Abstract
Background and Objective
AMG 986 is a first-in-class, novel apelin receptor small molecule agonist initially developed as a treatment for patients with heart failure (HF). Previously, a first-in-human study of AMG 986 was conducted in healthy and HF subjects; however, AMG 986 was not evaluated in Japanese subjects.
Methods
This was a phase I, open-label, single-dose, single-center study conducted to evaluate the safety and pharmacokinetics (PK) of AMG 986 200 mg and 400 mg in 12 healthy Japanese subjects. Six subjects received AMG 986 200 mg and six subjects received AMG 986 400 mg.
Results
Following oral administration, median time to maximum observed plasma concentration (tmax) was 1.0 h for both the AMG 986 200 mg and 400 mg groups, and mean terminal half-life (t½) was 15.1 h and 17.6 h, respectively. When comparing the AMG 986 200 mg and 400 mg groups, 1.33-fold and 1.18-fold higher maximum observed plasma concentration (Cmax) and AUC∞, respectively, were observed for the 2-fold increase in dose. AMG 986 exhibited an acceptable safety and tolerability profile; all adverse events were mild in severity.
Conclusion
AMG 986 exposure increased with increasing dose, and the increase was less than dose proportional in healthy Japanese subjects. The results of this study could facilitate the subsequent clinical development of AMG 986 for the treatment of Japanese patients with HF.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40268-022-00386-3.
Key Points
| AMG 986 exposure increased with increasing dose, and the increase was less than dose proportional. |
| The current study may inform the clinical development of AMG 986 for the treatment of Japanese patients with heart failure. |
Introduction
Chronic heart failure (HF) is a complex syndrome that results in inadequate systemic blood flow when neurohormonal mechanisms are no longer able to deliver an adequate physiological response [1]. The prognosis for patients with HF remains poor, demonstrating an unmet need for novel therapies [2]. The apelin receptor is a member of the G protein-coupled receptor gene family that binds the apelin and ELABELA/Toddler/Apela ligands and shares significant homology with the angiotensin II type 1 receptor [3–5]. AMG 986 is a first-in-class, novel apelin receptor small molecule agonist that binds and activates the apelin receptor to improve cardiac function by increasing cardiac contractility without affecting heart rate, initially developed as a treatment for patients with HF [6, 7]. In preclinical studies, AMG 986 increased cardiac contractile reserve, ejection fraction, and stroke volume. Furthermore, improvements in ventriculoarterial coupling and left ventricular contractile function were also observed (Amgen, data on file). The chemical structure of AMG 986 is presented in Fig. 1.
Fig. 1.
Chemical structure of AMG 986
Recently, a phase Ib, first-in-human study in healthy and HF subjects (NCT03276728) reported that AMG 986 exposure increased nonlinearly with increasing oral doses of 5–650 mg [7]. The oral bioavailability of AMG 986 ranged from 40 to 80% and the approximate terminal half-life (t½) of AMG 986 was 20 h [7]. Additional studies indicate AMG 986 was highly protein bound (99.6% bound in human plasma). Furthermore, the metabolism of AMG 986 in vitro was principally catalyzed by human cytochrome P450 (CYP) 3A. AMG 986 was an in vitro inducer of CYP3A4, as determined by increases in CYP3A4 messenger RNA levels in primary human hepatocytes, and AMG 986 was not an in vitro inhibitor of any of the major drug-metabolizing human CYP enzymes. Additionally, AMG 986 was characterized in vitro as a substrate of P-glycoprotein (P-gp) and organic anion-transporting polypeptide 1B3 (Amgen, data on file).
Broadly, it is recognized that genetic polymorphisms and ethnic differences can significantly alter the pharmacokinetic (PK) properties of drugs [8, 9], and, to date, AMG 986 has not been evaluated in Japanese subjects. The renal and biliary elimination of drugs is not generally influenced by ethnic differences [8–10]; however, interethnic variability is common when P-gp and CYP enzymes are involved in drug transport and metabolism, and in drugs with nonlinear PK properties [10]. This raises the possibility that interethnic variability may exist in the PK properties of AMG 986, potentially leading to differences in efficacy and/or toxicity in Japanese subjects [8, 9].
Therefore, the current study was conducted to characterize the PK, safety, and tolerability of AMG 986 in healthy Japanese subjects. Single oral doses of AMG 986 200 mg and 400 mg were selected for evaluation in the current study, based on safety and tolerability data available from the first-in-human study [7].
Methods
Study Design
This was a phase I, open-label, single-dose study to evaluate the safety, tolerability, and PK of AMG 986, conducted in healthy Japanese subjects at a single center in the US. The study consisted of a 28-day screening period, after which eligible subjects (n = 12) were enrolled to either receive a single dose of either AMG 986 200 mg (n = 6) or 400 mg (n = 6). On day 1, subjects received a single oral dose of AMG 986 after a fast of approximately 8 h (no food or liquids, except water) and refrained from consuming food and liquid (except water) for approximately 2 h after dose administration. AMG 986 was administered in a fasted condition to mitigate any potential food effects on drug absorption, and was administered as 25 mg tablets and swallowed whole with 240 mL of water. Subjects in the AMG 986 400 mg group were dosed at least 6 days after dosing in the AMG 986 200 mg group. The confinement period lasted 5 days, after which subjects were discharged from the clinical evaluation unit. Safety follow-up visits were conducted on days 15 and 30 (end of study).
Participants
Eligible subjects were healthy, first-generation Japanese males practicing effective birth control and women with non-childbearing potential aged 20–55 years who had not lived outside of Japan for more than 10 continuous years. Subjects had a body mass index of 18–32 kg/m2 and physical examinations, including vital signs, clinical laboratory values, and electrocardiogram (ECG) readings, that were clinically acceptable to the investigator. Exclusion criteria included concurrent or prior use of strong CYP3A4 inhibitors or strong CYP3A4 inducers within 14 and 28 days, respectively, of AMG 986 administration.
PK Sampling and Assessments
On day 1, blood samples for the PK analysis of AMG 986 were collected from each subject at predose and 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h postdose. The PK parameters estimated were maximum observed plasma concentration (Cmax) and dose-normalized Cmax (Cmax/dose), area under the plasma concentration-time curve (AUC) from time zero to the last quantifiable time point postdose (AUClast) and dose-normalized AUClast (AUClast/dose), AUC from time zero (i.e., time of administration of AMG 986) to infinity (AUC∞) and dose-normalized AUC∞ (AUC∞/dose), time to Cmax (tmax), and t½. All PK parameters were estimated using the noncompartmental analysis by Phoenix WinNonlin version 6.4 (Certara, Princeton, NJ, USA).
Plasma concentrations of AMG 986 were determined using validated high-performance liquid chromatography (HPLC) mass spectrometry. AMG 986 and internal standard (IS) 13C6-AMG 986 were extracted from 0.050 mL of human plasma by a protein precipitation extraction procedure. The extraction began by adding 50.0 μL of calibration standards, quality control samples, and study samples to appropriate wells of a 96-well plate, with 50.0 μL of blank human plasma added to each blank well. The samples were diluted with 50 μL of methanol/water (50/50, volume per volume [v/v]). Following the addition of 150 μL of IS to all appropriate wells (or 150 μL of acetonitrile to blank wells), the plate was covered, vortexed, and centrifuged. A Tomtec Quadra4™ (Tomtec Inc., Hamden, CT, USA) 96-well pipettor system was used to transfer 100 μL of the supernatant to a new 96-well plate. After 400 μL of acetonitrile/water (80/20, v/v) was added to all wells, the plate was covered and vortexed. The extracts were chromatographed under reverse-phase conditions on a Kinetex® C8 (Phenomenex Inc., Torrance, CA, USA) HPLC column using a gradient system with 0.1% formic acid in water and 0.1% formic acid in acetonitrile. The compounds were detected and quantified by tandem mass spectrometry in positive ion mode on an API 4000™ (AB Sciex, Framingham, MA, USA) equipped with a Turbo IonSpray® interface. The m/z transition values for AMG 986 and IS were 524.3 → 312.2 and 530.3 → 318.2, respectively. The assay had a lower limit of quantitation (LLOQ) of 10.0 ng/mL. Calibration curves were obtained by performing a linear regression (weighted 1/x2) on the calibration standards.
Safety Outcomes
The safety and tolerability of AMG 986 were assessed and included subject incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), clinical laboratory tests, 12-lead ECG, and vital signs. All adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Analysis
Concentrations below the LLOQ (10.0 ng/mL) were set to zero before data analysis. Descriptive statistics, including mean, median, range, and standard deviation (SD), were used to report the PK parameters, and no formal statistical hypothesis testing was performed. Dose-normalized PK parameters were calculated by dividing the mean value by the AMG 986 dose. Mean (SD) and individual AMG 986 concentration-time profiles were summarized by cohort and plotted by time postdose (in h). Safety results were summarized using descriptive statistics.
Results
Baseline Characteristics
Overall, 12 subjects were enrolled in the study—six subjects each in the AMG 986 200 mg and 400 mg groups. All subjects received a single dose of AMG 986 and completed the study. The mean (SD) age of subjects was 41.4 (8.9) years, and all subjects were Asian (Table 1).
Table 1.
Baseline characteristics
| Characteristic | AMG 986 200 mg [n = 6] |
AMG 986 400 mg [n = 6] |
|---|---|---|
| Age, years [mean (SD)] | 37.2 (6.7) | 45.7 (9.2) |
| Sex [n (%)] | ||
| Male | 6 (100.0) | 3 (50.0) |
| Female | 0 (0.0) | 3 (50.0) |
| Weight, kg [mean (SD)] | 65.3 (5.4) | 63.8 (14.2) |
| Ethnicity [n (%)] | ||
| Non-Hispanic/Latino | 6 (100.0) | 6 (100.0) |
| Race [n (%)] | ||
| Asian | 6 (100.0) | 6 (100.0) |
SD standard deviation
PK Results of AMG 986 200 mg and 400 mg
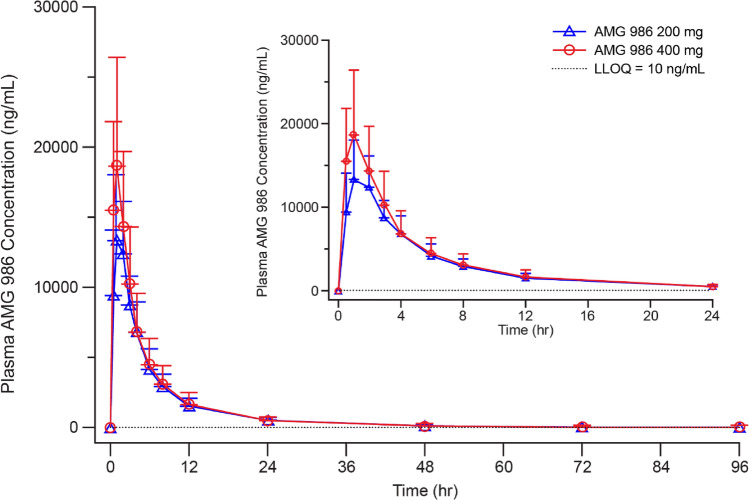
Mean (SD) AMG 986 plasma concentration-time profiles by dose after oral administration are shown in Fig. 2. Individual AMG 986 concentration-time profiles for the 200 mg and 400 mg doses are reported in electronic Supplementary Figs. 1 and 2, respectively. After a single oral dose of AMG 986 200 mg, mean (SD) Cmax and AUC∞ values were 15,800 (3640) ng/mL and 88,700 (22,900) h ng/mL, respectively. After a single oral dose of AMG 986 400 mg, mean (SD) Cmax and AUC∞ values were 21,000 (7300) ng/mL and 105,000 (44,900) h ng/mL, respectively. Median (range) tmax values were 1.0 (1.0–2.0) and 1.0 (0.50–1.0) h for the AMG 986 200 mg and 400 mg groups, respectively. Mean (SD) t½ was 15.1 (3.2) h and 17.6 (3.8) h for AMG 986 200 mg and 400 mg, respectively (Table 2).
Fig. 2.
Mean (SD) plasma concentration-time profiles for AMG 986 200 mg and 400 mg administered orally in healthy Japanese subjects (inset = time 0–24 h). LLOQ lower limit of quantitation, SD standard deviation
Table 2.
Pharmacokinetic parameter estimates for AMG 986 200 mg and 400 mg after oral administration in healthy Japanese subjects
| Cmax, ng/mL | Cmax/dose, ng/mL/mg | AUClast, h ng/mL | AUClast/dose, h ng/mL/mg | AUC∞, h ng/mL | AUC∞/dose, h ng/mL/mg | tmax, h | t½, h | |
|---|---|---|---|---|---|---|---|---|
| AMG 986 200 mg | ||||||||
| Mean (SD) | 15,800 (3640) | 79.2 (18.2) | 89,100 (20,500) | 445 (102) | 88,700 (22,900) | 444 (115) | – | 15.1 (3.2) |
| Median (range) | – | – | – | – | – | – | 1.0 (1.0–2.0) | – |
| Geometric mean (CV%) | 15,500 (23.2) | 77.5 (23.2) | 86,600 (28.2) | 433 (28.2) | 85,800 (31.4) | 429 (31.4) | 1.3 (37.4) | 14.8 (20.3) |
| AMG 986 400 mg | ||||||||
| Mean (SD) | 21,000 (7300) | 52.4 (18.3) | 104,000 (43,100) | 259 (108) | 105,000 (44,900) |
263 (112) |
– | 17.6 (3.8) |
| Median (range) | – | – | – | – | – | – | 1.0 (0.5–1.0) | – |
| Geometric mean (CV%) | 19,400 (50.0) | 48.6 (50.0) | 95,700 (48.2) | 239 (48.2) | 96,700 (49.1) | 242 (49.1) | 0.80 (36.3) | 17.2 (23.0) |
AUC∞ area under the plasma concentration-time curve from time zero to infinity, AUC∞/dose dose-normalized AUC∞, AUClast area under the plasma concentration-time curve from time zero to the last quantifiable time point postdose, AUClast/dose dose-normalized AUClast, Cmax maximum observed plasma concentration, Cmax/dose dose-normalized Cmax, CV% coefficient of variation, SD standard deviation, t½ terminal half-life, tmax time to maximum concentration
Safety
A total of seven subjects (58.3%) had TEAEs—two subjects (33.3%) and five subjects (83.3%) in the AMG 986 200 mg and 400 mg groups, respectively. By preferred term, TEAEs were catheter site pain (one subject), somnolence (three subjects), and contact dermatitis (four subjects). All TEAEs were Common Terminology Criteria for Adverse Events (CTCAE) grade 1 (mild) and not considered treatment related by the investigator. There were no SAEs, fatal adverse events, or an adverse event leading to the discontinuation of AMG 986. No clinically important changes from baseline in laboratory values, ECG parameters, or vital signs were observed.
Discussion
There is an unmet need for effective therapies to reduce the high hospitalization and mortality rates associated with HF. AMG 986 is a first-in-class, novel apelin receptor small molecule agonist, initially developed as a treatment for HF [6, 7]. To date, AMG 986 has not been evaluated in Japanese subjects. Therefore, the current phase I study was conducted to characterize the PK, safety, and tolerability of the 200 mg and 400 mg doses of AMG 986 in healthy Japanese subjects. The dose levels in the current study were selected to reflect those studied in healthy non-Japanese subjects in the first-in-human study [7].
Following a single oral administration of AMG 986, mean Cmax values were 15,800 ng/mL and 21,000 ng/mL, and mean AUC∞ values were 88,700 h·ng/mL and 105,000 h ng/mL, in the AMG 986 200 mg and 400 mg groups, respectively. While AMG 986 exposure increased with increasing dose, the observed increase was less than dose proportional. Specifically, the AMG 986 400 mg dose resulted in 33% and 18% increases in Cmax and AUC∞, respectively, relative to the 200 mg dose, along with lower dose-normalized Cmax and AUC∞ values. These results support the trend of decreasing dose-normalized exposures observed in the first-in-human study [7]. Absorption of AMG 986 was rapid, with a median tmax of 1.0 h for both the 200 mg and 400 mg doses, while the mean t½ ranged from 15.1 to 17.6 h; there were no clear dose-related trends observed in either tmax or t½. Overall, AMG 986 exhibited an acceptable safety and tolerability profile in Japanese subjects, and the most common TEAE was contact dermatitis (four subjects). All adverse events were CTCAE grade 1 and there were no deaths in the study.
In the current study, AMG 986 PK parameters (AUC, Cmax, tmax, and t½) in Japanese healthy subjects appeared similar to those in the non-Japanese population evaluated in the first-in-human study [7]. A possible explanation for the current findings may be similar CYP3A4 and P-gp activity between the Japanese subjects evaluated here and those in the first-in-human study, which included Black and Asian (non-Japanese) subjects in addition to Caucasian subjects [7]. Overall, the current results suggest an absence of intrinsic factors (e.g., ethnicity-related polymorphisms) in the Japanese population, demonstrating similar AMG 986 exposures compared with non-Japanese subjects; however, genetic polymorphisms were not tested in the current study and therefore further studies are needed to fully support this interpretation of the results.
There are limitations to the current study. In the first-in-human study, AMG 986 was evaluated with single and multiple daily ascending doses up to a 650 mg dose level [7]; however, the current study used a lower maximum single dose of 400 mg because the limited data available when this study was planned indicated that this dose had acceptable safety and tolerability. Additionally, formal statistical comparisons of PK parameters were not conducted between subjects in the current study and those in the first-in-human study [7], and therefore comparisons should be made with caution.
Conclusions
AMG 986 exposure increased with increasing dose from 200 mg to 400 mg in healthy Japanese subjects, and the increase was less than dose proportional. While formal statistical comparisons were not conducted, the PK properties of AMG 986 200 mg and 400 mg in Japanese healthy subjects appeared similar to those in non-Japanese subjects evaluated in the first-in-human study. AMG 986 exhibited an acceptable safety and tolerability profile. The results of this study may facilitate the subsequent clinical development of AMG 986 for the treatment of Japanese patients with HF.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by Liam Gillies, PhD, CMPP, of Cactus Life Sciences (part of Cactus Communications) and funded by Amgen Inc.
Declarations
Funding
This study was funded by Amgen Inc.
Conflicts of interest
AT, OM, SV, SH, JH, and EL are stockholders and employees of Amgen Inc.
Availability of data and material
Qualified researchers may request data from Amgen clinical studies; complete details are available at http://www.amgen.com/datasharing.
Code availability
Not applicable.
Author contributions
All authors provided substantial contributions to the interpretation of data for the work, drafted and revised the manuscript critically for important intellectual content, and approved the final version to be published.
Ethics approval
The study was conducted following ethical guidelines of the Declaration of Helsinki and Council for International Organizations of Medical Sciences, applicable Good Clinical Practice guidelines of the International Council for Harmonisation, and applicable local laws and regulations. Locally appointed ethics review boards approved the research protocol.
Consent to participate
Written informed consent was obtained from all subjects.
Consent for publication
Not applicable.
References
- 1.Francis GS. Pathophysiology of chronic heart failure. Am J Med. 2001;110(Suppl 7A):37S–46S. doi: 10.1016/S0002-9343(98)00385-4. [DOI] [PubMed] [Google Scholar]
- 2.Jones NR, Roalfe AK, Adoki I, Richard Hobbs FD, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21(11):1306–1325. doi: 10.1002/ejhf.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27(6):672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1–2):355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 5.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 6.Ason B, Chen Y, Guo Q, Hoagland KM, Chui RW, Fielden M, et al. Cardiovascular response to small-molecule APJ activation. JCI Insight. 2020;5(8):e132898. doi: 10.1172/jci.insight.132898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellawell J, Abbasi S, Trivedi A, Tsirtsonis K, Kaufman A. Safety, tolerability, pharmacokinetics, and pharmacodynamics of AMG 986, a novel small molecule apelin receptor agonist, in healthy subjects and heart failure patients. J Card Fail. 2020;26(10):S68. doi: 10.1016/j.cardfail.2020.09.200. [DOI] [Google Scholar]
- 8.Chen M-L. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45(10):957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
- 9.Xie HG, Kim RB, Wood AJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Annu Rev Pharmacol Toxicol. 2001;41:815–850. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- 10.ICH Harmonised Tripartite Guideline: Ethnic Factors in the Acceptability of Foreign Clinical Data. E5(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 5 Feb 1998. https://database.ich.org/sites/default/files/E5_R1__Guideline.pdf. Accessed Sep 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.