Abstract
Purpose
This study aims to evaluate the effects of Manilkara zapota (L) P. Royen fruit peel extract (EMZFP) and its fractions in ameliorating diabetes and its complications in alloxan and STZ-NA induced diabetes in Wistar rats.
Methods
Antidiabetic effects of EMZFP were assessed in alloxan (150 mg kg-1) induced diabetes in differently grouped rats (n=6). Diabetic rats were treated with EMZFP 150, 300, and 600 mg kg-1 while, glimepiride (0.09 mg kg-1) was used as a reference standard. Treated animals were assessed for various biological parameters i.e. blood glucose, serum lipids, nephroprotective markers, cardiovascular risk indices, liver glycogen, neuropathy, body weight, and histopathology of kidneys. However, for evaluating antidiabetic effects of fractions (chloroform, acetone, ethyl acetate, and remaining ethanol fraction) of EMZFP, diabetes was induced by streptozotocin (60 mg kg-1)–nicotinamide (120 mg kg-1/ml) in differently grouped male rats (n=6). Diabetic rats were treated with EMZFP fractions 200 mg kg-1 however; glibenclamide (10 mg kg-1) was a reference standard and evaluated for blood glucose, serum lipids, cardiovascular risk indices, and diabetic neuropathy.
Results
EMZFP 300 and 600 mg kg-1/day demonstrated significant antihyperglycemic effects with augmentation in glycogen content, perfection in serum lipid profile, cardiovascular risk indices, body weight enhancement, nephroprotective effects, beneficial in peripheral neuropathy, and histopathological evidence of reversal of glomerulosclerosis. EMZFP-Et and EMZFP-EA fractions depicted a significant improvement in blood glucose, serum lipid profile, cardiovascular risk indices, and peripheral neuropathy.
Conclusion
EMZFP and its Et and EA fractions ameliorated diabetes and its complications by improving glycemic control and associated biochemical alteration.
Highlights
• Manilkara Zapota (L.) P. Royen fruit peel 70% ethanolic extract exert antidiabetic effects
• EMZFP significantly ameliorated diabetic biochemical parameters and its complications.
• EMZFP-Et and EMZFP-EA fractions exert potential antihyperglycemic, hypolipidemic effects and significantly improved cardiovascular risk indices, and peripheral neuropathy.
• Studied MZFP can be used as promising natural herbal source of antidiabetic principles.
Keywords: Blood glucose, Cardiovascular risk indices, Diabetes mellitus, Diabetic complications, Diabetic Nephropathies, Diabetic neuropathy
Introduction
Diabetes mellitus (DM) is a chronic metabolic dysregulation with several causes and is manifested by elevated blood glucose levels with altered carbohydrate, lipid, and protein metabolism as a consequence of pancreatic β-cell dysfunction, distortion of the insulin signaling pathway and deprived of insulin resistance [1, 2]. All over the world Type 2 diabetes mellitus (T2DM) is the most common chronic ailment due to the practice of fast food habits and sedentary lifestyles. T2DM is specifically a consequence of insulin resistance and/or inadequate insulin synthesis [3], in chronic stage manifested by severe complications, such as retinopathy, cardiomyopathy, neuropathy, nephropathy, diabetic foot syndrome, and dyslipidemia [4].
Normally, feedback control of the antioxidant system keeps free radicals at a control level in the body. Persistent hyperglycemia resulted in oxidative stress and creates the key and collective events in the pathogenesis of elevated vascular complications such as advanced glycation end product, polyol pathway flux, cytokines, sorbitol, hexamine pathway flux, and activation of protein kinase C isoforms [5].
Accordingly, the current drug regimen focuses on refining the glycemic load to counteract the complications accompanying DM. The management of DM is done by three classes of drug interventions. The first one increases endogenous insulin availability- sulphonylureas, insulin analogs, glinides, dipeptidyl peptidase IV inhibitors, and glucagon-like peptide-1 agonists [6]. Second, drugs increasing the sensitivity of insulin- thiazolidinedione and biguanide [7] and the third one is the carbohydrate hydrolyzing enzyme inhibitors- α-amylase and α-glycosidase inhibitors [8]. Amongst these, sulphonylureas and glitazone may induce obesity, osteoporosis, and inhibition of hepatic regeneration [9]. While, a thiazolidinedione, may induce risk reduction of IGT conversion to T2DM and prolonged use is associated with noticeable weight gain and edema increasing cardiac threats [10]. Owing to these limits of available drug interventions phytotherapy or use of bioactive phytochemicals from traditionally known antidiabetic herbal resources are courtesy of diabetic management and treatment.
Realizing this, herbal extracts of food industry byproducts rich in a nutritionally valuable mixture of components, which may act additively or in synergy in the management of DM, attracted many researchers to investigate and evaluate their antidiabetic potentials [11]. The previous literature findings for extraction of herbal food byproducts were so far up to single-step extraction, which may limit the pulling out of bioactive phytoconstituents. Further fractionation with a specific type of solvent can enrich the extract consequently improving its bioactivity considerably [12].
Manilkara zapota (L) P. Royen fruit from the Sapotaceae family is widely consumed and cultivated nutritional fruit across the tropical reasons of the world. This fruit’s nonedible part like peel constitutes a higher amount of various biologically active phytochemicals as compared to its edible pulp portion [13]. Manilkara zapota (L) P. Royen fruit peel (MZFP) has been reported to the presence of antioxidant principles, enriched variety of phenolic compounds and flavonoids with phenolic hydroxyl groups which effectively scavenges reactive oxygen species [14, 15] and most of the flavonoids had already reported as antidiabetic, helps to minimize cardio-vascular complications [16–19]. 5-caffeoyl quinic acid conjugates, a phenolic compound identified in MZFP reported for treating different diseases and disorders [20, 21]. Lycopene- a carotenoid found in MZFP capable to eradicate the singlet oxygen and protects low-density lipoproteins, proteins, DNA, and lipid molecules from free radicals and performs a key role in shielding from many diseases [22–24]. Furthermore, other various bioactive phytoconstituents were also reported in MZFP, like hydroxybenzoic acids (gallic, ellagic, and p-hydroxybenzoic), flavanols (catechin, epicatechin, and quercetin), hydroxycinnamic acids (chlorogenic, ferulic, and trans-cinnamic), and kaempferol. All these phytochemicals highlight their role in antioxidant activity, antidiabetic effects, and in various other diseases where free radicals scavenging perform a key role in eradicating the disease and its complications [25, 26].
In hyperglycemic state glucose oxidation and non-enzymatic protein glycation causes the excessive free radical formation and this worsened free radical level is concurrently suppressed by an antioxidant defense mechanism, leading to critical impairment of cellular organelles and enzymes, rise in lipid peroxidation, and advances in insulin resistance; this concerned of oxidative stress can stimulate the advances and progression of DM complications [27]. Antioxidants of natural plant sources are capable of scavenging free radicals and considerably diminish the chance of progression of DM complications [28].
In our preliminary studies, we observed that MZFP possesses significant in-vitro free radical scavenging and α-glucosidase enzyme inhibition potentials when ascorbic acid and acarbose were taken as reference standards respectively [29]. Still, no studies were carried out regarding the in-vivo antidiabetic potentials of this fruit peel. Hence, in this presented investigation we further assessed EMZFP and its various fractions, to unfold the hindrance abilities for antidiabetic potentials with its complications like nephropathy, neuropathy, and dyslipidemia in alloxan and STZ-NA induced diabetic rat models respectively.
Materials and methods
Chemicals
All the chemicals used were of analytical grade. Pet ether, n-hexane, ether, chloroform, acetone, ethyl acetate, ethanol, Na-citrate buffer, formalin, Xylene (Merck Life Sci. Pvt. Ltd., Ind.). Alloxan monohydrate (Fine chem., Pvt. Ltd., Mumbai, Ind). Streptozotocin and nicotinamide (SRL diagnostics, Pvt. Ltd., Mumbai, Ind.). Glucose oxidase/peroxidase standard diagnostic kit (Reckon diagnostics, Pvt. ltd., Ind.). Hematoxylin and eosin-stains, urethane, trichloroacetic acid, anthrone reagent (Sigma Aldrich Pvt. Ltd., Ind.).
Collection and authentication of plant sample
Fresh and just ripened Manilkara zapota (L) P. Royen fruits were collected from the local farm area of Nanded, MS, India (latitude 19.130 N & longitude 77.320 E). For taxonomic identity and authentication, the plant herbarium sheet was prepared and deposited at the herbarium of Botanical Survey of India, Pune, India and voucher specimen number issued was BSI/WRC/100-1/TECH./2019/68/ specimen no. 1; dated 24 Dec 2019.
Sample preparation, extraction, and fractionation
The fruits peels were removed with the steel spoon and shade dried. Later it was pulverized to make it in coarse powder. The 1000 ml of 70% ethanol was added to 100 g of dried peel powder (pet ether defatted) and kept for shaking at 28°C for 2 h with 150 rpm on an orbital incubator shaker (Remi RIS 24+). After cooling to room temperature, centrifuged at 2500 rpm for 15 min and filtered by Whatman filter paper No 1. The filtrate was then evaporated for 3 days and obtained extract was put in storage in airtight dark bottles at 4°C before further analysis. [30, 31].
Further; EMZFP was dissolved in ethanol before mounting for column chromatography to separate it into fractions with chloroform, acetone, and ethyl acetate. Slurry of 150 g silica gel G (60-120 mesh size) mixed with 350 ml of n-hexane was prepared. A vertical borosilicate glass column (20 mm width × 500 mm length) was rinsed with acetone and well dried before packing, to the bottom a piece of glass wool was placed, and to its top sea sand (particle size 60) added up to 1 cm height. The prepared slurry was poured into this column up to 2/3rd of the column height and to the top 1 cm height level of sea sand was placed. This whole assembly was rinsed with solvent to aid the proper packing of the column. The dry powder sample for fractionation was prepared by evaporating a mixture of 10 g of EMZFP and 20 g of silica gel G in ethanol on the hot plate. The top of the prepared column was filled with this dry powder, to its top 1 cm height level sea sand was added, and the whole assembly was eluted with various solvents (chloroform, acetone, and ethyl acetate) by using the gradient elution method to get respective soluble fractions along with remaining ethanol fraction. A solvent level of 6 cm height above the extract was maintained to prevent drying of the column. At a flow rate of 5 ml/min, 50 ml of solvent was collected for each fraction. The obtained each fraction was stored in amber vials and was kept in a refrigerator at 4°C before further usage [32].
Experimental animals
Albino rats of Wistar inbred, 2-3 months old, weighing 200-220 g were selected and kept in ventilated cages with a standard pellet diet and water ad libitum for seven days before initiating the study. Animal care and all the animal experiments were performed and approved by the Institutional animal ethics committee (SNIOP/CPCSEA/IAEC/CP-PL/01-2021) following the CPCSEA guidelines for care and use of laboratory experimental animals in India. The blood samples (3 ml) for analysis were collected using 23 gauze needles from the lateral tail vein following restraint. All the dosing and collection of the biological sample were done between 9 am to 10 am to avoid circadian rhythm-induced changes [33].
Acute oral toxicity study
The acute oral toxicity studies were performed individually for EMZFP and its fractions in two individual stages in nulliparous and non-pregnant, 8-12 weeks old female mice, as per the OECD 423 guidelines. For the first stage, mice were grouped into three groups (n= 03) and given p.o. 05, 50, 300, and 2000 mg kg-1 b.w. doses of test extract. Then mice were witnessed for the next 24 h for any marks of toxicity, moribund status, and or death. The outcomes from the first stage recommended the doses for the second stage were 3000 and 5000 mg kg-1 b.w. doses of test extract were administered p.o. to the other three groups (n=03) of mice. However, for the each fraction in the second stage, the doses of 2000 mg kg-1 b.w were given p.o. Any sign of toxicity, moribund status, and or mortality was noted in the next 72 h for both EMZFP and its fractions.
Experimental induction of diabetes in Wistar rats
Before induction of diabetes, rats were kept for overnight fasting and prior injection of diabetogenic agent animals were anesthetized by light ether. For evaluation of antidiabetic effects of EMZFP, to induce and establish diabetic condition, a single i.p. dose of alloxan monohydrate (150 mg kg-1 b.w.) was given to the rats [34, 35].
While evaluating the antidiabetic effects of fractions of EMZFP, diabetes was induced by streptozotocin (STZ) –nicotinamide (NA) rat model. Due to the less sensitivity of female rats to STZ, male rats were preferred in this study. STZ causes pancreatic β-cell damage, whereas NA partially protects the β-cell against STZ, while the combination of these two makes the rats’ insulin-deficient but not insulin-resistance [36]. For induction of diabetes a single i.p. dose of NA (120 mg kg-1 b.w./ml) in saline was given 15 min before the inoculation of a single i.p. dose of 60 mg kg-1 b.w. of STZ to each rat [37, 38].
The alloxan monohydrate was dissolved in 0.9 % saline [39], while STZ was freshly prepared by solubilizing in 0.1 ml of cold Na-citrate buffer (0.1 M, pH 4.5) [40]. 1 ml syringe with 23 G needle was used for inoculations of all freshly prepared (alloxan, STZ, and NA) volumes of 1 ml kg-1 b.w., and inoculated within 5 min after its preparation to avoid degradation. Normal water fed was replaced with 5% glucose water for up to the next 48 h to counteract severe acute hypoglycemia in all induced rats. On day 3rd after inoculations, rats with fasting blood glucose level ≥ 200 mg/dl for alloxan and fasting blood glucose level ≥ 250 mg/dl for STZ- NA induced diabetes were well-thought-out as diabetic rats and utilized for further experiments [41, 42].
Experimental design
For evaluation of antidiabetic effects of EMZFP
After induction of diabetes, survived rats of either sex were randomly divided into different groups (n = 6), while for the control normal rats were selected, and fasting blood glucose levels of all rats were recorded. The treatment group animals were administered with EMZFP and blood glucose levels were recorded at 0th, 7th, 14th, and 21st days [33, 43, 44].
Normal control (Gr. I): Normal rats were received p.o. 1 ml kg-1 b.w. suspension of 1% gum acacia.
Diabetic control (Gr. II): Alloxan-induced diabetic untreated rats were received p.o. 1 ml kg-1 b.w. suspension of 1% gum acacia.
Glimepiride (Gr. III): Alloxan-induced diabetic rats were received p.o. glimepiride 0.09 mg kg-1 b.w. in suspension of 1% gum acacia.
EMZFP 150 (Gr. IV): Alloxan-induced diabetic rats were received p.o. EMZFP 150 mg kg-1 b.w., in suspension of 1% gum acacia.
EMZFP 300 (Gr. V): Alloxan-induced diabetic rats were received p.o. EMZFP 300 mg kg-1 b.w., in suspension of 1% gum acacia.
EMZFP 600 (Gr. VI): Alloxan-induced diabetic rats were received p.o. EMZFP 600 mg kg-1 b.w., in suspension of 1% gum acacia.
For evaluation of antidiabetic effects of fractions of EMZFP
STZ-NA induced diabetic, survived rats (male) were randomly divided into different groups (n = 6), while for the control group, normoglycemic rats were selected, and fasting blood glucose levels of all grouped animals were recorded. The treatment groups animals were administered p.o. fractions of EMZFP for 21 days [45, 46].
Normal control (Gr-I): Normal rats were received p.o. 1 ml kg-1 b.w. suspension of 1% gum acacia.
Diabetic control (Gr-II): STZ-NA induced diabetic untreated rats were received p.o. 1 ml kg-1 b.w. suspension of 1% gum acacia.
Glibenclamide (Gr-III): Diabetic rats were received p.o. glibenclamide 10 mg kg-1 b.w.
Treatment group (Gr-IV to Gr-VII): Diabetic rats were received a suspension of various fractions (chloroform, acetone, ethyl acetate, and remaining ethanol fraction) of EMZFP at the doses of 200 mg kg-1 b.w., prepared in 1% gum acacia solution.
Blood glucose level estimation
Antihyperglycemic effect of EMZFP extract was assessed by estimating blood glucose level on 1st, 7th, 14th, and 21st day, while, for fractions of EMZFP on 1st, and 21st day, by using Reckon diagnostic’s GOD/POD kits.
Body weight
Animals were weighed on the 7th, 14th, and 21st days to estimate any weight changes on treatment with EMZFP extract.
Estimation of Serum levels of Total Cholesterol, HDL, LDL, and Triglyceride
Standard diagnostic kits from Reckon diagnostics, Pvt., ltd., India were used to estimate serum levels of total cholesterol, HDL, LDL, and triglyceride on the 21st day of treatment with EMZFP and its fractions.
Assessment of the nephroprotective effect
a) Serum biochemical analysis
On the 21st day, serum levels of total protein, albumin, and creatinine were assessed for any possible effects of EMZFP on diabetic renal conditions. Standard diagnostic kits from Reckon diagnostics, Pvt., ltd., India were used.
b) Histological study
On the 21st day, any possible nephroprotective effect of EMZFP was studied by performing a histological study. Isolated kidneys from each rat belonging to each group were cleaned and kept in a 10% formalin solution. After embedding in paraffin wax, sliced with a rotary microtome in to about 5 μm thickness. Xylene and ethanol were used for deparaffinization. Further, hematoxylin and eosin-stained sections were microscopically examined for evaluation of histoarchitecture, hyalinization, and glomerulosclerosis [47].
Evaluation of liver glycogen content
On the 21st day, liver glycogen content was determined by the standard method described [48]. Animal livers were isolated after scarifying with a urethane overdose, cleaned with saline, and homogenized (4 min.) with 40 ml of 5% trichloroacetic acid in the homogenizer vessel. After centrifuging the homogenate for 10 min at 3500 rpm the supernatant was filtered by filter paper and the residue was again extracted with the same procedure to extract 98% of glycogen. To the 1 ml of filtrate 5 ml of 95% ethanol was added and kept overnight at room temperature for precipitation in a test tube. On the next day, this precipitated content was centrifuged for 15 min at 3000 rpm and supernatant clear content was decanted. The test tube containing the remaining pellet was kept in an inverted state (15 min) until getting a dry pellet. This pellet was then added with 10 ml of anthrone reagent, 2 ml of distilled water and shaken vigorously for 5 min. The tube was then kept in boiling water for 15 min, later on, cooled to room temperature.
The optical density was colorimetrically determined at 620 nm. 2 ml of standard glucose (0.1 mg) solution and anthrone reagent (10 mL) was used as a standard solution while, for blank solution 2 ml water with 10 ml anthrone reagent was used. Glycogen content was estimated by the formula;
Mg of glycogen in 100 g of tissue = DU/DS×0.1 ×Volume of extract/weight of tissue ×100×0.9
Where, DU is the optical density of unknown; DS is the optical density of standard.
Assessment of cardiovascular risk indices [49]
Various cardiovascular risk indices were calculated as;
Assessment of neuropathic pain
Post-treatment, on the 21st day hot plate-induced pain sensation ability of diabetic rats treated with EMZFP and its fractions was assessed. Paw licking responses of these diabetic rats were observed by placing them on a hot and cold plate apparatus set at the temperature of 50 °C with thermostat connected heating element of stainless steel (V J Enterprise). To prevent tissue damage a 10 s cutoff time was kept. The average of two times, for hot plate response latency for ipsilateral (uninjured) paw licking was measured, with 15 min time intervals [50].
Statistical analysis
The values presented were mean ± SEM (n=6). The presence of a significant difference between the mean values was calculated by two-way ANOVA followed by tukey's multiple comparison tests at 95% confidence interval with GraphPad Prism software version 9.2.0. While, the p-values < 0.05 were considered statistically significant.
Results
Acute oral toxicity study
During acute oral toxicity studies, in all cases when mice treated with EMZFP and its fractions behaved normally with no signs of any toxicity or moribund status. No death was observed at doses of 2000, 3000, and 5000 mg kg-1 b.w. with EMZFP and at 2000 mg kg-1 b.w. with fractions of EMZFP. In both the cases, no problematic clinical marks were detected in surviving mice (Table 1). As such, the minimal lethal dose (LD50) could be more than 5000 mg kg-1 b.w. for EMZFP; the therapeutic dose preferred was the 1/10th (300 mg kg-1 b.w.) of the safe dose (3000 mg kg-1 b.w.) for this extract. However, for fractions of EMZFP the therapeutic dose preferred was the 1/10th (200 mg kg-1 b.w.) of safe dose (2000 mg kg-1 b.w.).
Table 1.
The time course of signs of toxicity in mice treated with EMZFP-2000, 3000, and 5000 mg kg-1 b.w. and 2000 mg kg-1 b.w. for fractions of EMZFP.
| Observations | At 3 h | At 24 h | At 72 h |
|---|---|---|---|
| Physical activity | N | N | N |
| Skin and fur | N | N | N |
| Eyes and mucous membranes | N | N | N |
| Behavioral pattern | N | N | N |
| Respiratory | N | N | N |
| Circulatory | N | N | N |
| Autonomic profile | N | N | N |
| Neurologic profile | N | N | N |
| Sleep | N | N | N |
| Diarrhea | N | N | N |
| Tremors | N. O.* | N. O.* | N. O.* |
| Mortality | N. O.* | N. O.* | N. O.* |
N normal, N.O.* not observed
Effect of EMZFP on blood glucose
As predicted, rats from the untreated diabetic control group showed a continued increase in blood glucose levels from the 1st to 21st day. The glimepiride-treated diabetic animals exhibited a reduction in blood glucose level from the 14th day onwards when compared with diabetic control group animals (p < 0.001) (Table 2). The treatment of diabetic animals with EMZFP 150 mg kg-1 b.w. was exhibited a reduction in blood glucose level significantly on the 21st day (p<0.05), and treatment with EMZFP 300 mg kg-1 b.w. it was significantly reduced on the 14th day (p<0.05), and the 21st day (p<0.001). However, a highly significant reduction in blood glucose level was elucidated on treatment with EMZFP 600 mg kg-1 b.w. on the 14th day (p<0.01), and on the 21st day (p<0.001).
Table 2.
Effect of EMZFP on blood glucose level in alloxan-induced diabetic rats
| Groups | Blood glucose level (mg/dl) at | |||
|---|---|---|---|---|
| 1st day | 7th day | 14th day | 21st day | |
| Normal control | 72.45 ± 4.56 | 65.15 ± 3.87 | 78.89 ± 2.47 | 71.47 ± 2.73 |
| Diabetic control | 266.43 ± 12.87 $ | 273.36 ± 11.15 $ | 279.62 ± 10.28 $ | 285.92 ± 9.45 $ |
| Diabetic + Glimepiride | 271.67 ± 11.42 | 244.76 ± 10.74 | 192.91 ± 14.36 *** | 161.32 ± 10.28 *** |
| Diabetic + EMZFP 150 | 272.67 ± 8.96 | 253.58 ± 10.28 | 247.74 ± 11.24 | 228.58 ± 9.34 * |
| Diabetic + EMZFP 300 | 260.35 ± 10.12 | 248.64 ± 13.78 | 221.52 ± 14.82 * | 198.02 ± 11.29 *** |
| Diabetic + EMZFP 600 | 265.34 ± 14.47 | 229.56 ± 15.24 | 215.36 ± 9.68 ** | 173.83 ± 12.87 *** |
Data was represented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with diabetic control. Diabetic rats treated with EMZFP 600 mg kg-1 b.w. elucidated significant blood glucose lowering effects on the day 14th and 21st.
Effect of fractions of EMZFP on blood glucose
Treatment with the doses of 200 mg kg-1 b.w. of EMZFP fractions for 21 days in STZ-NA induced diabetic rats were elucidated notable blood glucose-lowering effects (Table 3). A fall in blood glucose level was recorded when treated with EMZFP-Et (230.57 ± 10.48 mg/dl) and EMZFP-EA (238.83 ± 8.87 mg/dl) at a significant level of p<0.01 and p<0.05 respectively, reveling the marked antihyperglycemic effects.
Table 3.
Effect of fractions of EMZFP on blood glucose level in STZ-NA induced diabetic rats
| Groups | Blood glucose level (mg/dl) at | |
|---|---|---|
| Day 1st | Day 21st | |
| Normal control | 66.35 ± 5.46 | 67.78 ± 4.12 |
| Diabetic control | 268.33 ± 14.24 $ | 290.48 ± 12.37 $ |
| Diabetic + Glibenclamide | 273.47 ± 12.65 | 158.44 ± 8.25 *** |
| Diabetic + EMZFP-C 200 | 285.76 ± 10.87 | 272.88 ± 9.18 |
| Diabetic + EMZFP-A 200 | 288.16 ± 14.39 | 264.95 ± 8.54 |
| Diabetic + EMZFP-EA 200 | 289.26 ± 9.52 | 238.83 ± 8.87 * |
| Diabetic + EMZFP-Et 200 | 286.18 ± 11.76 | 230.57 ± 10.48 ** |
(EMZFP-C: chloroform fraction; EMZFP-A: acetone fraction; EMZFP-EA: ethyl acetate fraction; EMZFP-Et: remaining ethanol fraction)
Data represented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with diabetic control.
Diabetic animals treated with EMZFP-Et 200 showed a significant reduction in the blood glucose levels (p < 0.01) on 21st day.
Body weight changes
Untreated diabetic rats showed a notable reduction in body weight. While the rats treated with EMZFP for 21 days showed a gain in body weight at the doses of 150 mg kg-1 b.w. (6.00 ± 3.92), 300 mg kg-1 b.w. (7.65 ± 1.92), and 600 mg kg-1 b.w. (7.71 ± 2.13), when compared with untreated diabetic rats (Table 4).
Table 4.
Effect of EMZFP on body weight changes in alloxan-induced diabetic rats
| Groups | Body weight changes (g) | ||
|---|---|---|---|
| 7th day | 14th day | 21st day | |
| Normal control | 05.12 ± 1.45 | 6.25 ± 2.14 | 7.34 ± 2.34 |
| Diabetic control | -9.12 ± 2.15 $ | -7.82 ± 2.78 $ | -6.72 ± 5.88 $ |
| Diabetic + Glimepiride | 1.56 ± 1.24 | 5.00 ± 2.28 * | 7.56 ± 1.88 ** |
| Diabetic + EMZFP 150 | -7.57 ± 1.82 | -6.64 ± 1.25 | 6.00 ± 3.92 * |
| Diabetic + EMZFP 300 | -5.45 ± 2.10 | 5.10 ± 1.23* | 7.65 ± 1.92 ** |
| Diabetic + EMZFP 600 | -2.65 ± 2.73 | 6.50 ± 1.16 ** | 7.71 ± 2.13 ** |
Values were presented as mean ± SEM (n=6), $p < 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. A Significant change in body weight was observed in diabetic rats treated with EMZFP 300 and 600 mg kg-1 b. w.
Effect of EMZFP on serum levels of total cholesterol, HDL, LDL, and triglyceride
Diabetic rats treated with EMZFP showed some protective effects, dosing with EMZFP-300 mg kg-1 b.w. showed a significant reduction in serum LDL, and triglyceride (p<0.05) and a notable rise in serum HDL levels when compared with diabetic control rats. However, rats treated with EMZFP-600 mg kg-1 b.w. indicated more decline in serum cholesterol (p<0.05), LDL and triglyceride levels (p<0.01), and perfection in the serum HDL level (p<0.05) when compared with diabetic control rats (Table 5).
Table 5.
Effect of EMZFP on serum lipids in alloxan-induced diabetic rats
| Groups | Serum cholesterol (mg/dl) | Serum HDL (mg/dl) | Serum LDL (mg/dl) | Serum Triglyceride (mg/dl) |
|---|---|---|---|---|
| Normal control | 83.45 ± 5.47 | 72.24 ± 3.41 | 43.54 ± 3.14 | 108. 28 ± 6.94 |
| Diabetic control | 176.72 ± 8.28 $ | 40.28 ± 2.14 $ | 118.45 ± 5.47 $ | 164.48 ± 6.82 $ |
| Diabetic + Glimepiride | 142.57 ± 5.26 *** | 72.94 ± 2.42 *** | 85.78 ± 3.98 *** | 120.67 ± 5.29 *** |
| Diabetic + EMZFP 150 | 170.65 ± 3.12 | 62.45 ± 2.45 | 98.62 ± 2.72 | 158.72 ± 8.27 |
| Diabetic + EMZFP 300 | 161.49 ± 2.45 | 63.72 ± 1.87 | 93.48 ± 3.38 * | 139.40 ± 2.86 * |
| Diabetic + EMZFP 600 | 151.90 ± 3.57 * | 65.28 ± 2.65 * | 89.43 ± 2.69 ** | 136.28 ± 7.62 ** |
Values were expressed as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and **p < 0.001 when compared with diabetic control. EMZFP 600 mg kg-1 b.w. elucidated a significant decline in serum cholesterol, triglyceride, and LDL level, furthermore a significant perfection in serum HDL level.
Effect of fractions of EMZFP on serum levels of total cholesterol, HDL, LDL, and triglyceride
Untreated STZ-NA induced diabetic rats exhibited a substantial upsurge in serum cholesterol, LDL, and triglyceride whereas, a decrease in HDL level. Diabetic rats treated with EMZFP-Et elucidated a significant (p<0.01) decline in serum level of cholesterol (140.90 ± 3.55), LDL (85.52 ± 3.92), and triglyceride (160.54 ± 1.75) but an increase in serum HDL (60.15 ± 2.24) when compared with diabetic control group rats. However, the treatment with EMZFP-EA has recorded a significant decrease in serum cholesterol (144.94 ± 2.42) at p<0.05 while, for LDL (87.04 ± 2.86), and triglyceride (161.98 ± 2.18) at p<0.01, and for increment in serum HDL (58.24 ± 2.65) at p<0.05 (Table 6).
Table 6.
Effect of fractions of EMZFP on serum lipids in STZ-NA induced diabetic rats.
| Groups | Serum cholesterol (mg/dl) | Serum HDL (mg/dl) | Serum LDL (mg/dl) | Serum Triglyceride (mg/dl) |
|---|---|---|---|---|
| Normal control | 92.62 ± 1.42 | 71.32 ± 2.36 | 45.38 ± 2.78 | 107.09 ± 4.72 |
| Diabetic control | 161.54 ± 3.57 $ | 41.58 ± 2.48 $ | 105.75 ± 3.48 $ | 180.75 ± 3.37 $ |
| Diabetic + Glibenclamide | 130.82 ± 2.52 *** | 64.15 ± 1.87 *** | 79.37 ± 4.58 *** | 145.42 ± 3.67 *** |
| Diabetic + EMZFP-C 200 | 158.52 ± 2.89 | 55.82 ± 3.25 | 95.67 ± 2.57 | 170.58 ± 3.48 |
| Diabetic + EMZFP-A 200 | 152.35 ± 3.24 | 57.65 ± 1.24 | 89.28 ± 4.22 * | 164.20 ± 2.65 * |
| Diabetic + EMZFP-EA 200 | 144.94 ± 2.42 * | 58.24 ± 2.65 * | 87.04 ± 2.86 ** | 161.98 ± 2.18 ** |
| Diabetic + EMZFP-Et 200 | 140.90 ± 3.55 ** | 60.15 ± 2.24 ** | 85.52 ± 3.92 ** | 160.54 ± 1.75 ** |
(EMZFP-C: chloroform fraction; EMZFP-A: acetone fraction; EMZFP-EA: ethyl acetate fraction; EMZFP-Et: remaining ethanol fraction)
Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and **p < 0.001 when compared with diabetic control. Diabetic rats treated with Et and EA fractions demonstrated a significant reduction in serum levels of cholesterol, LDL. triglyceride and increment in serum HDL level.
Effect of EMZFP on cardiovascular risk indices
Convinced cardiovascular protective effects were recorded in diabetic rats when treated with EMZFP (Table 7). A noteworthy decrease in cardiovascular risk indices viz. atherogenic, cardiac and coronary artery indices were demonstrated at 150, 300, and 600 mg kg-1 b.w. doses of EMZFP extracts when compared with diabetic control group animals.
Table 7.
Effect of EMZFP on cardiovascular risk indices in alloxan-induced diabetic rats
| Groups | Cardio-vascular risk indices (mg/dl) | ||
|---|---|---|---|
| Atherogenic Index | Cardiac Index | Coronary Artery Index | |
| Normal control | 0.472 ± 0.34 | 1.485 ± 0.72 | 0.646 ± 0.58 |
| Diabetic control | 5.276 ± 0.74 $ | 6.247 ± 0.62 $ | 3.981 ± 0.47 # |
| Diabetic + Glimepiride | 1.245 ± 0.47 ** | 2.254 ± 0.87 ** | 0.849 ± 0.76 * |
| Diabetic + EMZFP 150 | 2.314 ± 0.78 | 3.824 ± 0.35 | 1.476 ± 0.34 |
| Diabetic + EMZFP 300 | 2.200 ± 0.27 * | 3.110 ± 0.58 * | 1.143 ± 0.79 |
| Diabetic + EMZFP 600 | 1.746 ± 0.26 ** | 2.700 ± 0.76 ** | 0.901 ± 0.610 * |
Data represented as mean ± SEM (n=6), $p < 0.001, #p< 0.05 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control.
A significant reduction in cardiovascular risk indices viz. atherogenic, cardiac and coronary artery indices were demonstrated by EMZFP 600 mg kg-1 b.w. doses.
Effect of fractions of EMZFP on cardiovascular risk indices
Treatments of diabetic rats with the fractions of EMZFP were demonstrated cardiovascular protective effects when assessed for cardiovascular risk indices viz. atherogenic, cardiac, and coronary artery indices. EMZFP-EA fraction showed a notable (p < 0.05) decrease in cardiovascular risk indices compared to diabetic control rats. EMZFP-Et fraction demonstrated a significant (p < 0.05) reduction in cardiac and coronary artery index while, highly significant (p < 0.01) reduction in the atherogenic index when compared with diabetic control group animals (Table 8).
Table 8.
Effect of fractions of EMZFP on cardiovascular risk indices in STZ-NA induced diabetic rats.
| Groups | Cardio-vascular risk indices (mg/dl) | ||
|---|---|---|---|
| Atherogenic Index | Cardiac Index | Coronary Artery Index | |
| Normal control | 0.742 ± 0.65 | 1.511 ± 0.48 | 0.752 ± 0.37 |
| Diabetic control | 4.772 ± 0.46 $ | 5.010 ± 0.27 # | 4.343 ± 0.82 $ |
| Diabetic + Glibenclamide | 1.000 ± 0.64 ** | 1.612 ± 0.64 * | 0.912 ± 0.62 * |
| Diabetic + EMZFP-C 200 | 2.558 ± 0.38 | 3.388 ± 0.94 | 1.627 ± 0.78 |
| Diabetic + EMZFP-A 200 | 2.272 ± 0.75 | 2.268 ± 0.25 | 1.375 ± 0.61 |
| Diabetic + EMZFP-EA 200 | 1.601 ± 0.87 * | 1.813 ± 0.37 * | 1.200 ± 0.58 * |
| Diabetic + EMZFP-Et 200 | 1.201 ± 0.59 ** | 1.715 ± 0.72 * | 1.127 ± 0.23 * |
(EMZFP-C: chloroform fraction; EMZFP-A: acetone fraction; EMZFP-EA: ethyl acetate fraction; EMZFP-Et: remaining ethanol fraction)
All data represented as mean ± SEM (n=6), $p < 0.01, #p< 0.05 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Diabetic rats treated with Et and EA fractions elucidated a significant reduction in cardiovascular risk indices.
Assessment of the nephroprotective effect
After induction of diabetes in diabetes control group animals, a significant (p<0.001) decrease in serum total protein and albumin, while a noticeable (p<0.01) increase in serum creatinine was observed. However, diabetic animals when treated with EMZFP-600 mg kg-1 b.w., a significant (p<0.05) rise in serum total protein and albumin, and a decrease in serum creatinine was observed. Whereas, EMZFP-300 mg kg-1 b.w., administration recorded a considerable (p<0.05) decrease in serum creatinine, when compared with diabetic control group animals (Table 9).
Table 9.
Effect of EMZFP on kidney parameters in alloxan-induced diabetic rats
| Groups | Serum Total Protein (g/dl) | Serum Albumin (g/dl) | Serum Creatinine (mg/dl) |
|---|---|---|---|
| Normal control | 5.64 ± 0.87 | 6.15 ± 0.48 | 0.94 ± 0.36 |
| Diabetic control | 1.41 ± 0.55 $ | 2.14 ± 0.26 $ | 4.52 ± 0.39 # |
| Diabetic + Glimepiride | 5.10 ± 0.34 ** | 5.98 ± 0.37 ** | 1.28 ± 0.64 * |
| Diabetic + EMZFP 150 | 3.82 ± 0.28 | 3.18 ± 0.72 | 1.77 ± 0.87 |
| Diabetic + EMZFP 300 | 4.24 ± 0.72 | 4.85 ± 0.82 | 1.52 ± 0.72 * |
| Diabetic + EMZFP 600 | 4.62 ± 0.18 * | 5.33 ± 0.67 * | 1.40 ± 0.58 * |
Data represented as mean ± SEM (n=6), $p < 0.001, #p< 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Diabetic rats treated with EMZFP 600 mg kg-1 b.w. demonstrated a significant increment in serum total protein, albumin, and reduction in serum creatinine.
Histopathological findings of kidneys for control and EMZFP treated rats
Earlier histological investigations of kidneys reveal that prolonged DM may encourage severe pathological alterations, such as changes in kidney tubule segments, hyalinization, changes in the mesangial matrix, and nephritis (glomerulosclerosis). The untreated diabetic animals showed significant scripts of disruptions of kidney tubule segments with hyalinization and nephritis. However, histopathological findings from kidneys of diabetic animals treated with glimepiride and EMZFP-300, and EMZFP-600 showed the well-conserved segments of kidney tubules with a clear picture of interspersed blood capillaries. All the architecture of kidneys appears to be normal, with no signs of any histological findings for hyalinization, changes in the mesangial matrix, and nephritis (Photomicrograph 1).
Fig. 12.
Photomicrographs (100×) showing effects on 21 days administration (p.o.) of EMZFP on histoarchitecture of kidney in alloxan-induced diabetic rats. A) Control group: depicted normal glomerulus architecture. B) Diabetic control group: abnormal glomerulus appearance. C) Diabetic + Glimepiride group: glomerular capillaries appear to be normal, with no signs of hyalinization, changes in the mesangial matrix, and nephritis. D) Diabetic + EMZFP-150 group: shows few signs of hyalinization and glomerulosclerosis. E) Diabetic + EMZFP-300 and F) Diabetic + EMZFP-600 group: picture suggests normal histoarchitecture with an absence of matrix expansion and no marks of hyalinization and nephritis.
Analysis of liver glycogen
Untreated diabetic rats were observed with a significant (p<0.01) low liver glycogen content as compared to normal control group rats. While rats treated with EMZFP-600 mg kg-1 b.w. showed a significant (p<0.05) increase (14. 65 ± 0.47mg/100g) in liver glycogen content when compared with diabetic control group rats (Table 10).
Table 10.
Analysis of liver glycogen content in alloxan-induced diabetic rats post-treatment with EMZFP
| Groups | Liver Glycogen (mg/100 g) |
|---|---|
| Normal control | 16.25 ± 0.57 |
| Diabetic control | 11.18 ± 0.67 $ |
| Diabetic + Glimepiride | 15.17 ± 1.13* |
| Diabetic + EMZFP 150 | 12.64 ± 0.68 |
| Diabetic + EMZFP 300 | 13.92 ± 1.08 |
| Diabetic + EMZFP 600 | 14. 65 ± 0.47 * |
Diabetic rats when treated with EMZFP 600 mg kg-1 b.w. elucidated a significant increment in liver glycogen content. Values were presented as mean ± SEM (n=6), $ p < 0.01 when compared with normal control; *p < 0.05 when compared with diabetic control.
Effect of EMZFP extracts and its fractions on hot plate induced neuropathic pain in diabetic rats
Latency to foot licking was recorded and statistically analyzed for all grouped animals. A decreased sensitivity towards the thermal stimuli was observed with untreated diabetic control group rats. Alloxan-induced diabetic rats when treated with EMZFP-600 mg kg-1b.w. demonstrated a significant (p<0.01) improvement in pain sensitivity by licking the foot at an average time of 6.7 s from individual two readings (Table 11). However, STZ-NA induced diabetic rat’s treatment with EMZFP-EA and EMZFP-Et elucidate a significant (p<0.05) improvement in pain sensitivity by licking the foot at an average time of 6.8 s and 6.6 s from individual two readings respectively (Table 12).
Table 11.
Effect of EMZFP extracts on hot plate induced neuropathic pain in alloxan induced diabetic rats
| Groups | Foot licking response(s) |
|---|---|
| Normal control | 4.2 ± 0.5 |
| Diabetic control | 8.9 ± 0.2 $ |
| Diabetic + Glimepiride | 6.3 ± 0.4 ** |
| Diabetic + EMZFP 150 | 8.1 ± 0.5 |
| Diabetic + EMZFP 300 | 7.1 ± 0.3 * |
| Diabetic + EMZFP 600 | 6.7 ± 0.5 ** |
Values were presented as mean ± SEM (n=6), $ p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control.
Diabetic rats when treated with EMZFP 600 mg kg-1 b.w. were demonstrated a significant improvement in pain sensitivity.
Table 12.
Effect of fractions of EMZFP extracts on hot plate induced neuropathic pain in STZ-NA induced diabetic rats
| Groups | Foot licking response(s) |
|---|---|
| Normal control | 4.5 ± 0.4 |
| Diabetic control | 8.7 ± 0.3 $ |
| Diabetic + Glibenclamide | 6.9 ± 0.5 * |
| Diabetic + EMZFP-C 200 | 8.5 ± 0.3 |
| Diabetic + EMZFP-A 200 | 7.8 ± 0.3 |
| Diabetic + EMZFP-EA 200 | 6.8 ± 0.5 * |
| Diabetic + EMZFP-Et 200 | 6.6 ± 0.4 * |
(EMZFP-C: chloroform fraction; EMZFP-A: acetone fraction; EMZFP-EA: ethyl acetate fraction; EMZFP-Et: remaining ethanol fraction)
Diabetic rats when treated with Et and EA fractions were revealed a significant improvement in pain sensitivity. Values were presented as mean ± SEM (n=6), $ p < 0.001 when compared with normal control; *p < 0.05 when compared with diabetic control.
Discussion
The folkloric treatment with the use of ethnobotanicals has an extensive history for treating diabetes and thus it is continued to be an area of research for finding efficacious natural antidiabetic agents. Owing to enriched variety of phytocontents and antioxidant principles in EMZFP [51] it was selected to investigate its in-vivo antidiabetic potentials by evaluating various biochemical diabetic parameters and complications.
Insulin receptor or post-receptor defects with insulin resistance are a characteristic of T2DM. Various researchers reported that STZ-NA induced T2DM model is more suitable for evaluating the antidiabetic potentials of both pharmacological and natural plant sources on the course of DM [52]. Furthermore, this model has certain features like stable moderate hyperglycemia without any exogenous insulin to survive, glucose intolerance, and impairment in insulin secretion by glucose stimulation, reduction in pancreatic insulin stores, pancreatic β-cell reduction, receptiveness to sulphonylureas- glibenclamide [53].
Treatment with EMZFP for 21 days in alloxan-induced diabetic rats demonstrated significant dose-dependent serum glucose-lowering effects by EMZFP 600 mg kg-1 b.w. p.o. when compared with diabetic untreated rats (Fig. 1). Similarly, with 200 mg kg-1 b.w. p.o. doses of fractions EMZFP-Et and EMZFP-EA were also elucidated significant antihyperglycemic effects (Fig. 2). However, the blood glucose level in glimepiride and glibenclamide treated rats was lower than all EMZFP and its fractions treated groups. The gallic and ellagic acid [25, 26] had been accountable for enhanced uptake of glucose, α-glucosidase enzyme inhibition, lipid peroxidation, reduction of oxidative stress [54] and regeneration of β-cells and GLUT4 mediated glucose uptake [55]. Also, much literature had documented that gallic acid on oral administration in both the STZ and alloxan-induced diabetic animals had significantly reduced the blood glucose level, creatinine, and an increment in protein content [56, 57]. As MZFP possesses gallic and ellagic acid together with other rich variety of phytoconstituents like p-hydroxybenzoic acid, catechin, epicatechin, quercetin, etc., which might have enhanced the glucose tolerance, regulated carbohydrate metabolism key pathways, or insulin secretion. Thus, perfection in glycemic control and improvement in insulin secretion mechanism of remnant β-cells in diabetic animals might have lowered blood glucose level.
Fig. 1.
Effect of EMZFP on blood glucose level in alloxan-induced diabetic rats. Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with diabetic control. Diabetic animals treated with EMZFP 600 showed a significant reduction in the blood glucose levels (p < 0.01) from14th day onwards.
Fig. 2.
Effect of fractions of EMZFP on blood glucose level in STZ-NA induced diabetic rats. Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and ***p < 0.001 when compared with diabetic control. Diabetic animals treated with EMZFP-Et 200 showed a significant reduction in the blood glucose levels (p < 0.01) on 21st day.
Hyperglycemia in diabetic animals corresponds to failure in weight gain during 21 days study period. While, EMZFP 300 and 600 mg kg-1 b.w. treated diabetic rats demonstrated a significant weight gain slightly more than normal control rats (Fig. 3) which might be due to improvement in the synthesis of a structural protein and glycemic control [58].
Fig. 3.
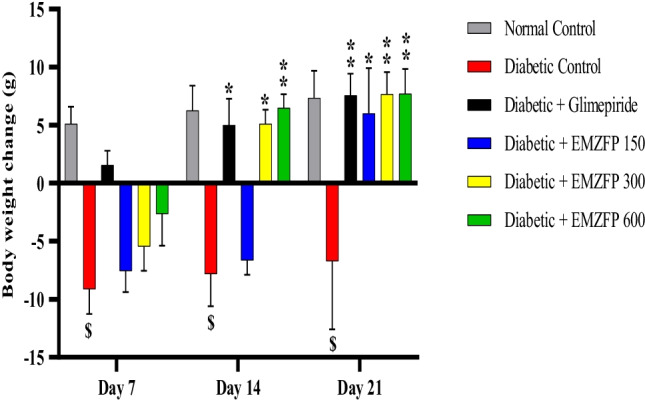
Effect of EMZFP on body weight changes in alloxan-induced diabetic rats. Bars showed with different superscripts for every profile are significantly different. Values were expressed as mean ± SEM (n=6), $p < 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control. Diabetic rats treated with EMZFP 300 and 600 mg kg-1 b.w. showed a significant change in body weight.
Hypertriglyceridemia and hypercholesterolemia are some of the etiological factors in DM complications like coronary heart diseases, atherosclerosis, and acute pancreatitis [59]. Depletion in insulin, grounds for insufficiency in lipoprotein lipase activity which considerably contributes to the triglycerides increment in DM [45]. While, hyperlipidemia contributes to a rise in cholesterol, triglycerides, LDL, VLDL, and a fall in HDL levels [60]. However, assessment of EMZFP extract and its fraction for dyslipidemia had demonstrated a significant fall in serum levels of cholesterol, triglycerides, LDL, and rise in HDL when treated with EMZFP-600 mg kg-1 b.w. (Fig. 4) and EMZFP-Et, EMZFP-EA respectively (Fig. 5). It is well postulated and has been reported by many researchers that, owing to impaired glucose metabolizing pathways leads to the generation of free radicals and progression in DM, furthermore; which can be well repealed by phytochemicals having worthy antioxidant stuff [61]. Thus, it is sensible to accomplish that the antioxidants [62] or active phytoconstituents in the EMZFP, EMZFP-EA, and remaining EMZFP-Et fractions normalized the serum lipids possibly by perfecting hydrolysis, selective uptake, and metabolism of certain lipoprotein, and attenuating lipid peroxidation [63]. Literature findings also suggested that within the intestine or in the liver a possibility of merging of flavonoids into lipoprotein and later ward it is conceded within lipoprotein particle. Thus flavonoids may perhaps preferably shield LDL from oxidation [64].
Fig. 4.

Effect of EMZFP on serum lipids in alloxan-induced diabetic rats. Bars showing different superscripts for every profile are significantly different. EMZFP 600 mg kg-1 b.w. elucidated a significant reduction in serum cholesterol, triglyceride, and LDL level, furthermore a significant enhancement in serum HDL level. Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p< 0.05, **p< 0.01, and ***p< 0.001 when compared with diabetic control.
Fig. 5.

Effect of fractions of EMZFP on serum lipids in STZ-NA induced diabetic rats. Bars showing different superscripts for every profile are significantly different. Diabetic rats treated with Et and EA fractions demonstrated a significant reduction in serum levels of cholesterol, LDL. triglyceride and increment in serum HDL level. Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control; *p < 0.05, **p < 0.01, and **p < 0.001 when compared with diabetic control.
Hyperlipidemia is one of the etiological factors for the progression of atherosclerosis under lipid peroxidation [65] however; prolonged hyperglycemia accelerates glycation and oxidation of small dense LDL particles, which leads to increases in the possibility of cardiovascular complications [66]. Treatment with EMZFP-600 mg kg-1 b.w (Fig. 6) and its fractions EMZFP-EA and EMZFP-Et have elucidated a more significant reduction in atherogenic, cardiac and coronary artery indices (Fig. 7). The direct possible mechanism for these observations may account for the enriched antioxidants and above-discussed phytoconstituents liable for repletion of hyperglycemia and hyperlipidemia, along with phenolic compounds, such as quercetin and kaempferol [67] present in MZFP.
Fig. 6.

Effect of EMZFP on cardio vascular risk indices in alloxan-induced diabetic rats. Bars showing different superscripts for every profile are significantly different. Diabetic rats treated with EMZFP 600 mg kg-1 b. w. demonstrated a significant reduction in cardiovascular risk indices viz. atherogenic, cardiac and coronary artery indices. Values were presented as mean ± SEM (n=6), $p < 0.001, #p< 0.05 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control
Fig. 7.

Effect of fractions of EMZFP on cardio vascular risk indices in STZ-NA induced diabetic rats. Effect of fractions of EMZFP on cardio vascular risk indices in STZ-NA induced diabetic rats treated for 21 days. Bars showing different superscripts for every profile are significantly different. Diabetic rats treated with Et and EA fractions demonstrated a significant reduction in cardiovascular risk indices. Values were presented as mean ± SEM (n=6), $p < 0.01, #p< 0.05 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control.
Progressive proteinuria signifies the reduction in total protein content in diabetic untreated rats [68]. In diabetic nephropathy, accumulation of urea nitrogen and an increment in uric acid is responsible for renal dysfunction with glomerulosclerosis [69]. Diabetic rats when treated with EMZFP 600 mg kg-1 b.w. had demonstrated a significant improvement in kidney parameters like serum protein, albumin, and decline in the serum creatinine level (Fig. 8), which was supported by histological findings from the kidneys showing evidence of reversal of glomerulosclerosis (Photomicrograph 1).
Fig. 8.

Effect of EMZFP on kidney parameters in alloxan-induced diabetic rats. Bars showing different superscripts for every profile are significantly different. Diabetic rats treated with EMZFP 600 mg kg-1 b.w. demonstrated a significant increment in serum total protein, albumin, and reduction in serum creatinine. Values were expressed as mean ± SEM (n=6), $p < 0.001, #p< 0.01 when compared with normal control; *p < 0.05, **p < 0.01 when compared with diabetic control.
In diabetic state defects in activation of synthase, phosphatase is responsible for depletion in glycogen content of liver and skeleton muscles [70]. After treatment with EMZFP-600 mg kg-1 b.w. to the diabetic rats showed a marked increase in liver glycogen elucidating that imperfect glycogen storage of diabetic state was moderately improved (Fig. 9). This effect may be attributable to the perfection in the activation of synthase phosphatase due to an increment in insulin response promoting glycogenesis and/or decline in hepatic glycogenolysis [71].
Fig. 9.

Analysis of liver glycogen content in alloxan-induced diabetic rats post-treatment of EMZFP. Analysis of liver glycogen content in alloxan-induced diabetic rats post-treatment of EMZFP extracts for 21 days. Bar superscripts indicates a significance difference. Values were presented as mean ± SEM (n=6), $ p < 0.01 when compared with normal control, *p < 0.05 when compared with diabetic control. A significant rise in liver glycogen content was observed when diabetic rats treated with EMZFP-600 were compared with diabetic control group rats.
Prolonged hyperglycemia increases the possibilities of diabetic neuropathy and is clinically intervened by nontraumatic lower-limb amputations in diabetic patients. Numerous data from preclinical and clinical studies stated the pathogenesis of diabetic peripheral neuropathy signifying the key role of hyperglycemia persuaded advanced glycation end products (AGEs) [72]. The non-enzymatic reaction of methylglyoxal and interaction of elevated glucose with lipids, nucleic acids, and proteins is responsible for the generation of AGEs [73]. Decreased antioxidant defenses, increase in AGEs, altered glucose metabolism, disruption of the polyol pathway, and oxidative stress are some illustrative associated contrivances in the pathogenesis of peripheral neuropathy [74]. Assessment of EMZFP and its fractions for diabetic peripheral neuropathic complications by the hot plate method demonstrated that, EMZFP-600 mg kg-1 b. w., (Fig. 10) EMZFP-EA and EMZFP-Et (Fig. 11) significantly lowered the foot licking response time and elucidated peripheral nerve protective property. EMZFP had a considerable antioxidant effect and radical-scavenging properties [29] which may account for protection against oxidative cell damage [75]. Thus, the amelioration in peripheral diabetic neuropathic pain by this extract and its fraction might be due to a part or large extent of polyphenolic and flavonoid contents and to the free radical scavenging or antioxidant potentials of Manilkara zapota peel.
Fig. 10.

Effect of EMZFP extracts on hot plate induced neuropathic pain in alloxan-induced diabetic rats. Effect of EMZFP extracts on hot plate induced neuropathic pain in alloxan-induced diabetic rats. Bar superscripts indicates a significance difference; Values were presented as mean ± SEM (n=6), $p < 0.001 when compared with normal control, *p < 0.05, **p < 0.01 when compared with diabetic control. A significant improvement in pain sensation was observed when diabetic rats treated with EMZFP-300 and EMZFP-600 was compared with diabetic control group rats.
Fig. 11.

Effect of fractions of EMZFP extracts on hot plate induced neuropathic pain in STZ-NA induced diabetic rats. Effect of fractions of EMZFP extracts on hot plate induced neuropathic pain in STZ-NA induced diabetic rats. Bar superscripts indicates a significance difference; Values represented as mean ± SEM (n=6), $p < 0.001 when compared with normal control, *p < 0.05 when compared with diabetic control. A significant improvement in pain sensation was observed, when diabetic rats treated with EMZFP-EA 200 and EMZFP- Et 200 were compared with diabetic control group rats.
Conclusions
Rats when treated with EMZFP, a noteworthy antihyperglycemic effects with augmentation in liver glycogen content, perfection in lipid profile and cardiovascular risk indices, enhancement in body weight, nephroprotective effects, beneficial effects in peripheral neuropathy, and histopathological evidence of reversal of glomerulosclerosis were observed. Although these results were not similar to that glimepiride effect, gradual positive augmentation in diabetic parameters is indicative of antidiabetic potentials of this peel extract when evaluated in the alloxan-induced diabetic rat model. However, during the antidiabetic evaluation of the EMZFP fractions in the STZ-NA induced diabetic rat model, EMZFP-EA and EMZFP-Et fractions contributed significant antihyperglycemic effects with perfection in lipid profile, cardiovascular risk indices, and positive peripheral neuropathic effects.
These preliminary study findings provide a basis for future research, where isolated phytoconstituents from this fruit peel should further be comprehensively evaluated pharmacologically and biochemically in exploring mechanism(s) for the antidiabetic outcome of this fruit peel. Further preclinical findings with isolated compounds could be supporting the traditional herbal medicine system for counting Manilkara zapota fruit peels in the dietary management of DM and likely prevention of its impediments.
Declaration of interest
The authors report no declarations of interest.
Consent for publication
Not applicable
Acknowledgments
The authors are thankful to the research center, School of Pharmacy and RUSA Centre for herbo medicinal studies at S.R.T.M. University, Nanded, India. Sincerely acknowledges S. N. Institute of Pharmacy, Pusad, India for providing laboratory animal research facilities. The authors are grateful to the Botanical Survey of India, Pune, India, for identification and authentication of plant material.
Abbreviations
- i.p.
Intraperitoneal
- p.o.
Administered orally
- b.w.
Body weight
- STZ
Streptozotocin
- NA
Nicotinamide
- MZFP
Manilkara zapota (L.) P. Royen fruit peel
- EMZFP
70% ethanolic extract of Manilkara zapota (L.) P. Royen fruit peel
- EMZFP-C
Chloroform fraction of EMZFP
- EMZFP-A
Acetone fraction of EMZFP
- EMZFP-EA
Ethyl acetate fraction of EMZFP
- EMZFP-Et
Remaining ethanol fraction of EMZFP
Author contribution
KPP: Conceptualization, methodology, investigation, data interpretation, editing & writing original draft. DSC: Methodology supervision. NVV: Formal analysis. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Availability of data on reasonable request to corresponding author
Declaration
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szkudelski T. Streptozotocin–nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med. 2012; 237(5):481–490. 10.1258/ebm.2012.011372 [DOI] [PubMed]
- 2.Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008;46(7):2376–83. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.El-Tantawy WH. Nutrition in the management of type 2 diabetes mellitus. Arch Physiol Biochem. 2019;28:1–8. doi: 10.1080/13813455.2019.1657899. [DOI] [PubMed] [Google Scholar]
- 4.Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47(10):2679–85. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Elangovan A, Subramanian A, Durairaj S, Ramachandran J, Lakshmanan DK, Ravichandran G, Nambirajan G, Thilagar S. Antidiabetic and hypolipidemic efficacy of skin and seed extracts of Momordica cymbalaria on alloxan induced diabetic model in rats. J Ethnopharmacol. 2019;15(241):111989. doi: 10.1016/j.jep.2019.111989. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nyström T, Norhammar A. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabetes Res Clin Pract. 2016;1(117):39–47. doi: 10.1016/j.diabres.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 7.Chinsembu KC. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J Herb Med. 2019;1(15):100230. doi: 10.1016/j.hermed.2018.09.001. [DOI] [Google Scholar]
- 8.Sun W, Zeng C, Liao L, Chen J, Wang Y. Comparison of acarbose and metformin therapy in newly diagnosed type 2 diabetic patients with overweight and/or obesity. Curr Med Res Opin. 2016;32(8):1389–96. doi: 10.1080/03007995.2016.1176013. [DOI] [PubMed] [Google Scholar]
- 9.Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2016;1(82):93–100. doi: 10.1016/j.bone.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB. Diabetes incidence and glucose tolerance after termination of pioglitazone therapy: results from ACT NOW. J Clin Endocrinol Metab. 2016;101(5):2056–62. doi: 10.1210/jc.2015-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanakis CM. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci Technol. 2012;26(2):68–87. doi: 10.1016/j.tifs.2012.03.003. [DOI] [Google Scholar]
- 12.Choudhary SK, Chhabra G, Sharma D, Vashishta A, Ohri S, Dixit A. Comprehensive evaluation of anti-hyperglycemic activity of fractionated Momordica charantia seed extract in alloxan-induced diabetic rats. Evid Based Complement Alternat Med. 2012;1:2012. doi: 10.1155/2012/293650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomathy K, Baskar R, Kumaresan K. Comparison of antioxidant potential in pulp and peel extracts of Manilkara zapota (L.) P. Royen. Afr J Biotechnol. 2013; 12(31):4936–4943.
- 14.Singh B, Singh JP, Kaur A, Singh N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res Int. 2017;1(101):1–6. doi: 10.1016/j.foodres.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22(5):749–60. doi: 10.1016/S0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 16.Xiao J, Capanoglu E, Jassbi AR, Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr. 2016;56(sup1):S29–45. doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit Rev Food Sci Nutr. 2017;57(9):1874–905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- 18.Khan H, Jawad M, Kamal MA, Baldi A, Xiao J, Nabavi SM, Daglia M. Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem Toxicol. 2018;1(119):355–67. doi: 10.1016/j.fct.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Yen GC, Duh PD, Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem. 1993;41(1):67–70. doi: 10.1021/jf00025a015. [DOI] [Google Scholar]
- 20.Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, Weinstein IB, Kennelly EJ. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla) J Nat Prod. 2003;66(7):983–6. doi: 10.1021/np020576x. [DOI] [PubMed] [Google Scholar]
- 21.Pontes PV, Moreira RF, Trugo LC, Maria CA. The content of chlorogenic acids in tropical fruits. J Sci Food Agric. 2002;82(10):1177–81. doi: 10.1002/jsfa.1163. [DOI] [Google Scholar]
- 22.da Silva LM, De Figueiredo EA, Ricardo NM, Vieira IG, De Figueiredo RW, Brasil IM, Gomes CL. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;15(143):398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Cmaj. 2000;163(6):739–44. [PMC free article] [PubMed] [Google Scholar]
- 24.Porrini M, Riso P, Brusamolino A, Berti C, Guarnieri S, Visioli F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br J Nutr. 2005;93(1):93–9. doi: 10.1079/BJN20041315. [DOI] [PubMed] [Google Scholar]
- 25.Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–66. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Can-Cauich CA, Sauri-Duch E, Betancur-Ancona D, Chel-Guerrero L, González-Aguilar GA, Cuevas-Glory LF, Pérez-Pacheco E, Moo-Huchin VM. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J Funct Foods. 2017;1(37):501–6. doi: 10.1016/j.jff.2017.08.028. [DOI] [Google Scholar]
- 27.Ahmed OM, Hassan MA, Abdel-Twab SM, Azeem MN. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother. 2017;1(94):197–205. doi: 10.1016/j.biopha.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 28.González-Molina E, Domínguez-Perles R, Moreno DA, García-Viguera C. Natural bioactive compounds of Citrus limon for food and health. J Pharm Biomed Anal. 2010;51(2):327–45. doi: 10.1016/j.jpba.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Karle PP, Dhawale SC, Navghare VV, Shivpuje SS. Optimization of extraction conditions and evaluation of Manilkara zapota (L.) P. Royen fruit peel extract for in vitro α-glucosidase enzyme inhibition and free radical scavenging potential. Futur J Pharm Sci. 2021;7(1):1. doi: 10.1186/s43094-021-00305-4. [DOI] [Google Scholar]
- 30.Woo PF, Yim HS, Khoo HE, Sia CM, Ang YK. Effects of extraction conditions on antioxidant properties of sapodilla fruit (Manilkara zapota) Int Food Res J. 2013;20(5):2065. [Google Scholar]
- 31.Duh PD, Yen GC. Antioxidant efficacy of methanolic extracts of peanut hulls in soybean and peanut oils. J Am Oil Chem Soc. 1997;74(6):745. doi: 10.1007/s11746-997-0212-z. [DOI] [Google Scholar]
- 32.Gini TG, Jeya Jothi G. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt J Basic Appl Sci. 2018;5(3):197–203. doi: 10.1016/j.ejbas.2018.05.010. [DOI] [Google Scholar]
- 33.Primarianti AU, Sujono TA. Antidiabetic activity of durian (Durio zibethinus Murr.) and rambutan (Nephelium lappaceum L.) fruit peels in alloxan diabetic rats. Procedia Food Sci. 2015;3:255–61. doi: 10.1016/j.profoo.2015.01.028. [DOI] [Google Scholar]
- 34.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22(4):359–70. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramya S, Narayanan V, Ponnerulan B, Saminathan E, Veeranan U. Potential of peel extracts of Punica granatum and Citrus aurantifolia on alloxan-induced diabetic rats. Beni-Suef Univ J Basic Appl Sci. 2020;9(1):1–1. doi: 10.1186/s43088-020-00049-9. [DOI] [Google Scholar]
- 36.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 37.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107(2):285–90. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Azad AK, Sulaiman WM. Antidiabetic effects of P. macrocarpa ethanolic fruit extract in streptozotocin-induced diabetic rats. Futur J Pharm Sci. 2020;6(1):1–2. doi: 10.1186/s43094-020-00073-7. [DOI] [Google Scholar]
- 39.Macdonald Ighodaro O, Mohammed Adeosun A, Adeboye Akinloye O. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina. 2017;53(6):365–74. doi: 10.1016/j.medici.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal R, Sethiya NK, Mishra SH. Antidiabetic activity of alkaloids of Aerva lanata roots on streptozotocin-nicotinamide induced type-II diabetes in rats. Pharm Biol. 2013;51(5):635–42. doi: 10.3109/13880209.2012.761244. [DOI] [PubMed] [Google Scholar]
- 41.Chika A, Bello SO. Antihyperglycaemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 2010;129(1):34–7. doi: 10.1016/j.jep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Bora NS, Bairy PS, Salam A, Kakoti BB. Antidiabetic and antiulcerative potential of Garcinia lanceifolia Roxb. bark. Futur J Pharm Sci. 2020;6(1):1–1. doi: 10.1186/s43094-020-00101-6. [DOI] [Google Scholar]
- 43.Nagarajan NS, Murugesh N, Kumaresan PT, Radha N, Murali A. Antidiabetic and antihyperlipemic effects of Clemeo felina. Fitoterapia. 2005;76(3–4):310–5. doi: 10.1016/j.fitote.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med. 2011;4(11):900–3. doi: 10.1016/S1995-7645(11)60215-0. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava AK, Mukerjee A, Tripathi A. Antidiabetic and antihyperlipidemic activities of Cucumis melo var. momordica fruit extract on experimental animals. Futur J Pharm Sci. 2020;6(1):1–9. doi: 10.1186/s43094-020-00116-z. [DOI] [Google Scholar]
- 46.Arunachalam K, Parimelazhagan T. Antidiabetic activity of Ficus amplissima Smith. bark extract in streptozotocin induced diabetic rats. J Ethnopharmacol. 2013;147(2):302–10. doi: 10.1016/j.jep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Soussi A, Gargouri M, El Feki A. Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicol Ind Health. 2018;34(7):469–80. doi: 10.1177/0748233718770293. [DOI] [PubMed] [Google Scholar]
- 48.Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956;220(2):583–93. doi: 10.1016/S0021-9258(18)65284-6. [DOI] [PubMed] [Google Scholar]
- 49.Kang MJ, Lee EK, Lee SS. Effects of two P/S ratios with same peroxidizability index value and antioxidants supplementation on serum lipid concentration and hepatic enzyme activities of rats. Clin Chim Acta. 2004;350(1–2):79–87. doi: 10.1016/j.cccn.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Boyce-Rustay JM, Jarvis MF. Neuropathic pain: models and mechanisms. Curr Pharm Des. 2009;15(15):1711–6. doi: 10.2174/138161209788186272. [DOI] [PubMed] [Google Scholar]
- 51.Pravin KP, Shashikant DC. Manilkara zapota (L.) Royen fruit peel: a phytochemical and pharmacological review. Syst Rev Pharm. 2019;10(1):11–4. doi: 10.5530/srp.2019.1.2. [DOI] [Google Scholar]
- 52.Weng Y, Yu L, Cui J, Zhu YR, Guo C, Wei G, Duan JL, Yin Y, Guan Y, Wang YH, Yang ZF. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J Ethnopharmacol. 2014;152(3):553–60. doi: 10.1016/j.jep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol Hung. 2014;101(4):408–20. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- 54.Arun KB, Jayamurthy P, Anusha CV, Mahesh SK, Nisha P. Studies on activity guided fractionation of pomegranate peel extracts and its effect on antidiabetic and cardiovascular protection properties. J Food Process Preserv. 2017;41(1):e13108. doi: 10.1111/jfpp.13108. [DOI] [Google Scholar]
- 55.Gandhi GR, Jothi G, Antony PJ, Balakrishna K, Paulraj MG, Ignacimuthu S, Stalin A, Al-Dhabi NA. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014;15(745):201–16. doi: 10.1016/j.ejphar.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 56.Ramkumar KM, Vijayakumar RS, Vanitha P, Suganya N, Manjula C, Rajaguru P, Sivasubramanian S, Gunasekaran P. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum Exp Toxicol. 2014;33(6):638–49. doi: 10.1177/0960327113504792. [DOI] [PubMed] [Google Scholar]
- 57.Garud MS, Kulkarni YA. Gallic acid attenuates type I diabetic nephropathy in rats. Chem Biol Interact. 2018;25(282):69–76. doi: 10.1016/j.cbi.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem Biol Interact. 2009; 182(1):67–72. 10.1016/j.cbi.2009.08.012 [DOI] [PubMed]
- 59.Mollazadeh H, Mahdian D, Hosseinzadeh H. Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. Phytomedicine. 2019;1(53):43–52. doi: 10.1016/j.phymed.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Udenze EC, Braide VB, Okwesilieze CN, Akuodor GC. Pharmacological effects of Garcinia kola seed powder on blood sugar, lipid profile and atherogenic index of alloxan-induced diabetes in rats. Pharmacologia. 2012;3(12):693–9. doi: 10.5567/pharmacologia.2012.693.699. [DOI] [Google Scholar]
- 61.Kapoor R, Singh S, Tripathi M, Bhatnagar P, Kakkar P, Gupta KC. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PloS One. 2014;9(2):e89124. doi: 10.1371/journal.pone.0089124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathe D. Dyslipidemia and diabetes: animal models. Diabete Metab. 1995;21(2):106–11. [PubMed] [Google Scholar]
- 63.Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006;98(1):97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 64.Mahmoud AM, Ahmed OM, Ashour MB, Abdel-Moneim A. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int J Diabetes Dev Countries. 2015;35(3):250–63. doi: 10.1007/s13410-014-0268-x. [DOI] [Google Scholar]
- 65.Rajanandh MG, Satishkumar MN, Elango K, Suresh B. Moringa oleifera Lam. A herbal medicine for hyperlipidemia: A pre–clinical report. Asian Pac J Trop Dis. 2012;2:S790–5. doi: 10.1016/S2222-1808(12)60266-7. [DOI] [Google Scholar]
- 66.Ferretti G, Rabini RA, Bacchetti T, Vignini A, Salvolini E, Ravaglia F, Curatola G, Mazzanti L. Glycated low density lipoproteins modify platelet properties: a compositional and functional study. J Clin Endocrinol Metab. 2002;87(5):2180–4. doi: 10.1210/jcem.87.5.8466. [DOI] [PubMed] [Google Scholar]
- 67.Metwally FM, Rashad HM, Ahmed HH, Mahmoud AA, Raouf ER, Abdalla AM. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac J Trop Biomed. 2017;7(3):214–21. doi: 10.1016/j.apjtb.2016.12.007. [DOI] [Google Scholar]
- 68.Latha RC, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact. 2011;189(1–2):112–8. doi: 10.1016/j.cbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Salil G, Nevin KG, Rajamohan T. Effect of dietary coconut kernel protein on the liver and pancreas of alloxan-induced diabetic rats: comparison with L-arginine and glibenclamide. Mediterr J Nutr Metab. 2012;5(2):127–33. doi: 10.3233/s12349-012-0090-2. [DOI] [Google Scholar]
- 70.Grover JK, Vats V, Yadav S. Effect of feeding aqueous extract of Pterocarpus marsupium on glycogen content of tissues and the key enzymes of carbohydrate metabolism. Mol Cell Biochem. 2002;241(1):53–9. doi: 10.1023/A:1020870526014. [DOI] [PubMed] [Google Scholar]
- 71.Ramkumar KM, Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Sujatha J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(12):3390–4. doi: 10.1016/j.fct.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 72.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14(10):953–61. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 73.Sharma A, Mittal S, Aggarwal R, Chauhan MK. Diabetes and cardiovascular disease: inter-relation of risk factors and treatment. Futur J Pharm Sci. 2020;6(1):1–9. doi: 10.1186/s43094-021-00395-0. [DOI] [Google Scholar]
- 74.Tavakoli M, Mojaddidi M, Fadavi H, Malik RA. Pathophysiology and treatment of painful diabetic neuropathy. Curr Pain Headache Rep. 2008;12(3):192–7. doi: 10.1007/s11916-008-0034-1. [DOI] [PubMed] [Google Scholar]
- 75.Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I. Antioxidants and human diseases. Clin Chim Acta. 2014;25(436):332–47. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data on reasonable request to corresponding author
Declaration





