Abstract
Purpose
Wounds with dead tissue, purulent wounds, and gangrene are good options for larval therapy. We aim to investigate the effect of larval therapy on diabetic wounds and compare it with traditional treatment.
Methods
The sterile larvae were used in wound treatment and the infection rate, Erythrocyte Sedimentation Rate (ESR), and wound size were measured and compared before and after the treatment.
Results
The scars of 40 patients in the larval therapy group were evaluated every 10 days and the mean size of the scar decreased from 38.5 cm (36.6 cm) before treatment to 5.0 cm (6.6 cm) after 60 days. ESR mean was decreased from 57.3 (18.3) before treatment to 15.8 (4.8) after treatment in the larval therapy group. These parameters were significantly decreased compared to the debridement group (p < 0.001).
Conclusions
Larval therapy is effective in diabetic wound healing. The size of the wound after larval treatment is smaller than before. There was no difference between the two groups for infection rate. ESR was significantly decreased in the larval therapy group that indicating the lower inflammation in this group.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-00973-w.
Keywords: Lucilia sericata, Diabetes, Chronic ulcers, Larva
Introduction
The first reports of larvae being used to treat wounds date back to ancient times. At that time, this method was widely used all over the world. From the sixteenth century onwards, various physicians on the battlefield reported the effects of fly larvae (maggot) in wound healing in 1556, 1827, 1860, and 1920 [1, 2]. For the first time in a year, a third of Henriʼs physicians in France reported the importance of fly larvae in treating diseases. In 1827, the head of Napoleon's army medical team reported the importance of fly larvae in healing wounds. In 1920, a U.S. Army medical officer who had been sent to the French front to treat the wounded during World War I observed the wounds of two soldiers while working and he saw many larvas in their wounds. There is no fever in their body; the wounds are not infected and there is a considerable acceleration in wound healing [3]. Even today, doctors use the miraculous effects of using the larvae of species of flies to treat some types of wounds. The first scientific studies on the use of maggots were started by a doctor named Dr. W.S. Bayer [4]. During the 1920s and 1930s, Bayer reported successful treatment of bone infections and chronic foot ulcers in more than 90 patients using maggots. The discovery of penicillin and sulfa drugs in the 1940s led to the cessation of maggot therapy [3].
But now, with the advent of antibiotic-resistant microbes, human medicine has turned to larval therapy again. The FDA approved the method in 2004. In addition to larval therapy, the new wave of this method has started in that country and is very common in countries such as the United States, England, and Germany, so that by 2004, 70,000 people in the United Kingdom were treated with this method [5]. One of the pioneers in the use of larval therapy in modern times is Dr. Sherman. He first tested the larval treatment method on himself; In this way, they created a large wound in his leg in the operating room, and then he personally placed the larvae of Lucilia sericata in the wound, while observing the activity of the larvae and their amazing effect in wound healing; Showed that larvae do not have the slightest adverse effect on the human body [6].
He mentions one of the most remarkable cases of larval therapy, in which a woman's intestine was perforated and a diffuse infection in the abdomen damaged the intestinal wall. Because surgery to remove dead intestinal tissue was so dangerous, doctors decided to try using larvae. The patient's abdomen was opened and after placing 2,000 larvae on the dead tissue, a dressing was performed. Two days later, the maggots were removed and no dead tissue remained. The woman recovered without the need for any other surgery [1]. However, the patient who uses maggot therapy does not need antibiotics, because the larvae themselves have the antibiotic allantoin, which spreads on the wound. Basically, wounds that do not respond to antibiotics or do not reach due to the presence of rotten tissue and antibiotic infection are good options for maggot therapy.
Allantoin is also present in snail mucus and is used as a skin product in burns and wounds [7]. The mechanism of action of larvae is as follows: 1- Allantoin antibiotic is present in the saliva of larvae, which is effective against a wide range of bacteria. 2- Larvae also produce ammonia, which has high antimicrobial properties and also makes the pH of wounds 8 and causes problems in the colonization of bacteria, and accelerates wound healing. 3- In the head of larvae, there are also a lot of sharp hairs that physical contact with microbes causes the destruction of a significant part of them. 4-The larvae also secrete substances similar to interleukins, which accelerate wound healing and the formation of fibroblasts. 5. The larvae also provide more movement to the site to which they give a gentle massage in the wound and improve the circulating of blood there. 6- X-rays do not have the slightest effect on the larvae and the activity of the larvae make the wound smaller moment by moment, while with the surgical method, due to the removal of the infected and necrotic part, a part of the healthy tissue is removed by the surgeon and eventually the wound gets bigger. 7- Larvae destroy dead tissue and activate the production of living and healthy tissue. 8- They clean the wound from bacteria, while not damaging the living and healthy tissue. 9- Larvae eat necrotic and infected tissue and then come out of the wound themselves [8–10].
It is a relatively easy and inexpensive method and, unlike antibiotics, will not cause any side effects. In our previous studies, the effect of honey on wound healing has been very effective in ulcerative colitis [11], the healing effects of honey can be mentioned in this study. We aimed the use sterile larvae on wounds and evaluate the effects of larval therapy on the infection rate, ESR, and wound size and the results compared with traditional debridement treatment.
Patients and methods
Production of sterile larvae
It is caught from different regions of Hamadan province and sent to the Institute of Entomology of Islamic Azad University, Malayer Branch, in tubes with appropriate labels. Information such as collection date and region, sampling method, and weather conditions were recorded. Identifying the Adult flies is based on the posterior airway and standard characteristics. Once identified, adult males and females will be transferred to new cages to ovulate. The average temperature will be 27° C, the humidity will be 5%, and the daylight / dark period will be 16: 8 h regulated for breeding. The liver is used to stimulate adult flies to lay eggs. After spawning in flies on the liver tissues, egg masses are isolated and after washing with 1% sodium hypochlorite. Then sterilize distilled water and finally wash with 70% of alcohol and finally with water and hold each for 15–20 s until they are sterilized. The sterilized eggs are placed in sterile containers at 37° C to hatch the first-stage larvae. These larvae are used to treat wounds and microbial culture performed before and after usage.
Study design
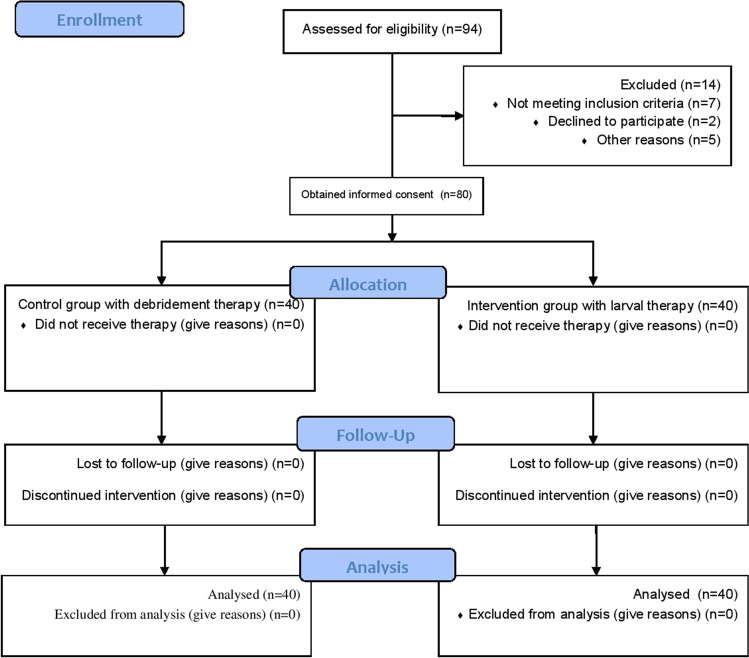
Wounds are treated for 80 people with chronic diabetic wounds, 40 in maggot therapy and 40 in the debridement group. The initial enrollment was 94 and 14 patients were excluded from the study and 80 were entered (Fig. 1). In maggot therapy, after rinsing the wound with saline serum, the area around the wound is covered with zinc oxide ointment. Depending on the extent of the infection and the size of the wound, a suitable number of sterile Lucilia sericata larvae are placed on the wound surface. And the cotton bandage will be covered. After performing the dressing and fixing it, the patient will be discharged and 48 h later he will return to change the dressing. If all the infected tissues have been removed from the wound surface, there is no need for larvae, but the larvae will be repeated if there are infected tissues. In the debridement group, the procedure was performed without larva. All patient information such as age, gender, occupation, level of education, underlying disease, ESR, blood sugar, urea, and creatinine are recorded before starting treatment. Also, before any treatment, patients will be evaluated for organ perfusion and the presence or absence of osteomyelitis. Evaluation and evaluation of wound size by sizing the wound site with a standard measure (stroactive) and the amount of inflammation and redness or infection in the wound site is reviewed and reported daily. Patients are examined directly under the supervision of the internal medicine specialist of Dr. Javaheri clinic and the results are sent to surgeon colleague.
Fig. 1.
CONSORT flow diagram of patients referred to and followed by the maggot therap
For larval therapy to be successful, maggots need to be free of any bacteria before being placed on the wound. Recently, using a sterilization method; the fly's eggs are washed with a dilute solution of sodium hypochlorite and then with sterile water. Then, they are then placed in 4% formaldehyde and after rinsing with water, they are placed in sterile containers for breeding and the larvae, which are between 1 and 3 mm long, are placed on the infected wound to remove the infected tissues [12, 13]. In human medicine, a layer of hydrocolloid is prepared to the size and shape of the wound and placed on the wound. This protects the skin around the wound from the larval proteolytic enzyme. Zinc paste can also be used instead. Ten larvae are placed on each square meter of the wound and the wound is bandaged with a mesh cover. An absorbent pad is placed on top of this mesh to absorb liquid secretions and dead tissue. This pad can be replaced if necessary. The larvae should be removed from the wound after 2 days. This can be easily done by removing the mesh dressing and washing the wound with a sterile saline solution. If necessary, this can be repeated [7]. For all patients wound culture and antibiogram were performed. The best antibiotic in the laboratory was chosen for two 10 days periods with two days off. Clindamycin is the best antibiotic in this regard.
Inclusion and exclusion criteria
Inclusion criteria were chronic ulcers in people with chronic diabetes over 15 years of age and people with informed consent to enter the study. Exclusion from the study 1- For people using concomitant drugs to be effective in wound healing or 2-Immunosuppressive patients who have difficulty in wound healing, such as hemophilia and liver disease. 3- The size of the wounds to be more than 5 cm. 4- Patients with osteomyelitis, cellulite, and cancer. 5-The patient needs amputation or flap surgery or a smoker or burger patient. 6- The patient with vascular problems that needs surgery. 7- The wound to be fresh because the chances of an infection increase or the wound to be under pressure. 8- Multiple wound or more than one wound. 9-The patient with cloadication and problems needs vascular grafts. 10- The HbA1C to be above 8 and 9 because it indicates a lack of blood sugar control. 11- ESR and CRP more than mild (+ 1). 12- Patients take anticoagulants or the patient to be afraid of insects. 13- The history of skin allergies and eczema or to be allergic to certain drugs. 14- The patient that received chemotherapy or radiotherapy.
Statistical analysis
The results before and after treatments are compared with paired t-test and analyzed in SPSS software, v.18. For describing the findings, descriptive statistics such as mean ± standard deviation and frequency were used. Due to the qualitative variables, Chi-square tests were used in the significance level of P < 0.05. The size of scare and ESR data were analyzed by repeated measurement test and after obtaining the results using post hoc power analysis, the ability of this sample size to show real differences was calculated. In this analysis, we considered the effect size to be at least seven-tenths with a 5% error to show the difference between the two groups. This sample volume provides us with a power of at least 80% (G * power 3.1 software).
Results
Age, education, and sex of patients were matched between the two groups and there were no differences between them (Tables 1 and 2). Larval therapy is effective in wound healing. The size of the wound after larval treatment is smaller than before (p < 0.001, Table 3). ESR was significantly decreased after 60 days (p < 0.001, Table 4). Demographic data and other systematic diseases are demonstrated in Table 1. The blood circulation was normal without any obstruction by sonographer detection. Table 2 showed the bacterial infection rate in both groups.
Table 1.
Demographic data and clinical variables of patients undergoing treatment with debridement and maggot therapy
| Parameters | Debridement group (N, %) | Larval therapy group (N, %) | p-value* | |
|---|---|---|---|---|
| Age (years) | 45–50 | 9 (22%) | 7 (17%) | 0.575 |
| 50–55 | 6 (15%) | 8 (20%) | 0.559 | |
| 55–60 | 17 (43%) | 20 (50%) | 0.533 | |
| 60–65 | 8 (20%) | 5 (13%) | 0.402 | |
| Education | High school Diploma and sub-diploma | 29 (72%) | 25 (62%) | 0.345 |
| Associate Degree | 1 (3%) | 4 (10%) | 0.207 | |
| Bachelor | 9 (22%) | 8 (20%) | 0.827 | |
| Masters | 1 (3%) | 3 (8%) | 0.330 | |
| Sex | Male | 22 (55%) | 21 (52%) | 0.789 |
| Female | 18 (45%) | 19 (48%) | ||
| Smoking history | Yes | 25 (62%) | 35 (87%) | 0.011 |
| No | 15 (38%) | 5 (13%) | ||
| History of drug abuse | Yes | 9 (22%) | 12 (30%) | 0.418 |
| No | 31 (78%) | 28 (70%) | ||
| Wound depth | Grade 2 | 10 (25%) | 9 (22%) | 0.753 |
| Grade 3 | 30 (75%) | 31 (78%) | ||
| Diabetes | Yes | 40 (100%) | 40 (100%) | 1.0 |
| No | 0 (0%) | 0 (0%) | ||
| Cardiac disease | Yes | 19 (48%) | 18 (45%) | 0.789 |
| No | 21 (52%) | 22 (55%) | ||
| Hypertension | Yes | 7 (17%) | 11 (27%) | 0.283 |
| No | 33 (83%) | 29 (73%) | ||
*Chi-squared test by MedCalc software
Table 2.
Culture Characteristics of maggot therapy and debridement Group in the culture of wound secretions
| Bacteria | Larval therapy group (N, %) | Debridement group (N, %) | p-value* | ||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| Staphylococcus aureus | 25 (62%) | 15 (38%) | 24 (60%) | 16 (40%) | 0.855 |
| Pseudomonas aeruginosa | 18 (45%) | 22 (55%) | 15 (37%) | 25 (63%) | 0.470 |
| Klebsiellaspp | 31 (77%) | 9 (23%) | 29 (72%) | 11 (28%) | 0.610 |
| Escherichia coli | 15 (37%) | 25 (63%) | 13 (32%) | 27 (68%) | 0.640 |
| Enterococcusspp. | 9 (22%) | 31 (78%) | 10 (25%) | 30 (75%) | 0.753 |
| Other | 8 (20%) | 32 (80%) | 11 (27%) | 29 (73%) | 0.463 |
*Chi-squared test by MedCalc software
Table 3.
Scare size (cm2) after treatment in groups by mean (SD), (n = 40)
| Group | 10 days | 20 days | 30 days | 40 days | 60 days | p-value * |
|---|---|---|---|---|---|---|
| larva | 57.97 (49.86) | 39.20 (37.32) | 21.62 (21.78) | 12.55 (14.95) | 5.05 (6.59) | < 0.001 |
| debridement | 33.90 (17.55) | 29.47 (16.62) | 22.35 (12.54) | 17.37 (11.06) | 10.60 (7.85) | |
| p-value# | 0.005 | 0.136 | 0.855 | 0.105 | 0.001 |
Table 4.
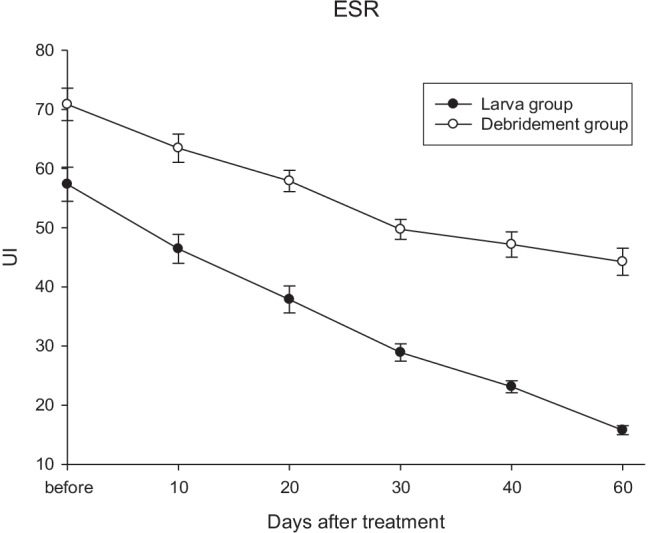
ESR (UI) after treatment in groups by mean (SD), (n = 40)
| Group | 10 days | 20 days | 30 days | 40 days | 60 days | p-value * |
|---|---|---|---|---|---|---|
| larva | 46.42 (15.47) | 37.87 (14.43) | 28.92 (9.24) | 23.12 (6.44) | 15.77 (4.77) | < 0.001 |
| debridement | 63.45 (15.13) | 57.90 (11.37) | 49.72 (10.70) | 47.17 (13.50) | 44.25 (14.56) | |
| p-value# | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
*Repeated measurement test; #t-test
The age of patients is categorized and compared with each other because age is an important factor in wound healing and there was no difference between the two groups in this factor (Table 1). Smoking history was different between the two groups (p = 0.011). Wound depth was not significantly different between the two groups and the rate of cardiac disease and hypertension were no differences between the two groups (Table 1). In 8 weeks, 14% patients wound with maggot therapy are completely closed, but people with conventional therapy whose wounds have not been closed after this period, this shows that larval therapy greatly increases the speed of wound healing. The scars of 40 patients in maggot therapy were evaluated every 10 days and the mean size of the scar decreased from 38.5 cm (36.6 cm) before treatment to 5.0 cm (6.6 cm) after 60 days of treatment (Figs. 2 and 3).
Fig. 2.
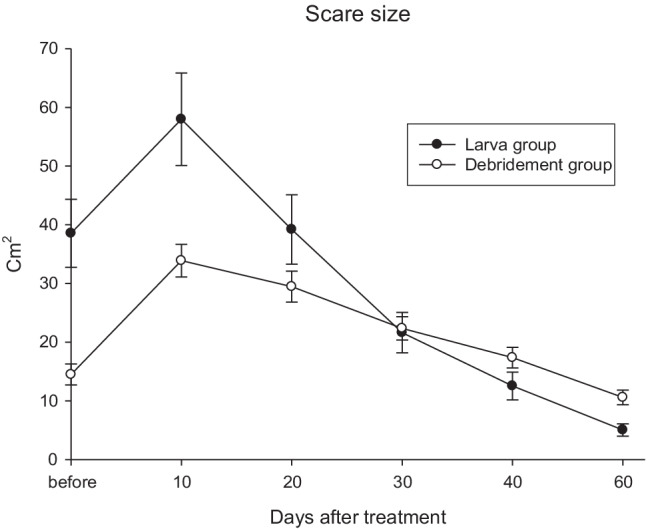
Scar size (cm2) after 60 days between two groups
Fig. 3.
ESR (UI) decrease after 60 days in both groups
ESR mean in maggot therapy was decreased from 57.3 (18.3) before treatment to 15.8 (4.8) after 60 days of treatment (Fig. 4).
Fig. 4.
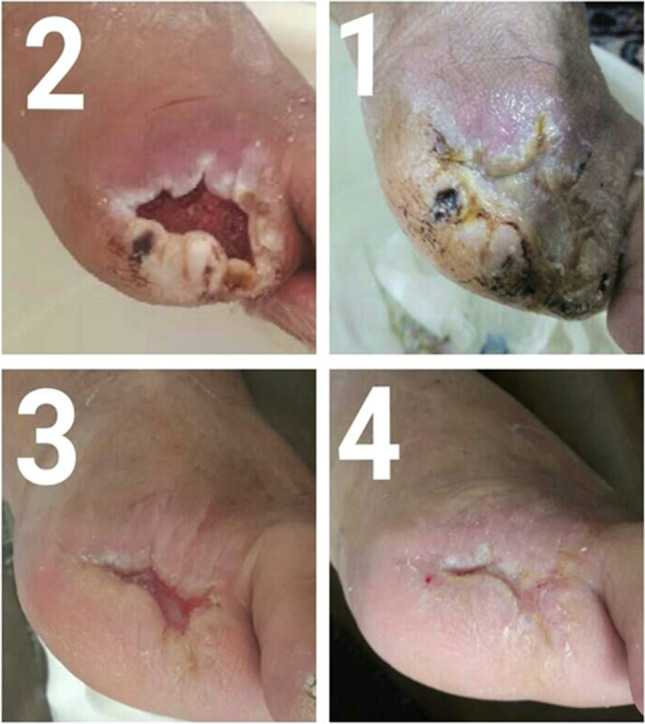
The larval treatment effects every 10 days
Discussion
The treatment of wounds is usually slow and hard; especially in diabetic patients because high blood glucose interferes with wound healing. In general, the use of larvae can kill 10 to 15 g of dead cells per day. The larvae kill the dead tissue by secreting digestive juices and then swallowing the tissue and the dissolved bacteria. The length of maggots increases from about 2 mm to 10 mm during this period. Other antibiotic treatment methods can be used simultaneously with larval treatment [13].
Larva therapy was introduced as an effective way to treat chronic ulcers. Larval treatment reduces the risk of amputation, reduces the use of antibiotics, prevents long-term hospitalization, and reduces the incidence of outpatient visits. Larval treatment can be used as the first-line treatment in patients with antibiotic resistance, infectious ulcers, immunosuppression, and diabetic disease [14].
Valachová et al. demonstrated that larval treatment is more effective than conventional treatments in the debridement of chronic ulcers that is consistent with the results of our study [15]. Lepage et al. investigated the effect of larval treatment in comparison with hydrogel in wound healing, which did not show any difference between the two groups. No antibiotic was used in this study [16]. Only changes in the wound culture were investigated. In this study that probably using antibiotics will increase the speed of recovery and also eliminate the necrotic parts faster [16]. Our findings showed there was no difference in infection rate between the two groups and the reason for the differences between the two studies can be attributed to the difference in the method of the two studies. In 8 weeks, 14% patients wound with maggot therapy are completely closed, but people with conventional therapy whose wounds have not been closed after this period, this shows that larval therapy greatly increases the speed of wound healing.
In terms of the depth of involvement in the Nezakat et al. study, 85% of patients had deep ulcers (Grade III) and only 10% of patients had Grade II ulcers [17]. In that study, 90% of patients had infectious ulcers while in our results, 78% of patients in the maggot therapy group had deep ulcers (Grade III), 22% had Grade II ulcers.
The difference between the results of this study and the present study may be due to the choice of the type of chronic ulcer and the mean age difference between the two groups.
In the study of Blueman and Bousfield, the frequency of smoking was 23% in the control group and 14% in the larval treatment group, while in our study 62% of patients in the debridement group and 87% in the larval therapy group were smokers (p = 0.011) [18]. Using cigarettes and opium can slow down wound healing and prolong the course of treatment while the larval therapy showed better results. Therefore, in the treatment of chronic ulcers, the condition of the patient and especially the use of cigarettes as a confounding factor for wound healing should always be considered [18]. The important point in our study was the examination of ulcers caused by cultures and smears that have not been addressed in other studies. Although debrided wounds decreased their size, the rate of wound infection was not significantly higher than that maggot therapy.
Mumcuoglu et al. [9] reported effective debridement for 24 of 27 non-healing wounds in 22 diabetic patients treated with an average of six maggot treatments over the course of two weeks; 12 wounds were debrided within just one week. Rayman et al. [19] and Fleischmann et al. [20] used maggot therapy as a valuable treatment for diabetic foot wounds. Wayman et al. [21] showed that maggot therapy was associated with lower cost and more rapid healing compared with hydrogel for the treatment of venous stasis ulcers. Based on a review of the related literature, maggot therapy like surgical intervention is a safe and effective method used to remove necrotic tissue, disinfection and faster tissue growth. The general benefit of larval therapy in larval therapy is the shortened treatment time and reduced the duration of antibiotic therapy with no need for hospitalization [22]. The mean therapy duration in larval therapy usually was 60 days and in normal debridement usually was 71–86 days. In our results, 60 days was enough time for the healing of wounds in two groups.
In previous studies, maggot therapy can effectively reduce the bacterial load in a wound [22]. Larvae therapy has a variety of effects on different bacterial species (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Enterococcus). Therefore, it is suggested that more extensive studies on the number of patients should be done in multicenter studies on the effects of larval treatment on bacterial growth in chronic ulcers. Wounds with dead tissue, purulent wounds, and gangrene are good options for larval therapy. In general, the use of larvae for a wound is a last resort usually after the patient has been unsuccessfully treated with antibiotics and surgery for months [2].
This study simply evaluated diabetic wounds that these chronic wounds could be healed better by another therapy and we should not consider maggot therapy only as a last resolve (an alternative to surgery). Clinical applications of larvae for the treatment of diabetic ulcers, bedsores, burns, scabies, and certain types of benign and malignant tumors, pimples, and boils where other therapies do not respond or are not suitable for treatment.
The results of this study demonstrate the use of larva in the treatment of chronic diabetic wounds could significantly improve wound healing and the decrease in scare size and ESR between the two groups were significant (p < 0.001). ESR showed the better improvement of inflammation was occurred in maggot therapy group and this method could influence four physiological phases of wound healing (homeostasis, inflammation, proliferation, and remodelling/maturing) [23]. Many questions remain unanswered, and a large prospective evaluation is warranted. A larger study, preferably with subjects whose disease is not as advanced, might better demonstrate the impact of maggot therapy on complete wound healing. In addition to issues of efficacy and safety, future studies also must address the cost-effectiveness of maggot therapy (e.g., at what measurable level of hypoperfusion is an extremity wound unlikely to respond to maggot therapy).
Conclusion
The present analysis demonstrated that maggot therapy is more effective and efficient in debriding non-healing foot and leg ulcers in diabetic male veterans than the typical conventional treatment currently prescribed. Maggot therapy was also associated with a more rapid decrease in wound size and an increase in granulation tissue, making the wounds ready for surgical closure. In our results, the wound healing was improved and the size of scares was smaller in larval therapy but there was no difference between the two groups for infection rate. ESR was significantly decreased in the larval therapy group that indicating the lower inflammation in this group. Antibiotic therapy is suggested after larval therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The research number was 99-01-69-22873 that supported by Shiraz University of Medical Sciences. We thank from technicians of the clinic in Hamadan for their assistance. Many thanks for the executive help of the Vice Chancellor for Treatment, Hamadan University of Medical Sciences.
Declarations
Competing interests
None declared.
Ethics statement
The study was approved by the Shiraz University of Medical Sciences ethics committee (IR.SUMS.REC.1399.826). The trial registration number was IRCT20201012049000N1 and people with informed consent to enter the study.
Footnotes
‘What is already known about this topic?’ The larvae also provide more movement to the site to which they give a gentle massage in the wound and improve the circulating of blood there. Larvae destroy dead tissue and activate the production of living and healthy tissue. Larvae eat necrotic and infected tissue and then come out of the wound themselves.
‘What does this study add?’ The sterile larvae were used in wound treatment and the effects of larval therapy on wounds were evaluated. Larval therapy is effective in diabetic wound healing. The size of the wound after larval treatment is smaller than before. There was no difference between the two groups for infection rate. ESR was significantly decreased in the larval therapy group that indicated the lower inflammation in this group.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sherman RA, Shimoda KJ (2004) Presurgical maggot debridement of soft tissue wounds is associated with decreased rates of postoperative infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 39 (7):1067-1070. 10.1086/423806 [DOI] [PubMed]
- 2.Thomas S, Jones M, Shutler S, Jones S. Using larvae in modern wound management. J Wound Care. 1996;5(2):60–69. doi: 10.12968/jowc.1996.5.2.60. [DOI] [PubMed] [Google Scholar]
- 3.Mulder JB. The medical marvels of maggots. J Am Vet Med Assoc. 1989;195(11):1497–1499. [PubMed] [Google Scholar]
- 4.Waters J. The benefits of larval therapy in wound care. Nurs Times. 1998;94(2):62–63. [PubMed] [Google Scholar]
- 5.Reed S, Bayly W. Toxicologic Diseases: Antibiotic-Associated Diarrhea-Pathogenesis. Equine Internal Medicine. Philadelphia: Saunders Company; 1998. [Google Scholar]
- 6.Sherman RA. Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 2009;3(2):336–344. doi: 10.1177/193229680900300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Mubarak MA, Lamari FN, Kontoyannis C. Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection. J Chromatogr A. 2013;1322:49–53. doi: 10.1016/j.chroma.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 8.Thomas S, Jones M, Shutler S, Andrews A (1996) Wound care. All you need to know about ... maggots. Nursing times 92 (46):63–66, 68, 70 passim [PubMed]
- 9.Mumcuoglu KY, Ingber A, Gilead L, Stessman J, Friedmann R, Schulman H, Bichucher H, Ioffe-Uspensky I, Miller J, Galun R, Raz I. Maggot therapy for the treatment of diabetic foot ulcers. Diabetes Care. 1998;21(11):2030–2031. doi: 10.2337/diacare.21.11.2030. [DOI] [PubMed] [Google Scholar]
- 10.Bunkis J, Gherini S, Walton RL. Maggot therapy revisited. The Western journal of medicine. 1985;142(4):554–556. [PMC free article] [PubMed] [Google Scholar]
- 11.Vistnes LM, Lee R, Ksander GA. Proteolytic activity of blowfly larvae secretions in experimental burns. Surgery. 1981;90(5):835–841. [PubMed] [Google Scholar]
- 12.Sherman RA, Hall MJ, Thomas S. Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol. 2000;45:55–81. doi: 10.1146/annurev.ento.45.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Pechter EA, Sherman RA. Maggot therapy: the surgical metamorphosis. Plast Reconstr Surg. 1983;72(4):567–570. doi: 10.1097/00006534-198310000-00032. [DOI] [PubMed] [Google Scholar]
- 14.Steenvoorde P, Jukema GN. The antimicrobial activity of maggots: in-vivo results. J Tissue Viability. 2004;14(3):97–101. doi: 10.1016/s0965-206x(04)43005-8. [DOI] [PubMed] [Google Scholar]
- 15.Valachová I, Bohová J, Pálošová Z, Takáč P, Kozánek M, Majtán J. Expression of lucifensin in Lucilia sericata medicinal maggots in infected environments. Cell Tissue Res. 2013;353(1):165–171. doi: 10.1007/s00441-013-1626-6. [DOI] [PubMed] [Google Scholar]
- 16.Lepage OM, Doumbia A, Perron-Lepage MF, Gangl M. The use of maggot debridement therapy in 41 equids. Equine Vet J Suppl. 2012;43:120–125. doi: 10.1111/j.2042-3306.2012.00609.x. [DOI] [PubMed] [Google Scholar]
- 17.Nezakati E, Hasani MH, Zolfaghari P, Rashidan M, Sohrabi MB. Effects of Lucilia sericata maggot therapy in chronic wound treatment: A randomized clinical trial. Chronic Wound Care Management and Research. 2020;7:11–17. doi: 10.2147/CWCMR.S248149. [DOI] [Google Scholar]
- 18.Blueman D, Bousfield C. The use of larval therapy to reduce the bacterial load in chronic wounds. J Wound Care. 2012;21(5):244–253. doi: 10.12968/jowc.2012.21.5.244. [DOI] [PubMed] [Google Scholar]
- 19.Rayman A, Stansfield G, Woollard T, Mackie A, Rayman G. Use of larvae in the treatment of the diabetic necrotic foot. Diabetic Foot. 1998;1:7–13. [Google Scholar]
- 20.Fleischmann W, Russ M, Moch D, Marquardt C (1999) [Biosurgery - maggots, are they really the better surgeons?]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 70 (11):1340–1346. 10.1007/s001040050790 [DOI] [PubMed]
- 21.Wayman J, Nirojogi V, Walker A, Sowinski A, Walker MA. The cost effectiveness of larval therapy in venous ulcers. J Tissue Viability. 2000;10(3):91–94. doi: 10.1016/s0965-206x(00)80036-4. [DOI] [PubMed] [Google Scholar]
- 22.Bazaliński D, Kózka M, Karnas M, Więch P (2019) Effectiveness of chronic wound debridement with the use of larvae of lucilia sericata. Journal of clinical medicine 8 (11). 10.3390/jcm8111845 [DOI] [PMC free article] [PubMed]
- 23.Gazi U, Taylan-Ozkan A, Mumcuoglu KY. The effect of Lucilia sericata larval excretion/secretion (ES) products on cellular responses in wound healing. Med Vet Entomol. 2021;35(3):257–266. doi: 10.1111/mve.12497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.