Abstract
Background:
Toxicology studies suggest that neonicotinoids may be associated with adiposity development via thyroid hormone disruption and increased oxidative stress. Prior epidemiological studies report mixed results for the association between neonicotinoids and adiposity measures.
Objective:
To examine the association between detectable concentrations of parent neonicotinoids (imidacloprid, acetamiprid, clothianidin) and neonicotinoid metabolites (5-hydroxy-imidacloprid, N-desmethyl-acetamiprid) with adiposity measures among US adults, and whether sex modifies the associations for metabolites.
Methods:
National Health and Nutrition Examination Survey (NHANES) 2015–2016 data was utilized to estimate covariate adjusted associations between detectable neonicotinoids and fat mass index (FMI), lean mass index (LMI), waist circumference, body fat percentage, and body mass index (BMI) using multiple linear regression. The odds of being overweight or obese with detectable neonicotinoids were estimated using logistic regression. Sampling strategies were accounted for in the regression models.
Results:
Detectable levels of acetamiprid were associated with a decrease in FMI (β =−3.17 kg/m2, 95% CI [−4.79, −1.54]), LMI (β =−3.17 kg/m2, 95% CI [−5.17, −1.17]), body fat percentage (β =−4.41, 95% CI [−8.20, −0.62]), waist circumference (β =−9.80 cm, 95% CI [−19.08, −0.51]), and BMI (β =−3.88kg/m2, 95% CI [−7.25, −0.51]) among adults. In contrast, detectable levels of 5-hydroxy-imidacloprid were associated with greater rates of being overweight/obese (IRR =1.11, 95% CI [1.04, 1.18)) and increased LMI (β =0.67 kg/m2, 95% CI [0.04, 1.29]). Sex modified the association between N-desmethyl-acetamiprid and LMI (pint =0.075) with a positive association among males (β =1.14 kg/m2, 95% CI [0.38, 1.90]), and an insignificant inverse ciation in females. Sex also modified the association for N-desmethyl-acetamiprid with FMI (pint =0.095) and body fat percentage (pint =0.072), with suggestive evidence showing positive associations for males and inverse associations for females.
Conclusion:
In this exploratory analysis, detectable concentrations of acetamiprid were inversely associated with adiposity, while there were mixed findings for 5-hydroxy-imidacloprid. Findings suggest sex differences, though results are not clear with regard to the directionality of the association by sex.
Introduction
Neonicotinoids are nicotine-based insecticides frequently used to protect agricultural crops from pest-related damage, and to kill other pests (Bass & Field, 2018). Neonicotinoids became widely popular after their introduction in 1991 since they were effective on pests that were resistant to other insecticides (Nauen et al., 2008). Bayer CropScience discovered the first neonicotinoid compound, imidacloprid, in 1991. It remains the most widely used neonicotinoid to date (Nauen et al., 2008). Subsequently, other neonicotinoid compounds such as clothianidin, acetamiprid, thiamethoxam, dinotefuran, and thiacloprid, were made commercially available (Ihara & Matsuda, 2018). Neonicotinoids have a long half-life in soil (6 months to 3 years), which leads to concerns regarding their exposure in humans (Goulson, 2013).
An environmental study showed that 72% of the fruits and 45% of the vegetables tested had detectable levels of multiple neonicotinoids, while freshwater sources in California showed the presence of imidacloprid in 89% of the samples, with 19% exceeding Environmental Protection Agency (EPA) toxicity limits (1.05 μg/L) (Chen et al., 2014; Starner & Goh, 2012). A study conducted using the National Health and Nutritional Examination Survey data (NHANES) showed that almost 50% of the participants had detectable concentrations of urinary metabolites of at least one type of neonicotinoid, with acetamiprid (35%) and imidacloprid metabolites (19.7%) being detected most frequently (Ospina et al., 2019). Hair samples collected from Chinese women in two cities indicated the presence of five different neonicotinoids (imidacloprid, acetamiprid, thiamethoxam, clothianidin, and thiacloprid), with imidacloprid detected in 99% of the samples (Peng et al., 2020), while a 250-fold increase in concentrations of neonicotinoids (imidacloprid, acetamiprid, dinotefuran, nitenpyram, nithiazine, clothianidin, thiacloprid, and thiamethoxam) was seen in urine samples of Japanese women from 1994 to 2011 (Ueyama et al., 2015). Among studies examining participants with occupational use of neonicotinoids, the presence of seven types of neonicotinoids was detected in urine samples of farmers in Japan, with thiamethoxam, dinotefuran, imidacloprid, and clothianidin present in 96–100% of the samples (Han et al., 2018).
Evidence suggests neonicotinoids may be associated with adverse health outcomes among adults (Hernandez et al., 2008; Marfo et al., 2015). Neonicotinoid concentrations among farmers have been associated with reduced total lung capacity and residual volume; neonicotinoids were also associated with 7 times higher likelihood of developing respiratory symptoms (Hernandez et al., 2008). Acetamiprid and thiamethoxam metabolites were positively associated with several neo-nicotinic symptoms, including memory loss, tremors, headaches, fatigue, chest pain, muscle spasms, and coughing, as compared to individuals displaying other or no symptoms (Marfo et al., 2015). Animal studies show that neonicotinoids have endocrine disruptive properties. Imidacloprid exposure in rats is positively related to reductions in testosterone, subsequently leading to infertility (Mikolic & Karaconji, 2018). Imidacloprid also reduces thyroid hormone levels in other species, which can disturb the hypothalamus-pituitary-thyroid axis (Leemans et al., 2019). Furthermore, neonicotinoids have been shown to suppress natural hormone function as well as disrupt regular hormone synthesis and metabolism (Mikolic & Karaconji, 2018). These mechanisms have been linked to the potential obesogenic actions of neonicotinoids. Toxicological studies have shown that imidacloprid disrupts steroid hormone as well as insulin regulation, which can potentiate metabolic changes leading to obesity (Mikolic & Karaconji, 2018). Disruption of steroid hormone regulation by EDCs, coupled with EDC induced dysfunction of growth hormone and estrogen can lead to an increase in size and number of adipocytes during developmental years (Grun & Blumberg, 2009). Epidemiological studies have shown a positive association between neonicotinoid compounds (imidacloprid and N-desmethyl-acetamiprid) and BMI in adults and children, respectively (Peng et al., 2020; Wang et al., 2020).
This study examined the association between detectable concentrations of neonicotinoids and measures of adiposity in adults using a representative sample from the 2015–2016 cycle of NHANES. This study also assessed whether sex modified the relationship between neonicotinoid metabolites and adiposity.
Methods
Data source and study population
This exploratory cross-sectional study utilized data from the 2015–2016 cycle of NHANES since neonicotinoids were only quantified in a subset of the population within this cycle. The 2015–2016 cycle sampled 15,327 people across 30 survey locations. Of these, 9,971 completed the interview and 9,544 were examined. Inclusion criteria for the study population in the present study were adults over the age of 19 years who had at least one reported urinary neonicotinoid measured (among imidacloprid, acetamiprid, clothianidin, 5-hydroxy-imidacloprid, and N-desmethyl-acetamiprid) along with at least one reported measure of adiposity (among fat mass, lean mass, body fat percentage, waist circumference, and body mass index (BMI)) (N=1699). One hundred and sixty-three pregnant women were excluded resulting in 1675 participants included in the study analyses.
Neonicotinoids
Neonicotinoids measured included imidacloprid, acetamiprid, clothianidin, and thiacloprid. Additionally, 5-hydroxy-imidacloprid (a metabolite of imidacloprid) and N-desmethyl-acetamiprid (a metabolite of acetamiprid) were measured. For each subject, 0.2 milliliters (ml) urine was used as a sample for analysis. Enzymatic hydrolysis of urinary conjugates of the target neonicotinoids was performed, followed by online solid phase extraction, reversed phase high-performance liquid chromatography separation, and isotope dilution-electrospray ionization tandem mass spectrometry detection (Baker et al., 2019). This method has a precision range between 3.7 to 10.2%, while its accuracy is between 91.2 to 116% (Baker et al., 2019). N-desmethyl-acetamiprid had the highest detection frequency (32.4%), followed by 5-hydroxy-imidacloprid (20%), clothianidin (7.7%), imidacloprid (4.1%), and acetamiprid (0.3%) (Supplemental table S1). Given relatively low detection frequencies, we analyzed neonicotinoids as “detect” versus “non-detect.” Thiacloprid was not examined given its low detection frequency (0.02%). Additionally, variables were created for: 1) any detectable parent neonicotinoid (imidacloprid, acetamiprid, or clothianidin); and 2) any detectable neonicotinoid metabolite (5-hydroxy-imidacloprid or N-desmethyl-acetamiprid). Participants who had concentrations above the limit of detection for either imidacloprid, acetamiprid, or clothianidin were considered to have detectable concentrations of a parent neonicotinoid. Those who had concentrations of imidcloprid, acetamiprid, and clothianidin that were all less than the limit of detection were considered as having non-detectable parent neonicotinoids. The same methodology was applied to define any detectable neonicotinoid metabolites with 5-hydroxy-imidacloprid and N-desmethyl-acetamiprid. About 11.6% of individuals in the study sample had detectable levels of any parent neonicotinoid, and 43% had detectable levels of neonicotinoid metabolites.
Adiposity measures
Height was measured at the Mobile Examination Center (MEC) by trained technicians using a stadiometer. A digital weighing scale was used to measure weight. Each participant was allowed to only wear underwear and a standard MEC examination gown while their weight was being measured. BMI was calculated by dividing weight in kilograms by height squared in meters, and then rounded off to one decimal point. BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (30 kg/m2 or more) (Centers for Disease Control [CDC], 2020).
A steel retractable measuring tape was used to record waist circumference. Before recording waist measurements, subjects were asked to clip their shirt gowns above the waist, while the measuring tape was aligned along the line of both iliac crests and wrapped around the waist to record a measurement (NHANES Anthropometry Procedures Manual, 2016). Dual energy X-ray absorptiometry (DXA) was used to calculate fat mass, lean mass, and total body fat. Based on these values, fat mass index (FMI) and lean mass index (LMI) were derived by dividing fat mass by height squared, and lean mass by height squared, respectively (Zhu et al., 2017). FMI and LMI are reported to be better measures of adiposity as compared to BMI, since they specifically assess fat and lean mass, respectively, while also serving as a better representation of central fat mass (Han et al., 2010; Kyle et al., 2003).
Statistical methods
All analyses were weighted to account for clustering of samples, measurement errors, oversampling, and missing measurements. Linear regression models were used to estimate βs and corresponding 95% confidence intervals (CIs) for the associations between detectable levels of neonicotinoids and continuous measures of adiposity, which included LMI, FMI, waist circumference, BMI, and body fat percentage. Poisson’s modified regression was used to estimate incidence rate ratios (IRRs) and corresponding 95% CIs for the associations between detectable levels of neonicotinoids and the odds of being overweight or obese, as well as separately for overweight and obese participants. In order to examine effect measure modification by sex, a product term between the detectable neonicotinoid metabolites and sex was included in the previously described models. Prior research has indicated that the statistical power to assess for interactions is lower than for the main effects (Aguinis, 1995). As such, we considered a P<0.10 as statistically significant for the interaction term in the effect measure modification analyses (Aguinis, 1995; Thiese et al., 2016). Covariates included in the final model were based on bivariate analysis with neonicotinoid compounds and measures of adiposity (P<0.20). These included age, sex, race/ethnicity, marital status, self-reported current health status, and thyroid issues. We completed a secondary analysis examining the association between neonicotinoids and adiposity measures by smoking status to determine whether associations differ.
The sensitivity analyses were adjusted for daily caloric intake, bisphenol A (BPA) concentrations, and physical activity. Additionally, associations were examined after removing participants whose FMI, LMI, BMI or waist circumference were beyond 3 standard deviations from the mean. Lastly, we checked all models for their robustness by examining how the main estimates for neonicotinioids behaved when regression specifications were modified. All statistical data analyses were completed using the STATA 16.1 software.
Results
Study participants
The participants were relatively evenly distributed by sex, with over one-third between the ages of 40–60 years (36.3%) (Table 1). A majority of the participants were Non-Hispanic white, married or living with a partner, and had a high monthly family income. Approximately 52% were physically active, and 41% self-reported either excellent or good health. Most of the participants did not have thyroid conditions (89%) and were non-smokers (76%). Among the study participants, 70% were overweight or obese, with a mean BMI of 29.3 kg/m2. Average waist circumference was 100.2 cm, with an average FMI of 9.7 kg/m2 and LMI of 18.3 kg/m2. Average body fat percentage was 32.6%. Males were significantly more likely than females to have greater waist circumference, lower FMI, higher LMI, and lower body fat percentage. Mexican-American/Other Hispanic participants were more likely to have a higher body fat percentage, while Non-Hispanic blacks were more likely to have a higher BMI, and a greater waist circumference. Participants with a higher BMI were more likely to self-report a poor or fair current health status and have a higher prevalence of thyroid-related issues. Those who were divorced or separated were more likely to have a higher body fat percentage compared to single or unmarried participants. A greater waist circumference was associated with being older, having a fair or poor current health status, and having thyroid related problems. Individuals reporting good health status were less likely to be overweight or obese, and had lower BMI, waist circumference, and FMI. Participants with detectable levels of any parent neonicotinoid were more likely to be Non-Hispanic white, while participants with detectable levels of neonicotinoid metabolites were more likely to be non-smokers.
Table 1.
Study population characteristics for neonicotinoids and adiposity measures among adults (20+ years), NHANES 2015–2016^
| Characteristics | n (%) | Overweight/obese n (%) | BMI (kg/m2) Mean (SD) | Waist circumference (cm) Mean (SD) | FMI (kg/m2) Mean (SD) | LMI (kg/m2) Mean (SD) | Fat percentage Mean (SD) | Detectable parent neonicotinoid n (%) | Detectable neonicotinoid metabolite n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Total | 1675 | 1226 (69.9) | 29.3 (6.9) | 100.2 (17.1) | 9.7 (4.3) | 18.3 (3.4) | 32.6 (8.4) | 193 (11.6) | 711 (42.9) |
| Sex | |||||||||
| P-value | 0.071 | 0.756 | <0.001* | <0.001* | <0.001* | <0.001* | 0.531 | 0.090 | |
| Male | 798 (48.8) | 588 (50.7) | 29.4 (6.6) | 102.8 (17.0) | 7.9 (3.5) | 19.8 (3.1) | 26.8 (6.3) | 95 (51.5) | 304 (45.0) |
| Female | 877 (51.2) | 638(49.3) | 29.3 (7.2) | 97.7 (16.8) | 11.5 (4.4) | 16.9 (3.0) | 38.3 (6.0) | 98 (48.5) | 407 (55.0) |
| Age (years) | |||||||||
| P-value | <0.001* | 0.067 | 0.006* | 0.006* | 0.065 | 0.005* | 0.139 | 0.923 | |
| 20–29 | 283 (18.1) | 167 (13.6) | 27.6 (7.9) | 92.6 (19.3) | 8.7 (4.7) | 17.7 (3.3) | 30.2 (9.6) | 35 (16.4) | 126 (18.6) |
| 30–39 | 277 (17.4) | 195 (17.1) | 29.8 (7.4) | 99.9 (16.7) | 9.9 (4.6) | 19.0 (3.6) | 32.0 (8.1) | 31 (18.2) | 128 (18.2) |
| 40–49 | 300 (17.8) | 233 (18.8) | 29.4 (6.1) | 100.6 (14.3) | 10.0 (3.7) | 18.3 (3.2) | 33.7 (7.2) | 44 (25.5) | 134 (18.1) |
| 50–59 | 270 (18.8) | 202 (19.4) | 29.7 (6.6) | 101.7 (15.6) | 10.4 (4.1) | 18.3 (3.3) | 34.5 (8.0) | 35 (20.9) | 101 (18.2) |
| 60–69 | 291 (15.7) | 231 (17.6) | 30.6 (7.2) | 105.5 (18.2) | - | - | - | 20 (7.9) | 116 (14.6) |
| >70 | 254 (12.3) | 198 (13.5) | 28.8 (5.4) | 103.0 (14.5) | - | - | - | 28 (11.1) | 106 (12.4) |
| Race/ethnicity | |||||||||
| P-value | <0.001* | 0.005* | 0.031* | 0.005* | 0.039* | 0.010* | 0.017* | 0.206 | |
| Non-Hispanic Asian/Other Race/Multiracial | 259 (10.1) | 136 (8.5) | 27.9 (6.5) | 96.1 (16.0) | 9.2 (3.4) | 17.7 (3.1) | 32.7 (6.7) | 47 (16.2) | 134 (12.0) |
| Non-Hispanic White | 523 (63.5) | 360 (61.2) | 28.9 (6.8) | 100.4 (17.4) | 9.4 (4.2) | 18.0 (3.3) | 32.2 (8.4) | 51 (58.3) | 197 (61.7) |
| Non-Hispanic Black | 390 (11.3) | 311 (12.7) | 31.2 (7.6) | 101.8 (18.1) | 10.5 (5.1) | 19.3 (3.4) | 32.5 (9.9) | 43 (10.9) | 168 (11.5) |
| Mexican-American/Other Hispanic | 503 (15.1) | 419 (17.6) | 30.7 (6.8) | 101.0 (15.2) | 10.7 (4.6) | 19.1 (3.4) | 33.9 (8.3) | 52 (14.6) | 212 (14.9) |
| Marital status | |||||||||
| P-value | 0.064 | 0.344 | 0.119 | 0.084 | 0.441 | 0.001* | 0.500 | 0.051 | |
| Married/Living with partner | 980 (63.6) | 733 (65.6) | 29.5 (6.9) | 101.1 (17.1) | 9.9 (4.2) | 18.5 (3.5) | 32.9 (8.0) | 115 (61.5) | 428 (67.3) |
| Widowed/Never married | 444 (23.1) | 300 (20.6) | 28.5 (7.2) | 97.8 (17.8) | 9.1 (4.7) | 18.0 (3.1) | 31.0 (9.4) | 56 (27.4) | 180 (20.5) |
| Divorced/Separated | 251 (13.3) | 193 (13.8) | 29.7 (6.6) | 100.5 (15.6) | 10.4 (4.1) | 18.3 (3.3) | 34.4 (7.9) | 22 (11.2) | 103 (12.2) |
| Monthly family income | |||||||||
| P-value | 0.358 | 0.340 | 0.634 | 0.172 | 0.557 | 0.479 | 0.970 | 0.194 | |
| High | 749 (63.0) | 534 (62.3) | 29.1 (6.8) | 100.2 (16.9) | 9.5 (4.1) | 18.2 (3.4) | 32.4 (7.9) | 95 (63.1) | 343 (66.4) |
| Medium | 235 (12.9) | 167 (12.5) | 29.0 (6.7) | 99.2 (17.3) | 10.0 (4.9) | 18.3 (3.2) | 32.8 (9.7) | 23 (12.5) | 93 (11.8) |
| Low | 568 (24.1) | 437 (25.2) | 30.2 (7.7) | 101.4 (18.1) | 10.3 (4.7) | 18.7 (3.4) | 33.3 (8.6) | 56 (24.5) | 218 (21.7) |
| Current health status | |||||||||
| P-value | <0.001* | <0.001* | <0.001* | <0.001* | 0.001* | <0.001* | 0.669 | 0.283 | |
| Excellent/Very good | 504 (41.7) | 304 (34.6) | 27.2 (5.7) | 94.9 (15.0) | 8.6 (3.7) | 17.5 (3.0) | 30.8 (8.4) | 63 (45.1) | 228 (45.0) |
| Good | 644 (41.4) | 490 (44.6) | 30.3 (7.1) | 103.2 (17.5) | 10.2 (4.5) | 18.7 (3.5) | 33.1 (8.2) | 69 (39.8) | 262 (40.3) |
| Fair/Poor | 390 (16.9) | 333 (20.8) | 32.3 (7.5) | 107.3 (17.5) | 11.7 (4.6) | 19.4 (3.5) | 35.6 (7.9) | 40 (15.1) | 157 (14.7) |
| Physical activity | |||||||||
| P-value | 0.607 | 0.431 | 0.667 | 0.680 | 0.837 | 0.580 | 0.533 | 0.489 | |
| Active | 438 (52.4) | 296 (51.1) | 28.2 (6.6) | 97.2 (17.4) | 9.1 (4.0) | 18.2 (3.3) | 31.3 (8.1) | 71 (55.3) | 216 (54.0) |
| Inactive | 382 (47.6) | 269 (48.9) | 28.7 (6.8) | 98.0 (16.0) | 9.3 (4.1) | 18.1 (3.2) | 31.9 (8.7) | 49 (44.7) | 185 (46.0) |
| Thyroid issues | |||||||||
| P-value | 0.007* | 0.006* | 0.019* | 0.016* | 0.394 | <0.001* | 0.796 | 0.948 | |
| Present | 178 (11.1) | 141 (13.0) | 31.1 (7.4) | 104.3 (17.2) | 12.1 (4.7) | 17.8 (3.4) | 38.3 (5.9) | 18 (11.6) | 73 (11.2) |
| Absent | 1496 (88.9) | 1084 (87.0) | 29.1 (6.8) | 99.7 (17.0) | 9.5 (4.2) | 18.4 (3.4) | 32.1 (8.4) | 175 (88.4) | 638 (88.8) |
| Calorie intake (kcal) | |||||||||
| P-value | 0.939 | 0.982 | 0.307 | 0.002* | 0.004* | <0.001* | 0.910 | 0.105 | |
| <1447 | 390 (21.2) | 288 (21.2) | 29.3 (6.9) | 99.0 (17.0) | 11.2 (4.6) | 18.0 (3.5) | 36.4 (7.2) | 47 (19.7) | 165 (20.9) |
| 1447–1975 | 386 (24.2) | 280 (23.7) | 29.2 (7.4) | 99.8 (18.1) | 10.1 (4.6) | 17.5 (3.3) | 34.4 (8.3) | 37 (24.0) | 153 (21.2) |
| 1976–2531 | 388 (27.7) | 294 (28.2) | 29.4 (6.4) | 100.9 (15.6) | 9.9 (4.1) | 18.3 (3.1) | 33.1 (8.1) | 41 (26.8) | 161 (26.4) |
| >2531 | 388 (26.9) | 278 (27.0) | 29.5 (6.9) | 101.7 (17.7) | 8.6 (3.8) | 19.3 (3.4) | 28.7 (7.9) | 49 (29.5) | 177 (31.5) |
| Smoking status | |||||||||
| P-value | 0.891 | 0.532 | 0.246 | 0.495 | 0.278 | 0.219 | 0.091 | <0.001* | |
| Smoker | 390 (24.2) | 274 (24.1) | 29.6 (7.3) | 101.4 (17.9) | 9.4 (4.3) | 18.7 (3.5) | 31.5 (8.4) | 36 (19.4) | 103 (15.4) |
| Non-smoker | 1222 (75.8) | 911 (75.9) | 29.2 (6.7) | 99.8 (16.7) | 9.7 (4.2) | 18.2 (3.3) | 32.8 (8.3) | 150 (80.6) | 580 (84.6) |
| BPA (μg/g cr) | |||||||||
| P-value | 0.098 | 0.140 | 0.036* | 0.373 | 0.287 | 0.258 | 0.836 | 0.920 | |
| <0.6 | 414 (24.4) | 309 (25.8) | 29.5 (6.5) | 101.7 (16.8) | 9.6 (4.0) | 18.7 (3.3) | 31.9 (7.8) | 55 (26.1) | 191 (25.1) |
| 0.6–0.99 | 417 (24.1) | 304 (23.1) | 29.2 (7.1) | 99.2 (17.7) | 9.7 (4.6) | 18.4 (3.3) | 32.2 (8.6) | 51 (23.5) | 181 (23.8) |
| 1–1.8 | 411 (25.6) | 290 (23.8) | 28.7 (6.7) | 98.3 (16.0) | 9.5 (4.4) | 18.0 (3.2) | 32.4 (8.8) | 46 (27.4) | 172 (24.7) |
| >1.8 | 413 (26.0) | 312 (27.4) | 30.0 (7.2) | 102.2 (17.5) | 10.3 (4.3) | 18.4 (3.6) | 33.9 (8.4) | 36 (23.0) | 160 (26.4) |
Percent, mean, and SD values presented are weighted to account for the NHANES complex survey design
Statistically significant at P<0.05 (using analysis of variance and Wald tests).
Neonicotinoids and measures of adiposity
Detectable levels of any parent neonicotinoid were not significantly associated with measures of adiposity (P>0.05) (Table 2). However, detectable levels of any neonicotinoid metabolite were associated with a 0.62 kg/m2 increase in LMI (95% CI [0.03, 1.21]). Significant inverse associations were seen between acetamiprid and FMI, LMI, body fat percentage, waist circumference, and BMI. Specifically, detectable levels of acetamiprid in urine were associated with approximately a 3 kg/m2 decrease in FMI (95% CI [−4.79, −1.54]) and LMI (95% CI [−5.17, −1.17]) compared to levels below the detection limit. Similarly, detectable levels of acetamiprid were associated with a decrease of approximately 4% in body fat percentage (95% CI [−8.20, −0.62]) and almost 4 kg/m2 in BMI (95% CI [−7.25, −0.51]). Additionally, detectable levels of acetamiprid were associated with a decrease of nearly 10 cm in waist circumference (95% CI [−19.08, −0.51]). Of the neonicotinoid metabolites, 5-hydroxy-imidacloprid had a positive association with LMI (β=0.67 kg/m2, 95% CI [0.04, 1.29]). There was no association between imidacloprid, clothianidin, and N-desmethyl-acetamiprid with any measures of adiposity observed. Detectable concentration of 5-hydroxy-imidacloprid was associated with 11% increase in incidence of being overweight or obese (95% CI [1.04, 1.18]) (Table 3). Although results for association with being overweight or being obese were not significant, a positive trend in association was observed for acetamiprid and clothianidin among overweight participants, while obese participants showed an inverse trend in this association. Subgroup analysis based on smoking status found that imidacloprid had a significant positive association with body fat percentage among smokers, while there was no association among non-smokers (Supplemental Table S2).
Table 2.
Estimated differences (β) and 95% confidence intervals (CI) in adiposity measures by detectable concentrations of urinary neonicotinoids in US adults, NHANES, 2015–2016^
| Fat mass index (N=822) | Lean mass index (N=844) | Body fat % (N=824) | Waist circumference (cm) (N=1462) | BMI (kg/m2) (N=1517) | |
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Any parent neonicotinoid | |||||
| Detect | −0.14 (−1.11, 0.82) | −0.45 (−1.35, 0.44) | 0.24 (−0.96, 1.44) | −1.67 (−4.74, 1.41) | −0.77 (−2.27, 0.73) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Acetamiprid | |||||
| Detect | −3.17 (−4.79, −1.54)* | −3.17 (−5.17, −1.17)* | −4.41 (−8.20, −0.62)* | −9.80 (−19.08, −0.51)* | −3.88 (−7.25, −0.51)* |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Clothianidin | |||||
| Detect | −0.10 (−1.21, 1.01) | −0.49 (−1.66, 0.68) | 0.40 (−1.27, 2.07) | −0.94 (−4.63, 2.74) | −0.71 (−2.37, 0.95) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Imidacloprid | |||||
| Detect | 0.05 (−1.40, 1.49) | −0.06 (−1.15, 1.02) | 0.28 (−1.64, 2.20) | −2.04 (−5.77, 1.69) | −0.53 (−2.61, 1.55) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Any neonicotinoid metabolite | |||||
| Detect | 0.24 (−0.37, 0.86) | 0.62 (0.03, 1.21)* | −0.15 (−1.01, 0.70) | −0.47 (−2.36, 1.42) | 0.15 (−0.65, 0.95) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 5-Hydroxy-imidacloprid | |||||
| Detect | 0.17 (−0.58, 0.93) | 0.67 (0.04, 1.29)* | −0.15 (−1.43, 1.13) | −0.61 (−2.94, 1.72) | −0.03 (−0.91, 0.84) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| N-Desmethyl-acetamiprid | |||||
| Detect | 0.30 (−0.36, 0.96) | 0.60 (−0.06, 1.26) | −0.05 (−0.90, 1.00) | −0.01 (−2.35, 2.33) | 0.28 (−0.60, 1.17) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
Adjusted by age, race, sex, marital status, current health status, and thyroid status.
Statistically significant at P<0.05
Table 3.
Incidence rate ratios (IRRs) and 95% confidence intervals (CI) in overweight/obese measures by detectable concentrations of urinary neonicotinoids in US adults, NHANES, 2015–2016^
| Overweight/Obese (N=1517) | Overweight (N=1517) | Obese (N=1517) | |
|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |
| Any parent neonicotinoid | |||
| Detect | 0.91 (0.69, 1.19) | 1.17 (0.79, 1.74) | 0.72 (0.50, 1.05) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Acetamiprid | |||
| Detect | 0.60 (0.33, 1.08) | 0.55 (0.20, 1.51) | 0.64 (0.20, 2.00) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Clothianidin | |||
| Detect | 0.85 (0.64, 1.12) | 1.13 (0.70, 1.83) | 0.67 (0.43, 1.04) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Imidacloprid | |||
| Detect | 1.04 (0.79, 1.37) | 1.24 (0.70, 2.17) | 0.87 (0.51, 1.46) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Any neonicotinoid metabolite | |||
| Detect | 1.04 (0.97, 1.11) | 1.03 (0.76, 1.40) | 1.04 (0.83, 1.31) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 5-Hydroxy-imidacloprid | |||
| Detect | 1.11 (1.04, 1.18)* | 1.08 (0.81, 1.46) | 1.13 (0.90, 1.42) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| N-Desmethyl-acetamiprid | |||
| Detect | 1.02 (0.93, 1.13) | 1.05 (0.77, 1.44) | 1.00 (0.78, 1.28) |
| Non-detect | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
Adjusted by age, race, sex, marital status, health status, and thyroid status.
Statistically significant at P<0.05.
Effect measure modification by sex
There was evidence of effect measure modification by sex for the association between N-desmethyl-acetamiprid and LMI (P for interaction = 0.075) (Figure 1). A significant positive association was observed between detectable urinary N-desmethyl-acetamiprid and LMI among males (β = 1.14 kg/m2, 95% CI [0.38, 1.90]), while a null association was observed in females (β = 0.14 kg/m2, 95% CI [−0.79, 1.07]). There was evidence of significant sex differences between N-desmethyl-acetamiprid and FMI (P for interaction = 0.095), and body fat percentage (P for interaction = 0.072). Males had a positive association while females had an inverse association, however the sex-specific regression estimates were not statistically significant (P>0.05). Effect measure modification by sex was insignificant for the association between neonicotinoid metabolites with waist circumference and BMI (Supplemental Table S3). Similar results were observed for the association with being overweight or obese (Supplemental Table S4).
Figure 1.
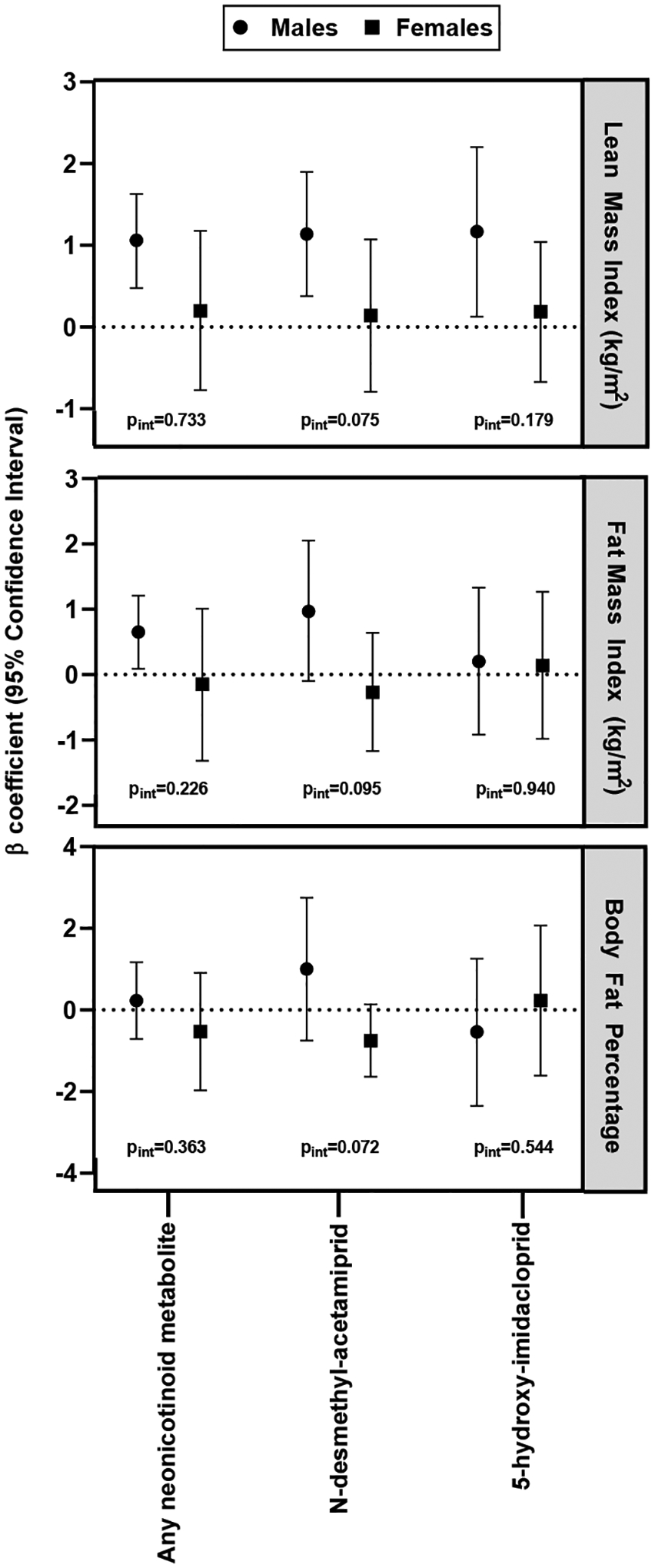
Association between detectable levels of neonicotinoid metabolites with adiposity measures in adults by sex. Adjusted by age, race, sex, marital status, current health status, and thyroid status.^
^ LMI (N=844); FMI (N=822); Body fat percentage (N=824).
Sensitivity analyses
Additional adjustment for BPA concentrations as well as removal of outliers for FMI, LMI, waist circumference, and BMI did not change the overall conclusions of the study (results not shown). However, after additionally adjusting for daily caloric intake, 5-hydroxy-imidacloprid was no longer associated with LMI (Supplemental Tables S5, S6). Lastly, adjusting for physical activity resulted in some findings that were no longer significant, including the inverse association between acetamiprid with body fat percentage, waist circumference, and BMI as well as the positive association between 5-hydroxy-imidacloprid and LMI (Supplemental Tables S7, S8). However, an inverse association was still observed between acetamiprid with FMI and LMI. Additionally, it was found that clothianidin had a significant inverse association with LMI and BMI. With regard to the effect measure modification analyses by sex, adjustment for daily kilocalorie intake showed that sex differences for the association between N-desmethyl-acetamiprid and FMI were no longer present (Supplementary Tables S9, S10). Similarly, adjustment for physical activity no longer showed evidence of sex differences between imidacloprid and N-desmethyl-acetamiprid with LMI (Supplemental Tables S11, S12). Lastly, our conclusions did not differ following the examination of the structural validity of the statistical models using robustness checks when regression specifications are modfied.
Discussion
The findings of this study were mixed. While it was observed that detectable levels of acetamiprid in urine were inversely associated with several adiposity measures in adults, detectable levels of 5-hydroxy-imidacloprid were significantly associated with greater odds of being overweight or obese. Further, any detectable neonicotinoid metabolite and 5-hydroxy-imidacloprid was associated with increased LMI. Evidence of effect measure modification by sex was present, however, the findings were inconsistent.
Previous toxicological studies in other mammalian species have shown mixed results with respect to the association between neonicotinoids and adiposity. Some studies have reported an inverse association between acetamiprid and obesity (Mosbah et al., 2018; Sheets et al., 2016; Terayama et al., 2016; Terayama et al., 2018). Terayama et al (2016, 2018) conducted a study in 8-week old mice by dividing into four group- a control group, a group given dimethyl sulfoxide, a group given 10 times greater than No Observed Adverse Effect Level (NOAEL) of acetamiprid, and a group given 100 times greater than NOAEL acetamiprid. They observed that mice administered acetamiprid at a level 100 times greater than the NOAEL showed a significant decrease in body weight as compared to the control group or the group given a dose of acetamiprid 10 times greater than NOAEL. Similarly, Mosbah et al (2018) reported that male Wistar rats aged 8–12 weeks old given 27 mg/kg/day acetamiprid in their diet over a 45 day period exhibited a greater decrease in weight as compared to the control group. Sheets et al (2016) divided 25–30 pregnant rats into four groups, where each group was administered a different dosage of acetamiprid. Outcome assessment was done on the 20th gestational day, where it was observed that there was a 4–5% decrease in body weight among the pregnant rats dosed with acetamiprid as compared to the control group. Additionally, they also observed that the offspring of these rats had a 5–10% lower body weight compared to offspring of the control group, when measured on the first postnatal day (PND). Imidacloprid had an inverse association with body weight in adult mice (Arfat et al., 2014; Burke et al., 2018) and female rats (Bhardwaj et al., 2010). However, Sun et al (2016, 2017) observed that imidacloprid was associated with an increase in body weight, with similar positive associations seen with clothianidin and body weight (Tanaka, 2012).
Neonicotinoids may be associated with decreased adiposity for a few reasons. Imidacloprid toxicity has been associated with reduction in appetite among mice. A study by Burke et al (2018) showed that mice fed an imidacloprid rich diet subsequently reduced their food intake, which led to a decrease in body weight. This was attributed to an increase in cholinergic activity secondary to chronic neonicotinoid exposure. An increase in cholinergic activity leads to changes in acetylcholine receptor (AchR) signaling frequency, which is partially responsible for appetite suppression (Herman et al., 2016). Imidacloprid has also been linked to a potential “anorexic” effect among exposed white- tailed songbirds as compared to the controls (Eng et al., 2019). Additionally, there is evidence to support alteration of gut microflora as a result of imidacloprid toxicity, specifically in Acetobacter and Lactobacillus genera (Yuan et al., 2019). Imidacloprid is chemically similar to acetamiprid, hence these mechanisms of action may explain the findings of our study as well.
Imidacloprid has been associated with the exacerbation of metabolic stress which may lead to weight gain (Sun et al., 2017). Disruptions in metabolic homeostasis are associated with an increased production of glucocorticoids, which are catalysts for an increased rate of adipocyte development and differentiation (Karalis et al., 2009). This mechanism is consistent with the development of obesity, which could explain the positive associations between 5-hydroxy-imidacloprid and LMI seen in this study.
Mixed findings in the present study for 5-hydroxy-imidacloprid may be partially attributed to the usage of BMI to categorize individuals as overweight and obese. Although BMI is a common measure used to assess obesity, it is not a good indicator of the type of body mass assessed and is unable to make a distinction between fat mass and lean mass (Weber et al., 2014). Additionally, the magnitude of the positive association seen with 5-hydroxy-imidacloprid and LMI is small, while it is associated with nearly double the odds of being overweight or obese. These findings show that 5-hydroxy-imidacloprid increases fat mass along with an increase in lean mass, which may explain its positive association with LMI and being overweight or obese.
While 5-hydroxy-imidacloprid showed a significant positive association with being overweight or obese, the parent neonicotinoid (imidacloprid) was not associated with any measures of adiposity. A potential reason could be related to a higher detection frequency observed for 5-hydroxy-imidacloprid (42.9%) as compared to imidacloprid (11.6%) among the study participants. Metabolism of imidacloprid in rats showed that it is not distributed to adipose tissue (Sheets, 2010). This might explain why imidacloprid demonstrated insignificant findings in this study.
Effect modifications by sex were observed for the associations for N-desmethyl-acetamiprid with FMI, LMI and body fat percentages, though it is unclear whether males or females are more sensitive to neonicotinoids. A significant positive association was observed between detectable urinary N-desmethyl-acetamiprid and LMI among males, while a null association was observed in females. Sex differences seen in the association between neonicotinoid metabolites and adiposity may be due to different biological mechanisms. Findings by Lukowicz et al (2018) indicate that increased oxidative stress in male mice may be responsible for the adipogenic effect of neonicotinoids. This generates higher levels of triglycerides in the liver, which may be responsible for weight changes. However, another study on male rats observed an increase in cholinergic activity induced by clothianidin, which subsequently increased intestinal motility, leading to reduction in body weight (Onaru et al., 2020). In female mice, findings indicated that neonicotinoids may be responsible for the activation of peroxisome proliferator-activated receptor (PPAR)-alpha (a prominent receptor involved in fatty acid metabolism) as well as the release of free radicals, which may be responsible for an increase in fat metabolism, subsequently leading to reduced body weight (Lukowicz et al., 2018). A protective effect generated by estrogen could also be a reason for reduced adiposity associated with neonicotinoid exposure (Sun et al., 2017). This could explain the mixed directionality of association seen among males and females in this study. However, Sun et al conducted two separate studies on male and female mice, both of which showed an increase in weight associated with imidacloprid, which led them to conclude that neonicotinoid associated weight gain is independent of sex (2016; 2017).
Epidemiological studies conducted have reported positive and null associations between neonicotinoids and adiposity (Makris et al., 2019; Peng et al., 2020; Wang et al., 2020). Peng et al (2020) observed that increased concentrations of imidacloprid in hair samples were positively associated with BMI among Chinese women aged 25–45 years. In contrast, a null association was observed between concentrations of a neonicotinoid metabolite (6-chloronicotinic acid) and BMI z-scores in a randomized control trial among 11–12-year-old children from 6 schools in Cyprus, where the control group was given an organic diet while the other group had a regular diet (Makris et al., 2019), while detectable levels of N-desmethyl-acetamiprid in urine were associated with greater odds of being obese among a cohort of 7–11-year-old children in Shanghai, China, although results were only borderline significant (Wang et al., 2020). The present study did not find a positive association between N-desmethyl-acetamiprid and obesity. A possible reason could be due to the differences in the detection frequencies of N-desmethyl-acetamiprid. This study had a detection frequency of 32.4% in urine for N-desmethyl-acetamiprid, while the study by Wang et al (2020) had a detection frequency of 62.4%.The higher detection frequency, along with differences related to geographic location may explain the difference in results.
This study had several strengths, including the usage of data from NHANES, a nationally representative population, which increases the generalizability of the findings. Second, the use of NHANES data made it possible to adjust for a wide range of potential confounders. Third, a number of sensitivity analyses were performed to ensure the final conclusions did not change, including additionally adjusting for BPA concentrations, daily kilocalorie intake, and weekly physical activity levels. Fourth, a comprehensive list of adiposity measures was used. Previous epidemiological studies only used BMI (Makris et al., 2019; Peng et al., 2020; Wang et al., 2020). Fifth, effect measure modification by sex was analyzed. This had not been examined in previous epidemiological studies to our knowledge, although some toxicological studies have reported on potential sex differences in the association with neonicotinoids and adiposity (Bhasker & Mohanty, 2014; Burke et al., 2018; Lukowicz et al., 2018; Tanaka, 2012).
This study had a number of limitations. First, since this was a cross-sectional exploratory study, a temporal association between neonicotinoids and adiposity could not be made. Second, neonicotinoids have a short half-life. Quantified concentrations of neonicotinoids may not reflect true exposure of neonicotinoids over time, which may have led to misclassification bias. Third, since a number of questions were self-reported, there could be a reporting bias. Fourth, a two-year cycle data may not be sufficient to study sex-specific associations. Fifth, detection frequencies of neonicotinoids were lower than in previous epidemiological studies, which may affect the results. Further, some findings should be interpreted with caution given the low detection frequencies of several neonicotinoid compounds. In particular, the inverse associations between detectable concentrations of acetamiprid with FMI, LMI, body fat percentage, waist circumference, and BMI as acetamiprid had a detection frequency of 0.3%. Finally, this study did not examine a dose related response since neonicotinoid measures were examined as “detect” versus “non-detect”.
Conclusion
The current study was the first to our knowledge to examine whether exposure to neonicotinoids is associated with adiposity in US adults. Findings were inconsistent since acetamiprid had an inverse association with adiposity measures, while 5-hydroxy-imidacloprid had a positive association with being overweight or obese, and a positive association with LMI. There was evidence of effect modification by sex in the association, however, results were inconsistent with regard to the directionality by sex. Future research should focus on examining the association between neonicotinoids and adiposity using a prospective study design. Additionally, further studies should examine this association in geographic locations with higher neonicotinoid use.
Supplementary Material
Highlights.
Neonicotinoid compounds may be associated with the pathogenesis of adiposity.
Acetamiprid was inversely associated with all the adiposity measures examined.
5-Hydroxy-imidacloprid was positively associated with being overweight/obese.
Sex differences were seen with N-desmethyl-acetamiprid and some adiposity measures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit Author Statement
All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept (AMG, AMV), design (AMG, AMV), analysis (AMG), interpretation of data (AMG, SM, CC, CZ, AC, AMV), writing (AMG), or revision of the manuscript (AMG, SM, CC, CZ, AC, AMV).
References
- Aguinis H (1995). Statistical power problems with moderated multiple regression in management research. J Manage, 21(6), 1141–1158. 10.1016/0149-2063(95)90026-8 [DOI] [Google Scholar]
- Arfat Y, Mahmood N, Tahir MU, Rashid M, Anjum S, Zhao F, Li DJ, Sun YL, Hu L, Zhihao C, Yin C, Shang P, & Qian AR (2014). Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicology Reports, 1, 554–561. 10.1016/j.toxrep.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Serafim AB, Morales-Agudelo P, Vidal M, Calafat AM, & Ospina M (2019). Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 411(3), 669–678. 10.1007/s00216-018-1481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, & Field LM (2018). Neonicotinoids. Current Biology: CB, 28(14), R772–R773. 10.1016/j.cub.2018.05.061 [DOI] [PubMed] [Google Scholar]
- Berheim EH, Jenks JA, Lundgren JG, Michel ES, Grove D, & Jensen WF (2019). Effects of neonicotinoid insecticides on physiology and reproductive characteristics of captive female and fawn white-tailed deer. Scientific Reports, 9(1), 4534. 10.1038/s41598-019-40994-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S, Srivastava MK, Kapoor U, & Srivastava LP (2010). A 90 days oral toxicity of imidacloprid in female rats: Morphological, biochemical and histopathological evaluations. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 48(5), 1185–1190. 10.1016/j.fct.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Bhaskar R, & Mohanty B (2014). Pesticides in mixture disrupt metabolic regulation: In silico and in vivo analysis of cumulative toxicity of mancozeb and imidacloprid on body weight of mice. General and Comparative Endocrinology, 205, 226–234. 10.1016/j.ygcen.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, & Main KM (2012). Thyroid effects of endocrine disrupting chemicals. Molecular and Cellular Endocrinology, 355(2), 240–248. 10.1016/j.mce.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Brunet JL, Badiou A, & Belzunces LP (2005). In vivo metabolic fate of [14C]-acetamiprid in six biological compartments of the honeybee, Apis mellifera L. Pest Management Science, 61(8), 742–748. 10.1002/ps.1046 [DOI] [PubMed] [Google Scholar]
- Burke AP, Niibori Y, Terayama H, Ito M, Pidgeon C, Arsenault J, Camarero PR, Cummins CL, Mateo R, Sakabe K, & Hampson DR (2018). Mammalian susceptibility to a neonicotinoid insecticide after fetal and early postnatal exposure. Scientific Reports, 8(1), 16639. 10.1038/s41598-018-35129-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2016). National Health and Nutrition Survey (NHANES) Anthropometry Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Anthropometry_Procedures_Manual.pdf
- CDC (2020). About Adult BMI. Healthy Weight, Nutrition, and Physical Activity. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html
- CDC (2020). How much physical activity do adults need? Physical Activity. https://www.cdc.gov/physicalactivity/basics/adults/index.htm
- Chen M, Tao L, McLean J, & Lu C (2014). Quantitative analysis of neonicotinoid insecticide residues in foods: Implication for dietary exposures. Journal of Agricultural and Food Chemistry, 62(26), 6082–6090. 10.1021/jf501397m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzguner V, & Erdogan S (2010). Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pesticide Biochemistry and Physiology, 97(1), 13–18. 10.1016/j.pestbp.2009.11.008 [DOI] [Google Scholar]
- Eng ML, Stutchbury B, & Morrissey CA (2019). A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science (New York, N.Y.), 365(6458), 1177–1180. 10.1126/science.aaw9419 [DOI] [PubMed] [Google Scholar]
- FDA (2020). How to Understand and Use the Nutrition Facts Label. Food. https://www.fda.gov/food/new-nutrition-facts-label/how-understand-and-use-nutrition-facts-label
- Goulson D (2013). Review: An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol, 50, 977–987. doi: 10.1111/1365-2664.12111 [DOI] [Google Scholar]
- Grün F, & Blumberg B (2009). Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology, 304(1–2), 19–29. 10.1016/j.mce.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Kim KW, Kim KI, Na KY, Chae DW, Kim S, & Chin HJ (2010). Lean mass index: A better predictor of mortality than body mass index in elderly Asians. Journal of the American Geriatrics Society, 58(2), 312–317. 10.1111/j.1532-5415.2009.02672.x [DOI] [PubMed] [Google Scholar]
- Han W, Tian Y, & Shen X (2018). Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere, 192, 59–65. 10.1016/j.chemosphere.2017.10.149 [DOI] [PubMed] [Google Scholar]
- Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, Tepe B, Carlson JC, Ung K, Selever J, Tong Q, & Arenkiel BR (2016). A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature, 538(7624), 253–256. 10.1038/nature19789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández AF, Casado I, Pena G, Gil F, Villanueva E, & Pla A (2008). Low level of exposure to pesticides leads to lung dysfunction in occupationally exposed subjects. Inhalation Toxicology, 20(9), 839–849. 10.1080/08958370801905524 [DOI] [PubMed] [Google Scholar]
- Hinton BJ, Fan B, Ng BK, & Shepherd JA (2017). Dual energy X-ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999–2004 with additional visualization methods. PloS One, 12(3), e0174180. 10.1371/journal.pone.0174180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JM, Igielnik R, & Kochhar R (2020, January 9). Pew Research Center, January 2020. Most Americans Say There Is Too Much Economic Inequality in the U.S., but Fewer Than Half Call It a Top Priority. Pew Research Center. https://www.pewresearch.org/social-trends/2020/01/09/most-americans-say-there-is-too-much-economic-inequality-in-the-u-s-but-fewer-than-half-call-it-a-top-priority/ [Google Scholar]
- Ihara M, & Matsuda K (2018). Neonicotinoids: Molecular mechanisms of action, insights into resistance and impact on pollinators. Current Opinion in Insect Science, 30, 86–92. 10.1016/j.cois.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Karalis KP, Giannogonas P, Kodela E, Koutmani Y, Zoumakis M, & Teli T (2009). Mechanisms of obesity and related pathology: Linking immune responses to metabolic stress. The FEBS Journal, 276(20), 5747–5754. 10.1111/j.1742-4658.2009.07304.x [DOI] [PubMed] [Google Scholar]
- Kumar S, & Kelly AS (2017). Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clinic Proceedings, 92(2), 251–265. 10.1016/j.mayocp.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Kyle UG, Schutz Y, Dupertuis YM, & Pichard C (2003). Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition (Burbank, Los Angeles County, Calif.), 19(7–8), 597–604. 10.1016/s0899-9007(03)00061-3 [DOI] [PubMed] [Google Scholar]
- Leemans M, Couderq S, Demeneix B, & Fini JB (2019). Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Frontiers in Endocrinology, 10, 743. 10.3389/fendo.2019.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowicz C, Ellero-Simatos S, Régnier M, Polizzi A, Lasserre F, Montagner A, Lippi Y, Jamin EL, Martin JF, Naylies C, Canlet C, Debrauwer L, Bertrand-Michel J, Al Saati T, Théodorou V, Loiseau N, Mselli-Lakhal L, Guillou H, & Gamet-Payrastre L (2018). Metabolic effects of a chronic dietary exposure to a low-dose pesticide cocktail in mice: Sexual dimorphism and role of the constitutive androstane receptor. Environmental Health Perspectives, 126(6), 067007. 10.1289/EHP2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris KC, Konstantinou C, Andrianou XD, Charisiadis P, Kyriacou A, Gribble MO, & Christophi CA (2019). A cluster-randomized crossover trial of organic diet impact on biomarkers of exposure to pesticides and biomarkers of oxidative stress/inflammation in primary school children. PloS One, 14(9), e0219420. 10.1371/journal.pone.0219420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfo JT, Fujioka K, Ikenaka Y, Nakayama SM, Mizukawa H, Aoyama Y, Ishizuka M, & Taira K (2015). Relationship between urinary N-desmethyl-acetamiprid and typical symptoms including neurological findings: A prevalence case-control study. PloS One, 10(11), e0142172. 10.1371/journal.pone.0142172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, & Shimomura I (2013). Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obesity Research & Clinical Practice, 7(5), e330–e341. 10.1016/j.orcp.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Mayes JS, & Watson GH (2004). Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity Reviews : An Official Journal of the International Association for the Study of Obesity, 5(4), 197–216. 10.1111/j.1467-789X.2004.00152.x [DOI] [PubMed] [Google Scholar]
- Mikolić A, & Karačonji IB (2018). Imidacloprid as reproductive toxicant and endocrine disruptor: investigations in laboratory animals. Arhiv Za Higijenu Rada I Toksikologiju, 69(2), 103–108. 10.2478/aiht-2018-69-3144 [DOI] [PubMed] [Google Scholar]
- Mosbah R, Djerrou Z, & Mantovani A (2018). Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug and Chemical Toxicology, 41(2), 206–212. 10.1080/01480545.2017.1337127 [DOI] [PubMed] [Google Scholar]
- Mullur R, Liu YY, & Brent GA (2014). Thyroid hormone regulation of metabolism. Physiological Reviews, 94(2), 355–382. 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey. (2018, June 18). https://www.cdc.gov/visionhealth/vehss/data/national-surveys/national-health-and-nutrition-examination-survey.html
- Nauen R, Jeschke P, & Copping L (2008). In focus: Neonicotinoid insecticides. Pest Management Science, 64(11), 1081. 10.1002/ps.1659 [DOI] [PubMed] [Google Scholar]
- Onaru K, Ohno S, Kubo S, Nakanishi S, Hirano T, Mantani Y, Yokoyama T, & Hoshi N (2020). Immunotoxicity evaluation by subchronic oral administration of clothianidin in Sprague-Dawley rats. The Journal of Veterinary Medical Science, 82(3), 360–372. 10.1292/jvms.19-0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina M, Wong LY, Baker SE, Serafim AB, Morales-Agudelo P, & Calafat AM (2019). Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 National Health and Nutrition Examination Survey. Environmental Research, 176, 108555. 10.1016/j.envres.2019.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN (2012). Thyroid hormone and obesity. Current Opinion in Endocrinology, Diabetes, and Obesity, 19(5), 408–413. 10.1097/MED.0b013e328355cd6c [DOI] [PubMed] [Google Scholar]
- Peng FJ, Hardy EM, Mezzache S, Bourokba N, Palazzi P, Stojiljkovic N, Bastien P, Li J, Soeur J, & Appenzeller B (2020). Exposure to multiclass pesticides among female adult population in two Chinese cities revealed by hair analysis. Environment International, 138, 105633. 10.1016/j.envint.2020.105633 [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, & Pechacek TF (2006). Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environmental Health Perspectives, 114(6), 853–858. 10.1289/ehp.8850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnak T, & Yilmaz Y (2013). Liver disease and malnutrition. Best Practice & Research. Clinical Gastroenterology, 27(4), 619–629. 10.1016/j.bpg.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, & Yadav UC (2016). Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sciences, 148, 183–193. 10.1016/j.lfs.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Sheets LP (2010). Chapter 95 - Imidacloprid: A neonicotinoid insecticide. Hayes’ Handbook of Pesticide Toxicology (Third Edition). Academic Press. https://doi-org.ezproxy.library.unlv.edu/10.1016/B978-0-12-374367-1.00095-1 [Google Scholar]
- Sheets LP, Li AA, Minnema DJ, Collier RH, Creek MR, & Peffer RC (2016). A critical review of neonicotinoid insecticides for developmental neurotoxicity. Critical Reviews in Toxicology, 46(2), 153–190. 10.3109/10408444.2015.1090948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner K, & Goh KS (2012). Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bulletin of Environmental Contamination and Toxicology, 88(3), 316–321. 10.1007/s00128-011-0515-5 [DOI] [PubMed] [Google Scholar]
- Sun Q, Qi W, Xiao X, Yang SH, Kim D, Yoon KS, Clark JM, & Park Y (2017). Imidacloprid promotes high fat diet-induced adiposity in female C57BL/6J mice and enhances adipogenesis in 3T3-L1 adipocytes via the AMPkα-mediated pathway. Journal of Agricultural and Food Chemistry, 65(31), 6572–6581. 10.1021/acs.jafc.7b02584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Xiao X, Kim Y, Kim D, Yoon KS, Clark JM, & Park Y (2016). Imidacloprid promotes high fat diet-induced adiposity and insulin resistance in male C57BL/6J mice. Journal of Agricultural and Food Chemistry, 64(49), 9293–9306. 10.1021/acs.jafc.6b04322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T (2012). Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Research. Part B, Developmental and reproductive Toxicology, 95(2), 151–159. 10.1002/bdrb.20349 [DOI] [PubMed] [Google Scholar]
- Terayama H, Endo H, Tsukamoto H, Matsumoto K, Umezu M, Kanazawa T, Ito M, Sato T, Naito M, Kawakami S, Fujino Y, Tatemichi M, & Sakabe K (2016). Acetamiprid accumulates in different amounts in murine brain regions. International Journal of Environmental Research and Public Health, 13(10), 937. 10.3390/ijerph13100937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama H, Qu N, Endo H, Ito M, Tsukamoto H, Umemoto K, Kawakami S, Fujino Y, Tatemichi M, & Sakabe K (2018). Effect of acetamiprid on the immature murine testes. International Journal of Environmental Health Research, 28(6), 683–696. 10.1080/09603123.2018.1504897 [DOI] [PubMed] [Google Scholar]
- Thiese MS, Ronna B, & Ott U (2016). P value interpretations and considerations. J Thorac Dis, 8(9), E928–E931. 10.21037/jtd.2016.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama J, Harada KH, Koizumi A, Sugiura Y, Kondo T, Saito I, & Kamijima M (2015). Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environmental Science & Technology, 49(24), 14522–14528. 10.1021/acs.est.5b03062 [DOI] [PubMed] [Google Scholar]
- Vincent HK, & Taylor AG (2006). Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. International Journal of Obesity (2005), 30(3), 400–418. 10.1038/sj.ijo.0803177 [DOI] [PubMed] [Google Scholar]
- Wang H, Yang D, Fang H, Han M, Tang C, Wu J, Chen Y, & Jiang Q (2020). Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: A biomonitoring-based study. Environment International, 143, 105918. Advance online publication. 10.1016/j.envint.2020.105918 [DOI] [PubMed] [Google Scholar]
- Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga MR, Yuan Z, & Martínez MA (2018). Mechanism of neonicotinoid toxicity: Impact on oxidative stress and metabolism. Annual Review of Pharmacology and Toxicology, 58, 471–507. 10.1146/annurev-pharmtox-010617-052429 [DOI] [PubMed] [Google Scholar]
- Weber DR, Leonard MB, Shults J, & Zemel BS (2014). A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. The Journal of Clinical Endocrinology and Metabolism, 99(9), 3208–3216. 10.1210/jc.2014-1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Pan Z, Jin C, Ni Y, Fu Z, & Jin Y (2019). Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere, 227, 425–434. 10.1016/j.chemosphere.2019.04.088 [DOI] [PubMed] [Google Scholar]
- Zacharewski T (1998). Identification and assessment of endocrine disruptors: Limitations of in vivo and in vitro assays. Environmental Health Perspectives, 106 Suppl 2(Suppl 2), 577–582. 10.1289/ehp.98106577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Hunter M, James A, Lim EM, Cooke BR, & Walsh JP (2017). Discordance between fat mass index and body mass index is associated with reduced bone mineral density in women but not in men: The Busselton Healthy Ageing Study. Osteoporosis international : A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 28(1), 259–268. 10.1007/s00198-016-3710-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


