Abstract
Obesity has been labeled as the global pandemic of the 21st century, resulting from a sedentary lifestyle and caloric excess. Nonalcoholic fatty liver disease (NAFLD), characterized by excessive hepatic steatosis, is strongly associated with obesity and metabolic syndrome and is estimated to be present in one-quarter of the world population, making it the most common cause of the chronic liver disease (CLD). NAFLD spectrum varies from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis. The burden of NAFLD has been predicted to increase in the coming decades resulting in increased rates of decompensated cirrhosis, hepatocellular carcinoma (HCC), and liver-related deaths. In the current review, we describe the pathophysiology of NAFLD and NASH, risk factors associated with disease progression, related complications, and mortality. Later, we have discussed the changing epidemiology of HCC, with NAFLD emerging as the most common cause of CLD and HCC. We have also addressed the risk factors of HCC development in the NAFLD population (including demographic, metabolic, genetic, dietary, and lifestyle factors), presentation of NAFLD-associated HCC, its prognosis, and the issue of HCC development in non-cirrhotic NAFLD. Lastly, the problems related to HCC screening in the NAFLD population, the remaining challenges, and future directions, especially the need to identify the high-risk individuals, will be discussed. We will conclude the review by summarizing the clinical evidence for treating fibrosis and preventing HCC in those at risk with NAFLD-associated HCC.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, hepatocellular carcinoma, metabolic syndrome, HCC screening
Introduction
The World Health Organization estimates that 39% of adults are overweight, while 13% are obese.1 The burden of obesity and associated comorbidities is expected to rise in the coming decades, especially since 40 million children were estimated to be overweight or obese in 2018.1,2 Obesity is a known risk factor for cardiovascular diseases.3,4 It is closely associated with metabolic complications, including diabetes mellitus (DM), hyperlipidemia (HLD), and nonalcoholic fatty liver disease (NAFLD).5 High body mass index (BMI) is associated with an increased risk of different types of cancers, including liver and colon cancer.6,7 More frightening is the fact that obesity doubles the mortality risk in patients with liver cancer.8
NAFLD is the most common cause of chronic liver disease (CLD). It is characterized by hepatic steatosis (HS) either by imaging or histology without secondary causes of hepatic fat accumulation, such as excessive alcohol consumption (daily ≥30 g for men and ≥20 g for women), medications, or other chronic liver diseases.9,10 NAFLD includes two pathologically distinct conditions: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is defined as the presence of ≥5% HS without evidence of hepatocellular injury in the form of hepatocyte ballooning. NASH is characterized by steatosis with inflammation and hepatocyte ballooning with or without liver fibrosis.11
Recently, it has been recommended to rename NAFLD as Metabolic dysfunction-associated fatty liver disease (MAFLD). This new definition has been proposed to better encapsulate the spectrum of fatty liver disease. MAFLD diagnosis is based on hepatic steatosis (based on histological, imaging, or blood biomarker) and one of the following three conditions: type 2 diabetes mellitus, overweight/obesity, or metabolic dysregulation.12 However, this manuscript will be limited to the prior definition of NAFLD rather the entire umbrella of MAFLD disease.
NAFLD is often labeled as the liver manifestation of metabolic syndrome and is strongly associated with obesity, type 2 diabetes mellitus (T2DM), HLD, and hypertension (HTN).13 There has been a significant rise in NAFLD prevalence in the last two decades. This trend mirrors a similar uptrend in the prevalence of obesity and diabetes and is thought to result from the sedentary lifestyle and changing dietary patterns.13,14 Insulin resistance is an essential component in the underlying pathophysiology of NAFLD. Expectedly, the prevalence of NAFLD has increased by three folds in patients with metabolic syndrome.14,15 Globally, the prevalence of NAFLD is estimated to be 25.24% (95% CI: 22.10–28.65), with the highest prevalence in the Middle East and South America and the lowest in Africa.14 A recent meta-analysis has put the burden of NAFLD may be even higher in Asia, with a prevalence of 30%.16 Modelling studies have indicated that the burden of NAFLD will continue to rise in the future.17,18 As the growing population of NAFLD patients ages, they will develop further complications associated with the disease. This will likely result in higher rates of decompensated cirrhosis, hepatocellular carcinoma (HCC), and liver-related deaths.17
Pathophysiology and Disease Progression in NAFLD
The original “two-hit” hypothesis proposed to explain the pathophysiology of steatohepatitis was perhaps an over-simplification of the process.19 It stated that steatosis was the first hit, which sensitized the liver, followed by the “second hit” of oxidative stress leading to necro-inflammation resulting in NASH.19 However, it is now believed that multiple factors are involved in fatty liver disease pathogenesis. A range of environmental factors, including but not limited to diet, lifestyle, insulin resistance, and dysbiosis of gut microbiota acting in a genetically or epigenetically susceptible host, modify the responses to caloric excess.20,21 Insulin resistance and hyperinsulinemia are central in this pathogenesis, leading to fatty acid accumulation. This accumulation may result in lipotoxicity and inflammation, causing cell damage and subsequent activation of progenitor cells resulting in fibrosis and disease progression.2,22
Not all patients with NAFLD are at an equal risk of developing complications. One study reported that 2.4–12.8% of patients with NAFLD would experience disease progression or liver-related mortality ranging over three to seven years follow-up.23 It is thus of paramount importance to identify patients who are at higher risk of developing these complications. Both NAFL and NASH can progress to advanced liver fibrosis.24–27 In one meta-analysis of 411 patients with biopsy-proven NAFL or NASH, 43% had stable disease, one-third had fibrosis progression, and 22% had disease improvement. The rate of fibrosis progression in NASH patients was double that of the patients with NAFL.25 Currently, it is not clear which NAFLD patients are likely to progress and develop advanced liver disease. However, patients presenting with lobular inflammation or hepatic fibrosis at the time of diagnosis are at higher risk of disease progression than those presenting with steatosis alone.24 Increasing age and uncontrolled metabolic risk factors are also associated with NAFLD disease progression.24
McPherson et al studied 108 patients (81 with NASH and 27 with NAFL) using paired liver biopsies. They found out that there was no difference in the proportion of patients exhibiting fibrosis progression between the two groups.27 A recent meta-analysis demonstrated that among NAFLD patients, the stage of liver fibrosis is a strong predictor of all-cause mortality and morbidity. Compared with no fibrosis, incremental rise in fibrosis stages was associated with a five to twelve-fold increased risk of death and liver-related events, including liver failure, HCC and, need for transplantation.28 This meta-analysis did not suggest any differential risk between NAFL and NASH patients, as the risk of mortality and morbidity associated with increasing fibrosis stage appeared similar in both the groups.28
However, this topic remains debatable as the differential diagnosis of NAFLD and NASH is a well-recognized clinical practice controversy.29 It is possible that some NASH patients may be wrongly labeled as non-NASH patients, as liver biopsy captures the snapshot of the clinical spectrum of NAFLD patients at only one point in time. Also, patients with advanced fibrosis may have lost some active NASH features when, in fact, NASH was the original causative factor of fibrosis.28
Our understanding of risk factors that promote fibrosis progression in NAFLD patients is still evolving. The presence of metabolic traits, such as DM, HTN, HLD, and obesity, have been associated with the risk of progression to cirrhosis30 and mortality.31 DM confers the most significant risk of fibrosis progression.30,32 Regardless of the driving mechanisms, fibrosis in NAFLD patients is associated with future disease progression. The degree of fibrosis is associated with increased liver-specific and all-cause mortality, independent of any other histopathological feature.33–37
HCC and NAFLD
Changing Epidemiology of HCC
Liver cancer is the fifth most common diagnosed cancer and the fourth leading cause of worldwide cancer deaths in 2018. Hepatocellular carcinoma (HCC) is the most common primary liver cancer comprising of 75%–85% of cases.38,39 There were 841,000 new cases reported in 2018, and it accounted for 782,000 deaths annually.38 Liver cancer has a male predominance with a mortality rate two to three times higher in men than women.38,40,41 When assessing the underlying etiology of liver disease in patients with HCC, women were more likely to have NASH cirrhosis compared to men. Women also tend to present more often with non-cirrhotic HCC than men and have less advanced disease at presentation with greater overall survival.42 HCC remains the second leading cause of cancer-related deaths in men. Globally, incident cases of liver cancer increased by 114.0% from 471,000 in 1990 to 1,007,800 in 2016. The overall age-standardized incidence rate increased by an average of 0.34% (95% CI 0.22%−0.45%) per year in this period.43 There is a considerable variation in the prevalence of HCC across the globe due to the difference in causative etiologies. A higher prevalence of HCC was observed in China, South East Asia, and sub-Saharan Africa, mirroring the prevalence of chronic hepatitis B virus infection in these regions. On the other hand, Japan, Italy, and France have a high prevalence of HCC because of the high prevalence of hepatitis C virus (HCV) infection.44 According to the global HCC BRIDGE study between 2005 and 2012, the most common risk factor for HCC was hepatitis C virus in North America, Europe, and Japan, while hepatitis B virus (HBV) accounted for the majority of cases in China, South Korea, and Taiwan.45
In recent years, there has been a change in the epidemiology of HCC. Recent innovations in antiviral therapies and successful implementation of vaccination have led to better control of HBV and HCV, in turn leading to a drop in incidence of HCC associated with these viruses in some regions around the world as highlighted in the Global Burden of Disease study; however, the study also noted an unfavorable overall uptrend in the incidence of HCC, including in countries with a high socio-demographic index like the Netherlands, the UK, and the USA.43
In the last decade, growing evidence has supported a trend toward NASH overtaking viral hepatitis as the leading cause of HCC in western countries.46–50 NASH is the second leading etiology of HCC leading to liver transplant in the US between 2002 and 2012,42 and the most rapidly growing indication of liver transplant in patients with HCC.46,47 NASH-related HCC increased from 8.3% in 2002 to 10.3% in 2007 and further rose to 13.5% in 2012. In the same periods, transplantation for HCC due to NASH increased fourfolds.46 NAFLD/NASH was reported to be the most common underlying etiologic risk factor (59%) for HCC development, followed by DM and HCV in another study.48 Recently, a study of 13,648 Medicare patients with HCC reported that while the overall incidence of HCC is increasing, mortality rates are declining. NAFLD remains the most important cause and an independent predictor of HCC in this patient cohort.49 Younossi et al corroborated similar findings in a study in which adult liver transplant candidates were included between 2002 and 2016. The proportion of NASH-related HCC cases increased 7.7-fold from 2.1% to 16.2%, and the prevalence of HCC in liver transplant (LT) candidates with NASH increased by 11.8-fold.47 Similar findings of an increase in the proportion of NASH related liver transplant and decline in HCV-related patients requiring transplant have been reported by Goldberg et al and in the analysis from the national Organ Procurement and Transplant Network (OPTN).50,51
Incidence of HCC in NAFLD
As previously discussed, the incidence and prevalence of NAFLD are increasing worldwide. In the largest meta-analysis of 8.5 million NAFLD patients, the annual incidence of HCC in NAFLD patients was 0.44 per 1000 person-years,14 much less than the incidence of HCC in chronic HBV infection.52 In the same meta-analysis, the incidence rate for HCC in NASH patients was significant at 5.29 cases per 1000 person-years.14 Ascha et al compared the incidence of HCC development in cirrhotic patients secondary to HCV or NASH. They noted that 12.8% of NASH cirrhotic and 20.3% of HCV-cirrhotic patients developed HCC over a median follow-up of 3.2 years.53 Yearly cumulative incidence of HCC was found to be 2.6% in patients with NASH-cirrhosis, compared with 4.0% in patients with HCV cirrhosis.53 Although the incidence of HCC development is lower in NASH patients, the overall burden of NAFLD and NASH patients would suggest that the absolute number of patients developing NAFLD-related HCC will continue to increase in the future.
Kanwal et al reported that the incidence of HCC in NAFLD patients was 0.21/1000 patient-years (PY), with patients having cirrhosis at the highest risk of 10.6/1000 PY. However, 20% of patients with NAFLD who developed HCC had no evidence of cirrhosis.54 A large cross-sectional study from Europe recently showed that patients with NASH are 60% more likely to develop HCC than patients without NASH after adjusting for other associated factors.55 In Asian patients with biopsy-proven NAFLD, liver-specific mortality was 2.34/1000 PY, and HCC incidence was 4.17/1000 PY. Liver fibrosis was the most significant predictor of liver-related but not overall mortality.56
Overall, the incidence and prevalence of NAFLD are increasing, and in the last three decades, NAFLD and alcohol-related liver disease are the two liver diseases with a growing prevalence. This is synchronous with the increasing rates of obesity and T2DM.57 These rising rates of obesity, DM, and metabolic syndrome contribute to ever-increasing numbers of NAFLD-related HCC.58 Although the relative risk of HCC associated with obesity, DM, and metabolic syndrome is not as high as the risk associated with HBV or HCV, the higher prevalence of these conditions account for higher population attributable fractions (PAF), with 36.6% for obesity and DM versus 22.4% for HCV and 6.3% for HBV.59,60 Table 1 summarizes incidence, trend and risk factors of HCC among NAFLD/NASH patients in few selected studies.
Table 1.
Incidence, Trend, and Risk Factors for HCC in NAFLD
| Study Author Year | Study Type | Population | Study Duration or Follow-Up in Years | Cumulative Incidence | Risk Factors and Other Findings |
|---|---|---|---|---|---|
| Cho et al, 2011180 | Retrospective cohort | 329 patients with non-B non-C, non-alcohol, or specific cause-related HCC | 2001–2010 | NR | Increased proportion of NAFLD related HCC increased from 3.8% to 12.2% |
| Dyson et al, 2014128 | Retrospective cohort | 632 patients with HCC | 2000–2010 | NR | The proportion of NAFLD HCC 21.5% (136/632) in 2010. 10 fold increase in NAFLD HCC over 10 years |
| Wong et al, 201446 | Retrospective cohort | UNOS registry; 61,868 adults with LT including 10,061 with HCC | 2002–2012 | NR | Increase in % of NASH related HCC 8.3% in 2002 versus 10.3% in 2007 versus 13.5% in 2012 |
| Younossi et al, 2015127 | Retrospective cohort | SEER registries, 4929 HCC cases 14,937 controls | 2004–2009 | NR | The proportion of NAFLDHCC: 14.1%; 9% annual increase of NAFLDHCC; |
| Park et al, 201545 | Retrospective cohort | BRIDGE, 14 countries, 18,031 HCC patients | 2005–2012 | NR | The proportion of NASHHCC: North America 12%, Europe 10%, China 5%, South Korea 6%, Japan 2% |
| Beste et al, 2015181 | Retrospective cohort | 129,998 cirrhosis, 21,326 HCC | 2001–2013 | NR | Incidence of NAFLDHCC increased from 2.63 to 5.14 per 100, 000 |
| Younossi et al, 201947 | Retrospective cohort | 158,347 adult LT candidates 26,121 HCC | 2002–2017 | NR | The proportion of NASH in HCC increased 7.7-fold from 2.1% to 16.2% |
| Hashimoto et al, 2009182 | Prospective Cohort | 137 NASH with advanced fibrosis | 1990–2007 | 5-year cumulative incidence of HCC was 7.6% | RF: Older age, AST level AST, low grade of histological activity, and advanced fibrosis stage. 5-year survival rate was 82.8% |
| Yatsuji et al, 2009183 | Prospective cohort | 68 patients with cirrhotic NASH 69 patients with HCV Cirrhosis |
1990–2006 | 5-year HCC rate was 11.3% for NASH | the 5-year survival rates were 75.2% |
| Ascha et al, 201053 | Prospective cohort | 195 NASH cirrhosis 315 HCV cirrhosis |
2003–2007 3.2 yrs. follow up | 2.6% yearly CI | 12.8% of NASH cirrhosis developed HCC RF: Older age and alcohol consumption |
| Kawamura et al, 201285 | Retrospective cohort | 6508 patients with NAFLD | 1997–2010 Median follow-up Yrs. | Cumulative rates of NAFLDHCC were 0.02% (year 4), 0.19% (year 8), 0.51% (year 12) | RF: AST level ≥40, platelet count ≤150, age ≥ 60 years and diabetes at baseline. |
| Amarapurkar et al 2013184 | Retrospective cohort | 585 patients with liver cirrhosis NASH-related cirrhosis 7%, cryptogenic cirrhosis 17.8% |
Cumulative follow-up 6.8 + 1.2 years | The annual rate of cirrhotic NASHHCC: 0.46% | The annual rate of cryptogenic cirrhosis HCC: 0.6% |
| Kodama et al, 2013185 | Retrospective cohort | 72 patients with NASH cirrhosis and 85 with ALD cirrhosis | 1990–2010 | 5 yrs. CI 10.5% in NASH-cirrhosis |
RF: older age, higher γ-GTP level, and higher Child-Pugh score |
| Younossi et al, 201614 | Meta-analysis | 86 studies with population of 8,515,431 | 1989–2015 | NAFLD-HCC incidence 0.44 per 1000 person-years | The global prevalence of NAFLD: 25.24% |
| Vilar-Gomez et al, 201835 | Retrospective cohort | 458 NAFLD patients, bridging fibrosis/ compensated cirrhosis | 1995–2016 mean follow-up time of 5.5 years | 1 yr CI. 0.2 F3 1.8 CPT A5 4.7 CPT A6 |
RF: Older age, male sex, diabetes, current smoking, (All cohort) Moderate alcohol consumption, current smoking (Cirrhosis cohort) 10 years accumulated NAFLDHCC rate: 9% |
| Marot et al, 2016186 | Retrospective cohort | 752 patients with cirrhosis (78 NAFLD, 145 HCV, 529 ALD) | 1995–2014 | Annual risks of NAFLDHCC: 3.1% | 10-year cumulative incidence rate: 23.7% |
| Bhala et al, 2011187 | Prospective/Retrospective cohort | 247 NAFLD patients (118 F3, 129 F4) | mean follow up of 7.1 yrs. | 2.4% of NAFLD patients developed HCC | No risk factors identified |
| Lee et al 201779 | Retrospective cohort | 18,080 noncirrhotic NAFLD | 6.3 years | 1 year (0.18) 5 years (1.03) 10 years (2.73) |
RF: Older age High ALT |
| Fuji et al 202256 | Retrospective cohort | 1398 patient with biopsy-confirmed NAFLD | Median follow-up period 4.6 years 8874 person-years | HCC incidence 4.17/1000 person-years (95% CI 3.02–5.75). | Liver-specific mortality 2.34/1000 person-years overall mortality 5.34/1000 person-years |
| Pinyopornpanish et al 202184 | Retrospective cohort | 392,800 adult patients with NAFLD. 367,690 non-cirrhotic NAFLD 25,110 cirrhotic NAFLD |
Median follow up 5 years 2015 to 2020 | HCC in non-cirrhotic NAFLD 4.6/10,000 persons HCC in cirrhotic NAFLD 374.4/10,000 persons |
RF for HCC in non-cirrhotic NAFLD Age > 65 DM ever had elevated ALT Male gender Smoking |
| Alexander et al 2019154 | Matched-cohort study | 136,703 patients with NAFLD/NASH | Median follow up of 3.3 years | Incidence of NAFLD-HCC 0.3 per 1000 person-years, | Hazard ratio for HCC among NAFLD patients was 3.51 DM at baseline strongest independent predictor of HCC |
| Kanwal et al 201854 | Retrospective cohort | 296,707 NAFLD patients with matched controls | 2004–2015 Mean follow up of 9 years | 0.21/1000 PY | 490 NAFLD patients developed HCC during a mean follow up of 9 years HR for HCC among NAFLD patients: 7.62 RF: Cirrhosis, Older Hispanics |
| Sanyal et al, 2006188 | Prospective cohort | 152 NASH cirrhosis | 10 years | 10 patients developed HCC over 10 years | No risk factors identified |
Abbreviations: NR, Not reported; RF, Risk factors.
HCC in Cryptogenic Cirrhosis
It is also important to mention that previously classified cryptogenic cirrhosis cases are now more and more recognized as “Burnt-out” NASH or advanced stage of NASH.26 Hence, the incidence of NAFLD-related HCC tends to be underquoted previously either because of misdiagnosis as cryptogenic cirrhosis or metabolic syndrome remaining under quantified in some regions of the world.61 A majority of patients with cryptogenic cirrhosis share many similarities with NASH, where both groups were strongly associated with DM and obesity.62–65 It has been reported that likely more than half of the patients with cryptogenic cirrhosis had prior histological or clinical features associated with NAFLD.62 Also, an increase of visceral fat area in cryptogenic HCC patients and the negative correlation with liver–spleen density ratio measurements also support the idea that cryptogenic cirrhosis is burnt-out NAFLD in which visceral fat remains but liver fat is depleted.63 Further clarification of this diagnosis is needed in the future to estimate the true incidence of HCC in patients with NAFLD.
HCC in Non-Cirrhotic NAFLD
Cirrhosis remains the most important cause for the development of HCC, but in NAFLD patients, HCC can develop even in the absence of cirrhosis. Several studies have shown that up to 50% of patients with NAFLD-related HCC had no clinical or histological evidence of cirrhosis.66–72 A multicenter prospective study from Italy showed that 50% of NAFLD-related HCC patients had no evidence of cirrhosis. After adjusting for confounders, the survival rate of NAFLD-related HCC patients was similar to HCV-related HCC patients.66 Ertle et al reported that among 162 patients with HCC, patients with NAFLD/NASH-related HCC exhibited a higher prevalence of metabolic features, and 41% had no features of cirrhosis.67 Similarly, another study found out that NAFLD risk factors are present in most patients with HCC in the non-cirrhotic liver.73 Alexander et al described the association of hepatic steatosis with the development of HCC in non-cirrhotic liver.74 The presence of NAFLD and components of metabolic syndrome, particularly obesity and DM, have been independently associated with HCC development and together contribute to the risk of HCC in the non-cirrhotic liver.75–77 Recently, Tobair et al have shown that among patients with non-cirrhotic NAFLD, male gender, alcohol consumption, and high FIB-4 index were risk factors for HCC development.78 Elevated ALT along with increasing age have been identified as independent risk factors for HCC development among NAFLD patients.79 Non-cirrhotic NAFLD-related HCC patients are more likely to present with advanced HCC falling outside the Milan criteria but have better overall survival as compared to cirrhotic patients developing HCC.80
Overall, non-cirrhotic NAFLD patients have 2.5-fold higher risk of developing HCC than other etiologies of CLD without cirrhosis.81 However, the overall risk of HCC development in non-cirrhotic NAFLD is not well quantified but is thought to be less than the 1.5% per year threshold for the efficacy of HCC surveillance (>0.25 life-years gained).54 Therefore, systematic HCC screening may not be prudent and is currently not recommended by the American Association for the study of liver disease (AASLD) and American gastroenterological Association (AGA) for HCC surveillance in non-cirrhotic NAFLD patients.82,83 Recently, one large study from the US has shown that prevalence of HCC in non-cirrhotic NAFLD is 4.6/10,000 persons and 374.4/10,000 among cirrhotic NAFLD patients. Age > 65 years, presence of DM, smoking, male gender, and even one time elevated ALT were the risk factors identified for HCC development in non-cirrhotic NAFLD patients. The presence of all these five factors increased the prevalence to 45.5/10,000 persons.84
In the absence of well-defined risk scoring systems for HCC development in non-cirrhotic NAFLD patients, it may be useful to consider surveillance for HCC in those at the highest possible risk, such as older male patients with alcohol use history, elevated ALT levels, diabetes, or advanced fibrosis as determined by non-invasive methods. However, this remains a controversial topic.
Overview of Risk Factors
The strongest risk factor for the development of HCC is the presence of liver cirrhosis, as more than two-thirds of HCC cases develop in patients with cirrhosis. The incidence rate is 25-fold higher in NAFLD patients with advanced fibrosis than patients without significant fibrosis.85 However, certain other demographic, metabolic, and genetic factors have been associated with the development of HCC in NAFLD. Here, we will briefly describe those risk factors.
Demographic Risk Factor
Increasing age and male gender are associated with the development of HCC in NASH patients with cirrhosis.35,53,79,85,86 Ascha et al examined a cohort of NASH cirrhosis patients for the development of HCC. They found out that older age was associated with the development of HCC in multivariate regression analysis while the male gender was associated with HCC in univariate analysis.53 These findings were also confirmed by Corey et al in a case-control study showing that older age, male gender, and non-Hispanic ethnicity were risk factors associated with HCC development in NASH cirrhosis patients.86 Vilar et al35 also found that in patients with NASH-related bridging fibrosis or cirrhosis, older age and male gender are independent risk factors for the development of HCC. Lee et al noticed that the 10-year cumulative HCC incidence was highest in older (age >55 years) patients with ALT elevation,79 while Kawamura showed that elderly NAFLD patients with DM, elevated serum AST, and thrombocytopenia are at the highest risk of HCC development.85 Male patients with NASH appear to be at higher risk of developing HCC,70 and several mechanisms have been proposed to explain this difference. Estrogen has been associated with HCC suppression in females via inhibiting the JAK1-STAT6 signaling pathway.87 Sex-specific differences in exposure to HCC risk factors may also contribute to the higher risk of HCC among men. Men are more likely to be infected with hepatitis B and C viruses, smoke more, consume more alcohol, and have increased liver iron stores compared to women.88 Lastly, androgens have been linked with upregulation of inflammation in men with elevated cytokine levels, particularly relevant in HCC related to HBV.89
Ethnicity also appears to play a role in NAFLD-related HCC. Hispanics are more likely than non-Hispanics to develop HCC90,91 and have an increased risk of NAFLD development.14 US-born Hispanics have a higher incidence of HCC development than Hispanics born outside the US, while the reverse is true for Asians, suggesting that socioeconomic, environmental, and cultural differences may play a more substantial role than genetics.92
Metabolic Risk Factors
Metabolic syndrome, obesity, and DM are linked with NAFLD development. They have also been associated with the development of HCC independently. Obesity has been linked with the development of multiple cancers and an increased risk of mortality overall.6,93 For HCC specifically, Nair et al studied patients who underwent liver transplants and demonstrated that obesity was an independent predictor for HCC in patients with alcohol-related cirrhosis and cryptogenic cirrhosis.94 In a meta-analysis of 11 cohort studies, Larsen et al demonstrated that the relative risks of liver cancer were 1.17 for overweight and 1.89 for obese compared with normal-weight persons.95 While elevated BMI is an independent risk factor, visceral or central adiposity specifically is associated with increased risk of HCC.96 Visceral adiposity is a risk factor for HCC recurrence after curative treatment in patients with suspected NASH.97 It has been demonstrated that intramuscular fat deposition, sarcopenia, and visceral adiposity rather than BMI alone are independent risk factors for a poor prognosis in HCC patients. The proportion of these body composition components is higher in obese and underweight patients than normal-weight patients. Sarcopenia and intramuscular fat deposition contribute to muscle weakness resulting in reduced activities associated with daily living. Also, they are associated with insulin resistance, vitamin D deficiency, and increased inflammatory cytokine levels, which may explain the increased risk of HCC.98
The prevalence of metabolic syndrome, as defined by the US National Cholesterol Education Program Adult Treatment Panel III criteria (complete definition in Table 2),99 is associated very strongly with NAFLD development and has also been linked with HCC development. In the Surveillance, Epidemiology, and End Results (SEER)-Medicare database study, which studied HCC cases between 1993 and 2005, metabolic syndrome was significantly associated with the increased risk of HCC and intrahepatic cholangiocarcinoma.100 Also, the presence of each additional metabolic trait (diabetes, hypertension, dyslipidemia, and obesity) in NAFLD patients increases the risk of HCC, with DM conferring the greatest risk.30 Metabolic disorders contributed more to the burden of HCC in this population as a risk factor than any other factors, including smoking, alcohol, and viral hepatitis.101 Chen et al has shown in a nationwide cohort that while metabolic syndrome alone without NAFLD does not increase the risk of HCC, it significantly increases the risk of hepatocarcinogenesis in patients with NAFLD.102
Table 2.
National Cholesterol Education Program Adult Treatment Panel III Definition for Metabolic Syndrome (2001)
| Three or More of the Following Risk Factors | |
|---|---|
| Fasting plasma glucose | >5.6 mmol/L (100 mg/dL) |
| Blood pressure | >130/≥85 mm Hg |
| Triglycerides | ≥1.7 mmol/L (150 mg/dL) |
| High-density lipoprotein cholesterol | Men: <1.03 mmol/L (40 mg/dL) Women: <1.29 mmol/L (50 mg/dL |
| Obesity | Men: waist circumference >102 cm Women: waist circumference >88 cm |
Notes: Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497.
The evidence linking DM with the development of HCC is robust.103–107 Raff et al showed that DM increases the risk of HCC in NAFLD and alcohol-related liver disease patients.103 Two separate meta-analyses have provided evidence that DM is associated with increased risk of HCC and HCC mortality.104,107 This association is independent of geographic location, alcohol consumption, history of cirrhosis, or infections with hepatitis B or C virus. Also, duration of DM, level of glycemic control, use of sulfonylureas, or insulin treatment possibly in combination increases the risk of HCC, while metformin has a protective effect on HCC development.104,108 Yang et al demonstrated similar results that DM is associated with increased risk of HCC in NAFLD-related cirrhosis patients.106
Genetic Risk Factors
Host genetic polymorphisms are involved in HCC pathogenesis and may serve as risk factors and predictive biomarkers.109 At least three common genetic variants in the patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), and membrane-bound O-acyltransferase domain-containing 7 (MBOAT7) genes have been robustly linked to NAFLD in the population. The study of these genes’ role highlights novel pathways in the development of NAFLD and their possible role in HCC development.110
The rs738409 C > G variant in the PNPLA3 gene, encoding an I148M mutation, is independently associated with NAFLD and is also associated with fibrosis progression and HCC development.111–115 Initially, the variant allele PNPLA3 (rs738409; I148M) was associated with increased hepatic fat levels and hepatic inflammation and was more prevalent among Hispanics.111 Later, it was shown that carriage of the PNPLA3 polymorphism is also associated with a greater risk of progressive steatohepatitis, fibrosis, and HCC.112 This association is independent of potential confounders, including age, gender, BMI, presence of DM, advanced fibrosis, or cirrhosis.112 Two separate meta-analyses have shown that PNPLA3 is associated with an increased risk of advanced fibrosis and is an independent risk factor for HCC among patients with nonalcoholic steatohepatitis or alcohol-related cirrhosis.113,114
The rs58542926 C > T variant in the TM6SF2 gene, encoding an E167K mutation, is associated with hepatic steatosis and an increased risk of liver fibrosis.116,117 Its role in HCC development, however, remains uncertain.118 The rs641738 C > T variant of the MBOAT7 gene has been shown to increase the risk of hepatic steatosis and progressive liver disease in NAFLD patients.119 It is also associated with an increased risk of HCC in non-cirrhotic patients with NAFLD.120 A genome-wide study of histologically proven NAFLD patients from Japan found a polymorphism in the DYSF gene to be associated with HCC in addition to the known PNPLA3 polymorphism.121
It is important to emphasize that the above studies were cross-sectional in design, thus carrying their known limitations. Given that NAFLD has a polygenic inheritance pattern, identifying a single genetic mutation predictive of HCC development is challenging. The data on the impact of selected single nucleotide polymorphisms (SNPs) on HCC occurrence is lacking, and the findings need to be validated in prospective cohorts before they can be incorporated in clinical and biochemical parameters to identify patients at the highest risk of HCC development who may benefit from surveillance.109,110
Alcohol and Smoking
Although significant alcohol intake is excluded from the definition of NAFLD, it has been shown that any level of alcohol intake is associated with a higher risk of HCC development in NASH patients compared to patients who have never consumed alcohol.53 Recently, a longitudinal study has shown that moderate alcohol consumption among compensated NASH-cirrhotic patients may exacerbate liver disease progression and increase the risk of hepatic decompensation, HCC, and death.35 Smoking has also been linked with the development of HCC in general,122 and has been shown to increase the risk of HCC development by two folds in one study.35 Based on these findings, NAFLD patients, especially those with advanced fibrosis, should be counseled for smoking cessation and advised against alcohol consumption.
Presentation of NAFLD-Related HCC
Patients with NAFLD-related HCC are older at presentation than patients with non-NAFLD-related HCC patients and are more likely to be men.10 NAFLD-related HCC patients have been shown to have more extrahepatic comorbidities but have a lower prevalence of cirrhosis, with only 66% of the HCC patients have underlying cirrhosis.10,66,123 Patients with NAFLD-related HCC have less severe liver dysfunction at the time of diagnosis, more often had the presence of metabolic syndrome, and had a lower model for end-stage liver disease scores.123,124 Compared to HCV-related HCC patients, NAFLD-related HCC patients tend to be diagnosed with smaller-sized tumors, but there is no difference in terms of the total number of lesions or the status of microvascular invasion or lymph node involvement.53,124 The size of the NAFLD-HCC tumor is inversely related to the extent of cirrhosis as determined by the Child-Pugh score.125 It has also been shown that NAFLD patients have slower tumor growth rate as compared to viral-related HCC.125 Trabecular carcinoma is the most common histopathological type of HCC in NAFLD patients.126
Prognosis of NAFLD-Related HCC
The data on the prognosis and survival of NAFLD-related HCC patients is conflicting. NAFLD-related HCC patients are diagnosed at more advanced stages due to less systematic surveillance and therefore may not be good candidates for all available treatment options.10 It has been shown that despite retained liver function, patients with NASH-related HCC had reduced overall survival123,127 and advanced tumor stage.127 However, the opposite trend has been reported in other studies showing that the survival rate of NAFLD-related HCC patients is at least the same as of HCV-HCC patients.66,126 Reddy et al showed improved survival in NASH-related HCC patients with recurrence rates the same as other causes.124 Despite NASH-related HCC patients being older at the time of presentation and having advanced disease, similar survival may be attributed to incidental presentation and lower cirrhosis prevalence.128 NAFLD-related HCC patients undergoing surgical resection have increased post-resection morbidity and 30-day mortality than HCV and HBV-related HCC patients.129,130 However, overall survival is comparable between the NAFLD-related HCC and Hepatitis virus-related HCC, with the NAFLD-related HCC group having better disease-free survival.69,126,127 A recent meta-analysis has also demonstrated that patients with NAFLD-related HCC have better disease-free and overall survival after liver resection than non-NAFLD-related HCC.132 When all forms of curative therapies were considered, overall survival was similar between the two groups, but disease-free survival favored the NAFLD-related HCC group.132 However, in cases of advanced HCC, one study showed that NAFLD-related HCC patients have reduced overall survival when treated with anti-PD1 (programmed death-1) or anti-PDL1 (programmed death ligand-1) agents as compared to HBV or HCV-related HCC. This reduced responsiveness of HCC to immunotherapy in NASH patients might be attributable to aberrant T cell activation resulting in tissue damage and impaired immune surveillance.133 However, the evidence on this topic is evolving rapidly, and further studies are needed to confirm or refute these observations.
NAFLD-Related HCC and Liver Transplant
NASH is the fastest growing indication for liver transplant in patients with HCC.46,47 Patients undergoing liver transplantation with NAFLD-related indications tend to fare the same as other conditions. In a meta-analysis, patients with NASH who underwent liver transplants had similar 1, 3, and 5 years survival rates as patients without NASH. However, patients with NASH were more likely to die from cardiovascular complications or sepsis.134 Specifically for NASH-related HCC patients, Sadler et al demonstrated that overall survival at 1, 3, and 5 years and tumor recurrence was the same for NASH-related HCC and non-NASH-related HCC patients, and NASH status appeared to have a protective effect for tumor recurrence among patients with tumors beyond Milan criteria.135
Post liver transplant, the incidence of recurrent NAFLD is 59%, 57%, 82% at 1, 3, and 5 years follow-up, similar to de novo NAFLD incidence. However, the incidence of recurrent NASH is significantly higher than de novo NASH; 53%, 57.4%, and 38% for recurrent NASH vs 1 3%, 16%, and 17%, for de novo NASH at 1, 3, and 5 years post liver transplant.136 Post-transplant, high BMI, hyperlipidemia, and alcohol abuse history are predictors of hepatic steatosis development.136,137 Hepatic steatosis, though extremely common after liver transplantation, does not seem to have an adverse effect on post liver transplant outcomes,138 but biopsy-proven NASH post liver transplant is a significant risk factor for lower long-term survival.139
Issue of Surveillance
Current American and European guidelines do not recommend separate surveillance guidelines for non-cirrhotic NAFLD-related HCC. However, there is considerable concern that some level of surveillance is needed for non-cirrhotic NAFLD-related HCC.140 As noted previously, patients with non-cirrhotic NAFLD-related HCC are more likely to be older, picked up at an advanced stage, have an incidental presentation, and have a higher prevalence of metabolic syndrome and cardiovascular diseases. Also, almost one-third of the patients with NAFLD-related HCC have no underlying cirrhosis.66,128 Given the ever-increasing incidence of NAFLD, the burden of NAFLD-related HCC will continue to increase in the future, highlighting the need to develop predictive biomarkers to identify specific high-risk subgroups. The most important issue is the non-invasive diagnosis of patients with advanced fibrosis, as fibrosis is the most important factor in NAFLD patients associated with adverse outcomes. Several serum biomarkers have shown good accuracy to identify patients with advanced fibrosis, who needs close followup and surveillance for the development of cirrhosis and HCC.10 NAFLD fibrosis score (NFS), Hepamet Fibrosis Score (HFS), and FIB-4 outperform other scores in the identification of fibrosis and prediction of long-term outcomes including mortality.141,142 NFS and FIB-4 are the best at predicting long-term liver-related events, while NFS is the best predictor of HCC.141 FIB-4 ≥ 2.67 is a strong predictor of both all-cause mortality and adverse liver-related outcomes, including progression to NASH, cirrhosis, end-stage liver disease, and transplantation.143 The Hepamet fibrosis score provides greater advantage in selecting NAFLD patients who should undergo liver biopsy.142
Patients with NAFLD-related cirrhosis are less likely to undergo HCC surveillance resulting in HCC being detected at a more advanced stage.66,128 Ultrasonography – the recommended surveillance modality – is operator-dependent and is challenging in centrally overweight patients with heterogeneous fatty liver.144 It has been shown that sensitivity of ultrasound (US) for HCC detection is significantly worse for obese patients, patients with NASH and advanced cirrhosis (Child C).145,146 Overall sensitivity of US for HCC detection is 82% compared to cross-sectional imaging, but it is reduced in obese patients (BMI ≥ 30 kg/m2) to 76% versus 86% for non-obese patients and in NASH patients to 59% versus 84% for non-NASH patients.146
Despite these limitations, guidelines have recommended using the US abdomen every six months with or without AFP.9,10 However, it has been recommended that the adequacy of US should be documented in assessing the liver parenchyma for mass lesions. When the adequacy of US is sub-optimal, computed tomography or magnetic resonance imaging with or without AFP should be used for future surveillance every six months.82 It has been shown that in select subgroup of cirrhosis patients who are at high risk of developing HCC (annual HCC risk >3%), MRI-based HCC screening is cost-effective and can increase early tumor detection.147
For patients with cirrhosis, different models are available to predict the risk of HCC. The Toronto HCC risk index is a validated scoring system to predict the 10-year risk of HCC in cirrhotic patients based on commonly available parameters such as age, sex, underlying etiology, and platelets.148 GALD and BALAD-2 models have shown good value for diagnosing HCC and predicting patient survival, respectively.149 However, they are not specific for NAFLD-related HCC patients. Ioannou et al have recently developed a simple model to estimate HCC risk in NAFLD cirrhosis patients. The model includes age, gender, DM, BMI, platelets, albumin, and AST to ALT ratio and exhibits an area under the receiver operating characteristic curve (AUROC) of 0.75. This risk-based screening was shown to have a net benefit over the standard approach.150 For NAFLD patients, validated HCC risk prediction models are needed to identify the patients at high risk who would benefit the most from surveillance.151
In practice, we agree with the approach suggested in the “AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with NAFLD” of using two non-invasive tests to assess advanced liver cirrhosis and fibrosis. Patients having unequivocal evidence of cirrhosis or advanced fibrosis (FIB-4 > 3.25, or transient elastography score >16.1 kPa or >5 kPa for magnetic resonance elastography) should be included in regular HCC surveillance.82 Patients with no or low-grade fibrosis (F0-F2) should be followed up with assessment of the fibrosis stage every 1–3 years. Although patients with F3 fibrosis have shown to have an increased risk of HCC compared to patients with stage F0 to F2 fibrosis,152 no recommendations can be made about the induction of such patients in regular HCC surveillance. However, we suggest that patients with F3 fibrosis who have additional risk factors for HCC development (older age, male gender, diabetes, any degree of alcohol intake) should be closely monitored.153
For evaluation of NAFLD after liver transplant, the patients are screened with hepatic imaging by post-transplant protocol. Patients with steatosis but no elevation of their serum transaminases are followed with standard protocol imaging. However, if liver enzymes are increased, either transient elastography (TE) or magnetic resonance elastography (MRE) to determine if patients have fibrosis. Patients without fibrosis can be followed with yearly TE to ensure no disease progression. If F3 fibrosis is noted, they are screened for HCC every six months with imaging. Lesions noted on imaging are further characterized via cross-sectional imaging and survey by standard protocol.
Prevention of NAFLD-Related HCC
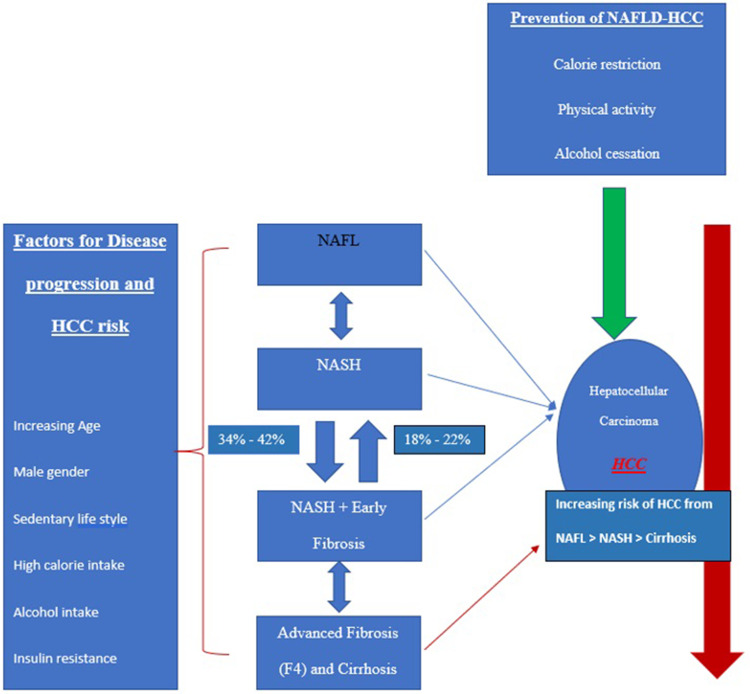
The presence of cirrhosis among NAFLD patients is the most significant risk factor for HCC development. In addition, DM has been linked with the risk of HCC among NAFLD patients.154 Apart from being linked with the development and progression of NAFLD, DM and obesity are independently associated with the development of HCC.7,155 Lifestyle and dietary interventions targeting the development of obesity, DM, and metabolic syndrome or their reversal on a population-based level would be a cost-effective way to reduce the HCC risk.38 The interplay of different pathophysiological states of hepatic steatosis from NAFLD to NASH to cirrhosis leading to HCC development, as well factors associated with disease progression and prevention are summarized in Figure 1. 2,10,22
Figure 1.
Pathophysiological states of NAFLD and HCC along with risk factors for disease progression and HCC prevention.2,10,23
Physical activity has also been shown to reduce the incidence of HCC by 36% in one study,156 with the level of reduction correlating with the level of physical activity.156,157
Daily consumption of fruits and vegetables is associated with decreased cancer risk.158 A meta-analysis noted that increased intake of vegetables, but not fruits, was associated with a lower risk for HCC. Every 100 g/day increase in vegetable intake decreased the risk of HCC by 8%.159 Among different dietary patterns, close adherence to the Mediterranean diet appears most protective against HCC.160 The beneficial effect of the Mediterranean diet and exercise seems to extend beyond the potential impact of weight loss and should be recommended to all patients with NAFLD/NASH.161
Alcohol cessation has also been shown to reduce HCC development risk by 6–7% a year.162 Among beverages, coffee consumption has been shown to reduce the risk of HCC. In one population-based prospective cohort study, people who drank 2–3 cups per day of coffee had a 38% reduction in the risk for HCC and a 46% reduction in risk of death from chronic liver disease than those who did not take coffee.163 A meta-analysis also confirmed that the risk of HCC is reduced by 40% for any coffee consumption.164
In addition to dietary and lifestyle interventions, the use of pharmacological therapy, such as statins and metformin, for the prevention of HCC appears to be interesting. Multiple studies have shown the protective effect of metformin against HCC development.79,165,166 A meta-analysis of 19 studies involving 550,882 diabetic subjects showed that metformin use reduced liver cancer risk by 48%. This association was independent of hepatitis B/C virus infection status, cirrhosis, obesity, behavioral factors, and time-related bias.165 The protective effect of metformin seems independent of the hypoglycemic effect since other hypoglycemic agents like sulfonylureas and exogenous insulin have been linked with increased risk of HCC development.167,168 Angiotensin-converting enzyme inhibitors (ACEIs) but not angiotensin receptor blockers (ARBs) have also been shown to decrease the risk of cirrhotic complications and HCC development, with greater benefit noted in CKD patients.169
For statins, dose-dependent reduction in incident cirrhosis and HCC has been demonstrated in both HCV and HBV infected patients.170,171 Atorvastatin and Fluvastatin seem to have the highest protective role among statins class.170,172
Anti-fibrotic therapies acting to halt and possibly reverse fibrosis progression in NAFLD patients are at various stages of development and, hopefully, will prevent the development of HCC when used in practice. Selonsertib, an ASK1 inhibitor, was shown in a Phase 2 trial to reduce liver fibrosis in patients with nonalcoholic steatohepatitis and stage 2–3 fibrosis,173 but failed to meet the primary efficacy endpoint of improvement in fibrosis stage in NASH and bridging fibrosis or compensated cirrhosis in two separate Phase III randomized trials.174 Cenicriviroc, a dual inhibitor of fibrosis-promoting CCR2/CCR5, showed improvement in fibrosis among NASH patients with no worsening of steatohepatitis in a phase 2 study.175 Currently, a Phase 3, multicenter, randomized, clinical trial of subjects with NASH and stage F2 or F3 fibrosis is undergoing to evaluate the efficacy and safety of Cenicriviroc (AURORA, NCT03028740).176
Another drug Elafibranor, a PPAR α/δ agonist, showed to halt fibrosis progression in non-cirrhotic NASH patients in a phase 2 trial.177 However, recently, in phase III trial RESOLVE-IT, elafibranor failed to meet its primary (NASH resolution) and secondary endpoints (improvement of fibrosis stage) (NCT02704403). Obeticholic acid (OCA), a nuclear Farnesoid X receptor (FXR) activator, has been shown to ameliorate NASH and improve fibrosis in the REGENERATE trial.178 However, pruritus is a significant side effect of the drug affecting clinical compliance, and long-term health effects of obeticholic acid are still for debate.179
The development of effective anti-fibrotic therapy is the need of the hour for NAFLD patients, as the severity of liver fibrosis is directly related to liver-associated morbidity and mortality. While it is thought that decreasing the presence of fibrosis and NASH may reduce the risk of developing HCC, none of the emerging therapies have shown a reduction in the incidence of HCC in NAFLD patients.
Conclusion
NAFLD is the most common cause of chronic liver disease, and the burden of NAFLD-associated liver disease is expected to continue to worsen in the future. NAFLD-related cirrhosis and HCC share the same risk factors, including DM, obesity, and metabolic syndrome. The incidence of HCC in NAFLD-related cirrhosis is estimated to be between 1% and 3% per year, exceeding the threshold of >1.5% per year, justifying regular HCC surveillance in this population.83 The need for HCC surveillance in non-cirrhotic NAFLD is not cost-effective currently, and more evidence is needed on this topic from more extensive prospective studies. Non-cirrhotic NAFLD patients have a threefold increased risk of HCC development compared to other causes of liver disease;81 however, the overall incidence remains low and undefined (2.7% at 10 years for non-cirrhotic NAFLD vs 15% at 10 years in NAFLD-related cirrhosis).140 While the current guidelines suggest against HCC surveillance in non-cirrhotic NAFLD patients, those with advanced fibrosis or cirrhosis suggested by non-invasive markers may warrant consideration for surveillance. Currently, the role of genetic risk factors and concurrent comorbid conditions is not well incorporated into surveillance guidelines. The development of predictive biomarker models and risk scores to identify the high-risk population remain an unmet clinical need. Clinical guidelines should consider this, and a risk score should be proposed based on genetic risk factors and comorbid conditions to identify the highest risk patients for HCC development in non-cirrhotic NAFLD.
Abbreviations
NAFLD, Nonalcoholic fatty liver disease; CLD, Chronic liver disease; NASH, Nonalcoholic steatohepatitis; HCC, Hepatocellular carcinoma; DM, Diabetes mellitus; HLD, Hyperlipidemia; BMI, Body mass index; HS, Hepatic steatosis; HTN, Hypertension; HCV, Hepatitis C virus; HBV, Hepatitis B virus; US, Ultrasound.
Disclosure
Dr David Victor is a member of advisory board in Eisai and Exelixis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Obesity and overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed March 20, 2020.
- 2.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428. doi: 10.1038/s41575-019-0145-7 [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 4.Kivimäki M, Kuosma E, Ferrie JE, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2(6):e277–e285. doi: 10.1016/S2468-2667(17)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malnick SDH, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–579. doi: 10.1093/qjmed/hcl085 [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08 [DOI] [PubMed] [Google Scholar]
- 7.Bhaskaran K, Douglas I, Forbes H. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. The Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Das A, Majumder K, et al. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer-related Mortality: a Systematic Review and Meta-Analysis. Am J Clin Oncol. 2018;41(9):874–881. doi: 10.1097/COC.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 10.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. - PubMed - NCBI. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27062661. Accessed March 27, 2020.
- 11.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268 [DOI] [PubMed] [Google Scholar]
- 12.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 13.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 15.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1 [DOI] [PubMed] [Google Scholar]
- 17.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 19.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98 [DOI] [PubMed] [Google Scholar]
- 20.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab Clin Exp. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 21.Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non-alcoholic fatty liver disease. Hepatol Int. 2019;13(6):688–694. doi: 10.1007/s12072-019-09995-8 [DOI] [PubMed] [Google Scholar]
- 22.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–4761. doi: 10.1210/jc.2006-0587 [DOI] [PubMed] [Google Scholar]
- 23.White DL, Kanwal F, El-Serag HB. Non-Alcoholic Fatty Liver Disease and Hepatocellular Cancer: a Systematic Review. Clin Gastroenterol Hepatol. 2012;10(12):1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59(3):550–556. doi: 10.1016/j.jhep.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis Progression in Nonalcoholic Fatty Liver versus Nonalcoholic Steatohepatitis: a Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654.e9. doi: 10.1016/j.cgh.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong S, Ting Y, Chan W. Epidemiology of non‐alcoholic fatty liver disease‐related hepatocellular carcinoma and its implications. JGH Open. 2018;2(5):235–241. doi: 10.1002/jgh3.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 28.Taylor RS, Taylor RJ, Bayliss S, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Gastroenterology. 2020;158(6):1611–1625.e12. doi: 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 29.Rinella ME, Loomba R, Caldwell SH, et al. Controversies in the Diagnosis and Management of NAFLD and NASH. Gastroenterol Hepatol (N Y). 2014;10(4):219–227. [PMC free article] [PubMed] [Google Scholar]
- 30.Kanwal F, Kramer JR, Li L, et al. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2019. doi: 10.1002/hep.31014 [DOI] [PubMed] [Google Scholar]
- 31.Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine. 2018;97:13. doi: 10.1097/MD.0000000000010214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(11):1224–1229, 1229.e1-2. doi: 10.1016/j.cgh.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 33.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 34.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: a Multi-National Cohort Study. Gastroenterology. 2018;155(2):443–457.e17. doi: 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 36.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: systematic Review and Meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. doi: 10.1016/j.jhep.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 38.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 39.Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011;38(4):201–205. [PMC free article] [PubMed] [Google Scholar]
- 40.Akinyemiju T, Abera S, Ahmed M, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balogh J, Iii DV, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocellular Carcinoma. 2015;1:254. doi: 10.2147/JHC.S61146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phipps M, Livanos A, Guo A, et al. Gender Matters: characteristics of Hepatocellular Carcinoma in Women From a Large, Multicenter Study in the United States. Am J Gastroenterol. 2020;115(9):1486–1495. doi: 10.14309/ajg.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683. doi: 10.1016/j.jhep.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Goh GBB, Chang PE, Tan CK. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol. 2015;29(6):919–928. doi: 10.1016/j.bpg.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. doi: 10.1002/hep.26986 [DOI] [PubMed] [Google Scholar]
- 47.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e3. doi: 10.1016/j.cgh.2018.05.057 [DOI] [PubMed] [Google Scholar]
- 48.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26(9):2183–2191. doi: 10.1185/03007995.2010.506375 [DOI] [PubMed] [Google Scholar]
- 49.Hester D, Golabi P, Paik J, Younossi I, Mishra A, Younossi ZM. Among Medicare Patients With Hepatocellular Carcinoma, Non-alcoholic Fatty Liver Disease is the Most Common Etiology and Cause of Mortality. J Clin Gastroenterol. 2019. doi: 10.1097/MCG.0000000000001172 [DOI] [PubMed] [Google Scholar]
- 50.Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Non-alcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152(5):1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Toy M. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010-2019. PLoS One. 2020;15(9):e0239393. doi: 10.1371/journal.pone.0239393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Do AL, Wong CR, Nguyen LH, Nguyen VG, Trinh H, Nguyen MH. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol. 2014;48(7):644–649. doi: 10.1097/MCG.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 53.Ascha MS, Hanouneh IA, Lopez R, Tamimi TAR, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi: 10.1002/hep.23527 [DOI] [PubMed] [Google Scholar]
- 54.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients with Non-alcoholic Fatty Liver Disease. Gastroenterology. 2018;155(6):1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asfari MM, Talal Sarmini M, Alomari M, Lopez R, Dasarathy S, McCullough AJ. The association of nonalcoholic steatohepatitis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2020. doi: 10.1097/MEG.0000000000001681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujii H, Iwaki M, Hayashi H, et al. Clinical Outcomes in Biopsy-Proven Nonalcoholic Fatty Liver Disease Patients: a Multicenter Registry-Based Cohort Study. Clin Gastroenterol Hepatol. 2022:S1542–3565. doi: 10.1016/j.cgh.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 57.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi: 10.1136/gutjnl-2019-318813 [DOI] [PubMed] [Google Scholar]
- 58.Arnold M, Razum O, Coebergh JW. Cancer risk diversity in non-western migrants to Europe: an overview of the literature. Eur J Cancer. 2010;46(14):2647–2659. doi: 10.1016/j.ejca.2010.07.050 [DOI] [PubMed] [Google Scholar]
- 59.Petrick JL, Braunlin M, Laversanne M, Valery P, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139(7):1534–1545. doi: 10.1002/ijc.30211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welzel TM, Graubard BI, Quraishi S, et al. Population-Attributable Fractions of Risk Factors for Hepatocellular Carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314–1321. doi: 10.1038/ajg.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seydel GS, Kucukoglu O, Altinbas A, et al. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. 2016;15(5):662–672. doi: 10.5604/16652681.1212316 [DOI] [PubMed] [Google Scholar]
- 62.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi: 10.1053/jhep.2002.36939 [DOI] [PubMed] [Google Scholar]
- 63.Lee SS, Jeong SH, Byoun YS, et al. Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer. 2013;13(1):335. doi: 10.1186/1471-2407-13-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Younossi Z, Stepanova M, Sanyal AJ, et al. The conundrum of cryptogenic cirrhosis: adverse outcomes without treatment options. J Hepatol. 2018;69(6):1365–1370. doi: 10.1016/j.jhep.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 65.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123(1):134–140. doi: 10.1053/gast.2002.34168 [DOI] [PubMed] [Google Scholar]
- 66.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63(3):827–838. doi: 10.1002/hep.28368 [DOI] [PubMed] [Google Scholar]
- 67.Ertle J, Dechêne A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128(10):2436–2443. doi: 10.1002/ijc.25797 [DOI] [PubMed] [Google Scholar]
- 68.Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11(1):18–27. doi: 10.1016/s1499-3872(11 [DOI] [PubMed] [Google Scholar]
- 69.Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594–601.e1. doi: 10.1016/j.cgh.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9(5):428–433. doi: 10.1016/j.cgh.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 71.Leung C, Yeoh SW, Patrick D, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol. 2015;21(4):1189–1196. doi: 10.3748/wjg.v21.i4.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10(4):632–639. doi: 10.1007/s12072-015-9679-0 [DOI] [PubMed] [Google Scholar]
- 73.Schütte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. doi: 10.1186/1471-230X-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28(5):848–854. doi: 10.1111/jgh.12116 [DOI] [PubMed] [Google Scholar]
- 75.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36(3):317–324. doi: 10.1111/liv.13031 [DOI] [PubMed] [Google Scholar]
- 76.Reeves HL, Zaki MYW, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61(5):1234–1245. doi: 10.1007/s10620-016-4085-6 [DOI] [PubMed] [Google Scholar]
- 77.Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol. 2016;111(11):1573–1580. doi: 10.1038/ajg.2016.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tobari M, Hashimoto E, Taniai M, et al. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35(5):862–869. doi: 10.1111/jgh.14867 [DOI] [PubMed] [Google Scholar]
- 79.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141(7):1307–1314. doi: 10.1002/ijc.30784 [DOI] [PubMed] [Google Scholar]
- 80.Pinyopornpanish K, Al-Yaman W, Dasarathy S, Romero-Marrero C, McCullough A. Hepatocellular Carcinoma in Patients Without Cirrhosis: the Fibrosis Stage Distribution, Characteristics and Survival. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07048-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48(7):696–703. doi: 10.1111/apt.14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: expert Review. Gastroenterology. 2020;158(6):1822–1830. doi: 10.1053/j.gastro.2019.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 84.Pinyopornpanish K, Khoudari G, Saleh MA, et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21(1):394. doi: 10.1186/s12876-021-01978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107(2):253–261. doi: 10.1038/ajg.2011.327 [DOI] [PubMed] [Google Scholar]
- 86.Corey KE, Gawrieh S, deLemos AS, et al. Risk factors for hepatocellular carcinoma in cirrhosis due to nonalcoholic fatty liver disease: a multicenter, case-control study. World J Hepatol. 2017;9(7):385–390. doi: 10.4254/wjh.v9.i7.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang W, Lu Y, Xu Y, et al. Estrogen Represses Hepatocellular Carcinoma (HCC) Growth via Inhibiting Alternative Activation of Tumor-associated Macrophages (TAMs). J Biol Chem. 2012;287(48):40140–40149. doi: 10.1074/jbc.M112.348763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061 [DOI] [PubMed] [Google Scholar]
- 89.Chiu CM, Yeh SH, Chen PJ, et al. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007;104(8):2571–2578. doi: 10.1073/pnas.0609498104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. doi: 10.1038/ajg.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Couto CA, Gelape CL, Calmet F, Martin P, Levy C. Effect of ethnicity on liver transplant for hepatocellular carcinoma. Exp Clin Transplant. 2013;11(4):339–345. doi: 10.6002/ect.2013.0008 [DOI] [PubMed] [Google Scholar]
- 92.Chang ET, Yang J, Alfaro-Velcamp T, So SKS, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3106–3118. doi: 10.1158/1055-9965.EPI-10-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 94.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36(1):150–155. doi: 10.1053/jhep.2002.33713 [DOI] [PubMed] [Google Scholar]
- 95.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97(7):1005–1008. doi: 10.1038/sj.bjc.6603932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlesinger S, Aleksandrova K, Pischon T, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132(3):645–657. doi: 10.1002/ijc.27645 [DOI] [PubMed] [Google Scholar]
- 97.Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58(6):839–844. doi: 10.1136/gut.2008.164053 [DOI] [PubMed] [Google Scholar]
- 98.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–140. doi: 10.1016/j.jhep.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 99.Panel On Detection, Evaluation, And Treatment Of High Blood Cholesterol In Adults E, Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 100.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54(2):463–471. doi: 10.1002/hep.24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757–1765. doi: 10.1002/cncr.29971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen YG, Yang CW, Chung CH, Ho CL, Chen WL, Chien WC. The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: a nationwide population-based cohort study. Hepatol Int. 2022. doi: 10.1007/s12072-021-10281-9 [DOI] [PubMed] [Google Scholar]
- 103.Raff EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol. 2015;3(1):9–16. doi: 10.14218/JCTH.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–122. doi: 10.1002/dmrr.1291 [DOI] [PubMed] [Google Scholar]
- 105.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. doi: 10.1136/gut.2004.052167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang JD, Ahmed F, Mara KC, et al. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71(3):907–916. doi: 10.1002/hep.30858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165 [DOI] [PubMed] [Google Scholar]
- 108.Kramer JR, Natarajan Y, Dai J, et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2021. doi: 10.1002/hep.32244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13(2):179–187. doi: 10.1080/17474124.2019.1549989 [DOI] [PubMed] [Google Scholar]
- 110.Pennisi G, Celsa C, Giammanco A, Spatola F, Petta S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: screening Issue and Future Perspectives. Int J Mol Sci. 2019;20:22. doi: 10.3390/ijms20225613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu YL, Patman GL, Leathart JBS, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. doi: 10.1016/j.jhep.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 113.Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(3):325–334. doi: 10.1038/ajg.2013.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trépo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: evidence from a meta-analysis of individual participant data. Hepatology. 2014;59(6):2170–2177. doi: 10.1002/hep.26767 [DOI] [PubMed] [Google Scholar]
- 115.Grimaudo S, Pipitone RM, Pennisi G, et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2020;18(4):935–944.e3. doi: 10.1016/j.cgh.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 116.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–356. doi: 10.1038/ng.2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falleti E, Cussigh A, Cmet S, Fabris C, Toniutto P. PNPLA3 rs738409 and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis. Dig Liver Dis. 2016;48(1):69–75. doi: 10.1016/j.dld.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 119.Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150(5):1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Donati B, Dongiovanni P, Romeo S, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7(1):4492. doi: 10.1038/s41598-017-04991-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawaguchi T, Shima T, Mizuno M, et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13(1):e0185490. doi: 10.1371/journal.pone.0185490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abdel-Rahman O, Helbling D, Schöb O, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245–254. doi: 10.1111/jebm.12270 [DOI] [PubMed] [Google Scholar]
- 123.Weinmann A, Alt Y, Koch S, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. doi: 10.1186/s12885-015-1197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809–1819. doi: 10.1002/hep.25536 [DOI] [PubMed] [Google Scholar]
- 125.Benhammou JN, Lin J, Aby ES, et al. Nonalcoholic fatty liver disease-related hepatocellular carcinoma growth rates and their clinical outcomes. Hepatoma Res. 2021;7:70. doi: 10.20517/2394-5079.2021.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tokushige K, Hashimoto E, Yatsuji S, et al. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45(9):960–967. doi: 10.1007/s00535-010-0237-1 [DOI] [PubMed] [Google Scholar]
- 127.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. doi: 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- 128.Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110–117. doi: 10.1016/j.jhep.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 129.Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15(8):1450–1458. doi: 10.1007/s11605-011-1540-8 [DOI] [PubMed] [Google Scholar]
- 130.Conci S, Cipriani F, Donadon M, et al. Hepatectomy for Metabolic Associated Fatty Liver Disease (MAFLD) related HCC: propensity case-matched analysis with viral- and alcohol-related HCC. Eur J Surg Oncol. 2021:S0748. doi: 10.1016/j.ejso.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 131.Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63(1):93–101. doi: 10.1016/j.jhep.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 132.Chin KM, Prieto M, Cheong CK, et al. Outcomes after curative therapy for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a meta-analysis and review of current literature. HPB. 2021:S1365. doi: 10.1016/j.hpb.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 133.Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi: 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12(3):394–402.e1. doi: 10.1016/j.cgh.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 135.Sadler EM, Mehta N, Bhat M, et al. Liver Transplantation for NASH-Related Hepatocellular Carcinoma Versus Non-NASH Etiologies of Hepatocellular Carcinoma. Transplantation. 2018;102(4):640–647. doi: 10.1097/TP.0000000000002043 [DOI] [PubMed] [Google Scholar]
- 136.Saeed N, Glass L, Sharma P, Shannon C, Sonnenday CJ, Tincopa MA. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: systematic Review and Meta-analysis. Transplantation. 2019;103(11):e345–e354. doi: 10.1097/TP.0000000000002916 [DOI] [PubMed] [Google Scholar]
- 137.Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: disease burden, current management and future challenges. JHEP Rep. 2020;2(6):100192. doi: 10.1016/j.jhepr.2020.100192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayanan P, Mara K, Izzy M, et al. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation. 2019;103(1):e14–e21. doi: 10.1097/TP.0000000000002317 [DOI] [PubMed] [Google Scholar]
- 139.Gitto S, de Maria N, Di Benedetto F, et al. De-novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol. 2018;30(7):766–773. doi: 10.1097/MEG.0000000000001105 [DOI] [PubMed] [Google Scholar]
- 140.Reig M, Gambato M, Man NK, et al. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation. 2019;103(1):39–44. doi: 10.1097/TP.0000000000002361 [DOI] [PubMed] [Google Scholar]
- 141.Younes R, Caviglia GP, Govaere O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021:S0168. doi: 10.1016/j.jhep.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 142.Ampuero J, Pais R, Aller R, et al. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2020;18(1):216–225.e5. doi: 10.1016/j.cgh.2019.05.051 [DOI] [PubMed] [Google Scholar]
- 143.Vieira Barbosa J, Milligan S, Frick A, et al. Fibrosis-4 Index as an Independent Predictor of Mortality and Liver-Related Outcomes in NAFLD. Hepatol Commun. 2021. doi: 10.1002/hep4.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Della Corte C, Colombo M. Surveillance for hepatocellular carcinoma. Semin Oncol. 2012;39(4):384–398. doi: 10.1053/j.seminoncol.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 145.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi: 10.1111/apt.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Samoylova ML, Mehta N, Roberts JP, Yao FY. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transpl. 2018;24(9):1171–1177. doi: 10.1002/lt.25202 [DOI] [PubMed] [Google Scholar]
- 147.Nahon P, Najean M, Layese R, et al. Early hepatocellular carcinoma detection using magnetic resonance imaging is cost-effective in high-risk patients with cirrhosis. JHEP Rep. 2022;4(1):100390. doi: 10.1016/j.jhepr.2021.100390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharma SA, Kowgier M, Hansen BE, et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2018;68(1):92–99. doi: 10.1016/j.jhep.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 149.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14(6):875–886.e6. doi: 10.1016/j.cgh.2015.12.042 [DOI] [PubMed] [Google Scholar]
- 150.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71(3):523–533. doi: 10.1016/j.jhep.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75(6):1476–1484. doi: 10.1016/j.jhep.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 152.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Eng J Med. 2021;385(17):1559–1569. doi: 10.1056/NEJMoa2029349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fassio E, Barreyro FJ, Pérez MS, et al. Hepatocellular carcinoma in patients with metabolic dysfunction-associated fatty liver disease: can we stratify at-risk populations? World J Hepatol. 2022;14(2):354–371. doi: 10.4254/wjh.v14.i2.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. doi: 10.1186/s12916-019-1321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 156.Behrens G, Matthews CE, Moore SC, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. Eur J Epidemiol. 2013;28(1):55–66. doi: 10.1007/s10654-013-9767-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wen CP, Lin J, Yang YC, et al. Hepatocellular Carcinoma Risk Prediction Model for the General Population: the Predictive Power of Transaminases. J Natl Cancer Inst. 2012;104(20):1599–1611. doi: 10.1093/jnci/djs372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sauvaget C, Nagano J, Hayashi M, Spencer E, Shimizu Y, Allen N. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki Life Span Study. Br J Cancer. 2003;88(5):689–694. doi: 10.1038/sj.bjc.6600775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147(5):1031–1042. doi: 10.1053/j.gastro.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 160.Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60(3):606–611. doi: 10.1016/j.jhep.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 161.Lange NF, Radu P, Dufour JF. Prevention of NAFLD-associated HCC: role of lifestyle and chemoprevention. J Hepatol. 2021;75(5):1217–1227. doi: 10.1016/j.jhep.2021.07.025 [DOI] [PubMed] [Google Scholar]
- 162.Heckley GA, Jarl J, Asamoah BO. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11(1):446. doi: 10.1186/1471-2407-11-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148(1):118–125. doi: 10.1053/j.gastro.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413–1421.e1. doi: 10.1016/j.cgh.2013.04.039 [DOI] [PubMed] [Google Scholar]
- 165.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine. 2017;96(19):e6888. doi: 10.1097/MD.0000000000006888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(7):2347–2353. doi: 10.1210/jc.2012-1267 [DOI] [PubMed] [Google Scholar]
- 167.Kawaguchi T, Taniguchi E, Morita Y, et al. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30(3):479–486. doi: 10.1111/j.1478-3231.2009.02191.x [DOI] [PubMed] [Google Scholar]
- 168.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(6):881–891. doi: 10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 169.Zhang X, Wong GLH, Yip TCF, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2021. doi: 10.1002/hep.32294 [DOI] [PubMed] [Google Scholar]
- 170.Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64(1):47–57. doi: 10.1002/hep.28506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–630. doi: 10.1200/JCO.2011.36.0917 [DOI] [PubMed] [Google Scholar]
- 172.Zhou YY, Zhu GQ, Wang Y, et al. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7(16):21753–21762. doi: 10.18632/oncotarget.7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67(2):549–559. doi: 10.1002/hep.29514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harrison SA, Wong VWS, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39. doi: 10.1016/j.jhep.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 175.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767. doi: 10.1002/hep.29477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anstee QM, Neuschwander-Tetri BA, Wong VWS, et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: aurora Phase 3 study design. Contemp Clin Trials. 2020;89:105922. doi: 10.1016/j.cct.2019.105922 [DOI] [PubMed] [Google Scholar]
- 177.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 178.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19 [DOI] [PubMed] [Google Scholar]
- 179.Smeuninx B, Boslem E, Febbraio MA. Current and Future Treatments in the Fight against Non-Alcoholic Fatty Liver Disease. Cancers. 2020;12(7):54. doi: 10.3390/cancers12071714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho EJ, Kwack MS, Jang ES, et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion. 2011;84(Suppl 1):17–22. doi: 10.1159/000333210 [DOI] [PubMed] [Google Scholar]
- 181.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471–1482.e5. doi: 10.1053/j.gastro.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 182.Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x [DOI] [PubMed] [Google Scholar]
- 183.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24(2):248–254. doi: 10.1111/j.1440-1746.2008.05640.x [DOI] [PubMed] [Google Scholar]
- 184.Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34(3):159–163. doi: 10.7869/tg.120 [DOI] [PubMed] [Google Scholar]
- 185.Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(Suppl 1):E247–252. doi: 10.1111/j.1530-0277.2012.01900.x [DOI] [PubMed] [Google Scholar]
- 186.Marot A, Henrion J, Knebel JF, Moreno C, Deltenre P. Alcoholic liver disease confers a worse prognosis than HCV infection and non-alcoholic fatty liver disease among patients with cirrhosis: an observational study. PLoS One. 2017;12(10):e0186715. doi: 10.1371/journal.pone.0186715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54(4):1208–1216. doi: 10.1002/hep.24491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–689. doi: 10.1002/hep.21103 [DOI] [PubMed] [Google Scholar]