Abstract
The hippocampal theta rhythm is critical for learning and memory. New research demonstrates that theta oscillations in freely moving humans are similar in frequency and function to those observed in rodents and are modulated by movement speed and exploratory behavior.
Going for an early morning run in El Moro Canyon is an unforgettable experience. Despite its familiarity, it somehow feels a tad different every time. The sensory barrage around each turn is a welcome change from the office setting that will occupy the rest of my day. Each point in the breathtaking landscape leaves a lasting memory, one that I myself help create as I move through the landscape. Much of our lives is spent moving and exploring in the same way, crafting experiences that are the result of our unique interactions with the world. In the evolutionary sense, we are constantly and actively sensing our environment to learn more about it and to make decisions that promote our survival. How does the brain transform this constant sensory flow into meaningful representations that guide memory, spatial navigation, and decision-making? One answer comes in the form of brain rhythms that synchronize activity within and across regions. A new study by Aghajan et al. [1], reported recently in Current Biology, demonstrates the prevalence of a fast theta rhythm (~8 Hz) in freely moving humans and suggests that the rhythm plays an important role in actively sensing and learning about the environment.
The hippocampal theta rhythm (4–12 Hz) is thought to regulate the hippocampal–cortical information exchange that enables the formation of memory representations [2]. While past studies have observed a robust hippocampal theta rhythm in moving rodents that increases with running speed [3], human evidence has been mixed [4], with virtual navigation and memory studies suggesting that human hippocampal theta may operate in a lower frequency band (4 Hz). A major concern for past neurophysiological recording studies in humans is that they have typically been conducted in patients while they were stationery, which diverges considerably from animal studies in which animals are typically free to move and explore. Recording in freely moving humans has historically been difficult, as epilepsy patients are typically tethered to the recording equipment. Advances in epilepsy monitoring and intervention with wireless recording and stimulation devices provide a unique opportunity for examining brain activity in untethered, freely moving humans.
In a sample of patients chronically implanted with the wireless NeuroPace Responsive Neurostimulator (RNS®), Aghajan et al. [1] found that theta oscillations were increased during movement compared to standing still, and further increased during fast movement compared to slow movement (Figure 1), suggesting a modulation of the theta rhythm by movement speed similar to results reported in rodents [3,5]. The observed theta was predominantly in the ~8 Hz range, which is comparable to results from rodent studies and contrasts sharply with previously reported functional relevance of a slower (3–4 Hz) theta in stationery humans in virtual navigation [6] and memory [7,8] tasks. Two other recent reports [9,10] also noted similar activity in the upper theta band in moving humans, providing convergent evidence that the 8 Hz rhythm is present and functionally relevant in humans. These recent studies also call into question models that functionally equate slow frequency (1–4 Hz) theta oscillations in humans with faster (6–12 Hz) theta oscillations in rodents [4].
Figure 1. Modulation of theta by speed of movement.
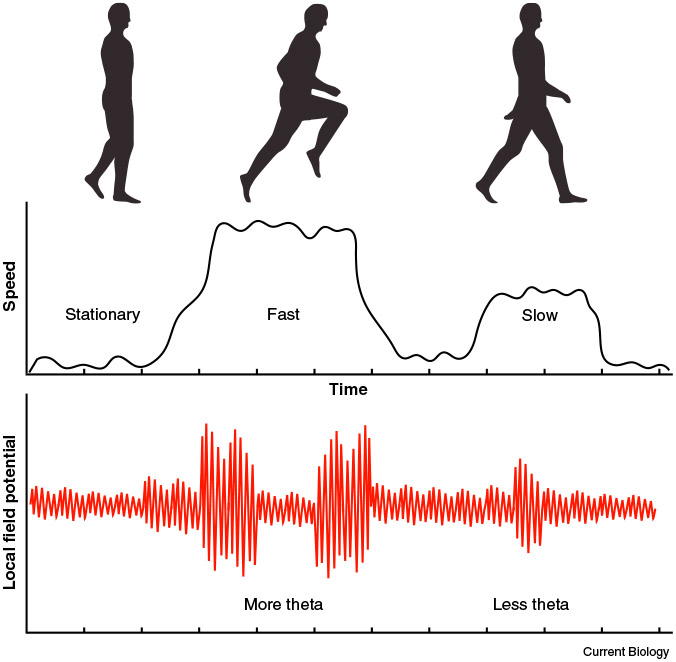
Aghajan et al. [1] find that, as movement speed increases (top graph), 8 Hz theta oscillations in the human hippocampus also increase in frequency (number of bouts), amplitude (the height of the peaks), as well as duration.
Despite the similarity of theta across species, Aghajan et al. [1] do report a striking divergence: they note that theta oscillations in moving humans, in contrast to those found in rodents, were not continuous but rather occurred in transient bouts. Interestingly, the bouts were higher in prevalence and duration in a congenitally blind participant who used a Hoover cane to navigate the environment. This observation raises the possibility that the discrepancy across species may be associated with the extent to which sampling and exploring the environment is a continuous multisensory experience in rodents compared to humans. It is possible that the rate of environmental exploration via somatosensory and path integration cues is higher in the blind participant than in sighted participants. While this possibility remains speculative, Aghajan et al. [1] additionally found that moving while head scanning from side to side was associated with a more prominent theta rhythm, suggesting that the amount of exploration may be a determinant of theta prevalence in humans. Studies in rodents have previously shown that head scanning is associated with faster formation and stability of place fields as well as higher theta power in the local field potential [11]. Taken together, these results suggest that theta plays an important role in actively sensing and forming representations of the experience.
While recordings in freely moving nonhuman primates are not readily feasible, eye movement tracking during virtual navigation has been used to assess the relationship between brain rhythms and recognition memory performance. Evidence from electrophysiological recording in the monkey hippocampus demonstrates a strong relationship between visual exploration by saccadic eye movements, memory performance, and theta oscillations [12]. The theta rhythm observed in monkeys is also in the upper theta band and exhibits a resetting that is locked to stimulus onset, suggesting an attentional role that is important for memory. Thus, saccadic eye movements, a chief exploratory mode for primates, are also associated with the theta rhythm and are linked to memory performance. In 1997, Gilchrist et al. [13] reported a case, A.I., who, due to a congenital condition, could not exhibit saccadic eye movements but was able to read at normal speeds, an unexpected finding given the known role of saccades in reading. Further testing indicated that A.I. compensated for her condition by moving her head in a saccadic pattern with an average fixation length of 200 ms, suggesting that she sampled text at around ~5 Hz. This further suggests that the theta rhythm may serve as an optimal oscillatory frequency for the brain to explore and sample the environment, even when it clearly comes at an energetic cost as in A.I.’s case.
The role of theta in building memory traces of the environment and the mechanisms by which this occurs remain subject to intense investigation, although interest in this area is anything but new. There is something oddly mysterious about the theta rhythm, one that has haunted scientists for decades but has been subject to inquiry by anthropologists for even longer. I will share an example that I find most fascinating. Scholars of circle drumming in indigenous cultures in Haiti and a number of regions in Africa suggest that the unusual behaviors and vivid hallucinations exhibited by dancers are due to a form of auditory entrainment to a frequency that is all too familiar [14]. While drumming may start slow at the beginning of a dance, it gradually speeds up to a tempo approaching 7–9 cycles per second (in the theta range), at which point the experience is heightened for the dancers and the audience.
Interestingly, auditory driving [15] using ‘theta drumming’ is thought to generate long-lasting memories of the experience [14]. Does it drive the hippocampal theta rhythm continuously to help encode the experience with rich detail? Is this type of theta activity distinct from the transient theta bouts reported by Aghajan et al. [1]? Does entrainment to a constant theta rhythm facilitate better encoding and retention? Of such rhythmic sensory stimulation, Walter and Walter in 1949 wrote that “… the hallucinations described by the subjects were of character so compelling that one subject was able to sketch them some weeks later” [16].
The idea is intriguing to say the least and is clearly not lost on commercial enterprises marketing consumer-grade products to sense brain waves and use auditory driving to induce ‘optimal states’. Whether the latter approach is of any real gain remains questionable but may be worth exploring in the laboratory. Until then, we should all seize the opportunity to actively explore the environment while we move through it, generating theta even if in transient bouts. It may be a nice break from walking while fixated on a small cell phone screen.
REFERENCES
- 1.Aghajan ZM, Schuette P, Fields T, Tran M, Siddiqui S, Hasulak N, Tcheng TK, Eliashiv D, Mankin EA, Stern J, Fried I, and Suthana N (2017). Theta oscillations in the human medial temporal lobe during real-world ambulatory movement. Curr. Biol 27, 3743–3751.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgin LL (2016). Rhythms of the hippocampal network. Nat. Rev. Neurosci. 17, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland WL, Teitelbaum H, and Hedges EK (1975). Relationship between hippocampal theta activity and running speed in the rat. J. Comp. Physiol. Psychol 88, 324–328. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs J (2014). Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos. Trans. R. Soc. Lond. B 369, 20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeewajee A, Barry C, O’Keefe J, and Burgess N (2008). Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, and Ekstrom AD (2013). A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 23, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lega BC, Jacobs J, and Kahana M (2012). Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22, 748–761. [DOI] [PubMed] [Google Scholar]
- 8.Lega BC, Burke J, Jacobs J, and Kahana MJ (2016). Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb. Cortex 26, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohbot VD, Copara MS, Gotman J, and Ekstrom AD (2017). Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat. Commun 8, 14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush D, Bisby JA, Bird CM, Gollwitzer S, Rodionov R, Diehl B, McEvoy AW, Walker MC, and Burgess N (2017). Human hippocampal theta power indicates movement onset and distance travelled. Proc. Natl. Acad. Sci. USA 114, 12297–12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monaco JD, Rao G, Roth ED, and Knierim JJ (2014). Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat. Neurosci 17, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutras MJ, Fries P, and Buffalo EA (2013). Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl. Acad. Sci. USA 110, 13144–13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist ID, Brown V, and Findlay JM (1997). Saccades without eye movements. Nature 390, 130–131. [DOI] [PubMed] [Google Scholar]
- 14.Neher A (1962). A physiological explanation of unusual behavior in ceremonies involving drums. Hum. Biol 34, 151–160. [PubMed] [Google Scholar]
- 15.Neher A (1961). Auditory driving observed with scalp electrodes in normal subjects. Electroencephalogr. Clin. Neurophysiol 13, 449–451. [DOI] [PubMed] [Google Scholar]
- 16.Walter VJ, and Walter WG (1949). The central effects of rhythmic sensory stimulation. Electroenceph. Clin. Neurophysiol 1, 57–86. [PubMed] [Google Scholar]


