Abstract
Diffusion tensor imaging is capable of resolving large fiber bundles (e.g. the corpus callosum) and has been quite informative in understanding the overall structural connectivity of the brain. Recent data has shown that traditional resolution limitations can be exceeded in humans in vivo to submillimeter resolution. This chapter discusses these new techniques, and specific applications to small pathways such as the perforant path in the medial temporal lobe. High-resolution diffusion tensor imaging is a promising new tool that can be used to discover novel biomarkers for Alzheimer’s disease and other disorders. It allows for a much more detailed investigation of brain white matter than previously possible, perhaps offering clues into the first signs of synaptic deterioration that may precede frank neuronl loss. Although these methods are still in their infancy and many challenges have to be overcome before they can be used in a clinical fashion, results so far have been promising. Challenges and future directions are discussed in detail.
Keywords: Hippocampus, perforant path, diffusion tensor imaging, biomarkers, Alzheimer’s disease, aging, entorhinal cortex
OVERVIEW OF DTI AND ITS APPLICATIONS TO ALZHEIMER’S DISEASE
Diffusion tensor imaging (DTI) is based on the principle that water molecules are constantly in motion. Although this motion is typically random, organized structures such as axons or dendrites constrain the mobility of water molecules making it less random. For example, water will diffuse more readily along the principal axis of an axon than perpendicular to it, thus its motion becomes more directional or anisotropic. In a broad sense, DTI attempts to quantify diffusion anisotropy in a meaningful way. The pioneering work of Basser and colleagues [1-4] first introduced a rigorous formulation of anisotropy in the diffusion tensor. Since the mid-nineties, DTI has evolved through many stages of development.
It is now possible to sample water movement in hundreds of directions (high-angular resolution diffusion imaging or HARDI) whereas original DTI studies only required six directions, the minimum number of directions required to estimate a diffusion tensor. Methods to account for fiber crossing, bending and twisting such as Q-ball imaging [5] and diffusion spectrum imaging (DSI) [6, 7] have been developed. Various sophisticated fiber-tracking techniques have now become commonplace tools in neuroimaging laboratories for mapping white matter connectivity [8-12].
Diffusion signals capture microstructural properties of white matter that cannot otherwise be captured on traditional structural MRI scans. For example, diffusion anisotropy is increased in regions of high axonal coherence, robust myelination, and tight packing and decreased in areas where white matter is not as organized [1, 4, 13]. Given the method’s increased sensitivity to microscopic changes in white matter, it has remarkable promise for detecting white matter abnormalities otherwise invisible to traditional structural MRI methods [14]. This makes it an especially attractive technology to use for investigating white matter changes in aging as well as Alzheimer’s disease (AD) and its prodromal state, mild cognitive impairment (MCI).
Studies of the aging brain using DTI have generally found decreased fractional anisotropy (FA) and increased mean diffusivity (MD) in frontal white matter, the anterior cingulum, the fornix, and the corpus callosum (c.f. [15]). These changes in anisotropy and diffusivity are generally attributed to fiber degeneration and demyelination with increasing age. On the other hand, diffusion imaging studies of MCI and AD patients have observed decreased anisotropy throughout the brain but most notably in the temporal lobes (see recent reviews [15] and [16]).
A large body of research has indicated that the medial temporal lobes (MTL), and in particular the hippocampus and entorhinal cortex, are the first to deteriorate in the course of AD [17-19]. Several studies have used DTI to investigate the MTL in particular in MCI and AD. For example, Fellgiebel and colleagues [20] observed decreased FA and increased MD in the left hippocampus in AD patients compared to controls. Mielke et al. [21] noted in AD patients decreased FA in the fornix and cingulum, the two major fiber bundles that connect the limbic lobes to the rest of the brain. They also observed less dramatic changes in individuals with MCI, suggesting that these microstructural alterations likely vary along a spectrum from MCI to AD.
Changes in parahippocampal white matter have been observed in AD patients using DTI, e.g. [22]. A recent study by Choo et al. [23] reported a dissociation within cingulum fibers that may distinguish MCI from AD. They observed decreased FA in parahippocampal cingulum fibers in individuals with MCI and decreased FA in posterior cingulate cingulum fibers in AD patients. This may indicate that there is a topological pattern of white matter degeneration that manifests at the early stages of AD with the parahippocampal white matter being the site of the earliest changes.
THE NEED FOR HIGH-RESOLUTION DTI OF THE MEDIAL TEMPORAL LOBE
Although traditional DTI has been helpful in evaluating the state of MTL white matter in general, it remains largely unable to distinguish among the many fiber pathways in the region. Very few attempts have been made to use DTI to investigate smaller pathways within the medial temporal lobe. Aside from the work I will discuss shortly, only one previous study attempted to quantify diffusion signals in the perforant path region in vivo. Kalus et al. [24] found reductions in intervoxel coherence in the perforant path zone in individuals with MCI compared to controls, possibly indicating synaptic loss in the region. However, since there are many crossing fibers in this region, it was not possible to uniquely attribute these signal losses to the perforant path itself.
High-resolution DTI offers a means to identify microstructural signals that can be uniquely attributed to individual fiber bundles. The increased resolution allows us to conduct a more detailed investigation where the contribution of each individual fiber bundle to an image voxel is magnified. As is the case with almost any imaging technique, increasing resolution is a substantial challenge. First, it is almost impossible to increase the resolution without sacrificing signal-to-noise ratio (SNR). Multiple averages are necessary to match traditional SNR levels. Second, one would need to apply parallel imaging techniques such as SENSE [25, 26] in order to reduce scan time to a clinically applicable range (~1 hr).
Third, the microstructural anatomical features reflected in high-resolution diffusion signals need to be adequately investigated. For example, at high resolution, white matter is not the only tissue in which anisotropic diffusion can be observed. Pyramidal cells have long apical dendrites, which also demonstrate remarkable anisotropy on high-resolution rodent DTI scans [27-29]. Thus, diffusion indices at high-resolution must be validated either by relating them to other structural and functional measures, to a relevant behavioral outcome, or to histological features.
If all of the above challenges can be addressed, one key question is the extent to which resolution can/must be enhanced. Here, it is important to keep in mind that the required resolution to resolve a small pathway like the perforant path may be different from the maximum resolution that can be achieved in a clinically practical scan time. The physiological limitation in diffusion imaging is imposed by water molecules. Thus, unlike functional MRI where optimal resolutions range around 1–1.5 mm (the approximate size of the capillary bed giving rise to the BOLD signal), the theoretical limitation on resolution in DTI is far smaller and is quite likely in the nanometer range. Our experiments with this technique in humans in vivo suggest that a resolution of ~0.5 mm sufficiently samples signals from small regions within the hippocampus and can index anisotropic diffusion in pyramidal dendrites as well as small fiber pathways [30]. It is also a sequence that can be performed in under forty minutes (twelve scans) and thus can be applied in a clinical setting.
HIGH-RESOLUTION DTI AND THE PERFORANT PATH
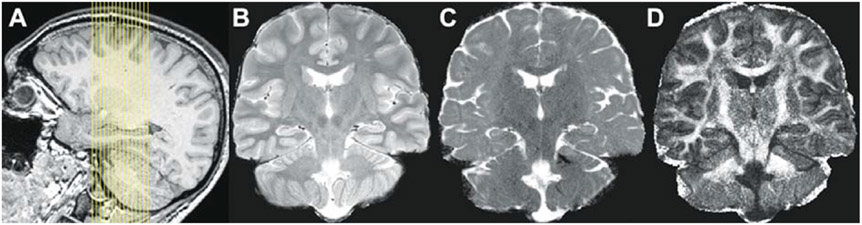
A novel high-resolution DTI sequence was recently introduced by Yassa et al. [30]. It is a 32-direction, limited field of view (FOV) scanning protocol at 0.66 mm x 0.66 mm in-plane, with a slice thickness of 3 mm. This is a sequence optimized for the MTL with the high-resolution plane corresponding to the coronal plane perpendicular to the principal axis of the hippocampus (Fig. 1A). Our choice of this plane was guided by tracer studies in primate tissue [31] suggesting that the coronal sections are the ones where the perforant path can be evaluated easily.
Fig. 1.
(A) Slice placement and field of view for high-resolution DTI scan, (B) High-resolution T2-weighted FSE scan acquired in the same geometry as the DTI scan, (C) single subject sample MD image based on 12 averages, and (D) single subject sample FA image based on 12 averages. B, C, and D are from the same subject. Alignment of C and D with B shows the limited effects of distortions on the region of interest.
The technical details of this sequence were published in [30] and are shown here in Table 1. Fig. 1B shows an in-plane T2 Fast Spin Echo scan acquired in the same resolution as the diffusion images for registration. Fig. 1C shows a sample mean diffusivity (MD) and Fig. 1D shows a sample fractional anisotropy (FA) image.
Table 1.
High-resolution DTI Scan Parameters
| Parameter | Value |
|---|---|
| FOV | 170 × 170 |
| Matrix size | 256 × 256 |
| Voxel size | 0.664 × 0.664 mm |
| Slice thickness | 3 mm (1 mm gap) |
| Number of slices | 15 |
| TR/TE | 2,717 ms/67 ms |
| Flip angle | 90° |
| SENSE factor | 2.5 |
| b-value | 1,200 s/mm2 |
| Directions | 32 |
| Averages | 12 |
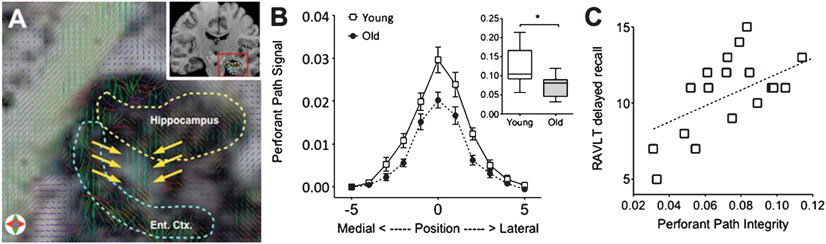
This sequence can be easily adapted to any Philips 3.0 Tesla scanner or any scanner that can make use of parallel imaging techniques (e.g. SENSE and GRAPPA). We used this sequence in order to visualize and quantify the perforant path (Fig. 2A) and assess its integrity in a sample of young and older adults. We developed a simple assessment method that calculated the magnitude of the diffusion signal along the canonical orientation of the perforant path. This quantity (Eq. 1 in [30]) was evaluated along the entire bank of the entorhinal cortex giving rise to a Gaussian-like curve that captured the perforant path from its most medial extent to its most lateral. This signal was operationalized as area-under-the-curve (AUC), which showed a significant difference between young and aged adults (Fig. 2B).
Fig. 2.
(A) Tensor visualization of the perforant path (indicated by yellow arrows) overlaid on a FA map. After Yassa et al., PNAS 2010; 107(28):12687-91, Fig. 1D, reprinted with permission. (B) comparison of perforant path diffusion signal in young vs. old showing a significant difference in area under the curve. After Yassa et al., PNAS 2010; 107(28):12687-91, Fig. 3, reprinted with permission, (C) correlation between perforant path integrity (operationalized as area under the curve) and delayed verbal recall in older adults. Adapted from Yassa et al., PNAS 2010; 107(28):12687-91 with permission.
To ascertain that this difference was unique to the perforant path, we conducted the same measurements in another hippocampal pathway, the temporoalvear pathway, which does not seem to degrade substantially with aging [32]. We found no evidence for a group difference in this “control” pathway suggesting that the perforant path findings are not evidence for global white matter differences between groups. We also found a strong relationship in the older adults between our measure of perforant path integrity and scores on the Rey Auditory Verbal Learning Test (RAVLT), a memory measure that is sensitive to hippocampal deficits (Fig. 2C), suggesting that this diffusion signal has a valid neurobiological basis. No other relationships with neuropsychological measures of intelligence, working memory, attention and executive function were found, strongly suggesting that perforant path deficits have specific functional implications to hippocampal-dependent memory.
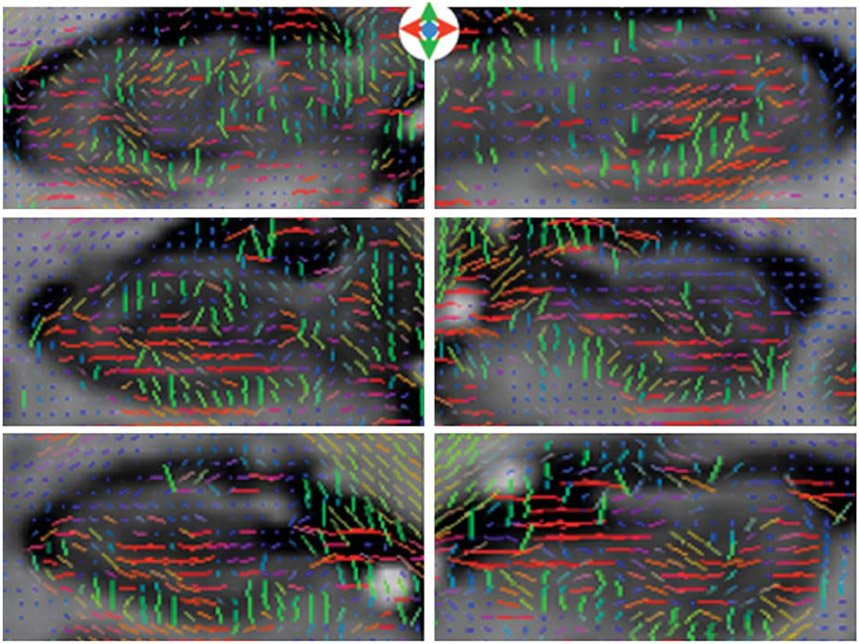
In addition, we showed that high-resolution DTI is sensitive to hippocampal diffusion signals likely arising from pyramidal dendrites, although we did not formally assess dendritic integrity in this study (Fig. 3). This investigation requires the parcellation of gray and white matter compartments inside the hippocampus using a combination of structural images and FA maps with the additional delineation of subfields. Preliminary data suggest that dendritic integrity can be assessed at the subfield level (Yassa M. and Stark C. unpublished data).
Fig. 3.
Hippocampal gray matter anisotropy. Note the change in tensor orientation from green (superior-inferior) to red (left-right) consistent with the changing orientation of the pyramidal cells in the folded hippocampal sheet. After Yassa et al., PNAS 2010; 107(28):12687-91, Fig. S2, reprinted with permission.
Overall, the results of this high-resolution DTI investigation in humans in vivo have been quite promising. They demonstrate that: (1) high-resolution DTI is possible in humans in clinically applicable scan durations (45–60 minutes), (2) high-resolution diffusion signals have the key advantage of being able to evaluate small diffuse pathways such as the perforant path as well as dendritic diffusion signals, and (3) high-resolution diffusion measures are associated with behavioral measures dependent on the integrity of the pathway or region in question.
RELATIONSHIP OF HIGH-RESOLUTION DIFFUSION INDICES TO HISTOLOGICAL FEATURES
A critical issue with diffusion imaging especially in the absence of frank pathology (e.g. stroke or lesion) is the validity of the signal. For example, what does it mean to have decreased fractional anisotropy? Is there a neurobiological basis for FA group differences? Although there have not been many formal investigations of the microstructural anatomical features that may give rise to differences in diffusion signals, recent work has provided some promising results.
For example, Pych and colleagues [33] used DTI microimaging to show that the size of the hippocampal dendritic compartment was tightly coupled to fractional anisotropy in a mouse model of AD. In fact, their initial experiments and the work by Mori and others [27-29] partly inspired our attempt to use in vivo high-resolution DTI to attempt to visualize and quantify pyramidal dendrites in the hippocampus.
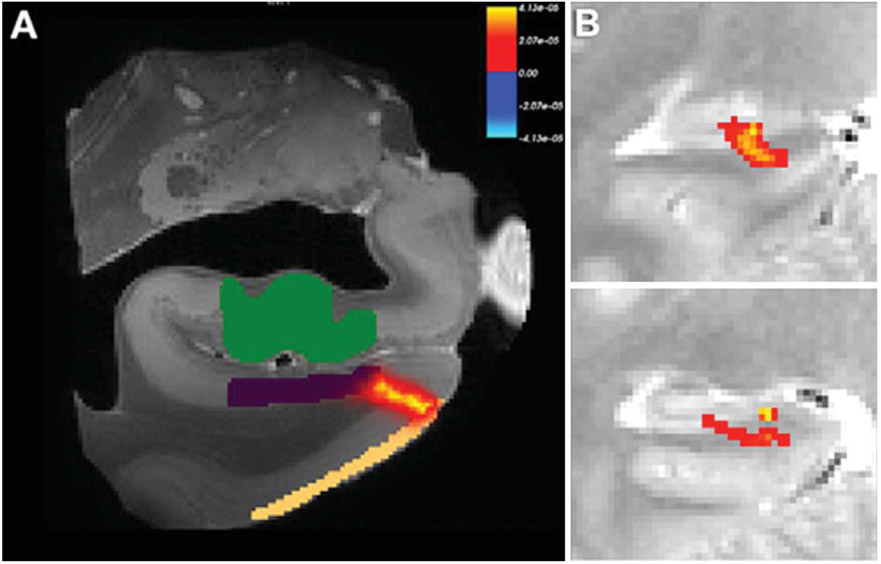
A recent investigation [34] used high-resolution DTI to visualize the perforant pathway in ex vivo human tissue. The authors used probabilistic tractography to map the perforant pathway from the entorhinal cortex to the hippocampus (Fig. 3A). We have also conducted a similar investigation using FSL’s bedpostX and probtrackX and found remarkably similar results (Fig. 3B). The authors validated the ex vivo tractography results using histology (Luxol Fast Blue myelin stain) which stained the perforant path in the same locations identified by the diffusion signals.
These studies were a necessary first step towards validation. However, other potential relationships between diffusion signals and synaptic markers remain to be investigated. For example, work by Smith et al. [35] suggests that synaptophysin immunoreactivity at the entorhinal-dentate perforant path synapse is reduced in aged rodents with spatial learning impairments. It would be interesting to see how well diffusion measures correlate with presynaptic markers such as synaptophysin. In addition, if these diffusion measures can be used to track dendritic changes, validation using dendritic markers such as synaptopodin and MAP2 may also be informative. These validation efforts can be conducted in animal models and can significantly inform neuroimaging efforts in the human.
ISOLATING BIOMARKERS FOR ALZHEIMER’S DISEASE
A logical question is whether high-resolution DTI can be used to identify early biomarkers for Alzheimer’s disease. AD biomarker discovery is one of the most active areas of research. Converging evidence across studies suggests that about a third of cognitively normal older adults have many of the traditional biomarker signatures present in AD, such as cortical amyloid binding [36-38] and cerebrospinal fluid-derived Aβ42 and phosphorylated tau [39, 40]. The above is also true for histopathological features (i.e., senile plaques and neurofibrillary tangles) identified upon autopsy [41]. There is an emerging consensus that these markers in nondemented adults represent a preclinical form of AD [37, 38, 41]. However, determining which cognitively normal individuals will ultimately develop AD remains a significant challenge.
There is an increasing need to develop biomarkers that reflect neural changes at the earliest stages of the disease in order to correctly target susceptible individuals. It is unlikely that any single biomarker will be 100% predictive. However, we can attempt to develop a “biomarker profile” that combines the most predictive probes in order to determine who will convert to AD with greater confidence.
High-resolution DTI of the hippocampus may offer novel contributions to this endeavor. One potential biomarker may be perforant path integrity. We have shown that perforant path integrity is coupled to behavioral performance on a hippocampus-dependent verbal learning test [30]. Although this investigation was conducted in nondemented older adults, the same approach can be applied to the earliest stages of dementia.
While AD affects the entire brain, the hippocampus and entorhinal cortex are the sites of the earliest pathological changes. It is possible that perforant path degradation is one of the earliest pathological features in the MTL that precedes frank cellular loss. Such a measure, if combined with other biomarkers, can increase our diagnostic accuracy and can also be used as an outcome measure to evaluate the efficacy of therapeutic interventions. Furthermore, since perforant path integrity is related to performance on a memory test, it is likely that it is part of a mechanistic dysfunction. If so, then intervention at this level may rescue memory deficits.
The terrain for Alzheimer’s disease biomarker discovery using high-resolution DTI is largely uncharted. This method presents novel and exciting opportunities. Unlike other structural and functional imaging methods high-resolution DTI could provide important insights into fiber/synaptic and dendritic integrity, making it possible to address numerous questions that could not previously be addressed.
REMAINING TECHNICAL CHALLENGES AND FUTURE DIRECTIONS
Although the first high-resolution DTI experiments have been largely successful, it is important to note that the technique is still in its infant stages. There are many challenges that remain to be addressed. For example, the use of single shot echoplanar imaging (ssEPI) is associated with geometric distortions (stretching and shearing) caused by local eddy currents in the gradient coils. We have avoided this problem largely by acquiring a small FOV around the medial temporal lobe. However, in order to more directly ameliorate this problem, multishot sequences can be used. These sequences are far more robust against distortion effects, however they are much more susceptible to motion artifacts. As a result, about 30–40% of all scans might be discarded due to motion effects.
Even if the subject holds perfectly still, the brain’s pulsatile motion can potentially induce artifacts. One way to address this is the use of cardiac or respiratory gating, which can compensate for pulsatile motion [42]. Of course, this comes at the cost of increasing scan time, but there may be a suitable tradeoff that can be reached. It is worthy of note that the effect of physiological noise on high-resolution scans is much higher than traditional resolution scans, since smaller voxels are more likely to be affected than larger voxels (where the same shifts are less likely to affect whole voxels).
Our current scan time is under an hour. However, in order to make this type of scan more clinically applicable, reducing this time to under a half hour is an important future direction. We are currently experimenting with increasing parallel imaging factors to achieve this goal. One additional benefit of decreasing the scan time is that scan fidelity would remain high as smaller amounts of motion correction have to be applied to the data before tensor solving.
Although our current in-plane resolution is relatively high, the thru-plane resolution is still on par with traditional DTI, thus leading to partial volume contamination. Despite the fact the hippocampus is a relatively straight structure that largely conforms to the plane of slice acquisition, it still does exhibit some inter-individual variation and bends upwards towards the tail. Thus, an important future goal in increasing our scan resolution is to use isotropic voxels and perhaps achieve a 0.5 mm isotropic resolution. This can be made possible by scanning at higher field strengths. As 7.0 Tesla magnets are becoming more commonplace across institutions in the country, this endeavor is becoming more feasible. Adapting the sequence we created for the 3.0 T scanner to 7.0 T should not be difficult but will requires additional pulse sequence programming to optimize it for the higher-strength magnet.
Another significant challenge is that the SNR in individual scans is still quite low and thus multiple averages are required in order to generate reasonable scalar maps. Although averaging may help with SNR, the low SNR in individual scans can induce a bias in the diffusion images that depends on the scan orientation and the gradients employed. We used a slightly higher b-value than is typical for traditional DTI (1200 s/mm2) to attempt to increase the individual scan SNR. It is possible that even higher b-values can be used, although this has to be balanced against the choice of voxel resolution, angular resolution, SNR and clinically feasible times. This compromise is unfortunately far from trivial.
The MTL and in particular the perforant path regions contain many crossing fibers. A fundamental flaw in the diffusion tensor model is that it assumes a single ellipsoid with a single orientation in each voxel, thus it is unable to capture physiological features of white matter tracts such as fiber crossing, bending and twisting. Other evolving methods may offer some promise in resolving these characteristics. For example, Q-ball imaging [5], a high-angular resolution diffusion imaging (HARDI) technique, uses a mathematical alternative to the tensor model that employs probability distributions and vector math to model anisotropy. By collecting high-angular resolution data (e.g., 256 directions) it is possible to identify several crossing tracts per voxel.
More recently, several methods to extract super-resolution features from diffusion data have been developed. One such method, Spatial HARDI [43] uses spatial information in a Bayesian framework to constrain the reconstruction of raw diffusion MRI data. This spatial regularization scheme when applied to spherical harmonic q-ball imaging improves SNR and better identification of crossing fibers leading to more accurate tractography. Another method, track-density imaging (TDI; [44]) uses post-processing methods based on diffusion MRI fiber-tracking to increase spatial resolution beyond the limitations of the single voxel. Each technique is not without its own challenges and limitations, however, using multiple techniques to arrive at similar conclusions is an essential component of neuroscientific inquiry.
Another significant challenge for using diffusion methods to investigate the integrity of the medial temporal lobe in Alzheimer’s disease is that this region undergoes substantial structural changes that could have profound effect on diffusion indices. For example, a decrease in fractional anisotropy could be caused by a decrease in white matter integrity or regional atrophy. It is likely that diffusion-based methods will be more helpful in elucidating subtle changes early in the course of the disease and not late. Comparison of these high-resolution methods with traditional DTI methods for investigating aging and AD is also necessary.
Aside from the technical challenges, much remains to be done to validate the measures and indices we are currently using. This can be done using cross-species investigations with histological validation. Augustinack et al., [34] and others have already initiated this important work. However, scanning an animal model in vivo and following up the scans with detailed histological analyses of pre- and post-synaptic integrity will also be necessary to determine the meaning of high-resolution diffusion signals.
In summary, high-resolution DTI and the application to perforant path imaging demonstrates the power and promise of diffusion imaging at high resolutions. Together with other functional and structural imaging tools, diffusion imaging may provide important clues and help discover novel biomarkers that can be used for diagnosis and intervention in Alzheimer’s disease and other brain disorders.
Fig. 4.
(A) Probabilistic tractography of the perforant path in ex vivo human tissue. After Augustinack et al., Frontiers in Human Neuroscience, 2010; 4(42),1-13 reprinted with author’s permission. (B) Sample probabilistic tractography of the perforant path in a human subject in vivo. In both cases, the perforant path clearly originates in the entorhinal cortex and terminates in the hippocampus. Both studies are consistent in terms of the location and approximate size of the perforant path.
REFERENCES
- [1].Basser PJ (1995) Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8, 333–344. [DOI] [PubMed] [Google Scholar]
- [2].Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103, 247–254. [DOI] [PubMed] [Google Scholar]
- [4].Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiolog 201, 637. [DOI] [PubMed] [Google Scholar]
- [5].Tuch DS (2004) Q-ball imaging. Magn Reson Med 52, 1358–1372. [DOI] [PubMed] [Google Scholar]
- [6].Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM (2005) Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54, 1377–1386. [DOI] [PubMed] [Google Scholar]
- [7].Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41, 1267–1277. [DOI] [PubMed] [Google Scholar]
- [8].Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies - a technical review. NMR Biomed 15, 468–480. [DOI] [PubMed] [Google Scholar]
- [9].Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tournier JD, Calamante F, King MD, Gadian DG, Connelly A (2002) Limitations and requirements of diffusion tensor fiber tracking: an assessment using simulations. Magn Reson Med 47, 701–708. [DOI] [PubMed] [Google Scholar]
- [11].Berman JI, Chung S, Mukherjee P, Hess CP, Han ET, Henry RG (2008) Probabilistic streamline q-ball tractography using the residual bootstrap. Neuroimage 39, 215–222. [DOI] [PubMed] [Google Scholar]
- [12].Iturria-Medina Y, Canales-Rodriguez EJ, Melie-Garcia L, Valdes-Hernandez PA, Martinez-Montes E, Aleman-Gomez Y, Sanchez-Bornot JM (2007) Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. Neuroimage 36, 645–660. [DOI] [PubMed] [Google Scholar]
- [13].Pierpaoli C and Basser PJ (1996) Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36, 893–906. [DOI] [PubMed] [Google Scholar]
- [14].Taylor WD, Hsu E, Krishnan KR, MacFall JR (2004) Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry 55, 201–207. [DOI] [PubMed] [Google Scholar]
- [15].Chua TC, Wen W, Slavin MJ, Sachdev PS (2008) Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol 21, 83–92. [DOI] [PubMed] [Google Scholar]
- [16].Stebbins GT, Murphy CM (2009) Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol 21, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344, 769–772. [DOI] [PubMed] [Google Scholar]
- [18].Gomez-Isla T, Price JL, McKeel DWJ, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer ii entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16, 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27, 1372–1384. [DOI] [PubMed] [Google Scholar]
- [20].Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P (2004) Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 18, 101–108. [DOI] [PubMed] [Google Scholar]
- [21].Mielke MM, Kozauer NA, Chan KC, George M, Toroney J, Zerrate M, Bandeen-Roche K, Wang MC, Vanzijl P, Pekar JJ, Mori S, Lyketsos CG, Albert M (2009) Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neuroimage 46, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD (2010) White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol Aging 31, 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, Youn JC, Kim SG, Kim KW, Jhoo JH, Woo JI (2010) Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 31, 772–779. [DOI] [PubMed] [Google Scholar]
- [24].Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C (2006) Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage 30, 713–720. [DOI] [PubMed] [Google Scholar]
- [25].Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, van Muiswinkel AM, Mori S, van Zijl PC, Valavanis A, Kollias S, Boesiger P (2004) SENSE-DTI at 31. Magn Reson Med 51, 230–236. [DOI] [PubMed] [Google Scholar]
- [26].Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42, 952–962. [PubMed] [Google Scholar]
- [27].Zhang J, Miller MI, Plachez C, Richards LJ, Yarowsky P, van Zijl P, Mori S (2005) Mapping postnatal mouse brain development with diffusion tensor microimaging. Neuroimage 26, 1042–1051. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J, Richards LJ, Yarowsky P, Huang H, van Zijl PC and Mori S (2003) Three-dimensional anatomical characterization of the developing mouse brain by diffusion tensor microimaging. Neuroimage 20, 1639–1648. [DOI] [PubMed] [Google Scholar]
- [29].Zhang J, van Zijl PC, Mori S (2002) Three-dimensional diffusion tensor magnetic resonance microimaging of adult mouse brain and hippocampus. Neuroimage 15, 892–901. [DOI] [PubMed] [Google Scholar]
- [30].Yassa MA, Muftuler LT, Stark CE (2010) Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A 107, 12687–12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Witter MP (2007) The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res 163, 43–61. [DOI] [PubMed] [Google Scholar]
- [32].Wilson IA, Gallagher M, Eichenbaum H, Tanila H (2006) Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci 29, 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pych JC, Venkatasubramanian PN, Faulkner J, Wyrwicz A (2008) IC-P2-161: Decreased hippocampal fractional anisotropy in Tg2576 Alzheimer’s disease model mice may reflect a reduction in dendrites. Alzheimer’s and Dementia 4, T71–T72. [Google Scholar]
- [34].Augustinack JC, Helmer K, Huber KE, Kakunoori S, Zollei L, Fischl B (2010) Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Front Hum Neurosci 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20, 6587–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC (2007) Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844. [DOI] [PubMed] [Google Scholar]
- [38].Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O’Keefe G, Masters CL, Rowe CC (2008) Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia 46, 1688–1697. [DOI] [PubMed] [Google Scholar]
- [39].De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ (2010) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64, 343–349. [DOI] [PubMed] [Google Scholar]
- [41].Price JL, McKeel DWJ, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chung S, Courcot B, Sdika M, Moffat K, Rae C, Henry RG (2010) Bootstrap quantification of cardiac pulsation artifact in DTI. Neuroimage 49, 631–640. [DOI] [PubMed] [Google Scholar]
- [43].Raj A, Hess C, Mukherjee P (2011) Spatial HARDI: improved visualization of complex white matter architecture with bayesian spatial regularization. Neuroimage 54, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Calamante F, Tournier JD, Jackson GD, Connelly A (2010) Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 53, 1233–1243. [DOI] [PubMed] [Google Scholar]