Abstract
As synaptic vesicles fuse, they must continually be replaced with new docked, fusion-competent vesicles to sustain neurotransmission. It has long been appreciated that vesicles are recruited to docking sites in an activity-dependent manner. However, once entering the sites, vesicles were thought to be stably docked, awaiting calcium signals. Based on recent data from electrophysiology, electron microscopy, biochemistry, and computer simulations, a picture emerges in which vesicles can rapidly and reversibly transit between docking and undocking during activity. This ‘transient docking’ can account for many aspects of synaptic physiology. In this review, we cover recent evidence for transient docking, physiological processes at the synapse that it may support, and progress on the underlying mechanisms. We also discuss an open question: what determines for how long and whether vesicles stay docked, or eventually undock?
Introduction
As soon as synaptic vesicle exocytosis was first hypothesized to be the basis for neurotransmitter release, it was clear that vesicles must continually flow towards the site of fusion during activity [1]. Decades before any fusion machinery had been identified, Bernard Katz imagined vesicles fluttering back and forth at the active zone before crashing into the membrane to fuse (Katz, Nobel Lecture, 1970). However, the view of this dynamic vesicle movement was later abandoned, owing to our understanding of the molecular state of a synaptic vesicle ready for fusion. To allow for fast, synchronous neurotransmitter release at each active zone, synaptic vesicles are tightly attached to the plasma membrane within sites of concentrated release machinery at which one vesicle may dock and fuse at a time: release sites [2–4]. Vesicles are primed, or docked, with a large supramolecular complex around a core assembled SNARE complex, awaiting calcium influx to trigger fusion [5]. This docking state is thought to be static, with these vesicles constituting most or all of the readily-releasable pool [6]. However, recent experiments have shown that vesicles rapidly transition between docked and undocked states, reviving Katz’s original vision (see [7] and [8] for recent reviews). Furthermore, the balance between docking and undocking can be shifted transiently based on activity levels to ultimately determine synaptic outputs. Here, we review the evidence for fast and reversible synaptic vesicle docking at mammalian central synapses, its potential key role in presynaptic plasticity, candidate molecular mechanisms, and processes that may govern the speed at which vesicles dock and undock.
The replacement site-docking site model and the discovery of transient docking
When a synaptic vesicle has entered a release site and become poised for fusion, it is referred to as ‘docked’ (Figure 1). In this state, the ternary SNARE complex has been formed and converted from a loose to a tight trans conformation [9,10]. Thus, the vesicle is docked and molecularly primed for calcium-triggered fusion. Since SNARE complex assembly and zippering is highly exergonic [5], this is often assumed to be a static state. However, docking, priming, and SNARE complex assembly/zippering are fundamentally reversible [11–13]. In fact, electrophysiological data have suggested that docked vesicles are in constant equilibrium with ‘replacement’ vesicles that replenish docked vesicles after activity-dependent fusion—a process which is accelerated by calcium [8,14–22]. This replacement site-docking site model predicts that vesicles are able to dock very quickly (as fast as several ms) and reversibly, and as such all these vesicles can be release-ready during activity, comprising the total readily-releasable pool [7,14,15].
Figure 1.
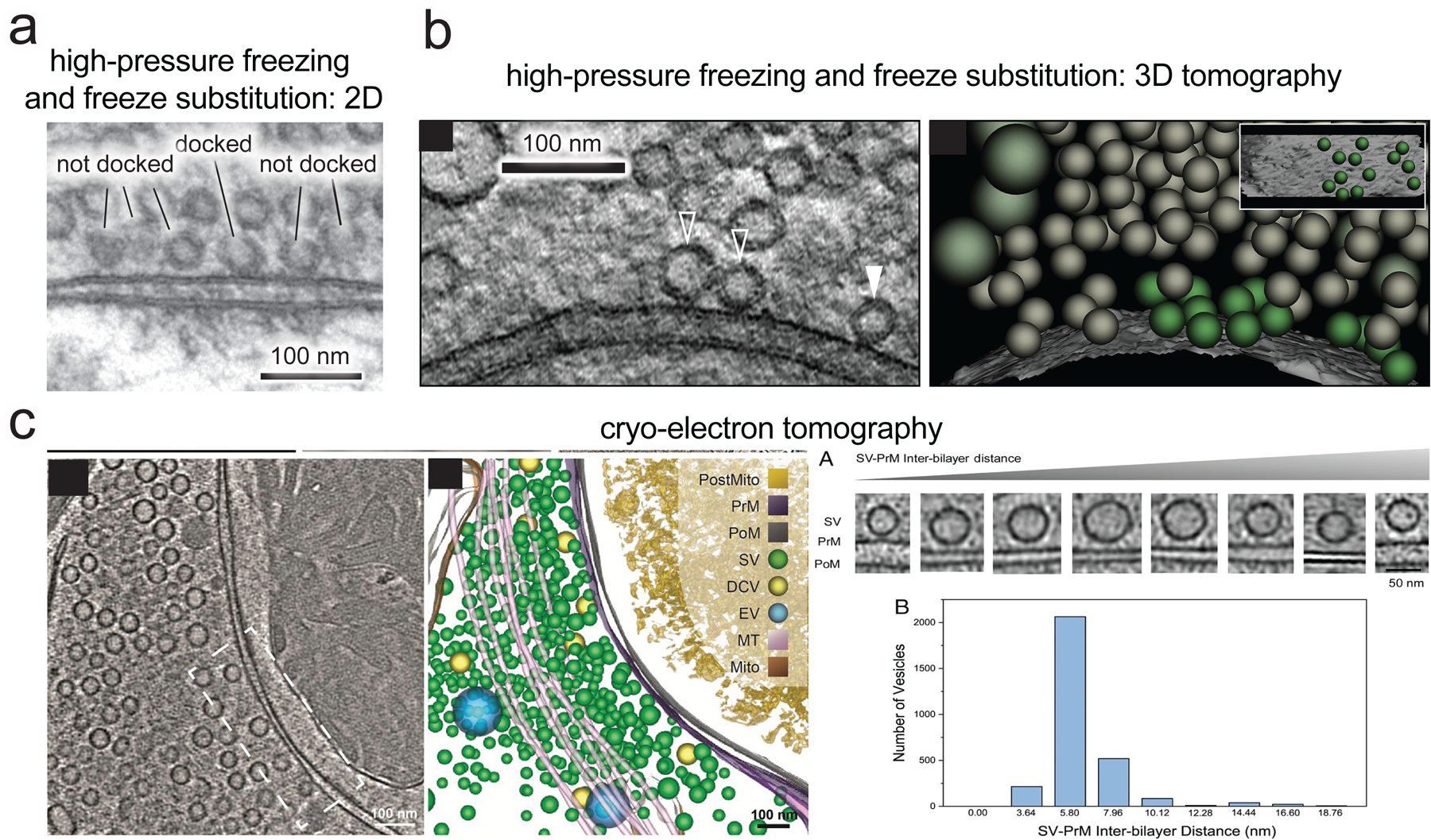
The strict definition of synaptic vesicle docking.
What is meant by ‘docking’ in synaptic ultrastructure varies from study to study. This term is often used for all vesicles within 30–40 nm of the plasma membrane at the active zone (measuring the nearest distance between the edge of the vesicle membrane and plasma membrane). Here, we refer to docking by a strict definition: structurally, docking is the closest synaptic vesicles can get to the plasma membrane at the active zone before fusion as observed by electron microscopy. (a) In high-pressure frozen and freeze-substituted samples, docked vesicles make a ‘point contact’ with the plasma membrane, visible in both (a) 2D thin sectioning EM and (b) 3D electron tomography (solid arrowheads indicate vesicles with visible plasma membrane contact in the tomograph slice shown, hollow arrowheads indicate vesicles that are docked and make contact with the plasma membrane, but the contact is not visible in this slice; green vesicles in the 3D rendering are docked), with no apparent space between vesicle membrane and plasma membrane down to the effective resolution of this technique (0–2 nm) [28,43]. (c) In cryo-electron tomography, which visualizes the native state of tissue under vitreous ice without any staining, dehydration, or fixation, the closest vesicles get to the plasma membrane in synapses at rest is ~5 nm [84–86], and by our definition these constitute docked vesicles. This means the apparent 0–2 nm distance in freeze substituted samples is likely an artifact. However, the two characteristic distances in these techniques are likely both meaningful and correspond to the same vesicles. ~75% of all vesicles within 20 nm of the plasma membrane accumulate at this closest distance in cultured hippocampal synapses, regardless of which technique is used [28,84]. [27,82]. Accumulation at this specific distance is unique to docking, as undocked vesicles within 100 nm are roughly evenly distributed in distance from the active zone. Only this closest stage of approach requires SNARE complex assembly [27,43], only docked vesicles are depleted by stimulation [26,28], and in cryo-electron tomography only these vesicles are connected to the membrane by a stereotyped protein density that may correspond to the docking/fusion machinery [84]. All these lines of evidence together strongly argue that docked vesicles, and only docked vesicles, are at the final stage of priming and readiness for fusion. Vesicles that are close to the plasma membrane, but not docked, we refer to simply as undocked or as ‘replacement vesicles’ (these vesicles are sometimes referred to as ‘tethered’). In terms of distance from the plasma membrane by EM, our definition of docking corresponds to the term ‘tightly docked’ often used in the field [7]. Note that any studies using traditional chemical fixation for electron microscopy, rather than fast freezing, cannot resolve the distinctions discussed here. Aldehyde fixation of living tissue causes severe deformations in cellular structures [87] and directly triggers synaptic vesicle exocytosis [88], making evaluation of fine structure near the active zone inaccurate. For example, under chemical fixation, preventing SNARE complex assembly has no apparent effect on docking [89].
(a) and (c) are reproduced, with permission, from [43] and [84], respectively. (b) is reproduced from [26].
In the last decade, methods have been developed to stimulate neurons then freeze them at precise time points for electron microscopy observation (referred to as flash-and-freeze for optical stimulation via channelrhodopsin and zap-and-freeze for electrical field stimulation). In such time-resolved electron microscopy experiments, docked vesicles are depleted by single and trains of action potentials, consistent with this being the final stage of readiness before fusion [23–29].
As predicted by the replacement site-docking site model, a wave of newly-docked vesicles replenish vacated docking sites. In flash-and-freeze experiments either using high extracellular calcium (4 mM) or trains of stimulation, docking is restored to the baseline on the order of seconds [26]. However, whether vesicles toggle between docked and undocked states was not clear, perhaps due to the temporal resolution in these studies. In fact, in a recent study using zap-and-freeze, which allows temporal precision of freezing relative to action potentials down to the millisecond, vesicle docking is reversible and occurs in two phases: milliseconds and seconds [28]. Docked vesicles are depleted by 40 % at 5 ms after an action potential – consistent with flash-and-freeze studies [26,27]. However, by 15 ms, docking is completely restored. Surprisingly, these vesicles undock within ~100 ms, and then, over the course of 10 s, docking is again restored. These data suggest that release sites can be replenished in two phases. Furthermore, in line with the replacement site-docking site model, they argue for the existence of fast and reversible ‘transient docking’ (Figure 2).
Figure 2.
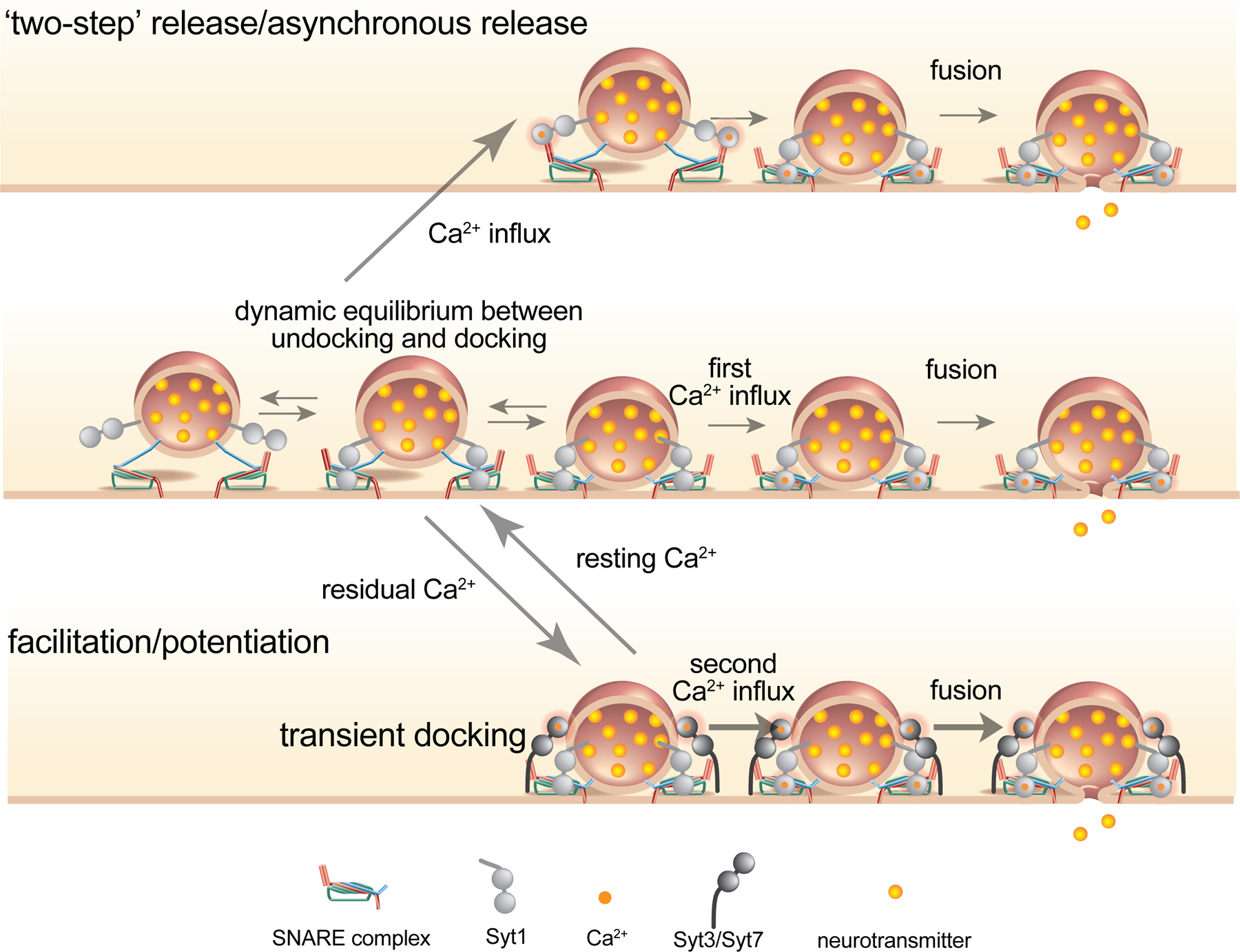
Proposed scheme for docking and undocking of synaptic vesicles at rest and during activity. Middle row: At steady state, vesicles reversible dock and undock as the trans-SNARE complex tightens and loosens, shuttling between a ‘docking site’ and ‘replacement site. Upon calcium binding to Syt1, docked vesicles fuse. Top row: in ‘two-step’ release, calcium binding to a calcium sensor(s) triggers docking then immediate fusion, perhaps giving rise to asynchronous release. While Syt1 is shown here, other higher-affinity calcium sensors may mediate docking or fusion during two-step release. Bottom row: docking is enhanced during activity as high-affinity calcium sensors such as Syt3 and Syt7 (and/or other signaling molecules) push vesicles into the dock state, or lock them there. Biasing the reaction coordinate towards docking makes more docked vesicles available for the next round of fusion, giving rise to synaptic potentiation and resistance to synaptic depression. For simplicity, only the SNARE complex, and not other essential parts of the docking machinery like Munc13, is shown. Adapted from an unpublished figure by Erik M. Jorgensen, with inspiration from [7]. Note that molecular structures are hypothetical.
Kinetics and calcium dependence of docking
The discovery that vesicles dock and undock immediately after action potentials raises two obvious questions. 1) What determines the speed at which vesicles dock and undock, and 2) what differentiates whether vesicles stably dock or ultimately undock?
Since residual calcium is the main trigger for transient docking [14,28,30], it is parsimonious to suggest that the dynamics of docking are dictated by the dynamics of calcium and the relevant calcium-sensing proteins (more discussion in Molecular mechanisms). In line with this, transient docking [27,28] after a single action potential follows a similar time course to residual calcium [31], peaking at ~15 ms and declining over ~100 ms, suggesting that calcium dynamics dictate the time course of transient docking.
In contrast, docking at the steady-state and recovery of docking to the steady-state after activity may not be entirely dependent on calcium. For example, raising the extracellular calcium concentration by almost 4-fold had no effect on docking at the steady state [28]. Even more convincingly, while loading cells with EGTA (which strongly lowers basal calcium) completely blocked transient docking, it had no effect on docking at rest, suggesting that calcium is not involved in steady-state docking. These findings conflict with electrophysiological data, which indicate that more docking sites are filled at higher basal calcium concentrations [21,30]. Nevertheless, it is clear that baseline docking takes place in the absence of calcium. Indeed, even activity-dependent recovery is itself not entirely calcium-dependent. Synapses always eventually recover from depression, they simply do so much more slowly without a sustained rise in calcium [16,32]. Therefore, while calcium clearly biases vesicles towards docking, there must be calcium-independent mechanisms setting baseline docking and undocking rates to which synapses naturally return after bouts of activity.
All this raises the question: what differentiates the recently-discovered transient rise in docking from the well-established recovery of docked vesicles [16,18]? For a synapse to eventually return to a baseline, the fate of newly-docked vesicles would be expected to be opposite: in transient docking, they must eventually undock, while in recovery, opposite is true. For now, what determines for how long a newly-docked vesicle will stay docked is a mystery.
Potential functions of transient docking: presynaptic plasticity and asynchronous release
Transient docking can make vesicles available for the next action potential on the order of milliseconds, and thus could be a major contributor to presynaptic potentiation. The fastest but most fleeting form of presynaptic enhancement is facilitation, in which a single stimulus engenders a calcium-dependent increase in release in response to subsequent stimuli [33–35]. Although docking observed in zap-and-freeze experiments only return to baseline, lacking the overshoot of baseline docking that would produce facilitation, changes in docking after an action potential still match paired-pulse responses at different time points: weak facilitation in release over the course of 50 ms (synapses of cultured hippocampal neurons do not facilitate strongly) and depression of release to ~40% over the course of 500 ms [36]. In replacement site-docking site models, transient docking accounts for facilitation patterns better than increasing the fusion probability of docked vesicles, and experimentally the same perturbations that block docking site filling also block facilitation [7,14,37]. A similar model has been applied to Drosophila neuromuscular junctions, where a combination of superresolution and electron microscopy suggested that facilitation can be only explained by activity-dependent inhibition of undocking or recruitment of new docking sites [38]. Thus, transient docking may support synaptic facilitation. This idea still needs to be tested more thoroughly, particularly the relative contributions of transient docking vs. fusion probability of already-docked vesicles to the balance of facilitation and depression.
While transient docking as described so far sets in after single stimuli and decays quickly, complex stimuli could induce a longer-lasting increase in docking to support longer-lasting forms of presynaptic potentiation. Each of these has a characteristic stimulation strength that induces them, time of onset, and decay time [34]. Mild trains of action potentials induce augmentation, an increase in release that sets in after several seconds and decays over the course of a minute [39,40]. The size of the readily-releasable pool does not change during augmentation [40,41]. Instead, the enhancement has been ascribed to ‘superpriming’ of synaptic vesicles [41,42]: the same number of vesicles fuse over the course of a high-frequency stimulus, but a greater proportion of ‘superprimed’ vesicles fuse early in the train. This apparent superpriming can be accounted for by a shift of vesicles from the replacement pool to the docked pool [15]. In fact, augmentation is driven by increased recruitment to the plasma membrane of Munc13 [41], a key docking/priming protein [43] (see molecular mechanisms). Thus, increased docking may be the basis for augmentation, but this needs to be tested directly.
Currently, the most direct evidence for an increase in vesicle docking as the basis for plasticity of neurotransmitter release is in post-tetanic potentiation (PTP). PTP is induced by intense stimuli, sets in over the course of ~30 s, and lasts for minutes [34,44]. Using flash-and-freeze of hippocampal mossy fiber synapses in acute slices, a PTP-inducing stimulus at first strongly depletes docked vesicles [25]. However, once PTP has set in after 20 s, docked vesicles not only recover but are 25% more abundant than at rest. This rise in docking, as well as an increase in the number of large docked vesicles characteristic of this synapse, corresponds to a larger readily-releasable pool [25].
Docking may also be involved in long-term forms of presynaptic plasticity that last hours or more. The readily-releasable pool grows larger during presynaptic homeostatic plasticity [45,46], docked vesicles increase in number during long-term potentiation [47], and a recently-discovered form of presynaptic enhancement triggered by mechanical stimuli is associated with greater assembly of trans-SNARE complexes [48]. A shared characteristic of many of these longer-lasting forms of potentiation is that, unlike in augmentation and facilitation, the size of the readily-releasable pool increases. This could correspond to an increase in both docked and replacement vesicles. Such slower changes could also result from building new release/docking sites [49], as opposed to changing the proportion of occupied docking sites. Nevertheless, docking has now been implicated in every known form of presynaptic plasticity.
All the possible contributions of docking to plasticity share a common principle: vesicles dock, but not fuse, so they are available for fusion upon a subsequent stimulus (Figure 2, facilitation and potentiation). But what if a vesicle docked and then immediately fused (Figure 2, ‘two-step release’)? In such a scenario, transient docking would supply vesicles for asynchronous release [50]. Indeed, slower and asynchronous release during high-frequency trains can be accounted for in simulations by the replacement site-docking site model [15,30], and the same perturbations (EGTA-AM and latrunculin) that block transient docking also block slow/asynchronous release [15]. This would help explain why asynchronous release is always more prominent during train stimulation than after single action potentials, since release during trains will be dominated by newly-docked vesicles [15]. This is all in line with the idea that the ‘readily-releasable pool’ comprises both docked vesicles and replacement site vesicles [14] (and maybe also vesicles upstream of the replacement site, which can also be quickly recruited [53]), since replacement site vesicles, while not at the final stage of fusion-competence, can dock and then fuse within milliseconds. Two-step release may account for the curious finding that, in mutants where the active zone is disrupted and there are almost no baseline docked vesicles, vesicles can still fuse and the readily-releasable pool is mostly intact [51]. It remains to be tested whether this two-step process is entirely responsible for asynchronous release, or if already-docked vesicles can also fuse asynchronously.
Molecular mechanisms of transient docking
As discussed in The discovery of transient docking, tightening and loosening of already-assembled trans-SNARE complexes probably accounts for the final docking/undocking step that underlies transient docking. Less is known about loading into the replacement site that vesicles transit through before docking. This would presumably correspond to initial assembly of the trans-SNARE complex, as well as steps of vesicle attachment to the active zone further upstream of SNARE complex assembly. It is clear that these steps are also reversible [12,13] and vesicles are loaded into the replacement site during activity [52,53]. This could explain why the increase in docked vesicles observed by electron microscopy during transient docking does not correspond to a loss of undocked vesicles close to the active zone [27,28]. Indeed, the pool of undocked vesicles close to the plasma membrane at the active zone (within 100 nm) seems very resistant to depletion, even during high-frequency stimulation [36]. This indicates that there must be a robust mechanism that sustains them. One exciting recent proposal is that all vesicles in the readily-releasable pool are captured in or attached to a phase-separated domain constituting the active zone [54,55].
But what drives transient docking? Vesicles dock during activity in a residual calcium-[14,15,28] and actomyosin-dependent [14,15] manner. Therefore, there must be mechanisms involving calcium-sensing and cytoskeleton-regulating proteins. Such mechanisms would not be required for baseline docking or fusion itself, but only for biasing the docked/undocked balance in favor of docking during activity. Here we discuss proteins that have been implicated in transient docking, either based on direct evidence or their role in short-term plasticity.
A variety of C2 domain-containing proteins, which bind membranes with increased affinity upon calcium binding, interact with the exocytic machinery and regulate neurotransmitter release in a calcium-dependent manner [56]. The most well-studied and essential of these is Synaptotagmin 1 (Syt1), the major calcium sensor for synchronous neurotransmitter release [57,58]. Baseline docking is reduced ~35% in Syt1 knockouts [27,43], although this has been attributed in some cases to an overall reduction in synaptic vesicles rather than a specific effect on docking [43]. This raises the question of whether Syt1 could promote docking, in addition to fusion, during activity. Indeed, the initial discovery of transient docking by electron microscopy was in the context of Syt1. Mutations that disrupt membrane/SNARE complex binding and baseline docking render Syt1 unable to efficiently trigger fusion, but in its place these mutants trigger transient docking [27]. Syt1’s function in baseline docking does not depend on calcium binding, but transient docking is completely absent when the mutant Syt1s cannot bind calcium. However, there are two key features of transient docking as measured in wild-type synapses that are inconsistent with Syt1 being the sole calcium sensor. First, Syt1 is a low-affinity calcium sensor, and due to its fast kinetics, its activity would not be expected to last as long as transient docking does [59]. However, vesicles undock over the course of ~100 ms in these mutants, similarly to the time course for transient docking in wild-type synapses, suggesting that Syt1 can operate on longer time scales or collaborate with other sensors that remain active for longer. Second, loading cells with the slow calcium chelator EGTA only minimally interferes with Syt1-driven processes like fast neurotransmitter release, but completely blocks transient docking [14,28]. Thus, while Syt1 contributes to synaptic vesicle docking at rest in a calcium-independent manner and may amplify this function after calcium binding, other C2 domain-containing proteins with slower kinetics that respond to lower calcium concentrations must also be involved.
Another candidate for transient docking is Munc13. Munc13 is the single most essential protein for synaptic vesicle exocytosis: without it docking, priming, and neurotransmitter release are absent [43,60–62]. Munc13 supports docking and priming through various means, most notably by templating SNARE complex assembly [63]. In addition to its indispensable constitutive function, Munc13 is also a convergence point for many forms of presynaptic plasticity, both through interactions with other proteins and its own domains that respond to calcium, phosphatidylinositol 4,5-bisphosphate, and diacylglycerol [60]. Relevant to transient docking, membrane-binding domains at either end of Munc13 have been proposed to bridge the synaptic vesicle membrane and plasma membrane [64–67]. One of these, the C2B domain, operates similarly to the synaptotagmins, binding membrane in response to calcium with high affinity [68]. A recent study using knock-in point mutations highlights the importance of this domain for short-term plasticity [69]. At the Calyx of Held, preventing calcium binding to the C2B domain does not affect single action potential responses but accelerates depression during trains and slows recovery from depression. A mutation that enhances calcium binding does the opposite, slowing train depression and accelerating recovery. This combination of docking, calcium-sensitive regulation of short-term plasticity, and membrane bridging makes the C2B domain of Munc13 a likely sensor for transient docking.
Calcium-sensing proteins that are not important for basal transmission, but critical for short-term plasticity, are ideal candidates for triggering transient docking. Synaptotagmin 7 (Syt7) has emerged in the last five years as the most important driver of synaptic facilitation [70,71]. Syt7 knockout causes more dire problems for short-term plasticity than any other known protein: normally facilitating synapses tend to strongly depress starting with a second stimulus and continue to depress more quickly than normal throughout a train [70,72,73]. Some non-facilitating synapses also depress more quickly and profoundly in the absence of Syt7 [32,74,75], and Syt7 can also support the slower process of recovery from train depression in some cases [32,72,74]. These functions, particularly in facilitation, have been ascribed to an increase in release probability of docked vesicles [35,70]. A mathematical model in which release-ready vesicles are present in two pools, one of which has very low initial release probability that increases during activity in a Syt7-dependent manner, could account for all these phenotypes [74]. As discussed in The discovery of transient docking and potential functions of transient docking, all these physiological phenomena: facilitation, resisting depression, recovery from depression, and apparent mobilization of reluctant or slow-releasing vesicles to a higher release probability pool, could be explained by transient docking. Critically, there is direct ultrastructural evidence for Syt7’s role in supporting docking during activity. By time-resolved electron microscopy, Syt7 knockouts have a normal complement of docked vesicles at baseline, but 30% fewer at 5 ms after both single and trains of action potentials. Furthermore, the second phase of docking that takes place over seconds is slower [36]. These data directly implicate Syt7 in activity-dependent docking at both millisecond and second timescales. This docking function could explain some, or all, of Syt7’s physiological roles.
Another high-affinity member of the synaptotagmin family, Syt3, has also been shown to be critical for facilitation, resistance to and recovery from train depression [76]. In mathematical models, the experimental data could be recapitulated by Syt3 promoting transient docking, but not by Syt3 increasing the fusion probability of already-docked vesicles. Unlike Syt1, Syt3 and Syt7 both act on the plasma membrane, not synaptic vesicles [32,36,76]. This raises the possibility that, like Munc13, they could function in part by bridging the membrane between synaptic vesicles and the plasma membrane upon calcium binding. In summary, these C2 domain-containing proteins may support facilitation, as well as resistance to and recovery from synaptic depression, by acting as calcium sensors for transient docking.
While dispensable for baseline docking and exocytosis, an intact actomyosin cytoskeleton is absolutely required for transient docking [14,15]. This is consistent with the well-known role of the cytoskeleton in synaptic recovery and short-term plasticity [77]. However, the nature and dynamics of actin networks that support docking are unknown, as the specific actin regulatory proteins involved have not been identified. Do actin dynamics help propel vesicles towards the docked state, or is it just a stable scaffold that is required? Several recent studies show that specific cytoskeleton proteins can regulate different steps of the synaptic vesicle’s journey to the active zone in unexpected ways [78,79], highlighting the importance of 1) disrupting individual proteins and 2) analyzing specific phenotypes. Future studies should focus on individual actin regulators and specifically test their role in transient docking. Some clues come from work in chromaffin cells, where the actin-regulating protein Intersectin-1 and the BAR-domain containing protein Endophilin A1 collaborate to maintain fusion by enhancing priming [80]. More such studies are needed at synapses before we can speculate on the mechanism by which actin controls transient docking.
We should point out that the different molecules that support activity-dependent docking are likely to vary between synapse types, not only in which are present but in their relative importance. For example, while both Syt3 and Syt7 are expressed at the Calyx of Held, deleting Syt3 has a potent effect [76], whereas Syt7 is less important [81] compared to at hippocampal synapses [70]. Conversely, expression of individual proteins seems to be necessary and sufficient in some cases. Increased expression of Syt7 during development is correlated with a change from depression to frequency invariance at Purkinje cell to deep cerebellar nuclei and vestibular synapses [74]. Further, introducing Syt7 via transgene expression at climbing fiber to Purkinje cell synapses, where it is not normally present, by itself converts these depressing synapses to facilitation [82]. Therefore, finding that a given protein is not required at a given synapse should not be taken to rule out its importance in general. Double and triple knockout studies will also be important to address redundancy and quantify relative contributions. This diversity makes functional sense given the broad tapestry of synapse types. The balance of docked and undocked vesicles at rest has been proposed as a basis for different plasticity patterns, for example in facilitating vs. depressing synapses [7,83]. Release at facilitating synapses could be dominated by replacement site vesicles, which do not fuse initially but transiently dock to boost release, whereas depressing synapses have many docked vesicles but few replacement site vesicles, so they exhaust their readily-releasable pool quickly [7,83]. Thus, molecular diversity in the control of transient docking could contribute to the wildly diverse plasticity patterns of synapses.
Conclusion
Within the last five years, the activity-dependent dynamics of vesicle docking have emerged as a key control point for neurotransmitter release. An important lesson from the progress made so far is to interpret physiological, ultrastructural, genetic, computational, and biochemical data in the context of each other. Soon after the replacement site-docking site model was proposed based on electrophysiology, just such a transient docking event had been identified and corresponding dynamics of the trans-SNARE complex verified in vitro. Looking forward, some open questions are obvious and can be readily addressed by current methods, such as the identity of calcium sensors and the diversity of docking dynamics at different synapse types and in different plasticity regimes. Others, such as how different stages of docking and recruitment correspond to different biochemical states, need new approaches. Ultimately, we should also keep an eye toward how the nanoscale fluttering back and forth of synaptic vesicles can help give rise to the function of neurons, circuits, and brains.
Highlights:
During neuronal activity, synaptic vesicles transition between docked and undocked states on time scales ranging from milliseconds to seconds.
Docking has been implicated in all known forms of presynaptic plasticity, as well as asynchronous release.
Residual calcium and the actin cytoskeleton are essential for transient docking; candidate calcium sensors include Syt1, Syt3, Syt7, and Munc13.
What determines kinetics and reversibility of docking during synaptic activity is still uncertain.
Acknowledgements
We thank Edwin R. Chapman for drawing our attention to Katz’s idea of “frequent transient collisions” by vesicles with the plasma membrane, and Skyler L. Jackman for raising the idea of vesicle-resident vs. plasma membrane-resident calcium sensors. S.W. was supported by start-up funds from the Johns Hopkins University School of Medicine, Johns Hopkins Discovery funds, Johns Hopkins Catalyst award, the National Science Foundation (1727260), and the National Institutes of Health (1DP2 NS111133-01 and 1R01 NS105810-01A1). S.W. is an Alfred P. Sloan fellow, a McKnight Foundation Scholar and a Klingenstein and Simons Foundation scholar. G.F.K. was supported by a grant from the National Institutes of Health to the Biochemistry, Cellular and Molecular Biology program of the Johns Hopkins University School of Medicine (T32 GM007445), a National Science Foundation Graduate Research Fellowship (2016217537), and is a Hay Fellow of the Johns Hopkins University School of Medicine Department of Cell Biology. T.H.O. was supported by a grant from the National Institutes of Health to the Biochemistry, Cellular and Molecular Biology program of the Johns Hopkins University School of Medicine (T32 GM007445) and is a National Science Foundation Graduate Research Fellow (2019241734).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
References
- 1.Curtis DR, Eccles JC: SYNAPTIC ACTION DURING AND AFTER REPETITIVE STIMULATION. J Physiol 1960, 150:374–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter AM, Böhme MA, Sigrist SJ: Vesicle release site organization at synaptic active zones. Neurosci Res 2018, 127:3–13. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Tang A-H, Blanpied TA, Burrone J, Holzbaur E: Subsynaptic spatial organization as a regulator of synaptic strength and plasticity. Curr Opin Neurobiol 2018, 51:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto H, Ariyoshi T, Kimpara N, Sugao K, Taiko I, Takikawa K, Asanuma D, Namiki S, Hirose K: Synaptic weight set by Munc13–1 supramolecular assemblies. Nat Neurosci 2018, 21:41–55. [DOI] [PubMed] [Google Scholar]
- 5.Jahn R, Fasshauer D: Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenmund C, Stevens CF: Definition of the Readily Releasable Pool of Vesicles at Hippocampal Synapses. Neuron 1996, 16:1197–1207. [DOI] [PubMed] [Google Scholar]
- 7.Neher E, Brose N: Dynamically Primed Synaptic Vesicle States: Key to Understand Synaptic Short-Term Plasticity. Neuron 2018, 100:1283–1291. [DOI] [PubMed] [Google Scholar]
- 8.Silva M, Tran V, Marty A: Calcium-dependent docking of synaptic vesicles. Trends Neurosci 2021, 44:579–592. [DOI] [PubMed] [Google Scholar]
- 9.Yavuz H, Kattan I, Hernandez JM, Hofnagel O, Witkowska A, Raunser S, Walla PJ, Jahn R: Arrest of trans-SNARE zippering uncovers loosely and tightly docked intermediates in membrane fusion. J Biol Chem 2018, 293:8645–8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkowska A, Heinz LP, Grubmüller H, Jahn R: Tight docking of membranes before fusion represents a metastable state with unique properties. Nat Commun 2021, doi: 10.1038/s41467-021-23722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy VN, Stevens CF: Reversal of synaptic vesicle docking at central synapses. Nat Neurosci 1999, 2:503–507. [DOI] [PubMed] [Google Scholar]
- 12.He E, Wierda K, Van Westen R, Broeke JH, Toonen RF, Cornelisse LN, Verhage M: Munc13–1 and Munc18–1 together prevent NSF-dependent de-priming of synaptic vesicles. Nat Commun 2017, doi: 10.1038/ncomms15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Bao H, Das D, Courtney NA, Jiang Y, Briguglio JS, Lou X, Roston D, Cui Q, Chanda B, Chapman ER: Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature 2018, 554:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a novel in vitro planar lipid bilayer electrophysiology setup, the authors measure the opening and closing of fusion pores by SNARE complexes with microsecond temporal resolution. They find that pores flicker frequently, and SNARE complexes are not stable even after they have opened a pore. This indicates that even after fusion, trans-SNARE complexes are dynamic and reversible. While this study focuses on the flickering of fusion pores, a step downstream of docking, these data are the most conclusive to date that trans-SNARE complexes at advanced stages of the reaction coordinate for fusion can still quickly and reversibly convert between states of loose and tight assembly, as well as disassemble completely. This likely serves as the molecular basis for fast and reversible docking.
- 14.**.Miki T, Malagon G, Pulido C, Llano I, Neher E, Marty A: Actin- and Myosin-Dependent Vesicle Loading of Presynaptic Docking Sites Prior to Exocytosis. Neuron 2016, 91:808–823. [DOI] [PubMed] [Google Scholar]; The authors use a sophisticated electrophysiology recording and analysis system to measure individual release events at single synapses during trains. They find that each synapse contains a discrete number of docking/release sites. Importantly, data combined with simulations suggest that these release sites are not always filled, and they propose that vesicles can rapidly dock in a calcium- and actomyosin-dependent manner to replace those that have fused or increase the total number of vesicles available. Thus, the readily-releasable pool does not correspond only to docked vesicles ready to fuse, but also ‘replacement site’ vesicles that can rapidly dock and become fusion-competent. This paper contains the original proposal of a millisecond-scale reversible transition between docking and undocking, as well as the idea that this, rather than changes in release probability, could be the basis for short-term plasticity
- 15.**.Miki T, Nakamura Y, Malagon G, Neher E, Marty A: Two-component latency distributions indicate two-step vesicular release at simple glutamatergic synapses. Nat Commun 2018, doi: 10.1038/s41467-018-06336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; An electrophysiological recording and analysis method for counting vesicle fusion events at single parallel fiber to molecular layer interneuron synapses is extended to measure the latencies of fusions, ie. exactly when after each action potential vesicles fuse. Fast, synchronous release is found to actually be composed of two time constants: one on the scale of hundreds of microseconds and the other on the scale of several milliseconds. The slower release becomes more dominant during train stimulation and depends on an intact actomysosin cytoskeleton. These data can be explained by the replacement site/docking site model, in which replacement site vesicles can quickly dock and then fuse. This study provides experimentally-determined time constant for transient docking and presents the new idea that, in addition to being available for subsequent stimuli thereby supporting short-term plasticity, transiently docked vesicles may also immediately fuse, thereby supporting asynchronous release.
- 16.Dittman JS, Regehr WG: Calcium Dependence and Recovery Kinetics of Presynaptic Depression at the Climbing Fiber to Purkinje Cell Synapse. J Neurosci 1998, 18:6147–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaba T, Neher E: Quantitative relationship between transmitter release and calcium current at the calyx of Held synapse. J Neurosci 2001, 21:462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LY, Kaczmarek LK: High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 1998, 394:384–388. [DOI] [PubMed] [Google Scholar]
- 19.Eshra A, Schmidt H, Eilers J, Hallermann S: Calcium dependence of neurotransmitter release at a high fidelity synapse. eLife 2021, doi: 10.7554/eLife.70408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saviane C, Silver RA: Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature 2006, 439:983–987. [DOI] [PubMed] [Google Scholar]
- 21.Malagon G, Miki T, Tran V, Gomez L, Marty A: Incomplete vesicular docking limits synaptic strength under high release probability conditions. eLife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doussau F, Schmidt H, Dorgans K, Valera AM, Poulain B, Isope P: A frequency-dependent mobilization of heterogeneous pools of synaptic vesicles shapes presynaptic plasticity. eLife 2017, doi: 10.1101/146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imig C, López-Murcia FJ, Maus L, García-Plaza IH, Mortensen LS, Schwark M, Schwarze V, Angibaud J, Nägerl UV, Taschenberger H, et al. : Ultrastructural Imaging of Activity-Dependent Synaptic Membrane-Trafficking Events in Cultured Brain Slices. Neuron 2020, 108:843–860.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges-Merjane C, Kim O, Jonas P: Functional Electron Microscopy (“Flash and Freeze”) of Identified Cortical Synapses in Acute Brain Slices. Neuron 2020, doi: 10.1016/j.neuron.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.*.Vandael D, Borges-merjane C, Zhang X, Jonas P: Short-Term Plasticity at Hippocampal Mossy Fiber Synapses Is Induced by Natural Activity Patterns and Associated with Vesicle Pool Engram Formation. Neuron 2020, 107:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Post-tetanic potentiation, a form of short-term plasticity induced by intense stimulation, is found to arise from an increase in the readily-releasable pool at hippocampal mossy fiber synapses. Flash-and-freeze electron microscopy found that induction of post-tetanic potentiation corresponded to an increase in the number of docked vesicles. This is the first report correlating an increase in docking directly measured by electron microscopy with a short-term plasticity paradigm in an intact circuit.
- 26.Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl-Kielczynski B, Rosenmund C, Jorgensen EM: Ultrafast endocytosis at mouse hippocampal synapses. Nature 2013, 504:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.**.Chang S, Trimbuch T, Rosenmund C: Synaptotagmin-1 drives synchronous Ca2+−triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment. Nat Neurosci 2018, 21:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Consistent with previous reports, knocking out synaptotagmin 1, the calcium sensor for synchronous release, leads to a ~35% decrease in baseline docked vesicles evaluated by electron microscopy at dissociated cultured hippocampal synapses. This effect is not phenocopied by mutations that block calcium binding, but is phenocopied by mutations of polybasic residues important for membrane binding and evoked release. Strikingly, in flash-and-freeze experiments, the polybasic syt1 mutants drive vesicles to the docked state within 20 ms of an action potential. These vesicles then undock over the course of 100–300 ms. This syt1-driven docking requires calcium influx and calcium binding by the C2 domains, as well as trans-SNARE complex assembly. In electrophysiology experiments, these mutants do not support efficient release after single stimuli but facilitate very strongly, suggesting that these transiently-docked vesicles are fusion-competent. This paper provides the first electron microscopy evidence of fast, reversible vesicle docking.
- 28.**.Kusick GF, Chin M, Raychaudhuri S, Lippmann K, Adula KP, Hujber EJ, Vu T, Davis MW, Jorgensen EM, Watanabe S: Synaptic vesicles transiently dock to refill release sites. Nat Neurosci 2020, 23:1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]; A new system, termed ‘zap-and-freeze’, allows synapses to be frozen at extremely precise time points after electrical field stimulation and examined by electron microscopy. Synapses frozen 5, 8, and 11 ms after single action potentials have visible ‘pits’ that correspond to exocytosed vesicles, and a 40% reduction in docked vesicles, consistent with previous reports using optogenetic stimulation (‘flash-and-freeze’). This massive loss in docked vesicles cannot be accounted for by the amount of exocytosis after a single action potential, raising the possibility that some vesicles ‘undock’ immediately after calcium influx. Remarkably, at 14 ms docked vesicles return to baseline in a calcium-dependent manner. These vesicles then undock over the course of ~100 ms, and it takes 10 s for docking to again be restored. This is and ultrastructural picture of predicted replacement site-docking site model, in which vesicles dock during activity in two phases: one on the scale of milliseconds and temporary and the other taking several seconds and more stable.
- 29.Watanabe S, Liu Q, Davis MW, Hollopeter G, Thomas N, Jorgensen NB, Jorgensen EM: Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. eLife 2013, 2013:e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.*.Blanchard K, Zorrilla J, Mart DS, Marty A, Llano I, Trigo FF: Differentially poised vesicles underlie fast and slow components of release at single synapses. J Gen Physiol 2020, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study applies the electrophysiology vesicle fusion counting method at single synapses to calcium uncaging experiments. Release was dominated more by vesicles that dock and then fuse at synapses where prior stimuli had triggered fusion, consistent with release sites having been vacated then filled by new docked vesicles. Conversely, subthreshold calcium stimuli that did not trigger release enhanced the amount of release by already-docked vesicles upon a subsequent suprathreshold stimulus, supporting the idea that a high-affinity calcium sensor triggers transient docking and not all release/docking sites are filled at baseline, such that an increase in docking can lead to synaptic potentiation. This study provides the most direct evidence to date that docked vesicle dynamics during activity represent a balance between vacating and calcium-driven filling of release sites
- 31.Regehr WG, Delaney KR, Tank DW: The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci 1994, 14:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Bai H, Hui E, Yang L, Evans CS, Wang Z, Kwon SE, Chapman ER: Synaptotagmin 7 functions as a Ca2+−sensor for synaptic vesicle replenishment. eLife 2014, doi: 10.7554/eLife.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eccles JC, Katz B, Kuffler SW: Nature of the “endplate potential” in curarized muscle. J Neurophysiol 1941, 4:362–387. [Google Scholar]
- 34.Regehr WG: Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackman SL, Regehr WG: The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94:447–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*.Vevea JD, Kusick GF, Chen E, Courtney KC, Watanabe S, Chapman ER: Synaptotagmin 7 is enriched at the plasma membrane through γ-secretase processing to promote vesicle docking and control synaptic plasticity in mouse hippocampal neurons. eLife 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]; Using iGluSnFR imaging of dissociated cultured hippocampal neurons, the authors evaluate the reported physiological functions of syt7: facilitation, asynchronous release, resisting depression during train stimulation, and recovery from depression. All of these phenotypes are found to be present at these synapses, although the effect on asynchronous release after single action potentials is very slight. They then use zap-and-freeze electron microscopy to test if docked vesicle dynamics could underly these phenotypes. While docking is unaffected at baseline, 5 ms after both single and high-frequency trains of action potentials, synapses lacking syt7 have ~25% fewer docked vesicles, and 5 s after the train docked vesicles recover more slowly than in the wild-type. These data indicate that syt7 helps dock vesicles during activity, which maybe the basis for its role in facilitation, resisting depression during trains, and recovery from depression.
- 37.Pulido C, Marty A: A two-step docking site model predicting different short-term synaptic plasticity patterns. 2018, doi: 10.1085/jgp.201812072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobbersmed JRL, Grasskamp AT, Jusyte M, Ditlevsen S, Sørensen JB, Walter AM: Rapid regulation of vesicle priming explains synaptic facilitation despite heterogeneous vesicle:Ca 2+ channel distances. eLife 2020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magleby KL, Zengel JE: Augmentation: a process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol 1976, 257:449–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens CF, Wesseling JF: Augmentation Is a Potentiation of the Exocytotic Process. Neuron 1999, 22:139–146. [DOI] [PubMed] [Google Scholar]
- 41.Xue R, Ruhl DA, Briguglio JS, Figueroa AG, Pearce RA, Chapman ER: Doc2-mediated superpriming supports synaptic augmentation. Proc Natl Acad Sci U S A 2018, 115:E5605–E5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JS, Ho W-K, Neher E, Lee S-H: Superpriming of synaptic vesicles after their recruitment to the readily releasable pool. Proc Natl Acad Sci U S A 2013, 110:15079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imig C, Min SW, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee JS, Brose N, Cooper BH: The Morphological and Molecular Nature of Synaptic Vesicle Priming at Presynaptic Active Zones. Neuron 2014, 84:416–431. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd DP: POST-TETANIC POTENTIATION OF RESPONSE IN MONOSYNAPTIC REFLEX PATHWAYS OF THE SPINAL CORD. J Gen Physiol 1950, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delvendahl I, Kita K, Müller M: Rapid and sustained homeostatic control of presynaptic exocytosis at a central synapse. Proc Natl Acad Sci U S A 2019, 8057:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller M, Liu KSY, Sigrist SJ, Davis GW: RIM controls homeostatic plasticity through modulation of the readily-releasable vesicle pool. J Neurosci 2012, 32:16574–16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung JH, Kirk LM, Bourne JN, Harris KM: Shortened tethering filaments stabilize presynaptic vesicles in support of elevated release probability during LTP in rat hippocampus. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ucar H, Watanabe S, Noguchi J, Morimoto Y, Iino Y: Mechanical actions of dendritic-spine enlargement on presynaptic exocytosis. Nature 2021, 600:686–689. [DOI] [PubMed] [Google Scholar]
- 49.Böhme MA, Mccarthy AW, Grasskamp AT, Beuschel CB, Goel P, Jusyte M, Laber D, Huang S, Rey U, Petzoldt AG, et al. : Rapid active zone remodeling consolidates presynaptic potentiation. Nat Commun 2019, doi: 10.1038/s41467-019-08977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaeser PS, Regehr WG: Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 2014, 76:333–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang SSH, Held RG, Wong MY, Liu C, Karakhanyan A, Kaeser PS: Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking. Neuron 2016, 91:777–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miki T, Midorikawa M, Sakaba T: Direct imaging of rapid tethering of synaptic vesicles accompanying exocytosis at a fast central synapse. Proc Natl Acad Sci U S A 2020, doi: 10.1073/pnas.2000265117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran V, Miki T, Marty A: Three small vesicular pools in sequence govern synaptic response dynamics during action potential trains. Proc Natl Acad Sci U S A 2022, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Ganzella M, Zhou J, Zhu S, Jahn R, Zhang M: Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol Cell 2020, doi: 10.1016/j.molcel.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 55.McDonald NA, Shen K: Finding functions of phase separation in the presynapse. Curr Opin Neurobiol 2021, 69:178–184. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro PS, Houy S, Sørensen JB: C2-domain containing calcium sensors in neuroendocrine secretion. J Neurochem 2016, 139:943–958. [DOI] [PubMed] [Google Scholar]
- 57.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC: Synaptotagmin I: A major Ca2+sensor for transmitter release at a central synapse. Cell 1994, 79:717–727. [DOI] [PubMed] [Google Scholar]
- 58.Chapman ER: A Ca2+Sensor for Exocytosis. Trends Neurosci 2018, 41:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER: Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci U S A 2005, 102:5210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dittman JS: Unc13: a multifunctional synaptic marvel. Curr Opin Neurobiol 2019, 57:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Augustin I, Rosenmund C, Südhof TC, Brose N: Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 263:457–461. [DOI] [PubMed] [Google Scholar]
- 62.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C: Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A 2002, 99:9037–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma C, Li W, Xu Y, Rizo J: Munc13 mediates the transition from the closed syntaxin –Munc18 complex to the SNARE complex. Nat Struct Mol Biol 2011, 18:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quade B, Camacho M, Zhao X, Orlando M, Trimbuch T, Xu J, Li W, Nicastro D, Rosenmund C, Rizo J: Membrane bridging by Munc13–1 is crucial for neurotransmitter release. eLife 2019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camacho M, Quade B, Trimbuch T, Xu J, Sari L, Rizo J, Rosenmund C: Control of neurotransmitter release by two distinct membrane- - binding faces of the Munc13–1 C1C2B region. eLife 2021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu J, Camacho M, Xu Y, Esser V, Liu X, Trimbuch T, Pan Y-Z, Ma C, Tomchick DR, Rosenmund C, et al. : Mechanistic Insights into Neurotransmitter Release and Presynaptic Plasticity from the Crystal Structure of Munc13–1 C 1 C 2 BMUN. eLife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Seven AB, Camacho M, Esser V, Xu J, Trimbuch T, Quade B, Su L, Ma C, Rosenmund C, et al. : Functional synergy between the Munc13 C-terminal C1 and C2 domains. eLife 2016, doi: 10.7554/eLife.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, MacHius M, Rizo J, et al. : Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol 2010, 17:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.*.Lipstein N, Chang S, Lin K-H, López-Murcia FJ, Neher E, Taschenberger H, Brose N: Munc13–1 is a Ca2+−phospholipid-dependent vesicle priming hub that shapes synaptic short-term plasticity and enables sustained neurotransmission. Neuron 2021, 109:3980–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Two new mouse models with point mutations in Munc13–1’s C2B domain that abolish or enhance it’s ability to bind calcium where assessed by electrophysiology at the Calyx of Held. The mutation that abolished calcium binding to the C2B domain accelerated depression during trains and slowed recovery from depression. A mutation that enhances calcium binding did the opposite, slowing train depression and accelerating recovery from depression. As measured by action potentials in postsynaptic neurons, altering these short-term plasticity processes profoundly altered the faithfulness and fidelity of transmission at these fast-firing synapses. These findings directly implicate Munc13 as a calcium sensor in short-term plasticity and provide unique evidence that bidirectionally tuning calcium sensing properties has bidirectional effects on short-term plasticity.
- 70.Jackman SL, Turecek J, Belinsky JE, Regehr WG: The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 2016, 529:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huson V, Regehr WG: Diverse roles of Synaptotagmin-7 in regulating vesicle fusion. Curr Opin Neurobiol 2020, 63:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen C, Satterfield R, Young SM, Jonas P: Triple Function of Synaptotagmin 7 Ensures Efficiency of High-Frequency Transmission at Central GABAergic Synapses. Cell Rep 2017, 21:2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turecek J, Regehr XWG: Synaptotagmin 7 Mediates Both Facilitation and Asynchronous Release at Granule Cell Synapses. J Neurosci 2018, 38:3240–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turecek J, Jackman SL, Regehr WG: Synaptotagmin 7 confers frequency invariance onto specialized depressing synapses. Nature 2017, 551:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Courtney KC, Vevea JD, Li Y, Wu Z, Zhang Z, Chapman ER: Synaptotagmin 1 oligomerization via the juxtamembrane linker regulates spontaneous and evoked neurotransmitter release. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.**.Weingarten DJ, Shrestha A, Kissiwaa SA, Spruston E, Jackman SL: Fast resupply of synaptic vesicles requires Synaptotagmin-3. bioRxiv 2021, [DOI] [PubMed] [Google Scholar]; This study shows that the high-affinity calcium sensor synaptotagmin 3 is important to resist depression during and recover from depression after train stimulation at the Calyx of Held and cerebellar climbing fiber synapses. Deleting syt3 or preventing calcium binding renders these processes calcium-insensitive. While these synapses normally depress, they can facilitate at subphsyiological external calcium concentrations; this facilitation also depends on syt3. Computational models in which syt3 accelerates vesicle docking can account for the wild-type and knockout data, but models in which syt3 increases release probability of docked vesicles cannot. These data reveal another high-affinity calcium sensor that may operate in tandem with or in place of others depending on the synapse, and are consistent with the idea that facilitation and resisting/recovering from depression are at least in part manifestations of the same process: calcium-accelerated docking.
- 77.Lee JS, Ho WK, Lee SH: Actin-dependent rapid recruitment of reluctant synaptic vesicles into a fast-releasing vesicle pool. Proc Natl Acad Sci U S A 2012, 109:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’neil SD, Rácz B, Brown WE, Gao Y, Soderblom EJ, Yasuda R, Soderling SH: Action potential-coupled rho gtpase signaling drives presynaptic plasticity. eLife 2021, doi: 10.7554/eLife.63756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maschi D, Gramlich MW, Klyachko VA: Myosin V functions as a vesicle tether at the plasma membrane to control neurotransmitter release in central synapses. eLife 2018, doi: 10.7554/eLife.39440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gowrisankaran S, Houy S, del Castillo JGP, Steubler V, Gelker M, Kroll J, Pinheiro PS, Schwitters D, Halbsgut N, Pechstein A, et al. : Endophilin-A coordinates priming and fusion of neurosecretory vesicles via intersectin. Nat Commun 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo F, Südhof TC: Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse. Neuron 2017, 94:826–839. [DOI] [PubMed] [Google Scholar]
- 82.Weyrer C, Turecek J, Harrison B, Regehr WG: Introduction of synaptotagmin 7 promotes facilitation at the climbing fiber to Purkinje cell synapse. Cell Rep 2021, 36:109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maus L, Lee C, Altas B, Brose N, Imig C, Cooper BH, Maus L, Lee C, Altas B, Sertel SM, et al. : Ultrastructural Correlates of Presynaptic Functional Heterogeneity in Hippocampal Synapses. Cell Rep 2020, 30:3632–3643.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radhakrishnan A, Li X, Grushin K, Krishnakumar SS, Liu J, Rothman JE: Symmetrical arrangement of proteins under release-ready vesicles in presynaptic terminals. Proc Natl Acad Sci 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lučić V: Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J Cell Biol 2010, 188:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tao C-L, Liu Y-T, Sun R, Zhang B, Qi L, Shivakoti S, Tian C-L, Zhang P, Lau P-M, Zhou ZH, et al. : Differentiation and characterization of excitatory and inhibitory synapses by cryo-electron tomography and correlative microscopy. J Neurosci 2018, 38:1548–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.JLAN Murk, G Posthuma, AJ Koster, HJ Geuze, AJ Verkleij, MJ Kleijmeer, BM Humbel: Influence of aldehyde fixation on the morphology of endosomes and lysosomes: Quantitative analysis and electron tomography. J Microsc 2003, 212:81–90. [DOI] [PubMed] [Google Scholar]
- 88.Smith JE, Reese TS: Use of aldehyde fixatives to determine the rate of synaptic transmitter release. J Exp Biol 1980, 89:19–29. [DOI] [PubMed] [Google Scholar]
- 89.Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET: Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol 2004, 6:1102–1108. [DOI] [PubMed] [Google Scholar]


