Abstract
Abstract
Type 1 diabetes mellitus (T1DM) is one of the most common chronic immune-mediated diseases. The prevalence is worldwide especially among children and young adults. The destruction of the pancreatic β-cells due to some abnormalities in the immune system characterizes T1DM. Considering the high burden of the disease and its impact on human health, researchers have made great efforts during the last decades; investigating the disease pathogenesis and discovering new strategies for its management. Fortunately, probiotics have been found as potential remedies for T1DM. This review aims to explore the potentialities of probiotics in managing T1DM and its complications. Based on the outcomes of human and animal studies carried out from 2016 to 2021, the review hopes to assess the effectiveness of probiotics in the prevention and treatment of T1DM and its complications. We first tried to explain the disease's pathogenesis, and highlighted the possible mechanisms involved in these potentialities of probiotics. We concluded that, probiotics can be used as possible therapeutic tools for the management of T1DM. Possible mechanisms of action of probiotics include; the modulation of the gut microbiota, the regulation of inflammation-related cytokines, the production of short chain fatty acids (SCFAs), and the regulation of GLP-1. However, we recommend further studies especially human trials should be carried out to investigate these potentialities of probiotics.
Highlights
• T1DM is highly prevalent worldwide, causing high morbidity and mortality especially among children and young adults
• Gut microbiota plays a significant role in the pathogenesis of T1DM via an interconnection with the immune system
• Probiotics can be used as possible therapeutic tools for the management of T1DM
• Possible mechanisms of action of probiotics include the modulation of the gut microbiota, the regulation of inflammation-related cytokines, the production of SCFAs, and the regulation of GLP-1
Keywords: Probiotics, Type 1 diabetes mellitus, Gut microbiota, Management, SCFAs
Introduction
Type 1 diabetes (T1DM) also known as insulin-dependent, juvenile, or childhood-onset, is a chronic autoimmune disease characterized by the insufficiency in insulin production due to selective destruction of pancreatic β-cells [1, 2]. It is an illness typically identified among young age group with insulin deficiency [3]. T1DM could be identified through the production of urine (polyuria), excess thirst (polydipsia), constant hunger, weight loss, changes in vision, and tiredness [1, 4]. It can be classified into two; the majority of T1DM cases can be attributed to autoimmune-mediated damage of beta cells ( known as type 1a) while the marginal cases are due to idiopathic damage or failure of beta cells to make insulin (type 1b)[5]. However, there is another exceptional subtype of T1DM called diabetes LADA (Latent Autoimmune Diabetes of Adults), who’s characteristic is slow β-cell damage [6]. T1DM does not only bring complications but also affects many organs such as the heart, blood vessels, nerves, eyes, and kidney, and can lead to a significant morbidity and mortality in the world. T1DM complications have been categorized as microvascular complications, which comprise retinopathy, nephropathy, and neuropathy; and macrovascular ones including cardiovascular disease, cerebrovascular diseases and peripheral arterial disease [7, 8].
In several countries, immune-mediated diseases, mainly allergic and autoimmune diseases such as T1DM seem to have augmented in prevalence and are leading causes of mortality especially among kids and young people [9]. At least 500,000 kids have T1DM globally over the last 50 to 60 years [10]. T1DM prevalence has augmented, especially in developed nations [11]. For instance, the yearly prevalence in Finland (one of the countries with high T1DM incidence) was 12 per 100,000 children under 15 during the 1950s and raised to 65 per 100,000 in 2006 [12, 13]. According to the data from the national diabetes statistics reports, T1DM accounts for 5 to 10% of all diabetes; 210,000 kids and teenagers aged under 20 years or 25 per 10,000 US youths are suffering from diabetes, and this involves 187,000 suffering from T1DM. About 1.4 million adults of 20 of age and more representing 5.2% of all US adults with diabetes reported suffering from T1DM and are under insulin treatment. During the last years, the overall prevalence of T1DM has risen considerably [14, 15]. Furthermore, data from the EuroDIAB (European Diabetes) study disclosed that the T1DM prevalence among kids (both sex) aged 0 to 4 raised by 3.7%, 5–9 years augmented by 3.4% among males and 3.7% among females and 10 to14 years age groups grown by 3.3% in males and 2.6% in females annually in that order, during the last 25 years [16]. In China, about 5018 new cases of T1DM have been diagnosed according to data from a recent survey. Contrary to the general trends, the great majority of cases are diagnosed among adults above 20 years of age while the prevalence among kids was among the lowest [17]. This prevalence is on the rise since there has been a significant rise in new cases during the last two decades in the country[18].
Environmental, viral and genetic factors are the main triggers of T1DM. An autoimmune illness that commonly manifests during infancy but can develop or be diagnosed at any age [19–21]. Other known risk factors for T1DM development include gender, age, race, geographic location, seasonality [5], chemicals, diet [22, 23], the use of antibiotics, vitamin D levels, exposure to toxins, birth weight, and birth delivery route [24]. Also, children with T1DM have been shown to have a high risk of developing other conditions such as periodontal diseases [25].
Given all the information mentioned above about T1DM, it is necessary to look for a management solution. Even though it represents only the minor part of all diabetes patients, it is a life-threatening disorder mainly for children and young adults. Concerning T1DM treatment strategies, about a century ago, insulin had been discovered and was being used till 50 years later when sulfonylureas and biguanides had been found [26]. However, insulin had been revealed as not only a friend but a foe in the occurrence of T1DM. According to a study, insulin functions as a friend since it promotes the compensation of β-cell. However, it becomes a foe since it also contributes to the decompensation of β-cell [27]. There is therefore, a need for a new treatment strategy. Interestingly, as a result of many studies (animals and humans), probiotics have been found to have great potentials in the modulation of the gut microbiota (GM) and management of diseases such as T1DM[28]. According to W.H.O, probiotics are "live microorganisms which, when administered in adequate amounts, confer a health benefit on the host" [29]. It is naturally referring to existing microorganisms that normally facilitate the health improvement on the host body when consumed in suitable amounts and a proper diet [30–33]. Probiotics have been documented for their numerous health-promoting roles and alleviating physiological and psychological hitches and pains, improving intestinal health and immune responses [34, 35]. Besides, probiotics have been recommended as interesting coadjuvent in managing associated metabolic diseases including T1DM. Nevertheless, the results are not consistent and their varied effects' mechanisms are understudied [36]. Numerous experiments (animal and humans) have been conducted to assess probiotics' health-promoting status during the previous years [37–39]. Recent researches have also evoked the efficacy of probiotics in the management of T1DM [40, 41], and positive results had been reported. However, recommendations are made on some other focuses such as the need to consider the investigations on the possible mechanisms of actions. Thus, the aim of this review is to explore and confirm the potentialities of probiotics in the management of T1DM and its complications. Moreover, this review aims to highlight the possible mechanisms through which probiotics act in the management of T1DM and its complications.
Understanding the Pathogenesis of T1DM
Overview: Immunologic cells and Molecules involved
T1DM is a long-term autoimmune disorder caused by the lack of insulin secretion due to the (β)-cell death in the islet of Langerhans [42]. Autoimmune diseases are diseases resulting from self-tissue attacks due to an individual's immune system [43]. Immunologically, T1DM is mediated through autoreactive lymphocytes activation, autoantibodies production, and the pancreatic β-cells destruction by T-cells in individuals with genetic predispositions [44]. The pathogenesis of T1DM results from multifarious contacts of the pancreatic β-cell with the innate and adaptive immune system cells [45]. Known immune cells implicated in the destruction of the (β)-cells are the lymphocytes CD4 + , CD8 + T cells, and macrophages, which act after their infiltration in the islets [46]. However, other immune cells such as lymphocytes B cells are also known as pivotal regulators of the immune system and autoimmunity, highly implicated in T1DM pathogenesis, more precisely in the autoimmune destruction of the pancreatic islets. B cells are known to be able to promote T1DM through the presentation of islet-derived peptides to the autoreactive T-cells, the production of autoantibodies against β‐cells antigens and the secretion of proinflammatory cytokines [47, 48]. The destruction of the (β)-cells is due to immune attacks mainly by Lymphocyte cytotoxic T-cells that suddenly become very active and destroy the healthy β‐cells. Next, this phenomenon is followed by the penetration of the other immune cells such as macrophages, causing the insulitis or the inflammation of the islets and consequently the deficiency in insulin production due to the β‐cells destruction [49]. The autoreactive immune reaction occurs in the pancreatic lymph nodes (PLNs) that continuously provide autoreactive lymphocyte T-cells. Acting as natural immune effectors, the remaining immune cells, mainly the macrophages and dendritic cells (DCs), are responsible for the activation of the cytotoxic T‐cells. Also, in the context of the Major Histocompatibility Complex class II (MHC II) molecules, these two mentioned immune cells act as antigen‐presenting cells (APCs) thereby, activating the T-cells and contributing to the pathogenesis of T1DM by the adaptive immune response pathway [49].
T1DM is also caused by the dysfunction of indoleamine 2, 3 dioxygenase 1 (IDO 1), the enzyme that metabolizes tryptophan and exerts potent immunoregulatory effects on DCs. The defect in the catabolism of tryptophan by IDO-1 leads to the wrong dysfunction of DCs resulting in an impaired tolerogenesis [50]. Also, β-cells death results from the secretion of granzymes or perforin by CD8 + T-cells, which have direct cytotoxicity effects on them [49].
Factors contributing to the occurrence of T1DM
The etiology of T1DM is multifaceted and can result from both environmental and genetic factors similarly to the other autoimmune diseases [51]. Apparently, the immune response against the β cells leading to the occurrence of T1DM are triggered by genetic and environmental factors [52].
Genetic factors
Genetic factors are linked to the activation of the cytotoxic T-cells. For instance, whole-genome screens have pointed out that more than 15 loci are linked to T1DM. Other two genes closely connected to T-cell activation have been recognized later [46]. An allele of the gene for a negative regulator of T-cell activation, cytotoxic T lymphocyte antigen 4 (CTLA4), present on chromosome 2q33, is the third predisposition locus for T1DM and linked to the abundance of regulatory T-cells [46]. Moreover, the candidate gene approach has studied susceptible genes for T1DM, and some key genes like HLA and insulin gene (INS) had been identified[53]. Findings from researches have effectively acknowledged numerous genes that are in charge of the occurrence of T1DM during the past few decades [51]. It has been further confirmed that the alteration or manipulation of these genes by gene therapy approach for example could possibly offer a more holistic disease management or even heal T1DM[51], hence genetic factors are involved in the pathogenesis of T1DM.
Environmental factors
Some environmental factors, mainly viral infections are known to be linked to the onset of T1DM. More precisely, enterovirus [45], rotavirus, and rubella [46] have been known as the most common viral infections associated with T1DM. Enteroviral major capsid protein VP1 and RNA were identified in islets of individuals newly diagnosed with T1DM along with high-expression of the MHC-I [45]. Furthermore, other environmental factors that have been mentioned to be linked to the onset of T1DM include the GM. The gastrointestinal tract, made of the greatest surface area in the human body, is heavily colonized by 500 to 1000 diverse bacterial species [54]. During the last years, many researchers have elucidated the relationship between the host and the GM about health and disease. Alteration in the GM composition had been emphasized by evidence from human studies to have a significant role in the progression of T1DM [55]. Moreover, T1DM sufferers have been found with more unstable and less diverse gut microbiome than healthy subjects. Similarly, changes in the ratio of Firmicutes to Bacteroidetes have been noticed in these T1DM sufferers, indicating the association of the GM to the immune system [54]. A case–control study evaluating the dissimilarities in the GM composition of kids with T1DM and healthy ones revealed similar findings. This study showed important disparities in the abundance of Bifidobacterium, lactobacillus, and clostridium among the two groups [56]. A systematic review assessing the relationship between the GM and T1DM disclosed that alterations in the gut microbiota composition are correlated to T1DM [57]. Moreover, the microbial environment influences the incidence of T1DM in NOD mice. This finding had supported the important role of the GM in the onset of T1DM [58]. Numerous environmental exposure, including infant and adult diet, sufficiency in vitamin D, early life exposure to some viruses such as enteroviruses, low diversity in the gut microbiome, are associated with the development of T1DM [59]. Therefore, the GM is incontestably one of the environmental factors involve in the pathogenesis of T1DM. However, there is one question remained unanswered; how does the GM is connected to the immune system and trigger T1DM?
Gut microbiota (GM), immune system and T1DM
About 60 phyla of bacteria are identified presently, including a quiet few, mainly Firmicutes, Bacteroides, Actinobacteria, Cyanobacteria, Spirochaetes Fusobacteria, Proteobacteria, and Verrucomicrobia exist in the human intestine [60]. The two predominant bacteria phyla in the human gut are Gram-negative Bacteroides and Grampositive Firmicutes (Lactobacillus spp., Bacillus spp Clostridium spp [61]. The largest phyla of bacteria are Firmicutes encompassing over 200 bacterial genera, while Bacteroides comprise about 20 [62]. GM as a pivotal factor in the occurrence of several disorders (namely allergic and autoimmune diseases including T1DM) and considered an endocrine organ implicated in the energy homeostasis maintaining and the host immunity [63, 64]. The bacteria residing in the gut are known to modulate the host’s innate immune response for the immune cells’ response to infections to occur more quickly [63]. The gut is the starting point of autoimmune stimulations; the external environment is in contact with the intestine and its microbiota. There is a multifaceted site for the interaction with the mucosal immune system and, reasonably, with the gut associated lymphoide tissus (GALT), which has a huge composite of immune cells [49]. Several immune niches are known to be contained by the intestine; these include the GALT, which serve as a site for the priming and differentiation of adaptative immune cells. On the other hand, the intestinal epithelium and lamina propria are the residence places for antigen-experienced lymphocytes and stay for the long term as devoted effectors or regulatory cells [65]. Furthermore, it has been found that the microbiota boosts the mucus layer on the intestinal mucosa throughout the direct competition for nutrients, and this process excites the immune system of the gut with the assist of the GALT [66]. More precisely, the immune system is known to be linked to a rise in the abundance of anaerobic bacteria in the gut when immunoglobulin-A is absent. Moreover, the components of the innate immune system are known to impact the GM [66]. Therefore, there is an interconnection between the gut microbiome and the immune cells.
As evidence, finding revealed that, concerning the gut composition, the non existence of Bifidobacterium species contrasting with a high amount of bacteria from genus Bacteroides in feces is positively correlated to the appearance of autoantibodies, which target the islet β-cells of the pancreas [67]. Curiously, many studies have pointed out that the great influence of the gut on various aspects of the host biology, such as the metabolism and the immune system [68]. It has been well documented that GM can have an interaction with non-enteral cells, namely immune cells, and hepatocytes, and produce molecules like short chain fatty acids (SCFAs), indole derivatives, polyamines, and secondary bile acid [69]. Findings from other studies disclosed that relating to the gut microbiome and T-cells, the maturation of particular subsets of lymphocytes is regulated by the local microbiota. For example, in the gut, some specific species of Clostridia contribute to the production of Th17 cells [70]. Other findings during the early post-natal period, the contacts between the immune system and the GM plays a key role in the immune system’s maturation, modulation, and response to self-antigens all along with the lifespan. Consequently, many have recommended that dysbiosis may have a role in the progression of disorders related to immune deregulation, especially allergies, autoimmune, and inflammatory diseases [69]. Furthermore, it is known that the mechanism through which GM modulates the immune system is the production of molecules with immunomodulatory and anti-inflammatory activity able to have an impact on the immune cells. In fact, in the T1DM pathogenesis, cytotoxic actions on the β-cells are induced by pro-inflammatory cytokines, namely IL-1β, IFN-γ, tumor necrosis factor (TNF)-α, released by macrophages, which consequently leads to β-cell death [49]. Therefore, with regards to the previously mentioned examples and findings, it is obvious that the GM is strongly implicated in the pathogenesis of T1DM. An imbalance in the GM composition may be the origin of the immune reactions leading to the β-cells damage in T1DM.
Probiotics for the management of T1DM: Possible Mechanisms of Action
Through the gut microbiome (gut microbiota)
The microbes colonizing humans and their genes are mostly found in the gastrointestinal tract are called gut microbiome or microbiota (GM) [71]. The GM composition can be highly impacted by several environmental factors such as diet and antibiotics [72]. Researchers found that probiotics wield their effects using diverse ways of action such as their aptitude to control the composition of the intestinal microbiota, manipulate the metabolic profile of intestinal microbiota, ameliorate the intestinal barrier function and integrity, and stimulate molecules with influences on humoral and cellular immune responses [67, 73]. Emergent studies have pointed out the importance of GM in human health; some probiotics such as yoghurt appear to interact with the gut microbiota [74]. A predictive model proposed that probiotics act on the systemic immune responses, guarantee the homeostasis of the normal microbiota in the intestinal mucosa, and consequently administered as an adjuvant remedy to manage immune-mediated illnesses. Suggested ways to reach that efficacy comprise mucus production, antimicrobial peptide making, the preservation of the activity of the gastrointestinal–epithelial barrier, securing sufficient connections between the GM and the mucosal immune cells, and lastly, in response to pathobionts, serving the activation of the host immune system [75]. The consumption of specific probiotics strains had been shown to result in numerous health beneifts such as the right control of gut membrane integrity and permeability, thus prevent gut leakiness and inflammation [70], which is strongly involved in T1DM pathogenesis. A study investigated the statement claiming that the probiotic Lactobacillus rhamnosus GG instigated the intestinal barrier's maturation through claudin-3 expression. Similarly, claudin had also been said to be induced by Lactobacillus johnsonii [54]. Findings include standardization of disturbed microbiota composition, intestinal maturation, diminished pathogenic load and infections, and a better immune response in kids who were administrated probiotics during some preclinical studies [36]. Carbohydrate fermentation and digestion, the polarization of specific immune responses, vitamin synthesis, and the prevention of colonization by pathobionts are the major contributions of the microbiota to the host. Studies in germ-free (GF) mice disclosed that the GM is essential in the right immune system mellowing, such as the GALT growth, known for its significant task in the gut mucosa, intolerance induction autoantigens [75].
Through the production of Short Chain Fatty Acids (SCFAs)
Also known as volatile fatty acids, SCFAs are organic acids mainly produced in the gastrointestinal tract in millimolar amounts and majorly in high quantity in predominant anaerobic microorganism areas. The most common SCFAs are acetic acid, propionic acid, and butyric acid. SCFAs symbolize the principal carbon flow from the diet to the host microbiome and an energy source for the host and gut bacteria [60, 76]. SCFAs are known for their important roles in maintaining the host's health, such as maintaining intestinal and immune homeostasis. SCFAs have been proven to have protective effects in the early onset of T1DM in a cohort TEDDY (The Environmental Determinants of Diabetes in the Young) study carried out among children [77]. Also, SCFAs derived from the GM can manipulate different types of cells, among which the immune cells and the pancreatic β-cells are implicated in T1DM pathogenesis [78]. Nevertheless, their production need adequate substrates such as dietary fibers, prebiotics, and probiotics. It is revealed that probiotics can boost the production of the SCFAs [60]. For instance, in fit adults, probiotic intake accelerates the production of SCFAs, fecal moisture, frequency of defecation, and quantity of stools. By acting so, probiotics influence the immune system [36]. Also, researches revealed that, in the mature gut, permeability is wrought by GM from the dilapidation and fermentation of carbohydrates into SCFAs. Butyrate is known for its capability to ameliorate the intestinal barrier by normalizing the assemblage of tight junctions [54]. Besides, other functions of SCFAs, their ability to restore and facilitate the functions of the intestinal mucosa barrier is prominent [79]. SCFAs promote intestinal epithelial cells' production by intestinal mucosin and mucus excretion, inhibit the pro-inflammatory factors production, and increase anti-inflammatory cytokines' production IL-10, then trigger Treg cell function [79]. SCFAs can also provide power to enterocytes and diminish the making of toxic substances and inflammation through the inhibition of the development of dangerous bacteria [79]. As described, probiotics are needed for the production of SCFAs, which play an important role in the delay of T1DM.
Through the control of inflammatory cytokines’ production
The beneficial role of probiotics in intestinal mucosa ranges from the control of the secretion of proinflammatory cytokines, such as IFN-γ, TNF-α, and IL-12, generally through the induction of the maturation and activity of regulatory T cells in the intestinal mucosa [32, 67]. Interestingly, the intake of probiotic strains has been revealed to decline the concentration of cytokines such as IL-6, IL-1β, and TNF-α whereas rising that of anti-inflammatory cytokines, such as transforming growth factor-β (TGF-β) and IL-10, which, as a reminder, are heavily involved in T1DM pathogenesis [49]. A randomized control trial carried out to investigate the effect of Lactobacillus casei 01 on inflammatory biomarkers found a significant decrease of proinflammatory cytokine level was in the probiotic fed group [80]. Also, untimely intake of VSL#3 was found to prevent in NOD mice diabetes. In that study, a low case of insulitis and a low rate of β-cell damage were observed among mice under test group. These positive effects were linked to an augmented making of IL-10 from Peyer’s patches and the spleen and increased IL-10 appearance in the pancreas, where IL-10-positive islet-infiltrating mononuclear cells were observed [81]. Similarly, another study revealed that the use of VSL#3 stops the expression of IL-1β, whereas it improves the production of protolerogenic constituents of the inflammasome, mainly IL-33 and indoleamine 2, 3-dioxygenase (IDO) [75]. Also, the use of several probiotics in a randomized clinical trial disclosed an improvement of inflammation biomarkers in subjects with diabetes [82]. Lactobacillus reuteri 6475 secretory components have been revealed with an anti-TNF-α effect [83]. Bacillus coagulans were also found to reduce serum amyloid level and diminish the proinflammatory cytokines TNF-α in the rat model [84]. Additionally, the intake of B. breve CNCM I-4035 was followed by an important augmentation in fecal IgA content and an augmentation of the plasmatic concentrations of IL-4 and IL-10, while the concentrations of IL-12 declined. L. rhamnosus and L. casei use also displayed the same outcomes [36]. IL-10 is an anti-inflammatory cytokine with numerous pleiotropic properties in immunoregulation and inflammation. Also, IL-10 is known for its ability to impede the activation, and the effector function of several immune cells such as T-cells and macrophages are highly implicated in the pathogenesis of T1DM [81, 85]. Similarly to the previously mentioned examples, many other probiotics strains such as lactobacillus plantarum 06CC2, Lactobacillus paracasei KW3110 have been revealed to have the ability to improve the activity of the cytokines such as interleukin-12 (IL-12), interferon-gamma (interferon-ɣ), thereby protecting against viral infections as one of the environmental factors contributing to the progression of T1DM [86]. The regulation of proinflammatory signaling pathways by suppressing TLR signaling is another mechanism of action for some probiotics. Toll-like receptors (TLRs) are a set of proteins that is not only important in the innate immune system but are also implicated in inflammatory processes [87, 88]. Therefore, suppressing the TLR signaling is a way to prevent inflammation, which is also involved in the T1DM pathogenesis.
Through the regulation of Glucagon-like peptide-1 (GLP-1) production
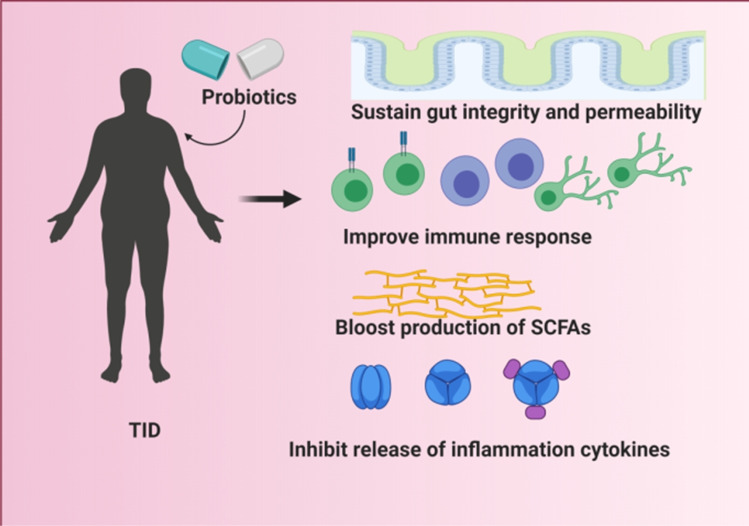
Derived from proglucagon, GLP-1 is a peptide of 36 or 37 amino acids. Its role is to improve the release of glucose-dependent insulin, suppress the secretion of Glucagon, decrease glycemia in T1DM patients and diminish food intake [89]. GLP-1 is majorly produced by the endocrine L-cells of the colon and distal small intestines [90]. It is known as a potential incretin hormone, able to regulate under some conditions, insulin production. Furthermore, it is known to have an impact on β-cells in normal physiological circumstances [91]. More precisely, GLP-1 is known to be able to normalize the β-cells growth. Interestingly, the probiotic strain lactobacillus kefiranofaciens M and lactobacillus kefiri K can impede the onset of T1DM by raising the GLP-1 synthesis [92]. Therefore, from the previously described role of GLP-1 in the context of T1DM, boosting its production may be one of the ways of preventing T1DM (Fig. 1).
Fig. 1.
Probiotics mechanism of actions in the management of T1DM. Probiotics help improve the health conditions of T1DM patients by sustaining the integrity and permeability of the gut microbiota resulting in an improvement of the immune response. Probiotics are also able to boost the production of SCFAs. Furthermore, they are able to inhibit the release of inflammatory cytokines
Probiotics as possible remedy to T1DM: Evidence from Experimental Studies
Animal studies
Mice models
For the effect of Akkermansia muciniphila a probiotic strain on T1DM in mice model, has capacities to induce not only the remodeling of the GM but also the regulation of islet autoimmunity in NOD (Non-Diabetic Obese) mice [93]. Furthermore, probiotic strains that belong to the probiotic families namely Bifidobacteriaceae and Lactobacillaceae and genus Streptococcus thermophilus had been revealed with the ability to improve T1DM conditions in NOD mice through an interesting organization of the gut microbiota formula. It also impacted the intestinal inflammation by keeping the gut immune homeostasis and hindering the IL-1β appearance [75]. Jing Zhang and his team demonstrated from their laboratory that in mice, Lactobacillus reuteri has the propriety of preventing T1DM-induced bone loss and marrow adiposity [83]. In a T1DM C57BL/6 mice model study, Amro Abdelazez and his team have demonstrated that the probiotic strains Lactobacillus brevis KLDS 1.0727 and KLDS 1.0373, have the capabilities to decrease glycemia [94]. Furthermore, the oral intake of L. kefiranofaciens M and L. kefiri K, which are probiotic strains, had been found efficacious in the induction of IL-10 synthesis in the pancreas. This was an animal study on STZ-induced C57BL/6 mice [92]. As a reminder, the synthesis of IL-10 is important to restrain the quantity of T-helper (Th) cell 1-associated cytokines (IL-1β, IL-6, and IL-2) and pro-inflammatory cytokines (TNF-α) available in the pancreas [92]. The probiotic strain Saccharomyces boulardii THT 500,101 used to treat STZ-diabetic mice had been revealed efficacious since the authors found that this strain can modify the gut microbiota, improve hyperglycemia, and dyslipidemia [95]. Probiotic strains of Bifidobacterium species (spp.) also were disclosed to be negatively linked to β-cell autoimmunity in a study on STZ-induced diabetic mice. It was found that the intake of Bifidobacterium spp induced a decrease in the expression of some interleukin and immune markers involved in autoimmunity [96]. The probiotic strain Leuconostoc mesenteroides EH-1 had also been revealed with potentialities to decrease blood glucose and IL-6 concentration and raise insulin level in Streptozotocin (STZ)-induced T1DM mice [78]. Clostridium butyricum CGMCC0313.1 daily administration to female NOD mice in a study was found with positive effects on T1DM such as mitigation of insulitis, delay of T1DM onset, and improvement of metabolic dysfunctions [97].
Rat models
Using rat model, a team of researchers investigated the influence of the intake of γ-aminobutyric acid (GABA)-producing Lactobacillus brevis DPC 6108 and pure GABA on the onset of diabetes in streptozotocin (STZ)-induced diabetic Sprague Dawley rats. The authors revealed that there had been an attenuation of the hyperglycemia caused by diabetes [98]. L. rhamnous MTCC5957, L. rhamnous MTCC5897, and L. fermentum MTCC5898 used to prepare the probiotic fermented milk (PFM) has been shown in STZ-induced diabetic rats experiment to be efficacious in the management of T1DM and its complications [99]. Also, another study in NOD mice reported that enteral intake of Lactobacillus lactis-expressing HSP65-6IA2P2 has the potentialities to postpone the occurrence of T1DM in NOD mice [100]. Administration of a combination of several probiotics to NOD mice for 36 weeks was found effective in reducing T1DM incidence and insulitis. There had been an increase in βcell mass and reduction in gut permeability [101]. Probiotic combination with other products such as soy milk and omega-3 had beneficial effects on T1DM complications in diabetic rats [102].
Human studies assessing the effects of probiotics on T1DM
Positive revelations were also made in human trials conducted to investigate the effects of probiotics intake on T1DM and its complications. A prospective cohort study conducted in ‘’the TEDDY study’’, with the purpose to investigate the consequences of the premature probiotic intake on islet autoimmunity among kids characterized as having high genetic risk for T1DM. The study outcomes disclosed that the early dietary intake (mainly within the 27 days after birth) of probiotics, fundamentally Lactobacillus and Bifidobacterium, may reduce the threat of islet autoimmunity in kids [103]. In a double-blinded randomized control trial carried out among 42 healthy subjects with an unknown risk factor for T1DM to assess their responses to the intake of L. johnsonii N6.2. From thier findings, the intake of L. johnsonii N6.2 considerably reduced the abdominal pain, indigestion, and cephalic syndromes. According to the authors, the data have supported the safety and possibility of L. johnsonii N6.2 use in prevention trials among individuals at risk for T1DM [104]. In a recent study carried out among children and young adults with T1DM to evaluate the effect of 3 months intake of Lactobacillus Rhamnosus GG on immune system functions, findings showed that the probiotic conferred important anti-inflammatory effects [2]. Furthermore, the use of probiotic had been revealed to be linked to a considerable better blood sugar control and diminution of odds of metabolic syndrome and its components in a cross-sectional assessment of the relationship between the use of probiotics-containing products and different health markers in a huge number of T1DM subjects [105]. Also, the use of the probiotic Lactobacillus rhamnosus GG has revealed efficacious in the metabolism of systemic tryptophan (whose catabolism occurs through the GM) and the restraining of proinflammatory cytokines in childhood T1DM [106]. Furthermore, the probiotic strain Lactobacillus brevis CD2 had been proven to alleviate risk factors related to caries and gingival health in T1DM children in a randomized clinical trial [40]. These mentioned defections or periodontal diseases are some of the consequences of T1DM in children [25]. Some studies have found interesting outcomes from the combination of probiotics with other products. For example, the combination of gliclazide to some hypoglycemic agents, namely probiotics and bile acids, had resulted in more pronounced effects than when used alone. Even though the molecular mechanisms of interactions are not fully understood, it had been disclosed that there is a good synergy of impact resulting in a diminution of the blood sugar and enhancement of diabetes-related complications [41]. Even more, some studies carried out assessing the impacts of probiotics on other autoimmune disorders are reassuring [107–109]. As listed, the revelations from these studies support that the probiotics intake (used alone or in a combination), whether at a very young age or in adulthood, looks promising in reducing T1DM risk or in the management of its complications. However, all the probiotic strains used in the different studies did not provide such promising results. A randomized double-blind placebo-controlled clinical trial was carried out to study the prevention of allergy. There was no impact of probiotic use on the T1DM onset among children aged 13 or on islet cell autoimmunity among children aged 5 [110].
Summary of major animal and human studies on probiotic interventions and their findings related to T1DM are listed in Table 1.
Table 1.
Summary of major animal and human studies on the use of probiotics in the management of T1DM as described in Sects. 3.1 and 3.2
| Probiotic strain | Model/Subjects/study type | Major findings or outcomes | References |
|---|---|---|---|
| Animal studies assessing the effects of probiotics on T1DM | |||
| -Akkermansia muciniphila | NOD (Non-Diabetic Obese) mice |
Induction of gut microbiota remodeling Control of islet autoimmunity |
[93] |
| Lactobacillus brevis KLDS 1.0727 and KLDS 1.0373 | C57BL/6 mice |
Considerable reduction of glycemia level Considerable reduction of blood plasma Histological assays of mice organs |
[94] |
| Probiotic strains belonging to families Bifidobacteriaceae, Lactobacillaceae, and genus Streptococcus thermophilus | NOD mice |
Improve the T1DM via positive modulation of GM composition Decrease the intestinal inflammation through the maintenance of gut immune homeostasis and inhibition of IL-1β expression |
[75] |
| Lactobacillus brevis DPC 6108 | Streptozotocin (STZ)-induced diabetic Sprague Dawley rats; | Attenuation of the hyperglycemia caused by the diabetes | [98] |
| Lactobacillus reuteri | Mice model |
Stops T1DM-induced bone loss and marrow adiposity Prevention of TNF-α-mediated suppression of Wnt10b and osteoblast maturation markers |
[83] |
| probiotic-fermented milk containing L. rhamnous MTCC5957, L. rhamnous MTCC5897, and L. fermentum MTCC5898 | STZ-induced diabetic rats |
A decrease in TNF-α and IL -6 concentrations Significant improvement of glucose metabolism, serum inflammation status, oxidative stress, serum lipid profile Significant reduction of mRNA expression of pick and g6pase genes coding the key enzymes of gluconeogenesis way |
[99] |
| Lactobacillus lactis-expressing HSP65-6IA2P2 | NOD mice |
Prevent hyperglycemia Ameliorate glucose tolerance Decrease insulitis Increase regulatory immune reactions Balance in Tcells levels |
[100] |
| L. kefiranofaciens M and L. kefiri K | STZ-induced C57BL/6 mice | Stimulate the production of IL-10 in the pancreas | [92] |
| Saccharomyces boulardii Tht 500,101 | STZ -diabetic mice |
Changes in the GM composition Amelioration of hyperglycemia, dyslipidemia Modulation of inflammatory profiles |
[95] |
| strains belonging to Bifidobacterium spp. | STZ-induced diabetic mice | Negatively associated with β-cell autoimmunity | [96] |
| Leuconostoc mesenteroides EH-1 | Streptozotocin (STZ)-induced T1DM mice |
Decrease of blood glucose and IL-6 concentration Augmentation of insulin level |
[78] |
| Clostridium butyricum CGMCC0313.1 | female NOD mice |
Mitigation of insulitis Delay of T1DM onset Improvement of metabolic dysfunctions |
[97] |
| Lactobacillus acidophilus, Lactobaciluscasei, Lactobacillus reuteri, Bifidobacterium bifidium, and Streptococcus thermophiles | NOD mice |
Reduction of T1DM incidence and insulitis Increase in βcell mass Reduction in gut permeability |
[101] |
| Lactobacillus casei + soy milk + omega-3 | T1DM rats |
Improvement of stereological changes in the tibia and vertebra Raise of antioxidant activity Amelioration of the redox homeostasis |
[102] |
| Human studies assessing the effects of probiotics on T1DM | |||
| Lactobacillus and Bifidobacterium, | TEDDY prospective cohort study with 8676 children genetically at increased risk for T1DM for 10 years | Reduction in the risk of islet autoimmunity | [103] |
| Lactobacillus johnsonii N6.2 | A double blinded randomized trial among 42 healthy individuals Participants receiving 1 capsule/day containing 108 colony-forming units(CFU) for 8 weeks |
Regulation of the penetration of monocytes, natural killer cells, circulating Teff, TH1 cells, and cytotoxic CD8 + T cells in the islets Reduction of the occurrence of abdominal pain, indigestion, and cephalic syndromes |
[104] |
| Lactobacillus rhamnosus GG | A prospective randomized and single-blind study among 87 pediatric patients with T1DM for 3-month | Induction of anti-inflammatory activity | [2] |
| probiotics-containing products | 1039 T1DM adult patients (mean age 46 ± 14 years, 45% men) for at least 8 weeks |
Maintaining better glycemic control ameliorate conditions of metabolic syndromes, such as high blood pressure, high TG levels, and HDL-C |
[105] |
| Lactobacillus rhamnosus GG | 61 young (aged 3–18 years) T1DM patients for 3-month |
Regulation of systemic tryptophan metabolism which occurs through the GM Restraining of proinflammatory profile |
[106] |
| lactobacillus brevis CD2 | Randomized clinical trial in 68 T1DM children for 60 days) | Amelioration of the risk factors related to caries and gingival health in | [40] |
| probiotic treatment in infancy | A cohort of 123 children with high risk of allergy in 13 year follow-up | No effect on the development of T1DM by the age of 13 years nor islet cell autoimmunity by the age of 5 years | [110] |
Conclusion
T1DM is an autoimmune disease due to a selective destruction of β-cells by Tcells, which highly prevalent worldwide especially among children and young adults. Evidence from animal and human studies carried out to assess the effects of probiotics on T1DM have proven that probiotics reflect their definition. Evidence from the experimental studies explored in this review is proof that probiotics can impact the autoimmunity or autoimmune and anti-inflammatory diseases such as T1DM. Even though all the study results are not positively correlated, it is worthy to state that probiotics can be an alternative, adjuvant or preventive therapy in the management of T1DM and its complications. The mechanisms of probiotics on T1DM include the normalization of GM composition, which is linked to the immune system since T1DM is an autoimmune disease. Also, probiotics act on T1DM through the regulation of inflammation-related cytokines, the production of SCFAs, and the regulation of GLP-1. Hence, the effects of probiotics on T1DM must not be undervalued; rather, they may be a new approach to fight autoimmune, metabolic diseases, and inflammations such as T1DM. This review recommends more human studies on T1DM and human studies should not only be focused on RCTs but also on cohort and case controls studies to assess whether the regular intake of probiotics can be linked to the delay or prevention of T1DM.
Abbreviations
- APCs
Antigen-presenting cells
- β
Beta
- DCs
Dendritic cells
- GALT
Gut-associated lymphatic tissues
- GM
Gut microbiota
- T1DM
Type 1 diabetes
- GLP-1
Glucagon-like peptide-1
- HLA
Human Leukocyte Antigen
- IDO
Indoleamine 2,3-dioxygenase
- IFN
Interferon
- TNF
Tumor necrosis factor
- IL-10
Interleukin 10
- L
Lactobacillus
- LjN6.2
Lactobacillus johsonii Strain N6.2
- Lr
Lactobacillus reuteri Strain
- MHC
Major Histocompatibility Complex
- TLRs
Toll-like receptors
- STZ
Streptozotocin
- SCFAs
Short-chain fatty acids
- TEDDY
The Environmental Determinants of Diabetes in the Young
Author Contributions
KSD and OB have conceived and drafted the manuscript, KSD wrote the manuscript, and IC and OB have critically reviewed it. All the authors have approved the final version submitted for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
N/A
Conflict of interests
The authors declare that they have no conflict interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diabetes [https://www.who.int/news-room/fact-sheets/detail/diabetes]. Accessed 10 Sep 2021.
- 2.Bianchini S, Orabona C, Camilloni B, Berioli MG, Argentiero A, Matino D, Alunno A, Albini E, Vacca C, Pallotta MT, et al. Effects of probiotic administration on immune responses of children and adolescents with type 1 diabetes to a quadrivalent inactivated influenza vaccine. Hum Vaccin Immunother. 2020;16(1):86–94. doi: 10.1080/21645515.2019.1633877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Huang E, Gao S. Type 1 Diabetes Mellitus and Cognitive Impairments: A Systematic Review. Journal of Alzheimer's disease : JAD. 2017;57(1):29–36. doi: 10.3233/JAD-161250. [DOI] [PubMed] [Google Scholar]
- 4.Groele L, Szajewska H, Szypowska A. Effects of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: protocol of a randomised controlled trial. BMJ open. 2017;7(10):e017178. doi: 10.1136/bmjopen-2017-017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salgaço MK, Oliveira LGS, Costa GN, Bianchi F, Sivieri K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl Microbiol Biotechnol. 2019;103(23–24):9229–9238. doi: 10.1007/s00253-019-10156-y. [DOI] [PubMed] [Google Scholar]
- 7.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):625–640. doi: 10.1016/j.ecl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Clinic M: Type 1 diabetes. 2020. https://www.mayoclinic.org/diseases-conditions/type-1-diabetes/symptoms-causes/syc-20353011. Accessed 29 June 2021.
- 9.Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Ruparelia A, Devani P, Trivella M, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS medicine. 2018;15(2):e1002507. doi: 10.1371/journal.pmed.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanta A, Lyka E, Koufakis T, Zebekakis P, Kotsa K. Prevention strategies for type 1 diabetes: a story of promising efforts and unmet expectations. Hormones (Athens) 2020;19(4):453–465. doi: 10.1007/s42000-020-00207-9. [DOI] [PubMed] [Google Scholar]
- 11.Insel R, Knip M. Prospects for primary prevention of type 1 diabetes by restoring a disappearing microbe. Pediatr Diabetes. 2018;19(8):1400–1406. doi: 10.1111/pedi.12756. [DOI] [PubMed] [Google Scholar]
- 12.Knip M, Honkanen J. Modulation of Type 1 Diabetes Risk by the Intestinal Microbiome. Curr DiabRep. 2017;17(11):105. doi: 10.1007/s11892-017-0933-9. [DOI] [PubMed] [Google Scholar]
- 13.Kondrashova A, Hyöty H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int Rev Immunol. 2014;33(4):284–295. doi: 10.3109/08830185.2014.889130. [DOI] [PubMed] [Google Scholar]
- 14.National Diabetes Statistics Report, 2020 [https://www.cdc.gov/diabetes/data/statistics-report/index.html]. Accessed 29 Aug 2021.
- 15.Rewers M, Hyöty H, Lernmark Å, Hagopian W, She JX, Schatz D, Ziegler AG, Toppari J, Akolkar B, Krischer J. The Environmental Determinants of Diabetes in the Young (TEDDY) Study: 2018 Update. Curr DiabRep. 2018;18(12):136. doi: 10.1007/s11892-018-1113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–417. doi: 10.1007/s00125-018-4763-3. [DOI] [PubMed] [Google Scholar]
- 17.Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, Mu Y, Jia W. Incidence of type 1 diabetes in China, 2010–13: population based study. Bmj. 2018;360:j5295. doi: 10.1136/bmj.j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johanna S. Prevalence of Type 1 Diabetes on the Rise in China. 2018. https://www.medindia.net/news/prevalence-of-type-1-diabetes-on-the-rise-in-china-175999-1.htm. Accessed 30 Aug 2021.
- 19.Yang J, Tamura RN, Uusitalo UM, Aronsson CA, Silvis K, Riikonen A, Frank N, Joslowski G, Winkler C, Norris JM, et al. Vitamin D and probiotics supplement use in young children with genetic risk for type 1 diabetes. Eur J Clin Nutr. 2017;71(12):1449–1454. doi: 10.1038/ejcn.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. 2013;37(4):269–276. doi: 10.1016/j.jcjd.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Type 1 Diabetes [https://www.cdc.gov/diabetes/basics/diabetes.html]. Accessed 1 Sept 2021.
- 22.Bibbò S, Dore MP, Pes GM, Delitala G, Delitala AP. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann Med. 2017;49(1):11–22. doi: 10.1080/07853890.2016.1222449. [DOI] [PubMed] [Google Scholar]
- 23.Uusitalo U, Lee HS, Andrén Aronsson C, Vehik K, Yang J, Hummel S, Silvis K, Lernmark Å, Rewers M, Hagopian W, et al. Early Infant Diet and Islet Autoimmunity in the TEDDY Study. Diabetes Care. 2018;41(3):522–530. doi: 10.2337/dc17-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu C, Wong FS, Wen L. Type 1 diabetes and gut microbiota: Friend or foe? Pharmacol Res. 2015;98:9–15. doi: 10.1016/j.phrs.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh-Hüsgen P, Meissner T, Bizhang M, Henrich B, Raab WH. Investigation of the oral status and microorganisms in children with phenylketonuria and type 1 diabetes. Clin Oral Invest. 2016;20(4):841–847. doi: 10.1007/s00784-015-1564-7. [DOI] [PubMed] [Google Scholar]
- 26.Aschner P. Insulin Therapy in Type 2 Diabetes. Am J Ther. 2020;27(1):e79–e90. doi: 10.1097/MJT.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 27.Rachdaoui N. Insulin: The Friend and the Foe in the Development of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020; 21(5):1770. 10.3390/ijms21051770. [DOI] [PMC free article] [PubMed]
- 28.Livia Bordalo Tonucci KODS, Ferreira Celia Lucia De Luces Fortes, Ribeiro Sonia Machado Rocha, Oliveira Leandro Licursi De, Martino Hercia Stampini Duarte. Clinical Application of Probiotics in Diabetes Mellitus: Therapeutics and New Perspectives. Critical Reviews in Food Science and Nutrition. 2015;57(11):1549–7852. doi: 10.1080/10408398.2014.934438. [DOI] [PubMed] [Google Scholar]
- 29.Probiotics Definition by the World Health Organization [https://www.probiotics-lovethatbug.com/probiotics-definition.html]. Accessed 22 Jul 2021
- 30.Camacho F, Macedo A, Malcata F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar Drugs. 2019; 17(6). 10.3390/md17060312. [DOI] [PMC free article] [PubMed]
- 31.Kothari D, Patel S, Kim SK. Probiotic supplements might not be universally-effective and safe: A review. Biomed Pharmacother. 2019;111:537–547. doi: 10.1016/j.biopha.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Alookaran JJ, Rhoads JM. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients. 2018;10(10). 10.3390/nu10101537. [DOI] [PMC free article] [PubMed]
- 33.Azad MAK, Sarker M, Li T, Yin J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res Int. 2018;2018:9478630. doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesika P, Sivamaruthi BS, Chaiyasut C. Do Probiotics Improve the Health Status of Individuals with Diabetes Mellitus? A Review on Outcomes of Clinical Trials. Biomed Res Int. 2019;2019:1531567. doi: 10.1155/2019/1531567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes & metabolic syndrome. 2019;13(1):175–182. doi: 10.1016/j.dsx.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of Action of Probiotics. Advances in nutrition (Bethesda, Md) 2019;10(suppl_1):S49–s66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, Kim HB, Lee JH. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J Microbiol Biotechnol. 2019;29(9):1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 38.Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96(3):170–178. [PubMed] [Google Scholar]
- 39.Abraham BP, Quigley EMM. Probiotics in Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46(4):769–782. doi: 10.1016/j.gtc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Lai S, Lingström P, Cagetti MG, Cocco F, Meloni G, Arrica MA, Campus G. Effect of Lactobacillus brevis CD2 containing lozenges and plaque pH and cariogenic bacteria in diabetic children: a randomised clinical trial. Clin Oral Invest. 2021;25(1):115–123. doi: 10.1007/s00784-020-03342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikov M, Đanić M, Pavlović N, Stanimirov B, Goločorbin-Kon S, Stankov K, Al-Salami H. Potential Applications of Gliclazide in Treating Type 1 Diabetes Mellitus: Formulation with Bile Acids and Probiotics. Eur J Drug Metab Pharmacokinet. 2018;43(3):269–280. doi: 10.1007/s13318-017-0441-y. [DOI] [PubMed] [Google Scholar]
- 42.Lamichhane S, Ahonen L, Dyrlund TS, Siljander H, Hyöty H, Ilonen J, Toppari J, Veijola R, Hyötyläinen T, Knip M, et al. A longitudinal plasma lipidomics dataset from children who developed islet autoimmunity and type 1 diabetes. Scientific data. 2018;5:180250. doi: 10.1038/sdata.2018.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74–85. doi: 10.1111/cei.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zununi Vahed S, Moghaddas Sani H, Rahbar Saadat Y, Barzegari A, Omidi Y. Type 1 diabetes: Through the lens of human genome and metagenome interplay. Biomed Pharmacother. 2018;104:332–342. doi: 10.1016/j.biopha.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 45.Barnett R. Type 1 diabetes. Lancet (London, England) 2018;391(10117):195. doi: 10.1016/S0140-6736(18)30024-2. [DOI] [PubMed] [Google Scholar]
- 46.Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ Canadian Medical Association Journal Journal de l'Association Medicale Canadienne. 2006;175(2):165–170. doi: 10.1503/cmaj.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, Bassi R, D'Addio F, Schatton T, Abdi R, et al. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes. 2015;64(1):158–171. doi: 10.2337/db13-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelhamid L, Luo XM. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients. 2018;10(8):1016. 10.3390/nu10081016. [DOI] [PMC free article] [PubMed]
- 49.Mishra SP, Wang S, Nagpal R, Miller B, Singh R, Taraphder S, Yadav H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms. 2019;7(3):67. 10.3390/microorganisms7030067. [DOI] [PMC free article] [PubMed]
- 50.Mondanelli G, Albini E, Pallotta MT, Volpi C, Chatenoud L, Kuhn C, Fallarino F, Matino D, Belladonna ML, Bianchi R, et al. The Proteasome Inhibitor Bortezomib Controls Indoleamine 2,3-Dioxygenase 1 Breakdown and Restores Immune Regulation in Autoimmune Diabetes. Front Immunol. 2017;8:428. doi: 10.3389/fimmu.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chellappan DK, Sivam NS, Teoh KX, Leong WP, Fui TZ, Chooi K, Khoo N, Yi FJ, Chellian J, Cheng LL, et al. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–1200. doi: 10.1016/j.biopha.2018.09.138. [DOI] [PubMed] [Google Scholar]
- 52.Krischer JP, Lynch KF, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B. Genetic and Environmental Interactions Modify the Risk of Diabetes-Related Autoimmunity by 6 Years of Age: The TEDDY Study. Diabetes Care. 2017;40(9):1194–1202. doi: 10.2337/dc17-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikegami H, Noso S, Babaya N, Kawabata Y. Genetics and pathogenesis of type 1 diabetes: prospects for prevention and intervention. J Diabetes Investig. 2011;2(6):415–420. doi: 10.1111/j.2040-1124.2011.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gülden E, Wong FS, Wen L. The gut microbiota and Type 1 Diabetes. Clinical immunology (Orlando, Fla) 2015;159(2):143–153. doi: 10.1016/j.clim.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111–118. doi: 10.1016/j.diabres.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuño MI. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamshidi P, Hasanzadeh S, Tahvildari A, Farsi Y, Arbabi M, Mota JF, Sechi LA, Nasiri MJ. Is there any association between gut microbiota and type 1 diabetes? A systematic review Gut pathogens. 2019;11:49. doi: 10.1186/s13099-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world–recent facts and figures. Immunol Today. 1993;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 59.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet (London, England) 2018;391(10138):2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12(4):1107. 10.3390/nu12041107. [DOI] [PMC free article] [PubMed]
- 61.Yang L, Bajinka O, Jarju PO, Tan Y, Taal AM, Ozdemir G. The varying effects of antibiotics on gut microbiota. AMB Express. 2021;11(1):11662. doi: 10.1186/s13568-021-01274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kassaian N, Aminorroaya A, Feizi A, Jafari P, Amini M. The effects of probiotic and synbiotic supplementation on metabolic syndrome indices in adults at risk of type 2 diabetes: study protocol for a randomized controlled trial. Trials. 2017;18(1):148. doi: 10.1186/s13063-017-1885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut microbes. 2018;9(4):308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajinka O, Darboe A, Tan Y, et al. Gut microbiota and the human physiological changes. Ann Microbiol. 2020;70:65. doi: 10.1186/s13213-020-01608-2. [DOI] [Google Scholar]
- 65.Fenton TM, Jørgensen PB, Niss K, Rubin SJS, Mörbe UM, Riis LB, Da Silva C, Plumb A, Vandamme J, Jakobsen HL, et al. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity. 2020;52(3):557–570.e556. doi: 10.1016/j.immuni.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bajinka O, Darboe A, Tan Y, Abdelhalim KA, Cham LB. Gut microbiota and the human gut physiological changes. Annals of Microbiology. 2020;70(1):65. doi: 10.1186/s13213-020-01608-2. [DOI] [Google Scholar]
- 67.Sales-Campos H, Soares SC, Oliveira CJF. An introduction of the role of probiotics in human infections and autoimmune diseases. Crit Rev Microbiol. 2019;45(4):413–432. doi: 10.1080/1040841X.2019.1621261. [DOI] [PubMed] [Google Scholar]
- 68.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92(1087):286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 69.Bajinka O, Tan Y, Abdelhalim KA, Özdemir G, Qiu X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express. 2020;10(1):130. doi: 10.1186/s13568-020-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152(1):1–12. doi: 10.1111/imm.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harsch IA, Konturek PC. The Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabetes Mellitus: New Insights into "Old" Diseases. Med Sci (Basel). 2018;6(2):32. 10.3390/medsci6020032. [DOI] [PMC free article] [PubMed]
- 72.Hampe CS, Roth CL. Probiotic strains and mechanistic insights for the treatment of type 2 diabetes. Endocrine. 2017;58(2):207–227. doi: 10.1007/s12020-017-1433-z. [DOI] [PubMed] [Google Scholar]
- 73.Homayouni A, Bagheri N. Mohammad-Alizadeh-Charandabi S, Kashani N, Mobaraki-Asl N, Mirghafurvand M, Asgharian H, Ansari F, Pourjafar H: Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr Diabetes Rev. 2020;16(6):538–545. doi: 10.2174/1573399815666190712193828. [DOI] [PubMed] [Google Scholar]
- 74.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, Clementi M, Canducci F, Falcone M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:7569431. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caesar R. Pharmacologic and Nonpharmacologic Therapies for the Gut Microbiota in Type 2 Diabetes. Can J Diabetes. 2019;43(3):224–231. doi: 10.1016/j.jcjd.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark Å, Hagopian WA, Rewers MJ, She JX, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traisaeng S, Batsukh A, Chuang TH, Herr DR, Huang YF, Chimeddorj B, Huang CM. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci Rep. 2020;10(1):7928. doi: 10.1038/s41598-020-64916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother. 2020;125:109914. doi: 10.1016/j.biopha.2020.109914. [DOI] [PubMed] [Google Scholar]
- 80.Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, Sharif SK, Vaghef-Mehrabany L, Asghari-Jafarabadi M, Nakhjavani MR, Mohtadi-Nia J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. 2014;17(5):519–527. doi: 10.1111/1756-185X.12333. [DOI] [PubMed] [Google Scholar]
- 81.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48(8):1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 82.Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J Clin Nutr. 2018;27(3):581–591. doi: 10.6133/apjcn.082017.03. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156(9):3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abhari K, Shekarforoush SS, Hosseinzadeh S, Nazifi S, Sajedianfard J, Eskandari MH. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr Res. 2016;60:30876. doi: 10.3402/fnr.v60.30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 86.Takeda S, Kawahara S, Hidaka M, Yoshida H, Watanabe W, Takeshita M, Kikuchi Y, Bumbein D, Muguruma M, Kurokawa M. Effects of oral administration of probiotics from Mongolian dairy products on the Th1 immune response in mice. Biosci Biotechnol Biochem. 2013;77(7):1372–1378. doi: 10.1271/bbb.120624. [DOI] [PubMed] [Google Scholar]
- 87.Kordjazy N, Haj-Mirzaian A, Haj-Mirzaian A, Rohani MM, Gelfand EW, Rezaei N, Abdolghaffari AH. Role of toll-like receptors in inflammatory bowel disease. Pharmacol Res. 2018;129:204–215. doi: 10.1016/j.phrs.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Lin PP, Hsieh YM, Kuo WW, Lin YM, Yeh YL, Lin CC, Tsai FJ, Tsai CH, Tsai CC, Huang CY. Suppression of TLR-4-related inflammatory pathway and anti-fibrosis effects of probiotic-fermented purple sweet potato yogurt in hearts of spontaneously hypertensive rats. Chin J Physiol. 2013;56(3):174–183. doi: 10.4077/CJP.2013.BAB118. [DOI] [PubMed] [Google Scholar]
- 89.Information, National Center for Biotechnology. Glucagon-like peptide 1. 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Glucagon-like-peptide-1. Accessed 28 June 2021.
- 90.Sun J, Li H, Jin Y, Yu J, Mao S, Su KP, Ling Z, Liu J. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson's disease via gut microbiota-GLP-1 pathway. Brain Behav Immun. 2021;91:703–715. doi: 10.1016/j.bbi.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 91.Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. Glucagon-like peptide 1 (GLP-1) Molecular metabolism. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shih-HungWei Y-P. Ming-JuChen: Selecting probiotics with the abilities to enhance GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. Journal of Functional Foods. 2015;18:473–486. doi: 10.1016/j.jff.2015.08.016. [DOI] [Google Scholar]
- 93.Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67(8):1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- 94.Abdelazez A, Abdelmotaal H, Evivie SE, Melak S, Jia FF, Khoso MH, Zhu ZT, Zhang LJ, Sami R, Meng XC. Screening Potential Probiotic Characteristics of Lactobacillus brevis Strains In Vitro and Intervention Effect on Type I Diabetes In Vivo. Biomed Res Int. 2018;2018:7356173. doi: 10.1155/2018/7356173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albuquerque R, Brandão ABP, De Abreu I, Ferreira FG, Santos LB, Moreira LN, Taddei CR, Aimbire F, Cunha TS. Saccharomyces boulardii Tht 500101 changes gut microbiota and ameliorates hyperglycaemia, dyslipidaemia, and liver inflammation in streptozotocin-diabetic mice. Benef Microbes. 2019;10(8):901–912. doi: 10.3920/BM2019.0056. [DOI] [PubMed] [Google Scholar]
- 96.Le TK, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le TH, Pham XD. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomedical research (Tokyo, Japan) 2015;36(1):63–70. doi: 10.2220/biomedres.36.63. [DOI] [PubMed] [Google Scholar]
- 97.Jia L, Shan K, Pan LL, Feng N, Lv Z, Sun Y, Li J, Wu C, Zhang H, Chen W, et al. Clostridium butyricum CGMCC0313.1 Protects against Autoimmune Diabetes by Modulating Intestinal Immune Homeostasis and Inducing Pancreatic Regulatory T Cells. Frontiers in immunology. 2017;8:1345. doi: 10.3389/fimmu.2017.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marques TM, Patterson E, Wall R, O'Sullivan O, Fitzgerald GF, Cotter PD, Dinan TG, Cryan JF, Ross RP, Stanton C. Influence of GABA and GABA-producing Lactobacillus brevis DPC 6108 on the development of diabetes in a streptozotocin rat model. Benef Microbes. 2016;7(3):409–420. doi: 10.3920/BM2015.0154. [DOI] [PubMed] [Google Scholar]
- 99.Yadav R, Dey DK, Vij R, Meena S, Kapila R, Kapila S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb Pathog. 2018;125:454–462. doi: 10.1016/j.micpath.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 100.Liu KF, Liu XR, Li GL, Lu SP, Jin L, Wu J. Oral administration of Lactococcus lactis-expressing heat shock protein 65 and tandemly repeated IA2P2 prevents type 1 diabetes in NOD mice. Immunol Lett. 2016;174:28–36. doi: 10.1016/j.imlet.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 101.Kim TK, Lee JC, Im SH, Lee MS. Amelioration of Autoimmune Diabetes of NOD Mice by Immunomodulating Probiotics. Front Immunol. 1832;2020:11. doi: 10.3389/fimmu.2020.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bayat M, Dabbaghmanesh MH, Koohpeyma F, Mahmoodi M, Montazeri-Najafabady N, Bakhshayeshkaram M. The Effects of Soy Milk Enriched with Lactobacillus casei and Omega-3 on the Tibia and L5 Vertebra in Diabetic Rats: a Stereological Study. Probiotics Antimicrob Proteins. 2019;11(4):1172–1181. doi: 10.1007/s12602-018-9482-z. [DOI] [PubMed] [Google Scholar]
- 103.Uusitalo U, Liu X, Yang J, Aronsson CA, Hummel S, Butterworth M, Lernmark Å, Rewers M, Hagopian W, She JX, et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 2016;170(1):20–28. doi: 10.1001/jamapediatrics.2015.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marcial GE, Ford AL, Haller MJ, Gezan SA, Harrison NA, Cai D, Meyer JL, Perry DJ, Atkinson MA, Wasserfall CH, et al. Lactobacillus johnsonii N6.2 Modulates the Host Immune Responses: A Double-Blind, Randomized Trial in Healthy Adults. Frontiers in immunology. 2017;8:655. doi: 10.3389/fimmu.2017.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahola AJ, Harjutsalo V, Forsblom C, Freese R, Mäkimattila S, Groop P-H. The self-reported use of probiotics is associated with better glycaemic control and lower odds of metabolic syndrome and its components in Type 1 Diabetes. Journal of Probiotics & Health. 2017;5(4). 10.4172/2329-8901.1000188.
- 106.Mondanelli G, Orecchini E, Volpi C, Panfili E, Belladonna ML, Pallotta MT, Moretti S, Galarini R, Esposito S, Orabona C. Effect of Probiotic Administration on Serum Tryptophan Metabolites in Pediatric Type 1 Diabetes Patients. International journal of tryptophan research : IJTR. 2020;13:1178646920956646. doi: 10.1177/1178646920956646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Håkansson Å, Andrén Aronsson C, Brundin C, Oscarsson E, Molin G, Agardh D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019;11(8):1925. 10.3390/nu11081925. [DOI] [PMC free article] [PubMed]
- 108.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet (London, England) 2003;361(9372):1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 109.Liu XJ, Yu R, Zou KF. Probiotic Mixture VSL#3 Alleviates Dextran Sulfate Sodium-induced Colitis in Mice by Downregulating T Follicular Helper Cells. Current medical science. 2019;39(3):371–378. doi: 10.1007/s11596-019-2045-z. [DOI] [PubMed] [Google Scholar]
- 110.Savilahti E, Härkönen T, Savilahti EM, Kukkonen K, Kuitunen M, Knip M. Probiotic intervention in infancy is not associated with development of beta cell autoimmunity and type 1 diabetes. Diabetologia. 2018;61(12):2668–2670. doi: 10.1007/s00125-018-4738-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.