Abstract
We evaluated strategies to identify and recruit a racially/ethnically diverse cohort of women at high-risk for breast cancer to a randomized controlled trial (RCT). We enrolled 300 high-risk women and 50 healthcare providers to a RCT of standard educational materials alone or in combination with web-based decision support tools. We implemented five strategies to identify high-risk women: 1) recruitment among patients previously enrolled in a study evaluating breast cancer risk; 2) automated breast cancer risk calculation using information extracted from the electronic health record (EHR); 3) identification of women with atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS) using ICD9/10 diagnostic codes; 4) clinical encounters with enrolled healthcare providers; 5) recruitment flyers/online resources. Breast cancer risk was calculated using either the Gail or Breast Cancer Surveillance Consortium (BCSC) models. We identified 6,229 high-risk women and contacted 3,459 (56%), of whom 17.2% were identified from prior study cohort, 37.5% through EHR risk information, 14.8% with AH/LCIS, 29.0% by clinical encounters, and 1.5% through recruitment flyers. Women from the different recruitment sources varied by age and 5-year invasive breast cancer risk. Of 300 enrolled high-risk women, 44.7% came from clinical encounters and 27.3% from prior study cohort. Comparing enrolled to not enrolled participants, there were significant differences in mean age (57.2 vs. 59.1 years), proportion of non-Whites (41.5% vs. 54.8%), and mean 5-year breast cancer risk (3.0% vs. 2.3%). We identified and successfully recruited diverse high-risk women from multiple sources. These strategies may be implemented in future breast cancer chemoprevention trials.
Keywords: breast cancer chemoprevention, clinical decision support tools, high risk for breast cancer, recruitment strategies, racial/ethnic minorities
Introduction
Chemoprevention with anti-estrogens, including selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs), has been shown in randomized controlled trials to reduce the incidence of invasive breast cancer by up to 50–65% among women at high risk for developing breast cancer.1–6 Multiple national medical organizations, including the U.S. Preventive Services Task Force and the American Society of Clinical Oncology, recommend that physicians discuss anti-estrogen chemoprevention with high-risk women.7,8 However, uptake of breast cancer chemoprevention among high-risk women is estimated to be less than 15%.9–11 Use of chemoprevention is particularly low among racial/ethnic minorities, which can lead to increased health disparities in these populations.12 Barriers to chemoprevention uptake include lack of routine breast cancer risk assessment, insufficient clinician and patient knowledge about chemoprevention, and concerns about side effects.10,13,14 There is therefore a clear need to identify strategies to improve chemoprevention uptake among women at high risk for breast cancer.
Integration of breast cancer risk assessment into the clinical workflow and the development of innovative approaches to communicate breast cancer risk could improve chemoprevention uptake among high-risk women. In particular, decision support tools for patients and providers could facilitate breast cancer risk assessment and shared decision-making regarding breast cancer prevention. We have developed web-based decision support tools, RealRisks and BNAV (Breast cancer risk NAVigation) for patients and healthcare providers, respectively, that include interactive educational modules on breast cancer risk assessment, genetic testing, screening, chemoprevention and other prevention strategies.15,16 We evaluated these tools in a randomized controlled trial among a racially/ethnically diverse group of women, with the hypothesis that RealRisks and BNAV would improve accuracy of breast cancer risk perception, facilitate referrals to specialty clinics, and increase chemoprevention uptake among high-risk women.17
In addition to evaluating these outcomes, our randomized controlled trial evaluating these decision support tools provided a unique opportunity to examine recruitment strategies that can identify a large, racially/ethnically diverse population of women at high risk for breast cancer. Such strategies could also be utilized for other clinical studies targeting high-risk women to improve recruitment yield, including among racial/ethnic minorities. We evaluated five strategies to identify women at high risk for breast cancer for recruitment to our trial, and compared recruitment yield and patient demographics of each strategy.
Materials and Methods
Study Design of Parent Trial
We enrolled a total of 300 high-risk women and 50 healthcare providers to a randomized controlled trial (RCT) of chemoprevention decision support from November 2016 to March 2020 at Columbia University Irving Medical Center (CUIMC) in New York, NY.17 Women were eligible for enrollment if: 1) age 35 to 75 years; 2) at high risk for breast cancer either because they had a history of lobular carcinoma in situ (LCIS) or had an estimated five-year invasive breast cancer risk ≥1.67% according to the Gail18 or Breast Cancer Surveillance Consortium (BCSC)18 models and/or a lifetime risk ≥20% according to the Gail model; 3) no prior history of breast cancer or ductal carcinoma in situ (DCIS); 4) no prior use of a SERM or AI for breast cancer chemoprevention; 5) no prior bilateral mastectomies; 6) English or Spanish speaking; 7) had a healthcare provider at CUIMC; 8) had access to the internet; 9) had access to text messaging and/or email; and 10) able to provide informed consent. Potential participants were first screened to determine eligibility, and then underwent informed consent in person, over the phone, or online in either English or Spanish. Providers were identified and approached for recruitment after one of their patients was identified as being potentially eligible for the study. Enrolled healthcare providers were primary care providers (internists, family medicine providers, gynecologists), as well as specialists (breast surgeons, medical oncologists, nurse practitioners) who practiced at CUIMC. The study was approved by the Institutional Review Board (IRB) at CUIMC. The study team obtained written informed consent from patient prior to enrollment, and the studies were conducted in accordance with recognized ethical guidelines (i.e. the Declaration of Helsinki).
Patients completed a baseline survey including questions on perceived breast cancer risk, chemoprevention knowledge, and breast cancer worry, and then were randomized 1:1 to either receive access to the web-based decision support tool RealRisks in addition to standard educational materials related to breast cancer risk and chemoprevention (intervention arm), or receive access to standard educational materials alone (control arm). If an enrolled provider’s patient was randomized to the intervention arm, he or she was given access to the BNAV tool. Patients were administered surveys at 1 month, 6 months, and after the next clinical encounter with their healthcare providers at CUIMC.
Strategies to Identify Eligible Women at High-Risk for Breast Cancer
Women at high risk for breast cancer were identified and recruited using five recruitment strategies, as described below (Figure 1). Strategies 1 to 3 aimed to identify patients using existing databases and the electronic health record (EHR), while Strategy 4 identified patients through clinical encounters with enrolled healthcare providers and Strategy 5 relied on patient self-referral. Women who were identified through multiple strategies were characterized using a hierarchy: Referrals by enrolled healthcare providers (Strategy 4) > Self-referrals (Strategy 5) > Recruitment from the KYRAS study (Strategy 1) > Diagnosis of AH/LCIS (Strategy 3) > Breast cancer risk assessment from the EHR (Strategy 2). Women were therefore only considered as having been identified using one category during analyses.
Figure 1.

Five Strategies to Identify and Recruit Women at High-Risk for Breast Cancer to a Randomized Controlled Trial.
This figure illustrates the five recruitment strategies utilized in our study, and how they related to each other.
Abbreviations:
AH = atypical hyperplasia
BCSC = Breast Cancer Surveillance Consortium
CUIMC = Columbia University Irving Medical Center
EHR = electronic health record
ICD = international classification of diseases
KYRAS = Know Your Own Risk: Assessment at Screening
LCIS = lobular carcinoma in situ
1). Recruitment among patients previously enrolled in a study evaluating breast cancer risk.
We previously enrolled women to the Know Your Risk: Assessment at Screening (KYRAS) study, an observational study at CUIMC that evaluated self-reported breast cancer risk factors among women at the time of screening mammography.19 Women were approached for enrollment to the KYRAS study by a member of the study team during their routine screening mammography at the Avon Breast Imaging Center at CUIMC, and participants completed a one-time comprehensive survey on demographic characteristics and breast cancer risk factors that was used to calculate 5-year and lifetime breast cancer risk according to the Gail model. A subset of patients also agreed to be recontacted for future studies. Among the 2,019 women enrolled in the KYRAS study between November 2014 and December 2015, 76.8% identified as Hispanic and 17.7% met high-risk criteria based upon an estimated 5-year invasive breast cancer risk ≥1.67% according to the Gail model.19 For the chemoprevention decision support RCT, the study team specifically assessed eligibility among high-risk women enrolled in the KYRAS study who had agreed to be recontacted, and then contacted them via email, mailed letter, or telephone.
2). Breast cancer risk calculation using data extracted from the electronic health record (EHR).
Information related to established breast cancer risk factors, including age, race/ethnicity, first-degree family history of breast cancer, benign breast biopsies and mammographic density, was extracted from the EHR for women undergoing screening mammography at CUIMC, as previously described.20 Estimated 5-year risks of invasive breast cancer were calculated using the BCSC model. Among 9,514 women, 1,443 (15.2%) met high risk criteria based upon a 5-year invasive breast cancer risk ≥1.67% according to the BCSC model. Of note, this automated EHR-based strategy might have underestimated risk in some patients through inaccurate or missing information. Approximately 88% of women had unknown family history of breast cancer and 95% did not have breast pathology reports in the CUIMC EHR system, which was classified as none or unknown for breast cancer risk calculation. After contact was made with potential participants, we clarified and collected any missing breast cancer risk information by self-report to confirm eligibility for the trial.
3). Identification of women with atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS) through diagnostic codes.
Women with a diagnosis of AH or LCIS between 2007 and 2015 were identified from the ICD-9/10 codes in the EHR (610.9/N60.99 and 233.0/D05/.90, respectively), including outpatient medical records at CUIMC and the New York Presbyterian Hospital (NYPH) tumor registry.21 Manual review of the EHR was done in patients with LCIS to confirm the diagnosis, as LCIS and DCIS share the same ICD-9/10 codes. For women with more than one diagnosis, the most advanced lesion was used, with LCIS considered more advanced than AH. Breast cancer risk was calculated according to the BCSC model using patient information collected from aggregate data extraction from the EHR. Similar to Strategy 2, once the study team made contact, we collected additional breast cancer risk information by self-report.
4). Clinical encounters with enrolled healthcare providers.
Healthcare providers enrolled in the RCT consented to allow research staff to approach their high-risk patients for potential enrollment. Any high-risk women identified through the three recruitment strategies above were merged with clinic schedule data for enrolled healthcare providers, including primary care providers as well as specialists providing high-risk consultations (breast surgeons and medical oncologists). An email was sent to enrolled providers by the study team informing them that a high-risk patient was scheduled to see them in clinic the following week and requesting permission to contact the patient. Women were contacted to determine their eligibility by the study team prior to their clinic visit. Providers then had an opportunity to discuss enrollment in the study during the clinic visit. Enrolled healthcare providers could also refer patients who met high-risk criteria for breast cancer directly to the study team, without prior identification by the study team. Tying recruitment efforts to the clinical encounter therefore allowed providers to encourage participation of their high-risk patients.
5). Self-referrals via recruitment flyers and online resources.
Recruitment flyers providing information about the study and contact information for the study team were distributed throughout the medical campus community along with online distribution via the RecruitMe platform (https://recruit.cumc.columbia.edu/), an online recruitment tool at CUIMC that provides information about current clinical trials. Flyers contained information including eligibility criteria, study intervention, evaluations, and compensation and were posted throughout the CUIMC campus before recruitment started. Women who responded were contacted by phone and pre-screened to determine their eligibility, with calculation of breast cancer risk according to the Gail model using patient-reported risk factor data collected during screening. Women without a medical provider at CUIMC were excluded from the study.
Contact and Recruitment of Eligible Patients
For women identified as potentially eligible for the trial, initial contact was made by email (if available) or mailed letter that included brief information about the RCT and contact information for the study team. Women with available e-mail addresses were prioritized for contact, given that we hoped to enrich for younger women at high risk for breast cancer with access to the internet. This initial contact attempt was followed by up to two follow-up phone calls using phone numbers either provided by patients (if enrolled in KYRAS) or listed in the EHR. Of note, our study team included two coordinators bilingual in English and Spanish, allowing for discussion and consent in a patient’s preferred language. Patients who enrolled in the study and completed the study evaluations received up to $95 as an incentive for participation.
Statistical Analysis
We conducted descriptive statistics to compare sociodemographic characteristics across the five recruitment sources, limiting our sample to women who had received at least one contact from the study team in the form of email, letter, and/or phone call. The frequency distribution of age, race/ethnicity, and mean 5-year invasive breast cancer risk were compared across the five recruitment sources using Chi-squared tests and 2-sample t-tests for categorical and continuous variables, respectively. We also performed descriptive analyses to compare the distribution of age, race/ethnicity, and mean breast cancer risk scores among contacted high-risk women who enrolled in the RCT versus those who did not enroll. Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.
Results
We identified a total of 6,229 high-risk women, of whom 3,663 were contacted via email, mailed letter, and/or telephone for participation (Figure 2). Of those contacted, 204 women did not meet eligibility criteria on preliminary screening: 127 were not between 35 and 75 years old, 38 did not have a medical provider at CUIMC, 25 had previously undergone bilateral mastectomy or breast augmentation (which did not allow breast cancer risk calculation according to the BCSC model), and 14 had a history of breast cancer. Of the 3,459 high-risk women (94.4%) who were contacted and eligible for enrollment, 16.3% were identified through prior enrollment in the KYRAS study, 37.8% through breast cancer risk data from the EHR, 15.0% through a diagnosis of AH/LCIS identified in the EHR, 29.3% through clinical visits with enrolled healthcare providers, and 1.6% through the recruitment flyers distributed in the community or through online recruitment. All of these 3,459 were contacted by email or letter, while approximately 19% had one phone contact attempt and 43% had two phone contact attempts from the study team. Ultimately, 300 women (8.7% of eligible women contacted) were enrolled in the study and underwent randomization.
Figure 2.
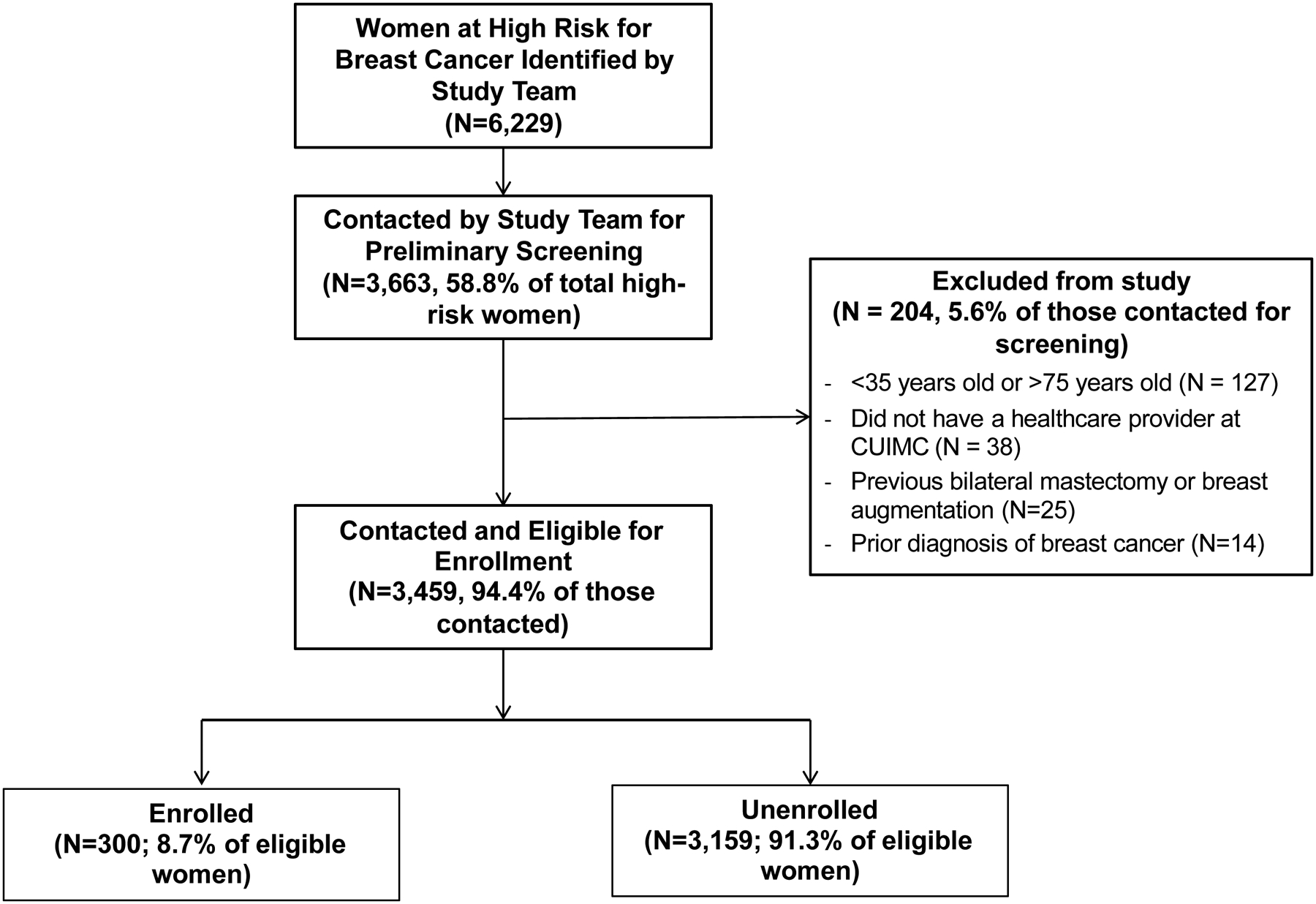
CONSORT Diagram
The CONSORT diagram illustrates patient enrollment, including number of high risk patients, number of patients contacted by study team, number of eligible patients (and reasons for exclusion of contacted patients), and number of patients enrolled on study.
The baseline characteristics of eligible high-risk women who were contacted (n=3,459), stratified by recruitment source, are shown in Table 1. The mean age of eligible women was 58.7 years (standard deviation [SD], 9.7), and 47% of women were non-Hispanic White, 15% non-Hispanic Black, 29% Hispanic, 5% Asian, and 20% other/unknown. Age was significantly different among women based upon recruitment source (p<0.001); for example, the mean age of women recruited through recruitment flyers and online recruitment was 47.4 years (SD, 8.8) compared to 63.9 years (SD, 7.6) for women recruited from the KYRAS study. Race/ethnicity also varied significantly by recruitment source (p<0.001); for example, the majority (54.9%) of women recruited from the KYRAS study were Hispanic, while the majority of women identified through breast cancer risk information in the EHR or diagnoses of AH/LCIS were non-Hispanic White. There were also significant differences in the mean 5-year risk of invasive breast cancer among women based upon recruitment source, with highest mean 5-year risk among those women with AH/LCIS (3.51%, SD 2.75%).
Table 1.
Participant characteristics among eligible women at high risk for breast cancer (n=3459), stratified by recruitment source.
| Patient Characteristics | Recruitment from KYRAS study N= 564 (16.3%) |
Breast cancer risk data from EHR N= 1309 (37.8%) |
Women with AH/LCIS N= 517 (15.0%) |
Clinic visits with enrolled providers N= 1015 (29.3%) |
Flyers/online recruitment N= 54 (1.6%) |
Total N= 3459 |
P-value |
|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | 63.9 (7.6) | 61.7 (6.5) | 57.7 (9.1) | 53.2 (11.2) | 47.4 (8.8) | 58.7 (9.7) | <0.001 |
| Unknown | 5 (0.9%) | 280 (21.4%) | 1(0.2%) | 246 (24.2%) | 3 (5.6%) | 535 (15.5%) | |
| Mean 5-year risk of invasive breast cancer, % (SD) | 2.03 (1.11) | 2.56 (1.03) | 3.51 (2.75) | 1.81 (1.49) | 1.37 (0.91) | 2.37 (1.62) | <0.001 |
| Yield from recruitment source, No. enrolled/Total from recruitment source (%) | 82/564 (14.5%) | 35/1309 (2.7%) | 31/517 (6.0%) | 134/1015 (13.2%) | 18/54 (33.3%) | 300/3459 (8.7%) | |
| Percentage of total enrolled participants (%) | 82 (27.3%) | 35 (11.7%) | 31 (10.3%) | 134 (44.7%) | 18 (6.0%) | 300 |
AH = atypical hyperplasia
EHR = electronic health record
KYRAS = Know Your Risk: Assessment at Screening
LCIS = lobular carcinoma in situ
Of the 300 women enrolled in the study, the greatest proportion of participants (44.7%) was identified through clinical encounters with enrolled healthcare providers, while 27.2% were recruited via the KYRAS study, 11.7% through breast cancer risk information in the EHR, 10.3% through diagnoses of AH/LCIS, and 6.0% through flyers and online recruitment. However, flyers and online recruitment had the highest enrollment yield, with 33.3% of women contacted through that method enrolling in the study. Recruitment yields were 14.5% for recruitment from the KYRAS study, 13.2% for clinical encounters with enrolled healthcare providers, 6.0% for diagnoses of AH/LCIS, and 2.7% for women identified through breast cancer risk information from the EHR.
We also compared characteristics among eligible high-risk women who were enrolled and not enrolled (Table 2). Enrolled patients were younger than those not enrolled, with a mean age of 57.3 years (SD, 10.1) versus 58.9 years (SD, 9.7), respectively. Enrolled patients were also more likely to be non-Hispanic White than not enrolled patients (58.5% vs. 45.4%, respectively) and had a higher mean 5-year invasive breast cancer risk (3.02% vs. 2.31%, respectively). Overall, we were able to enroll a racially/ethnically diverse high-risk population, including 13.7% non-Hispanic Black, 22.4% Hispanic, and 4.7% Asian.
Table 2.
Baseline characteristics of 3459 eligible high-risk women, stratified by enrollment status.
| Patient Characteristics | Enrolled N= 300 (8.7%) |
Not enrolled N= 3159 (91.3%) |
Total N= 3459 |
P-value |
|---|---|---|---|---|
| Mean age, years (SD) | 57.3 (10.1) | 58.9 (9.7) | 58.7 (9.7) | 0.006 |
| Unknown | 1 (0.3%) | 534 (16.9%) | 535 (15.5%) | |
| Mean 5-year risk of invasive breast cancer, % (SD) | 3.02 (1.57) | 2.31 (1.61) | 2.37 (1.62) | <0.001 |
Discussion
We were able to identify and recruit a large cohort of women at high risk for breast cancer using five strategies, which included recruitment of patients previously enrolled in a clinical study evaluating breast cancer risk, identification of high-risk women using clinical information extracted from the EHR, identification of women with AH or LCIS through diagnostic codes, clinical encounters with enrolled healthcare providers, and patient self-referral through recruitment flyers. Through these strategies, we enrolled a racially/ethnically diverse cohort of women to our study evaluating decision support tools for women at high risk for breast cancer, with over 40% of enrolled women from under-represented minority groups. While most eligible patients were identified through data in the EHR, the majority of enrolled patients were recruited through clinical encounters with enrolled providers or from a cohort of high-risk women previously enrolled in a clinical study. Although the highest recruitment yield came from flyers and online recruitment, this strategy only accounted for a small fraction of the women screened and enrolled. Recruited patients had higher mean 5-year breast cancer risk by the BCSC model than patients who were not enrolled, and patients with AH or LCIS had the highest breast cancer risk, consistent with the known at least 4-fold increased risk of breast cancer associated with these breast lesions22 and the inclusion of AH and LCIS in the BCSC model.18
Overall, we contacted over 3000 high-risk women in order to enroll 300 participants to our randomized controlled trial of web-based decision support tools. Therefore, we needed to screen a large high-risk population to meet our accrual goal. While we were able to meet our recruitment goals, the overall recruitment processes varied with level of efficiency, and could present a challenge to trials with limited resources and time for accrual. A yield of 10% study participation is consistent with low accrual to prevention trials when compared with accrual to trials for patients with active disease, such as diabetes,23 and highlights the ongoing need to identify efficient and effective recruitment methods for trials among healthy, high-risk populations.
Our finding that the majority of enrolled patients were recruited through direct discussion with and referrals from their healthcare providers is in agreement with previous studies demonstrating that provider recommendations increased the likelihoods both of chemoprevention uptake and participation in chemoprevention studies.11,24,25 For example, in one survey of women at high risk for breast cancer, those who were advised to enroll in a chemoprevention trial by their primary physicians were 13 times more likely to participate.24 In a systematic review of 63 studies (with 1681 adult patients) that evaluated barriers and facilitators to clinical trial participation for diseases including cancer and chronic medical conditions, major themes of patient-reported barriers to clinical trial participation included skepticism, fear, and mistrust as well as a lack of awareness of opportunities.26 However, major facilitators described by patients included trust in medical staff and information from their providers about clinical trials, as well as a sense of altruism and sense of belonging and connectedness. While automated methods can identify a large number of high-risk women, strategies involving direct communication with patients either by their providers or study team remained important in our study to achieve enrollment targets and should be utilized in future studies among chemoprevention and other studies to enhance recruitment yield. In addition, the high yield among patients who self-referred through flyers and online recruitment might reflect patient motivation to participate. While our current study did not capture patient or provider perspectives on enrollment, this is a potential area of study in future studies in order to better evaluate barriers and facilitators to enrollment among a population of high-risk women.
We were able to recruit a racially/ethnically diverse cohort of high-risk women, which is representative of the predominantly Hispanic population of women undergoing screening mammography at our medical center19 but also underscores the need to utilize a variety of recruitment strategies for more inclusive clinical trial enrollment. The National Surgical Adjuvant Breast and Bowel Project (NSABP) Study of Tamoxifen and Raloxifene (STAR) trial, in which high-risk women were randomized to tamoxifen or raloxifene, similarly utilized multiple strategies to improve minority accrual, including community outreach programs, presentations at national medical organization meetings, and workshops addressing the importance of minority accrual.27 These efforts resulted in enrollment of a more diverse cohort of women than previous breast cancer chemoprevention trials, with 21% of participants identifying as racial/ethnic minorities compared to less than 4% in the NSABP Breast Cancer Prevention Trial.28 For our study, having a diverse research team, including research staff who were bilingual Spanish/English speakers was useful in addressing language barriers, building trust, and recruiting minority women. Previous studies have demonstrated that a shared facilitator of clinical trial participation among racial/ethnic minorities is the presence of culturally-matched research personnel, study materials in patients’ languages, as well as discussion with providers they knew and trusted.29 Increasing representation of racial/ethnic minorities in clinical trials investigating breast cancer prevention strategies is critical to evaluate their efficacy in these populations with the goal of addressing health disparities. This underscores the importance of avoiding a “one size fits all” approach to patient recruitment, with the goal of facilitating inclusive clinical trial enrollment.
We also were able to identify a large group of women at high-risk for breast cancer using automated extraction of EHR data for risk calculation. While recruitment yield was low for this source, automated breast cancer risk calculation could help to identify women at high risk for breast cancer by overcoming barriers to routine breast cancer risk calculation including time constraints during clinical encounters and insufficient knowledge of breast cancer risk among primary care providers.14 We previously demonstrated that there is moderate agreement between breast cancer risk calculated using EHR-derived information and patient self-report, and also that EHR might identify more women at high risk for breast cancer because of pathologic risk factors including AH/LCIS compared to patient self-report.20 However, given the potential for missing or incomplete information in the EHR for risk factors including race/ethnicity, family history of breast cancer and prior breast biopsy, breast cancer risk estimates using EHR-derived data could serve as an initial screening tool to identify high-risk patients that can guide more comprehensive breast cancer risk assessment during clinical encounters. We are currently evaluating the use of automatically-populated breast cancer risk data from the EHR in our RealRisks decision aid30, including among high-risk women with AH or LCIS enrolled in an ongoing multicenter cluster RCT, “Making Informed Choices on Incorporating Chemoprevention into Care” (MiCHOICE, SWOG 1904) (NCT04496739).
Strengths of our study include our large cohort of eligible women, racially/ethnically diverse patient population, evaluation of multiple recruitment strategies, and use of EHR data to efficiently identify eligible high-risk women for enrollment. Limitations include that it was a single center study at an academic medical center, which might limit the generalizability of our findings. We had incomplete information on reasons for refusal to enroll in our study, which could have informed future efforts to reduce barriers to enrollment. We also did not collect additional patient information for all patients identified through our recruitment strategies, including information such as health insurance, employment status/income, and distance from healthcare facilities, limiting our ability to evaluate patient-level factors beyond age, race/ethnicity, and breast cancer risk that predicted enrollment. In addition, comparison of breast cancer risk of women across the five recruitment strategies might be limited by incomplete and missing information for breast cancer risk factors including family history of breast cancer and prior breast biopsy, particularly for EHR-based identification strategies. This missing data could have led to underestimation of breast cancer risk in potentially eligible patients, and therefore our strategies relying on automated EHR-based methods might have failed to identify potential participants, including racial/ethnic minorities. This could limit the application of these strategies as the primary source of potential participants in larger scale trials, without the use of additional strategies relying on patient-reported breast cancer risk factors.
In conclusion, we were able to identify and successfully recruit a large cohort of racially/ethnically diverse high-risk women from multiple recruitment sources to a randomized controlled trial evaluating the web-based decision support tools, RealRisks and BNAV. We are currently conducting a larger multicenter cluster randomized controlled trial, “Making Informed Choices on Incorporating Chemoprevention into Care” (MiCHOICE, SWOG S1904) evaluating the effect of RealRisks and BNAV on chemoprevention informed choice among women with high-risk breast lesions including AH or LCIS (NCT04496739). The recruitment strategies described here could be useful for future breast cancer chemoprevention trials to allow for more efficient identification of high-risk women and improve enrollment yields.
Prevention Relevance.
We describe five strategies to identify and successfully recruit a large cohort of racially/ethnically diverse high-risk women from multiple sources to a randomized controlled trial evaluating interventions to increase chemoprevention uptake. Findings could inform recruitment efforts for future breast cancer prevention trials to increase recruitment yield of high-risk women.
Funding:
The study was supported by the National Institutes of Health, National Cancer Institute NIH R01 CA226060-01A1 (K.D. Crew, R. Kukafka), R01 CA177995-04S1(K.D. Crew, R. Kukafka), R38 CA231577 (K.D. Crew), P30 CA013696 P30 (K.D. Crew), UL1TR001873(K.D. Crew). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Wickerham DL, Costantino JP, Vogel VG, Cronin WM, Cecchini RS, Ford LG, et al. The use of tamoxifen and raloxifene for the prevention of breast cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 2009;181:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the ibis-i breast cancer prevention trial. Lancet Oncol 2015;16:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (star) p-2 trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. New England Journal of Medicine 2011;364:2381–91. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (ibis-ii): An international, double-blind, randomised placebo-controlled trial. Lancet 2014;383:1041–8. [DOI] [PubMed] [Google Scholar]
- 6.Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor–positive ductal carcinoma in situ: A study based on nsabp protocol b-24. Journal of Clinical Oncology 2012;30:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: A systematic review for the u.S. Preventive services task force. Ann Intern Med 2013;158:604–14. [DOI] [PubMed] [Google Scholar]
- 8.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. Use of endocrine therapy for breast cancer risk reduction: Asco clinical practice guideline update. Journal of Clinical Oncology 2019;37:3152–65. [DOI] [PubMed] [Google Scholar]
- 9.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among u.S. Women. Cancer Epidemiol Biomarkers Prev 2010;19:443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: A systematic review and meta-analysis. J Clin Oncol 2010;28:3090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann Oncol 2016;27:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan CP, Haas JS, Pérez-Stable EJ, Gregorich SE, Somkin C, Des Jarlais G, et al. Breast cancer risk reduction options: Awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev 2006;15:162–6. [DOI] [PubMed] [Google Scholar]
- 13.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prevention Research 2010;3:686–8. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan CP, Haas JS, Pérez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among california physicians. Prev Med 2005;41:7–15. [DOI] [PubMed] [Google Scholar]
- 15.Coe AM, Ueng W, Vargas JM, David R, Vanegas A, Infante K, et al. Usability testing of a web-based decision aid for breast cancer risk assessment among multi-ethnic women. AMIA Annu Symp Proc 2016;2016:411–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Kukafka R, Fang J, Vanegas A, Silverman T, Crew KD. Pilot study of decision support tools on breast cancer chemoprevention for high-risk women and healthcare providers in the primary care setting. BMC Med Inform Decis Mak 2018;18:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crew KD, Silverman TB, Vanegas A, Trivedi MS, Dimond J, Mata J, et al. Study protocol: Randomized controlled trial of web-based decision support tools for high-risk women and healthcare providers to increase breast cancer chemoprevention. Contemp Clin Trials Commun 2019;16:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 1999;91:1541–8. [DOI] [PubMed] [Google Scholar]
- 19.McGuinness JE, Ueng W, Trivedi MS, Yi HS, David R, Vanegas A, et al. Factors associated with false positive results on screening mammography in a population of predominantly hispanic women. Cancer Epidemiol Biomarkers Prev 2018;27:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X, McGuinness JE, Sin M, Silverman T, Kukafka R, Crew KD. Identifying women at high risk for breast cancer using data from the electronic health record compared with self-report. JCO Clin Cancer Inform 2019;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD. Chemoprevention uptake among women with atypical hyperplasia and lobular and ductal carcinoma in situ. Cancer Prev Res (Phila) 2017;10:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, et al. Stratification of breast cancer risk in women with atypia: A mayo cohort study. J Clin Oncol 2007;25:2671–7. [DOI] [PubMed] [Google Scholar]
- 23.Cooper CL, Hind D, Duncan R, Walters S, Lartey A, Lee E, et al. A rapid review indicated higher recruitment rates in treatment trials than in prevention trials. J Clin Epidemiol 2015;68:347–54. [DOI] [PubMed] [Google Scholar]
- 24.Kinney AY, Richards C, Vernon SW, Vogel VG. The effect of physician recommendation on enrollment in the breast cancer chemoprevention trial. Prev Med 1998;27:713–9. [DOI] [PubMed] [Google Scholar]
- 25.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: Clinical and psychological factors. J Clin Oncol 2004;22:4951–7. [DOI] [PubMed] [Google Scholar]
- 26.Natale P, Saglimbene V, Ruospo M, Gonzalez AM, Strippoli GF, Scholes-Robertson N, et al. Transparency, trust and minimizing burden to increase recruitment and retention in trials: A systematic review. J Clin Epidemiol 2021;134:35–51. [DOI] [PubMed] [Google Scholar]
- 27.McCaskill-Stevens W, Wilson JW, Cook ED, Edwards CL, Gibson RV, McElwain DL, et al. National surgical adjuvant breast and bowel project study of tamoxifen and raloxifene trial: Advancing the science of recruitment and breast cancer risk assessment in minority communities. Clin Trials 2013;10:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the national surgical adjuvant breast and bowel project p-1 study. JNCI: Journal of the National Cancer Institute 1998;90:1371–88. [DOI] [PubMed] [Google Scholar]
- 29.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among african americans, latinos, asian americans, and pacific islanders. Am J Public Health 2014;104:e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuinness JE, Zhang TM, Cooper K, Kelkar A, Dimond J, Lorenzi V, et al. Extraction of electronic health record data using fast healthcare interoperability resources for automated breast cancer risk assessment. AMIA Annu Symp Proc 2021:843–52. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.


