Abstract
Single-copy gene fusions between the lacZ reporter gene and Escherichia coli strains containing promoters induced by cold shock (cspA), cytoplasmic stress (ibp), or protein misfolding in the cell envelope (P3rpoH) were constructed and tested to determine their ability to detect antibacterial agents while simultaneously providing information on their cellular targets. Antibiotics that affect prokaryotic ribosomes selectively induced the cspA::lacZ or ibp::lacZ gene fusion, depending on their mode of action. The membrane-damaging peptide polymyxin B induced both the P3rpoH::lacZ and ibp::lacZ fusions, while the β-lactam antibacterial agent carbenicillin activated only the P3rpoH promoter. Nalidixic acid, a compound that causes DNA damage, downregulated β-galactosidase synthesis from P3rpoH but had little effect on expression of the reporter enzyme from either the cspA or ibp promoter. All model antibiotics could be identified over a wide range of sublethal concentrations with signal-to-noise ratios between 2 and 11. A blue halo assay was developed to rapidly characterize the modes of action of antibacterial agents by visual inspection, and this assay was used to detect chloramphenicol secreted into the growth medium of Streptomyces venezuelae cultures. This simple system holds promise for screening natural or combinatorial libraries of antimicrobial compounds.
As a result of antibiotic use and misuse, bacterial drug resistance has become an increasing concern in human medicine. Compounding this problem is the fact that no new chemical entity has been approved by the United States Food and Drug Administration for the treatment of bacterial diseases for more than 20 years (28). The appearance of enterococcal strains resistant to all available drugs (7) and the lag in the discovery of new antibiotics have fueled a renewed search for compounds effective against bacteria that exhibit multiple drug resistance.
The initial step in the drug discovery process is the identification of promising lead compounds that warrant further development. Lead compounds are typically selected by screening large libraries compiled from natural sources, combinatorial approaches, or rational drug design. Almost all antibiotics currently in use are derived from natural products (e.g., secondary metabolites from Streptomyces spp.) and interfere with cell wall synthesis or macromolecular biosynthesis, including DNA, RNA, and protein synthesis (7).
Traditional methods for screening potential antimicrobial agents rely on inhibition of bacterial growth and often require high concentrations of the compound being tested. As a result, substances that possess antibacterial activity but are found in concentrations that are too low to cause growth inhibition may be overlooked. Furthermore, growth inhibition-based assays provide no information on the mode of action of candidate antibiotics, which is useful for developing a lead compound into a final, marketable drug. The development of “smart” assays that include a highly sensitive method for screening potential antibiotics and simultaneously provide clues about their cellular targets is required to guarantee that identification of lead compounds is not the rate-limiting step in drug discovery (32).
Living cells have evolved remarkable mechanisms for maintaining homeostasis under adverse growth conditions. In the enteric bacterium Escherichia coli, temperature upshifts and other types of stress induce the synthesis of heat shock proteins belonging to the ς32 regulon if misfolded proteins accumulate in the cytoplasm and to the ςE regulon if damage is sustained by the outer membrane or in the periplasm (16). In contrast, exposure to low temperatures leads to a transient shutdown of general protein synthesis and high-level accumulation of cold shock proteins whose Eς70-synthesized transcripts contain characteristic 5′ untranslated regions that play a central role in posttranscriptional regulation (27). In 1990, Van Bogelen and Neidhardt reported that when antibiotics targeting the prokaryotic ribosome were added to the growth medium of E. coli cultivated at 37°C, induction of either heat shock proteins or cold shock proteins was observed depending on whether the A site of the ribosome was empty or occupied (29). Antibiotics that induced a heat shock response (e.g., streptomycin and neomycin) were designated H-group antibiotics, while antibiotics that induced a cold shock response (e.g., chloramphenicol and tetracycline) were designated C-group antibiotics.
Gene fusions between stress promoters and reporter genes, such as lacZ, lux, or gfp, are powerful tools for detecting sublethal concentrations of pollutants and compounds that have cytotoxic or genotoxic effects (2–5, 23, 30). Orser and coworkers have shown that antibiotics that cause DNA damage induce gene fusions between certain stress promoters and lacZ (23). Specifically, mitomycin C activates the gyrA, zwf, clpB katF, and dinD promoters, while nalidixic acid induces the dinD and micF promoters (23). Oh et al. have also reported that the membrane-damaging agent polymyxin B activates a lux fusion to the osmoinducible osmY promoter (22). To date, however, the full potential of stress-inducible promoters for detecting and screening antibacterial agents has not been exploited. In this work, we took advantage of recent advances in our understanding of stress responses in E. coli in order to design a simple, sensitive, and selective microbial assay for the detection and categorization of all major classes of antibacterial compounds. We also demonstrated that this system is suitable for identifying antibiotics from natural sources.
MATERIALS AND METHODS
Plasmids and plasmid construction.
Plasmid pCSBG, a pBR322 derivative encoding E. coli lacZ under transcriptional control of cspA, the major cold shock promoter, has been described previously (31). Plasmid pIBPBG, which encodes lacZ under transcriptional control of the ibp heat shock promoter, was constructed in a similar manner. Briefly, the ibp promoter region, followed by the authentic ribosome binding site and the first 21 nucleotides of the ibpA open reading frame, was obtained on a 226-bp fragment by PCR amplification of plasmid pMON18003 (1). Forward primer 5′-GCCCCCTCAGTGCATGCAATAGACC-3′ was used to introduce an SphI site upstream of the −35 region, and reverse primer 5′-GAACGTAAAGCGTCGACAAATCAAAG-3′ was used to insert a SalI site downstream of the ibpA initiation codon. The amplified promoter fragment was subcloned into pT7Blue (Novagen), sequenced, and recovered following SphI-SalI digestion. The ibp promoter was ligated to the SphI-SalI backbone of pTBGM, a pBR322 derivative encoding a promoterless lacZ gene (31), which yielded pIBPBG.
Strain construction.
AB734, a wild-type E. coli K-12 strain containing a lacZ mutation and lacking antibiotic resistance (9), was obtained from the E. coli Genetic Stock Center. The cspA::lacZ and ibp::lacZ fusions encoded by pCSBG and pIBPBG were moved to the attλ site of AB734 through homologous recombination of the bla and lacZ genes by using λRS45 and the method of Simons et al. (26). High-titer lysates were prepared and spot titration was performed as described previously (25). The presence of single-copy fusions on the AB734 chromosome was verified by PCR amplification of chromosomal DNA performed with forward primers hybridizing to either the cspA (5′-CGTACAGACAATTGAAGCAGTG-3′) or ibp (5′-GCCGATGAGGATCCGCCTGATGG-3′) promoter region and a reverse primer hybridizing to the lacZ gene (5′-CGCGGAAACCGACATCGCAG-3′). An AB734 lysogen containing the λφ[ibp::lacZ] fusion and an AB734 lysogen containing the λφ[cspA::lacZ] fusion were designated ADA110 and ADA310, respectively. Phage λφ[P3rpoH::lacZ] was isolated from CAG16037 (19) and was used to infect AB734. The resulting lysogen was designated ADA410.
Culture and induction conditions.
Shake flasks (volume, 500 ml) containing 100 ml of Luria-Bertani (LB) medium were inoculated at a 1:50 dilution by using overnight samples, and cells were grown at 30°C (ADA110 and ADA410) or 37°C (ADA310). When the absorbance at 600 nm was ∼0.4, 30-ml portions of cultures were transferred to two preheated 125-ml shake flasks. One flask received an appropriate volume of an antibiotic stock solution, while the second received no additive or (for experiments involving chloramphenicol and tetracycline) an equal volume of 100% ethanol. All experiments were performed in triplicate. Stock solutions of chloramphenicol (3.4 mg/ml) and tetracycline (4.125 mg/ml) were prepared in 100% ethanol. Streptomycin sulfate (5 mg/ml), neomycin sulfate (5 mg/ml), carbenicillin (10 mg/ml), nalidixic acid (15 mg/ml), and polymyxin B sulfate (10 mg/ml) were dissolved in double-distilled H2O. All antibiotics were purchased from Sigma.
β-Galactosidase assays.
Culture samples (2 ml) were obtained immediately before cultures were divided and at different times (see below), and the absorbance at 600 nm of each sample was recorded. Cells were sedimented by centrifugation at 6,500 × g for 8 min, resuspended in an equal volume of 50 mM monobasic potassium phosphate (pH 6.5), and lysed with a French press at 10,000 lb/in2. Following centrifugation at 10,000 × g for 10 min, aliquots of clarified lysate were assayed for β-galactosidase activity in duplicate by using the chromogenic substrate o-nitrophenyl-β-d-galactopyranoside as described previously (20).
Antibiotic disk assays.
Cultures of ADA110 (5 ml) that had been grown overnight at 30°C in LB medium were centrifuged at 6,500 × g for 5 min, and the pellets were resuspended in 2.5 ml of 10 mM MgSO4. A 100-μl aliquot of cells was mixed with 4 ml of LB top agar that had been melted at 50°C and was poured into a preheated LB agar plate spread with 25 μl of a solution containing 50 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml in dimethyl fluoride. Antibiotic disks (Difco) were placed on the solidified layer. The plates were incubated overnight at 30°C.
Preparation of Streptomyces venezuelae extracts.
Wild-type Streptomyces venezuelae ISP5230, a chloramphenicol producer, and Cml-11, an isogenic mutant blocked in chloramphenicol production (10), were generous gifts from Leo Vining. Vegetative inocula (50 ml) in GNY medium (18) supplemented with 2% glycerol were prepared as described previously (6) and sedimented by centrifugation at 10,000 × g for 10 min. Shake flasks (volume, 250 ml) containing 25 ml of W-salts (6) supplemented with 0.75% isoleucine and 3% glucose were inoculated with 1-ml portions of wild-type and mutant cultures. The preparations were incubated at 26°C with shaking at 220 rpm. On day 6, 15 ml of each wild-type or Cml-11 culture was filtered through a 0.22-μm-pore-size membrane filter, and the supernatants were stored at 4°C. Chloramphenicol production was quantified by thin-layer chromatography (TLC) as follows. Aliquots (500 μl) of culture supernatants were extracted with an equal volume of ethyl acetate, vortexed, and centrifuged at 10,000 × g for 5 min. The upper layer of each preparation was transferred to a clean glass tube, dried by evaporation, and resuspended in 50 μl of ethyl acetate. Portions (3 μl) of samples and standards (containing 50, 100, and 200 μg of chloramphenicol per ml in ethyl acetate) were loaded with a capillary tube onto a Whatman AL SIL G/UV TLC plate. TLC was performed in chloroform-methanol (87.5:12.5, vol/vol). The chloramphenicol spots were viewed under UV illumination and were compared to standards in order to estimate sample concentrations. No chloramphenicol spot was observed with samples obtained from the supernatant of strain Cml-11, while the typical concentration of chloramphenicol in the supernatant of wild-type S. venezuelae was 5 μg/ml. In the experiment whose results are shown in Fig. 3, 1.5-ml portions of filtrates from wild-type and mutant cultures were extracted with ethyl acetate, resuspended in 10 μl of the same solvent after drying, and transferred to sterile Whatman filter paper disks that were approximately 7 mm in diameter. The estimated concentration of chloramphenicol transferred to each wild-type disk was 7.5 μg/ml. Disk assays were performed by using ADA310 cells as described above, except that the plates were incubated at 37°C.
FIG. 3.
Detection of chloramphenicol secreted into the growth medium of S. venezuelae cultures. A 1.5-ml aliquot of supernatant from cultures of wild-type S. venezuelae was extracted with ethyl acetate and transferred to a filter paper disk. Top agar supplemented with AB7341λφ[cspA::lacZ] cells was poured over an LB agar plate spread with X-Gal, and the disk was placed on the surface. The plate was incubated overnight at 37°C. The disk contained ∼7.5 μg of chloramphenicol, as estimated by TLC.
RESULTS AND DISCUSSION
Construction of single-copy stress promoter-lacZ fusions.
Among the various members of the E. coli ς32 regulon, the ibp operon, which encodes the bacterial small heat shock proteins IbpA and IbpB, undergoes the highest level of transcriptional induction following a temperature upshift (8). CspA, the major E. coli cold shock protein (15), is virtually undetectable at 37°C because of extreme transcript instability conferred by its 5′ untranslated region (11, 13, 14). However, the cspA mRNA is stabilized by 2 orders of magnitude at low temperatures, and each cell dedicates more than 10% of its synthetic capacity to CspA production shortly after it is transferred to 10°C (11, 13–15). The P3 promoter of the rpoH gene is one of five promoters known to be activated by protein misfolding in the cell envelope and is exclusively transcribed by the EςE holoenzyme (21, 24, 33). Because of their strength and specificity, the set of promoters described above appeared to be well suited to form the basis of a microbe-based assay for the detection and categorization of antibacterial agents that induce a heat shock, cold shock, or extracytoplasmic stress response in E. coli. Single-copy fusions between the ibp, cspA, or P3rpoH promoter and the lacZ reporter gene were therefore constructed and/or transferred to the attλ site of AB734, a K-12 strain lacking antibiotic markers but containing a lacZ mutation.
Induction of stress promoter-lacZ fusions by model antibiotics.
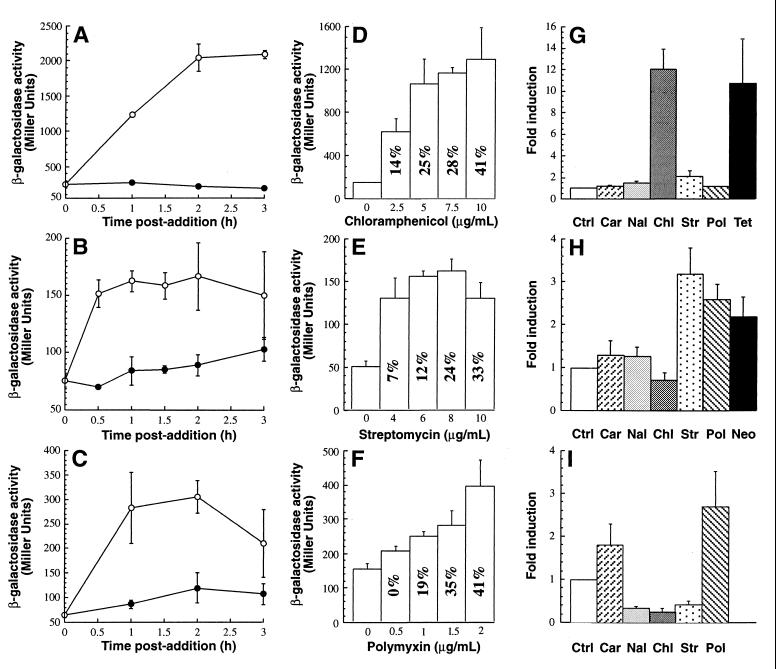
To demonstrate proof of principle, we characterized the responses of the lysogens to representative antibiotics belonging to the classes which they were hypothesized to detect by assaying cultures for β-galactosidase activity for up to 3 h after an antibiotic was added to the growth medium. The highest levels of enzymatic activity in AB734 λφ[cspA::lacZ] cultures supplemented with 5 μg of the C-group antibiotic chloramphenicol per ml were observed 3 h after addition and were about 11-fold greater than the background levels (Fig. 1A). Maximum induction of the λφ[ibp::lacZ] lysogen by 8 μg of the H-group antibiotic streptomycin per ml occurred 1 h after treatment, and the levels of β-galactosidase activity in antibiotic-supplemented cultures were approximately twice the levels in control cells (Fig. 1B). To determine if the P3rpoH::lacZ fusion was activated by antibacterial agents that affect cell integrity, mid-exponential-phase cultures of λφ[P3rpoH::lacZ] lysogens were supplemented with 1 μg of polymyxin B, an antimicrobial peptide that disrupts the outer membrane of gram-negative cells (12), per ml. Figure 1C shows that although maximal induction (∼3.2-fold) occurred 1 h after polymyxin B was added, there was a significant variation in the enzymatic activity at this time. Lower-level (∼2.5-fold) but more reliable induction was observed after 2 h. Overall, the results described above indicate that single-copy lacZ fusions to the cspA, ibp, and P3rpoH promoters provide an acceptable signal-to-noise ratio for detecting model antibiotics that affect the ribosomes or cell envelope of E. coli.
FIG. 1.
Induction characteristics and specificity of AB734 λφ[cspA::lacZ] (A, D, and G), AB734 λφ[ibp::lacZ] (B, E, and H), and AB734 λφ[P3rpoH::lacZ] (C, F, and I) lysogens. In the time courses experiments (A through C), mid-exponential-phase cultures were supplemented with 5 μg of chloramphenicol per ml (A), 8 μg of streptomycin per ml (B), or 1 μg of polymyxin B per ml (C), and enzymatic activities were determined at different times (○). Control cultures (●) received an equal volume of 100% ethanol (A) or no additive (B and C). In the concentration dependence experiments (D through F), enzymatic activities were determined at the time of maximal induction, as follows: 3 h after antibiotic was added for AB734 λφ[cspA::lacZ] (D), 1 h after antibiotic was added for AB734 λφ[ibp::lacZ] (E), and 2 h after antibiotic was added for AB734 λφ[P3rpoH::lacZ] (F). The values inside the bars are the percentages of growth inhibition relative to control cultures at the time of sample collection. In the specificity experiments (G through I), mid-exponential-phase cultures were supplemented with no additive (Ctrl), 8 μg of carbenicillin (Car) per ml, 50 μg of nalidixic acid (Nal) per ml, 5 μg of chloramphenicol (Chl) per ml, 8 μg of streptomycin (Str) per ml, 1 μg of polymyxin B (Pol) per ml, 5 μg of tetracycline (Tet) per ml, or 16 μg of neomycin (Neo) per ml, and enzymatic activities were determined at the time of maximal induction. AB734 λφ[cspA::lacZ] cultures were grown at 37°C, while AB734 λφ[ibp::lacZ] and λφ[P3rpoH::lacZ] cultures were grown at 30°C. The typical levels of growth inhibition 1 h after treatment in the experiments whose results are shown in panels G through I were 3% for carbenicillin, 7% for nalidixic acid, 20% for streptomycin and neomycin, 28% for chloramphenicol, 30% for polymyxin B, and 45% for tetracycline.
Dependence of induction levels on antibiotic concentration.
To obtain additional information concerning the sensitivity and robustness of the assay, mid-exponential-phase cultures of the lysogens were supplemented with various concentrations of model antibiotics, and the enzymatic activities and levels of growth inhibition were determined at the time of maximal induction (Fig. 1D through F). In all cases, a threshold concentration of antibiotic was required to obtain significant induction; 1.5- to 2.5-fold increases in enzymatic activity were observed when as little as 2.5 μg of chloramphenicol per ml, 4 μg of streptomycin per ml, and 1 μg of polymyxin B per ml were added to AB734 λφ[cspA::lacZ], AB734 λφ[ibp::lacZ], and AB734 λφ[P3rpoH::lacZ] cultures, respectively (Fig. 1D through F; data not shown). For all lysogens, the level of induction increased with the amount of antibiotic added to the growth medium and remained high over a wide range of concentrations (Fig. 1D through F). However, β-galactosidase activities declined at very high antibiotic concentrations, and growth inhibition was severe (Fig. 1E; data not shown). Therefore, the cspA::lacZ, ibp::lacZ, and P3rpoH::lacZ fusions are suitable for detecting model antibiotics over a wide range of sublethal concentrations.
Selective induction of stress promoter-lacZ fusions.
To determine if the set of strains described above could be used to obtain information concerning the mechanism of action of antibacterial agents, we quantified the responses of the lysogens to compounds that are known to affect the cell wall (carbenicillin), the outer membrane (polymyxin B), and DNA replication (nalidixic acid) and to additional C-group (chloramphenicol, tetracycline) and H-group (streptomycin, neomycin) antibiotics that target ribosomes. As expected, AB734 λφ[cspA::lacZ] cultures underwent high-level (>10-fold) induction when they were treated with the C-group antibiotics chloramphenicol and tetracycline (Fig. 1G). None of the other antibacterial agents tested led to a significant increase in the level of β-galactosidase activity, indicating that the cspA::lacZ fusion is a highly specific probe to detect C-group antibiotics that target prokaryotic ribosomes.
The λφ[ibp::lacZ] lysogen responded to the presence of the H-group antibiotics streptomycin and nemoycin with two- to threefold increases in enzymatic activity compared with background levels. Neither chloramphenicol nor nalidixic acid significantly activated the ibp promoter (Fig. 1H). However, polymyxin B, an antibacterial agent that damages the outer membrane of gram-negative cells by displacing divalent ions (12), caused a 2.5-fold increase in β-galactosidase activity when it was added to the growth medium of AB734 λφ[ibp::lacZ]. In contrast, the β-lactam antibiotic carbenicillin, which interferes with cell wall synthesis (12), had a much more modest (if any) inducing effect (Fig. 1H). Although the ibp operon is transcribed at a high level by the Eς32 holoenzyme (1, 8), it has been hypothesized that EςE may recognize an additional promoter (17). This potential ςE dependency, together with the difference in the abilities of the two antibiotics to trigger extracytoplasmic stress (see below), may explain our results. Alternatively, the ibp promoter may specifically respond to outer membrane damage through an indirect pathway that involves Eς32. In either case, our data suggest that λφ[ibp::lacZ] lysogens are well suited for detecting antibacterial agents belonging to the H-group of ribosome-targeting agents, as well as membrane-damaging compounds.
In addition to polymyxin B, another antibiotic that targets the cell envelope, carbenicillin, induced the P3rpoH::lacZ fusion (Fig. 1I). However, while addition of 1 μg of polymyxin B per ml to AB734 λφ[P3rpoH::lacZ] cultures resulted in a 2.7-fold increase in enzymatic activity, adding 8 μg of carbenicillin per ml to the medium resulted in only 1.7-fold induction compared with background levels. Although the reasons for the difference in the levels of induction remain unclear, polymyxin B may upregulate members of the ςE regulon more efficiently than carbenicillin does by triggering a more severe extracyoplasmic stress response. In agreement with this idea, it has been suggested that after polymyxin B penetrates the outer membrane, it induces mixing of anionic phospholipids between the outer layer of the cytoplasmic membrane and the inner layer of the outer membrane (22). This situation should lead to considerable stress in the periplasm.
Finally, it should be noted that the levels of β-galactosidase activity in AB734 λφ[P3rpoH::lacZ] cultures supplemented with either nalidixic acid, chloramphenicol, or streptomycin were reproducibly 60 to 70% lower than the levels of activity in control cells (Fig. 1I), suggesting that these antibiotics downregulate lacZ transcription from the P3rpoH promoter. Although the decrease in enzymatic activity is significant enough to warrant the use of λφ[P3rpoH::lacZ] lysogens for detection of antibacterial compounds that affect the ribosomes or cause DNA damage, promoters that are specifically activated by DNA-damaging agents (e.g., dinD [23]) may be better suited for detecting the latter class of compounds.
Blue halo assay for determining the mode of action of antibiotics.
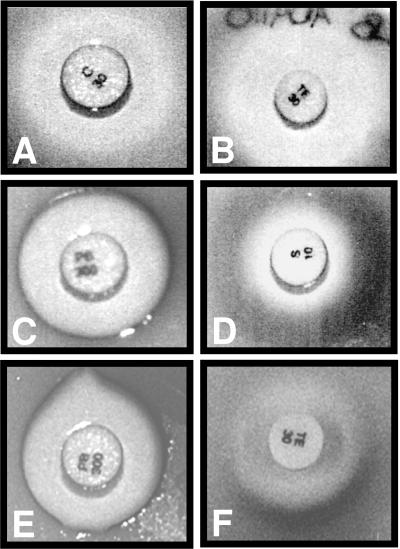
Formation of a nondiffusible blue pigment after cleavage of X-Gal by β-galactosidase has been used extensively in histochemistry and for clonal selection. To assess the usefulness of this chemical indicator for elucidating the mechanism of action of antibiotics by visual inspection, top agar containing AB734 λφ[ibp::lacZ] cells was layered onto a thin LB agar plate spread with X-Gal. Commercial antibiotic disks (Difco) impregnated with 10 μg of streptomycin, 30 μg of chloramphenicol, 30 μg of tetracycline, or 300 U of polymyxin B were placed on the surface, and the plate was incubated overnight at 30°C. Figure 2A through D show that the growth inhibition zones surrounding the streptomycin and polymyxin B disks were framed by an intense blue ring, while the boundary remained white in the case of chloramphenicol and tetracycline. These results are in agreement with the results of activity assays (Fig. 1H) and confirm that, in addition to H-group antibiotics, polymyxin B induces the ibp::lacZ fusion. Selective formation of blue halos around antibiotic disks containing antibacterial agents that induce the P3rpoH::lacZ and cspA::lacZ fusions was also observed when the appropriate lysogens were mixed with top agar (Fig. 2E and F; data not shown).
FIG. 2.
Blue halo formation restricted to specific lysogen-antibiotic combinations. Top agar supplemented with AB734 λφ[ibp::lacZ] (A through D), AB734 λφ[P3rpoH::lacZ] (E), or AB734 λφ[cspA::lacZ] (F) cells was poured over LB agar plates spread with X-Gal. Antibiotic disks (Difco) impregnated with 30 μg of chloramphenicol (A), 30 μg of tetracycline (B and F), 300 U of polymyxin B (C and E), or 10 μg of streptomycin (D) were placed on the surfaces of the plates. The plates were incubated overnight at 30°C (A through E) or 37°C (F).
Detection of antibiotic activity in natural extracts.
To assess the usefulness of stress promoter-lacZ fusions for identifying antibacterial compounds from natural sources, filtered fractions obtained from supernatants of cultures of S. venezuelae, a chloramphenicol producer, and of an isogenic mutant that is not able to synthesize this antibiotic were extracted with ethyl acetate. Filter paper disks were impregnated with the extracts and placed on top agar containing AB734 λφ[cspA::lacZ] cells above an LB agar bottom layer spread with X-Gal. Following overnight incubation at 37°C, a blue ring surrounded the disk containing the extract from wild-type S. venezuelae despite the fact that little growth inhibition had taken place (Fig. 3). In contrast, no halo was visible around the disk containing the extract from the mutant strain (data not shown).
Conclusions.
Our results show that when used in combination, the λφ[cspA::lacZ], λφ[ibp::lacZ], and λφ[P3rpoH::lacZ] lysogens form the basis of a minimal assay that can be used to detect and categorize the mechanisms of action of all major classes of antibacterial agents. The fact that growth inhibition and, therefore, high concentrations of antimicrobial compounds are not required for promoter activation, together with ease of automation, should make this system a valuable tool for identifying and characterizing new antibacterial agents from natural or combinatorial sources.
ACKNOWLEDGMENTS
We are grateful to Carol Gross, Alan Easton, and Kelly Hughes for providing E. coli strains, plasmids, and bacteriophages. We thank Leo Vining for providing Streptomyces strains and advice concerning their cultivation.
A.A.B. was the recipient of a GAANN fellowship from the Department of Education. This work was supported by NSF award BES-9501212 and by research project grant MBC-99-335-01 from the American Cancer Society.
REFERENCES
- 1.Allen S P, Polazzi J O, Gierse J K, Easton A M. Two novel heat shock genes-encoding proteins produced in response to heterologous protein expression in Escherichia coli. J Bacteriol. 1992;174:6938–6947. doi: 10.1128/jb.174.21.6938-6947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkin S, Smulski D R, Dadon S, Vollmer A C, Van Dyk T K, LaRossa R A. A panel of stress-responsive luminous bacteria for the detection of selected classes of toxicants. Water Res. 1997;31:3009–3016. [Google Scholar]
- 3.Belkin S, Van Dyk T K, Vollmer A C, Smulski D R, LaRossa R A. Monitoring subtoxic environmental hazards by stress-responsive luminous bacteria. Environ Toxicol Water Qual. 1996;11:179–185. [Google Scholar]
- 4.Ben-Israel O, Ben-Israel H, Ulitzur S. Identification and quantification of toxic chemicals by use of Escherichia coli lux genes fused to stress promoters. Appl Environ Microbiol. 1998;64:4346–4352. doi: 10.1128/aem.64.11.4346-4352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha H J, Srivastava R, Vakharia V N, Rao G, Bentley W E. Green fluorescent protein as a noninvasive stress probe in resting Escherichia coli cells. Appl Environ Microbiol. 1999;65:409–414. doi: 10.1128/aem.65.2.409-414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S, Vining L C, Westlake D W S. Nutritional requirements for chloramphenicol biosynthesis in Streptomyces venezuelae. Can J Microbiol. 1983;29:247–253. [Google Scholar]
- 7.Chu D T W, Plattner J J, Katz L. New directions in antibacterial research. J Med Chem. 1996;39:3853–3874. doi: 10.1021/jm960294s. [DOI] [PubMed] [Google Scholar]
- 8.Chuang S E, Burland V, Plunkett III G, Daniels D L, Blattner F R. Sequence analysis of four new heat-shock genes constituting the hslTS/ibpAB and hslVU operons in Escherichia coli. Gene. 1993;134:1–6. doi: 10.1016/0378-1119(93)90167-2. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt S K, Adelberg E A. Transduction of the attached sex factor of Escherichia coli. J Bacteriol. 1962;83:673–678. doi: 10.1128/jb.83.3.673-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doull J, Ahmed Z, Stuttard C, Vining L C. Isolation and characterization of Streptomyces venezuelae mutants blocked in chloramphenicol biosynthesis. J Gen Microbiol. 1985;131:97–104. doi: 10.1099/00221287-131-1-97. [DOI] [PubMed] [Google Scholar]
- 11.Fang L, Jiang W, Bae W, Inouye M. Promoter-independent cold-shock induction of cspA and its derepression at 37°C by mRNA stabilization. Mol Microbiol. 1997;23:355–364. doi: 10.1046/j.1365-2958.1997.2351592.x. [DOI] [PubMed] [Google Scholar]
- 12.Franklin T J, Snow G A. Biochemistry of antimicrobial action. New York, N.Y: Chapman and Hall Ltd.; 1989. [Google Scholar]
- 13.Goldenberg D, Azar I, Oppenheim A B. Differential mRNA stability of the cspA gene in the cold-shock response of Escherichia coli. Mol Microbiol. 1996;19:241–248. doi: 10.1046/j.1365-2958.1996.363898.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg D, Azar I, Oppenheim A B, Brandi A, Pon C L, Gualerzi C O. Role of Escherichia coli cspA promoter sequences and adaptation of translational apparatus in the cold shock response. Mol Gen Genet. 1997;256:282–290. doi: 10.1007/s004380050571. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein J, Pollitt N S, Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- 17.Laskowska E, Wawrzynow A, Taylor A. IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie. 1996;78:117–122. doi: 10.1016/0300-9084(96)82643-5. [DOI] [PubMed] [Google Scholar]
- 18.Malik V S, Vining L C. Metabolism of chloramphenicol by the producing organism. Can J Microbiol. 1970;16:173–179. doi: 10.1139/m70-030. [DOI] [PubMed] [Google Scholar]
- 19.Mescas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 21.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh J-T, Van Dyk T K, Cajal Y, Dhurjati P S, Sasser M, Jain M K. Osmotic stress in viable Escherichia coli as the basis for the antibiotic response by polymyxin B. Biochem Biophys Res Commun. 1998;246:619–623. doi: 10.1006/bbrc.1998.8682. [DOI] [PubMed] [Google Scholar]
- 23.Orser C S, Foong F C F, Capaldi S R, Nalezny J, MacKay W, Farr S B. Use of prokaryotic stress promoters as indicators of the mechanisms of chemical toxicity. In Vitro Toxicol. 1995;8:71–85. [Google Scholar]
- 24.Rouviere P E, De Las Penas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 26.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 27.Thieringer H A, Jones P G, Inouye M. Cold shock and adaptation. Bioessays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Trias J, Gordon E M. Innovative approaches to novel antibacterial drug discovery. Curr Opin Biotechnol. 1997;8:757–762. doi: 10.1016/s0958-1669(97)80131-0. [DOI] [PubMed] [Google Scholar]
- 29.Van Bogelen R A, Neidhardt F C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dyk T K, Majarian W R, Konstantinov K B, Young R M, Dhurjati P S, LaRossa R A. Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl Environ Microbiol. 1994;60:1414–1420. doi: 10.1128/aem.60.5.1414-1420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasina J A, Baneyx F. Recombinant protein expression at low temperature under the transcriptional control of the major Escherichia coli cold shock promoter cspA. Appl Environ Microbiol. 1996;62:1444–1447. doi: 10.1128/aem.62.4.1444-1447.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdine G L. The combinatorial chemistry of nature. Nature. 1996;384(Suppl.):11–13. doi: 10.1038/384011a0. [DOI] [PubMed] [Google Scholar]
- 33.Yura T, Nagai H, Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]