Abstract
The sense of touch is ubiquitous in vertebrates, and relies upon the detection of mechanical forces in the skin by the tactile end-organs of low-threshold mechanoreceptors. Significant progress has been made in understanding the mechanism of tactile end-organ function using mammalian models, but the detailed mechanics of touch sensation in Meissner and Pacinian corpuscles, the principal detectors of transient touch and vibration, remain obscure. The avian homologues of these corpuscles present an opportunity for functional study of mechanosensation in these structures, due to their relative accessibility and high abundance in the bill skin of tactile foraging waterfowl. Here, we review the current knowledge of mechanosensory end-organs in birds, and highlight the utility of the avian model to understand general principles of touch detection in glabrous skin of vertebrates.
Introduction
The sensation of non-painful touch in vertebrates is mediated by low threshold mechanoreceptors (LTMRs), pseudounipolar somatosensory neurons originating from the dorsal root ganglia or the trigeminal ganglia. Somatosensory afferents projecting from LTMRs detect mechanical stimuli in the skin and transmit tactile information through the corresponding somatosensory pathways in the central nervous system. In glabrous skin, such as that covering the human palm, LTMRs form four types of terminal end-organs: the Pacinian corpuscle, the Meissner corpuscle, the Ruffini corpuscle, and the Merkel cell-neurite complex. Comprehensive reviews on these mechanoreceptors and mechanotransduction in mammals can be found elsewhere [1–4]. Here we will focus on the current knowledge of these mechanoreceptors in birds, with an emphasis on the avian homologues of Pacinian and Meissner corpuscles, the principal detectors of transient touch and vibration. Furthermore, we will highlight the potential of avian model organisms to contribute to our understanding of each tactile end-organ and vertebrate mechanosensation as a whole.
While much of what is known about the structure and function of LTMRs has been acquired from research on mammals, birds provide a particularly useful and underutilized model to study tactile sensation. Many mechanosensory mechanisms and structures are conserved across vertebrates. Similar to mammalian mechanoreceptors, avian LTMRs can be classified as slowly-adapting (SA) or rapidly-adapting (RA) receptors, depending on their firing pattern in response to mechanical stimulation. The most prominent and well-studied end-organs in birds are Grandry and Herbst corpuscles, which are innervated by RA-LTMRs, and are structurally and functionally analogous to, respectively, Meissner and Pacinian corpuscles found in mammals [5–7]. Merkel cell-neurite complexes and Ruffini corpuscles, which are innervated by SA-LTMRs, are also found in birds, although their structure, location, and density may vary among species [8].
Mechanosensory end-organs are complex structures composed of the LTMR afferent and non-neuronal cells, such as Merkel cells or various types of lamellar cells. Traditionally, the afferent has been thought of as the sole mechanoreceptive entity within end-organs, whereas the non-neuronal components have been thought to play supportive and regulatory roles [9]. However, studies in mice have revealed that Merkel cells detect touch and modulate the function of the afferent [10–12], challenging this traditional view. Meanwhile, the role of the non-neuronal components in Meissner and Pacinian corpuscles is less clear, because these structures are poorly accessible to direct functional studies in mouse skin. In precocial birds, such as ducks, the development of the somatosensory system largely completes before hatching [13,14], which permits electrophysiological analysis of the non-neuronal components of mechanosensory end-organs using the skin of late-stage embryos [7]. Because of this advantage, birds provide a unique opportunity to study LTMRs and understand the basic principles of touch detection in vertebrates.
Mechanosensation in avian species
The sense of touch is indispensable to the survival of many forms of life, and birds are no exception. Just as some fish, reptiles, and mammals have evolved specialized mechanosensory organs [15–17], some birds have done so as well. In many avian species, the bill or beak has emerged as this tactile organ, and a fine sense of touch may extend to the tongue [18–21] and oropharynx [22]. In many waterfowl of the Anatidae family, which includes ducks (Figure 1a), swans, and geese, Grandry and Herbst corpuscles are present at an exceptionally high density in the skin covering the bill (Figure 1b–c), tongue and oral cavity, and are thought to underlie sophisticated tactile-based foraging [17,23–25]. In the Mallard duck (Anas platyrhynchos), bill-localized mechanosensation is required for effective feeding in a dark environment, where the sense of touch dominates foraging behavior [26]. Similarly, the New Zealand kiwi (Apteryx mantelli) relies upon tactile foraging in lieu of visually-guided foraging, utilizing the mechanoreceptors and corpuscles located in its bill [27,28]. Behavioral and anatomical evidence for a specialized mechanosensory bill/beak also exists in other birds, including emus, ostriches [22], finches [29–31], ibises [32], parrots [33], and various shorebirds [34], but mechanosensation may be enhanced in some species compared to others. Indeed, tactile specialist birds have a significantly higher proportion of mechanoreceptors in their trigeminal ganglia compared to visual foragers [35], and display a larger volume of the trigeminal nucleus, the brainstem region that perform the initial processing of sensory information from trigeminal LTMRs [36,37]. Consequently, in many of these specialist species, especially tactile-foraging ducks, the mechanosensory function of the bill can be considered equivalent to that of the human hand. As most of our understanding of the physiology of peripheral mechanotransduction in the skin of birds comes from tactile specialist waterfowl, we will focus on them when discussing the function of cutaneous LTMRs.
Figure 1. The structure and function of Grandry and Herbst corpuscles within the mechanosensory bill of waterfowl.
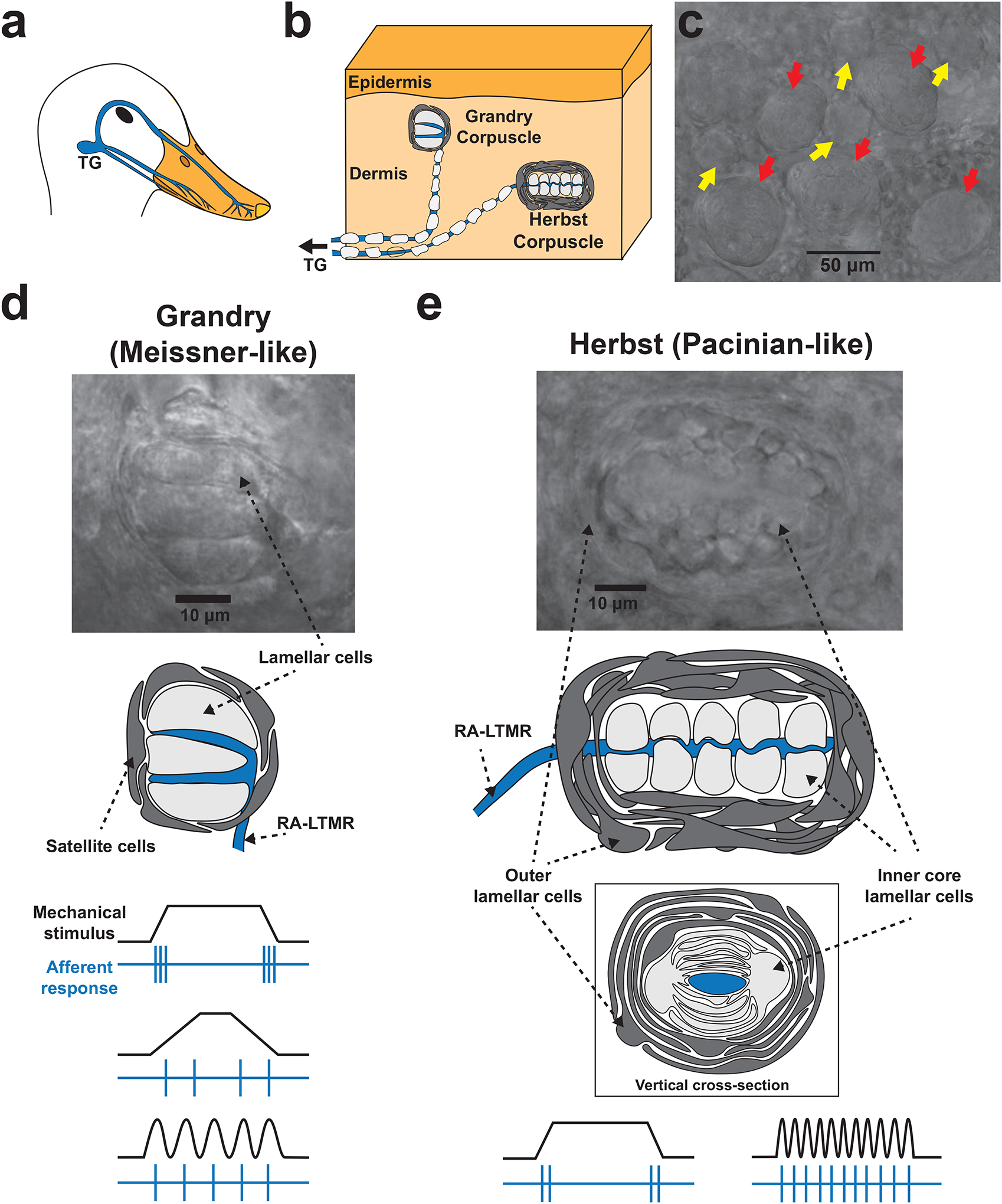
(a) The neuroanatomy underlying tactile sensation in the bill. LTMRs from the trigeminal ganglia (TG) project to the skin of the bill. (b) Trigeminal LTMRs form terminal end-organs in the bill dermis, most of which are Grandry and Herbst corpuscles. (c) Image of the bill dermis under a brightfield microscope. Grandry (yellow arrows) and Herbst (red arrows) corpuscles are present at a cumulative density of up to 200 corpuscles per square millimeter of skin, and can be easily distinguished by size and morphology. (d) Higher magnification image and diagram of a Grandry corpuscle. The Grandry corpuscle is composed of 2–12 lamellar cells which are layered above and below the terminals of the RA-LTMR. The structure is encapsulated by satellite cells. Below the diagram, example stimuli (black) and LTMR afferent responses (blue) are shown. The LTMR of the Grandry corpuscle detects changes in transient force, low frequency vibration, and velocity; the impulses/second of the LTMR response increase with increasing velocity of the mechanical stimulus. (e) Higher magnification image and diagram of a Herbst corpuscle. The Herbst corpuscle is composed of an outer capsule formed by outer core lamellar cells, which encloses an inner core comprised of inner core lamellar cells. The outer core and inner core lamellar cells form concentric lamellae surrounding the mechanoreceptor afferent. Below the diagram, example stimuli (black) and LTMR afferent responses (blue) are shown. The LTMR of Herbst corpuscles detects transient force and high frequency vibration.
Importantly, the major molecular mechanism of mechanotransduction in LTMRs appears to be conserved between birds and mammals. In Pekin ducks, the domesticated descendants of the Mallard, the mechanosensitive ion channel Piezo2 [38] mediates a portion of the excitatory mechanically activated (MA) current in somatosensory neurons [14], and is likely responsible for mechanotransduction in a subset of avian LTMRs, as it is in mice [39]. There is a significant increase in the duration of Piezo2-mediated MA current in duck mechanoreceptors compared to mice [14], the consequences of which are not yet clear, but it is possible that the longer-lasting MA current increases the chance of action potential firing in response to a light mechanical stimulus. When assayed in heterologous cells, most functional properties of duck Piezo2 appear to be highly conserved with mammalian orthologues, including fast inactivation [14] and cold-induced potentiation [40] of MA current. Additionally, Piezo2 is expressed in a large proportion of duck TG neurons, which display larger MA currents compared to other birds and mice [35,41]. This likely reflects the incredibly high density of trigeminal RA-LTMRs and associated sensory corpuscles in the bill (Figure 1b,c). Because of these conserved mechanisms and physiological advantages, investigation into the LTMRs and end-organs of avian tactile specialists may yield valuable insight into the function of these structures across vertebrates.
Grandry (Meissner-like) corpuscle
Grandry corpuscles are ovoid end-organs innervated by RA-LTMRs that detect velocity and low frequency vibration (Figure 1d) [5,6]. These structures are found in the bill of waterfowl at a density up to 65 corpuscles per square millimeter [23]. Grandry corpuscles contain as few as two lamellar cells that surround the nerve ending [7], although this number can be as high as 12 in some species [6]. The lamellar cells are derived from Schwann cells and form a stacked column in which nerve endings are sandwiched between pairs of cells, altogether encapsulated by satellite cells [13,23,42]. Similar formations called “Merkel corpuscles” have been previously characterized in nonaquatic birds, but these often bear a striking morphological resemblance to Grandry and Meissner corpuscles [31] and they are likely variations of the same structure [43] which are distinct from Merkel cell-neurite complexes. As stated previously, Grandry corpuscles are analogous to the Meissner corpuscle, the type II RA-LTMR of mammals.
Similar to the Grandry corpuscle, Meissner corpuscles detect velocity and low frequency vibration [44,45]. These structures are found in the glabrous skin of the human and primate hands [2,46] and mouse paws [47,48]. The nerve ending of the Meissner corpuscle is also surrounded by several lamellar cells of Schwann cell origin [9,49,50]. Interestingly, Meissner corpuscles are often innervated by more than one type of neuron [44,51,52], which can have different responses to the same mechanical stimulus [44]. It remains to be seen whether this multifaceted innervation and divergent physiology is present in the Grandry corpuscle of birds. Additionally, mammalian Meissner corpuscles are innervated by RA-LTMR afferents that express the mechanically gated ion channel Piezo2 [39,53]. Even though Piezo2 is expressed in ~70% of trigeminal neurons of the tactile specialist Pekin duck [14], whether Grandry corpuscles are innervated by Piezo2-expressing afferents remains to be tested.
Lamellar cells in Grandry and Meissner corpuscles are thought to be important for their function, though their exact role is currently obscure. The afferent is typically considered the sole entity within corpuscles that detects touch, whereas the role of lamellar cells is thought be auxiliary and supportive. A recent study showed USH2A, a putative tether protein which is implicated in human hearing and inner ear function [54,55], is not only expressed in lamellar cells of Meissner corpuscles, but necessary for vibration detection and proper function of the mechanoreceptor in mice [45]. This suggests that the lamellar cell may act as an anchor that supports mechanotransduction in the nerve ending by a yet unknown mechanism. Alternatively, or in addition, lamellar cells could actively participate in touch detection, and shape the response of the afferent, similar to the paradigm discovered in Merkel cell-neurite complexes, whereby both the Merkel cells and the afferent detect touch [10–12]. This idea is supported by a recent electrophysiological investigation of the lamellar cells of Grandry corpuscles in duck bill skin [7]. It was shown that mechanical stimulation triggers depolarization in lamellar cells via opening of mechanically gated ion channels of a yet unidentified type. This leads to generation of action potentials in lamellar cells via R-type voltage-gated calcium channels. Although lamellar cells detect touch, it remains unclear how, if at all, they communicate the tactile information with the afferent. Numerous large dense-core vesicles in these cells can be detected in the cytosol of lamellar cells using electron microscopy [7,56], raising the possibility that the lamellar cells help transduce mechanical forces and induce/modulate the afferent response via a chemical secretory mechanism, similar to the Merkel cell-neurite complex characterized in mice [57,58].
Herbst (Pacinian-like) corpuscle
In many birds, including tactile specialist waterfowl, Herbst corpuscles are the most common end-organ encountered in the skin. Herbst corpuscles are present throughout the class Aves [6,18,22,23,29,30,32,34,59–61], but as mentioned previously, have an incredibly high density in the bills of ducks [23] and geese [6], up to 140 corpuscles per square millimeter. Herbst (and Pacinian) corpuscles are ellipsoid structures composed of multiple layers of lamellar cells that surround the terminal of RA-LTMRs (distinct from Grandry-innervated RA-LTMRs), which detect high frequency vibration (Figure 1e). An outer capsule is formed by several concentric lamellae made up of flattened cells referred to as “outer core lamellar cells.” This capsule surrounds the inner core, a bilateral row of Schwann cell-derived “inner core lamellar cells” which extend complex, interdigitating lamellae around the nerve terminal at the center of the corpuscle [56].
Similarly, the mammalian Pacinian corpuscle has an encapsulated, lamellar structure and is present at varying densities in glabrous skin and other tissues, depending on the species. These corpuscles are found in the human hand [2,62], the mesentery of cats [63], and the foot/paw of many other mammals, including dogs [64], raccoons [65], and elephants [66]. However, Pacinian corpuscles are restricted to the joints [67] and periosteum [68] of rodents and are less experimentally accessible compared to the Herbst corpuscles in the bill of waterfowl [6,23]. Physiological data and computational models show that despite differences in size and number of outer lamellae, both the mammalian Pacinian corpuscle and avian Herbst corpuscle are rapidly adapting and tuned to high frequency vibration [5,69–71]. Thus, though there is some structural variation between the end-organs, their basic microanatomical features and functional roles are very similar.
The lamellar cells of the Herbst and Pacinian corpuscles are thought to be an integral passive component responsible for shaping the mechanical forces experienced by the nerve at the center of the structure. Removing the outer layers of these cells from the end-organ prolongs the generator potential produced by mechanically gated channels in the neuron of cat Pacinian corpuscles [72]. At the same time, lamellar cells possibly play a more active role in lamellated corpuscle signal transduction. Potential evidence for such a role exists in the Herbst corpuscle; lamellar cells of the outer layers of this corpuscle respond to touch in the form of MA current [7]. However, these lamellar cells lack voltage-gated ion channels and are located far from the neuron, so the functional consequences of their mechanosensitivity is unclear. It is possible that the MA current in these cells is important for mechanotransduction of the corpuscle as a whole, but this remains to be shown. Further work in this system is warranted to investigate the functional role of these lamellar cells in Herbst/Pacinian corpuscles and identify the molecule(s) responsible for their MA current.
The lamellar cells of the inner core of the Herbst corpuscle are also potentially critical: they form close junctions with the afferent via a complex network of thin lamellae. Additionally, these inner core cells express various calcium binding proteins [73,74] and the ion channels Trpv4 and Asic2 [75], which could have some physiological purpose within the corpuscle. Immunohistochemical and functional studies suggest a possible mechanochemical synaptic-like communication between inner core cells and the afferent in Pacinian corpuscles [76,77], but direct evidence for active touch detection in inner core cells is missing. Importantly, the structure of the inner core is mostly complete at embryonic days 24–28 in ducks [78], the time at which Herbst corpuscles are functional. At this stage, electron micrographs show vesicles and high density junctions located near membranes of the inner core, though it is unclear if these structures are located in the nerve terminal or lamellae of inner core cells [79]. While the outer lamellar cells could previously be removed from the cat Pacinian corpuscle, it was impossible to remove all of the inner core structure [80], and thus it was impossible to test the function of the inner core cells. The Herbst corpuscle may act as a more flexible experimental medium for investigating the inner core given the corpuscle’s accessibility and density in the duck bill. It would be worthwhile to know whether inner core cells can detect touch, and whether they play a role in shaping afferent responses, given their intimate association with the nerve terminal. However, electrophysiological properties of inner core lamellar cells have not been reported in any species.
Ruffini corpuscle and the Merkel cell-neurite complex
Ruffini corpuscles are traditionally considered to be innervated by type II SA-LTMRs, though this association is questionable due to discrepancies between physiological and anatomical data [81]. These structures are rare and difficult to identify in many species, including humans [81,82], and thus pose a challenge to investigate in any organism. Merkel cell-neurite complexes, on the other hand, are well characterized from studies in mammals, and are known to be innervated by type I SA-LTMRs. Recent work has revealed that in both hair follicles and touch domes of mice, Merkel cells detect mechanical stimuli and help shape the responses of corresponding afferents to touch [10–12]. A chemical synapse between the Merkel cell and the afferent mediates this interaction. In mice, this synapse has been reported to be either serotonergic or adrenergic [57,58]. It is unclear if avian Merkel cells perform a similar function, due to the absence of functional knowledge of these receptors in birds.
While physiological investigation of Ruffini corpuscles and Merkel-cell neurite complexes in birds is lacking, some anatomical and structural studies have been performed. Histological and ultrastructural evidence of Ruffini corpuscles and Merkel cells has been found in bill/beak skin and oral mucosa of the Muscovy duck (Cairina moschata) and Japanese quail (Corturnix coturnix japonica) [8,83], though both end-organs were rarer in the former. Confusion exists around the existence and identity of the avian Merkel cell-neurite complex due to the presence of previously mentioned “Merkel corpuscles,” which more closely resemble mammalian Meissner corpuscles and Grandry corpuscles of waterfowl [31]. Merkel cells can be found independent of these structures in the duck [84]; these cells may be more closely related to the Merkel cell-neurite complex seen in mammals. In the shoulder joint of the domestic pigeon (Columba livia domestica), Ruffini corpuscles were also identified [61], but it is unclear how common they are in other anatomical locations such as the beak. Within the avian Ruffini corpuscle, the nerve terminal forms a complex branching structure surrounded by specialized terminal Schwann cells and fibrous elements [82,83], components also seen in the Pacinian, Meissner, Herbst, and Grandry corpuscles. The function of these Schwann-like cells of Ruffini corpuscles and Merkel cells in birds is still unknown. Further investigation of the Ruffini corpuscle and the Merkel cell-neurite complex across different species may provide insight into evolutionarily conserved mechanisms of mechanotransduction and physiological properties of these mechanoreceptors.
Concluding remarks
Many questions remain about the precise physiological workings of vertebrate LTMRs. Of particular interest is the role of lamellar cells in the Herbst (Pacinian) and Grandry (Meissner) corpuscles. Thorough functional studies will be required to dissect the cellular and molecular mechanisms by which the neuronal and non-neuronal components of these end-organs interact with each other and transduce mechanical stimuli. Because of the high density and experimental accessibility of Grandry and Herbst corpuscles in tactile-foraging birds, these organisms remain powerful experimental models with which to study rapidly adapting mechanoreceptors. Avian species will be further useful for exploring conserved mechanisms of mechanosensation found throughout various vertebrate organisms and touch receptors.
Highlights.
The neurophysiology of touch sensation is conserved across birds and mammals
Bird mechanoreceptors innervate Grandry (Meissner) and Herbst (Pacinian) corpuscles
Grandry and Herbst corpuscles densely populate the bill skin of tactile foragers
Grandry and Herbst corpuscles detect transient touch, velocity, and vibration
Lamellar cells are critical to sensory corpuscle structure and function
Acknowledgements
This work was supported by NSF grant 1754286 to E.O.G., NSF grants 1923127, and NIH grant 1R01NS097547 to S.N.B. We thank Dr. Yury Nikolaev for contributing microscopy images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared.
References
- 1.Cobo R, García-Piqueras J, García-Mesa Y, Feito J, García-Suárez O, Vega JA: Peripheral Mechanobiology of Touch—Studies on Vertebrate Cutaneous Sensory Corpuscles. IJMS 2020, 21:6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobo R, García-Piqueras J, Cobo J, Vega JA: The Human Cutaneous Sensory Corpuscles: An Update. J Clin Med 2021, 10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Handler A, Ginty DD: The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci 2021, 22:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]; The most recent comprehensive review of the current knowledge on the structure and function of mammalian LTMRs in both hairy and glabrous skin. Mechanisms of mechanotransduction are also discussed.
- 4.Moehring F, Halder P, Seal RP, Stucky CL: Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 2018, 100:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottschaldt K-M: The physiological basis of tactile sensibility in the beak of geese. J Comp Physiol 1974, 95:29–47. [DOI] [PubMed] [Google Scholar]
- 6.Gottschaldt K-M, Lausmann S: The peripheral morphological basis of tactile sensibility in the beak of geese. Cell Tissue Res 1974, 153:477–96. [DOI] [PubMed] [Google Scholar]
- 7**.Nikolaev YA, Feketa VV, Anderson EO, Schneider ER, Gracheva EO, Bagriantsev SN: Lamellar cells in Pacinian and Meissner corpuscles are touch sensors. Sci Adv 2020, 6:abe6393. [DOI] [PMC free article] [PubMed] [Google Scholar]; By gaining electrophysiological access to lamellar cells of mechanosensory corpuscles in embryonic duck bill skin, the study reveals that outer core lamellar cells of Herbst/Pacinian corpuscles are mechanosensitive, whereas lamellar cells of Grandry/Meissner corpuscles are mechanosensitive and excitable.
- 8.Soliman SA, Madkour FA: A comparative analysis of the organization of the sensory units in the beak of duck and quail. Histology, Cytology and Embryology 2017, 1:1–16. [Google Scholar]
- 9.Cobo R, García-Mesa Y, García-Piqueras J, Feito J, Martín-Cruces J, García-Suárez O, Vega JA: The Glial Cell of Human Cutaneous Sensory Corpuscles: Origin, Characterization, and Putative Roles. IntechOpen; 2020. [Google Scholar]
- 10.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG: Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 2014, 157:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo S-H, Ranade S, Patapoutian A, et al. : Epidermal Merkel Cells are Mechanosensory Cells that Tune Mammalian Touch Receptors. Nature 2014, 509:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. : Piezo2 is required for Merkel cell mechanotransduction. Nature 2014, 509:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxod R: Development of Cutaneous Sensory Receptors Birds. In Development of Sensory Systems. Edited by Bate CM, Carr VMcM, Graziadei PPC, Hirsch HVB, Hughes A, Ingle D, Leventhal AG, Monti Graziadei GA, Rubel EW, Saxod R, et al. Springer; 1978:337–417. [Google Scholar]
- 14**.Schneider ER, Anderson EO, Mastrotto M, Matson JD, Schulz VP, Gallagher PG, LaMotte RH, Gracheva EO, Bagriantsev SN: Molecular basis of tactile specialization in the duck bill. Proc Natl Acad Sci U S A 2017, 114:13036–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors present late-stage duck embryos as a model to study mechanosensation, and demonstrate that Piezo2 underlies a portion of MA current in duck trigeminal neurons.
- 15.Amey-Özel M, von der Emde G, Engelmann J, Grant K: More a finger than a nose: the trigeminal motor and sensory innervation of the Schnauzenorgan in the elephant-nose fish Gnathonemus petersii. J Comp Neurol 2015, 523:769–789. [DOI] [PubMed] [Google Scholar]
- 16.Catania KC: Tactile sensing in specialized predators - from behavior to the brain. Curr Opin Neurobiol 2012, 22:251–258. [DOI] [PubMed] [Google Scholar]
- 17.Schneider ER, Gracheva EO, Bagriantsev SN: Evolutionary Specialization of Tactile Perception in Vertebrates. Physiology (Bethesda) 2016, 31:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbasabadi BM, Sayrafi R: Histomorphological features of the tongue of the Eurasian teal (Anas crecca). Anatomia, Histologia, Embryologia 2018, 47:119–123. [DOI] [PubMed] [Google Scholar]
- 19.Crole MR, Soley JT: Morphology of the tongue of the emu (Dromaius novaehollandiae). II. Histological features. Onderstepoort J Vet Res 2009, 76:347–361. [DOI] [PubMed] [Google Scholar]
- 20.Skieresz-Szewczyk K, Jackowiak H: Morphofunctional study of the tongue in the domestic duck (Anas platyrhynchos f. domestica, Anatidae): LM and SEM study. Zoomorphology 2016, 135:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackowiak H, Skieresz-Szewczyk K, Godynicki S, Iwasaki S, Meyer W: Functional morphology of the tongue in the domestic goose (Anser anser f. domestica). Anat Rec (Hoboken) 2011, 294:1574–1584. [DOI] [PubMed] [Google Scholar]
- 22.Crole MR, Soley JT: Comparative Distribution and Arrangement of Herbst Corpuscles in the Oropharynx of the Ostrich (Struthio camelus) and Emu (Dromaius novaehollandiae). The Anatomical Record 2014, 297:1338–1348. [DOI] [PubMed] [Google Scholar]
- 23.Berkhoudt H: The Morphology and Distribution of Cutaneous Mechanoreceptors (Herbst and Grandry Corpuscles) in Bill and Tongue of the Mallard (Anas Platyrhynchos L.). Netherlands Journal of Zoology 1980, 30:1–34. [Google Scholar]
- 24.Zweers GA: Structure, Movement, and Myography of the Feeding Apparatus of the Mallard (Anas Platyrhynchos L.) a Study in Functional Anatomy. Netherlands Journal of Zoology 1973, 24:323–467. [Google Scholar]
- 25.Avilova KV, Fedorenko AG, Lebedeva NV: The Mechanoreceptor Organs of the Lamellirostral Birds (Anseriformes, Aves). Biol Bull Russ Acad Sci 2018, 45:51–60. [Google Scholar]
- 26.Avilova K: The feeding behavior of the Mallard (Anas platyrhynchos) in the darkness. Sensory systems 2017, 31:139–143. [Google Scholar]
- 27.Martin GR, Wilson K-J, Martin Wild J, Parsons S, Fabiana Kubke M, Corfield J: Kiwi Forego Vision in the Guidance of Their Nocturnal Activities. PLoS One 2007, 2:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham S, Castro I, Alley M: A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. Journal of Anatomy 2007, 211:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genbrugge A, Adriaens D, De Kegel B, Brabant L, Van Hoorebeke L, Podos J, Dirckx J, Aerts P, Herrel A: Structural tissue organization in the beak of Java and Darwin’s finches. J Anat 2012, 221:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoshima K, Seta Y, Shimamura A: Fine Structure of the Herbst Corpuscles in the Lingual Mucosa of the Finch, Lonchura striata. Arch Histol Cytol 1992, 55:321–331. [DOI] [PubMed] [Google Scholar]
- 31.Toyoshima K, Shimamura A: Ultrastructure of Merkel corpuscles in the tongue of the finch, Lonchura striata. Cell Tissue Res 1991, 264:427–436. [Google Scholar]
- 32.Cunningham SJ, Alley MR, Castro I, Potter MA, Cunningham M, Pyne MJ: Bill Morphology of Ibises Suggests a Remote-Tactile Sensory System for Prey Detection - La Morfología del Pico de los Ibises Sugiere un Sistema Sensorial Táctil Remoto para la Detección de las Presas. The Auk 2010, 127:308–316. [Google Scholar]
- 33.Demery ZP, Chappell J, Martin GR: Vision, touch and object manipulation in Senegal parrots Poicephalus senegalus. Proc Biol Sci 2011, 278:3687–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham SJ, Corfield JR, Iwaniuk AN, Castro I, Alley MR, Birkhead TR, Parsons S: The Anatomy of the bill Tip of Kiwi and Associated Somatosensory Regions of the Brain: Comparisons with Shorebirds. PLOS ONE 2013, 8:e80036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Schneider ER, Anderson EO, Feketa VV, Mastrotto M, Nikolaev YA, Gracheva EO, Bagriantsev SN: A Cross-Species Analysis Reveals a General Role for Piezo2 in Mechanosensory Specialization of Trigeminal Ganglia from Tactile Specialist Birds. Cell Rep 2019, 26:1979–1987.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparison across numerous species shows that an increased proportion of mechanoreceptors in TG, and concurrent decrease in nociceptors and thermoreceptors, correlates with higher Piezo2 expression in somatosensory neurons of tactile specialists compared to visual foragers.
- 36.Gutiérrez-Ibáñez C, Iwaniuk AN, Wylie DR: The Independent Evolution of the Enlargement of the Principal Sensory Nucleus of the Trigeminal Nerve in Three Different Groups of Birds. BBE 2009, 74:280–294. [DOI] [PubMed] [Google Scholar]
- 37.Iwaniuk AN, Wylie DR: Sensory systems in birds: What we have learned from studying sensory specialists. Journal of Comparative Neurology 2020, 528:2902–2918. [DOI] [PubMed] [Google Scholar]
- 38.Syeda R: Physiology and Pathophysiology of Mechanically Activated PIEZO Channels. Annu Rev Neurosci 2021, 44:383–402. [DOI] [PubMed] [Google Scholar]
- 39.Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, et al. : Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng W, Nikolaev YA, Gracheva EO, Bagriantsev SN: Piezo2 integrates mechanical and thermal cues in vertebrate mechanoreceptors. Proc Natl Acad Sci U S A 2019, 116:17547–17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN: Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Natl Acad Sci U S A 2014, 111:14941–14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idé C, Munger BL: A cytologic study of Grandry corpuscle development in chicken toe skin. J Comp Neurol 1978, 179:301–324. [DOI] [PubMed] [Google Scholar]
- 43.Saxod R: Ontogeny of the cutaneous sensory organs. Microscopy Research and Technique 1996, 34:313–333. [DOI] [PubMed] [Google Scholar]
- 44**.Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang Q, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, et al. : Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 2020, 368:eabb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mice deficient in Meissner corpuscles exhibit severe deficits in detecting gentle touch in glabrous skin, and show compromised sensory motor control. Dual innervation of Meissner corpuscles in mice is shown; distinct Ret+ and TrkB+ afferents intermingle with lamellar cells.
- 45**.Schwaller F, Bégay V, García-García G, Taberner FJ, Moshourab R, McDonald B, Docter T, Kühnemund J, Ojeda-Alonso J, Paricio-Montesinos R, et al. : USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans. Nat Neurosci 2021, 24:74–81. [DOI] [PubMed] [Google Scholar]; The mammalian tether-like protein USH2A is necessary in the lamellar cells of Meissner corpuscles for detection of low frequency vibrations. Additionally, USH2A is expressed in hair follicles and is important for vibration detection in these structures as well.
- 46.Verendeev A, Thomas C, McFarlin SC, Hopkins WD, Phillips KA, Sherwood CC: Comparative analysis of Meissner’s corpuscles in the fingertips of primates. J Anat 2015, 227:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walcher J, Ojeda-Alonso J, Haseleu J, Oosthuizen MK, Rowe AH, Bennett NC, Lewin GR: Specialized mechanoreceptor systems in rodent glabrous skin. J Physiol 2018, 596:4995–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wai V, Roberts L, Michaud J, Bent LR, Clark AL: The Anatomical Distribution of Mechanoreceptors in Mouse Hind Paw Skin and the Influence of Integrin α1β1 on Meissner-Like Corpuscle Density in the Footpads. Frontiers in Neuroanatomy 2021, 15:628711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Piqueras J, Cobo R, Cárcaba L, García-Mesa Y, Feito J, Cobo J, García-Suárez O, Vega JA: The capsule of human Meissner corpuscles: immunohistochemical evidence. Journal of Anatomy 2020, 236:854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ide C: Histochemical study of lamellar cell development of Meissner corpuscles. Arch Histol Jpn 1982, 45:83–97. [DOI] [PubMed] [Google Scholar]
- 51.Cauna N: Nerve supply and nerve endings in Meissner’s corpuscles. American Journal of Anatomy 1956, 99:315–350. [DOI] [PubMed] [Google Scholar]
- 52.Paré M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL: The Meissner Corpuscle Revised: A Multiafferented Mechanoreceptor with Nociceptor Immunochemical Properties. J Neurosci 2001, 21:7236–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Mesa Y, García-Piqueras J, García B, Feito J, Cabo R, Cobo J, Vega JA, García-Suárez O: Merkel cells and Meissner’s corpuscles in human digital skin display Piezo2 immunoreactivity. J Anat 2017, 231:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al. : Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 1998, 280:1753–1757. [DOI] [PubMed] [Google Scholar]
- 55.Adato A, Lefèvre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C: Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet 2005, 14:3921–3932. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe I, Usukura J, Yamada E: Electron microscope study of the Grandry and Herbst corpuscles in the palatine mucosa, gingival mucosa and beak skin of the duck. Arch Histol Jpn 1985, 48:89–108. [DOI] [PubMed] [Google Scholar]
- 57**.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG: Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA 2016, 113:E5491–E5500. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration that mechanical stimulation of Merkel cells in mouse whisker follicles triggers serotonin release, which binds to ionotropic and metabotropic 5-HT receptors on the LTMR ending, driving afferent spikes in the static and (to a lesser extent) dynamic phases of the stimulus.
- 58**.Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo S-H, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA: MERKEL CELLS ACTIVATE SENSORY NEURAL PATHWAYS THROUGH ADRENERGIC SYNAPSES. Neuron 2018, 100:1401–1413.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that activation of Merkel cells by mechanical stimuli releases neurotransmitter via synaptic vesicles, and adrenergic signaling is required for normal function of Merkel-cell neurite complexes in touch domes in mice.
- 59.Wight PAL, Siller WG, Mackenzie GM: The distribution of herbst corpuscles in the beak of the domestic fowl. British Poultry Science 1970, 11:165–170. [DOI] [PubMed] [Google Scholar]
- 60.Zelená J, Halata Z, Szeder V, Grim M: Crural Herbst corpuscles in chicken and quail: numbers and structure. Anatomy and Embryology 1997, 196:323–333. [DOI] [PubMed] [Google Scholar]
- 61.Halata Z, Munger BL: The ultrastructure of Ruffini and Herbst corpuscles in the articular capsule of domestic pigeon. The Anatomical Record 1980, 198:681–692. [DOI] [PubMed] [Google Scholar]
- 62.Stark B, Carlstedt T, Hallin RG, Risling M: Distribution of human Pacinian corpuscles in the hand. A cadaver study. J Hand Surg Br 1998, 23:370–372. [DOI] [PubMed] [Google Scholar]
- 63.Gray JAB, Malcolm JL, Brown GL: The initiation of nerve impulses by mesenteric Pacinian corpuscles. Proceedings of the Royal Society of London Series B - Biological Sciences 1950, 137:96–114. [DOI] [PubMed] [Google Scholar]
- 64.Rico B, Solas MT, Clément J, Suárez I, Fernández B: Ultrastructural study of the Pacinian corpuscles in the newborn and adult dog forefoot. Eur J Morphol 1996, 34:311–320. [DOI] [PubMed] [Google Scholar]
- 65.Rice FL, Rasmusson DD: Innervation of the digit on the forepaw of the raccoon. Journal of Comparative Neurology 2000, 417:467–490. [DOI] [PubMed] [Google Scholar]
- 66.Bouley DM, Alarcón CN, Hildebrandt T, O’Connell-Rodwell CE: The distribution, density and three-dimensional histomorphology of Pacinian corpuscles in the foot of the Asian elephant (Elephas maximus) and their potential role in seismic communication. J Anat 2007, 211:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi S: Pacinian corpuscles in the articular capsule of the mouse knee joint, with special reference to postnatal development. Hokkaido Igaku Zasshi 1995, 70:159–173. [PubMed] [Google Scholar]
- 68.Zelená J: Rapid degeneration of developing rat Pacinian corpuscles after denervation. Brain Research 1980, 187:97–111. [DOI] [PubMed] [Google Scholar]
- 69.Bell J, Bolanowski S, Holmes MH: The structure and function of pacinian corpuscles: A review. Progress in Neurobiology 1994, 42:79–128. [DOI] [PubMed] [Google Scholar]
- 70.Gregory JE: An electrophysiological investigation of the receptor apparatus of the duck’s bill. J Physiol 1973, 229:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Quindlen-Hotek JC, Bloom ET, Johnston OK, Barocas VH: An inter-species computational analysis of vibrotactile sensitivity in Pacinian and Herbst corpuscles. Royal Society Open Science 2020, 7:191439. [DOI] [PMC free article] [PubMed] [Google Scholar]; Computational models of lamellated corpuscles in 19 different species are generated, and the varying morphologies of each corpuscle are compared. Despite these differences, each model predicts a similar range of frequencies at which the LTMR responds to vibratory stimuli.
- 72.Loewenstein WR, Mendelson M: Components of receptor adaptation in a Pacinian corpuscle. J Physiol 1965, 177:377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valle MED, Ciriaco E, Bronzetti E, Albuerne M, Naves FJ, Germana G, Vega JA: Calcium-binding Proteins in Avian Herbst and Grandry sensory corpuscles. The Anatomical Record 1995, 243:272–281. [DOI] [PubMed] [Google Scholar]
- 74.Chouchkov C, Palov A, Dandov A: Ultrastuctural immunocytochemistry of calcium-binding proteins in rapidly-adapting avian mechanoreceptors. Acta Histochemica 2002, 104:311–320. [DOI] [PubMed] [Google Scholar]
- 75.Cabo R, Gálvez A, Laurà R, José IS, Pastor JF, López-Muñiz A, García-Suárez O, Vega JA: Immunohistochemical Detection of the Putative Mechanoproteins ASIC2 and TRPV4 in Avian Herbst Sensory Corpuscles. The Anatomical Record 2013, 296:117–122. [DOI] [PubMed] [Google Scholar]
- 76.Pawson L, Pack AK, Bolanowski SJ: Possible glutaminergic interaction between the capsule and neurite of Pacinian corpuscles. Somatosens Mot Res 2007, 24:85–95. [DOI] [PubMed] [Google Scholar]
- 77.Pawson L, Prestia LT, Mahoney GK, Güçlü B, Cox PJ, Pack AK: GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J Neurosci 2009, 29:2695–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saxod R: Etude au microscope électronique de l’histogenèse du corpusculé sensoriel cutané de Herbst chez le Canard. Journal of Ultrastructure Research 1970, 33:463–482. [DOI] [PubMed] [Google Scholar]
- 79.Nafstad PHJ, Andersen AE: Ultrastructural investigation on the innervation of the Herbst corpuscle. Z Zellforsch 1970, 103:109–114. [DOI] [PubMed] [Google Scholar]
- 80.Loewenstein WR, Rathkamp R: Localization of Generator Structures of Electric Activity in a Pacinian Corpuscle. Science 1958, 127:341–341. [DOI] [PubMed] [Google Scholar]
- 81.Paré M, Behets C, Cornu O: Paucity of presumptive ruffini corpuscles in the index finger pad of humans. J Comp Neurol 2003, 456:260–266. [DOI] [PubMed] [Google Scholar]
- 82.Cobo R, García-Mesa Y, Cárcaba L, Martin-Cruces J, Feito J, García-Suárez O, Cobo J, García-Piqueras J, Vega JA: Verification and characterisation of human digital Ruffini’s sensory corpuscles. Journal of Anatomy 2021, 238:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halata Z, Grim M: Sensory nerve endings in the beak skin of Japanese quail. Anat Embryol (Berl) 1993, 187:131–138. [DOI] [PubMed] [Google Scholar]
- 84.Toyoshima K: Are Merkel and Grandry Cells Two Varieties of the Same Cell in Birds? Archives of Histology and Cytology 1993, 56:167–175. [DOI] [PubMed] [Google Scholar]


