1119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman M, Kamul-Hasan A, Islam M, Hasan A, Chowdhury F, Miah O, et al. Frequency and risk factors of diabetic retinopathy among patients with type 2 diabetes mellitus: a single-center study from Bangladesh. Mymensingh Med J. 2020;29(4):804–14. [PubMed] [Google Scholar]
- 16.Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation. 2014;129(9):999–1008. doi: 10.1161/CIRCULATIONAHA.113.002529. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Li T, Li P, Ma Y, Liu M, Shan Q, et al. Early assessment of the risk factors for diabetic retinopathy can reduce the risk of peripheral arterial and cardiovascular diseases in type 2 diabetes. Ophthalmic Res. 2018;59(4):221–7. doi: 10.1159/000479931. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zhang R-Y, Chen R-P, Sun J, Yang R, Ke X-Y, et al. Prevalence and risk factors for diabetic retinopathy in a high-risk Chinese population. BMC Public Health. 2013;13(1):633. doi: 10.1186/1471-2458-13-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanouchi M, Mori M, Hoshino J, Kinowaki K, Fujii T, Ohashi K, Furuichi K, Wada T, Ubara Y. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care. 2019;7(1):e000726. 10.1136/bmjdrc-2019-000726. [DOI] [PMC free article] [PubMed]
- 20.Hung C-C, Lin HY-H, Hwang D-Y, Kuo I-C, Chiu Y-W, Lim L-M, et al. Diabetic retinopathy and clinical parameters favoring the presence of diabetic nephropathy could predict renal outcome in patients with diabetic kidney disease. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]