Abstract
Background
The inflammatory potential of diets is associated with several diseases and can affect bone health. We aimed to systematically review and pool the current evidence on the association of DII with bone health in observational studies.
Methods
We searched PubMed and NLM Gateway (for Medline), Web of Science, Scopus and EMBASE up to December 16, 2020 for studies that examined the relationship between DII score and bone mineral density (BMD) or fracture. All observational studies were included in this meta-analysis. Heterogeneity between studies was evaluated using Cochran Q-statistic and I2 statistics. Random effect meta-analysis method was used to pool the effect size. Stratified meta-analysis according to the type of study (cohort/ non-cohort) was performed to assess the relationship of DII with BMD and fracture.
Results
In total, 13 articles were included in the present systematic review, including five cohorts, five cross-sectional, and three case-control studies. The total sample size of these studies was 211,938 individuals aged 5 to 85 years. According to random-effect meta-analysis, DII was associated with increased odds of fracture in non-cohort studies (pooled OR=1.42, 95%CI: 1.17, 1.67), but this association was not statistically significant in cohort studies (pooled OR=1.03, 95%CI: 0.97, 1.09). Moreover, only in non-cohort studies, the mean of BMD in subjects in the highest DII category was significantly lower than those in the lowest DII category (SMD: -9.59, 95%CI: -10.84,-8.33).
Conclusions
Our findings showed that high score of DII can have devastating effects on bone health. Further longitudinal studies are necessary to confirm these findings among more diverse populations.
Keywords: Dietary inflammatory index, Bone mineral density, Fracture, Meta-analysis, Osteoporosis
Introduction
Osteoporosis is an age-related metabolic bone disease characterized by bone mass reduction and alteration of bone architecture, which is the main risk factor of bone fragility and fracture [1]. The increasing prevalence of osteoporosis is a major concern of public health worldwide because of the increase in life expectancy and old age population [2]. It has been estimated that the number of people suffering from osteoporosis are more than 200 million in the world. According to the recent International Osteoporosis Foundation (IOF) report, 1 in 2 women over the age of 50 years and 1 in 5 men will experience osteoporotic fractures in their lifetime [3]. Osteoporosis and its related fracture impose a growing economic burden on affected individuals and the healthcare system [4].
Numerous risk factors including genetic and environmental susceptibility factors have been proposed to increase osteoporosis and fracture risk. Dietary pattern and nutrients intake are the modifiable non-pharmacologic risk factors that improve bone health [5].
Chronic low grade of inflammation in the body is associated with increased risk of disorders including obesity [6], cancer [7], diabetes and metabolic syndrome [8], as well as poor bone health and age-related sarcopenia [9]. Dietary inflammatory index (DII) is a literature-based scoring system that determines the potential inflammatory status of an individual’s diet based on the pro-and anti-inflammatory status of many specific foods and nutrients in the diet. A higher DII score represents a more pro-inflammatory diet, while a lower DII score indicates a more anti-inflammatory diet [10]. The first time, DII scores were introduced to the medical literature by Cavicchina [11] and colleges and then updated by Shivappa in 2017 [10]. In the generation of DII score, more than 1900 relevant articles published in the peer-reviewed journals were assessed to find the positive or negative association of nutrients and foods with specific inflammatory markers such as CRP [12], TNF-alpha [13], IL-1beta, IL-4, IL-6 and IL-10 [10, 14].
Although some previous studies reported an inverse association of high DII with lower BMD or increasedd fracture risk, other studies showed no association. In addition, there is inconsistency regarding the relationship between DII score and bone health status between men and women. Resent umbrella review confirmed that although several systematic review and meta-analysis studies have been assessed the relationship between DII and health outcomes [15], none of them evaluated the association of DII with bone health outcomes. Therefore, the current study conducted for systematically reviewing the evidence and a meta-analysis to pool the findings.
Methods
Search strategy
To identify the eligible studies for this systematic review, relevant studies were selected through searching Web of Science, PubMed and NLM Gateway (for Medline), Scopus, and before December 16, 2020. We used the following keywords in this review: (“dietary inflammatory index” OR “pro-inflammatory diet” OR “anti-inflammatory diet” OR “pro-inflammatory dietary pattern” OR “anti-inflammatory dietary pattern” or “Inflammatory potential of diet”) AND (“osteoporosis” or “bone mineral density” or “BMD” or “bone mass” or “osteopenia” or “fracture risk” or “fracture”). The search had no restriction on publication date or language. In addition, the reference lists of relevant publications were reviewed to avoid missing any published data. The study protocol was registered in the international prospective register of systematic reviews database [http://www.crd.york.ac.uk/ PROSPERO, registration no: 2018CRD42018104324].
Inclusion and exclusion criteria
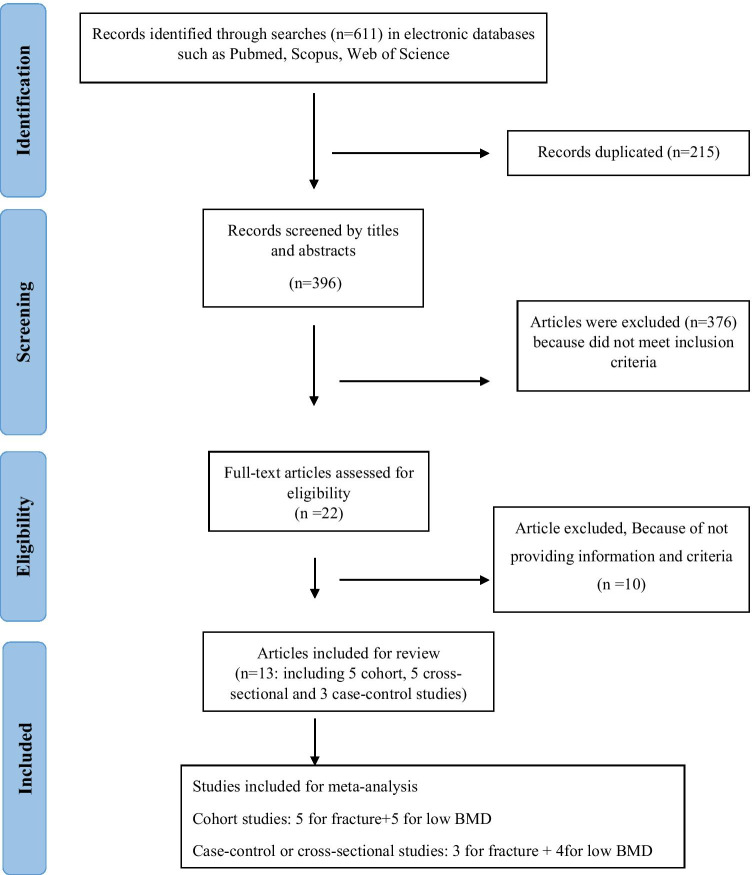
The present study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) protocol [16]. All observational studies that investigated the association between DII and bone health status, including BMD and fracture were eligible for inclusion. Studies that reported correlation coefficient, odds ratio (OR), hazard ratio (HR), standardized mean difference (SMD), along with 95% confidence interval (CI) for the association of DII with BMD changes or fracture were included in our meta-analysis. We excluded letters, comments, narrative reviews, and studies on nonhumans, and duplicated studies. The details of the study selection process are shown in Fig. 1.
Fig. 1.
Flow diagram of the study selection process
Data extraction
Two reviewers independently assessed the titles, abstracts and full texts of articles retrieved from the systematic searches. All articles that clearly did not meet the inclusion criteria were rejected. The selected articles were analyzed by reading the full text, and the eligible ones were then identified. In all refinement steps if there was disagreement between the two reviewers during the study selection process, the issue was resolved through discussion or in consultation with a third reviewer.
Data were extracted using a checklist, which was included the following information: first author, country, mean age, gender, sample size, study population, type of study, duration of follow up (for cohort studies), the tool for assessing DII, unit of comparison of DII (categorical/continuous), bone health status (BMD/fracture) and methods of measurement, relevant effect size (OR, HR, Beta coefficient, Pearson correlation and SMD), and adjusted confounders. If a study reported both outcomes (BMD and fracture), it was considered as two separated studies in the meta-analysis.
Assessment of study quality
The quality of studies was assessed using the Newcastle-Ottawa Quality Assessment Scale designed for cohort, case-control, or cross-sectional studies [17]. This scale consists of three portions of the selection, comparability, and outcomes/exposures, and studies earned maximum nine points. According to this scale, nine stars can be allocated to each study. In the present study, the publications with Newcastle-Ottawa scale ≥ 7 were considered high quality.
Statistical analysis
Heterogeneity between studies was assessed using Chi-square-based Q test and I2 statistics. Due to severe heterogeneity between studies, random effect meta-analysis proposed by Der-Simonian and Laird was used to pool the effect size. OR and HR with their 95% CI were used as effect size for the association of DII with fracture. Association of DII with BMD was presented as SMD with 95% CI. Publication bias was evaluated using Egger’s tests, and the results of Egger’s test were statistically significant at P < 0.1. Stratified meta-analysis according to the type of study (cohort/ non-cohort), sex, and definition of DII (categorical/ continuous) was performed to assess the association of DII with BMD and fracture. Sensitivity analysis was performed to assess the effect of removing any of the studies or group of studies on the pooled estimate. All the Analyses were conducted using the statistical software STATA 11.0 (Stata Corp., College Station, TX, USA) for the meta-analysis.
Results
Findings from the systematic review
A flow diagram for the process of studies selection is presented in Fig. 1. Out of 611 articles in the initial search, after excluding 215 articles for duplication, 396 articles were screened by title and abstracts. Of these, finally, 13 articles fulfilled our inclusion criteria, including five cohorts, five cross-sectional, and three case-control studies in the present systematic review. These studies included 211,938 individuals with the age range of 5 to 85 years. The selected articles were published between 2016 and 2019. The duration of cohort studies to investigate the association of DII and fracture varied from 7.9 to 11.4 years, while this duration for DII and BMD varied 5 months to 10 years. Out of these studies, five studies were conducted in the USA, two in Korea, two in China, one in Australia, one in Brazil, one in Spain, and one in Iran. Eight studies were performed on both males and females, and four studies on females. Four studies reported results for the association of DII score with fracture, three studies for BMD, and one study provided data for the association of DII with both fracture and BMD. In addition, three studies mentioned other markers of bone health status, including knee osteoarthritis, QUS of the right calcareous, and cortical bone peripheral. In most studies, BMD was measured by X-ray absorptiometry (DXA), except in one study in which BD was measured by QUS. The fracture was assessed in the hip, lower arm, wrist, spine, non-vertebral, and total.
Regarding the studied population, two studies have been conducted on postmenopausal women, four studies on healthy adults, one study on healthy adolescents, two studies on older adults, one study on subjects at risk of osteoarthritis, and 1 study on lactating women. The score of DII was assessed by food frequency questionnaire (FFQ) with different number items, including 48-item FFQ to 168-items FFQ in eight studies. Three studies used 24-hour recall, and two studies used 72- hours recall questionnaire to calculate the DII score. In all included studies, DII was categorized as tertile, quartile, and quintile. However, in five studies, DII was considered as continuous value.
Finding from meta-analysis
As noted above, four cohort studies were regarding the association of DII and fracture. Orchard et al., measured risk of fracture in total, hip and lower arm and Kim et al., study that measured risk of total fracture in females and in both gender were considered as three and two separate studies, respectively. In Verones et al., study the association of fracture were assessed with DII as per 1SD and as comparison Q5to Q1 of DII in males, females and both gender. Therefore, the results of this study were included in the meta-analysis as six separate studies. Cervo et al., the study evaluated the risk of fracture in two bone sites in males and females. Finally, 15 studies were included in systematic review. The total sample size of included studies was 174,882 subjects in cohort studies and 22,687 subjects in non-cohort studies. However, the association between DII and risk of fracture was not statistically significant in the cohort (pooled OR=1.03, 95%CI: 0.97-1.09, I2=75.5, P<0.001), in non-cohort studies, DII was associated with an increased risk of fracture (pooled OR=1.42, 95%CI: 1.17-1.67, I2=55.9, P=0.04). The analysis of DII score as a categorical variable also showed that individuals with the highest score of DII were 53% more prone to experience fracture than subjects who had the lowest DII score (Tables 1, 2, 3 and 4).
Table 1.
Studies included in systematic review and meta-analysis of DII and fracture
| First author (year) |
country | Mean (SD)age (range) |
gender | Sample size | Study population (health status) | Type of study | Follow up (number of incident case) | Dietary inflammatory index tool | Unit of *comparison | Bone health status (methods of measurement) |
Effect size measure (95%CI) |
Adjusted for* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
T.Orchard (2017)[18] |
USA |
63 (50-79) |
F | 10,290 | Postmenopausal women | Cohort |
11.4±3.3 y (total=47,974 hip=3738) |
122- item FFQ |
Q4 vs. Q1 (2.98 vs. -3.63) |
Hip fracture (self-reported or medical record) Lower arm fracture (self-reported or medical record) Total fracture (self-reported or medical record) |
HR= 1.02 (0.92-1.14) HR*= 0.92 (0.86-0.98) HR*= 0.95 (0.92-0.98) |
1,2,3,4,5,6,7,8,9, 10,11,12,13,14,15, 16,17,18,19 |
|
N.Veronese (2017)[19] |
USA |
60.6 (9.1) 45-79 |
F/M |
3648 M=1577 F=2071 |
With or at risk of knee osteoarthritis | Cohort |
8 y (560= 198 M, 362 F) |
70- item FFQ |
Per 1-SD increment DII (1.68 points) Q5 vs. Q1 |
Fracture (self- reported) |
F HR*= 1.14 (1.02–1.27) M HR= 0.95 (0.82–1.11) Overall HR=1.22 (0.91-1.64) F HR*=1.46 (1.02-2.11) M HR=0.91 (0.54-1.54) |
1, 2, 3, 4, 21, 24, 26,27,28,29 |
|
H.S kim (2018)[20] |
Korea |
52.34(8.24) 40-79 |
F/M |
159,846 M=57,740 F=102,106 |
Healthy adults | Cohort |
7.9 years (2572= 148 M, 2424 F) |
106- item FFQ |
Q5vs.Q1 (-9.12 to -0.98) vs. (2.1762 to 7.1055) |
Fracture (self –report ) |
Total HR*= 1.33 (1.12-1.58) F HR*= 1.33 (1.11- 1.59) M HR=1.32 (0.64-2.71) |
1, 3, 4, 5, 14, 26,30, 31 |
|
MM.Cervo (2019)[21] |
Australia |
63.0 (7.5) 51-79 |
F/M |
1098 M=559 F=538 |
Non-institutionalized older adults | Cohort |
10 years (total=566) |
74-item FFQ | 1- unit increase in E-DII |
Any fracture (self–report) Non-vertebral fracture (self-reported) |
M HR*= 1.090 (1.011-1.017) F HR*=0.878 (0.800-0.964) M HR= 1.074 (0.995-1.159) F HR=0.911 (0.827-1.003) |
1,4,31,32,34,35 |
|
ZQ. Zhang (2017)[22] |
70.62 (7.55) 52-83 |
F/M |
2100 F=781 M=269 |
Elders |
Case- control (case=1050 control=1050) |
NA | 79- item FFQ | Q4 vs. Q1 |
Hip fracture (self –report) |
Total OR*= 2.44 (1.73-3.45) Female OR*= 2.08 (1.38-3.12) Male OR*=4.30 (1.89-9.80) |
3, 4, 5, 8, 21, 23, 27,32 | |
|
M. Morimoto (2019)[23] |
Brazil |
57.9 (13.5) ≥40 |
F/M |
2269 F=1585 M=684 |
Healthy adults | Cross-sectional | NA | 24-hr recall |
Q4 vs.Q1 (>1.89 vs. ≤ 0.49) |
low impact fracture (self–report) |
OR: 0.98 (0.8-1.21) |
|
|
M. Mazidi (2017)[24] |
USA | 47.43 (0.27) | F/M |
18,318 F= 8921 M=9397 |
Healthy adults | Cross-sectional | NA | 24-h diet recall | Q4 vs. Q1 |
Hip fracture* Wrist* spine* |
OR;1.00(0.94-1.06) OR;1.03(0.97-1.09) OR;1.00(0.94-1.07) |
1,2,3,4,5,21,33 |
QUS: Quantitative ultrasonometry, Y: years, F: females, M: males, FFQ: food frequency questionnaire, HR: hazard ratio, OR: odds ratio, Q: quartile, T: tertile, DII: dietary inflammatory index, E-DII: Energy adjusted dietary inflammatory index, BD: Bone density
*1-age, 2-race, 3- BMI, 4-smoking, 5- physical activity, 6-DII (baseline),7-CT(clinical trial assignment), 8-parental history of fracture, 9-personal history of fracture at age 55 years or older, 10-region, 11-diabetes, 12-female hormone use, 13-NSAID use, 14-total calcium intake, 15- corticosteroid use (screening), 16-inflammatory bowel disease,17- rheumatoid arthritis, 18-weight, 19-height, 20- parity, 21- education, 22- fragility fracture history, 23- supplement intake, 24-antiresoptive drug use, 25- age at menarche, 26- total energy intake, 27-yearly income,28-Charlson comorbidityIndex,29-physical activity scale for the elderly, 30-gender, 31-alcohol consumption, 32- calcium supplement, 33-C-reactive protein, 34-percnetage body fat, 35- step per day,36- baseline T-score, 37- feeding modes, 38- time of complementary foods, 39-stage of sexual maturation, 40-muscle cross-sectional area,, 41-postmenopausal period, 42- vitamin D, 43- CES-D, 44-use of medications for Knee OA
Table 2.
Studies included in systematic review and meta-analysis of DII and BMD
| First author (year) |
country | Mean age (range) |
gender | Sample size | Study population (health status) | Type of study | Follow up (number of incident case) | Dietary inflammatory index tool |
Unit of comparison | Bone health status (methods of measurement) |
Effect size measure (95%CI) |
Adjusted for* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
T. Orchard (2017)[18] |
USA |
63 (50-79) |
F | 8303 | Postmenopausal women | Cohort | 6 years | 122- item FFQ | Q4 vs. Q1 |
BMD (DXA) Hip |
After multivariable adjustment, women with the least inflammatory dietary pattern (Q1) had a more positive overall change in hip BMD ( p for linear trend < 0.001) |
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 |
|
MM.Cervo (2019)[21] |
Australia |
63.0 (7.5) 50-79 |
F/M | 1098 | Non-institutionalized older adults | Cohort | 10 years | 74-item FFQ | 1- unit increase in E-DII |
BMD (DXA) Femoral neck Total Hip lumbar spine (DXA) |
M B= -0.001 (-0.008 to 0.006) F B=-0.002 (-0.009 to 0.005) M B= -0.009 (-0.017 to -0.000) F B=-0.007 (-0.016 to 0.001) M B=-0.013 (-0.024 to -0.002) F B=-0.009 (-0.019 to 0.001) |
1,4,31,32,34,35 |
| Y.Zhou (2019)[25] | china | 31.72 (4.50) | F | 150 | Lactating women | Cohort | 5 months | 48-item FFQ | DII Tertiles |
BD (QUS) |
Mean (SD) BD changes T1: 0.05 (0.3) T2: 0.00 (0.3) T3: -0.1 (0.4) |
3,5,21,26,36,37,38 |
|
N.Shivappa (2016)[26] |
Iran |
60 (8.4) 50-85 |
F | 160 | Postmenopausal women | Cross-sectional | NA |
168- item FFQ |
DII (continuous) DII > −0.06 vs. DII ≤ −0.06 |
BMD(DXA) lumbar spine femoral neck lumbar spine femoral neck |
OR*=1.64 (1.11- 2.43) OR=1.29 (0.86- 1.93) OR*=2.30 (1.05- 5.07) OR=1.22 (0.55- 2.72) |
1,3,4,5,9, 12, 20, 21,23,24,25 |
|
M. Mazidi (2017)[24] |
USA | 47.43 (0.27) | F/M |
18,318 M=9397 F=8921 |
Healthy adults | Cross-sectional | NA | One 24-h diet recall | Q1vs Q4 |
BMD (DXA) |
Standardized mean difference of BMD | 1,2,3,4,5,21,33 |
| W.Na (2019)[27] | Korea |
63.65 (8.44) ≥50 |
F | 2778 | Postmenopausal women | cross-sectional study | NA | 24-h dietary recall |
DII Tertiles T1 ( -5.15 to 0.84) vs. T3 (3.05 to 6.35) |
BMD (DXA) Total femur Femur neck Lumbar spine (L1-L4) |
OR= 1.27 (1.00-1.62) OR*= 1.43 (1.10-1.86) OR= 1.11 (0.87-1.49) |
1,3,4,5,12,27,32,41,42 |
Y: years, F: females, M:males, FFQ: food frequency questionnaire, HR: hazard ratio, OR: odds ratio, Q: quartile, T: tertile, DII: dietary inflammatory index, E-DII:Energy adjusted dietary inflammatory index, BD: Bone density
QUS: Quantitative ultrasonometry
* 1-age, 2-race, 3- BMI, 4-smoking, 5- physical activity, 6-DII (baseline),7-CT(clinical trial assignment), 8-parental history of fracture, 9-personal history of fracture at age 55 years or older, 10-region, 11-diabetes, 12-female hormone use, 13-NSAID use, 14-total calcium intake, 15- corticosteroid use (screening), 16-inflammatory bowel disease,17- rheumatoid arthritis, 18-weight, 19-height, 20- parity, 21- education, 22- fragility fracture history, 23- supplement intake, 24-antiresoptive drug use, 25- age at menarche, 26- total energy intake, 27-yearly income,28-Charlson comorbidityIndex,29-physical activity scale for the elderly, 30-gender, 31-alcohol consumption, 32- calcium supplement, 33-C-reactive protein, 34-percnetage body fat, 35- step per day,36- baseline T-score, 37- feeding modes, 38- time of complementary foods, 39-stage of sexual maturation, 40-muscle cross-sectional area,, 41-postmenopausal period, 42- vitamin D, 43- CES-D, 44-use of medications for Knee OA
Table 3.
Quality assessment of included studies on dietary inflammatory index and bone health
| Cohort Studies | ||||||||||
| Author/year | Representativeness of exposed cohort | Selection of no exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Study controls for age, sex, and marital status | Study controls for any additional factors | Assessment of outcome |
Followed up long enough for outcome to occur (> 15 years) |
Adequacy of follow- up of cohort | Total score |
|
T. Orchard (2017)[18] |
* | * | * | * | - | * | * | - | * | 7 |
|
N. Veronese (2018)[19] |
* | * | * | - | * | * | * | - | * | 7 |
|
H.S kim (2018)[20] |
* | * | * | * | * | * | * | - | * | 8 |
|
Yalin Zhou (2019) |
- | * | * | * | * | * | * | - | - | 6 |
|
N. Veronese (2018) [19] |
* | - | * | * | * | * | * | - | - | 6 |
|
MM.Cervo (2019)[21] |
* | - | * | * | * | * | * | - | * | 7 |
| Zhou (2019)[25] | - | * | * | * | * | * | * | - | - | 6 |
| Case-Control Studies | ||||||||||
| Adequate case definition | Representativeness of cases | Selection of controls | Definition of controls | Study control for age | Study controls for any additional factor | Assessment of exposure | Same method of ascertainment for cases and controls | Same non-response rate for both groups | Total score | |
|
Zhang ZQ (2017)[22] |
* | * | * | * | - | * | * | * | - | 7 |
| Cross-Sectional Studies | ||||||||||
| Author/year | Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure (risk factor) | The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled | Assessment of the outcome | Statistical test | Total score | ||
| W.Na (2019)[27] | * | * | - | ** | ** | ** | * | 7 | ||
| Morimoto M (2019)[23] | * | * | - | ** | * | - | * | 6 | ||
| Mazidi M (2017)[24] | * | * | - | ** | ** | ** | * | 9 | ||
| Coheley L.M (2019)[28] | - | * | - | ** | - | ** | * | 6 | ||
| kim (2018)[20] | - | * | - | ** | * | ** | * | 6 | ||
| Shivappa (2016)[26] | - | * | - | ** | ** | ** | * | 8 | ||
Table 4.
Meta-analysis of dietary inflammatory index and fracture
| Impairment | Number of study | Sample size | Pooled Effect size % (95% CI) | Model | Heterogeneity assessment | ||
|---|---|---|---|---|---|---|---|
| I2 % | Q test | P-value | |||||
| Fracture in Cohort studies | |||||||
| Overall | 15 | 174882 |
1.03 (0.97-1.09) |
Random | 75.5 | 57.19 | <0.001 |
| By sex | |||||||
| Male | 5 | 59876 |
0.97 (0.85-1.09) |
Random | 65.4 | 11.57 | 0.021 |
| Female | 8 | 115005 |
1.02 (0.95-1.09) |
Random | 79.7 | 34.49 | <0.001 |
| Both sex | 2 | 163494 |
1.29 (1.10-1.49) |
Random | ---- | ---- | ---- |
| Definition of DII | |||||||
| Categorical | 9 | 173784 |
1.06 (0.97-1.15) |
Random | 71.5 | 28.03 | <0.001 |
| Continuous | 6 | 4746 |
1.006 (0.92- 1.09) |
Random | 80.6 | 25.73 | <0.001 |
| Fracture in non-Cohort studies | |||||||
| Overall | 7 | 22687 |
1.42 (1.17-1.67) |
Random | 55.9 | 13.60 | 0.04 |
| By sex | |||||||
| Male | 1 | 269 |
4.30 (0.3-48.25) |
Random | ---- | ---- | ---- |
| Female | 1 | 781 |
2.08 (1.21- 2.95) |
Random | ---- | ---- | ---- |
| Both sex | 5 | 22687 |
1.33 (1.12-1.56) |
Random | 52.5 | 8.41 | 0.03 |
| Definition of DII | |||||||
| Categorical | 6 | 20418 |
1.53 (1.20- 1.86) |
Random | 55.1 | 11.14 | 0.07 |
| Continuous | 1 | 2269 |
1.18 (0.96-1.40) |
Random | ---- | ---- | ---- |
The results of the Egger test for association of DII with fracture show that publication bias does not exist (coefficient: 0.96; P = 0.25), and the funnel plot was symmetric.
Moreover, according to random-effect meta-analysis, the mean of BMD in subjects in the highest DII category was significantly lower than those in the lowest DII category (SMD: -9.59, 95%CI: -10.84,-8.33) in non-cohort studies. In cohort studies this association was not statistically significant (SMD: 0.141, 95%CI: -0.08, 0.36).
Quality assessment
The quality assessment of included studies was performed by two independent reviewers using Newcastle–Ottawa Scale (NOS). Any discrepancy between reviewers was resolved by a third reviewer. The qualitative assessment results showed that six studies had a high quality, and the remains had a moderate quality. Also, no low quality studies were observed.
Sensitivity analysis
Sensitivity analysis showed that excluding any individual studies could not significantly change the pooled estimate of DII association with fracture and BMD.
Discussion
The results of our study revealed that DII was associated with bone health outcomes (fracture and BMD). The risk of fracture was significantly higher in individuals who had the most pro-inflammatory diet (highest DII score) in comparison with those with an anti-inflammatory diet (lowest DII score). Moreover, the mean of BMD in subjects in the highest DII category was significantly lower than those in the lowest DII category. Dietary intake is one of the main environmental determinants of the inflammatory status of the body. DII is the literature-derived tool, which is generated by the association of nutrients and inflammatory markers, including pro- and anti-inflammatory foods.
By evaluating the other indexes of healthy dietary patterns such as Mediterranean diet, DASH, or HEI by using the pro-and anti-inflammatory categories of foods in DII, it is revealed that individuals with low DII score more probably consume healthy dietary patterns with more anti-inflammatory and antioxidant nutrients [29–31]. In evaluating of DII score, foods including red or processed meat, French fries, hydrogenated fats, which are known as unhealthy foods, are considered in pro-inflammatory group. Moreover, healthy foods such as fruits, vegetables and fish oils are categories as anti-inflammatory foods [32]. In considering single nutrients or foods individually, the results of a National survey on the Korean population showed that fat consumption (pro- inflammatory item) was an independent predictor of osteoporosis [33]. In another study, a low chance of having low BMD was observed in adolescents with high consumption of milk and cereals in their dietary pattern [34]. In other studies, low dietary intake of folate, total fibers, vitamin B6, potassium, vitamin A and foods such as milk and cereals were correlated with high likelihood of having low BMD [35, 36].
Other studies have reported that better adherence to the Mediterranean diet was associated with a lower risk of bone fracture [37]. Similar results were found regarding DASH and HEI with decreasing the risk of fracture [38].
There is a growing body of literature reporting positive correlation between pro-inflammatory foods such as red or processed meat, butter, and saturated fats or oils with the increased blood circulation of inflammatory markers including C-reactive proteins (CRP), TNF-alpha, E-selectin, soluble vascular cell adhesions molecules [39, 40]. It is well established that rheumatoid arthritis [41] and cystic fibrosis as chronic inflammatory diseases negatively affect bone health status [42]. In addition, as we know, low grade inflammation in the body potentially increases the risk of chronic disorders such as diabetes, insulin resistance, cancer, metabolic syndrome, asthma, cardiovascular disease, and also osteoporosis which is the main risk factor of bone fracture [43]. Consistent with this hypothesis, previous studies have shown that serum level of TNF-gamma is positively and the neutrophil/lymphocyte ratio (NLR) is negatively correlated with BMD [44]. Inflammatory cytokines such as TNF-alpha, CRP, increase the activity of osteoclasts. In addition, systematic inflammation in the body may elevate the osteoclast activities by endorsing ligand-RANK release [45]. As we know, calcium and vitamin D are the main dietary factors that influence the health status of bones. It was demonstrated that increased inflammation in the intestinal decreases the absorption of calcium and phosphorous by up regulating the synthesis of 1,25(OH)2D and suppressing the expression of vitamin D receptors [46].
To the best of our knowledge, this study was the first meta-analysis that assessed the relationship between DII score and bone health outcomes, including low BMD and fracture risk. This study has some limitations. In this review, we included all types of studies and analysis separately as cohort and non-cohort studies. The first limitation of this review measured the low BMD and risk of fracture in the different bone sites in included studies.
Conclusions
Our findings showed that high score of DII can have devastating effects on bone health. Further longitudinal studies are necessary to confirm these findings among more diverse populations. This knowledge could have implications for dietary advice provided to those at risk of osteopenia or osteoporosis. Further research is warranted to confirm these findings among more diverse populations.
Authors’ contributions
HA and MQ contributed to the conceptualization of the systematic review. ET, AM and JM conducted the data sereaching. HA, ET, JM and AM conducted the data screening and quality assessment. ET and HA conducted the data extraction and drafted the manuscript. MQ performed meta-analysis and drafted the manuscript. All authors provided critical review of drafts and have read and approved the final manuscript.
Funding
This study was supported by National Institute for Medical Research Development (NIMAD), Grant No. 977192.
Data availability
No additional data are available.
Declarations
Ethics approval and consent to participate
This systematic review and meta-analysis study was not performed on human or animal subjects. The Ethics council of National Institute for Medical Research Development (NIMAD), Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
Ehsaneh Taheri, Armita Mahdavi-Gorab, Jalal Moloudi, Hamid Asayesh, Mostafa Qorbani declare that they have no conflict of interest.
Footnotes
Hamid Asayesh and Mostafa Qorbani equally contributed as corresponding authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamid Asayesh, Email: hasayesh@gmail.com.
Mostafa Qorbani, Email: mqorbani1379@gmail.com.
References
- 1.Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–39. doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen VH. Community health programs and services for osteoporosis and osteoporotic fracture prevention: A population health perspective. Int J Healthc Manag. 2019;12(2):87–9. doi: 10.1080/20479700.2017.1313481. [DOI] [Google Scholar]
- 3.Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer S, Kapinos KA, Mulcahy A, Pinto L, Hayden O, Barron R. Estimating the long-term functional burden of osteoporosis-related fractures. Osteoporos Int. 2017;28(10):2843–51. doi: 10.1007/s00198-017-4110-4. [DOI] [PubMed] [Google Scholar]
- 5.Maslin K, Dennison E. Diet and bone health. Analysis in nutrition research. Amsterdam: Elsevier; 2019. p. 337–54.
- 6.Cooke AA, Connaughton RM, Lyons CL, McMorrow AM, Roche HM. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016;785:207–14. doi: 10.1016/j.ejphar.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Evans M. Investigating the effects of chronic low-grade inflammation on cancer immunotherapy. Dunedin: University of Otago; 2018.
- 8.Castro-Costa E, Diniz BS, Firmo JO, Peixoto SV, de Loyola Filho AI, Lima‐Costa MF, et al. Diabetes, depressive symptoms, and mortality risk in old age: The role of inflammation. Depress Anxiety. 2019;36(10):941–9. doi: 10.1002/da.22908. [DOI] [PubMed] [Google Scholar]
- 9.Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts intervalchanges in serum high-sensitivity C-reactive protein. J Nutr Biochem. 2009;139:2365–72. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS) Public Health Nutr. 2014;17:1825–33. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausalwomen. Ann Epidemiol. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. 2015;113:665–71. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F-H, Liu C, Gong T-T, Gao S, Sun H, Jiang Y-T, et al. Dietary inflammatory index and health outcomes: an umbrella review of systematic review and meta-analyses of observational studies. Front Nutr. 2021;8:190. doi: 10.3389/fnut.2021.647122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. Jama. 2018;319(4):388–96. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 17.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5(4):80–4. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 18.Orchard T, Yildiz V, Steck SE, Hébert JR, Ma Y, Cauley JA, et al. Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: results from the women’s health initiative. J Bone Min Res. 2017;32(5):1136–46. doi: 10.1002/jbmr.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese N, Stubbs B, Koyanagi A, Hébert JR, Cooper C, Caruso MG, et al. Pro-inflammatory dietary pattern is associated with fractures in women: an eight-year longitudinal cohort study. Osteoporos Int. 2018;29(1):143–51. doi: 10.1007/s00198-017-4251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Sohn C, Kwon M, Na W, Shivappa N, Hébert JR, et al. Positive association between dietary inflammatory index and the risk of osteoporosis: Results from the KoGES_Health Examinee (HEXA) cohort study. Nutrients. 2018;10(12):1999. doi: 10.3390/nu10121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervo MM, Shivappa N, Hebert JR, Oddy WH, Winzenberg T, Balogun S, et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr. 2020;39(2):516–23. doi: 10.1016/j.clnu.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z-q, Cao W-t, Shivappa N, Hebert JR, Li B-l, He J, et al. Association between diet inflammatory index and osteoporotic hip fracture in elderly Chinese population. J Am Med Dir Assoc. 2017;18(8):671–7. doi: 10.1016/j.jamda.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto M, Shivappa N, Genaro PdS, Martini LA, Schuch NJ, Hebert JR, et al. Lack of association between dietary inflammatory index and low impact fractures in the Brazilian population: the Brazilian Osteoporosis Study (BRAZOS). Adv Rheumatol. 2019;59(1):16. [DOI] [PubMed]
- 24.Mazidi M, Shivappa N, Wirth M, Hebert J, Vatanparast H, Kengne A. The association between dietary inflammatory properties and bone mineral density and risk of fracture in US adults. Eur J Clin Nutr. 2017;71(11):1273–7. doi: 10.1038/ejcn.2017.133. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhu X, Zhang M, Li Y, Liu W, Huang H, et al. Association between dietary inflammatory index and bone density in lactating women at 6 months postpartum: a longitudinal study. BMC Public Health. 2019;19(1):1–9. doi: 10.1186/s12889-018-6343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivappa N, Hébert JR, Karamati M, Shariati-Bafghi S-E, Rashidkhani B. Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur J Nutr. 2016;55(2):561–8. doi: 10.1007/s00394-015-0875-4. [DOI] [PubMed] [Google Scholar]
- 27.Na W, Park S, Shivappa N, Hébert JR, Kim MK, Sohn C. Association between inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: data from Fourth and Fifth Korea National Health and Nutrition Examination Surveys. Nutrients. 2019;11(4):885. doi: 10.3390/nu11040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coheley L, Shivappa N, Hebert J, Lewis R. Dietary inflammatory index® and cortical bone outcomes in healthy adolescent children. Osteoporos Int. 2019;30(8):1645–54. doi: 10.1007/s00198-019-04946-3. [DOI] [PubMed] [Google Scholar]
- 29.Neale EP, Batterham MJ, LC T. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutrition research (New York, NY). 2016;36(5):391-401. [DOI] [PubMed]
- 30.Mayer HL., Thomas C, Tierney AC, Kucianski T, George ES, Ruiz-Canela M, et al. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvment in dietary inflammatory index score in patients with coronary heart disease. Nutr Res (New York, NY). 2018;55:97–107. [DOI] [PubMed]
- 31.Dias JA, Wirfalt E, Drake I, Gullberg B, Hedblad B, Persson M, et al. A high quality diet is associated with reduced systemic inflammation in middleaged individuals. Atherosclerosis. 2015;238(1):38–44. doi: 10.1016/j.atherosclerosis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Wirth MD, Hébert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, et al. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res (New York, NY). 2016;36(3):214-9. [DOI] [PMC free article] [PubMed]
- 33.Kwon YM, Kim GW, Yim HW, Paek YJ, KS. L. Association between dietary fat intake and bone mineral density in Korean adults: data from Korea National Health and Nutrition Examination Survey IV (2008 approximately 2009). Osteoporos Int. 2015;26:969–76. [DOI] [PubMed]
- 34.Shin S, Kim SH, Joung H. Milk-cereal and whole-grain detary patterns protect against low bone mineral density among male adolescents and young adults. Eur J Clin Nutr. 2017;71(9):1101–7. doi: 10.1038/ejcn.2017.81. [DOI] [PubMed] [Google Scholar]
- 35.Karamati M, Yousefian-Sanni M, Shariati-Bafghi S-E, B R. Major nutrient patterns and bone mineral density among postmenopausal Iranian women. Calcif Tissue Int. 2014;94(6):648–58. doi: 10.1007/s00223-014-9848-5. [DOI] [PubMed] [Google Scholar]
- 36.Kruger MC, Brough L, Ilesanmi-Oyelere BL, Coad J, N NR. The Relationship between Nutrient Patterns and Bone Mineral Density in Postmenopausal Women. Nutrients. 2019;11(1262):1–10. doi: 10.3390/nu11061262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra-Majem L, Bosque-Prous M, Palomeras-Vilches A, Viñals-Mayolas E, Bou-Mias CM, Jordà-Castro A, et al. Adherence to the mediterranean diet and bone fracture risk in middle-aged women: a case control study. Nutrients. 2019;11(2508):1–15. doi: 10.3390/nu11102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahriarpour Z, Nasrabadi B, Shariati-Bafghi S-E, Karamati M, Rashidkhani B. Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women. Osteoporosis Int. 2020;31(11):2179–2188. doi: 10.1007/s00198-020-05450-9. [DOI] [PubMed] [Google Scholar]
- 39.Chai W, Morimoto Y, Cooney RV, Franke AA, Shvetsov YB, Marchand LL, et al. Dietary Red and processed meat intake and markers of adiposity and inflammation: the multiethnic cohort study. J Am Coll Nutr. 2017;36(5):378–85. doi: 10.1080/07315724.2017.1318317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telle-Hansen VH, Christensen JJ, Ulven SM, k H. Does dietary fat affect inflammatory markers in overweight and obese indivituals? a review of randomized controlled trials feom 2010 to 2016. Genes Nutr. 2017;12(1):26. [DOI] [PMC free article] [PubMed]
- 41.Shim JH, Stavre Z, G EM. Bone loss in rheumatoid arthritis: basic mechanisms and clinical implications. Clacif Tissue Int. 2018;102(5):533–46. doi: 10.1007/s00223-017-0373-1. [DOI] [PubMed] [Google Scholar]
- 42.Marquette M, CS H. Bone health and disease in cystic fibrosis. Pediatr Respir Rev. 2016;20:2–5. doi: 10.1016/j.prrv.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, E F. Inflammageing: chronic inflamation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):502–22. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Utsal L, Tillmann V, Zilmer M, Mäestu J, Purge P, Saar M, et al. Serum interferon gamma concentration is associated with bone mineral density in overweight boys. J Endocrinol Invest. 2014;37(2):175–80. doi: 10.1007/s40618-013-0029-6. [DOI] [PubMed] [Google Scholar]
- 45.Brien WO, Fissel BM, Gravallese EM, Maeda Y, Aliprantis AO, Yan J, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 2016;68(12):2889–900. doi: 10.1002/art.39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solak B, Dikicier BS, Celik HD, T E. Bone mineral density, 25-OH vitamin D and inflammation in patients with psoriasis. Photodermatol Photoimmunol Photomed. 2016;32(3):153–60. doi: 10.1111/phpp.12239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.