Abstract
Background
Chronic kidney disease (CKD) happens due to decreasing kidney function. Inflammation and oxidative stress have been shown to result in the progression of CKD. Quercetin is widely known to have various bioactivities including antioxidant, anticancer, and anti-inflammatory activities.
Objective
To evaluate the activity of quercetin to inhibit inflammation, stress oxidative, and fibrosis on CKD cells model (mouse mesangial cells induced by glucose).
Methods and Material
The SV40 MES 13 cells were plated in a 6-well plate with cell density at 5,000 cells/well. The medium had been substituted for 3 days with a glucose-induced medium with a concentration of 20 mM. Quercetin was added with 50, 10, and 5 µg/mL concentrations. The negative control was the untreated cell. The levels of TGF-β1, TNF-α, and MDA were determined using ELISA KIT. The gene expressions of the SMAD7, SMAD3, SMAD2, and SMAD4 were analyzed using qRT-PCR.
Results
Glucose can lead to an increase in inflammatory cytokines TNF-α, TGF-β1, MDA as well as the expressions of the SMAD2, SMAD3, SMAD4, and a decrease in SMAD7. Quercetin caused the reduction of TNF-α, TGF-β1, MDA as well as the expression of the SMAD2, SMAD3, SMAD4, and increased SMAD7.
Conclusion
Quercetin has anti-inflammation, antioxidant, antifibrosis activity in the CKD cells model. Thus, quercetin is a promising substance for CKD therapy and further research is needed to prove this in CKD animal model.
Keywords: Chronic kidney disease, Quercetin, Antioxidant, Anti-inflammation
Introduction
Chronic kidney disease (CKD) is a disorder in which the function of the kidneys is harmed. This is indicated by a lower glomerular filtration rate (GFR) or less than 60 mL/min per 1.73 m2. CKD is a global health concern, with a 1.8% increase in frequency in Indonesia between 2013 and 2018 (Webster et al., 2017). The main causes of CKD are diabetes and hypertension. One of the characteristics of CKD is fibrosis. Renal fibrosis is a long-term, progressive condition that affects kidney function during CKD disease (Chen et al., 2018a; Chen et al., 2018b). The reason of renal fibrosis is that extracellular matrix (ECM) proteins such as collagens, fibronectin, and laminin accumulated in the diabetic nephropathy (DN) condition (Zeng, Xiao & Sun, 2019). Pathogenesis of fibrosis of CKD implicates oxidative stress and inflammatory reactions (Popolo et al., 2013). Inflammation and oxidative stress have been shown to result in the development of CKD (Qian, 2017). Nuclear factor-kappaB (NF-kB) and nuclear factor-erythroid-2-related factor 2 (Nrf2) were triggered by inflammation and oxidative stress (Luo et al., 2021).
Patients with CKD usually suffer from chronic inflammation (Morena et al., 2002), and have severely damaged antioxidant systems that gradually worsen with renal failure (Libetta et al., 2011). Tumor Necrosis Alpha (TNF-α) and Transforming Growth Factor β1 (TGF-β1) are important cytokines in the inflammation process. Meanwhile, malondialdehyde (MDA) is a valuable parameter for oxidative stress (Webster et al., 2017). SMADs are the primary signaling mechanism by which TGF-β1 mediates CKD (Rapa et al., 2020). Thus, in uremic syndrome, modulated inflammation cytokines and oxidative stress are of primary importance for treating CKD (Rapa et al., 2020).
The most common therapies to treat the progression of CKD are anti-inflammatory drugs, antioxidants, and renin-angiotensin system (RAS) inhibitors (Zhou et al., 2015). However, those therapies are not adequate to prevent the disease. As a result, scientists around the world are still looking for new and innovative therapies on CKD (Li et al., 2019a; Li et al., 2019b). Natural product is an alternative therapy for CKD and anti-fibrosis (Chen et al., 2018a; Chen et al., 2018b). A previous study showed that Epigallocatechin-3-gallate from green tea (EGCG) treatment could inhibit fibrosis by TGF-B/SMADs pathway, also known for having strong antioxidant and anti-inflammation properties (Wang et al., 2015). Emodin is a natural component of Rhei Radix et Rhizoma, a Chinese herbal medicine with anti-inflammatory, antioxidant, anti-fibrotic, and antibacterial properties (Xu et al., 2021). In Indonesian herbal medicine, several flavonoids have been effectively found from Phyllanthus niruri, such as quercetin, which has been shown to have significant antioxidant and chelating properties (Rusmana et al., 2017).
Quercetin is a major flavonoid content in most plant extracts (Junior et al., 2018). Quercetin is found in apples, red grapes, kales, broccoli, onions, berries, and cherries (Xu et al., 2019). Like other flavonoids, quercetin has various bioactivities such as antibacterial, antioxidant, anti-inflammatory, and antivirus activities (Perdhana & Suzana, 2019; Prahastuti et al., 2019). Quercetin has been found in previous research to protect the kidneys by reducing glucose levels (Vera et al., 2018). By enhancing the antioxidant capacity of cells, quercetin can help minimize oxidative stress (Wu et al., 2017). Moreover, by suppressing inflammatory factors, quercetin can lessen inflammatory stimulation (Tan et al., 2020). Quercetin inhibits the transformation of renal tubular epithelial cells, which can postpone fibrosis and modulate macrophage polarization (Lu et al., 2018; Yang et al., 2018). Moreover, it has been proven that quercetin could protect human renal proximal tubular cells against the toxicity of radiocontrast medium in a recent study (Andreucci et al., 2018). The previous study showed that quercetin could improve renal function and reduce oxidative stress factors, serum level of Fibroblast Growth Factor 23 (FGF23), renal inflammation, and renal tubular damage on rat adenine-induction CKD model (Yang et al., 2018). However, further research is needed to see if quercetin can help in CKD treatment. Present study evaluated the potency of quercetin for CKD therapy with in vitro study in a cell model of CKD by modulating the production of TNF-α, TGF-β1, and MDA as inflammation cytokines, fibrosis, and oxidative stress marker and SMAD2, SMAD3, SMAD4 and SMAD7 as inflammation and fibrosis genes.
Materials and Methods
Cell culture and CKD treatment
Cell lines of mouse mesangial kidney SV40 MES 13 cells (ATCC® CRL-1927TM) were received from Aretha Medika Utama, Biomolecular and Biomedical Research Centre, Bandung, West Java, Indonesia, and plated in a 6-well plate (3516; Costar, Wujiang, Jiangsu, China) with a total of 5,000 cells per well. The cell media used were Dulbecco’s Modified Eagle Medium (DMEM) (L0103-500; Biowest, Riverside, MO, USA) and F12-K Mix Nutrient (L0135-500; Biowest, Riverside, MO, USA) with a ratio of 1:3 which had been substituted for 3 days with a glucose-induced medium with a concentration of 20 mM. Quercetin (Q4951-10G; Sigma Aldrich, Saint Louis, MO, USA) was added with 50 and 10, and 5 µg/mL in concentration. The positive control was mesangial cell induced by glucose without using quercetin, and negative control was the untreated cell (Sandhiutami et al., 2017; Widowati et al., 2018; Hidayat et al., 2022).
Total protein assay
The bovine serum albumin (BSA) (A9576, Lot. SLB2412; Sigma, St. Louis, MO, USA) 2 mg was used to get standard solution. The 20 µL of standard solutions, quercetin, and 200 µL Quick Start Dye Reagent 1 × (5000205; Bio-Rad, Hercules, CA, USA) were added briefly into each well. The plate was then incubated at room temperature for 5 min. The absorption was measured at 595 nm with the microplate reader (Multiskan GO Microplate Spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA) (Lister et al., 2020).
TGF-β1, TNF-α, MDA using ELISA method
The treated mesangial cells condition medium was used as the sample. Mouse TGF-β1 ELISA Kit (E-EL-M0051; Elabscience, Houston, Texas, USA), Mouse TNF-α (E-EL-M0049; Elabscience, Houston, Texas, USA), and MDA (E-EL-0060; Elabscience, Houston, Texas, USA) according to the protocol of manufacture with modified method were conducted. The absorbance was read at 450 nm using microplate reader (Sandhiutami et al., 2017; Widowati et al., 2018; Lister et al., 2020; Ginting et al., 2021; Prahastuti et al., 2019; Hidayat et al., 2022).
Gene expression of SMAD7, SMAD2, SMAD3, SMAD4 using RTPCR method
TRI reagent (R2050-1-200; Zymo, Irvine, CA, USA) was used to extract the RNA of cell (1 × 106 cell/mL) and RNA isolation KIT (R2073, Zymo, Irvine, CA, USA) was used to purify the RNA. These procedures were in accordance with the manufacturer’s protocol. Following that, the created technique was utilized to synthesize complementary-DNA using iScript Reverse Transcription Supermix for RT-PCR (170-8841; Bio-Rad, Hercules, CA, USA). The gene’s expression was determined using SsoFast Evagreen Supermix and qRT-PCR (GTC96S, Clever) in triplicate (172-5200; Bio-Rad, Gladesville, NSW, Australia) (Lister et al., 2020; Hidayat et al., 2022). Table 1 shows the primer sequence (Macrogen) and Table 2 shows the RNA concentration/purity.
Table 1. Primer sequences of GAPDH, SMAD2, SMAD3, SMAD4, SMAD7 genes.
| Gene |
Primer sequence (5′–3′)
Upper strand: sense Lower strand: antisense |
Product size (bp) | Annealing (°C) | Cycle | Reference |
|---|---|---|---|---|---|
| GAPDH | TCAAGATGGTGAAGCAG ATGTAGGCCATGAGGTCCAC |
217 | 59 | 40 | NCBI Reference Sequence: NM_001289726 |
| SMAD2 MOUSE | ATTACATCCCAGAAACACCAC TAGTATGCGATTGAACACCAG |
196 | 59 | 40 | NCBI Reference Sequence: NM_001252481.1 |
| SMAD3 MOUSE | GTAGAGACGCCAGTTCTACCT CATCTTCACTCAGGTAGCCAG |
178 | 59 | 40 | NCBI Reference Sequence: NM_016769.4 |
| SMAD4 MOUSE | GAGAACATTGGATGGACGAC ACATACTTGGAGCATTACTCTG |
242 | 54 | 40 | NCBI Reference Sequence: NM_001364967.1 |
| SMAD7 MOUSE | ACTCTGTGAACTAGAGTCTCCC CTCTTGGACACAGTAGAGCCT |
241 | 59 | 40 | NCBI Reference Sequence: NM_001042660.1 |
Table 2. RNA concentration and purity.
| Sample | Concentration of RNA (ng/µL) | RNA Purity (260/280 nm) |
|---|---|---|
| Negative Control | 43.37 | 2.0776 |
| Positive Control | 60.16 | 2.2706 |
| Quercetin 50 µg/mL | 18.65 | 1.9768 |
| Quercetin 10 µg/mL | 53.87 | 1.7291 |
| Quercetin 5 µg/mL | 52.07 | 1.7816 |
Mesangial cell’s morphology observation
The mesangial cells morphology was observed using an inverted microscope (CKX41-F32FL, Olympus). The observed parameter included cells proliferation that was indicated by the density of the cells. The morphology of the treated mesangial cell group was compared to the non-treated mesangial cell as the control group.
Statistical analysis
To verify the results of different treatments, one-way Analysis of Variance (ANOVA) was employed, followed by Tukey HSD, and Dunnet-T3 post-hoc test to confirm significant differences for all treatments (P < 0.05). The non-parametric test was evaluated by Mann-Whitney test (p < 0.05). The data were analyzed using the Statistical Package for the Social Sciences (SPSS) software version 16.
Results
TGF-β1 level
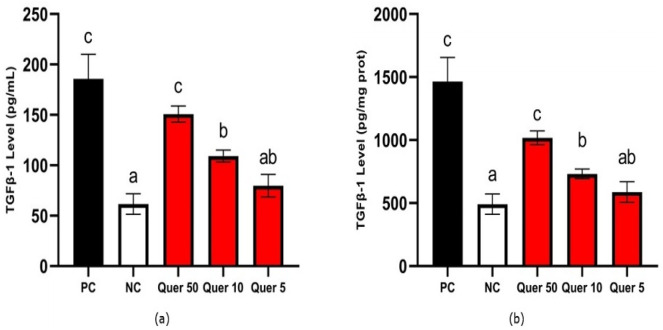
The level of TGF-β1 of quercetin treatments is shown in Fig. 1. The lowest TGF-β 1 (79.77 pg/mL) was the CDK cells model treated with 5 µg/mL of quercetin. Quercetin reduced the TGF-β1 level significantly compared to the CDK cells model according to the statistical analysis (p < 0.05).
Figure 1. TGF-β1 level of quercetin treatment on CKD cell model.
(A) TGF-β1 level (pg/mL) on CKD cell model, (B) TGF-β1 level (pg/mg protein) on CKD cell model. *The data is shown in from a mean value with a standard deviation, n = 3. PC: Positive control/glucose-induced mesangial cells; NC: Negative control/untreated mesangial cells, Quer 50: Positive control + Quercetin 50 µg/mL, Quer 10: Positive control + Quercetin 10 µg/mL, Quer 5: Positive control + Quercetin 5 µg/mL Different letter (a,ab,b,c) show significant difference among treatment on TGF-β1 level pg/mL (Fig. 1A), different letter (a,ab,b,c) indicate significant difference among treatment on TGF-β1 level pg/mg protein (Fig. 1B) based on Tukey’s HSD post hoc test (p < 0.05).
TNF-α level
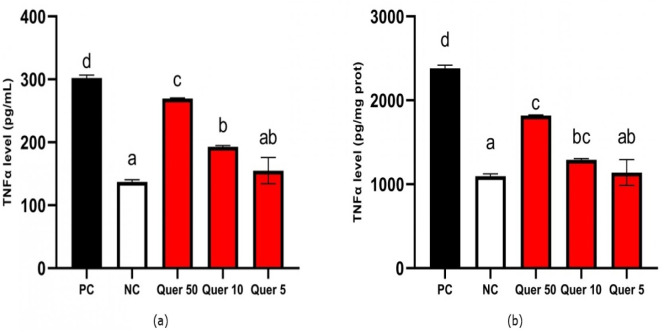
The result data show that quercetin decreased TNF-α level significantly from the positive control (Fig. 2). The lowest TNF-α level (154.97 pg/mL) was obtained when the CDK cells model was treated with 5 µg/mL of quercetin. Quercetin reduced TNF-α levels significantly compared to CKD cells model (p < 0.05).
Figure 2. TNF-α level of quercetin treatment on CKD cell model.
(A) TNF-α level (pg/mL) on CKD cell model. (B) TNF-α level (pg/mg protein) on CKD cell model. *The data is shown in from a mean value with a standard deviation, n = 3. PC: Positive control/glucose-induced mesangial cells; NC: Negative control/untreated mesangial cells, Quer 50: Positive control + Quercetin 50 µg/mL, Quer 10: Positive control + Quercetin 10 µg/mL, Quer 5: Positive control + Quercetin 5 µg/mL. Different letter (a,ab,b,c,d) show significant difference among treatment on TNF-α level pg/mL (Fig. 2A), different letter (a,ab,bc,c,d) indicate significant difference among treatment on TNF-α level pg/mg protein (Fig. 2B) based on Dunnett T3 post hoc test (p < 0.05).
MDA level
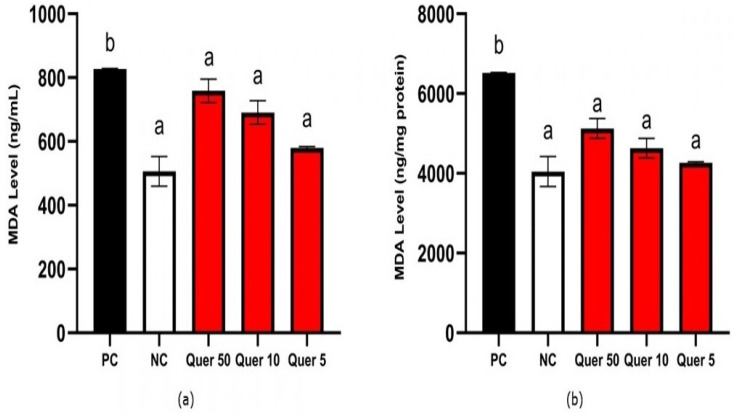
Figure 3 shows MDA level of quercetin treatment. Quercetin could reduce MDA levels to 758.72, 690.76, and 579.70 ng/mL at 50, 10, and 5 µg/mL concentrations, respectively. Quercetin reduced MDA level significantly compared to the CKD cells model according to the statistical analysis (p < 0.05).
Figure 3. MDA level of quercetin treatment on CKD cell model.
(A) MDA level (ng/mL) on CKD cell model. (B) MDA level (ng/mg protein) on CKD cell model. *The data is shown in from a mean value with a standard deviation, n = 3. PC: Positive control/glucose-induced mesangial cells; NC: Negative control/untreated mesangial cells, Quer 50: Positive control + Quercetin 50 µg/mL, Quer 10: Positive control + Quercetin 10 µg/mL, Quer 5: Positive control + Quercetin 5 µg/mL Different letter (a,b) indicate significant difference among treatment on MDA level pg/mL (Fig. 3A), pg/mg protein (Fig. 3B) based on Mann Whitney post hoc test (p < 0.05).
Gene expression of SMAD7, SMAD3, SMAD2, SMAD4
Figure 4 show the results of gene expression values. The results indicated that quercetin for all concentrations tests (50, 10, and 5 µg/mL, respectively) significantly decreased (p < 0.05) the relative ratio of SMAD2 gene expression to 13.06, 7.84, and 5.42 compared to positive control at 27.76. The treatment decreased the relative ratio of SMAD3 gene expression to 22.93, 15.98, and 7.91 compared to positive control at 45.32. Moreover, quercetin decreased the relative ratio of SMAD4 gene expression to 1.82, 1.66, and 1.15 compared to positive control at 2.23. Meanwhile, quercetin at 10 mg/mL and 5 mg/mL concentrations tests increased the relative ratio of SMAD7 gene expression to 0.71, and 0.92 from the positive control at 0.49.
Figure 4. SMADs gene expression of quercetin treatment on CKD cell model.
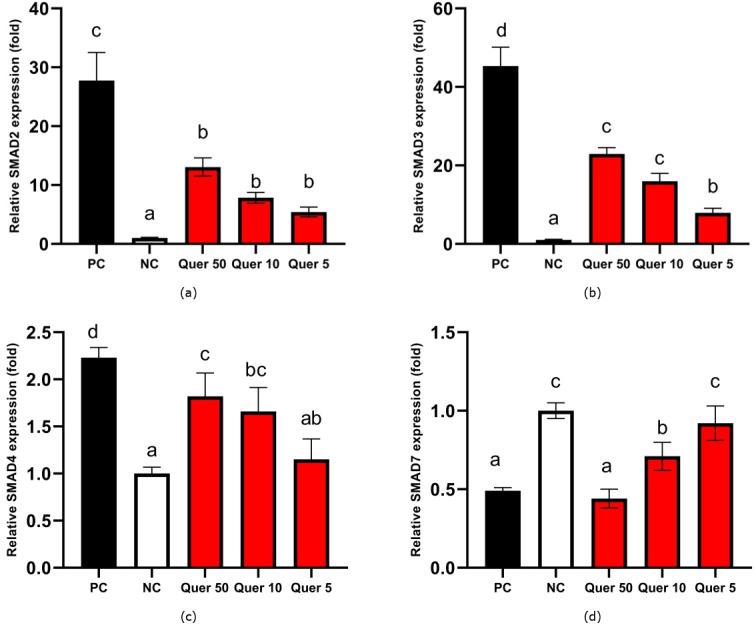
(A) SMAD2 gene expression on glucose-induced mesangial cells. (B) SMAD3 gene expression on glucose-induced mesangial cells. (C) SMAD4 gene expression on glucose-induced mesangial cells. (D) SMAD7 on glucose-induced mesangial cells. An asterisk (*) indicates that the data is shown from a mean value with a standard deviation, n = 3. PC: Positive control/glucose-induced mesangial cells; NC: Negative control/untreated mesangial cells, Quer 50: Positive control + Quercetin 50 µg/mL, Quer 10: Positive control + Quercetin 10 µg/mL, Quer 5: Positive control + Quercetin 5 µg/mL Different lowercase letters (a, b, c) indicate significant difference among treatment on SMAD2 gene expression (Fig. 4A), different lowercase letters (a, b, c, d) indicate significant difference among treatment on SMAD3 gene expression (Fig. 4B), different lowercase letters (a, ab, bc, c, d) indicate significant difference among treatment on SMAD4 gene expression (Fig. 4C), different lowercase letters (a, b, c) indicate significant difference among treatment on SMAD7 gene expression (Fig. 4D) based on Tukey HSD post hoc test (p < 0.05).
Mesangial cell’s morphology
The morphology of mesangial cells in this research is shown in Fig. 5. The glucose-induced mesangial cells showed differences between positive control and negative control (normal cell). The research showed that mesangial cells induced by glucose (positive control) caused visible high proliferating cells compared to mesangial cells without glucose induction (negative control). The treatment with quercetin showed that CKD cells model treated with quercetin was close to negative control cell’s condition which was characterized by normal cell growth.
Figure 5. Morphology of quercetin treatment on CKD cell model.
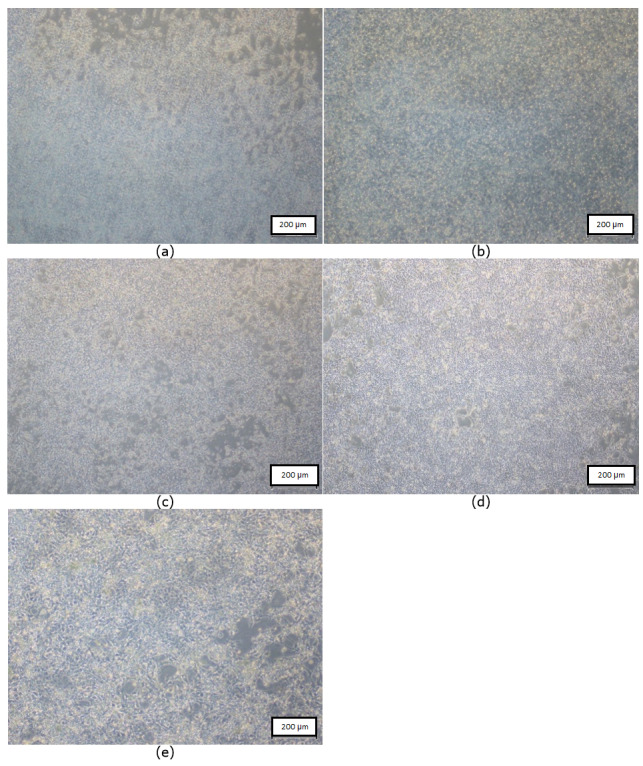
(A) Negative control: untreated mesangial cells (B) Positive control: glucose-induced mesangial cells (C) Quer 5: Positive control + quercetin 5 µg/mL (D) Quer 10: Positive control + quercetin 10 µg/mL (E) Quer 50: Positive control + quercetin 50 µg/mL.
Discussion
CKD is a condition when the function of the kidney is reduced. Quercetin is a natural compound having a wide range of biological effects. Quercetin has been shown to have antioxidant, anticancer, anti-aggregation, anti-inflammatory, and vasodilation activities. The goal of this study is to see if quercetin can help in CKD treatment (Rusmana et al., 2017; Lu et al., 2018, Yang et al., 2018, Perdhana & Suzana, 2019).
TNF-α is a powerful pro-inflammatory cytokine that aids the immune system during periods of inflammation (Nair et al., 2006). Pro-inflammatory cytokine influenced a lot in chronic inflammatory disorders by oxidative stress, and one of the highest is TNF-α. The previous study has shown that quercetin inhibits the production of TNF-α affected by the inhibition of NF-kB immunomodulatory cytokine transcription (Asni et al., 2009). Flavonoids can influence the immune response. Quercetin has anti-inflammatory properties by a decrease in the TNF-α production (Nair et al., 2006). The result data show that quercetin in all concentrations reduced TNF-α levels in CKD cells model.
MDA is a chemical that can be used to determine how free radicals behave in cells. MDA is usually used as a free radical indicator (Hojs et al., 2020; Ginting et al., 2021). Another research confirms this argument by claiming that the MDA mediator is a final fat peroxidation product that can define the degree of oxidative stress as a biological biomarker of fat peroxidation (Tualeka et al., 2019). The result shows that the level of MDA was decreased by quercetin treatment in CKD cell model. Quercetin has antioxidant activity by regulating glutathione (GSH). Previous researches have found a substantial association between GSH and MDA, and a reciprocal interaction between the two are backwards (Lin et al., 2017; Hojs et al., 2020). Furthermore, quercetin modulates enzymes or antioxidant substances to enhance antioxidant properties by affecting signal transduction pathways, thus preventing free radical production (Lu et al., 2018).
TGF-β1 is a potent cytokine in playing a driving role in the development of fibrosis by promoting myofibroblasts formation. TGF-β signals mediate the SMADS protein family, namely SMAD2, SMAD3, and SMAD4 (Meng, Chung & Lan, 2013). Except for SMAD7 gene expression, glucose stimulation in the positive control boosts all SMADS gene expression. The SMAD2, SMAD3, and SMAD4 genes, notably SMAD2, are upregulated in glucose-induced mesangial cells. Both SMAD2 and SMAD3 are extensively proof-activated in fibrotic kidneys in people and animal models with CKD (Quezada et al., 2012). The expression of SMAD3, SMAD2, and SMAD4 genes increases with increasing TGF-β (Wang et al., 2018). This is in line with the findings of this investigation, which is increasing the expression of SMAD2, SMAD3, and SMAD4 genes in accordance with increasing levels of TGF-β. Previous studies have shown that poricoic acid compound can inhibit the phosphorylation of SMAD3 induced by TGF-β induction (Wang et al., 2018). Poricoic acid also protects the kidneys during the progression of Acute Kidney Injury (AKI) to CKD by regulating the Gas6/AxlNFkB/Nrf2 pathway. Poricoic acid upregulates Gas6/Axl signaling in AKI, reducing oxidative stress and inflammation, and reducing renal fibrosis by downregulating Gas6/Axl signaling in CKD (Chen et al., 2019). Poricoic acid ZA further suppresses TGF-β1/SMADs pathway through inhibiting SMAD2/3 phosphorylation by blocking SMAD2/3-TGF-β RI protein interaction (Wang et al., 2017). This result is in accordance with previous studies that found decreases in TGF-β1, SMAD2, SMAD3, and SMAD4 after the treatment. TGF-β1 is inhibited by SMAD7, which is a nonspecific antagonist. SMAD7 is considered to block TGF signaling by competing with SMAD2/3 for type I receptor binding, increasing SMAD complex ubiquitination, and suppressing SMAD/DNA complexes in the nucleus (Chen et al., 2018a; Chen et al., 2018b). Thus, decreasing TGF-β1 causes an increase the SMAD7 gene expression (Hidayat et al., 2022). Quercetin inhibits fibrosis incidence through TGF-β /SMADs pathway signaling.
As explained before, the anti-inflammatory drug and antioxidant could be used as CKD therapy. Tripterygium Wilfordii Polyglycosides (TWPs) is the main compound of Tripterygium Wilfordii Hook. F. (TWHF) showing anti-fibrosis activity by reducing IL-1, IL-17, interferon-γ (IFN-γ), and TNF-α (Liu et al., 2021). Previous studies show that Abelmoschus manihot reduces proteinuria and lessens kidney damage and tubulointerstitial fibrosis so that it improves kidney function. These beneficial effects are related to the anti-inflammatory and antioxidant properties of A. manihot (Cai et al., 2017; Li et al., 2019a; Li et al., 2019b; Liao et al., 2019). Compounds that have good anti-inflammatory and antioxidant properties can act as antifibrosis in CKD therapy. According to the present study, quercetin has antioxidant activity by decreasing MDA level and has anti-inflammatory activity by decreasing TNF-α level. Therefore, quercetin can act as anti-fibrosis with its anti-inflammation and antioxidant activities.
The cells morphology shows that negative control had a confluence of 70–80% for the glucose-induced high proliferation of mesangial cells. Quercetin treatment could reduce cells proliferation. It was indicated by the lower density of the cells compared to that of positive control. The higher quercetin concentration showed higher inhibition to cell proliferation. Quercetin 10 µg/mL was more active to inhibit mesangial cells compared to quercetin 5 µg/mL; while the highest inhibition was shown by quercetin 50 µg/mL. That was indicated by the cell’s density being higher compared to negative control with cells density of 70–80% confluence. The previous studies showed that TGF-β1 could induce fibroblast proliferation (Kamejima et al., 2019; Prahastuti et al., 2019). The results of this study proved that quercetin was able to inhibit the cells proliferation of CKD cells model by reducing the TGF-β1 level. The proposed pathway of quercetin which prevents CKD by regulating inflammation and TGF-β1/SMADS pathway can be seen at Fig. 6.
Figure 6. Proposed pathway of quercetin in preventing CKD.
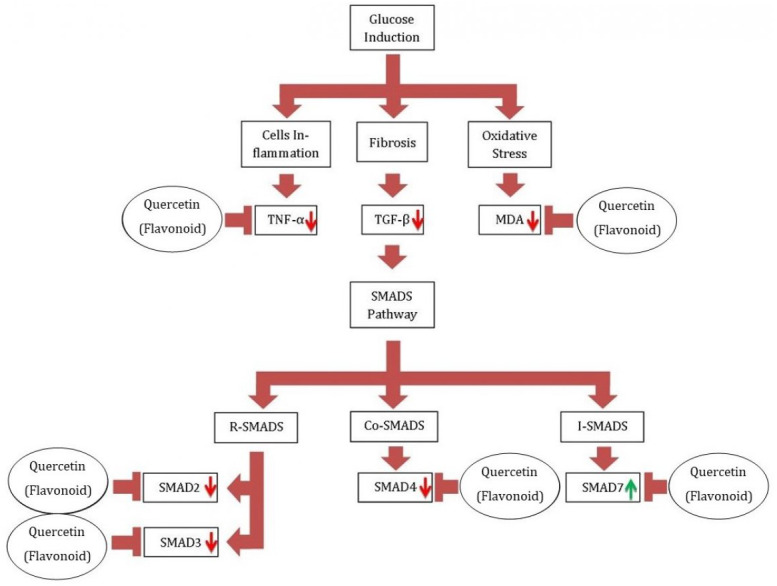
*Glucose induction could cause the inflammation and oxidative stress on mesangial cells. Cells inflammation induce the level of TNF-α, as oxidative stress increases the level of MDA on the cells, and cells fibrosis increase the level of TGF-β1. The increase of TGF-β1 level induce the expression of R-SMADS and Co-SMADS, while repress the I-SMADS expression. On glucose-induced mesangial cells, quercetin therapy reduced the levels of TGF-B1, TNF-A, and MDA. As it represses TGF-1, quercetin also reduces SMAD2, SMAD3, SMAD4, and increases SMAD7 expression. R-SMADS: receptor-regulated SMADS (SMAD 1, 2, 3, 5, and 8). C0-SMADS: common-mediator SMADS (SMAD4). I-SMADS: Inhibitor SMADS (SMAD 6 and 7).
Conclusion
In conclusion, this work has proven that quercetin has anti-inflammation, antioxidant, and antifibrosis properties in the CKD cells model. Thus, quercetin is a promising compound for CKD therapy and further research in CKD animal models is suggested to be examined.
Significance
Previous researches have demonstrated that quercetin suppressed a TNF-α production. Quercetin has been shown to have antioxidant properties by modulating GSH. The antioxidant activity of quercetin was found to lower MDA levels in CKD models in this investigation. Our findings also reveal new information on quercetin’s role in preventing fibrosis through TGF/SMAD signaling.
Supplemental Information
Acknowledgments
We extend appreciation to Meganita Marthania, Cahyaning Riski Wijayanti, Agung Novianto, and Afif Yati for valuable help as research assistants.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wahyu Widowati conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Sijani Prahastuti conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Rita Tjokropranoto conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Philips Onggowidjaja conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Hanna Sari Widya Kusuma analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Ervi Afifah performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Seila Arumwardana performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Muhamad Aldi Maulana performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Rizal Rizal analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.
References
- Andreucci et al. (2018).Andreucci M, Faga T, Pisani A, Serra R, Russo D, De Sarro G, Michael A. Quercetin protects against radiocontrast medium toxicity in human renal proximal tubular cells. Journal of Cellular Physiology. 2018;233(5):4116–4125. doi: 10.1002/jcp.26213. [DOI] [PubMed] [Google Scholar]
- Asni et al. (2009).Asni E, Harahap IP, Prijanti AR, Wanandi SI, Jusman SWA, Sadikin M. Pengaruh hipoksia berkelanjutan terhadap kadar malondialdehid, glutation tereduksi dan aktivitas katalase ginjal tikus. Majalah Kedokteran Indonesia. 2009;59(12):595–600. [Google Scholar]
- Cai et al. (2017).Cai HD, Su SL, Qian DW, Guo S, Tao WW, Cong XD, Tang R, Duan JA. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. Journal of Ethnopharmacology. 2017;206:152–159. doi: 10.1016/j.jep.2017.02.046. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018a).Chen DQ, Feng YL, Cao G, Zhao YY. Natural products as a source for antifibrosis therapy. Trends in Pharmacological Sciences. 2018a;39(11):937–952. doi: 10.1016/j.tips.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen DQ, Feng YL, Chen L, Liu JR, Wang M, Vaziri ND, Zhao YY. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2 axis. Free Radical Biology and Medicine. 2019;134:484–497. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018b).Chen F, Wei G, Xu J, Ma X, Wang Q. Naringin ameliorates the high glucose-induced rat mesangial cell inflammatory reaction by modulating the NLRP3 inflammasome. BMC Complementary and Alternative Medicine. 2018b;18(1):192. doi: 10.1186/s12906-018-2257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginting et al. (2021).Ginting CN, Lister INE, Girsang E, Artie DS, Aviani JK, Widowati W. Apoptotic, MDA, and FGF2 level of quercitrin treatment on hypoxic-induced EA.hy926 cell line. Journal of Reports in Pharmaceutical Sciences. 2021;10(1):22–28. doi: 10.4103/jrptps.JRPTPS_70_20. [DOI] [Google Scholar]
- Hidayat et al. (2022).Hidayat M, Prahastuti S, Afifah E, Widowati W, Yusuf M, Hasan K. The role of green peas protein hydrolysate in TGF/SMAD signaling to prevent renal fibrosis. Journal of King Saud University –Science. 2022;34(2022):101920. doi: 10.1016/j.jksus.2022.101920. [DOI] [Google Scholar]
- Hojs et al. (2020).Hojs NV, Bevc S, Ekart R, Hojs R. Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants. 2020;9(10):1–22. doi: 10.3390/antiox9100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junior et al. (2018).Junior SD da C, Santos JV de O, Campos LA de A, Pereira MA, Magalhães NSS, Cavalcanti IMF. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. International Journal of Environment, Agriculture and Biotechnology. 2018;3(5):1948–1958. doi: 10.22161/ijeab/3.5.50. [DOI] [Google Scholar]
- Kamejima et al. (2019).Kamejima S, Tatsumi N, Anraku A, Suzuki H, Ohkido I, Yokoo T, Okabe M. Gcm1 is involved in cell proliferation and fibrosis during kidney regeneration after ischemia–reperfusion injury. Scientific Reports. 2019;9(7883):1–13. doi: 10.1038/s41598-019-44161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019a).Li H, Chen W, Chen Y, Zhou Q, Xiao P, Tang R, Xue J. Neferine attenuates acute kidney injury by inhibiting NF-κB signaling and upregulating Klotho expression. Frontiers in Pharmacology. 2019a;10(October):1–13. doi: 10.3389/fphar.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019b).Li W, He W, Xia P, Sun W, Shi M, Zhou Y, Zhu W, Zhang L, Lui B, Zhu J, Zhu Y, Zhou E, Sun M, Gao K. Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Frontiers in Pharmacology. 2019b;10:567. doi: 10.3389/fphar.2019.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2019).Liao Z, Zhang J, Wang J, Yan T, Xu F, Wu B, Xiao F, Bi K, Niu J, Jia Y. The anti-nephritic activity of a polysaccharide from okra (Abelmoschus esculentus (L.) Moench) via modulation of AMPK-Sirt1-PGC-1α signaling axis mediated anti-oxidative in type 2 diabetes model mice. International Journal of Biological Macromolecules. 2019;140:568–576. doi: 10.1016/j.ijbiomac.2019.08.149. [DOI] [PubMed] [Google Scholar]
- Libetta et al. (2011).Libetta C, Sepe V, Esposito P, Galli F, Dal A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clinical Biochemistry. 2011;44(14–15):1189–1198. doi: 10.1016/j.clinbiochem.2011.06.988. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2017).Lin E, Kuo PH, Liu YL, Yang AC, Tsai SJ. Transforming growth factor-β signaling pathway-associated genes SMAD2 and TGFBR2 are implicated in metabolic syndrome in a Taiwanese population. Scientific Reports. 2017;7(1):1–8. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister et al. (2020).Lister INE, Ginting CN, Girsang E, Nataya ED, Azizah AM, Widowati W. Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency. Saudi Pharmaceutical Journal. 2020;28(10):1182–1189. doi: 10.1016/j.jsps.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2021).Liu P, Zhang J, Wang Y, Shen Z, Wang C, Chen DQ, Qiu X. The active compounds and therapeutic target of Tripterygium wilfordii Hook. f. in attenuating proteinuria in diabetic nephropathy: a review. Frontiers in Medicine. 2021;8:747922. doi: 10.3389/fmed.2021.747922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2018).Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, Wu S, Jin G, Bai Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochemical Pharmacology. 2018;154:203–212. doi: 10.1016/j.bcp.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2021).Luo LP, Suo P, Ren LL, Liu HJ, Zhang Y, Zhao YY. Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting I κB/NF-κB and Keap1/Nrf2 signaling pathways. Frontiers in Pharmacology. 2021;12:800522. doi: 10.3389/fphar.2021.800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Chung & Lan (2013).Meng XM, Chung AC, Lan HY. Role of the TGF-β /BMP-7/Smad pathways in renal diseases. Clinical Science. 2013;124(4):243–254. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- Morena et al. (2002).Morena M, Cristol JP, Senécal L, Leray-Moragues H, Krieter D, Canaud B. Oxidative stress in hemodialysis patients: is NADPH oxidase complex the culprit? Kidney International, Supplement. 2002;61(80):109–114. doi: 10.1046/j.1523-1755.61.s80.20.x. [DOI] [PubMed] [Google Scholar]
- Nair et al. (2006).Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clinical and Vaccine Immunology. 2006;13(3):319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdhana & Suzana (2019).Perdhana I, Suzana D. Peran kuersetin terhadap ekspresi Nrf2 pada stres oksidatif akibat penyakit ginjal kronik. Informatika Kedokteran : Jurnal Ilmiah. 2019;2:27–36. [Google Scholar]
- Popolo et al. (2013).Popolo A, Autore G, Pinto A, Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radical Research. 2013;47(5):346–356. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- Prahastuti et al. (2019).Prahastuti S, Hidayat M, Hasiana ST, Widowati W, Amalia A, Qodariah RL, Rizal R, Kusuma HSW, Khoiriyah Z. Ethanol extract of jati belanda (Guazuma ulmifolia L.) as therapy for chronic kidney disease in in vitro model. Journal of Reports in Pharmaceutical Sciences. 2019;8(2):229–235. doi: 10.4103/jrptps.JRPTPS_41_18. [DOI] [Google Scholar]
- Qian (2017).Qian Q. Inflammation: A key contributor to the genesis and progression of chronic kidney disease. In: Ronco C, editor. Expanded hemodialysis-innovative clinical approach in dialysis. Vol. 191. Contribution to Nephrology; Basel: 2017. pp. 72–83. [DOI] [PubMed] [Google Scholar]
- Quezada et al. (2012).Quezada M, Wang J, Hoang V, McGee EA. Smad7 is a transforming growth factor-beta-inducible mediator of apoptosis in granulosa cells. Fertility and Sterility. 2012;97(6):1452–1459. doi: 10.1016/j.fertnstert.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Rapa et al. (2020).Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. International Journal of Molecular Sciences. 2020;21(1):263. doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusmana et al. (2017).Rusmana D, Wahyudianingsih R, Elisabeth M, Maesaroh B, Widowati W. Antioxidant activity of Phyllanthus niruri extract, rutin and quercetin. Indonesian Biomedical Journal. 2017;9(2):84–90. doi: 10.18585/inabj.v9i2.281. [DOI] [Google Scholar]
- Sandhiutami et al. (2017).Sandhiutami NMD, Moordiani M, Laksmitawati DR, Fauziah N, Maesaroh M, Widowati W. In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264, 7 murine macrophage cell line. Iranian Journal of Basic Medical Sciences. 2017;20:99–106. doi: 10.22038/ijbms.2017.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan et al. (2020).Tan RZ, Wang C, Deng C, Zhong X, Yan Y, Luo Y, Lan HY, He T, Wang L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytotherapy Research. 2020;34(1):139–152. doi: 10.1002/ptr.6507. [DOI] [PubMed] [Google Scholar]
- Tualeka et al. (2019).Tualeka AR, Martiana T, Ahsan A, Russeng SS, Meidikayanti W. Association between malondialdehyde and glutathione (L-gamma-glutamyl-cysteinyl-glycine/GSH) levels on workers exposed to benzene in Indonesia. Open Access Macedonian Journal of Medical Sciences. 2019;7(7):1198–1202. doi: 10.3889/oamjms.2019.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera et al. (2018).Vera M, Torramade-Moix S, Martin-Rodriguez S, Cases A, Cruzado JM, Rivera J, Escolar G, Palomo M, Diaz-Ricart M. Antioxidant and anti-inflammatory strategies based on the potentiation of glutathione peroxidase activity prevent endothelial dysfunction in chronic kidney disease. Cellular Physiology and Biochemistry. 2018;51(3):1287–1300. doi: 10.1159/000495540. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, Vaziri ND, Guo Y, Zhao YY. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β -catenin pathway against renal fibrosis. British Journal of Pharmacology. 2018;175(13):2689–2708. doi: 10.1111/bph.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang M, Chen DQ, Wang MC, Chen H, Chen L, Liu D, Zhao H, Zhao YY. Poricoic acid ZA, a novel RAS inhibitor, attenuates tubulo-interstitial fibrosis and podocyte injury by inhibiting TGF-β /Smad signaling pathway. Phytomedicine. 2017;36:243–253. doi: 10.1016/j.phymed.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N. Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. Journal of Histochemistry and Cytochemistry. 2015;63(4):270–279. doi: 10.1369/0022155414568019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster et al. (2017).Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. The Lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- Widowati et al. (2018).Widowati W, Laksmitawati DR, Wargasetia TL, Afifah E, Amalia A, Arinta Y, Rizal R, Suciati T. Mangosteen peel extract (Garcinia mangostana L.) as protective agent in glucose-induced mesangial cell as in vitro model of diabetic glomerulosclerosis. Iranian Journal of Basic Medical Sciences. 2018;21(9):972–977. doi: 10.22038/IJBMS.2018.29349.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2017).Wu W, Yang JJ, Wan YG, Tu Y, Shi G, Han WB, Liu BH, Yao J. Pathogenesis and treatment of insulin resistance in chronic kidney disease and interventional effects of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica. 2017;42(1):49–55. doi: 10.19540/j.cnki.cjcmm.20161222.054. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2019).Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 21. 2019;24(6):1123–1138. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2021).Xu Q, Wang M, Guo H, Liu H, Zhang G, Xu C, Chen H. Emodin alleviates severe acute pancreatitis-associated acute lung injury by inhibiting the Cold-Inducible RNA-Binding Protein (CIRP)-mediated activation of the NLRP3/IL-1β /CXCL1 signaling. Frontiers in Pharmacology. 2021;23:655372. doi: 10.3389/fphar.2021.655372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2018).Yang H, Song Y, Liang YN, Li R. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2018;24:4760–4766. doi: 10.12659/MSM.909259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Xiao & Sun (2019).Zeng LF, Xiao Y, Sun L. A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Renal Fibrosis: Mechanisms and Therapies. 2019;4:9–79. doi: 10.1007/978-981-13-8871-2_4. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2015).Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. American Journal of Pathology. 2015;185(12):3211–3223. doi: 10.1016/j.ajpath.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.