Abstract
Neurons possess a complex morphology spanning long distances and a large number of subcellular specializations such as presynaptic terminals and dendritic spines. This structural complexity is essential for maintenance of synaptic junctions and associated electrical as well as biochemical signaling events. Given the structural and functional complexity of neurons, neuronal endoplasmic reticulum is emerging as a key regulator of neuronal function, in particular synaptic signaling. Neuronal endoplasmic reticulum mediates calcium signaling, calcium and lipid homeostasis, vesicular trafficking and proteostasis events that underlie autonomous functions of numerous subcellular compartments. However, based on its geometric complexity spanning the whole neuron, endoplasmic reticulum also integrates the activity of these autonomous compartments across the neuron and coordinates their interactions with the soma. In this article, we review recent work regarding neuronal endoplasmic reticulum function and its relationship to neurotransmission and plasticity.
Introduction
Neurons are polarized cells of the nervous system that specialize in the transfer, processing and storage of information. They are the only nervous system cells with excitable membranes. Via synchronized opening of voltage gated ion channels, neurons can generate electrical signals, action potentials, that travel along the cell and its processes. When the action potential reaches the presynaptic boutons in the axon, it triggers calcium influx and subsequent release of small molecules called neurotransmitters, through regulated fusion of synaptic vesicles with the plasma membrane (Figure 1). Neurotransmitters then bind to specific receptors in the juxtaposed postsynaptic membrane at dendrites, and initiate electrical and/or biochemical signaling in the next neuron. Axon and dendrites can reach lengths that are several orders of magnitude longer than the size of the neuronal cell body or soma, and they can also present complex branching patterns, all of this makes neuronal plasma membrane (PM) 3–4 orders of magnitude larger than the membrane of most cells in peripheral organs. Neurons spend a considerable proportion of their ATP production in maintaining the molecular properties of this massive amount of membrane. Moreover, the endoplasmic reticulum (ER) in neurons is continuous and it spans the whole cell volume, from the soma to the most distal dendrites and the complete length of the axon [1–7] (Figure 1); although reversible fragmentation of the ER has been proposed to occur in response to neuronal activity [8, 9]. Thus, the neuronal ER constitutes one of the largest organelles in biology. Here, we review our current understanding of the roles the ER plays in regulating neurotransmission in mature neurons via two central mechanisms: synthesis and trafficking of membrane components and regulation of calcium signaling.
Figure 1. Endoplasmic reticulum and trafficking organelles at a model excitatory synapse.
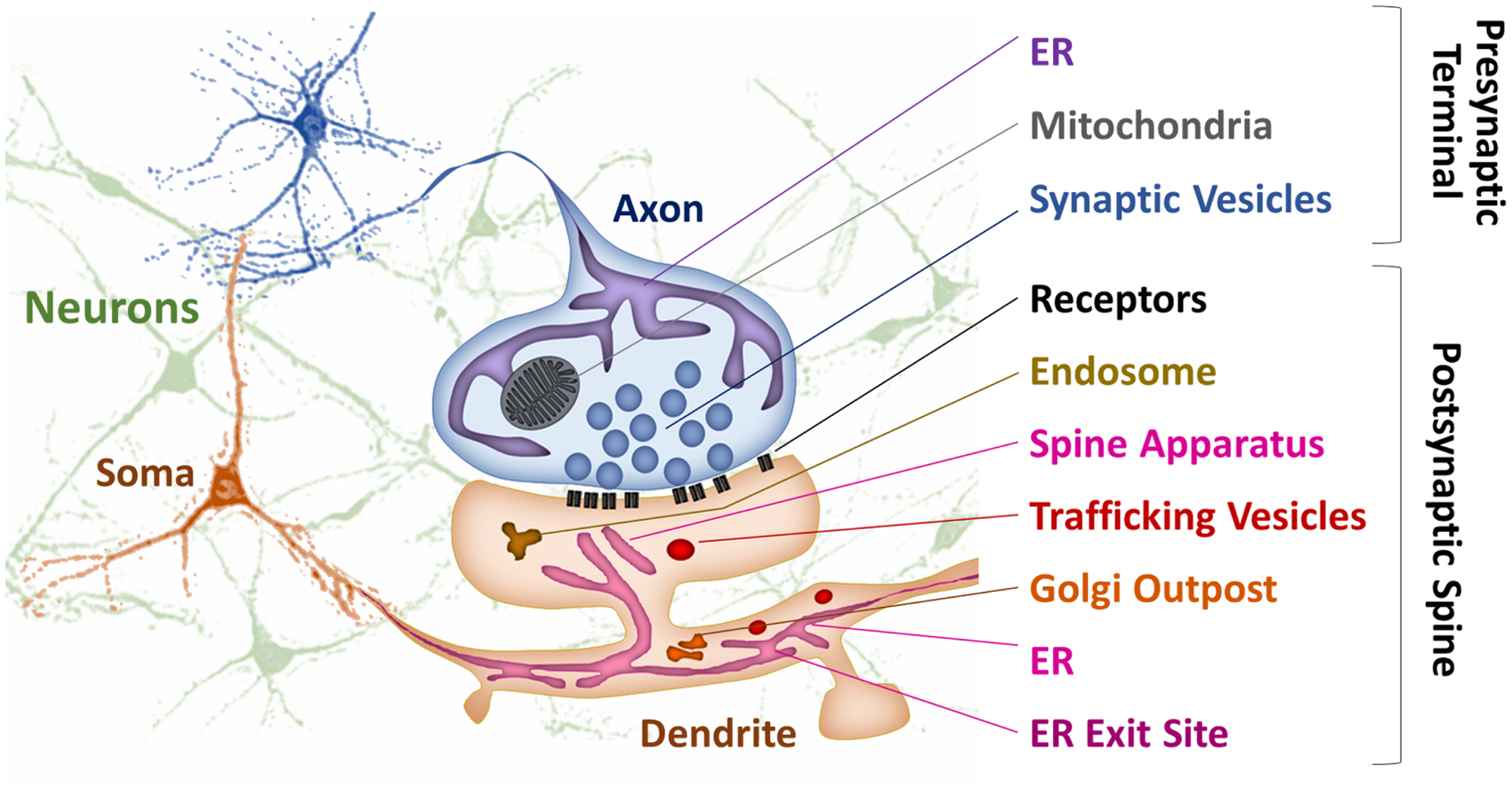
Presynaptic terminals in the axon (in blue) contain tubular ER and are filled with synaptic vesicles, small trafficking organelles that are filled with neurotransmitters. Dendrites (in orange) contain ER, ER exit sites that mediate the delivery of proteins and lipids to the plasma membrane, spine apparatus, endosomes and Golgi satellites and outposts.
The neuronal ER and membrane trafficking for synaptic specifications
Proteins and lipids may travel distances that range from millimeters to centimeters or even up to a meter (e.g. in motor neurons) in order to reach the synapse from the cell body. Moreover, the molecular composition of the soma, the presynaptic axon terminals and postsynaptic dendrites is different [10]. Thus, the enormous distances together with the complex molecular, structural and functional compartmentalization of neurons poses a challenge for the secretory pathway. Neurons overcome this challenge using localized autonomous trafficking pathways at dendrites and axons, which are able to produce, sort, maintain and recycle proteins and lipids independently of the soma (see [11–13]) (Figure 1).
The dendritic secretory pathway and its role in neurotransmission and plasticity
The levels of electrical activity in neurons can shape not only the morphology and composition of the dendritic PM but also the dynamics of internal organelles. In mammalian central synapses, only a fraction of dendritic spines contain ER at any given time point (15–50%; [3, 14]) this dendritic ER however is highly dynamic and over time it will transiently enter and explore most of the spines [15]. The mobility of the ER is positively regulated by neuronal activity and vice versa, manipulating the mobility of the ER can strengthen synapses influencing their capacity to undergo long-term potentiation (LTP) and depression (LTD) in the rodent hippocampus [15]. The ER-mediated modulation of LTP involves a mechanism dependent on the small GTPase Ras and the phosphatidylinositol 3-kinase (PI3K [16]; also see calcium-dependent mechanisms in the next section). Lysosomes, in turn, may modulate LTD at spines via a different pathway [16]. Moreover, spine volume and synapse size become highly correlated after LTP specially at spines that contain ER [17], indicating that the presence of ER can determine the plastic properties of dendritic spines in response to neuronal activity.
The dendritic ER is an important local source of molecules for structural plasticity. The dendritic ER volume decreases after LTP in the rat hippocampus as a consequence of membrane trafficking to the surface to support the generation of new spines [18]. Synapses are enlarged preferentially at spines that contain ER and poli-ribosomes after LTP, which also correlates with the appearance of the spine apparatus [19]. The spine apparatus is an enlargement of the ER that takes the form of stacked sacs separated by dense plates and is enriched in large dendritic spines in mature neurons [6, 20] (Figure 1). Formation and stabilization of the spine apparatus is dependent on the protein synaptopodin, and thus synaptopodin influences spine stability, neuron excitability and memory-related processes [21–23]. The mechanism of spine apparatus remodeling via synaptopodin involves actin cytoskeleton and calcium [24]. However, the molecular mechanisms of spine apparatus remodeling and its role in synaptic plasticity and memory formation remain largely unclear.
Proteins diffuse rapidly along the dendritic ER and accumulate at ER exit sites present at branching points and near spines (Figure 1), due to the increased morphological complexity of the ER at those locations [25]. These local export sites positively regulate dendrite branching and local protein delivery, including surface levels of glutamate ionotropic receptors (specifically α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, AMPAR) [25] thus influencing dendritic morphology and synaptic strength. The ER also supports local translation of synaptic proteins (reviewed in [26–28]) allowing the dynamic modulation of the dendritic proteome in response to different forms of neuronal activity and pathological processes. After synthesis, synaptic proteins in dendrites may follow an atypical, Golgi-independent trafficking pathway. Proteins including AMPAR subunits and the cell-adhesion molecule neuroligin 1, accumulate at recycling endosomes after exiting the dendritic ER and these recycling endosomes mediate their delivery to the surface [12, 29]. Different subunits of the AMPAR, namely GluA1–4, are thought to follow different exit routes from the dendritic ER and also their removal from the PM via endocytosis may be independently modulated (reviewed in [20, 21]). AMPAR receptor assembly occurs in the ER [30, 31], however how receptors of different subunit composition traffic to the dendritic PM or whether assembly or reassembly can also happen at the dendritic PM remains elusive. The atypical secretory pathway at work in dendrites causes the surface N-glycosylation pattern of neuronal proteins to be different, more “immature” compared to other cells (e.g. lacking sialic acid) [10]. However, the Golgi apparatus can fragment and disperse into dendrites in response to neuronal excitation, generating small Golgi satellites that can locally modify glycoproteins and deliver them to the PM [32] and suggesting that the functional properties of surface neuronal glycoproteins can be modulated by changing their glycosylation pattern in response to neuronal activity [12] thus influencing plasticity-related processes.
Less is known about the role of dendritic ER in local lipid metabolism. In highly branched neurons from Drosophila, both the development and maintenance of dendritic morphology and complexity depend on lipid synthesis [33, 34]. Whether local lipid synthesis occurs at dendrites and if it has any impact on neurotransmission remains unknown.
The axonal ER and local synthesis and trafficking of membranes
Similar to dendrites, the lipidic and protein composition of axons differs from the cell body. In neurons from the dorsal root ganglia, axons show a higher protein to lipid ratio and an enrichment in cholesterol relative to other lipids [10]. Axons only contain tubular smooth-looking, anastomosed ER [3] (Figure 1). In peripheral neurons, phospholipids can be synthesized in the axonal ER but not cholesterol, which is mainly produced by glial cells [35, 36] and then incorporated from the extracellular space via lipoproteins [37]. Bioactive lipids such as the endocannabinoid anandamide have also been found to be synthesized and degraded at the ER membrane in hippocampal neurons [38, 39]. Little is known about the occurrence and role of local phospholipid synthesis in axons during neurotransmission, although several lines of work have shown this process to be key for axonal growth and regeneration [40, 41]. A recent study found that blockade of phospholipid biosynthesis, specifically phosphatidylethanolamine and phosphatidylcholine, leads to activity-dependent axonal degeneration and loss of synaptic vesicles in Drosophila photoreceptors [42], emphasizing the importance of lipid synthesis for maintenance of axonal integrity. Biosynthesis of cholesterol has been proposed to be more efficient in developing neurons and restricted to the somatic ER, while mature neurons may need supplementation from surrounding astrocytes [35, 36, 43, 44]. In central and peripheral axons, cholesterol is necessary for proper action potential propagation along the axonal membrane and for the consequent release of neurotransmitters at the synapse [45–48]. Cholesterol-dependent domains at the plasma membrane mediate the clustering of ion channels and receptors, and can modulate their opening probability and conductance [49, 50] (reviewed in [51]). Cholesterol levels modulate SNARE (Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor) mediated fusion [52] and the endocytosis of synaptic vesicle proteins [53]. Furthermore, while cholesterol depletion reduces action potential driven neurotransmitter release, it increases spontaneous neurotransmission in mammalian neurons [48, 54] as well as in other organisms [45, 47], indicating that axonal lipids play a central role in balancing different forms of neurotransmission.
Local protein synthesis at axons has been historically more controversial, mainly due to lack of rough ER at axons. However, a variety of mRNAs are present in axons in the peripheral and central nervous system [55–57]. Ribosomes and the ER molecular machinery for protein translocation, folding and modification have also been found in axons [55, 56], and the pattern of translation at axons can be dynamically modified by neuronal activity [55, 57] indicating that it may serve important roles in information transfer and storage in the brain. Moreover, retrograde axon to soma signaling of proteins locally synthesized at axons may contribute to neurodegeneration [58]. Not only the machinery for translation is present at axons, recent work has shown that newly synthesized proteins, specifically ion channels, can be assembled and trafficked to the plasma membrane in peripheral nerves [59, 60], suggesting that the secretory pathway is present and functional at axons. More research is still necessary to uncover the specific location and molecular mechanism underlying protein synthesis and delivery at axons, and its relevance for the maintenance of neurotransmission. Another open question is whether posttranslational modifications and processing of proteins can occur at axons, including glycosylation.
The neuronal ER and calcium signaling
Neurons have the largest and most complex ER, which can connect the whole cell and at the same time mediate highly localized signals that are segregated from the rest of the neuron [61]. The ER behaves as a calcium sink and a calcium source (see below), clearing or releasing calcium in different circumstances and maintaining homeostatic calcium levels. The ER has the capacity to modulate calcium signals that are involved in different aspects of presynaptic neurotransmitter release and postsynaptic function and thus, it is a key player in neuronal physiology.
Intracellular calcium stores and dendritic functional and morphological plasticity
At mammalian hippocampal glutamatergic synapses, activation of N-methyl-D-aspartate receptors (NMDARs) by glutamate leads to calcium influx into the dendritic spine which in turn triggers calcium-induced calcium release (CICR) from the ER [62, 63]. At rest, activation of this pathway by spontaneous glutamate release blocks the synthesis of AMPARs and thus maintains homeostatic levels of neurotransmission [62]. Spontaneous inhibitory neurotransmission can also modulate calcium signaling at rest at excitatory synapses, leading to changes in gene expression and tuning excitatory synaptic strength [64], indicating that calcium signals can propagate and convey information independently of action potentials. Opening of NMDARs and the consequent CICR during evoked, action potential-driven neurotransmission leads to a reduction in calcium levels in the ER [63]. Depletion of ER calcium activates the ER resident Stromal Interaction Molecule (STIM) which then aggregates at ER-PM junctions and triggers the clustering and opening of Orai calcium channels (also known as CRAC – calcium release-activated channel –) [65]. This mechanism is mediated by the store operated calcium entry (SOCE) pathway. NMDAR and STIM1-dependent SOCE activation at glutamatergic spines as a consequence of CICR leads to inhibition of postsynaptic voltage-gated calcium channels (VGCCs) and gene transcription, regulating the ER content of spines in a frequency-dependent manner [63]. STIM proteins actively modulate neurotransmission by directly activating AMPARs and inhibiting VGCCs and NMDARs [66], they also regulate AMPAR trafficking [67] and can increase the number and stability of mushroom spines via a calcium/calmodulin-dependent protein kinase II (CaMKII) and end-binding protein 3 (EB3)/microtubules dependent pathway [68]. These effects may be primarily driven by STIM2 and Orai1, influencing LTP, LTD and other memory-related processes in the hippocampus [67, 69–73], while STIM1 responds to calcium fluctuations during neuron development and regulates dendritic maturation [74]. Voltage-gated potassium channels and Ryanodine Receptors (RyRs) also colocalize at somatic ER-PM junctions of hippocampal neurons [75], suggesting that multiple calcium and neuronal signaling pathways might interact and crosstalk at these locations.
While RyRs have been implicated in fast calcium sparks in dendrites, CICR via the coincidence detectors IP3Rs can lead to longer lasting calcium signals that can propagate in waves [76]. These dendritic calcium waves can reach very high concentrations (higher than AP-driven calcium) and travel long distances, although they rarely reach the nucleus [76]. Interestingly, calcium waves have mainly been observed in cortical and hippocampal pyramidal neurons, and it remains unknown what role they play in neuronal physiology (for a review see [77]). For example, a recent report showed that propagating calcium signals from dendrites to the cell body are necessary for gene expression in cultured neurons, but calcium waves and CICR were not involved [78].
The dendritic ER is both a source of calcium and a calcium sink that can remove cytoplasmic calcium resulting from neuronal activity via the sarcoendoplasmic reticulum calcium transport ATPase (SERCA) [79].Recent mathematical modeling has suggested that the spine apparatus acts as an important calcium sink at spines [80]. Calcium release from the dendritic ER via inositol 1,4,5-trisphosphate receptors (IP3Rs) enhances postsynaptic responses and it can unsilence synapses via a protein kinase C (PKC) and CaMKII mediated mechanism in hippocampal neurons [81]. In Purkinje cells, CICR via IP3Rs leads to LTD [82]. Interestingly, RyRs and STIM1 also participate in this process [83, 84]. A recent model predicts that SERCA-dependent calcium sequestration determines the type of plasticity that glutamatergic spines will undergo [85], suggesting that the combination of stimulation frequency and duration, probability of neurotransmitter release, opening of inositol 1,4,5-trisphosphate receptors (IP3Rs) and RyRs, together with the level of saturation of SERCA determine if a particular synapse will undergo postsynaptic LTP or LTD (also see [86]). All these findings point to the existence of a dynamic network connecting postsynaptic glutamate receptors, CICR, SOCE and calcium buffers allowing multiple outcomes depending on the type, intensity and duration of neurotransmission.
Little information is available about the role of the dendritic ER in calcium signaling at other, in particular non-glutamatergic, types of synapses. Activation of neurons in the paraventricular nucleus of the hypothalamus by norepinephrine and adrenergic receptors requires CICR [87]. Calcium release from internal stores and SOCE do not seem to modulate spontaneous inhibitory neurotransmission [88–90], although it can potentiate action potential-driven presynaptic GABA and dopamine release in different neuron types and organisms (see next section).
The axonal ER and calcium modulation of neurotransmitter release
Early work proposed that calcium released from internal stores, including the ER and lysosomes, can potentiate neurotransmitter release at hippocampal synapses [91, 92]. Since then, numerous studies have shown that CICR and SOCE amplify calcium signals at axons augmenting spontaneous, synchronous and asynchronous evoked release of glutamate, GABA, dopamine and other neurotransmitters in different regions of the nervous system and model organisms [90, 93–100] (Figure 2). Specific forms of neurotransmission appear to be coupled to different calcium sources and use different calcium sensors (also see [101] and [102]). For example, the glycoprotein reelin activates presynaptic ApoER2 receptors leading to calcium efflux from the ER via IP3Rs and selectively mobilizing a VAMP7-containing pool of synaptic vesicles [103]. This pathway only augments spontaneous neurotransmission but not evoked release. At the zebrafish neuromuscular junction, while synchronous evoked neurotransmission depends on opening of VGCC, asynchronous release is maintained by a “propagating intracellular calcium source” along the axon [100], which appears similar to the IP3R-dependent calcium waves observed in multiple cellular systems (see [77]). Activation of nicotinic acetylcholine receptors in hippocampal neurons triggers CICR via RyRs and leads to synchronized glutamate release and firing of the postsynaptic neuron even in the absence of action potentials [104]. CICR also modulates synaptic vesicle trafficking to maintain tonic activity of auditory hair cells [105]. STIM2-dependent SOCE augments spontaneous release of glutamate but not GABA via the selective activation of the calcium sensor synaptotagmin-7 [90], while STIM1B-mediated SOCE mobilizes the reserve pool of synaptic vesicles to maintain evoked neurotransmission during sustained activity [99]. Spontaneous release of GABA from Purkinje cells is enhanced by calcium release through RyRs [106], while both RyRs and IP3Rs trigger dopamine release from nigrostriatal neurons, independently of influx of extracellular calcium [94] (also see [93] for IP3R-mediated dopamine release in Drosophila). Similarly, calcium release from internal stores also increases the size of the readily releasable pool of synaptic vesicles at glycinergic interneurons of the retina [95]. Accordingly, by regulating the content of tubular ER at axons, autophagy can modulate calcium signaling and glutamate release from neurons [107]. The ER in turn is crucial to provide the components for autophagosome formation (see [108]) suggesting that autophagosomes and ER work together to set axonal properties. Additionally, endogenous mobile calcium buffers differ among glutamatergic and GABAergic presynaptic boutons and may account for differences in the probability of release and levels of asynchronous fusion of synaptic vesicles [109]. Besides the direct boost in calcium caused by opening of ER ionic channels, there are other contributing factors to the potentiation of neurotransmission. SOCE activation, for example, can enhance the membrane depolarization increasing neuron excitability [110, 111] and modulating the synchrony of neuron networks and interictal spikes during seizures [112]. In conclusion, accumulating evidence points to the coupling of different axonal ER-related calcium sources (Figure 2) to segregated forms of neurotransmitter release, and this may vary among different types of synapses. More research is needed to understand the molecular basis and the relevance of these pathways in information processing at the circuit level.
Figure 2. Calcium stores in an excitatory presynaptic terminal.
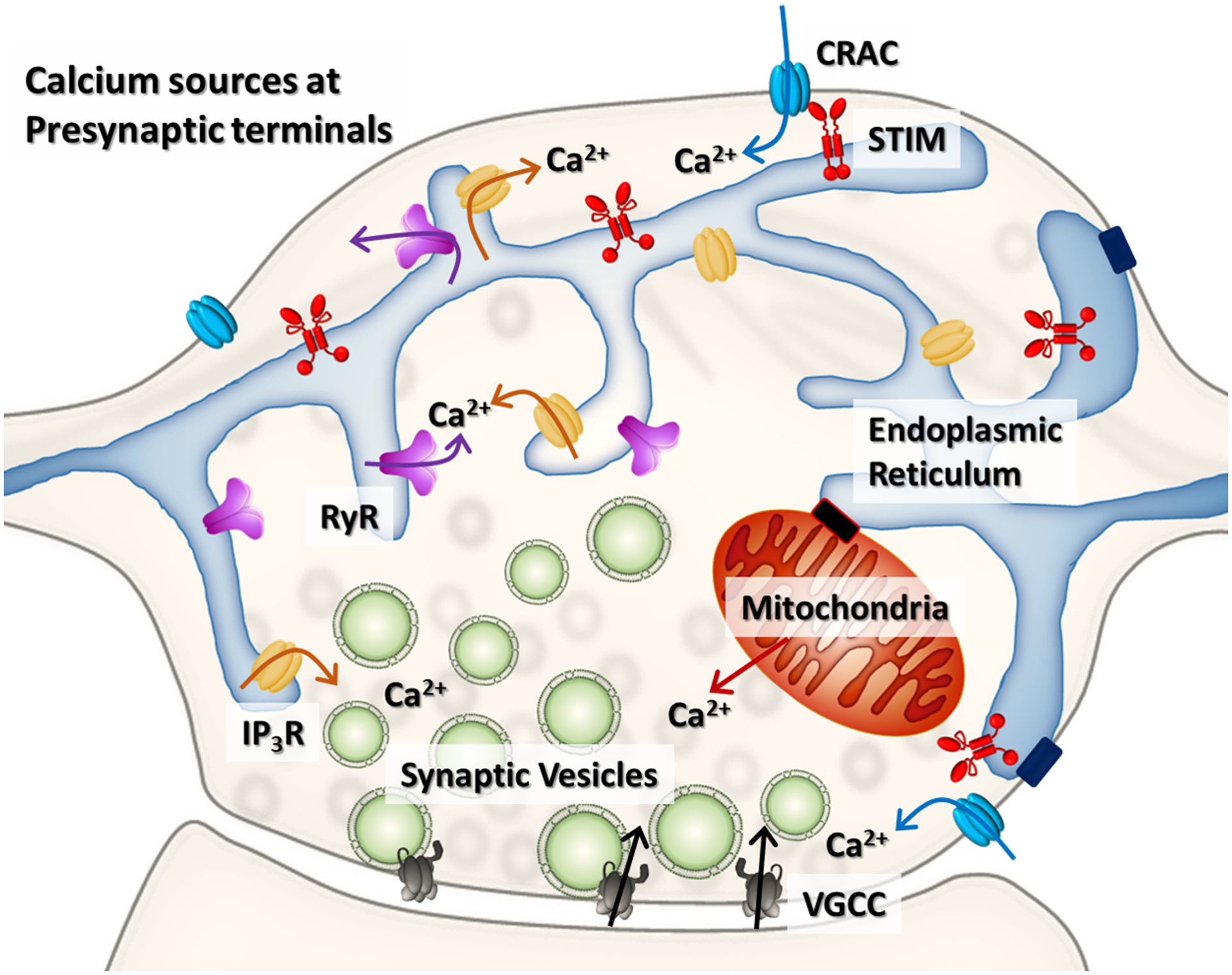
Action potentials gate the opening of VGCC with the consequent influx of extracellular calcium and the synchronized exocytosis of synaptic vesicles. The ER is the main intracellular source of calcium in axons. Calcium can be released via IP3Rs and RyRs during CICR, amplifying action potential-driven signals and neurotransmitter release. The SERCA sequesters calcium into the ER modulating calcium levels in the bouton. When calcium is depleted in the lumen of the ER, SOCE is triggered via STIM-CRAC interaction resulting in calcium influx into the terminal, which augments neurotransmitter release. Mitochondria can also work as a calcium source and a calcium sink (not discussed in this article).
Conclusion
Physiological studies to date have been largely focused on neuronal functional events associated with cellular excitability and synaptic transmission. However, as the emerging and increasingly rich phenomenology of neuronal ER indicates, neurons also harbor an extensive network of intracellular membranous organelles that maintains and integrates signaling events across their complex morphology. Nevertheless, mechanistic details of these ER-associated intracellular neuronal signaling events remain poorly understood. Recent advances in development of super-resolution imaging approaches, novel molecular probes and identification of molecular components that maintain neuronal ER will bring the study of neuronal ER-mediated signaling on par with classical neurophysiology and uncover its essential role in nervous system health and disease.
Acknowledgements
This work was supported by a grant from the National Institute of Mental Health (MH066198) to ETK and a NARSAD young investigator award to NLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no competing interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wang J, Fourriere L, and Gleeson PA, Local Secretory Trafficking Pathways in Neurons and the Role of Dendritic Golgi Outposts in Different Cell Models. Front Mol Neurosci, 2020. 13: p. 597391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy MJ and Hanus C, Architecture and Dynamics of the Neuronal Secretory Network. Annu Rev Cell Dev Biol, 2019. 35: p. 543–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, et al. , Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A, 2017. 114(24): p. E4859–E4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenzuela JI and Perez F, Diversifying the secretory routes in neurons. Front Neurosci, 2015. 9: p. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terasaki M, et al. , Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci U S A, 1994. 91(16): p. 7510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spacek J and Harris KM, Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci, 1997. 17(1): p. 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MK, Petersen OH, and Tepikin AV, The endoplasmic reticulum as one continuous Ca(2+) pool: visualization of rapid Ca(2+) movements and equilibration. EMBO J, 2000. 19(21): p. 5729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindhout FW, et al. , VAP-SCRN1 interaction regulates dynamic endoplasmic reticulum remodeling and presynaptic function. EMBO J, 2019. 38(20): p. e101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucharz K and Lauritzen M, CaMKII-dependent endoplasmic reticulum fission by whisker stimulation and during cortical spreading depolarization. Brain, 2018. 141(4): p. 1049–1062. [DOI] [PubMed] [Google Scholar]
- 10.Calderon RO, Attema B, and DeVries GH, Lipid composition of neuronal cell bodies and neurites from cultured dorsal root ganglia. J Neurochem, 1995. 64(1): p. 424–9. [DOI] [PubMed] [Google Scholar]
- 11.Luarte A, et al. , The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev Neurobiol, 2018. 78(3): p. 181–208. [DOI] [PubMed] [Google Scholar]
- 12.Hanus C and Ehlers MD, Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol, 2016. 39: p. 8–16. [DOI] [PubMed] [Google Scholar]
- 13.Radler MR, Suber A, and Spiliotis ET, Spatial control of membrane traffic in neuronal dendrites. Mol Cell Neurosci, 2020. 105: p. 103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris KM, Structural LTP: from synaptogenesis to regulated synapse enlargement and clustering. Curr Opin Neurobiol, 2020. 63: p. 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Alvarez A, et al. , Endoplasmic reticulum visits highly active spines and prevents runaway potentiation of synapses. Nat Commun, 2020. 11(1): p. 5083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Perez-Alvarez and colleagues use organotypic hippocampal cultures and live imaging to monitor the dynamics of the ER in dendritic spines of CA1 neurons. They found that ER tubules are very dynamic and invade all dendrites over time. The frequency of these visits correlates with synaptic activity and blocking ER motility induces LTD. The authors propose that transient ER visits to spines helps maintain synaptic strength and prevents runaway potentiation.
- 16.Zhang L, et al. , Ras and Rap Signal Bidirectional Synaptic Plasticity via Distinct Subcellular Microdomains. Neuron, 2018. 98(4): p. 783–800 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borczyk M, et al. , Neuronal plasticity affects correlation between the size of dendritic spine and its postsynaptic density. Sci Rep, 2019. 9(1): p. 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulik YD, et al. , Structural plasticity of dendritic secretory compartments during LTP-induced synaptogenesis. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chirillo MA, et al. , Local resources of polyribosomes and SER promote synapse enlargement and spine clustering after long-term potentiation in adult rat hippocampus. Sci Rep, 2019. 9(1): p. 3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray EG, Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat, 1959. 93: p. 420–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Yap K, et al. , The actin-modulating protein synaptopodin mediates long-term survival of dendritic spines. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (•) Yap and colleagues use 2-photon time-lapse fluorescence microscopy to analyze the morphology of dendritic spines of granule cells in the adult mouse dentate gyrus. They show that the spine apparatus is mainly present at large spines and it confers them higher stability. Spine loss is preceded by spine apparatus loss. The authors propose that spine apparatus presence is a better correlate of spine stability than spine size.
- 22.Deller T, et al. , Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A, 2003. 100(18): p. 10494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aloni E, et al. , Increased excitability of hippocampal neurons in mature synaptopodin-knockout mice. Brain Struct Funct, 2021. 226(7): p. 2459–2466. [DOI] [PubMed] [Google Scholar]
- 24.Konietzny A, et al. , Caldendrin and myosin V regulate synaptic spine apparatus localization via ER stabilization in dendritic spines. EMBO J, 2022. 41(4): p. e106523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui-Wang T, et al. , Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell, 2012. 148(1–2): p. 309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher C and Ramos A, Joining the dots - protein-RNA interactions mediating local mRNA translation in neurons. FEBS Lett, 2018. 592(17): p. 2932–2947. [DOI] [PubMed] [Google Scholar]
- 27.Biever A, Donlin-Asp PG, and Schuman EM, Local translation in neuronal processes. Curr Opin Neurobiol, 2019. 57: p. 141–148. [DOI] [PubMed] [Google Scholar]
- 28.Van Driesche SJ and Martin KC, New frontiers in RNA transport and local translation in neurons. Dev Neurobiol, 2018. 78(3): p. 331–339. [DOI] [PubMed] [Google Scholar]
- 29.Bowen AB, et al. , Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pick JE and Ziff EB, Regulation of AMPA receptor trafficking and exit from the endoplasmic reticulum. Mol Cell Neurosci, 2018. 91: p. 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buonarati OR, et al. , Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci Signal, 2019. 12(562). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govind AP, et al. , Activity-dependent Golgi satellite formation in dendrites reshapes the neuronal surface glycoproteome. Elife, 2021. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Govind and colleagues show that cultured cortical neurons have their Golgi machinery concentrated in the cell body and thus most newly synthesized dendritic proteins bypass the Golgi and are exported to the plasma membrane via ER exit sites. This causes an atypical N-glycosylation pattern of surface proteins in dendrites. After stimulation, the number of Golgi satellites in dendrites increases leading to glycosylation of surface proteins with potential significance for synaptic plasticity and pathology.
- 33.Ziegler AB, et al. , Cell-Autonomous Control of Neuronal Dendrite Expansion via the Fatty Acid Synthesis Regulator SREBP. Cell Rep, 2017. 21(12): p. 3346–3353. [DOI] [PubMed] [Google Scholar]
- 34.Meltzer S, et al. , Phospholipid Homeostasis Regulates Dendrite Morphogenesis in Drosophila Sensory Neurons. Cell Rep, 2017. 21(4): p. 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krivoi II and Petrov AM, Cholesterol and the Safety Factor for Neuromuscular Transmission. Int J Mol Sci, 2019. 20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieweg K, Schaller H, and Pfrieger FW, Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem, 2009. 109(1): p. 125–34. [DOI] [PubMed] [Google Scholar]
- 37.de Chaves EI, et al. , Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J Biol Chem, 1997. 272(49): p. 30766–73. [DOI] [PubMed] [Google Scholar]
- 38.Maccarrone M, Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front Mol Neurosci, 2017. 10: p. 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyilas R, et al. , Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci, 2008. 28(5): p. 1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcucci H, et al. , Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J Biol Chem, 2010. 285(33): p. 25382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy D and Tedeschi A, The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after CNS Injury and Disease. Cells, 2021. 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai JW, et al. , Transcriptional Feedback Links Lipid Synthesis to Synaptic Vesicle Pools in Drosophila Photoreceptors. Neuron, 2019. 101(4): p. 721–737 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Tsai and colleagues disrupted the biosynthesis of phosphatidylcholine and phosphatidylserine in Drosophila photoreceptors. They found that this blockade of lipid production impaired synaptic vesicle recycling leading to activity and calcium dependent degeneration of axons. The authors also found that the manipulation activated SREBP, a transcription factor that regulates genes involved in lipid metabolism. SREBP in turn downregulated a group of tetraspanins which caused a reduction in the number of synaptic vesicles, contributing to the defects in neurotransmission. The authors suggest that lipid synthesis may occur locally at axons and it may be essential for maintenance of neuronal structure and function.
- 43.Genaro-Mattos TC, et al. , Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chem Neurosci, 2019. 10(8): p. 3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J and Liu Q, Cholesterol metabolism and homeostasis in the brain. Protein Cell, 2015. 6(4): p. 254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamir O and Charlton MP, Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol, 2006. 571(Pt 1): p. 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrov AM, et al. , The role of cholesterol in the exo- and endocytosis of synaptic vesicles in frog motor nerve endings. Neurosci Behav Physiol, 2010. 40(8): p. 894–901. [DOI] [PubMed] [Google Scholar]
- 47.Petrov AM, Yakovleva AA, and Zefirov AL, Role of membrane cholesterol in spontaneous exocytosis at frog neuromuscular synapses: reactive oxygen species-calcium interplay. J Physiol, 2014. 592(22): p. 4995–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korinek M, et al. , Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci Rep, 2020. 10(1): p. 12651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (•) Korinek and colleagues used methyl-β-cyclodextrin to deplete cholesterol in cultured hippocampal neurons. They found that cholesterol plays key roles in both, the presynaptic and postsynaptic compartments. Presynaptically, cholesterol facilitates the propagation of action potentials and blocks spontaneous neurotransmitter release. Postsynaptically, cholesterol regulates the subcellular localization and opening probability of NMDARs. This works replicates and expands previous work reinforcing the essential role of cholesterol in maintaining the physicochemical properties of neuronal membranes.
- 49.Rahbek-Clemmensen T, et al. , Super-resolution microscopy reveals functional organization of dopamine transporters into cholesterol and neuronal activity-dependent nanodomains. Nat Commun, 2017. 8(1): p. 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taverna E, et al. , Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. J Biol Chem, 2004. 279(7): p. 5127–34. [DOI] [PubMed] [Google Scholar]
- 51.Levitan I, Singh DK, and Rosenhouse-Dantsker A, Cholesterol binding to ion channels. Front Physiol, 2014. 5: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreutzberger AJ, Kiessling V, and Tamm LK, High cholesterol obviates a prolonged hemifusion intermediate in fast SNARE-mediated membrane fusion. Biophys J, 2015. 109(2): p. 319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dason JS, et al. , Cholesterol and F-actin are required for clustering of recycling synaptic vesicle proteins in the presynaptic plasma membrane. J Physiol, 2014. 592(4): p. 621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wasser CR, et al. , Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol, 2007. 579(Pt 2): p. 413–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hafner AS, et al. , Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science, 2019. 364(6441). [DOI] [PubMed] [Google Scholar]; (••) Hafner and colleagues used a combination of techniques to demonstrate the presence of mRNAs and transcription machinery in axons and dendrites in different brain regions. They also show that active translation occurs in these compartments and that it can be dynamically modulated by different agents used to trigger plasticity (BDNF, DHPG, ACEA). The authors conclude that local protein synthesis adds spatial and temporal precision for proteome remodeling that can be exploited to rapidly modify synapses in specific subcellular compartments.
- 56.Merianda TT, et al. , A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci, 2009. 40(2): p. 128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostroff LE, et al. , Axon TRAP reveals learning-associated alterations in cortical axonal mRNAs in the lateral amgydala. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baleriola J, et al. , Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell, 2014. 158(5): p. 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez C, et al. , Axons provide the secretory machinery for trafficking of voltage-gated sodium channels in peripheral nerve. Proc Natl Acad Sci U S A, 2016. 113(7): p. 1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathur C, et al. , Demonstration of ion channel synthesis by isolated squid giant axon provides functional evidence for localized axonal membrane protein translation. Sci Rep, 2018. 8(1): p. 2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berridge MJ, The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium, 2002. 32(5–6): p. 235–49. [DOI] [PubMed] [Google Scholar]
- 62.Reese AL and Kavalali ET, Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dittmer PJ, et al. , STIM1 Ca(2+) Sensor Control of L-type Ca(2+)-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. Cell Rep, 2017. 19(2): p. 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horvath PM, et al. , A subthreshold synaptic mechanism regulating BDNF expression and resting synaptic strength. Cell Rep, 2021. 36(5): p. 109467. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Horvath and colleagues use a combination of electrophysiology, live imaging and molecular biology in cultured hippocampal neurons to test the role of inhibitory spontaneous neurotransmission in neuronal homeostatic properties. They found that subthreshold GABAergic transmission can generate calcium signals that are propagated to the nucleous to activate gene transcription leading to changes in BDNF secretion and modulating the strength of excitatory, glutamatergic synapses. This is a direct effect of inhibitory signals that do not dependen on excitatory/inhibitory balance and reveal a key role for spontaenous GABAergic neurotranmission in determining homeostatic synaptic properties.
- 65.Soboloff J, et al. , STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol, 2012. 13(9): p. 549–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruszczynska-Biegala J, et al. , STIM Protein-NMDA2 Receptor Interaction Decreases NMDA-Dependent Calcium Levels in Cortical Neurons. Cells, 2020. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]; (•) Gruszczynska-Biegala and colleagues expand on previous findings showing that STIM1 and 2 can interact with and modulate several plasma membrane calcium channels. In this work the authors show that STIM1 and 2 bind to NR2 subunit of NMDARs inhibiting calcium influx. SOCE and NMDARs are part of a negative feedback loop where activation of either of them blocks calcium flow via the other, uncovering a novel regulatory pathway of dendritic calcium signaling.
- 67.Yap KA, et al. , STIM2 regulates AMPA receptor trafficking and plasticity at hippocampal synapses. Neurobiol Learn Mem, 2017. 138: p. 54–61. [DOI] [PubMed] [Google Scholar]
- 68.Pchitskaya E, et al. , Stim2-Eb3 Association and Morphology of Dendritic Spines in Hippocampal Neurons. Sci Rep, 2017. 7(1): p. 17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Alvarez G, et al. , STIM2 regulates PKA-dependent phosphorylation and trafficking of AMPARs. Mol Biol Cell, 2015. 26(6): p. 1141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Alvarez G, et al. , Impaired spatial memory and enhanced long-term potentiation in mice with forebrain-specific ablation of the Stim genes. Front Behav Neurosci, 2015. 9: p. 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maneshi MM, et al. , Orai1 Channels Are Essential for Amplification of Glutamate-Evoked Ca(2+) Signals in Dendritic Spines to Regulate Working and Associative Memory. Cell Rep, 2020. 33(9): p. 108464. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Maneshi and colleagues show that Orai1 channels are activated during synaptic transmission contributing to the calcium influx in dendritic spines. This calcium signaling is necessary for the establishment of LTP and thus it positively modulates different types of memory processes. The authors suggest that Orai1 channels can be relevant molecular targets for the cognitive decline seen in neurodegenerative diseases and following brain injuries.
- 72.Korkotian E and Segal M, Orai1 regulates calcium entry into dendritic spines. Channels (Austin), 2017. 11(2): p. 99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tshuva RY, Korkotian E, and Segal M, ORAI1-dependent synaptic plasticity in rat hippocampal neurons. Neurobiol Learn Mem, 2017. 140: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 74.Kushnireva L, Korkotian E, and Segal M, Calcium Sensors STIM1 and STIM2 Regulate Different Calcium Functions in Cultured Hippocampal Neurons. Front Synaptic Neurosci, 2020. 12: p. 573714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (•) Kushnireva and colleagues use optical methods to measure the localization and dynamics of STIM1 and 2 at different maturation stages of cultured neurons. They show that STIM1 is expressed in developing neurons and it mediates formation of spines associated with local and spontaneous calcium signals. STIM2 is more prominent in mature neurons and it mediates SOCE in dendritic spines.
- 75.Kirmiz M, et al. , Remodeling neuronal ER-PM junctions is a conserved nonconducting function of Kv2 plasma membrane ion channels. Mol Biol Cell, 2018. 29(20): p. 2410–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyazaki K and Ross WN, Ca2+ sparks and puffs are generated and interact in rat hippocampal CA1 pyramidal neuron dendrites. J Neurosci, 2013. 33(45): p. 17777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ross WN, Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci, 2012. 13(3): p. 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wild AR, et al. , Synapse-to-Nucleus Communication through NFAT Is Mediated by L-type Ca(2+) Channel Ca(2+) Spike Propagation to the Soma. Cell Rep, 2019. 26(13): p. 3537–3550 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pozzo-Miller LD, et al. , Activity-dependent calcium sequestration in dendrites of hippocampal neurons in brain slices. J Neurosci, 1997. 17(22): p. 8729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bell M, et al. , Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium. J Gen Physiol, 2019. 151(8): p. 1017–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly PT, et al. , Postsynaptic IP3 receptor-mediated Ca2+ release modulates synaptic transmission in hippocampal neurons. Brain Res Mol Brain Res, 2005. 135(1–2): p. 232–48. [DOI] [PubMed] [Google Scholar]
- 82.Miyata M, et al. , Local calcium release in dendritic spines required for long-term synaptic depression. Neuron, 2000. 28(1): p. 233–44. [DOI] [PubMed] [Google Scholar]
- 83.Hartmann J, et al. , STIM1 controls neuronal Ca(2)(+) signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron, 2014. 82(3): p. 635–44. [DOI] [PubMed] [Google Scholar]
- 84.Kohda K, Inoue T, and Mikoshiba K, Ca2+ release from Ca2+ stores, particularly from ryanodine-sensitive Ca2+ stores, is required for the induction of LTD in cultured cerebellar Purkinje cells. J Neurophysiol, 1995. 74(5): p. 2184–8. [DOI] [PubMed] [Google Scholar]
- 85.Singh N, et al. , Presynaptic endoplasmic reticulum regulates short-term plasticity in hippocampal synapses. Commun Biol, 2021. 4(1): p. 241. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Singh and colleagues hypothesized that calcium buffering via SERCA contributes to presynaptic plasticity in CA3-CA1 synapses. Using previously published experimental data, the authors developed a mathematical model and show that that the level of saturation of SERCA and thus, its buffering capacity is what determines whether there will be accumulation of calcium after consecutive calcium influx events during repeated stimulation and in this way, it mediates facilitation. The authors also explore the contribution of IP3Rs and RyRs suggesting that they may have a relevant role only after long trains of stimulus. This work elegantly links the local content of smooth ER in presynaptic boutons, the calcium buffering properties via SERCA, release probability and presynaptic short-term plasticity.
- 86.Friedhoff VN, et al. , Stochastic reaction-diffusion modeling of calcium dynamics in 3D dendritic spines of Purkinje cells. Biophys J, 2021. 120(11): p. 2112–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Friedhoff and colleagues model calcium signals in individual dendrites of Purkinje neurons. Their simulations uncover that the kinetics or timing of calcium buffering combined with the activation/inactivation of IP3Rs and calcium influx due to synaptic activity is what determines if the spine will undergo LTP or LTD. This model allows for the understanding of how changes in timing can lead to diverse outcomes when multiple neuronal inputs are combined. It also emphasizes a central role of the ER in shaping calcium signals in synapses.
- 87.Milanick WJ, et al. , Activation of alpha-1 adrenergic receptors increases cytosolic calcium in neurones of the paraventricular nucleus of the hypothalamus. J Neuroendocrinol, 2019. 31(10): p. e12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim R, et al. , Glycinergic mIPSCs in mouse and rat brainstem auditory nuclei: modulation by ruthenium red and the role of calcium stores. J Physiol, 2003. 546(Pt 3): p. 691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maciag F, et al. , Behavioral and electrophysiological changes in female mice overexpressing ORAI1 in neurons. Biochim Biophys Acta Mol Cell Res, 2019. 1866(7): p. 1137–1150. [DOI] [PubMed] [Google Scholar]
- 90.Chanaday NL, et al. , Presynaptic store-operated Ca(2+) entry drives excitatory spontaneous neurotransmission and augments endoplasmic reticulum stress. Neuron, 2021. 109(8): p. 1314–1332 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Chanaday and colleagues investigate the molecular mechanism linking SOCE to neurotransmitter release at excitatory synapses. They show that STIM2-dependent SOCE can potentiate glutamatergic spontaneous neurotransmission through calcium sensor synaptotagmin7. In contrast, synaptotagmin1 suppresses this SOCE-mediated spontaneous release. Finally, they demonstrate that activation of this mechanism during chronic ER stress exacerbates and propagates the neuronal damage via increased glutamate release.
- 91.Emptage NJ, Reid CA, and Fine A, Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron, 2001. 29(1): p. 197–208. [DOI] [PubMed] [Google Scholar]
- 92.McGuinness L, Bardo SJ, and Emptage NJ, The lysosome or lysosome-related organelle may serve as a Ca2+ store in the boutons of hippocampal pyramidal cells. Neuropharmacology, 2007. 52(1): p. 126–35. [DOI] [PubMed] [Google Scholar]
- 93.Sharma A and Hasan G, Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (••) Sharma and Hasan generated an IP3R dominant negative mutant in Drosophila. Selective expression of the mutant in dopaminergic neurons allowed them to uncover that IP3Rs regulate dopamine release and membrane excitability modulating the maturation and function of a circuit that controls feeding motivated flight. Taken in the context of this review, this work suggests that the dynamics and role of IP3Rs and other ER-related calcium sources may differ among different neuron types.
- 94.Patel JC, et al. , Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci, 2009. 29(20): p. 6568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meadows MA, et al. , Glycine Release Is Potentiated by cAMP via EPAC2 and Ca(2+) Stores in a Retinal Interneuron. J Neurosci, 2021. 41(46): p. 9503–9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warrier A, et al. , Calcium from internal stores triggers GABA release from retinal amacrine cells. J Neurophysiol, 2005. 94(6): p. 4196–208. [DOI] [PubMed] [Google Scholar]
- 97.Mathew SS and Hablitz JJ, Calcium release via activation of presynaptic IP3 receptors contributes to kainate-induced IPSC facilitation in rat neocortex. Neuropharmacology, 2008. 55(1): p. 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szikra T, et al. , Depletion of calcium stores regulates calcium influx and signal transmission in rod photoreceptors. J Physiol, 2008. 586(20): p. 4859–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramesh G, et al. , A short isoform of STIM1 confers frequency-dependent synaptic enhancement. Cell Rep, 2021. 34(11): p. 108844. [DOI] [PubMed] [Google Scholar]; (••) Ramesh and colleagues studied a neuron-specific splice variant of STIM1, STIM1B, and found that it preferentially localizes to the presynaptic ER where it can counteract synaptic depression during high-frequency trains of stimulus. STIM1B mediates this enhancement via accumulation in calcium due to its slower kinetics. The authors propose that STIM1 splicing fine-tunes synaptic efficacy and may have a role in neurodegeneration.
- 100.Wen H, et al. , Synchronous and asynchronous modes of synaptic transmission utilize different calcium sources. Elife, 2013. 2: p. e01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Courtney NA, et al. , Excitatory and Inhibitory Neurons Utilize Different Ca(2+) Sensors and Sources to Regulate Spontaneous Release. Neuron, 2018. 98(5): p. 977–991 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams CL and Smith SM, Calcium dependence of spontaneous neurotransmitter release. J Neurosci Res, 2018. 96(3): p. 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bal M, et al. , Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron, 2013. 80(4): p. 934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma G and Vijayaraghavan S, Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron, 2003. 38(6): p. 929–39. [DOI] [PubMed] [Google Scholar]
- 105.Castellano-Munoz M, Schnee ME, and Ricci AJ, Calcium-induced calcium release supports recruitment of synaptic vesicles in auditory hair cells. J Neurophysiol, 2016. 115(1): p. 226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Llano I, et al. , Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci, 2000. 3(12): p. 1256–65. [DOI] [PubMed] [Google Scholar]
- 107.Kuijpers M, et al. , Neuronal Autophagy Regulates Presynaptic Neurotransmission by Controlling the Axonal Endoplasmic Reticulum. Neuron, 2021. 109(2): p. 299–313 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (•) Kuijpers and colleagues show that autophagy in neuronal axons is a negative regulator of the content of smooth ER and thus it influences calcium release through RyRs and the strength of excitatory neurotransmission. The authors propose that neuronal autophagy controls axonal ER calcium stores and in turn regulates neurotransmission.
- 108.Stavoe AKH and Holzbaur ELF, Autophagy in Neurons. Annu Rev Cell Dev Biol, 2019. 35: p. 477–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Timofeeva Y and Volynski KE, Calmodulin as a major calcium buffer shaping vesicular release and short-term synaptic plasticity: facilitation through buffer dislocation. Front Cell Neurosci, 2015. 9: p. 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dou Y, et al. , Orai1 Plays a Crucial Role in Central Sensitization by Modulating Neuronal Excitability. J Neurosci, 2018. 38(4): p. 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei D, et al. , Orai1 and Orai3 Mediate Store-Operated Calcium Entry Contributing to Neuronal Excitability in Dorsal Root Ganglion Neurons. Front Cell Neurosci, 2017. 11: p. 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinbeck JA, et al. , Store-operated calcium entry modulates neuronal network activity in a model of chronic epilepsy. Exp Neurol, 2011. 232(2): p. 185–94. [DOI] [PubMed] [Google Scholar]


