Abstract
Although obligate syntrophic reactions cannot proceed without hydrogenotrophs, it has been unclear from the literature whether potential improvements are achievable with higher concentrations of hydrogenotrophs. In this study, the relative importance of formate-/H2-utilizing and acetate-utilizing trophic groups in the anaerobic degradation of butyrate and propionate was assessed by adding various proportions of these enriched cultures to a mixed anaerobic seed inoculum. The improvement resulting from the additional acetate-utilizing cultures was much greater than with formate/H2 utilizers. Furthermore, formate/H2 utilizers did not improve propionate utilization significantly, suggesting the importance of optimum utilization of hydrogenotrophic capacity. During most of the volatile fatty acid (VFA) degradation period, the system responded with characteristic hydrogen levels to maintain the Gibbs free energy of oxidation approximately constant for both butyrate (−6 kJ) and propionate (−14 kJ). These free-energy values were independent of methanogenic activity, as well as the volume of the seed inoculum and the VFA concentrations present. By comparing the experimental results with kinetic and mass transfer models, it was postulated that the diffusional transfer of reducing equivalents was the major limiting factor for efficient VFA degradation. Therefore, for optimum utilization of the hydrogenotrophs, low acetate concentrations are vital to enable the system to respond with higher formate/H2 levels, thus leading to improved transfer of reducing equivalents. Due to the small number of propionate utilizers (and hence their limited surface area) and low bulk liquid concentrations, the additional formate/H2 utilizers were of minimal use for improving the degradation rate further. The butyrate degradation rates strongly correlated with the cumulative activity of hydrogenotrophs and acetotrophs over the experimental range studied, indicating the need to model obligate syntrophic reactions as a dependent function of methanogenic activity.
In methanogenic environments, acetogenesis is a key process in the mineralization of organic waste, with propionate and butyrate being the major volatile fatty acids (VFAs) produced (16). It is known from thermodynamics as well as experimental studies that the rate of VFA degradation is severely constrained by the free energy available for these organisms. Since both hydrogen and acetate are the final products of VFA degradation, their accumulation significantly lowers the free energy available for the organisms carrying out these reactions (Table 1). Recently, formate has also been suggested as an alternative electron carrier and was postulated to be more important than hydrogen in suspended systems due to mass transfer considerations (2, 30, 31, 33). Nevertheless, the maximum hydrogen or formate concentrations that can be tolerated by the VFA degraders are extremely low. As a result, while degrading VFAs, these obligate syntrophs can only grow in the presence of a hydrogen- or formate-consuming partner, usually a methanogen. A characteristic of this cooperation is the equalization of growth rates with a tight coupling of growth rates and rate-limiting substrate concentrations, while the ratio of these partner organisms is dependent upon the type of syntrophic reaction (13, 23, 29).
Since these syntrophic cultures act as a hypothetical composite species, the rate of VFA degradation is proportional to the H2-consuming activity. Both Schmidt and Ahring (27, 28) and Dwyer et al. (5) demonstrated this by the addition of hydrogen utilizers to the VFA degraders, as well as by inhibiting the hydrogenotrophs. Although these studies highlighted the critical importance of hydrogenotrophs for VFA degradation, they were not entirely clear about the quantitative improvements possible with further addition of hydrogenotrophs to mixed-culture inocula. For example, Schmidt and Ahring (27, 28) observed substantial improvements after addition of hydrogen utilizers to disintegrated granules while improvements with propionate utilizers were much smaller than those obtained with a butyrate-degrading culture, although comparatively, propionate utilizers suffer more severe inhibition due to H2. Since most of these product inhibition experiments were done primarily with pure cultures of VFA degraders and methanogens (1, 5), it would be useful to understand the influence of methanogens on a mixed-culture digester inoculum, as the ratio of VFA consumers to hydrogenotrophs could be different, and also this changes with time under shock loads. Such quantification and understanding of the rate-limiting mechanism would help in controlling digesters more efficiently. Although acetate is a potential inhibitor of VFA degradation, its role has often been underestimated. Similarly, most of the designs and control strategies suggested in the literature are concerned mainly with hydrogen and no attention has been paid to the control of acetate (9, 22).
Therefore, the objective of this work was to gain a better understanding of the relative contribution of acetate and formate/H2 utilizers added to a mixed-culture digester seed degrading VFAs. A second objective was to analyze the thermodynamic, kinetic, and mass transfer implications of acetate regulation and its impact on VFA degradation in order to improve digester design and control.
MATERIALS AND METHODS
Design of experiments.
Experiments were performed in 160-ml serum bottles in batch mode. Fixed volumes of inocula (20 ml) from a digester (operating at steady state with 4 g of chemical oxygen demand (COD) per liter of sucrose-based feed at a 15-day HRT) were supplemented with varying volumes of enriched formate and acetate cultures into 30% CO2–70% N2-flushed serum bottles. The concentration of volatile suspended solids in this inoculum was 1.67 g/liter, while the Sauter mean diameter of sludge flocs operating with a similar feed was ∼18 μm (34, 35). The enrichment cultures were added in portions of 10 to 30 ml by volume (see Tables 2 and 3). In two treatments, 30 ml of an enriched formate and acetate culture mixture was added at ratios of 2:1 and 1:2. To assess the initial population of VFA utilizers, in some treatments the inoculum volume was also varied. The total volume of the culture medium was 55 ml, and this was achieved by adding various proportions of the anaerobic medium of Owen et al. (19). After preparation of the culture medium, the serum bottles were inoculated in a 35°C water bath and left for 2 days to exhaust the residual carbon source in the cultures. The batch experiment was started by spiking the serum bottles with 5 ml of a concentrated VFA solution as sodium salt to result in a final concentration of approximately 2,500 mg/liter in the bottle. The degradation was then monitored by measuring the headspace hydrogen and the liquid-phase VFAs.
Analytical methods.
The VFAs were analyzed by high-performance liquid chromatography, and the gas-phase hydrogen was measured by using GMI’s exhaled-H2 monitor (34). All experimental treatments were done in duplicate, and the mean value was reported. The deviation observed between duplicates was, in general, less than 20% (mostly less than 10%), but at lower VFA concentrations (<200 mg/liter), 50 to 100% deviations were sometimes observed. The Gibbs free-energy values were calculated by using the standard free-energy values after correcting for temperature (3, 36).
Enrichment cultures. (i) Formate.
Enriched formate cultures were selected for several reasons: firstly, because both hydrogen and formate are possible electron transfer carriers for syntrophic degradation, and growth on formate is relatively simple and the activity can be quantified more easily; secondly, because in suspended systems the transport of formate seems to be more important than that of hydrogen (2, 30, 31). Finally, during step shock load, as well as pulse load, experiments, use of this sludge fed with 4 g of COD per liter resulted in greater amounts of formate than hydrogen being accumulated (35). Nevertheless, the formate-enriched cultures used in this study were able to use both H2 and formate. Throughout this work, it was assumed that the formate utilizers will use H2 if it is the main mode of electron transport (at least at the same rate as formate). This appears to be a valid assumption, as Schmidt and Ahring (27, 28) did not find any differences in the stimulation effect on either butyrate or propionate degradation with cultures that could use only hydrogen or both formate and hydrogen.
The formate cultures were enriched in 160-ml serum bottles through serial dilution (5-ml inocula in 45 ml of anaerobic medium) from a mixed-culture inoculum obtained from an anaerobic baffled reactor treating a sucrose-based feed (34). Formic acid (50 mg) was injected daily into the serum bottles as a 1-ml concentrated solution. The concentrated formic acid solution was prepared by adding 2 ml of formic acid (BDH) to 50 ml of a filter-sterilized effluent from a digester treating a sucrose-based feed. The COD of this effluent was less than 400 mg/liter (much lower than the formic acid COD in 1 ml, i.e., a COD of 17 versus 0.4 mg/liter), and this solution was used to avoid any nutrient deficiency. After 10 days of batch operation, the seed from the serum bottles was diluted to 10% with anaerobic medium and the enrichment was repeated four times in this manner. The typical daily gas production was 24 ml, with almost stoichiometric amounts of CH4. The ability of these organisms to consume hydrogen was tested by adding a 5% H2–30% CO2–65% N2 gas mixture, and methane production was measured by gas chromatography.
(ii) Acetate.
The acetate enrichments were developed in a 4-liter stirred tank reactor while continuously feeding acetate at an HRT of 8 days. The feed consisted mainly of sodium acetate, while peptone and meat extract were added as nutrient sources. Five liters of feed contained 27.33 g of sodium acetate, 3.0 g of peptone, 1.0 g of meat extract, 0.6 g of K2HPO4, 1.62 g of KH2PO4, 1.803 g of Na2HPO4, 0.0357 g of CoCl2 · 6H2O, 0.2355 g of FeCl2 · 4H2O, 0.01125 g of MnCl2 · 4H2O, 0.01125 g of Na2MoO4 · 2H2O, 0.0135 g of NiCl2 · 6H2O, 0.05 g of cysteine, and 1.25 g of Na2S. A nutrient solution consisting of S4 (3 ml/liter of feed) and vitamin solution S7 (10 ml/liter of feed) (19) was also added. All of the trace nutrients were injected separately daily, while the feed, buffer, peptone, and meat extract were autoclaved and fed continuously. To quantify the contamination due to VFA and formate utilizers, separate serum bottles were set up with spikes of formate, butyrate, and propionate for both enriched acetate and formate cultures. The formate utilization capacity (milligrams of formate per day per milliliter of culture) of enriched acetate utilizers was at least 10 times lower than the enriched formate utilizer activity (during a 24-h period). Less than 30 mg of acetate was consumed by enriched formate utilizers in 7 days. Approximately 300 and 70 mg of butyrate was consumed during 5 days of operation, and 580 and 840 mg of propionate was consumed in a 16-day period by enriched acetate and formate cultures, respectively (on a 1-liter basis).
Product inhibition model.
To compare the influence of trophic groups on butyrate degradation, a product inhibition model based on thermodynamics was selected from the literature (11). It was expected, from the work of Dwyer et al. (5), that the butyrate consumers would initially grow exponentially but that after a short time, the butyrate consumption would be linear. By neglecting the growth of the organisms during the linear period, the butyrate consumption rate (rB) can be described (11):
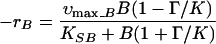 |
1 |
where Γ = [A]2[H2]2/[B], K = 2.1e − 8 (equilibrium constant), and the rate of acetate accumulation is
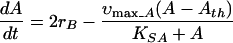 |
2 |
where υmax_A and υmax_B are the maximum acetate uptake and VFA uptake (in the absence of thermodynamic inhibition) and the term Γ/K accounts for product inhibition due to acetate and hydrogen. [B] and [A] are the molar butyrate and acetate concentrations, while H2 is the hydrogen partial pressure in atmospheres. KSA and KSB are the half-saturation constants for acetate and butyrate (30 and 7 mg/liter), and Ath is the threshold acetate concentration (0.6 mg/liter) (21). H2 metabolism was not incorporated into the model equations; instead, the dynamic variation of hydrogen was incorporated through a polynomial equation fitted to the experimentally measured H2 values over time. The model equations were integrated by using the modelling and simulation package gPROMS, and υmax_A and υmax_B were estimated by using a parameter estimation package, gEST (18).
RESULTS AND DISCUSSION
Butyrate degradation.
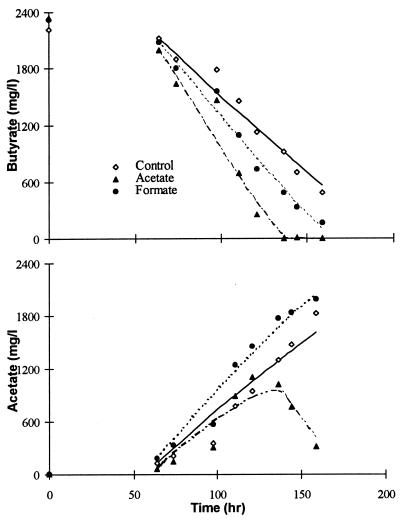
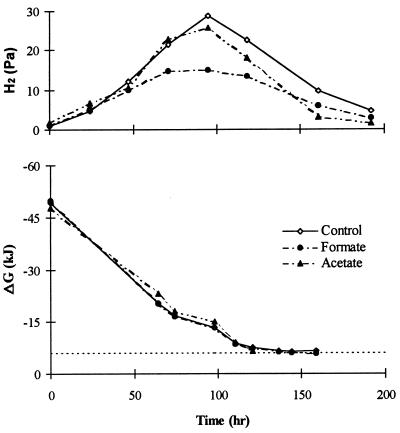
Figure 1 shows the butyrate and acetate dynamics during the batch degradation of butyrate with the addition of enriched formate and acetate cultures in comparison to the control (experiments 2, 6, and 9 in Table 2). The theoretical-model results are also presented in the same graphs as continuous lines. It can be seen from Fig. 1 that there was a significant improvement in VFA degradation, in comparison to the control, when acetate and formate utilizers were added, and this improvement was much greater in the presence of acetate utilizers than with formate utilizers. Butyrate was consumed completely in the presence of acetate utilizers after only 135 h, whereas it was nearly 500 mg/liter in the control and 160 mg/liter with formate utilizers, even after 160 h. Figure 2 shows the headspace H2 variation in the control and in the presence of acetate enrichment cultures, which were quite similar (maximum H2 of 26 to 28 Pa). In contrast, with formate utilizers, the maximum H2 levels reached were only around 15 Pa. However, these H2 levels did not exactly reflect the observed butyrate degradation rates, as the highest rates were noted with the acetate utilizers. This is in contrast to the results of Schmidt and Ahring (27, 28), who suggested that the rate of VFA degradation is inversely related to headspace hydrogen levels. Nevertheless, these hydrogen levels resulted in similar free-energy values for all of these treatments, which were independent of methanogenic activity and VFA concentrations. During most of the linear butyrate consumption period, the free-energy values were between −10 and −6 kJ and were almost stable at −6 kJ after 100 h (Fig. 2). However, this time period would have been much shorter if hydrogen mass transfer limitations were accounted for. Pauss et al. (20) suggested that with poorly soluble compounds like hydrogen, the liquid-phase concentrations will often be significantly higher than the headspace levels due to the slow mass transfer from the liquid to the gas phase. Interestingly, these stable ΔG′ values were quite similar in all of the treatments and close to the values reported in the literature (5, 10). Similar “energetic homeostasis” has also been reported earlier for the syntrophic H2 producer during ethanol oxidation (29). Under energetic homeostasis, the H2 producers work close to the thermodynamic limit of their survival through close feedback control of the product concentrations (5).
FIG. 1.
Butyrate degradation and acetate accumulation in the control and with the addition of formate and acetate utilizers (see experiments 2, 6, and 9 in Table 2). The simulation results are shown as continuous lines from 65 h onward.
FIG. 2.
Headspace hydrogen and Gibbs free-energy variations in serum bottles during the batch degradation of butyrate with the control and the addition of formate and acetate utilizers (see experiments 2, 6, and 9 in Table 2).
Variation of υmax with formate/H2 and acetate removal.
The equilibrium thermodynamic model (equation 1) described the experimental data quite closely (Fig. 1). However, as the ΔG′ values during the degradation period were almost the same, the model predicted the different responses by varying the υmax value of butyrate degradation.
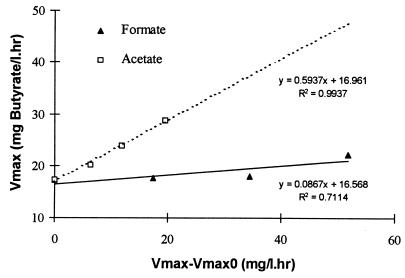
Figure 3 shows the variation in υmax_B in the bottles with formate and acetate utilizer addition and the control. The increase in the formate (Table 2, column 6, the numerical values of experiments 4 to 6 minus the control experiment 2) and acetate (Table 2, column 7, the numerical values of experiments 7 to 9 minus the control experiment 2) maximum uptake rates over the control, after the additions, is plotted on the abscissa; the υmax for formate uptake was determined experimentally before the commencement of these experiments. The υmax values for both acetate and butyrate were obtained from the parameter estimation results (columns 7 and 8, Table 2). From the slopes of the regression lines, it can be seen that the improvement in υmax_B is quite small when the number of formate/H2 utilizers is increased in comparison to the acetate utilizers (υmax_B values used for acetate enrichments were modified to compensate for slight formate contamination). From the thermodynamics, it could be expected that lowering of the formate and hydrogen levels would improve VFA degradation, as acetate is only inhibitory at very high levels, comparatively. It was surprising that the improvement with increasing amounts of acetate utilizers was much greater than with the formate/H2 utilizers. Interestingly, the υmax_B values fitted through parameter estimation (equation 1) correlated reasonably well with the predicted υmax_B values for all of the batch experiments (Table 2). The average error observed between fitted and estimated υmax_B values was less than 6%, with a correlation of 0.97 (with 12 datum points). This result clearly demonstrates the functional dependency of VFA degradation activity on both of the methanogens and the importance of acetate regulation. Typically, VFA degradation is modelled with a separate υmax value although it is known that VFA degraders are obligate syntrophs, and hence the VFA consumption rate is stoichiometrically related to their product removal rate. As these results show, the maximum VFA uptake rate is governed by the capacity of both the acetate and hydrogen utilizers and, in fact, varies over time as the concentration of these organisms changes with growth. Moreover, equation 1 is adequate only in describing the system behavior with acetate or hydrogen inhibition (11). However, it cannot describe the dynamics of the variations in methanogenic activity (Table 2). Hence, it is important to incorporate these changes in order to describe the kinetic capacity of VFA utilizers more accurately under shock loads, as well as under normal conditions. The same argument can probably be used to explain why propionate degradation is considered to be the rate-limiting step under shock loads (17) while the kinetic turnover rates under normal conditions suggest that acetate degradation is the rate-limiting step (12).
FIG. 3.
Variation in butyrate υmax with additional formate and acetate utilizers.
Comparison of simulated and experimental responses.
The greater improvements with acetate utilizers can be explained as due to either improved kinetics of formate/H2 uptake or reduced diffusional resistance. The following section analyses both of these possibilities through a simple theoretical model.
Since the H2/formate concentrations are extremely low, it was assumed that these concentrations could be estimated from thermodynamic relationships. This is a reasonable assumption, as many syntrophs work close to a characteristic free-energy level under steady-state conditions and even in batch operation (5, 10, 29); in our experiments, this value was around −6 kJ. By analogy, the analysis presented for hydrogen should also be applicable to formate. Under energetic homeostasis,
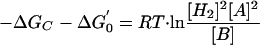 |
3 |
where −ΔGc is the stable minimum free energy of the VFA degrader, ΔG0′ is the standard free energy of reaction, R is the gas constant, and T is the temperature, and
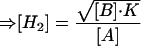 |
4 |
where
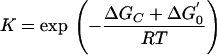 |
5 |
The hydrogen levels estimated through equation 4, in general, represent the qualitative trend of the headspace measurements and are quite similar during the later stages of the experiment. By neglecting hydrogen accumulation, the rate of butyrate degradation is related to hydrogen uptake under kinetic as well as mass transfer limiting conditions as follows:
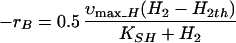 |
6 |
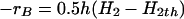 |
7 |
where h is the average mass transfer coefficient for the diffusional transfer of reducing equivalents in a unit volume of reactor and H2th is the threshold hydrogen concentration of the methanogens (0.3 Pa) (21). The acetate accumulation is computed by using equation 2.
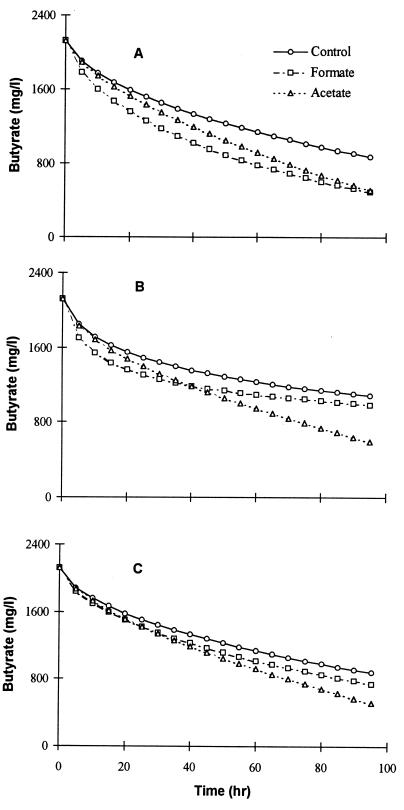
Figure 4 presents the simulated batch degradation profiles in the control and the treatments with formate and acetate utilizer addition under three different controlling mechanisms. The model parameters (h and υmax values) used in these simulations were estimated separately for each mechanism by using the control data, while the influence of formate/H2 and acetate utilizer addition was simulated by proportionately increasing these parameters using their activity data (Table 2). Figure 4A was simulated by assuming that hydrogen was the main mode of electron transport and hydrogen kinetics were the limiting factor (KSH = 5 μm) (25). In contrast to the experimental results of all of the batch degradation tests, the simulated degradation rates increased more with the addition of H2/formate utilizers than with the addition of acetate utilizers.
FIG. 4.
Simulation results of butyrate degradation in the control and the treatments with formate and acetate utilizer addition under kinetic and mass transfer limitations. (A) H2 as the main mode of transport. (B) Formate as the main mode of transport. (C) Diffusion of H2/formate as the rate-limiting step.
The predictions were more similar to the experimental data if formate was the electron transport mechanism between syntrophic partners (Fig. 4B). The KS and threshold formate concentration used were 0.22 mM and 10 μM (26). With formate utilizers, the simulated degradation rate was much higher during the initial period. However, this soon reduced dramatically due to a faster reduction of the concentration term (equation 6). In contrast, acetate regulation helped the system to respond with higher formate levels through lower acetate concentrations, thereby enhancing butyrate degradation. Figure 4C shows the simulated batch degradation profiles obtained by assuming that diffusion of formate/hydrogen was the rate-limiting step. The addition of formate utilizers improves the mass transfer rates by reducing the interbacterial distance, thus influencing the mass transfer coefficient value, h. However, the improvements with the additional formate utilizers were not substantial, as the interbacterial distance is reduced by the cubic root of the number of organisms. For example, to double the VFA degradation rate, the number of formate utilizers required is eight times the initial mass. In the above simulation, the mass transfer coefficient was increased by 1.3 times to account for the addition of formate utilizers while with acetate utilizers the same value as the control was used. It can be seen from Fig. 4C that the improvement obtained with hydrogen utilizers quickly diminished and almost reached the control, whereas acetate regulation helped the system to reach higher fluxes through an improved concentration gradient, even at lower butyrate levels.
Qualitatively, the models based on either formate kinetics or diffusion explained the experimental data. However, neither model predicted the experimental data well with a unique set of parameters, as the mechanisms of transport and the concentrations at the cell surfaces were unknown. Moreover, the thermodynamic relationship used in the simulation study (equation 4) assumed a constant [H+] (pH) but this changes with butyrate degradation, thus affecting the equilibrium concentrations.
It was found that to obtain a good fit with the kinetic model, a very low KS value (0.003 μM) was necessary. The υmax_H values fitted to the control and the formate and acetate utilizer additions were 0.39, 0.51, and 0.67 mmol of H2/liter · h, respectively. If enhanced kinetics were the reason for the improvements in VFA degradation, the υmax_H value obtained with formate utilizer addition should have been twice that of the control, and with acetate utilizers it should have been the same as the control. Interestingly, these values can be explained in a more rational way with mass transfer limitations; i.e., doubling of the formate utilizers improved butyrate degradation 1.3 times, whereas the improvements obtained with acetate utilizers were inversely related to their average acetate accumulation during the degradation period (500 mg/liter with acetate utilizers versus 850 mg/liter in the control).
The analysis presented here is subject to the limitations that batch systems are not stationary and their conditions vary over time. In the present study, the growth of biomass during the linear degradation period was not considered, although as a percentage change it should not be significant. Ideally, the experiments carried out as described above should have been done in continuous culture since the data might have been more reliable and easier to interpret. Furthermore, in batch culture, the cell-cell interactions could have changed when artificially altering the activity by dilution, whereas this problem would be less important in continuous culture systems. Ultimately, batch systems were used since the workload involved using continuous cultures would have been prohibitive. Nevertheless, this should not undermine the analysis here as butyrate degradation was almost linear with time (correlation coefficients obtained for seven datum points were >0.97) and hence it could be restricted to a small time period. Furthermore, the experimentally estimated improvement coefficients predicted reasonably well for all of the other treatments tested (Table 2), suggesting that acetate utilizers had a greater influence than formate utilizers. During the exponential phase, approximately 250 mg of butyrate was consumed; with this, the hydrogen utilizer yield could have increased by 10 to 20% over the initial control amount (assuming that the yield is independent of the hydrogen concentration). However, the actual yield could have been lower than this due to the concentration dependency of the Gibbs free energy (15). Nevertheless, the improvement obtained with formate utilizers was in the same range as that of Schmidt and Ahring (28), in whose study the batch degradation period was relatively short (<10 h). Furthermore, if mass transfer limitations controlled butyrate degradation, propionate should be influenced even more as propionate degradation is feasible at much lower concentrations.
Propionate degradation.
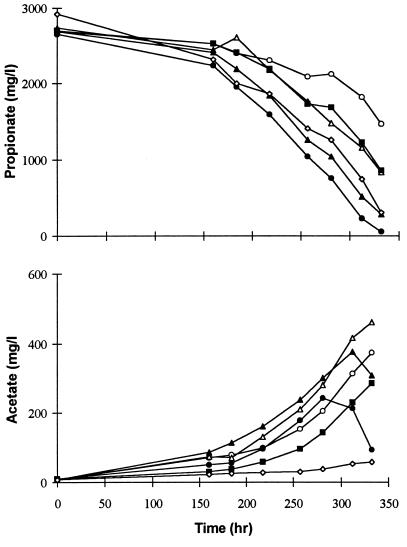
Figure 5 shows the propionate and acetate dynamics during the batch degradation of propionate with the addition of enriched formate and acetate cultures in comparison to the controls (experiments 1 to 4, 7, and 10 in Table 3). Propionate degradation was quite similar to butyrate consumption; after a brief lag phase, propionate was consumed in a linear fashion. The linear degradation gave a 0.98 correlation (with five points) for all of the treatments tested (except treatment 1, where four points were used from time 257 h, for which the correlation obtained was 0.91). The rates of degradation during the linear period were not substantially different in any of the treatments tested (Table 3), but the greatest improvements were observed in the treatments with acetate utilizers added or doubling of the inoculum size (treatments 4, 9, and 12). The addition of formate utilizers (treatments 5 to 7) resulted in a response similar to that of the control (treatment 2), but this decreased slightly at higher concentrations. The improvement obtained by increasing the initial inoculum size by four times was only a factor of 1.7. This was not entirely surprising, as such minimal improvements were reported earlier with H2/formate utilizer addition (27, 28). The headspace hydrogen values were quite similar during the entire period, with the average values ranging from 6 to 9 Pa. Similar to butyrate degradation during the linear degradation period, the free-energy values in all of the treatments were quite constant (approximately −14 kJ; Table 3).
FIG. 5.
Propionate degradation and acetate accumulation with different inoculum sizes and treatments with formate and acetate utilizer addition. Symbols: ○, control (10 ml); ▵, control (20 ml); ▴, control (30 ml); ●, control (40 ml); ■, plus formate utilizers (30 ml); ◊, plus acetate utilizers (30 ml). Linear degradation rates were noted from 218 h.
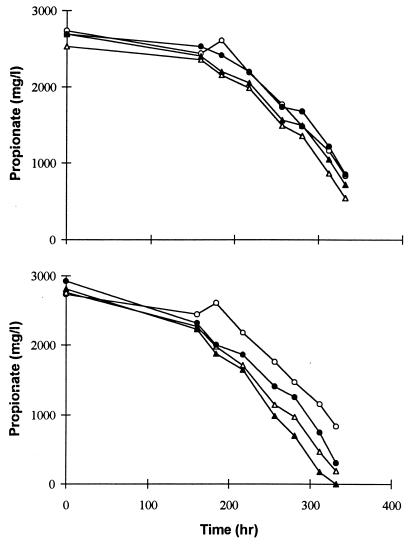
Unlike butyrate degradation, the improvements did not increase linearly with acetate and formate utilizer addition. Figure 6 shows the propionate degradation profiles with the addition of various proportions of acetate and formate utilizers; for comparison, control data are also shown. It can be seen from these graphs that the improvements were greater with smaller acetate or formate culture additions, while higher concentrations of these microorganisms started reducing the stimulatory effect. These differences were not due to experimental errors, as duplicate samples gave almost identical responses, with an average deviation of less than 10%. These differences can be explained through mass transfer limitations during syntrophic reactions.
FIG. 6.
Propionate degradation with different amounts of formate (top) and acetate (bottom) utilizers. Symbols: ○, control (20 ml); ●, plus 10-ml enrichment; ▴, plus 20-ml enrichment; ▵, plus 30-ml enrichment.
Mass transfer during syntrophic reactions.
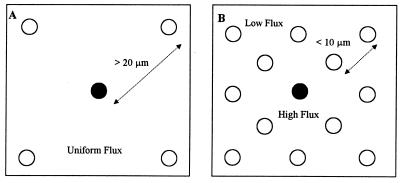
Theoretically, the rate of obligate syntrophic compound degradation is dependent on the kinetic capacity of the syntrophic partner if diffusion of intermediate metabolites is not the limiting factor. However, Boone et al. (2) suggested that both formate and hydrogen utilizers suffer severe mass transfer limitations when their bulk concentrations are very low, thus leading to underutilization of their kinetic capacity. Their mathematical model predicted sharp concentration gradients of formate or hydrogen around the methanogens responsible for their catabolism and, hence, the diffusion of these substrates. Formate or H2 concentrations are lowest at the cell surface of the methanogen and increase to bulk liquid concentrations at a distance of about 10 μm from the cell, assuming a single-cell diameter of 1 μm with dilute cell concentrations (106 to 107 cells/ml) (2). By comparing the literature (31) with similar feed strength and methane production rates, the cell concentration in the present study could be between 109 and 1010 cells/ml (average distance of less than 10 μm). At these high concentrations, the cells can no longer be assumed to exist as individual cells and the metabolite fluxes will vary significantly due to the influence of neighboring H2-/formate-consuming or -producing cells. Figure 7 shows the local flux variations that could develop when a methanogen is surrounded by formate, H2, or acetate consumers at low and high cell concentrations. In Fig. 7A, the electron flux will be uniform for all of the methanogens as the distance between the cells is large compared to the size of the concentration boundary layer (10 μm). In contrast, at shorter interbacterial distances, the flux will be higher with methanogens that are close to the VFA consumers compared to the cells which are farthest away from them. Hence, at higher cell concentrations, the diffusional transfer of electrons from VFA consumers, and hence the reduced flux from the bulk liquid, becomes the rate-limiting factor, thus severely constraining the stimulatory effects obtainable with additional methanogens. Using the same rationale, it could be hypothesized that the layered structure of aggregates suggested by Macleod et al. (14) is more efficient for VFA consumption as low acetate concentrations could be maintained with minimal interference to syntrophic partners due to aceticlastic or other organisms’ presence.
FIG. 7.
Possible variations in electron fluxes from VFA consumer to methanogens (open symbols) when interbacterial distances are large (A) and small (B).
Grotenhuis et al. (8) reported that in granular sludge the highest percentage of hydrogenotrophs was present when treating propionate-fed, compared to ethanol- or sucrose-fed, granules due to unfavorable propionate thermodynamics. Since the cell surface area of propionate oxidizers is limited, addition of extra methanogens is of little use as the mass transfer rates are low due to the low concentration gradient from the bulk liquid to the methanogens. In the present study, the greatest improvements (25%) were noted when acetate utilizers were added, and this was despite the fact that, unlike butyrate, the influence of acetate on propionate degradation was less dramatic, suggesting that mass transfer was the main limiting factor for further improvements. But this was reduced to 15% at higher concentrations of aceticlastic methanogens, possibly due to the shielding of the electron flux from producer to consumer by aceticlasts. Typically, the enriched cultures tended to have higher KS values than their syntrophically growing methanogenic counterparts. The reduced propionate degradation rates with higher concentrations of formate utilizers may be again due to the shielding of the electron flux by less efficient methanogens. In the present case, there was not much acetate production due to slow propionate degradation. However, Dong et al. (4) reported that the propionate degradation rate was almost doubled after addition of acetate utilizers.
In contrast, with butyrate, a 30% improvement in degradation was possible by doubling of the methanogens, and this was probably due to a reduced interbacterial distance. The arguments for mass transfer limitations presented in this report can be further strengthened by the observation that in a recent modelling study involving syntrophic perturbation experiments, the investigator had to consider local concentration variations through mass transfer to improve the model prediction capability (6). Similarly, the half-saturation constant for H2 in the absence of mass transfer limitations was found to be very low (7), suggesting that for most of the time H2 utilizers are saturated kinetically. However, this observation contradicts the experimental results of Kasper and Wuhrmann (12), who reported that in normal digesters the capacity of H2 utilizers was only exploited to a maximum of 1%. Nevertheless, if mass transfer is the main limitation for H2 metabolism, it provides a better explanation for both a low KS value and a high surplus activity of H2 utilizers. However, the possibility that different enzymes (depending on the H2 concentration [24]), are responsible for such variations should not be ruled out.
TABLE 1.
Reactions involved in the anaerobic syntrophic degradation of butyrate and propionate
| Compound | Reaction | ΔG′0 (kJ/mol)a |
|---|---|---|
| Butyrate | CH3CH2CH2COO− + 2H2O → 2CH3COO− + H+ + 2H2 | 48.1 |
| CH3CH2CH2COO− + 2HCO3− → 2CH3COO− + H+ + 2HCOO− | 45.5 | |
| Propionate | CH3CH2COO− + 3H2O → CH3COO− + HCO3− + CO2 + 3H2 | 76.5 |
| CH3CH2COO− + 2HCO3− → CH3COO− + H+ + 3HCOO− | 72.4 | |
| Hydrogen | 4H2 + CO2 → CH4 + 2H2O | −135.6 |
| Formate | 4HCOO− + H+ + H2O → CH4 + 3HCO3− | −130.1 |
ΔG′0 values are from reference 32.
TABLE 2.
Combination of populations used in the butyrate degradation batch experiment
| Expt no. | Digester inoculum vol (ml) | Formate enrichment vol (ml) | Acetate enrichment vol (ml) | Anaerobic medium (ml) | Initial formate activity (mg/liter · h) | Acetate activitya υmax_A (mg/liter · h) | Fitteda υmax_B (mg/liter · h) | Estimatedb υmax_B (mg/liter · h) |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 45 | 21 | 4.5 | 16.1 | 13.4 | ||
| 2 | 20 | 35 | 42 | 7.0 | 17.3 | 16.7 | ||
| 3 | 30 | 25 | 63 | 10.5 | 22.6 | 20.7 | ||
| 4 | 20 | 10 | 25 | 59 | 8.3 | 17.7 | 19.0 | |
| 5 | 20 | 20 | 15 | 77 | 7.6 | 18.0 | 20.1 | |
| 6 | 20 | 30 | 5 | 94 | 9.2 | 22.2 | 22.6 | |
| 7 | 20 | 10 | 25 | 43 | 13.2 | 20.2 | 20.5 | |
| 8 | 20 | 20 | 15 | 45 | 18.7 | 24.2 | 23.9 | |
| 9 | 20 | 30 | 5 | 46 | 26.5 | 29.4 | 28.7 | |
| 10 | 20 | 10 | 20 | 5 | 62 | 20.1 | 27.2 | 26.3 |
| 11 | 20 | 20 | 10 | 5 | 78 | 14.9 | 25.8 | 24.5 |
The butyrate and acetate activities are best-fit parameters obtained with equations 1 and 2.
Estimated υmax_B = 16.72 + 0.5937 × (υmax_A − 6.97) + 0.0867 × (formate activity − 42).
TABLE 3.
Combination of populations used in the propionate degradation batch experimenta
| Expt no. | Digester inoculum vol (ml) | Formate enrichment vol (ml) | Acetate enrichment vol (ml) | Anaerobic medium vol (ml) | Initial formate activity (mg/liter · h) | Acetate activity (mg/liter · h) | Measured Propionate activity (mg/liter · h) | ΔG′ (kJ) |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 45 | 21 | 3.9 | 8.3 | 13.4 ± 1.2 | ||
| 2 | 20 | 35 | 42 | 7.9 | 11.5 | 12.7 ± 2.0 | ||
| 3 | 30 | 25 | 63 | 11.8 | 13.6 | 12.4 ± 1.7 | ||
| 4 | 40 | 15 | 84 | 15.7 | 14.2 | 12.7 ± 2.1 | ||
| 5 | 20 | 10 | 25 | 50 | 7.9 | 12.2 | 12.4 ± 1.3 | |
| 6 | 20 | 20 | 15 | 59 | 7.9 | 11.1 | 12.8 ± 1.7 | |
| 7 | 20 | 30 | 5 | 67 | 7.9 | 11.1 | 13.2 ± 2.7 | |
| 8 | 20 | 10 | 25 | 43 | 13.9 | 13.1 | 13.0 ± 0.7 | |
| 9 | 20 | 20 | 15 | 45 | 19.9 | 14.4 | 15.5 ± 1.1 | |
| 10 | 20 | 30 | 5 | 46 | 25.9 | 13.1 | 18.5 ± 2.3 | |
| 11 | 20 | 20 | 10 | 5 | 53 | 13.9 | 13.6 | 14.9 ± 1.7 |
| 12 | 20 | 10 | 20 | 5 | 60 | 19.9 | 14.4 | 16.6 ± 2.1 |
Acetate activities were estimated from the average values obtained from the previous batch experiment, and propionate degradation was calculated from the experimental data.
ACKNOWLEDGMENTS
R.K.V. to acknowledges the financial support of the Association of Commonwealth Universities and Engineers India Ltd., New Delhi, for granting him study leave.
REFERENCES
- 1.Beaty P S, McInerney M J. Effects of organic acid anions on the growth and metabolism of Syntrophomonas wolfei in pure culture and defined consortia. Appl Environ Microbiol. 1989;55:977–983. doi: 10.1128/aem.55.4.977-983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boone D R, Johnson R L, Liu Y. Diffusion of the interspecies electron carriers hydrogen and formate in methanogenic ecosystems, and implications in the measurement of KM for H2 or formate uptake. Appl Environ Microbiol. 1989;55:1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad R, Wetter B. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch Microbiol. 1990;155:94–98. [Google Scholar]
- 4.Dong X, Plugge C M, Stams A J M. Anaerobic degradation of propionate by a mesophilic acetogenic bacterium in coculture and triculture with different methanogens. Appl Environ Microbiol. 1994;60:2834–2838. doi: 10.1128/aem.60.8.2834-2838.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer D F, Weeg-Aerssens E, Shelton D R, Tiedje J M. Bioenergetic conditions of butyrate metabolism by a syntrophic, anaerobic bacterium in coculture with hydrogen-oxidizing methanogenic and sulfidogenic bacteria. Appl Environ Microbiol. 1988;54:1354–1359. doi: 10.1128/aem.54.6.1354-1359.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furumai H. Modeling of hydrogen behavior and reduced product formation following perturbation with hydrogenic substrate in a methanogenic reactor. Water Sci Technol. 1997;36:255–262. [Google Scholar]
- 7.Giraldo-Gomez E, Goodwin S, Switzenbaum M S. Influence of mass transfer limitations on determination of the half saturation constant for hydrogen uptake in a mixed culture CH4 producing enrichment. Biotechnol Bioeng. 1992;40:768–776. doi: 10.1002/bit.260400704. [DOI] [PubMed] [Google Scholar]
- 8.Grotenhuis J T C, Smit M, Plugge C M, Xu Y, van Lammeren A A M, Stams A J M, Zehnder A J B. Bacteriological composition and structure of granular sludge adapted to different substrates. Appl Environ Microbiol. 1991;57:1942–1949. doi: 10.1128/aem.57.7.1942-1949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper S R, Pohland F G. Recent developments in hydrogen management during anaerobic biological wastewater treatment. Biotechnol Bioeng. 1986;28:585–602. doi: 10.1002/bit.260280416. [DOI] [PubMed] [Google Scholar]
- 10.Hickey R F, Switzenbaum M. Thermodynamics of volatile fatty acid accumulation in anaerobic digesters subject to increases in hydraulic and organic loading, Res. J Water Pollut Control Fed. 1991;63:141–144. [Google Scholar]
- 11.Hoh C Y, Cord-Ruwisch R. A practical kinetic model that considers end product inhibition in an anaerobic digestion process by including the equilibrium constant. Biotechnol Bioeng. 1996;51:597–604. doi: 10.1002/(SICI)1097-0290(19960905)51:5<597::AID-BIT12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Kasper H F, Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978;36:1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreikenbohm R, Bohl E. A mathematical model of syntrophic cocultures in the chemostat. FEMS Microbiol Ecol. 1986;38:131–140. [Google Scholar]
- 14.Macleod F A, Guiot S R, Costerton J W. Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microbiol. 1990;56:1598–1607. doi: 10.1128/aem.56.6.1598-1607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty P L. Energetics and bacterial growth in organic compounds in aquatic environments. New York, N.Y: Marcel Dekker Inc.; 1971. pp. 71–94. [Google Scholar]
- 16.McCarty P L. One hundred years of anaerobic treatment. In: Hughes D E, Stafford D A, Wheaty B I, Badder W, Lettinga G, Nyns E J, Verstrate W, editors; Hughes D E, Stafford D A, Wheaty B I, Badder W, Lettinga G, Nyns E J, Verstrate W, editors. Anaerobic digestion 1981. Amsterdam, The Netherlands: Elsevier; 1982. pp. 3–22. [Google Scholar]
- 17.McCarty P L, Mosey F E. Modelling of anaerobic digestion processes (a discussion of concepts) Water Sci Technol. 1991;24:17–33. [Google Scholar]
- 18.Oh M, Pantelides C C. A modelling and simulation language for combined lumped and distributed parameter systems. Comput Chem Eng. 1996;20:611–633. [Google Scholar]
- 19.Owen W F, Stuckey D C, Healy J B, Jr, Young L Y, McCarty P L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979;13:485–492. [Google Scholar]
- 20.Pauss A, Andre G, Perrier M, Guiot S R. Liquid-to-gas mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl Environ Microbiol. 1990;56:1636–1644. doi: 10.1128/aem.56.6.1636-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlostathis S G, Giraldo-Gomez E. Kinetics of anaerobic treatment: a critical review. Crit Rev Environ Control. 1991;21:411–490. [Google Scholar]
- 22.Poels J, van Assche P, Verstraete W. Influence of H2 stripping on methane production in conventional digesters. Biotechnol Bioeng. 1985;27:1692–1698. doi: 10.1002/bit.260271210. [DOI] [PubMed] [Google Scholar]
- 23.Powell G E. Equalisation of specific growth rates for syntrophic associations in batch culture. J Chem Technol Biotechnol. 1984;34B:97–100. [Google Scholar]
- 24.Reeve J N, Morgan R M, Nolling J. Environmental and molecular regulation of methanogenesis. Water Sci Technol. 1997;36:6–7. [Google Scholar]
- 25.Robinson J A, Tiedje J M. Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediment. Appl Environ Microbiol. 1982;44:1374–1384. doi: 10.1128/aem.44.6.1374-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schauer N L, Brown D P, Ferry J G. Kinetics of formate metabolism in Methanobacterium formicium and Methanospirillum hungatei. Appl Environ Microbiol. 1982;44:540–554. doi: 10.1128/aem.44.3.549-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt J E, Ahring B K. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl Environ Microbiol. 1993;59:2546–2551. doi: 10.1128/aem.59.8.2546-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt J E, Ahring B K. Interspecies electron transfer during propionate and butyrate degradation in mesophilic granular sludge. Appl Environ Microbiol. 1995;61:2765–2767. doi: 10.1128/aem.61.7.2765-2767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seitz H J, Schink B, Pfennig N, Conrad R. Energetics of syntrophic ethanol oxidation in defined chemostat cocultures 1. Energy requirement for H2 production and H2 consumption. Arch Microbiol. 1990;155:82–88. [Google Scholar]
- 30.Stams A J M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie van Leeuwenhoek. 1994;66:271–294. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- 31.Stams A J M, Dong X. Role of formate and hydrogen in the degradation of propionate and butyrate by defined suspended cocultures of acetogenic and methanogenic bacteria. Antonie van Leeuwenhoek. 1995;68:281–284. doi: 10.1007/BF00874137. [DOI] [PubMed] [Google Scholar]
- 32.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiele J H, Zeikus J G. Handbook on anaerobic fermentations. New York, N.Y: Marcel Dekker, Inc.; 1988. Interaction between hydrogen- and formate-producing bacteria and methanogens during anaerobic digestion; pp. 132–159. [Google Scholar]
- 34.Voolapalli R K, Stuckey D C. Stability enhancement of anaerobic digestion through membrane gas extraction under organic shock loads. J Chem Technol Biotechnol. 1998;73:153–161. [Google Scholar]
- 35.Voolapalli, R. K., and D. C. Stuckey. The utility of hydrogen monitoring for methanogenic reactors—influence of formate production and H2 kinetics. Submitted for publication.
- 36.Westermann P. The effect of incubation temperature on steady state concentrations of hydrogen and volatile fatty acids during anaerobic degradation in slurries from wetland sediments. FEMS Microbiol Ecol. 1994;13:295–302. [Google Scholar]