Abstract
While aggression is often conceptualized as a highly stereotyped, innate behavior, individuals within a species exhibit a surprising amount of variability in the frequency, intensity, and targets of their aggression1. While differences in genetics are a source of some of this variation across individuals (estimates place the heritability of behavior at around 25–30%)2, a critical driver of variability is previous life experience. A wide variety of social experiences, including sexual, parental, and housing experiences can facilitate “persistent” aggressive states, suggesting that these experiences engage a common set of synaptic and molecular mechanisms that act on dedicated neural circuits for aggression. It has long been known that sex steroid hormones are powerful modulators of behavior, and also, that levels of these hormones are themselves modulated by experience. Several recent studies have started to unravel how experience-dependent hormonal changes during adulthood can create a cascade of molecular, synaptic, and circuit changes that enable behavioral persistence through circuit level remodeling. Here, we propose that sex steroid hormones facilitate persistent aggressive states by changing the relationship between neural activity and an aggression “threshold”.
Although aggression is a ubiquitous, highly conserved social behavior, individuals will vary substantially in their degree of aggressivity. This can be manifested as differences in the duration of individual attacks, the length of the attack bouts, and the time between bouts, in some cases leading to epochs with sustained or persistent aggression that can last many minutes (Figure 1A). In addition to these acute changes, individuals may exhibit more long-term signatures of persistence, characterized by the frequency that they enter these high aggression states. These sustained periods of aggression have had many names across decades of research, including “aggressive arousal”, “attack-readiness”, and “temporal persistence”3–5. The common phenomenon that these terms all attempt to capture is that the likelihood of attack at any given moment is highly dependent on a fluctuating internal state that represents both experiential and motivational variables, relative to a threshold for attack. Neural activity in relevant nodes for aggression may explicitly encode this fluctuating internal state and the threshold represents the “readout” mechanism of this activity, providing a lower bound for enabling attack-triggering mechanisms. Changes in an individual’s aggressive internal state may bring an individual closer to this threshold, and make repeated attacks bouts more likely. Alternatively, decreases in motivational or internal state move an individual away from the threshold, making attack less likely. Acute increases in activity may arise from moment-to moment changes social-sensory-independent internal motivational state6 or through access to specific sensory cues Social sensory information can be acquired without self-motion (an intruder approaches), or can be actively acquired (an intruder is approached), and during these encounters, aggression-relevant sensory information may be the “trigger” for attack by pushing an individual across the threshold. Ethologists frequently use the term “releasing stimulus” to describe sensory stimuli that overwhelmingly facilitate the initiation of a stereotyped behavior7.
Figure 1. Experience-dependent changes in neural encoding of aggression.
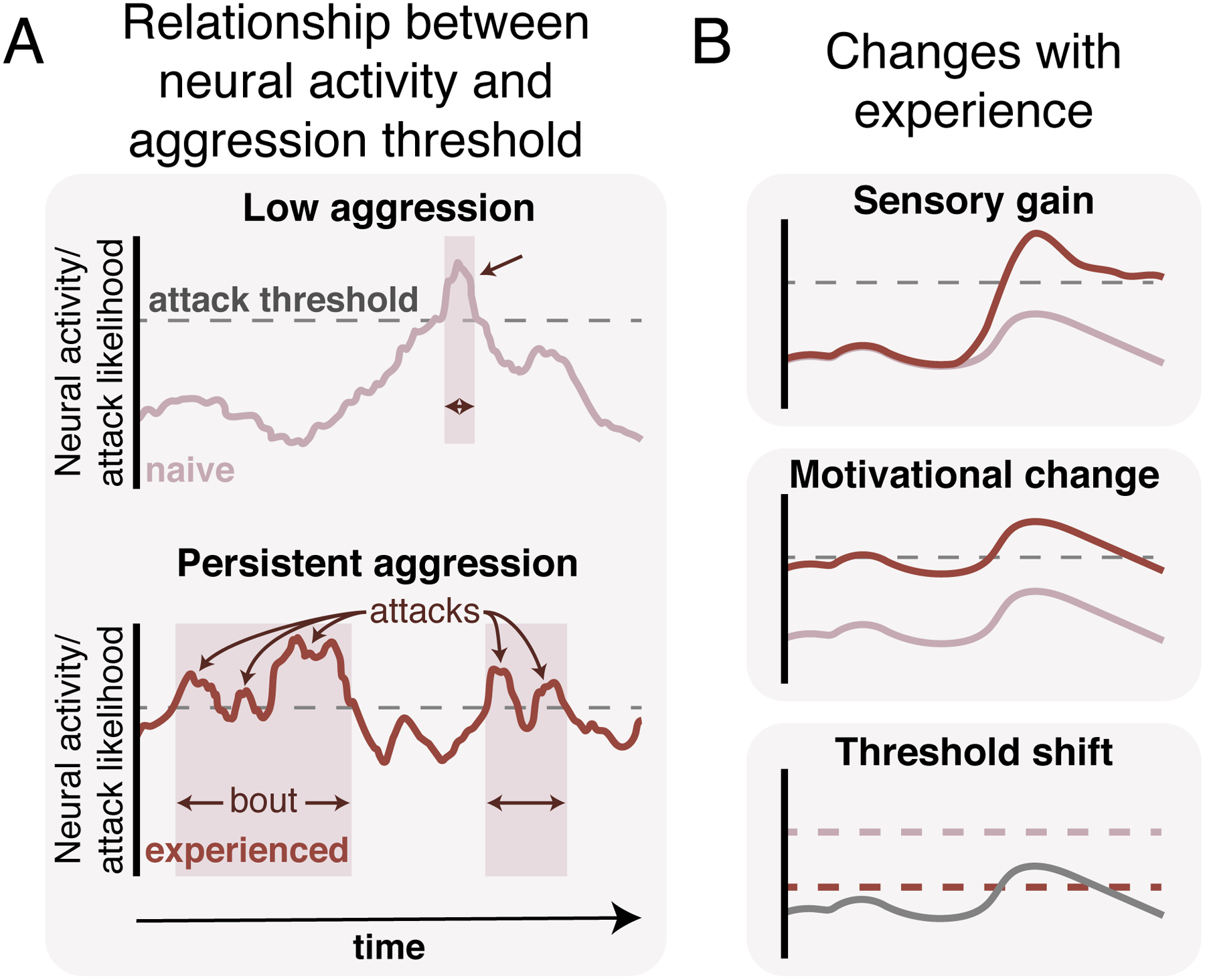
(A) We propose that neural activity within individual nodes of the social behavior network may represent attack likelihood. Increases in activity bring the animal closer to a theoretical attack threshold (dotted lines): once neural activity crosses this threshold an attack is triggered in an all-or-none manner (arrows). Multiple attacks in quick succession constitute bouts (shaded background). (B) Different manners in which experience may change neural encoding of attack likelihood. Top: Changes in sensory gain where the same stimulus will drive an increased neural response following experience. Middle: Changes in a motivational internal state will drive a shift in overall activity levels, bringing an animal closer or further from the attack threshold. Bottom: Experience can lower or raise the attack threshold itself leading to a decrease or increase in the amount of input needed to “release” attack.
In addition to these short timescale changes in sensory and motivational state, other, more long timescale variables may also modify an individual’s internal state and change the likelihood of attack. In particular, previous experience strongly affects the likelihood of aggression, decreasing the latency to first attack, as well the duration and frequency of attack. Animals may be “primed” for attack, either through previous attack8 or merely through exposure to sensory information. For example, investigating an anesthetized conspecific decreases attack latency to the next animal encountered9,10, demonstrating that sensory information may have long-lasting effects on the motivational state of the animal. These data suggest that prior exposure brings neural activity closer to a threshold, such that a similar level of sensory input on the second exposure is more likely to cross the threshold. This concept of an aggression threshold also has a literal analog in stimulation experiments that target aggression-promoting brain regions. When animals are behaviorally “primed” with previous access to aggression, the intensity of electrical stimulation required to evoke attack is decreased11,12, suggesting that this priming may push activity closer to the threshold such that less sensory activation is required.
One framework for understanding how experience can lead to behavioral persistence is that experience can change the relationship between neural activity that reflects the individual’s internal state and the attack threshold. We propose that this might happen in three separate, but not mutually exclusive ways (Figure 1B). First, increased aggression-relevant sensory input might generate attack by driving activity across the threshold. This might occur either from changes in the tuning or gain modulation of sensory circuits, or in changes to sensory acquisition behaviors. Second, changes in internal or motivational state might keep neural activity hovering close to the threshold, increasing the likelihood of crossing the threshold. Lastly, changes in the threshold itself might lead to persistent aggression. In this case, if the threshold itself was “lowered”, less input would be required to generate an attack. Here, we will explore recent data that points to a generalizable mechanism to implement this experience-dependent change and speculate about the brain regions that might perform these computations.
Neural circuits for aggression
While the patterning of social actions is, in many cases, a brainwide phenomenon, there exist dedicated circuits within the brain that have a clear role in the generation and maintenance of an aggressive state (Figure 2A). Critical nodes in this circuit exist as part of the “social behavior network” (SBN), a highly recurrent set of subcortical regions, largely in the hypothalamus, amygdala, and midbrain, whose functions and connectivity are extremely conserved throughout evolution13,14. The known functions and connectivity of these circuits in mammals has been extensively reviewed elsewhere15, so we will not detail the role of each node here. Briefly, key regions for aggression in both sexes include reciprocally connected regions of the hypothalamus (especially the ventromedial hypothalamus, ventrolateral area [VMHvl], ventral premammillary area, medial preoptic area [MPOA], and anterior hypothalamus), septal and amygdalar regions (including the medial and posterior amygdalae [MeA, PA], lateral septum [LS], and bed nucleus of the stria terminalis [BNST]), midbrain structures with connectivity to downstream motor pathways (most importantly, the periaqueductal gray [PAG]), and a newly implicated region in the basal forebrain, the substantia innominata16. Several regions within this network have been shown to be sensitive to specific social stimuli and aggression-promoting cues17–19. Aggressive information in this circuit may be somewhat hierarchically organized: for example, neural activity in the PAG exhibits higher selectivity to the motor aspects of attack, rather than the sensory or preparatory signals20. However, due to strong recurrent connectivity between network nodes, the aggression network defies simple feedforward descriptions, and instead, many regions conjunctively encode information about the sensory world, actions performed, and the individual’s internal state18,21.
Figure 2. Circuit mechanisms underlying hormone- and experience-dependent development of persistent aggression.
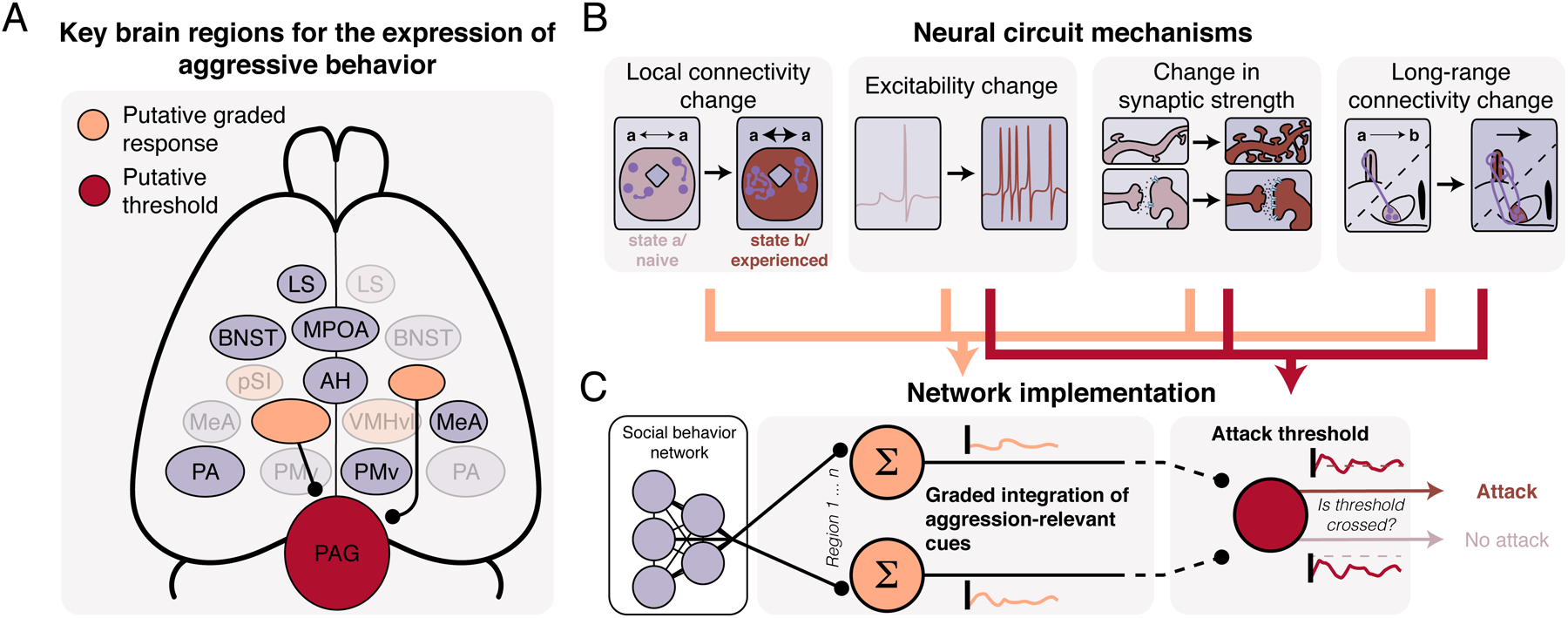
(A) Brain regions with identified roles in aggressive behavior. Putative graded response and threshold regions highlighted. Shading of contralateral regions done for display purposes. (B) Hormone- and experience-driven circuit changes within the overlapping aggressive and social behavior networks. (C) Proposed relationship between circuit plasticity and neural encoding of attack likelihood. Some circuit changes are more likely to affect graded representation of attack likelihood whereas others are more likely to change the relationship between neural activity and putative attack threshold. Abbreviations: LS, lateral septum; MPOA, medial preoptic area; BNST, bed nucleus of the stria terminalis; AH, anterior hypothalamus; pSI, posterior substantia innominata; VMHvl, ventromedial hypothalamus, ventrolateral area; MeA, medial amygdala; PA, posterior amygdala; PMv, ventral premammillary area; PAG, periaqueductal gray.
Beyond being anatomically conserved, brain regions in this circuit express an evolutionarily conserved constellation of hormone receptors13,22,23. In particular, receptors for circulating sex hormones (including androgens, estrogens, and progesterone) are expressed at varying levels in nearly every node in this circuit22,24,25. This ability to be responsive to network-wide changes in circulating hormones that are broadcast after specific social events likely bestows this network with the ability to be updated with experience. While hormonal influences have long been known to have a bidirectional relationship to aggression, how hormonal activation changes the computations performed by this circuit to facilitate attack remain broadly unknown.
Gonadal hormones are required for experience-dependent change in aggression
Since their initial identification in the early 20th century26–30, decades of classic research has demonstrated the powerful control that the sex steroid hormones estradiol (E2), testosterone (T), and progesterone (P4) exert on animal behavior27,31–35. Comparative work across metazoan phyla points to conserved roles for these molecules across species27, showcasing their broad evolutionary benefits. In contrast to previous literature suggesting that sex hormones exclusively organize neural circuits during early critical periods in development33,36, long-term changes in hormone milieu (including changes to both circulating hormone levels and receptors) during adulthood can shift neural gene expression, and consequently neural circuit connectivity, function, and ultimately behavior.
Hormones exert their primary control over neural function via intracellular hormone receptors. Additionally, steroid hormones bind to and signal via numerous membrane receptors, including the G-protein coupled estrogen receptor (GPR-30/GPER), the membrane associated estrogen receptor (mER), various classes of membrane associated androgen receptor (mAR), the estrogen receptor-X, and the GABAA receptor37–41, endowing them with the capacity for both acute and long-term change. Intracellularly, E2 acts at estrogen receptor alpha (ERα) and beta (ERβ); T acts at the androgen receptor (AR); and, P4 acts at the progesterone receptor (PR). Further, T is converted to E2 in the brain by aromatase, enabling it to act both directly at ARs and indirectly at ERα and ERβ. These hormone receptors are transcription factors and following binding to their hormone ligand, promote and repress transcription of entire sets of genes42,43. Thus, a change in the hormonal state (here defined as the current concentrations of serum sex hormones and their relative proportion to each other), will induce transcriptional changes. In turn, these hormonally regulated transcriptome dynamics will alter the protein composition of hormone-sensitive neurons, leading to changes in their connectivity, activity, and function42–44. Consistent with the role of sex hormones in modulating social behavior, the corresponding nuclear hormone receptors are found throughout the SBN and associated brain circuits25,45–47. For example, a recent mouse in situ hybridization study showed that neural populations in mouse MPOA and BNST often coexpress ERs, ARs, PRs, and aromatase, but at different levels and in different combinations across cell types22. Similar findings have been observed in hamsters with subsets of SBN neural populations coexpressing ERs and ARs48. Further, recent research focused on ERα, AR, PR, and aromatase-expressing neurons throughout SBN nodes demonstrates a clear role of these hormone-sensitive neurons in controlling a variety of social behaviors across many contexts49–54. Collectively, these data suggest a complex and flexible interplay between hormone milieu and the function of distinct neural populations to promote social behavior expression according to the given sensory and hormonal state of the individual.
Hormones and behavior exert a bidirectional influence over each other during aggression. Numerous social experiences lead to persistent increases in aggressive behavior. These include housing and sexual experience with the opposite sex, pregnancy, parturition and lactation, pup exposure, social isolation or overcrowding, repeated aggressive experience, and competition14,55–58. Although gonadal hormones are required for experience-induced changes in aggressive behavior in males and females, the specific relationship between T or E2 levels and this persistent increase remain unclear. In classic experiments to determine the relationship between serum T levels and sexual experience-induced increases in aggression, orchiectomized rats were treated with a range of T doses59. Aggression was abolished in orchiectomized rats but present in orchiectomized rats with T treatment, increasing as a function of T concentration up to physiological T levels: rats treated with supraphysiological T showed equivalent aggression as rats with physiological T treatment. These data suggest that while basal T is required for the experience-induced increase in aggression, T spikes above the average physiological range do not further increase aggression.
In a recent update to this finding, Stagkourakis and colleagues58 probed the role of T in the persistent aggression increases that follow repeated aggressive experience. They exposed subject mice to five consecutive days of intruders and recorded aggression towards intruder mice. Consistent with recent work demonstrating that aggression is rewarding in a subset of male mice6, they found that only a subset of aggressor mice show increases in aggression following repeated experience. Strikingly, Stagkourakis et al. show that aggressor mice have higher basal T compared to non-aggressors both prior to and after training and that aggressive experience increases T levels in aggressor but not non-aggressor mice. Consistent with the notion that a hormone concentration threshold is required for aggression, Stagkourakis et al. found that one week of T treatment was sufficient to elicit aggression in non-aggresssors. It is of note that the daily fluctuations in T in male rats exceed the experience-induced increases in T that are associated with changes in social behavior55,60. For example, whereas sensory exposure to a rat in estrus leads to a doubling of T levels in male rats within half an hour, spontaneous daily T fluctuations reach peaks 10–20 times baseline levels within the same time period55. Finally, orchiectomized rats require greater hypothalamic stimulation to evoke conspecific attack compared to intact controls, or orchiectomized rats with T replacement61. These data demonstrate that basal T levels may reorganize SBN circuits to facilitate aggression. Further, these data show that social sensory and behavioral experience can increase T levels; however the effects of these increases remain unclear. Taken together, this suggests sex steroid hormones are capable of adjusting the relationship between input and motor output and may contribute to social experience induced circuit plasticity.
Changes in the aggression threshold may be mediated by hormone-dependent plasticity
How do changes in hormone level result in changes in the relationship between neural activity and an aggression threshold? Recent data from across a variety of social contexts provides strong evidence that activation by steroid hormones drives neural plasticity through alterations in synaptic connectivity, excitability, and neural activity itself (Figure 2B).
Hormone-mediated changes in synaptic strength may serve to amplify incoming sensory information. For example, in the VMHvl of male mice, aggression is correlated with dendritic spine density58. Consistent with the hypothesis that basal T levels organize SBN connectivity to enable or promote aggression, androgenic manipulations that influence adult aggressive behavior also affect VMHvl synaptic density: orchiectomized rats show a decreased number of VMHvl spine and shaft synapses compared to intact males62,63 and perinatal T supplementation increases VMHvl synapse density in intact females63. T also promotes synapse formation outside the VMHvl, suggesting a potentially broad role across the social behavior network for androgens in synapse formation. For example, in the MPOA of male hamsters, T promotes local dendritic spine density: gonadally intact and orchietomized males with T replacement show greater spine density than orchietomized males without replacement64. In addition to T, E2 also regulates spine density and dendrite complexity in the VMHvl65,66. During proestrus, when ovarian E2 production peaks, VMHvl neurons from female mice show greater spine density and dendritic branching than VMHvl neurons from female mice in diestrus (when E2 levels are low), or from male mice66. Further, E2 treatment in Ovx mice increases spine density to levels seen in intact females. Beyond spine density increases, E2 promotes increased VMHvl neural expression of neurosteroid and neuropeptide receptors, including the progesterone receptor, mu-opioid receptor, cholecystokinin receptor A, and melanocortin-4 receptor44. E2 regulates spine density across the social behavior network as well with exogenous treatment leading to increases in LS and PAG spine density67,68. In addition, a recent study examining E2 binding and ERα-mediated gene expression within the BNST found that E2 stimulated ERα in BNST induces expression of genes associated with neurotransmitter receptors, synaptic organization, and synaptic plasticity43. Nevertheless, the relationship between E2-induced changes in SBN synaptic connectivity and the expression of specific social behaviors, including aggression, remains unexplored and fertile ground for future research.
Recent work has also demonstrated that changing basal levels of hormones can directly alter the sensory representations in the social behavior network69. In an elegant study that combined hormone manipulation and multiphoton cellular resolution imaging, McHenry et al showed that high levels of E2 increased the neural response in females to male related cues in the MPOA. These data suggest hormone levels control the “gain” on this sensory input. It is likely hormones may mediate similar computational changes in aggression circuits following experience-dependent hormonal change.
The most direct evidence for hormone-dependent changes to an aggression circuit comes from a recent paper, where the authors identified a glutamatergic projection from the posterior amygdala (PA) to VMHvl ERα+ neurons that undergoes long-term synaptic potentiation in aggressor mice following aggression experience58. The authors manipulated the strength of the synapse by inducing LTP and LTD in vivo and demonstrated that strengthening or weakening this synapse facilitates or abolishes the effects of aggression experience, indicating that amygdalo-hypothalamic synaptic strength underlies the experience-induced increase in aggression. Additionally, the authors found that whereas LTP could not be induced in control, non-aggressor mice, T treatment of non-aggressors permitted the induction of LTP at the PA→VMHvlERα synapse ex vivo and in vivo in these animals. This has the functional effect of increasing the gain of VMHvlERα neuronal responses to PA input, bringing them closer to a theoretical attack threshold. Taken together, these data suggest that T acts as a permissive gate on plasticity and may point to a potential mechanism whereby experience-dependent hormones facilitate the maintenance of a persistent state.
Beyond changes in local spine density which may serve to amplify sensory information, recent data has demonstrated that hormones have the power to remodel local and long-range circuits in the social behavior network. For example, in their study of E2 regulation of ERα mediated BNST gene expression, Gegenhuber et al. found that E2-driven ERα activation promoted the expression of a variety of neurite wiring genes in BNST neurons of adult mice across sexes, such as Brinp2, Unc5b, and Enah43. Additionally, VMHvl Progesterone Receptor expressing (PR+) neurons in females increase the density of their presynaptic sites to the anteroventral periventricular hypothalamus (AVPV) during estrus, following the E2 surge during proestrus54. Using optogenetic-assisted circuit mapping, Inoue et al. demonstrated that this increased projection density was correlated with an increased excitatory postsynaptic current response in AVPV neurons following VMHvlPr stimulation. These data indicate that the increased density of the presynaptic site was associated with a functional strengthening of the VMHvlPr→AVPV synapse. Next, Inoue and colleagues demonstrated that this strengthened VMHvlPr→AVPV synaptic connectivity required the action of E2 at ERα in VMHvlPr neurons. Optogenetic inhibition of the VMHvlPr→AVPV projection in E2 and P4 treated Ovx mice abolished lordosis in response to sexually motivated male mice.54
These data support the idea that multi-day changes in hormonal state can lead to large-scale circuit remodeling, changing the routing of information and the computational properties of the larger circuit. Through both local and long-range circuit remodeling, neurons in the aggression network may become more tightly coupled, requiring less sensory input to drive attack.
Where does the threshold “live” in the brain?
While the precise neural implementation of the aggression threshold is not yet known, several recent studies have provided hints about brain areas that are involved in driving overall levels of aggressive arousal (Figure 2C). In particular, neural recordings from the VMHvl and the pSI have suggested that activity in these areas may approximate a unidimensional attack-likelihood signal. In both cases, neural activity during the sensory phase of aggression that does not lead to attack is lower than activity that does result in attack, suggesting that if activity does not cross a threshold, attack is not initiated16,18. The VMHvl, which is well-positioned to integrate information from many nodes in the social behavior network70, increases activity both to aggression relevant sensory information, such as conspecific urine, but also increases during sensory-independent motivation prior to aggression6,71. Both regions send major projections to the PAG, in particular the lateral PAG, suggesting that the PAG may be the neural implementation of the threshold itself. Using simultaneous recordings of glutamatergic neurons in the VMHvl and the PAG, Falkner et al. showed that the VMHvl is sensitive to sensory and preparatory signals prior to attack, while the PAG has its peak response during attack itself20. The PAG, which appears to have a role in the gating of other innate behaviors, including vocalization and escape72,73, lacks this graded response and instead exhibits an all-or-none response during the action, consistent with that of a threshold. The role of hormone receptors, which are also richly expressed in the lateral PAG in particular25,46, likely have a role in determining the precise relationship between the graded inputs and the all-or-none behavioral output.
Future Directions
Overall, the effects of experience-dependent hormonal changes provide a mechanistic implementation of how experience may lead to persistent aggressive states by facilitating attack likelihood. Given the density of hormone receptor expression and interconnectivity of the social behavior network, it is likely that the effects of T and E2 extend network-wide, taking advantage of circulating hormones’ ability to act as a broadcast signal. New tool development for multisite monitoring of neural activity dynamics74,75 and modeling of inter-nodal relationships76,77 will be crucial for uncovering how these synaptic connectivity alterations affect the network-wide computations and functions critical for behavioral expression.
Given this framework, several critical open questions remain. First, while it is clear that hormonal modulation can remodel neural circuits on several timescales, we do not yet know whether these events are specific to hormone sensitive circuits. For example, do changes in synaptic connectivity modulate the strength of specific hormone-sensitive subnetworks? Beyond this, how do the actions of different hormones, broadcast in response to different behavioral events, change crosstalk in this network? In addition, our formulation of aggression arousal or attack-likelihood as a unidimensional signal here is almost certainly oversimplified, and new models78, which explicitly quantify how internal state shapes sensory-motor transformation during behavior, will be critical to interpreting neural activity.
We hope that new models of behavior that take into account hormone-mediated synaptic change will usher in a new science of computational neuroendocrinology. While the ability of researchers to monitor blood serum hormone levels over time has been limited by the small body mass of lab rodents, the successful development of novel fluorescent indicators for neuromodulatory molecules raises the possibility of future tracking of in vivo sex hormone dynamics across broad timescales. Such tool development would enable the design of experiments to quantify the relationship between short-term and longitudinal sex hormone dynamics and ongoing neural activity and behavior, allowing new insight. Researchers could pair these tools with manipulations to inactivate hormone-sensitive neural populations, knock down identified gene targets in hormone-sensitive neural populations, or block sex hormone receptors in these populations. Such manipulations would allow for the testing of the hypotheses that shifting hormonal state drives gene expression changes in distinct neural populations to alter neural activity patterns. This will change the likelihood of a given sensory stimulus to “release” attack or the likelihood of experience to generate persistent changes in neural computations and, consequently, social behavior.
Highlights.
Social experience generates persistent aggressive states.
Changes in experience-dependent sex hormones remodel local and long-range circuits in the brain’s “social behavior network”.
Circuit-level remodeling may change the relationship between neural activity and an aggression “threshold”.
Acknowledgements
NIMH DP2MH126375 (ALF), NIH R01MH126035 (ALF), Klingenstein-Simons Fellowship (ALF), a Siminos Pilot Grant (ALF) and an Alfred P. Sloan Fellowship (A.L.F.). Annegret Falkner. is a New York Stem Cell Foundation—Robertson Investigator. NIMH F32MH1125652 (EMG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potegal M & Knutson JF The Dynamics of Aggression: Biological and Social Processes in Dyads and Groups. (Psychology Press, 2013). [Google Scholar]
- 2.Dochtermann NA, Schwab T, Anderson Berdal M, Dalos J & Royauté R The Heritability of Behavior: A Meta-analysis. J. Hered 110, 403–410 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Potegal M Time course of aggressive arousal in female hamsters and male rats. Behav. Neural Biol 58, 120–124 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Hinde RA Animal behaviour: A synthesis of ethology and comparative psychology, 2nd ed. 2, 876 (1970). [Google Scholar]
- 5.Heiligenberg W The interaction of stimulus patterns controlling aggressiveness in the cichild fish Haplochromis burtoni. Anim. Behav 24, 452–458 (1976). [Google Scholar]
- 6.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K & Lin D Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci 19, 596–604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess EH, Hodges AW, Lorenz KZ, Lorenz K & Kickert RW The foundations of ethology. Am. J. Psychol 95, 521 (1982). [Google Scholar]
- 8.Potegal M & TenBrink L Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus). J. Comp. Psychol 98, 66–75 (1984). [Google Scholar]
- 9.Grant EC, Mackintosh JH & Lerwill CJ The effect of a visual stimulus on the agonistic behaviour of the golden hamster. Z. Tierpsychol 27, 73–77 (2010). [Google Scholar]
- 10.Mackintosh JH & Grant EC A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour 21, 246–259 (1963). [Google Scholar]
- 11.Kruk MR Hypothalamic attack: a wonderful artifact or a useful perspective on escalation and pathology in aggression? A viewpoint. Curr. Top. Behav. Neurosci 17, 143–188 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Kruk MR, van der Poel AM & de Vos-Frerichs TP The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour 70, 292–322 (1979). [DOI] [PubMed] [Google Scholar]
- 13.O’Connell LA & Hofmann HA The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol 519, 3599–3639 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Lischinsky JE & Lin D Neural mechanisms of aggression across species. Nat. Neurosci (2020) doi: 10.1038/s41593-020-00715-2. [DOI] [PubMed] [Google Scholar]
- 15.Wei D, Talwar V & Lin D Neural circuits of social behaviors: Innate yet flexible. Neuron 109, 1600–1620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; *Excellent review of the roles of individual nodes in the social behavior network during aggression
- 16.Zhu Z et al. A substantia innominata-midbrain circuit controls a general aggressive response. Neuron 109, 1540–1553.e9 (2021). [DOI] [PubMed] [Google Scholar]; **The authors identify a novel node in the aggression network that responds to a wide variety of aggression stimuli and contexts.
- 17.Chen A-X et al. Specific Hypothalamic Neurons Required for Sensing Conspecific Male Cues Relevant to Inter-male Aggression. Neuron 108, 763–774.e6 (2020). [DOI] [PubMed] [Google Scholar]; *The authors show the first in vivo recordings of a critical but understudied node in the aggression network and show that neurons respond to aggression-relevant stimuli.
- 18.Falkner AL, Dollar P, Perona P, Anderson DJ & Lin D Decoding ventromedial hypothalamic neural activity during male mouse aggression. J. Neurosci 34, 5971–5984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergan JF, Ben-Shaul Y & Dulac C Sex-specific processing of social cues in the medial amygdala. Elife 3, e02743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falkner AL et al. Hierarchical Representations of Aggression in a Hypothalamic-Midbrain Circuit. Neuron (2020) doi: 10.1016/j.neuron.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; **The authors show that VMHvl inputs to the lPAG convey sensory and action information during aggression while the lPAG encodes motor-related signals during attack.
- 21.Karigo T et al. Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589, 258–263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffitt JR et al. Molecular, spatial and functional single-cell profiling of the hypothalamic preoptic region. Science (2018) doi: 10.1126/science.aau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D-W et al. Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 179, 713–728.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juntti SA et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simerly RB, Chang C, Muramatsu M & Swanson LW Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol 294, 76–95 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Gallagher TF & Koch FC THE TESTICULAR HORMONE. J. Biol. Chem 84, 495–500 (1929). [Google Scholar]
- 27.Allen E & Doisy EA AN OVARIAN HORMONE: PRELIMINARY REPORT ON ITS LOCALIZATION, EXTRACTION AND PARTIAL PURIFICATION, AND ACTION IN TEST ANIMALS. JAMA 81, 819–821 (1923). [DOI] [PubMed] [Google Scholar]
- 28.Hartmann M & Wettstein A Zur Kenntnis der Corpus luteum-Hormone (2. Mitteilung.). Helv. Chim. Acta 17, 1365–1372 (1934). [Google Scholar]
- 29.Slotta KH, Ruschig H & Fels E Reindarstellung der Hormone aus dem Corpus luteum. (Vorläuf. Mitteil.). Ber. dtsch. Chem. Ges. A/B 67, 1270–1273 (1934). [Google Scholar]
- 30.Allen WM & Wintersteiner O CRYSTALLINE PROGESTIN. Science 80, 190–191 (1934). [DOI] [PubMed] [Google Scholar]
- 31.Beach FA & Holz AM Mating behavior in male rats castrated at various ages and injected with androgen. J. Exp. Zool 101, 91–142 (1946). [DOI] [PubMed] [Google Scholar]
- 32.Beach FA Bisexual mating behavior in the male rat: effects of castration and hormone administration. Physiological Zoölogy 18, 390–402 (1945). [Google Scholar]
- 33.Phoenix CH, Goy RW, Gerall AA & Young WC Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 (1959). [DOI] [PubMed] [Google Scholar]
- 34.Levine S & Mullins R Jr. ESTROGEN ADMINISTERED NEONATALLY AFFECTS ADULT SEXUAL BEHAVIOR IN MALE AND FEMALE RATS. Science 144, 185–187 (1964). [DOI] [PubMed] [Google Scholar]
- 35.Davis PG & Barfield RJ Activation of feminine sexual behavior in castrated male rats by intrahypothalamic implants of estradiol benzoate. Neuroendocrinology 28, 228–233 (1979). [DOI] [PubMed] [Google Scholar]
- 36.Arnold AP & Breedlove SM Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm. Behav 19, 469–498 (1985). [DOI] [PubMed] [Google Scholar]
- 37.Prossnitz ER & Barton M The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol 7, 715–726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vail G & Roepke TA Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids 142, 77–83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas P Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors. Endocrinology 160, 772–781 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Toran-Allerand CD et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci 22, 8391–8401 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belelli D & Lambert JJ Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci 6, 565–575 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Xu X et al. Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gegenhuber B, Wu MV, Bronstein R & Tollkuhn J Regulation of neural gene expression by estrogen receptor alpha. bioRxiv 2020.10.21.349290 (2020) doi: 10.1101/2020.10.21.349290. [DOI] [Google Scholar]; **The authors used the novel CUT&RUN technique to demonstrate that E2 stimulation of BNST ERα in both adult male and female mice activates and represses a network of genes for synapse organization, neurite wiring, membrane-associated ion channels, and neuromodulator receptors.
- 44.Krause WC et al. Oestrogen engages brain MC4R signaling to drive physical activity in female mice. Nature 599, 131–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; *The authors show that E2 acts at VMHvl ERα to increase melanocortin-4 receptor expression, driving increased VMHvl neural activity and overall energy expenditure.
- 45.Akesson TR, Simerly RB & Micevych PE Estrogen-concentrating hypothalamic and limbic neurons project to the medial preoptic nucleus. Brain Res. 451, 381–385 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Mitra SW et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144, 2055–2067 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Pfaff D & Keiner M Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol 151, 121–158 (1973). [DOI] [PubMed] [Google Scholar]
- 48.Wood RI & Newman SW Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology 62, 487–497 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Bayless DW et al. Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell 176, 1190–1205.e20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remedios R et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashikawa K et al. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci 20, 1580–1590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang Y-Y, Yamaguchi T, Song SC, Tritsch NX & Lin D A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 98, 192–207.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue S et al. Periodic Remodeling in a Neural Circuit Governs Timing of Female Sexual Behavior. Cell 179, 1393–1408.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; **Here, the authors show that increases in E2 during estrus promote circuit rewiring within the social behavior network such that VMHvl Pr+ neurons increase their projections to the anteroventral periventricular hypothalamus. The authors further show that this axonal outgrowth leads to a functional strengthening of the VMHvlPr→AVPV synapse and that this projection is required for the expression of lordosis behavior.
- 55.Albert DJ, Jonik RH & Walsh ML Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci. Biobehav. Rev 16, 177–192 (1992). [DOI] [PubMed] [Google Scholar]
- 56.Hashikawa K, Hashikawa Y, Lischinsky J & Lin D The Neural Mechanisms of Sexually Dimorphic Aggressive Behaviors. Trends Genet. 34, 755–776 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Lee CR, Chen A & Tye KM The neural circuitry of social homeostasis: Consequences of acute versus chronic social isolation. Cell 184, 1500–1516 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stagkourakis S, Spigolon G, Liu G & Anderson DJ Experience-dependent plasticity in an innate social behavior is mediated by hypothalamic LTP. Proc. Natl. Acad. Sci. U. S. A 117, 25789–25799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this elegant study, the authors provide a direct link between T signaling and circuit plasticity changes underlying experience-dependent changes in aggression. In particular, Stagkourakis et al. demonstrate that T levels gate the plasticity of the PA→VMHvlERα synapse. If T levels are too low, the PA→VMHvlERα synapse is unable to undergo plasticity; however, if T levels are sufficiently high, LTP and LTD could be induced to facilitate or block the ability of experience to drive a persistent increase in aggressive behavior.
- 59.Albert DJ, Jonik RH, Watson NV, Gorzalka BB & Walsh ML Hormone-dependent aggression in male rats is proportional to serum testosterone concentration but sexual behavior is not. Physiol. Behav 48, 409–416 (1990). [DOI] [PubMed] [Google Scholar]
- 60.Koolhaas JM, Schuurman T & Wiepkema PR The organization of intraspecific agonistic behaviour in the rat. Prog. Neurobiol 15, 247–268 (1980). [DOI] [PubMed] [Google Scholar]
- 61.Bermond B, Mos J, Meelis W, van der Poel AM & Kruk MR Aggression induced by stimulation of the hypothalamus: effects of androgens. Pharmacol. Biochem. Behav 16, 41–45 (1982). [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto A Synaptogenic action of sex steroids in developing and adult neuroendocrine brain. Psychoneuroendocrinology 16, 25–40 (1991). [DOI] [PubMed] [Google Scholar]
- 63.Matsumoto A & Arai Y Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology 42, 232–236 (1986). [DOI] [PubMed] [Google Scholar]
- 64.Garelick T & Swann J Testosterone regulates the density of dendritic spines in the male preoptic area. Horm. Behav 65, 249–253 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Carrer HF & Aoki A Ultrastructural changes in the hypothalamic ventromedial nucleus of ovariectomized rats after estrogen treatment. Brain Res. 240, 221–233 (1982). [DOI] [PubMed] [Google Scholar]
- 66.Madeira MD, Ferreira-Silva L & Paula-Barbosa MM Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J. Comp. Neurol 432, 329–345 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Chung SK, Pfaff DW & Cohen RS Estrogen-induced alterations in synaptic morphology in the midbrain central gray. Exp. Brain Res 69, 522–530 (1988). [DOI] [PubMed] [Google Scholar]
- 68.Miyakawa M & Arai Y Synaptic plasticity to estrogen in the lateral septum of the adult male and female rats. Brain Res. 436, 184–188 (1987). [DOI] [PubMed] [Google Scholar]
- 69.McHenry JA et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci 20, 449–458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lo L et al. Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc. Natl. Acad. Sci. U. S. A 116, 7503–7512 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin D et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans DA et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tschida K et al. A Specialized Neural Circuit Gates Social Vocalizations in the Mouse. Neuron 103, 459–472.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim CK et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sych Y, Chernysheva M, Sumanovski LT & Helmchen F High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat. Methods 16, 553–560 (2019). [DOI] [PubMed] [Google Scholar]; **Novel methodology for performing multi-region fiber photometry.
- 76.Perich MG & Rajan K Rethinking brain-wide interactions through multi-region ‘network of networks’ models. Curr. Opin. Neurobiol 65, 146–151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perich MG et al. Inferring brain-wide interactions using data-constrained recurrent neural network models. bioRxiv 2020.12.18.423348 (2020) doi: 10.1101/2020.12.18.423348. [DOI] [Google Scholar]
- 78.Calhoun AJ, Pillow JW & Murthy M Unsupervised identification of the internal states that shape natural behavior. Nat. Neurosci 22, 2040–2049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


