Abstract
Purpose
The aim of this study was to assess the impact of Ramadan intermittent fasting on metabolic and inflammatory profiles in type 2 diabetic patients (T2D).
Methods
It was a prospective study including 55 T2D patients treated with oral hypoglycemic drugs, who intended to observe Ramadan fasting in 2019. All participants underwent a questionnaire, a physical examination, laboratory investigations, and a cardiovascular risk assessment using the Framingham score before Ramadan (T0), immediately after Ramadan (T1), and two months after Ramadan (T2).
Results
The mean age of participants was 54.5 ± 10.1 years. The number of fasted days was 29.3 ± 2.3 days. The mean total daily calorie intake decreased significantly by 19% during Ramadan (p < 10–3). A significant decrease in weight (79.8 ± 12.9 vs 78.4 ± 13.3 kg, p = 0.003), body mass index (29.8 ± 5.4 vs 29.2 ± 5.4 kg/m2, p = 0.004), waist circumference (98.2 ± 9.6 vs 96.3 ± 10.2 cm, p = 0.015), fat body mass (24.3 ± 9.4 vs 23.5 ± 9.7 kg, p = 0.043) was observed at T1. The weight loss was significantly correlated with the number of fasting days (r = 0.348, p = 0.009) and was maintained at T2. Serum fructosamine increased at T1 (303.6 ± 46 vs 333.49 ± 59.49 µmol/L, p < 10–3) and returned to its baseline levels at T2. A significant decrease in insulin (9.7 ± 5.5 vs 7.98 ± 5.05 mIU/L, p = 0.043), fibrinogen (3.7 ± 0.8 vs 3.4 ± 0.6 g/L, p = 0.003), and hs-CRP (4.8 ± 5.7 vs 3.7 ± 4.5 mg/L, p = 0.058) levels was observed at T1. Homocysteine level was significantly higher after Ramadan (12.2 ± 6.2 vs 13.5 ± 6.4 µmol/L, p = 0.001). However, no significant changes were found in blood pressure, fasting blood glucose, HOMA-IR, uric acid, lipids, and white blood cells count. The mean Framingham score decreased insignificantly after Ramadan.
Conclusions
Ramadan fasting in T2D patients seems to have a favorable impact on anthropometric parameters and inflammatory profile. However, it may cause a transient worsening of glycemic control.
Keywords: Type 2 diabetes, Intermittent fasting, Anthropometric parameters, Glycemic control, Lipid profile, Inflammation, Cardiovascular risk
Introduction
Ramadan fasting represents a model of intermittent fasting that stands for abstaining from eating, drinking, smoking, and oral medication use from predawn to sunset for one month. It is practiced by approximately 50 million diabetic patients worldwide [1].
The glycemic control in diabetic patients who observe Ramadan fasting may be affected by several factors such as the duration of the fast, lasting from 10 to 19 h, the changes in physical activity and diet, and sleep disturbances. The Epidemiology of Diabetes and Ramadan (EPIDIAR) study showed that 79% of type 2 diabetic patients fast Ramadan [2]. Fasting increased the risk of severe hypoglycemia by 7.5- fold and the risk of hyperglycemia by five fold [2]. However, many patients insist on fasting, sometimes against the advice of their physicians. In these cases, physicians should undertake precautionary measures to reduce as far as possible the risk of complications and to support patients during the fasting month.
Risk stratification has been established by the American Diabetes Association [1]. Patients with well-controlled diabetes treated with lifestyle therapy, metformin, acarbose, thiazolidinediones, incretin-based therapies, and/or short-acting insulin secretagogues are considered to have a low to moderate risk of hypoglycemia during fasting. Ramadan fasting may even lead to an improvement in the metabolic and inflammatory profiles of these patients. Although the cardiometabolic effects of Ramadan fasting in type 2 diabetic patients are of great interest, literature data are limited and controversial. Some authors showed beneficial effects with a reduction in blood pressure, body mass index (BMI), and HbA1c levels [3, 4]. Nevertheless, others did not [5]. According to Bouguerra et al. [6], Ramadan fasting significantly alters the glycemic and lipid profiles in poorly controlled type 2 diabetic patients but it seems to have only slight effects in well-controlled patients.
The aim of this study was to assess the effects of Ramadan fasting on metabolic, inflammatory, and cardiovascular profiles in patients with type 2 diabetes.
Methods
Study design and participants
A prospective study including consecutive type 2 diabetic patients was conducted in the outpatient clinic of the Department of Endocrinology, La Rabta university hospital, Tunis, Tunisia. Patients were enrolled two months before the month of Ramadan 2019. This month lasted 30 days from May 06th to June 04th. Fasting started at predawn, 3–3:30 a.m., and ended at sunset, 7:10- 7:35 p.m., with an average duration of 16 h and 20 min each 24 h. The average temperature was 20° Celsius with extremes of 10 and 32° Celsius.
The study was approved by the ethics committee of the hospital and was carried out following the ethical standards of the institutional and the national research committee and the 1964 Helsinki declaration. All enrolled patients provided written informed consent before starting the study.
The inclusion criteria were: patients with type 2 diabetes, men and women, treated with oral antidiabetic drugs and lifestyle therapy, with an HbA1c ≤ 10%, and intending to fast the month of Ramadan 2019, in the absence of major contraindications to fasting. The diagnosis of type 2 diabetes was based on a body of clinical and biological arguments: family history of type 2 diabetes, woman personnel history of gestational diabetes or macrosomia, age at diabetes diagnosis ≥ 35 years, absence of ketonuria at diabetes diagnosis, the incidental discovery of diabetes degenerative complications and/or the coexistence of acanthosis nigricans, overweight, dyslipidemia, and/or arterial hypertension.
The non-inclusion criteria were: HbA1c > 10%, insulin therapy, pregnancy, lactation, infectious and/or inflammatory diseases, major organ damage, adrenal insufficiency, poorly controlled hypertension, Cushing’s syndrome, primary hyperaldosteronism, primary hyperparathyroidism, uncontrolled dysthyroidism, neoplasia, anemia, and the use of corticosteroids, non-cardioselective beta-blockers, betamimetics and/or high doses of diuretics. The exclusion criteria were: unwillingness to continue the study, occurrence of one of the non-inclusion criteria, a number of fasting days < 20, anti-diabetic drugs discontinuation, capillary glucose level > 16.5 mmol/L (3 g /L) and/or < 3.85 mmol/L (0.7 g /L), and/or the occurrence of hypoglycemia with either adrenergic or neuroglycopenic symptoms (> 3 episodes).
Assessments were done during three sessions visits: T0: before the month of Ramadan (from April 8thto May 4th), T1: immediately after the month of Ramadan, and T2: two months after Ramadan.
Clinical and biochemical analysis
All patients were questioned about age, gender, smoking habits, duration of diabetes, and associated comorbidities, the number of fasting days, eating habits, type, sleeping hours, the occurrence of complications during the fast, type, duration, and frequency of physical activity, whether or not the Tarawih prayer was practiced during the month of Ramadan. An active person was defined as practicing at least 150 min of moderate-intensity aerobic physical activity or at least 75 min of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate and vigorous-intensity activity throughout the week.
Anthropometric parameters (weight, height, waist circumference) and blood pressure measurements were determined at each visit. Height was measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 kg, using a mechanical column scale (Detecto*). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Waist circumference (WC) was measured midway between the lowest border of the rib cage and the upper border of the iliac crest, at the end of normal expiration.
Body composition (body fat, lean body mass, and total body water) was determined using an impedance meter (OMEGATEST MC*). The dietary survey was carried out by a dietician at T0 and during the month of Ramadan using the 7-day dietary assessment method. The daily caloric intake and its composition in macronutrients and micronutrients were calculated using the BILNUT software.
Venous blood samples were collected in the morning, after at least 12 h of fasting for the determination of blood glucose (Enzymatic/hexokinase method), insulinemia (Chemiluminescence), fructosamine (Enzymatic method), total cholesterol (Cholesterol oxidase), triglycerides (UV endpoint/Lipase glycerol kinase), HDL-cholesterol (UV endpoint/ DSBmT), uric acid (Enzymatic method), high sensitive C-reactive protein(hs-CRP) (Latex-Enhanced/ Immunoturbidimetry), ferritinemia (Chemiluminescence), complete blood count (CBC) (Automates Sysmex Analyzer), and fibrinogen (Functional analysis).
The HOMA-IR index (Homeostasis Model Assessment of insulin resistance) which allows assessing insulin resistance was calculated using the following formula: HOMA-IR index = Fasting insulinemia (mIU/L) x Fasting blood glucose (mmol/L) / 22.5.
To assess cardiovascular risk, the Framingham score was calculated for all participants at T0, T1, and T2.
Before the month of Ramadan, a therapeutic education was provided to the participants by the same investigator and included advice on the diet, meal schedules, the anti-diabetic drugs intake during the month of Ramadan, the blood glucose monitoring in patients owning a glucometer (at least 3 measurements/day at 10–11 a.m, before and 2 h after the dinner, and in the presence of abnormal symptoms), the break of the fast in case of a capillary glucose level lower than 3.85 mmol/L or higher than 16.5 mmol/L or in case of undercurrent illness and how to react in case of acute complications. The telephone number of the investigator was given to all the participants.
Statistical analysis
Data were analyzed using SPSS (Statistical Package for the Social Sciences) version 23 (SPSS Inc., Chicago, IL). Categorical variables were presented as percentages and continuous variables as means ± standard deviations. Quantitative variables were compared using Student’s T-test for two paired samples. Qualitative variables were compared using the Mac Nemar test. Correlations were studied using the bivariate correlation test. The level of significance was set at 0.05.
Results
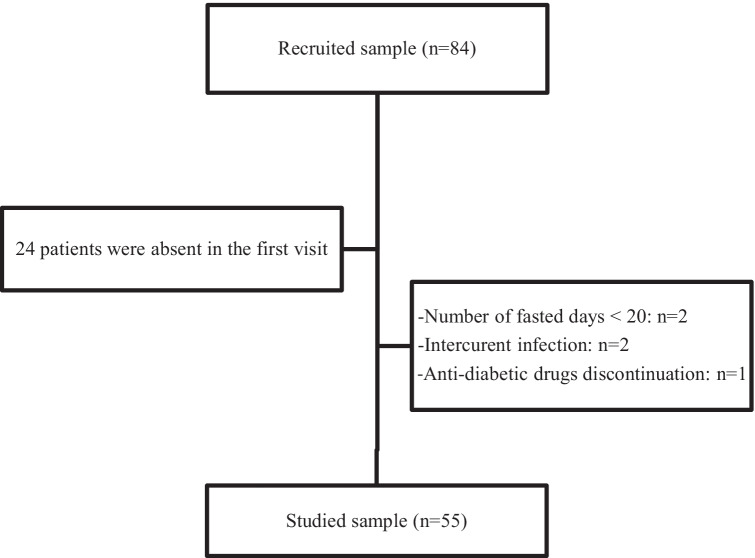
Eighty-four type 2 diabetic patients were initially enrolled but only 55 patients participated in the study (Fig. 1). Their demographic characteristics are shown in Table 1.
Fig. 1.
The flow chart of the study
Table 1.
Demographic characteristics of the participants
| Patients results | ||
|---|---|---|
| Age (years), mean ± SD | 54.5 ± 10.1 | |
| Gender | Men, n (%) | 26 (47) |
| Women, n (%) | 29 (53) | |
| Duration of diabetes (years), median | 2.2 | |
| Overweight, n (%) | 29 (53) | |
| Obesity, n (%) | 20 (36) | |
| Hypertension, n (%) | 24 (43) | |
| Dyslipidemia, n (%) | 25 (45) | |
| Diabetes complications, n (%) | macroangiopathy | 8 (15) |
| Microangiopathy | 1 (2) | |
| Diabetes treatment, n (%) | Metformin | 39 (71) |
| Metformin + sulphonylurea | 14 (25) | |
| Sulphonylurea | 2 (4) | |
SD: standard deviation, n: number of patients
The mean HbA1c level before the month of Ramadan was 7.1 ± 0.9% [5.4–10]. Diabetes was well-controlled in 74% of the cases.
The mean number of fasting days during the month of Ramadan was 29.3 ± 2.3 days. Forty-nine patients fasted for the whole month. The changes in lifestyle and food intake during the month of Ramadan are shown in Table 2. During Ramadan fasting, the daily calorie intake was reduced by 19% with a significant decrease in carbohydrate and protein intake.
Table 2.
Habits and food intake before and during the fasting month
| Before (T0) | During Ramadan | p-value | |
|---|---|---|---|
| Physically active (%) | 78 | 67 | 0.38 |
| Tarawih prayer (%) | - | 52 | – |
| Smoker (%) | 19 | 19 | – |
| Sleep duration (hours/day), mean ± SD | 6.9 ± 1.7 | 6.1 ± 1.8 | 0.001 |
| Fastfood consumption (%) | 26 | 5 | 0.008 |
| Number of meals/day, mean ± SD | 3.73 ± 0.82 | 2.57 ± 0.65 | < 10–3 |
| Daily calorie intake (Kcal/day), mean ± SD | 2443.0 ± 798.2 | 1984.5 ± 578.1 | < 10–3 |
| Carbohydrate intake (g/day), mean ± SD | 338.4 ± 130.2 | 253.9 ± 101.4 | < 10–3 |
| Saccharose intake (g/day), mean ± SD | 53.6 ± 39.7 | 30.0 ± 19.2 | 0.001 |
| Lipid intake (g/day), mean ± SD | 90.1 ± 28.2 | 82.3 ± 23.1 | 0.102 |
| Cholesterol intake (mg/day) | 173.6 ± 182.2 | 254.2 ± 128.9 | 0.022 |
| Protein intake (g/day), mean ± SD | 67.9 ± 22.6 | 55.5 ± 16.2 | < 10–3 |
| Fiber intake (g/day), mean ± SD | 22.6 ± 8.6 | 17.9 ± 23.9 | 0.173 |
| Sodium intake (mg/day), mean ± SD | 1612.3 ± 867.9 | 983.8 ± 687.9 | < 10–3 |
| Folate intake (µg/day), mean ± SD | 185.6 ± 81.9 | 119.2 ± 54.6 | < 10–3 |
| Water intake (mL/day), mean ± SD | 2146.1 ± 647.7 | 1538.4 ± 595.1 | < 10–3 |
SD Standard deviation
Statistical methods: binomial distribution / Student T-Test
During the fasting, two patients (4%), treated with glimepiride, experienced adrenergic symptoms but only one of them measured the concomitant blood glucose which was 0.5 g/L. No severe complications were reported.
Changes in anthropometric parameters, body composition, and blood pressure are shown in Table 3. Weight loss induced by Ramadan fasting was correlated with the number of fasting days (r = 0.348, p = 0.009). No significant correlations were observed between weight loss and other parameters.
Table 3.
Anthropometric parameters, body composition, and blood pressure before and after Ramadan fasting
| T0 | T1 | p1 | T2 | p2 | |
|---|---|---|---|---|---|
| Body weight (Kg), mean ± SD | 79.8 ± 12.9 | 78.4 ± 13.3 | 0.003 | 79.6 ± 13.8 | 0.047 |
| BMI (Kg/m2), mean ± SD | 29.8 ± 5.4 | 29.2 ± 5.4 | 0.004 | 29.5 ± 5.0 | 0.05 |
| WC (cm), mean ± SD | 98.2 ± 9.6 | 96.3 ± 10.2 | 0.015 | 97.1 ± 8.0 | 0.557 |
| Body fat (Kg), mean ± SD | 24.3 ± 9.4 | 23.5 ± 9.7 | 0.043 | 23.8 ± 9.4 | 0.248 |
| Lean body mass(Kg), mean ± SD | 55.8 ± 7.4 | 55.3 ± 7.1 | 0.059 | 55.8 ± 7.4 | 0.455 |
| Body water (mL), mean ± SD | 36,500 ± 8498 | 36,718 ± 7888 | 0.793 | 36,709 ± 8597 | 0.844 |
| SBP (mmHg), mean ± SD | 130.0 ± 15.5 | 127.9 ± 19.9 | 0.479 | 129.1 ± 22.1 | 0.549 |
| DBP (mmHg), mean ± SD | 77.8 ± 9.4 | 76.7 ± 10.3 | 0.547 | 78.2 ± 9.4 | 0.654 |
BMI Body mass index; DBP Diastolic blood pressure; p1 T0-T1; p2 T0-T2; SBP Systolic blood pressure; T0 before Ramadan; T1 Immediately after Ramadan; T2 Two months after Ramadan; WC Waist circumference
Statistical method: Student T-Test
The levels of metabolic and inflammatory parameters before and after the fasting are indicated in Table 4.
Table 4.
Biochemical parameters before and after Ramadan fasting
| T0 | T1 | p1 | T2 | p2 | |
|---|---|---|---|---|---|
| FBG (mmo/L), mean ± SD | 7.7 ± 2.2 | 7.7 ± 0.25 | 0.794 | 7.9 ± 0.25 | 0.344 |
| Fructosamine (µmol/L), mean ± SD | 303.6 ± 46 | 333.49 ± 59.49 | < 10–3 | 303.03 ± 50.62 | 0.735 |
| Fasting insulin (mIU/L), mean ± SD | 9.7 ± 5.5 | 7.98 ± 5.05 | 0.043 | 7.72 ± 3.58 | 0.015 |
| HOMA-IR, mean ± SD | 3.4 ± 2.7 | 2.80 ± 2.58 | 0.248 | 2.67 ± 1.23 | 0.081 |
| Cholesterol (mmol/L), mean ± SD | 4.49 ± 0.80 | 4.42 ± 0.77 | 0.305 | 4.37 ± 0.87 | 0.122 |
| Triglycerides (g/L), mean ± SD | 1.59 ± 0.85 | 1.62 ± 0.79 | 0.619 | 1.59 ± 0.77 | 0.719 |
| HDLc (g/L), mean ± SD | 1.16 ± 0.33 | 1.14 ± 0.25 | 0.475 | 1.03 ± 0.20 | < 10–3 |
| LDLc (g/L), mean ± SD | 2.61 ± 0.67 | 2.51 ± 0.69 | 0.178 | 2.58 ± 0.74 | 0.776 |
| Uric-acid(mg/L), mean ± SD | 49.8 ± 12.7 | 48 ± 14.4 | 0.275 | 52 ± 12 | 0.127 |
| Hs-CRP (mg/L), mean ± SD | 4.8 ± 5.7 | 3.7 ± 4.5 | 0.058 | 3.7 ± 3.9 | 0.137 |
| Homocysteine (µmol/L), mean ± SD | 12.2 ± 6.2 | 13.5 ± 6.4 | 0.001 | 14.1 ± 7.3 | < 10–3 |
| Ferritinemia (µg/L), mean ± SD | 73.9 ± 69.5 | 70.1 ± 67.7 | 0.450 | 64.8 ± 4 | 0.003 |
| Fibrinogen (g/L), mean ± SD | 3.7 ± 0.8 | 3.4 ± 0.6 | 0.003 | 3.5 ± 0.6 | 0.607 |
| WBC(elements/mm3), mean ± SD | 7097.7 ± 2042.1 | 6771.1 ± 1559.3 | 0.085 | 6660.3 ± 1880.4 | 0.028 |
| Hematocrit (%),mean ± SD | 39.8 ± 2.7 | 38.9 ± 2.8 | 0.003 | 39.3 ± 2.8 | 0.234 |
FBG Fasting blood glucose; SD Standard deviation; HOMA-IR Homeostasis Model Assessment of insulin resistance index; n Number of patients; p1 T0-T1; p2 T0-T2; T0 Before Ramadan; T1 Immediately after Ramadan; T2 Two months after Ramadan; WBC White blood cells
Statistical method: Student T-Test
Serum fructosamine increased at T1 (p < 10–3) and returned to its baseline levels at T2. No significant changes occurred in fasting blood glucose. Insulin levels decreased significantly after Ramadan (p = 0.043). However, no significant changes were observed in HOMA-IR. Fibrinogen and hs-CRP levels decreased significantly at T1, while the decrease in blood cells count and ferritinemia was insignificant. Homocysteine levels were significantly higher after Ramadan (p = 0.001). However, no significant changes were found in uric acid and lipids.
Table 5 shows the evolution of the Framingham score throughout the study. A reduction of the cardiovascular risk score was noted in 53% of patients at T1 and in 47% of cases at T2.
Table 5.
The Framingham score before and after Ramadan fasting
| T0 | T1 | p1 | T2 | p2 | |
|---|---|---|---|---|---|
| Framingham score (%), mean ± SD | 18.5 ± 9.7 | 16.7 ± 8.7 | 0.063 | 18.3 ± 9,1 | 0.361 |
| Patients with high CVR (%) | 49 | 32 | 0.109 | 44 | 0.219 |
SD Standard deviation; CVR Cardiovascular risk; n Number of patients; p1 The p-value between T0 and T1; p2 The p-value between T0 and T2; T0 Before Ramadan; T1 Immediately after Ramadan; T2 Two months after Ramadan
Statistical methods: Student T-test / Binomial distribution
At T1, BMI (r = -0.392, p = 0.013), fasting insulin level (r = -0.430, p = 0.007) and the HOMA-IR index (r = -0.337, p = 0.032) were negatively correlated with the sleep duration during Ramadan.
Discussion
This study aimed to evaluate the impact of Ramadan fasting on metabolic, inflammatory, and cardiovascular risk profiles in patients with type 2 diabetes. Our results showed that daylight fasting during Ramadan seems to confer favorable effects on anthropometric parameters, fasting insulin level, hs-CRP, ferritinemia, and fibrinogen. However, a significant increase in fructosamine and homocysteine was observed. Blood pressure, fasting blood glucose, lipid profile, and uric acid showed no significant changes.
The influence of Ramadan fasting on body weight and body composition was investigated in many studies with conflicting results. Many authors reported a weight loss after Ramadan fasting [7, 8] that was explained by the decrease in meal frequency and the reduction of energy intake [9]. In our study, there was a significant weight loss that was only correlated with the number of fasting days. There was also a significant decrease in body fat as in previous studies [10]. Some studies reported no significant changes [11–15] or even an increase in weight [16, 17].
A meta-analysis of 70 publications that studied the effect of Ramadan fasting on weight and body composition in adults showed a consistent reduction in weight and fat mass, especially in patients with overweight or obesity even though no advice was provided on lifestyle [18].
The influence of Ramadan fasting on blood pressure in diabetic patients is not well established. In the study of Erdem et al. [19], intermittent fasting was associated with a significant decrease in systolic and diastolic blood pressure in patients with newly diagnosed hypertension or prehypertension. In diabetic patients, Bener et al. [20] reported a significant decrease in systolic and diastolic blood pressures after Ramadan fasting. This finding was partly related to the reduction of sodium consumption during the fasting period [19]. In addition to the reduced-calorie and sodium intake, other mechanisms may contribute to the decrease of blood pressure during intermittent fasting such as the enhancement of parasympathetic nervous system activity, norepinephrine, and natriuretic peptides excretion, and insulin sensitivity [21].
In our patients, blood pressure did not show appreciable changes despite the reduction in sodium and water intake and body weight during Ramadan. Similar findings were reported in other studies [5, 22].
Glycemic control in diabetic patients observing Ramadan fasting may be affected by several factors such as changes in food quantity and quality, physical activity, and sleep disturbance with the risk of hypoglycemia according to the used antidiabetic drugs.
Ramadan fasting was reported to deteriorate, improve, or show no changes in glycemic control in diabetic patients. In a systemic review of 22 studies, Almulhem et al. [23] reported a favorable effect of fasting on glycemic parameters in five studies with a significant reduction in HbA1c and blood glucose. The other studies reported a worsening of glycemic control after Ramadan [6, 15]. Shahin et al. [5] did not find any significant changes in glycemic control. In our study, fasting glucose level and HOMA-IR index did not change but fructosamine level increased significantly. Despite the decrease in daily calorie and carbohydrate intake and the weight loss observed in our patients, these negative effects of Ramadan fasting on glycemic control could be related to sleep disorders. We noted that sleep duration decreased significantly during the fasting month and was negatively correlated with the BMI, the fasting insulin level, and the HOMA-IR index after Ramadan fasting. Disrupted sleep was also associated with increased ghrelin and reduced leptin and adiponectin which are also associated with insulin resistance and hyperglycemia [24–26].
The most fearful risk of Ramadan fasting in diabetic patients is hypoglycemia. In the present study, two patients (4%) presented with adrenergic symptoms.
The prevalence of hypoglycemia in diabetic patients fasting the month of Ramadan is different from one study to another. Tiboura et al. [11], M’guil et al. [13], and Bouguerra et al. [6] did not record any hypoglycemia in their patients. Zargar et al. [17] reported hypoglycemic events in 2.2% of diabetic patients treated with oral antidiabetic drugs during Ramadan compared to 3.7% of cases before Ramadan. In the study of Aravind et al. [27], 19.7% of diabetic patients experienced at least one hypoglycemic event during fasting. In the EPIDIAR study, the prevalence of severe hypoglycemia in diabetic patients treated with oral antidiabetic drugs was 3% compared to 0.4% before fasting [2]. These discordant results may be explained by the difference in antidiabetic medication taken by the patients [28]. A Systematic review and meta-analysis showed that incretin-mimetics were associated with the lowest risk of hypoglycemia in type 2 diabetic patients fasting Ramadan, followed by insulin glargine, and meglitinides [29]. In addition, educational sessions can reduce the risk of hypoglycemia [30, 31].
The impact of Ramadan fasting on the lipid profile is unclear. In our study as in other studies [22], no significant changes were observed after Ramadan fasting. However, other studies indicated a significant improvement in lipid profile after Ramadan fasting with a significant decrease in total cholesterol [7, 8, 12], triglycerides [7, 12], and LDLc levels [8], as well as a significant increase in HDLc levels [32]. On the other hand, many other studies reported a significant increase in total cholesterol [9], LDLc [7, 9], and triglyceride levels [9], and a decrease in HDLc [5, 7, 11]. These discrepancies may be explained by the difference in the quantity and the quality of lipid intake during Ramadan, culinary habits, and the differences in the physical activity between populations’ studies.
There was no effect of Ramadan fasting on uric acid levels in our study. Some studies showed a significant decrease in uric acid levels after fasting [12] whereas others reported a significant increase [7]. These different results may be explained by differences between patients’ studies such as weight changes (the weight loss induces DNA degradation), the occurrence of dehydration, and quantity of protein intake.
Chronic inflammation is common in type 2 diabetic patients and represents a key factor in the pathogenesis of atherosclerosis. Studies on the impact of Ramadan fasting on inflammatory and oxidative stress are limited and their results are conflicting. In a meta-analysis including 311 healthy participants, Fares et al. [33] demonstrated some short-term protection against elevated inflammatory and oxidative stress markers. Several mechanisms were evoked to explain this finding such as weight loss and the decrease in insulin level and insulin resistance [33]. In our study, the impact of Ramadan fasting on inflammation was evaluated by the variation of inflammatory markers (Hs-CRP, fibrinogen, ferritinemia, and weight blood cells count). We noted a transient decrease of fibrinogen and hs-CRP levels, and a delayed decrease in ferritinemia and white blood cells suggesting a protective impact of fasting on inflammatory profile.
Type 2 diabetes is a common risk factor for cardiovascular disease which is the most prevalent cause of morbidity and mortality in this population. Diabetic patients observing Ramadan fasting are at a higher risk of dehydration with increased levels of hematocrit and plasma osmolality. Consequently, blood viscosity may increase leading to a high risk of thrombosis and stroke. In addition, hypoglycemia has been associated with an increased risk of cardiovascular events [34]. In a meta-analysis of 22 studies, Almulhem et al. [18], concluded that there was insufficient evidence to link Ramadan fasting to an increased or reduced incidence of cardiovascular events in diabetic patients. No cardiovascular events were observed in our patients during the fasting month.
To investigate the effect of Ramadan fasting on cardiovascular risk among type 2 diabetic patients, we assessed patients’ Framingham risk scores and homocysteine levels before and after the fasting month. There was a decrease in the score immediately after Ramadan fasting. However, this score regained its previous levels one to two months later. Forty-nine percent of our patients had a high cardiovascular risk before fasting versus 32% at T1 and 44% at T2. Nematy et al. [32] reported a significant improvement in the Framingham score after Ramadan in 82 patients with at least one cardiovascular risk factor. Conversely, homocysteine levels increased significantly. This could be explained by the significant decrease in folates intake during the month of Ramadan as was observed in our study. In healthy people, a significant decrease in homocysteine levels after Ramadan fasting was reported [35, 36].
The limitations of our study are the small sample size, the absence of a control group, and the investigation period in which the high temperature and the long fasting period may have influenced dietary habits and lifestyle. In addition, patients were consecutively enrolled which limits the ability to generalize our findings to the population of type 2 diabetic patients observing Ramadan fasting. Despite these limitations, this study presents important strength points such as the use of fructosamine rather than HbA1c as it better reflects short-term changes in glycemic control, the carrying out of dietary surveys, and a relatively prolonged follow-up. Further studies involving larger sample sizes with prolonged follow-up would be useful to confirm our findings. Finally, to disclose the full potential benefits of such intervention, it is necessary to carry out a continued follow-up for a longer period after Ramadan month.
Conclusions
Ramadan fasting seems to confer beneficial effects on anthropometric parameters and inflammatory profile but a transient worsening of glycemic control in type 2 diabetic patients. It can be as safe, as there were no serious adverse medical effects of fasting. Individualized dietary education and a re-scheduled anti-diabetic regimen are necessary to optimize the beneficial effects of intermittent fasting.
Acknowledgements
The authors thank Pr. Raja Serairi Beji (École Supérieure des Sciences et Techniques de la Santé de Tunis. Université Tunis El Manar) for her help in the interpretation of the dietary data.
Author contribution
I. Oueslati, M. Chihaoui, M. Feki, B. Hammami, and N. Ben Romdhane contributed to the conception and design of the research; I. Oueslati, A. Kardi, F. Boukhayatia, B. Hammami, M. Cheikh, N. Ben Romdhane, and M. Yazidi contributed to the acquisition, interpretation, and analysis of the data; I. Oueslati and A. Kardi drafted the manuscript; M. Chihaoui critically revised the article for important intellectual content; All authors revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Funding
This work was sponsored by La Rabta university hospital, Tunis, Tunisia.
Data availability
Data are available from the authors upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of La Rabta university hospital (CEBM.EPS.HR/15/2019). All enrolled patients provided written informed consent before starting the study.
Competing interests
Authors state no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alarouj M, Assaadkhalil S, Buse J, Fahdil I, Fahmy M, Hafez S, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010;33(8):1895–1902. doi: 10.2337/dc10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27(10):2306–2311. doi: 10.2337/diacare.27.10.2306. [DOI] [PubMed] [Google Scholar]
- 3.Hassanein M, Al Sifri S, Shaikh S, Raza SA, Akram J, Pranoto A, et al. A real-world study in patients with type 2 diabetes mellitus treated with gliclazide modified-release during fasting: DIA-RAMADAN. Diabetes Res Clin Pract. 2020;163:108154. doi: 10.1016/j.diabres.2020.108154. [DOI] [PubMed] [Google Scholar]
- 4.Syed F, Arif MA, Ramzan A, Niazi R, Murtaza MI, Javed A. DAR-GRACE: Diabetes and Ramadan: glycaemic control, physician counselling and patient practices-experience from a tertiary care hospital in Pakistan. J Pak Med Assoc. 2020;70(11):1990–1995. doi: 10.5455/JPMA.42922. [DOI] [PubMed] [Google Scholar]
- 5.Sahin SB, Ayaz T, Ozyurt N, Ilkkilic K, Kirvar A, Sezgin H. The impact of fasting during Ramadan on the glycemic control of patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2013;121(9):531–534. doi: 10.1055/s-0033-1347247. [DOI] [PubMed] [Google Scholar]
- 6.Bouguerra R, Jabrane J, Maâtki C, Bensalem L, Hamzaoui J, Elkadhi A, et al. Lapratique du jeûne du mois de Ramadan chez le diabétique de type 2. Ann Endocrinol. 2006;67(1):54–59. doi: 10.1016/S0003-4266(06)72541-0. [DOI] [PubMed] [Google Scholar]
- 7.Bener A, Alhamaq AOAA, Öztürk M, Çatan F, Haris PI, Rajput KU, et al. Effect of Ramadan fasting on glycemic control and other essential variables in diabetic patients. Ann Afr Med. 2018;17(4):196–202. doi: 10.4103/aam.aam_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassanein M, Alawadi FF, Elhadidy KES, Ali SS, Echtay A, Djaballah K, et al. The characteristics and pattern of care for the type 2 diabetes mellitus population in the Middle East and North Africa region during Ramadan: An international prospective study (DAR-MENA T2DM) Diabetes Res Clin Pract. 2019;151:275–284. doi: 10.1016/j.diabres.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Khaled MB, Belbraouet S. Ramadan fasting diet entailed a lipid metabolic disorder among type 2 diabetic obese women. Am J Appl Sci. 2009;6(3):471–477. doi: 10.3844/ajassp.2009.471.477. [DOI] [Google Scholar]
- 10.Yeoh ECK, Zainudin SB, Loh WN, Chua CL, Fun S, Subramaniam T, et al. Fasting during Ramadan and associated changes in glycaemia, caloric intake and body composition with gender differences in singapore. Ann Acad Med Singapore. 2015;44(6):202–206. [PubMed] [Google Scholar]
- 11.Tiboura G, Khaled BM, Diaf M, Semeria S, Bouanani G. Effect of Ramadan fasting on serum glucose and lipid profile among algerian type 2 diabetes patients. Romanian J Diabetes Nut rMetab Dis. 2015;22(4):385–392. doi: 10.1515/rjdnmd-2015-0045. [DOI] [Google Scholar]
- 12.M’guil M, Ragala MA, Elguessabi L, Fellat S, Chraibi A, Chabraoui L, et al. Is Ramadan fasting safe in type 2 diabetic patients in view of the lack of significant effect of fasting on clinical and biochemical parameters, blood pressure, and glycemic control? Clin Exp Hypertens. 2008;30(5):339–57. doi: 10.1080/10641960802272442. [DOI] [PubMed] [Google Scholar]
- 13.Hassanein M, Hanif W, Malik W, Kamal A, Geransar P, Lister N, et al. Comparison of the dipeptidyl peptidase-4 inhibitor vildagliptin and the sulphonylurea gliclazide in combination with metformin, in muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the vector study. Curr Med Res Opin. 2011;27(7):1367–1374. doi: 10.1185/03007995.2011.579951. [DOI] [PubMed] [Google Scholar]
- 14.Karatoprak C, Yolbas S, Cakirca M, Cinar A, Zorlu M, Kiskac M, et al. The effects of long term fasting in Ramadan on glucose regulation in type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2013;17(18):2512–2516. [PubMed] [Google Scholar]
- 15.Vasan SK, Karol R, Mahendri NV, Arulappan N, Jacob JJ, Thomas N. A prospective assessment of dietary patterns in muslim subjects with type 2 diabetes who undertake fasting during Ramadan. Indian J Endocrinol Metab. 2012;16(4):552. doi: 10.4103/2230-8210.98009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zargar AH, Siraj M, Jawa AA, Hasan M, Mahtab H. Maintenance of glycaemic control with the evening administration of a long acting sulphonylurea in male type 2 diabetic patients undertaking the Ramadan fast. Int J Clin Pract. 2010;64(8):1090–1094. doi: 10.1111/j.1742-1241.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- 17.Mnif F, Slama CB, Chaieb L, Blouza S, Hsairi M, Abid M. Observational study of the tunisian diabetic patients’ profile during the fasting of the holy month of Ramadan. J Endocrinol Diabetes. 2016;3(1):1–8. [Google Scholar]
- 18.Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients. 2019;11(2):278. doi: 10.3390/nu11020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdem Y, Özkan G, Ulusoy Ş, Arıcı M, Derici Ü, Şengül Ş, et al. Turkish Society of Hypertension and Renal Diseases. The effect of intermittent fasting on blood pressure variability in patients with newly diagnosed hypertension or prehypertension. J Am Soc Hypertens. 2018;12(1):42–49. doi: 10.1016/j.jash.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Bener A, Yousafzai MT. Effect of Ramadan fasting on diabetes mellitus: a population-based study in Qatar. J Egyptian Public Health Associa. 2014;89(2):47–52. doi: 10.1097/01.EPX.0000451852.92252.9b. [DOI] [PubMed] [Google Scholar]
- 21.Di Daniele N, Marrone G, Di Lauro M, Di Daniele F, Palazzetti D, Guerriero C, et al. Effects of Caloric Restriction Diet on Arterial Hypertension and Endothelial Dysfunction. Nutrients. 2021;13(1):274. doi: 10.3390/nu13010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul AK, Khan MA, Fariduddin M. Effect of Ramadan fasting on anthropometric measures and metabolic profiles among type 2 diabetic subjects. J Enam Med Coll. 2015;5(2):93–98. doi: 10.3329/jemc.v5i2.23382. [DOI] [Google Scholar]
- 23.Almulhem M, Susarla R, Alabdulaali L, Khunti K, Karamat MA, Rasiah T, et al. The effect of Ramadan fasting on cardiovascular events and risk factors in patients with type 2 diabetes: A systematic review. Diabetes Res Clin Pract. 2020;159:1–21. doi: 10.1016/j.diabres.2019.107918. [DOI] [PubMed] [Google Scholar]
- 24.Cornelissen G. Metabolic Syndrome, Adiponectin, Sleep, and the Circadian System. E Bio Med. 2018;33:20–21. doi: 10.1016/j.ebiom.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh J. The effect of circadian and sleep disruptions on obesity risk. J Obes Met Synd. 2018;27(2):78. doi: 10.7570/jomes.2018.27.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albrecht U. The circadian clock, metabolism and obesity. Obes Rev. 2017;18(1):25–33. doi: 10.1111/obr.12502. [DOI] [PubMed] [Google Scholar]
- 27.Aravind SR, Khaled AT, Shaiful BI, Naim S, Ghaida K, Rose L, et al. Hypoglycaemia in sulphonylurea-treated subjects with type 2 diabetes undergoing Ramadan fasting: a five-country observational study. Curr Med Res Opin. 2011;27(6):1237–1242. doi: 10.1185/03007995.2011.578245. [DOI] [PubMed] [Google Scholar]
- 28.Jabbar A, Hassanein M, Beshyah SA, Boye KS, Yu M, Babineaux SM. CREED study: hypoglycaemia during Ramadan in individuals with type 2 diabetes mellitus from three continents. Diabetes Res ClinPract. 2017;132:19–26. doi: 10.1016/j.diabres.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Lee SWH, Lee JY, Tan CSS, Wong CP. Strategies to make Ramadan fasting safer in type 2 diabetics: a systematic review and network meta-analysis of randomized controlled trials and observational studies. Medicine. 2016;95(2):1–9. doi: 10.1097/MD.0000000000002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen LN, Ibrahim M, Ali NM, Assaad KSH, Tantawi HR, Nasr G, et al. Impact of an individualized type 2 diabetes education program on clinical outcomes during Ramadan. BMJ Open Diabetes Res Care. 2015;3(1):1–8. doi: 10.1136/bmjdrc-2015-000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tourkmani AM, Hassali MA, Alharbi TJ, Alkhashan HI, Alobikan AH, Bakhiet AH, et al. Impact of Ramadan focused education program on hypoglycemic risk and metabolic control for patients with type 2 diabetes. Patient Prefer Adherence. 2016;10:1709–1717. doi: 10.2147/PPA.S113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nematy M, AlinezhadNamaghi M, Rashed MM, Mozhdehifard M, Sajjadi SS, Akhlaghi S, et al. Effects of Ramadan fasting on cardiovascular risk factors: a prospective observational study. Nutr J. 2012;11:69. doi: 10.1186/1475-2891-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faris’ AE, Jahrami HA, Obaideen AA, Madkoure MI. Impact of diurnal intermittent fasting during Ramadan on inflammatory andoxidative stress markers in healthy people: Systematic review and meta-analysis. J Nutrition Interm Metab. 2019;15:18–26. doi: 10.1016/j.jnim.2018.11.005. [DOI] [Google Scholar]
- 34.Hanefeld M, Frier BM, Pistrosch F. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care. 2016;39:S205–S209. doi: 10.2337/dcS15-3014. [DOI] [PubMed] [Google Scholar]
- 35.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21(5):1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, c-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann NutrMetab. 2007;51(1):88–95. doi: 10.1159/000100954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request.