Abstract
Objective
To investigate the feasibility, safety, and clinical application value of single photon emission computed tomography/computed tomography (SPECT/CT)-guided bone marrow biopsy (BMB) in breast cancer (BC) patients with suspected bone metastases (BM) and compare its diagnostic performance for detection of BM with SPECT/CT.
Methods
The records of breast cancer patients referred for bone scintigraphy (BS), SPECT/CT and SPECT/CT-guided BMB from January of 2018 to June of 2021 in our hospital were retrospectively reviewed. 49 Patients were consecutively included in this study, all 49 specimens were analyzed by pathological and immunohistochemical studies.The biopsy success rate, total examination time, biopsy operation time, complications, CT radiation dose, and pathological and immunohistochemical results were recorded. The diagnostic performance based on SPECT/CT and SPECT/CT-guided BMB were compared with pathological, immunohistochemical examinations and the results of subsequent follow-up.
Results
Bone samples of the sites with high uptake were obtained in all 49 patients under BMB. No severe postoperative complications occurred. Among all 49 cases, 34 specimens were positive for metastatic breast cancer (69%, 34/49), and positive for benign tissue in 15 cases (31%, 15/49). 1 case of 15 cases was subsequently diagnosed as metastatic breast cancer according to the follow-up result. SPECT/CT-guided BMB demonstrated significantly higher negative predictive value (NPV) when compared to SPECT/CT (p = 0.021 < 0.05). Patients with differential expression of ER, PR, and HER-2 between primary lesions and metastatic lesions accounted for 12, 17, and 5 cases, respectively, and the changing rates were 35.2% (12/34), 50% (17/34), and 14.7% (5/34), respectively. Molecular subtype changes occurred in 7 patients, accounting for 47% (16/34) of metastatic patients.
Conclusion
It is insufficient to evaluate BM in BC patients using SPECT/CT imaging. SPECT/CT-guided BMB provided significantly higher sensitivity and NPV than SPECT/CT for detection of BM in BC patients. Our research redefines a new approach which can confirm diagnosis and potential molecular subtype changes for suspected bone metastatic lesions in BC patients, which can offer important opportunities for precision treatment and improved quality of life of BC patients with BM.
Keywords: Bone metastasis, Breast cancer, Biopsy, Tomography, Mission computer, Single photon, 99Tcm-methyl diphosphonate
Background
Breast cancer (BC) is the most common malignant tumour among women [1]. Bone is the most common location of metastases for BC. Bone metastases (BM) count approximately 60–70% of all metastatic BC and more than 70% of patients showed bone metastases during autopsy [2]. BM significantly affects both quality of life and survival of the breast cancer patient. Clinically, complications secondary to BM include pain, pathologic fractures, spinal cord compression, and hypercalcemia of malignancy [3]. Therefore, early diagnosis and treatment of BM in breast cancer patients has important significance.
Bone marrow biopsy (BMB) is the “gold standard” of diagnosis of BM in BC. According to relevant practice guidelines of the National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO)and China Anti-cancer Association (CACA) [4, 5], re-biopsy for suspected metastatic lesions in patients with late-stage BC is considered to confirm diagnosis. The evaluation of BM status is of critical importance in BC, as it is re-evaluated to confirm potential molecular subtype changes. The results may directly change the treatment plan.
However, It is not always performed in routine practice due to expertise unavailable and the lack of special technologie. In addition to the difficulties performing a re-biopsy in a location that is difficult to access, conventional imaging such as CT may not identify a metastasis and thus a biopsy will not be performed as a routine practice [6].
Nuclear medicine molecular imaging has unique advantages with respect to target area selection. In previous study, PET/CT-guided targeted BMB was confirmed to be a safe and feasible technique for the appraisal of advanced lung cancer and lymphomas [6, 7]. Zhao et al. applied SPECT/CT for thoracic tumor biopsy and confirmed its safety and reliability [8]. However, there are no studies examining the utility of SPECT/CT-guided targeted BMB.
After mastering PET/CT-guided percutaneous biopsy technology, this technology was introduced into SPECT/CT to perform SPECT/CT-guided BMB to target suspected bone metastatic lesions in breast cancer and test its feasibility and clinical value. The results are reported below.
Methods
Patients
The records of women with biopsy-proven breast cancer referred for routine clinical work-up with 99Tcm-methyl diphosphonate (MDP) bone scintigraphy (BS) and SPECT/CT from January of 2018 to June of 2021 in our hospital were retrospectively reviewed. Patients were consecutively included in this retrospective study if positive lesions were identified on SPECT/CT imaging and SPECT/CT-guided BMB were performed, and patients with a second malignancy were excluded. SPECT/CT scans were performed within 7 days before BMB. The findings from SPECT/CT and SPECT/CT-guided BMB were compared with the results of subsequent imaging follow-up and pathological and immunohistochemical examinations.
99mTc-MDP SPECT/CT technique and imaging
A GE Discovery NM/CT670 combined with a low energy high resolution collimator, with an energy window of 20% and energy peak of 140 keV, was used. 99mTc-MDP 740–1110 MBq was intravenously injected, and anterior and posterior full-body images and SPECT/CT fusion images were collected after 3 h. After determining the location of scanning field, SPECT tomography was performed first, with a matrix of 128 × 128, continuous acquisition of 360°, rotation of two probes of 180°, 12 s for each frame, a total of 32 frames were collected. Then, CT scanning was performed automatically with a matrix of 256 × 256 and scanning layer thickness of 5 mm(CT scanning parameters: 120 kV, 80 m A, 25 mm/ s entering speed, layer thickness 3.75 mm). Volumetrix MI Evolution for Bone was used for image reconstruction and fusion, without attenuation correction, Butterworth filter function, cutoff frequency 6.0, and transverse, sagittal, coronal and 3D images of SPECT, CT and their fusion were obtained directly. The matrix, pixel size and effective frame number of reconstructed SPECT and CT images are identical.
Image analysis
BS and SPECT-CT images were independently analyzed by two experienced nuclear medicine physicians on the work station. The readers were blinded to patients’ clinical information including previous therapy, previous BS findings, and the findings of other imaging modalities. Only the lesions that were not clearly defined on BS were evaluated. In case of any discrepancy regarding the findings of planar and SPECT images, a consensus was reached after mutual discussion. Malignant lesions were suggested by the presence of lytic, sclerotic, or mixed lytic-sclerotic changes on CT images. The presence of osteophytes, spondyloarthropathy, subchondral sclerosis, or narrowing of the joint space was regarded as a clear sign of the benign nature of the lesion. We also identified the location and pattern of bone lesions that was recorded.
SPECT/CT-guided targeted BMB
99mTc-MDP SPECT/CT fused images were used to determine the appropriate puncture site with high uptake of MDP, and the biopsy needle was introduced stepwise under fused SPECT/CT image and CT guidance. After conventional disinfection, draping, and local anaesthesia to the periosteum using 1% lidocaine, a bone puncture needle (BMT-B 2.4 × 70, Shanghai SA Medical Technology) was pressed, rotated, and inserted in accordance with the plan. Scanning was performed again to confirm that the needle tip was located at the edge of the target area (120 kV, 20 mA, image fusion using VMI software). The needle was connected to a spare casing tube and inserted into the needle core. The needle was forcefully pressed and rotated clockwise. When the fusion image confirmed that the needle tip passed through the target area, tissues were obtained after the needle was rotated counterclockwise and withdrawn. One or two samples were obtained for each patient, and the lengths of samples all were 1.5 or 2.2 cm. The BMB specimens were fixed in 10% formaldehyde solution and analyzed by morphological and immunohistochemical studies. The pathological results of all BMBs were validated by review of the individual pathology reports. After the biopsy procedure was finished, patients were kept for observation for at least 30 min after the in a recovery room and were allowed to leave when there were no adverse reactions.
Molecular subtyping of groups
Based on estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2(HER2) status, patients were classified following the recommendations of the 12th International Breast Conference [9]. The five patient groups were: 1) Luminal A: ER( +) and/or PR( +), HER2(-),Ki-67 low (< 14%); 2) Luminal B-HER2(-): ER( +) and/or PR( +),HER2(-), and Ki-67 high (> 14%); 3) Luminal B-HER2( +): ER( +)and/or PR( +), HER2( +), and any Ki-67 index; 4) HER2( +): ER(-),PR(-), and HER2( +); 5) Basal: ER(-), PR(-), and HER2(-).
Follow-up and reference standard
All the previous clinicopathological data of 49 patients have been followed up as soon as possible and their molecular subtypes have been classified. We derived the final diagnoses from histopathology and clinical/imaging follow-up (CT, MRI, PET -CT, SPECT -CT) over at least 6 months. It was considered positive for a tumor if there is an increase in size or a change of nature under treatment, whereas benign if lesions had unchanged size and character over 6 months without therapy [10].
Statistical analysis and ethics
The total examination time, biopsy operation time, complications, CT radiation dose, biopsy success rate and the changing rate were recorded. The changing rate was equal to the number of patients with altered expression of ER, PR, HER2 or molecular subtype divided by the total number of patients undergoing immunohistochemistry. The total dose-length product (DLP; mGy) of each scan was used as the CT radiation dose that patients received. The effective radiation dose (DLP × weighting factor κ; 0.019 mSv•mGy-1•cm-1 for the chest and 0.016 mSv•mGy-1•cm-1 for the abdomen and pelvis) was calculated. According to the the final diagnoses from histopathology and clinical/imaging follow-up, the diagnosis of SPECT/CT and SPECT/CT-guided BMB were classified as true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were is calculated according to the number of TN, TP, FP, FN, and determined on the basis on number of patients, not number of lesions. McNemar test was used to test differences in the sensitivity and specificity between SPECT/CT and SPECT/CT-guided BMB. Chi-square test was used to test differences in the NPV and PPV between SPECT/CT and SPECT/CT-guided BMB. All statistical analysis was performed using SPSS, version 26 software. A p-value < 0.05 was considered significant. This retrospective evaluation of collected data was approved by the ethics committee of our institution. This study was approved by the Ethics Committee of our hospital (approval number: YXLL-2020–033).
Results
SPECT/CT-guided BMB
Bone samples of the sites with high uptake of MDP were obtained in all 49 patients under BMB. Of these, biopsy tissue was successfully obtained in 10% of patients (5/49) despite the absence of morphological lesions on CT images. The average total examination time was (42.3 ± 10.8) min, the average biopsy operation time was (24.5 ± 6.2) min, and the effective radiation dose was (1.9 ± 0.8) mSv. No postoperative complications such as infection, pneumothorax, massive bleeding, or nerve damage occurred.
The sites and pattern of biopsy lesions are summarized in Table 1.
Table 1.
Sites and CT pattern of evaluated lesions
| Vriable | n | % |
|---|---|---|
| Sites | 49 | 100 |
| Vertebrae | 13 | 26 |
| Pelvis | 12 | 24 |
| Scapula | 3 | 6 |
| Sternum | 11 | 22 |
| Ribs | 6 | 12 |
| Clavicle | 4 | 8 |
| Characters | ||
| Lytic | 26 | 53 |
| Sclerotic | 18 | 36 |
| Unchanged | 5 | 11 |
Pathological diagnosis of BM
Among all 49 cases, 34 specimens were positive for metastatic breast cancer (69%, 34/49), and positive for benign tissue in 15 cases (31%, 15/49).
Biopsy identified 15 benign bone lesions, including fracture and bone marrow tissues (n = 10), fibrous tissues (n = 2), inflammatory cell infiltration (n = 3), myofibroblastoma (n = 1). To avoid potentially false negative of the 15 cases, they were further evaluated by clinical and imaging follow-up: 14 cases were confirmed as benign (inflammatory, myofibroblastoma, and lymph node hyperplasia) and 1 cases were subsequently diagnosed as metastatic breast cancer.
Typical images are shown in Figs. 1, 2 and 3.
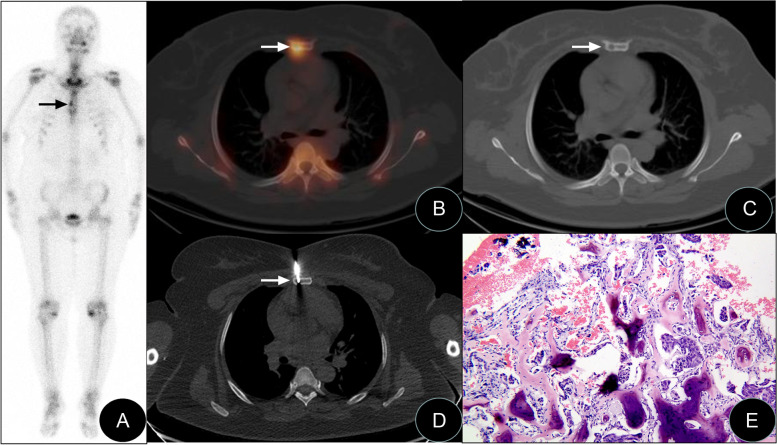
Fig. 1.
SPECT/CT-guided targeted bone marrow biopsy in the sternal body. Anterior bone scintigraphy, SPECT/CT fusion tomography image, biopsy image, and biopsy pathology image of a patient with invasive ductal carcinoma of the left breast (female, 44 years old). A The anterior bone scintigraphy suggested increased focal abnormal metabolism in the sternal body (black arrow). B, C SPECT/CT fusion tomography showed bone destruction in a location of increased metabolism in the sternal body. D SPECT/CT-guided percutaneous biopsy of the biological target in the sternal body. E Pathological examination results suggested metastatic breast invasive ductal carcinoma, and light microscopy results revealed the nested distribution of tumour cells in the space between bones and dead bones, with obvious cell atypia combined with nuclear hyperchromic malformation (HE × 100)
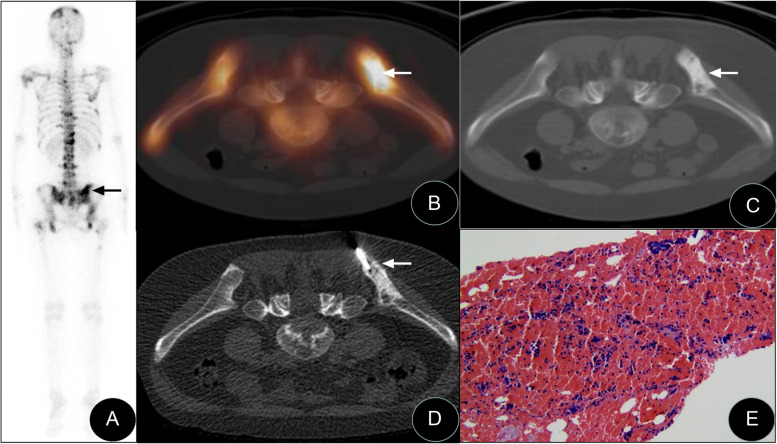
Fig. 2.
SPECT/CT-guided targeted bone marrow biopsy in the right ilium. Posterior bone scintigraphy, SPECT/CT fusion tomography image, biopsy image, and biopsy pathology image of a patient with invasive ductal carcinoma of the right breast 3 years after surgery (female, 29-year-old). A. The posterior bone scintigraphy suggested multiple abnormal increases in bone metabolism in the whole body and an abnormal increase in metabolism of the right ilium (black arrow). B, C SPECT/CT fusion tomography results indicated that the bone density in the right ilium that exhibited increased metabolism had significantly increased. D SPECT/CT-guided percutaneous biopsy of the biological target in the right ilium. E Pathological examination results suggested metastatic breast invasive ductal carcinoma, and light microscopy results revealed metastatic adenocarcinoma cells in blood clots (HE × 100)
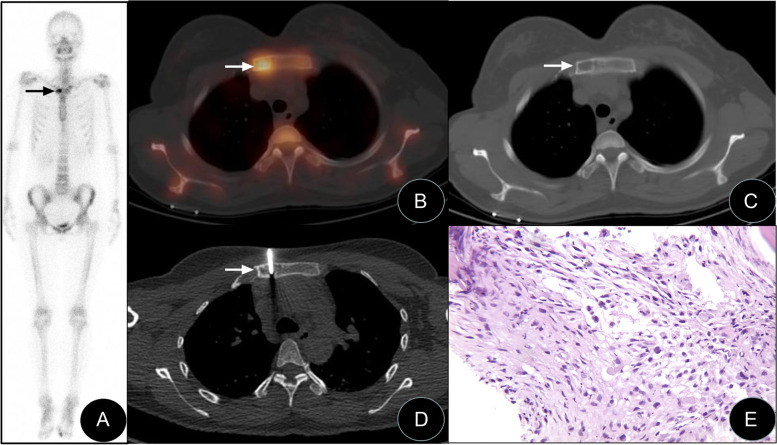
Fig. 3.
SPECT/CT-guided targeted bone marrow biopsy in the manubrium. Anterior bone scintigraphy, SPECT/CT fusion tomography image, biopsy image, and biopsy pathology image of a patient with invasive ductal carcinoma of the right breast 2 years after surgery (female, 30-year-old). A The anterior bone scintigraphy suggested abnormally increased bone metabolism on the right side of the manubrium (black arrow). B, C SPECT/CT fusion tomography results showed bone destruction in the location of increased metabolism on the right side of the sternal body. D SPECT/CT-guided percutaneous biopsy of the biological target in the sternal body. E Pathology and immunohistochemistry results suggested myofibroblastoma. Light microscopy results revealed a small amount of bone and dead bone tissues, polygonal cells between bone trabeculae, and nucleoli (HE × 100)
Diagnostic performance of SPECT/CT vs. SPECT/CT-guided BMB
According to the pathological results and follow-up results, BM were confirmed in 35 of the 49 patients (71%). SPECT/CT was positive for disease in 27, yielding a sensitivity of 77% (27/35). Because of 1 case was false negative, the sensitivity of SPECT/CT-guided BMB was 97.5% (34/35). SPECT/CT-guided BMB exhibited significantly higher sensitivity when compared to SPECT/CT for determination of BM (p = 0.016 < 0.05) (Table 2).
Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of SPECT/CT, and SPECT/CT-guided BMB in diagnosing metastasis
| Parameter | SPECT/CT | SPECT/CT-guided BMB | P value |
|---|---|---|---|
| Sensitivity | 77 | 97.5 | 0.016* |
| Specificity | 71 | 100 | |
| PPV | 87 | 100 | |
| NPV | 55.5 | 93.3 | 0.021* |
| Accuracy | 79 | 98 |
CT Computed Tomography, NPV Negative Predictive Value, PPV Positive Predictive Value, SPECT Single-photon Emission Computed Tomography
SPECT/CT was true negative for BM in 10 of 14 patients, yielding a specificity of 71% (10/14), whereas the specificity was of SPECT/CT-guided BMB (14/14,100%). (Table 2).The PPV and NPV of SPECT/CT were 87% (27/31) and 55.5% (10/18) respectively, while PPV and NPV of SPECT/CT-guided BMB were 100% (34/34) and 93.3% (14/15) respectively. SPECT/CT-guided BMB demonstrated significantly higher NPV when compared to SPECT/CT (p = 0.021 < 0.05) (Table 2).
Molecular classification & molecular subtyping shifts
Immunohistochemical examinations of ER, PR, and HER-2 were performed for all 34 metastatic tumors. Changes in ER expression in metastatic lesions and primary lesions were discovered in 12 patients (7 patients converted from positive to negative, and 5 patients converted from negative to positive), and the changing rate was 35.2%. Shifts in PR expression were discovered in 17 patients (13 patients converted from positive to negative, and 4 patient converted from negative into positive), and the changing rate was 50%. Conversions in HER-2 expression were discovered in 5 patients (4 patients converted from positive to negative, and 1 patient converted from negative to positive), and the changing rate was 14.7%. According to diverse combinations of ER, PR and HER-2 status, the total molecular subtype rate of molecular subtype shifts was up to 47% (16/35) (Table 3).
Table 3.
Molecular subtyping shifts of 34 breast cancer patients
| Molecular subtype shifts | datum | % |
|---|---|---|
| Unchanged | 19 | 55.8 |
| Changed | 16 | 47.0 |
| Total | 34 | 100 |
Discussion
To our knowledge, this study is the first to propose the notion that SPECT/CT-guided targeted BMB, which can add pathological confirmation and monitor potential molecular subtyping shifts if positive bone metastatic lesions were identified on SPECT/CT imaging. Based on the above data that we achieved, SPECT/CT-guided targeted BMB has gradually become routine practice at our institution.
Breast cancer is an evolutionary heterogeneous tumour, and its molecular subtype can convert between bone metastatic lesions and primary tumors [11, 12]. Therefore, it is significant that early detection of BM and personalized treatment based on molecular subtypes, which can preserve or improve long-term quality of life and functional independence of BC patients with BM [13].
Relevant practice guidelines of the National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO) and China Anti-cancer Association (CACA) all recommend re-biopsy for suspected metastatic lesions in patients with late-stage breast cancer to confirm diagnosis. After metastasis is confirmed, biological indicators are re-evaluated to confirm potential molecular subtype changes. The results may directly change the treatment plan.
CT guided-BMB is currently the main guidance method for bone biopsy. J. F. Hilton assessed the samples of patients underwent CT-guided biopsy of a radiologically evident BM from breast cancer. Positive samples for metastatic breast cancer were 21/39(52.5%) and sufficient tumor cells for hormone receptor analysis were available in 19/39(48.8%) [14]. However, there are some limitations [15]: (1) CT cannot confirm the sampling target for tumors that do not have lesions with abnormal morphology and structure at the early stage. (2) Systemic staging information cannot be obtained, the safest target area cannot be selected.
Nuclear medicine molecular imaging, including SPECT/CT and PET/CT, can display anatomic and metabolic information concurrently and has unique advantages with respect to target area selection.
Bone scintigraphy (BS),which is different from anatomical imaging,is a kind of imaging examination based on its own function. Metastatic bone tumors are usually detected 3 to 6 months earlier than CT. BS plays an irreplaceable role in the screening and early diagnosis of BM [16]. SPECT/CT has realized the organic combination of metabolic imaging and anatomical imaging, which has important clinical value in differentiating benign and malignant bone lesions [17, 18]. For staging of the skeleton, because of the greater contrast resolution of SPECT coupled with the correlation with the morphologic appearance of lesions on CT, further gains in sensitivity and, especially, in specificity and diagnostic confidence were apparent with SPECT/CT [10, 19, 20].
Many studies showed that PET/CT for guidance or guiding biopsy is feasible and may optimize the diagnostic yield of image-guided interventions [21, 22]. Wei et al. reported that PET/CT-guided percutaneous FDG-avid target biopsies offers a new integrated precise re-biopsy algorithm, which can improve precise individual therapy and prolong survival [6]. Her previous research also confirmed it is an effective and safe method in the evaluation of hypermetabolic bone lesions in patients with suspected advanced lung cancer [23]. Bing et al. drew a conclusion that PET/CT-guided targeted BMB may complement the results of possible false-positive PET/CT and false-negative iliac crest biopsy findings for evaluation of bone marrow involvement in newly diagnosed lymphomas [7].
However, there are fewer PET/CT apparatuses, thus their application is restricted. Furthermore, SPECT/CT is more common. Owing to that SPECT/CT is organic fusion of metabolic imaging and anatomical imaging, using SPECT/CT-guided biopsy can theoretically increase the accuracy and success rates and can be extensively promoted.There are fewer reports of SPECT/CT-guided biopsy. Zhao et al. applied SPECT/CT for thoracic tumour biopsy and confirmed its safety and reliability [8].
This study applied SPECT/CT to guide biopsy for suspected bone metastatic lesions in breast cancer. In our study, the relatively safer puncture site that is suspicious on SPECT/CT images was preferred consideration. Thus, the biopsy success rate was 100%, there were no serious complications, and an adequate amount of tissue was obtained in all 49 patients. 5 of 49 patients had no morphological changes on CT and were thus not suitable for CT-guided biopsy, while SPECT/CT identified an accurate biopsy site. Statistical analysis demonstrated that SPECT/CT-guided BMB showed significantly higher sensitivity and NPV when compared to SPECT/CT for determination of BM. Methods, results, advantages and potential limitations of reported previous studies to evaluate metastasis involvement in breast cancer and the present study are summarized in Table 4.
Table 4.
Methods, results, advantages and potential limitations of reported previous studies to evaluate metastasis involvement in breast cancer and the present study
| Reference First author, year |
Methods | Positive samples for metastatic breast cancer | Samples with adequate number of tumor cells for receptor analysis | Potential limitations |
|---|---|---|---|---|
| J. F. Hilton, 2011 [14] | CT-guided bone biopsy | 21/39(52.5%) | 19/39(48.8%) | Cannot confirm the location for tumors at the early stage |
| Wei Guo,2018 [6] | PET/CT guided biopsy | 46/54(85.2%) | 23/46(50%) | More expensive and less equipment |
| This study | SPECT/CT guided bone biopsy | 34/39(69%) | 34/34(100%) | 99mTc-MDP is not a specific tracer for metastatic disease |
The possible reasons lie in the principle of 99mTc-MDP and the limitations of SPECT/CT. Abnormal accumulation of 99mTc-MDP is related to changes in local blood flow and osteoblastic activity, but does not reflect the true tumor burden in the bone marrow. The mechanism of accumulation means that the uptake of 99mTc-labeled diphosphonates is not specific for metastatic disease [24]. BS and SPECT/CT sometimes fail to distinguish BM from benign disease, including trauma, inflammation and primary tumor of bone [10].
The additional radiation dose in this study was from positioning CT. Because of the advantage of fusion images, the tube current and tube voltage were only 20 mA and 120 mV, respectively, and the effective radiation dose was approximately (1.9 ± 0.8) mSv, which was lower than the dose for one-time chest CT scans and doses in literature reports [25].
In our study, the changing rates between metastatic lesions and primary lesions for ER, PR, and HER-2 expression in 34 metastatic tumors were 32.3% (11/34), 47% (16/34), and 14.7% (5/39) respectively, and the changing rate in the molecular subtype was 54.3% (19/39), a finding that was basically consistent with that in literature reports [6, 26].
In summary, this study suggested that SPECT/CT-guided bone biopsy was safe and feasible and did not significantly increase the radiation dose. It provides breast cancer patients with an opportunity for accurate pathological and heterogeneous diagnosis of suspicious BM. It has high clinical value and is worthy of extensive clinical application.
The limitations of our study were inherent to its retrospective design. In addition, conventional decalcification in bone biopsy histopathology might influence immunohistochemical results, therefore, the ER, PR, and HER-2 expression results might not be accurate.
Conclusion
It is insufficient to evaluate BM in BC using 99mTC-MDP SPECT/CT imaging. It is recommended that SPECT/CT-guided BMB be performed if positive lesions were identified on SPECT/CT imaging, which could offer significantly higher sensitivity and NPV when compared to SPECT/CT for detection of BM in BC patients. In addition, our study initially showed that SPECT/CT guidance provided a new integrated approach for bone metastatic BC patients, which include diagnosis of bone lesions, as well as a new SPECT/CT-guided BMB method to achieve tissue samples for monitoring potential molecular subtyping shifts of BC.
The integrated approach that includes SPECT/CT and SPECT/CT-guided BMB, which can be performed in one stop in nuclear medicine department, can offer important opportunities for precision treatment and improved quality of life of breast cancer patients with BM.
Acknowledgements
We are indebted to Nuclear department of Shanxi Bethune Hospital for providing technical assistance. We also thank all the patients for allowing us to analyze their data.
Abbreviations
- BC
Breast cancer
- BM
Bone metastases
- BMB
Bone marrow biopsy
- NCCN
National Comprehensive Cancer Network
- ESMO
European Society for Medical Oncology
- CACA
China Anti-cancer Association
- MDP
99Tcm-methyl diphosphonate
- BS
Bone scintigraphy
- ER
Based on estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- DLP
Dose-length product
- PPV
Positive predictive value
- NPV
Negative predictive value
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xiaomin Li, Caixia An and Wanchun Zhang. The first draft of the manuscript was written by Xiaomin Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Shanxi Province key research and development project (grant no. 201903D321202).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principlesof the1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Human Research Ethics Committee of Shanxi Bethune Hospital, Taiyuan, China (YXLL-2020–033). Written informed consent was obtained from each participant.
Consent to publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaomin Li, Email: lxmxman@163.com.
Wanchun Zhang, Email: zhang_wanchun@126.com.
References
- 1.Marazzi F, et al. Diagnosis and treatment of bone metastases in breast cancer: radiotherapy, local approach and systemic therapy in a guide for clinicians. Cancers (Basel) 2020;12(9):2390. doi: 10.3390/cancers12092390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi L, et al. State of art and advances on the treatment of bone metastases from breast cancer: a concise review. Chin Clin Oncol. 2020;9(2):18. doi: 10.21037/cco.2020.01.07. [DOI] [PubMed] [Google Scholar]
- 3.Sathiakumar N, et al. Accuracy of medicare claim-based algorithm to detect breast, prostate, or lung cancer bone metastases. Med Care. 2017;55(12):e144–e149. doi: 10.1097/MLR.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breast Cancer Professional Committee of Chinese Anticancer Association Chinese expert consensus on the clinical diagnosis and treatment of advanced breast carcinoma (2018) Chin J Oncol. 2018;9(40):703. doi: 10.3760/cma.j.issn.0253-3766.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Guo W, et al. Early re-staging and molecular subtype shift surveillance of locally recurrent or metastatic breast cancer: A new PET/CT integrated precise algorithm. Cancer Lett. 2018;418:221–229. doi: 10.1016/j.canlet.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Hao B, et al. Is it sufficient to evaluate bone marrow involvement in newly diagnosed lymphomas using (18)F-FDG PET/CT and/or routine iliac crest biopsy? A new approach of PET/CT-guided targeted bone marrow biopsy. BMC Cancer. 2018;18(1):1192. doi: 10.1186/s12885-018-5104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao YJ, Ni JM, Tang P, et al. SPECT/CT-guided percutaneous transthoracic needle biopsy of thoracic masses. Chin J Nucl Med Mol Imaging. 2018;4(38):238-42.
- 9.Goldhirsch A, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, et al. Bone scintigraphy in breast cancer: added value of hybrid SPECT-CT and its impact on patient management. Nucl Med Commun. 2012;33(2):139–147. doi: 10.1097/MNM.0b013e32834e3b14. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Vicente AM, et al. Molecular subtypes of breast cancer: metabolic correlation with (1)(8)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40(9):1304–1311. doi: 10.1007/s00259-013-2418-7. [DOI] [PubMed] [Google Scholar]
- 12.Haynes B, et al. Breast cancer complexity: implications of intratumoral heterogeneity in clinical management. Cancer Metastasis Rev. 2017;36(3):547–555. doi: 10.1007/s10555-017-9684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang H, et al. Preclinical advances in theranostics for the different molecular subtypes of breast cancer. Front Pharmacol. 2021;12:627693. doi: 10.3389/fphar.2021.627693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilton JF, et al. Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Res Treat. 2011;129(3):761–765. doi: 10.1007/s10549-010-1264-6. [DOI] [PubMed] [Google Scholar]
- 15.Working Committee on Molecular Imaging mediated Accurate Diagnosis of Nuclear Medicine Branch of Chinese Medical Association. Chinese Society of Nuclear Medicine 2016 expert consensus document on PET/CT-guided percutaneous biopsy. Chin J Nucl Med Mol Imaging. 2016;6(36):542-5.
- 16.Iagaru A, Minamimoto R. Nuclear medicine imaging techniques for detection of skeletal metastases in breast cancer. PET Clin. 2018;13(3):383–393. doi: 10.1016/j.cpet.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Zacho HD, et al. Three-minute SPECT/CT is sufficient for the assessment of bone metastasis as add-on to planar bone scintigraphy: prospective head-to-head comparison to 11-min SPECT/CT. EJNMMI Res. 2017;7(1):1. doi: 10.1186/s13550-016-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh P. The role of SPECT/CT in skeletal malignancies. Semin Musculoskelet Radiol. 2014;18(2):175–193. doi: 10.1055/s-0034-1371019. [DOI] [PubMed] [Google Scholar]
- 19.Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med. 2016;57(Suppl 1):27S–33S. doi: 10.2967/jnumed.115.157867. [DOI] [PubMed] [Google Scholar]
- 20.Mahaletchumy T, AbAziz A. Incremental value of single-photon emission computed tomography-computed tomography for characterization of skeletal lesions in breast cancer patients. World J Nucl Med. 2017;16(4):303–310. doi: 10.4103/1450-1147.215496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaeser B, et al. PET/CT-guided biopsies of metabolically active bone lesions: applications and clinical impact. Eur J Nucl Med Mol Imaging. 2010;37(11):2027–2036. doi: 10.1007/s00259-010-1524-z. [DOI] [PubMed] [Google Scholar]
- 22.Cerci JJ, et al. The impact of coaxial core biopsy guided by FDG PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2013;40(1):98–103. doi: 10.1007/s00259-012-2263-0. [DOI] [PubMed] [Google Scholar]
- 23.Guo W, et al. PET/CT-guided percutaneous biopsy of FDG-avid metastatic bone lesions in patients with advanced lung cancer: a safe and effective technique. Eur J Nucl Med Mol Imaging. 2017;44(1):25–32. doi: 10.1007/s00259-016-3455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rager O, et al. Accuracy of whole-body HDP SPECT/CT, FDG PET/CT, and their combination for detecting bone metastases in breast cancer: an intra-personal comparison. Am J Nucl Med Mol Imaging. 2018;8(3):159–168. [PMC free article] [PubMed] [Google Scholar]
- 25.Chintapalli KN, et al. Radiation dose management: part 1, minimizing radiation dose in CT-guided procedures. AJR Am J Roentgenol. 2012;198(4):W347–W351. doi: 10.2214/AJR.11.7958. [DOI] [PubMed] [Google Scholar]
- 26.Zardavas D, et al. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–394. doi: 10.1038/nrclinonc.2015.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.