Abstract
Washed cell suspensions of the facultative methylotroph strain IMB-1 grown on methyl bromide (MeBr) were able to consume methyl chloride (MeCl) and methyl iodide (MeI) as well as MeBr. Consumption of >100 μM MeBr by cells grown on glucose, acetate, or monomethylamine required induction. Induction was inhibited by chloramphenicol. However, cells had a constitutive ability to consume low concentrations (<20 nM) of MeBr. Glucose-grown cells were able to readily oxidize [14C]formaldehyde to 14CO2 but had only a small capacity for oxidation of [14C]methanol. Preincubation of cells with MeBr did not affect either activity, but MeBr-induced cells had a greater capacity for [14C]MeBr oxidation than did cells without preincubation. Consumption of MeBr was inhibited by MeI, and MeCl consumption was inhibited by MeBr. No inhibition of MeBr consumption occurred with methyl fluoride, propyl iodide, dibromomethane, dichloromethane, or difluoromethane, and in addition cells did not oxidize any of these compounds. Cells displayed Michaelis-Menten kinetics for the various methyl halides, with apparent Ks values of 190, 280, and 6,100 nM for MeBr, MeI, and MeCl, respectively. These results suggest the presence of a single oxidation enzyme system specific for methyl halides (other than methyl fluoride) which runs through formaldehyde to CO2. The ease of induction of methyl halide oxidation in strain IMB-1 should facilitate its mass culture for the purpose of reducing MeBr emissions to the atmosphere from fumigated soils.
Concern over the integrity of the stratospheric ozone layer has focused attention on the transport of halogenated substances into the atmosphere from both natural and anthropogenic sources. Initial attention was drawn to the unreactive chlorofluorocarbons, which have long atmospheric residence times (29). These anthropogenic substances are now banned from usage under the auspices of the Montreal Protocol. However, other, more reactive, halocarbons also enter the stratosphere and contribute to ozone degradation. Methyl iodide (MeI), methyl chloride (MeCl), and methyl bromide (MeBr) are monohalogenated methanes which originate from a combination of anthropogenic and natural sources (3, 15, 18, 21). Of these methyl halides, MeBr has the highest ozone degradation potential owing to the high reactivity of Br with O3, as well as MeBr’s relatively long atmospheric residence time (∼0.8 year) when compared with those of other brominated methanes or methyl halides (35, 36, 38, 47). In addition to its oxidation by tropospheric OH radicals (25), other biogeochemical sinks for MeBr include its dissolution from the atmosphere into the oceans, where it is destroyed by chemical and/or biological processes (12, 21, 48), and its consumption by bacteria in soils (16, 37, 38). Methyl halide-oxidizing bacteria have been isolated from soils (5, 9, 28), from seawater (12), and, most recently, from forest leaf litter (7).
The largest anthropogenic use of MeBr is as a preplanting, biocidal agricultural fumigant. Lesser amounts are used in the preservation of structures, for the storage of grains, and to prevent the unintended importation of biological pests (2). Continued international use of MeBr will be constrained and eventually eliminated by the year 2010 under the auspices of the Montreal Protocol (41). However, technologies which drastically reduce the emissions of MeBr to the atmosphere are being pursued in the hope of finding ways to continue employing MeBr as a preplanting fumigant without endangering the ozone layer. Proposed emission controls include improved physical constraints, such as use of impermeable tarps to cover fumigated fields (44), and bacterial oxidation of the injected MeBr (5).
The facultative methylotroph strain IMB-1 was isolated from fumigated soil and is a member of the alpha subgroup of the class Proteobacteria. It is able to grow on methyl halides, methylated amines, and non-C1 compounds such as glucose, acetate, and pyruvate (5, 28). MeBr oxidation in soils is greatly enhanced by addition of these cells to the soils, and it was also noted that oxidation is faster with cells grown on MeBr than with cells grown on glucose (5). Cells grown on methyl halides as well as on non-C1 compounds (e.g., glucose) have the capacity to oxidize [14C]MeBr to 14CO2, but it was not clear whether the enzymes responsible for methyl halide oxidation were inducible or constitutive. Previous work by Connell Hancock et al. (5) suggested that the enzyme for methyl halide oxidation was constitutive. The issue of inducibility is of considerable practical value because it is far easier to grow cells to high density on glucose than on toxic methyl halides.
In this paper we report on further aspects of the physiology and substrate affinities of strain IMB-1. Our results indicate that it has a constitutive methyl halide oxidation system capable of degrading low (nanomolar) levels of MeBr, MeCl, and MeI, and we report the different substrate affinities (Ks) for these substances. Induction of methyl halide oxidation at high (micromolar) concentrations occurs rapidly, a factor which has considerable practical significance for its mass culture.
MATERIALS AND METHODS
Cell suspension experiments.
Strain IMB-1 was grown in the mineral salts medium of Doronina et al. (9), which consisted of the following (in grams per liter): KH2PO4 (2.0), (NH4)2SO4 (2.0), MgSO4 · 7H2O (0.125), and FeSO4 · 7H2O (0.002) plus 1.0 ml of SL-10 trace minerals (45). Growth substrates included the following: glucose (30 mM), Na acetate (10 mM), and monomethylamine (15 mM). Growth on MeCl or MeBr was achieved by sterile, pulsed additions of the individual methyl halides (typically 250 μM per pulse, for a cumulative concentration of ∼1 mM over the incubation) to the medium during growth in order to avoid the toxicity associated with high initial concentrations (5, 28). Cells were grown in the dark at 22°C with constant rotary shaking (200 rpm). Aerobic growth on methyl halides was tested in crimp-sealed vessels which consisted of either 25-ml “Balch”-type test tubes (10 ml of medium) or serum bottles, ranging from 159 ml (70 ml of medium) to 1 liter (500 ml of medium). Growth on the nonvolatile substrates was tested either in test tubes (see above) or in 500- or 1,000-ml conical flasks which contained 250 or 500 ml of medium. Flasks were sealed with sterile cotton plugs to facilitate aeration.
Cells were harvested during late exponential phase and washed twice with medium lacking substrates, trace elements, and (NH4)2SO4. Washed cell suspensions (20 ml) were placed in serum bottles and air sealed with butyl rubber stoppers (5). Either concentrated or diluted cell suspensions were used, with densities ranging from 3.3 × 105 to 3.4 × 109 cells/ml as determined by acridine orange direct counting (17). The coefficient of variation for bacterial counts per field was typically 10 to 30%. Low cell densities were obtained by diluting cultures with the mineral salts medium described above in order to slow down the kinetic experiments so that accurate methyl halide uptake data could be obtained (see below). High cell densities were employed in the inhibitor and 14C-substrate experiments.
The effect of various methyl halides upon the consumption of MeBr by MeBr-grown cells was investigated by resuspending the washed cells in 57-ml serum bottles which were crimp-sealed under air and injected with ∼0.4 mM MeBr plus various concentrations (see Results) of MeCl, MeI, or methyl fluoride (MeF). The reverse experiment, namely the effect of MeBr upon the consumption of MeCl, was also performed. The effect of other halogenated methanes, including difluoromethane (DFM), dichloromethane (DCM), and dibromomethane (DBM), on consumption of MeBr was also tested. The effect of 1, 5, and 20 μM propyl iodide (PpyI) on uptake of 70 μM MeBr in the dark was examined by injecting the PpyI into cell suspensions which were actively consuming MeBr. All liquid halocarbons (MeI, PpyI, DBM, DCM) were added either “neat” or as dilutions made in deionized water in sealed vials which lacked a gas phase to prevent phase partitioning. The effect of chloramphenicol (20 or 250 μg/ml) on uptake of MeBr, MeCl, or MeI by cells grown on glucose, acetate, monomethylamine, MeCl, or MeBr was investigated with washed cell suspensions in order to determine whether methyl halide oxidation required de novo protein synthesis.
Kinetic experiments were conducted on MeBr-grown cells in an effort to determine the Michaelis-Menten parameters associated with the oxidation of MeBr, MeCl, and MeI. Washed cell suspensions were diluted by a factor of ∼100 for MeBr and MeI in an effort to slow the reactions. No dilution was needed for MeCl. Because of the frequent sampling of the gas phase, it was necessary to conduct these experiments with larger-volume serum bottles (119 or 159 ml). Rates of methyl halide degradation were determined by using a least-squares linear fit and were normalized to cell number. Kinetic parameters were calculated by a nonlinear least-squares fit to the Michaelis-Menten equation (S-Plus v4.5 software). Apparent first-order rate constants were calculated from the following equation: kapp = Vmax/Ks.
Experiments with 14C-labeled substrates.
Glucose-grown cells were divided into two equal portions; one was exposed to 18 μM MeBr for 6 h, and one was not. Cells were harvested and washed, and cell suspensions (3 ml) were placed into 13-ml serum bottles, which were crimp-sealed. The following 14C-labeled substrates were employed: 14C-MeBr (0.65 μCi added; NEN, Boston, Mass.) (specific activity [sp. act.] = 49 mCi/mmol; purity = 99.9%), 14C-methanol (0.45 μCi added; Sigma Chemicals Inc., St. Louis, Mo.) (sp. act. = 61.2 mCi/mmol; purity ≥98%), Na [14C]formaldehyde (0.43 μCi added; NEN) (sp. act. = 53 mCi/mmol). Substrates were injected, and the suspensions were allowed to incubate at 22°C in darkness for 7 or 15 min. Final concentrations of the radioisotopes were equivalent to 2.7 (±0.1) μM for the various substrates. The incubation was halted by acidification (0.25 ml of 6 N HCl), driving mineralized carbon to CO2. Transfer of 14CO2 to the gas phase was facilitated by shaking samples for 20 h (5).
Analytical.
Halocarbon concentrations were determined by syringe sampling of the gas phase of sealed vessels followed by gas chromatography using either a flame ionization or a 63Ni-electron capture detector (12, 28). Dissolved halocarbon concentrations were calculated with dimensionless Henry’s Law coefficients (KH) for distilled water applied to a gas/liquid phase volume partitioning equation (33). Only concentrations of dissolved halocarbons are given in the figures in this paper, and they do not include the amount of material present in the gas phase. KH values used were as follows: MeBr, 0.24; MeCl, 0.36; MeI, 0.20; MeF, 0.70; DFM, 1.28; DCM, 0.09; and DBM, 0.03 (1, 13, 19, 27, 40, 46). The 14CO2 concentration in the headspace of acidified serum vials was determined by gas chromatography in series with gas proportional counting (8). The iodide concentration was determined by ion chromatography (5).
RESULTS
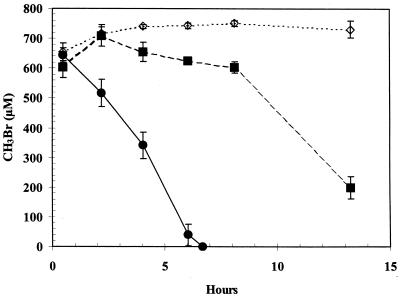
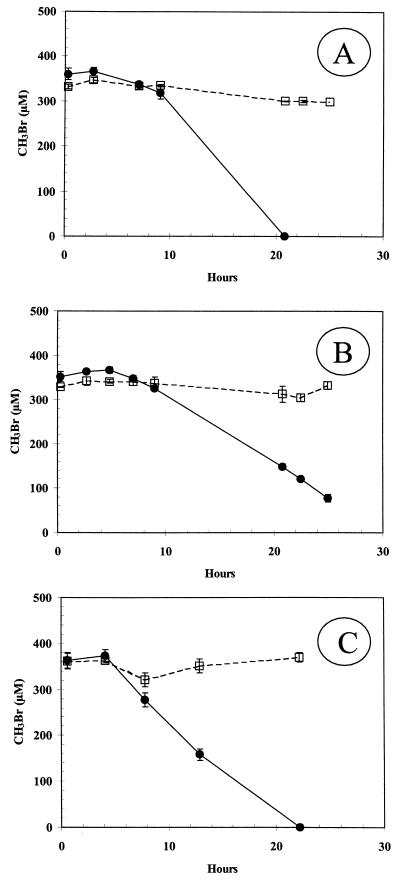
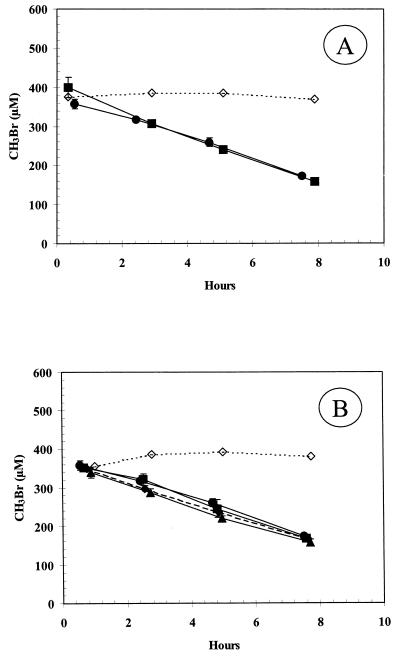
Glucose-grown cells demonstrated an 8-h lag before uptake of 650 μM MeBr was noted (Fig. 1). No uptake occurred in the control, which consisted of a medium blank. In contrast, cells growing on glucose which had been exposed overnight to ∼440 μM MeBr prior to washing immediately consumed MeBr. Consumption of ∼350 μM MeBr also lagged 6 to 8 h for cells grown on acetate (Fig. 2A), monomethylamine (Fig. 2B), or glucose (Fig. 2C). No significant removal of MeBr was observed for cells which were grown on glucose, acetate, or methylamine and then exposed to chloramphenicol (20 μg/ml) prior to injection of MeBr. Chloramphenicol did not affect consumption of MeBr by cells grown on either MeBr or MeCl. Chloramphenicol also did not affect consumption of MeCl (400 μM) or MeI (40 μM and 300 μM) by cells grown on MeBr (data not shown).
FIG. 1.
Consumption of MeBr by washed suspensions of glucose-grown cells. Symbols: ●, cells exposed to MeBr (cell density = 3.0 × 109 cells/ml); ■, cells not previously exposed to MeBr (cell density = 1.7 × 109 cells/ml); ◊, sterile medium. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation.
FIG. 2.
Consumption of MeBr by washed suspensions of cells incubated with or without chloramphenicol that were grown on acetate (A) (cell density = 8.4 × 108 cells/ml), monomethylamine (B) (cell density = 1.8 × 108 cells/ml), or glucose (C) (cell density = 3.4 × 109 cells/ml). Symbols: ●, untreated cell suspensions; □, cell suspensions incubated with chloramphenicol. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation. Absence of bars indicates that the error was smaller than the symbols.
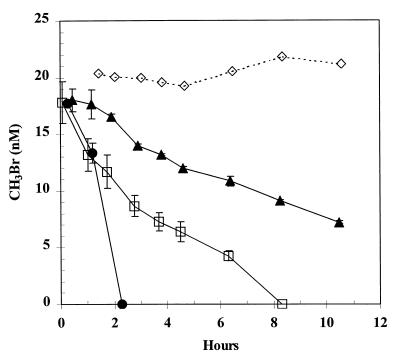
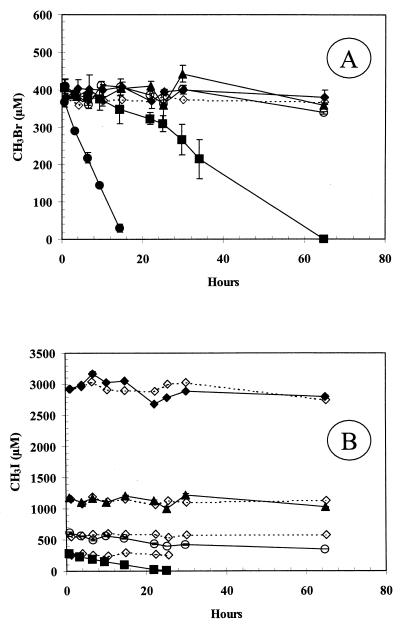
Consumption of MeBr occurred more rapidly at lower MeBr concentrations. For example, injection of MeBr into cells growing on glucose resulted in the complete consumption of 100 nM MeBr within 1.5 h, and in another experiment 7 nM MeBr was consumed within 2 h (data not shown). These cell lines had been taken through four previous growth transfers on glucose without exposure to MeBr, and so there was no possibility of a spurious induction associated with the transfer of the culture. We next examined the effects of chloramphenicol upon the consumption of comparably low concentrations of MeBr (13 to 18 nM) by glucose-grown cells. Consumption of 18 nM MeBr was immediate in uninhibited cells, while cells incubated with chloramphenicol at 250 μg/ml demonstrated only a partial inhibition, as seen in the form of slower MeBr removal rates (Fig. 3). The inhibition of the rate of MeBr consumption by cells increased with the duration of prior exposure to chloramphenicol. Thus, cells with only 2 h of prior exposure demonstrated a rate that was only ∼23% that of the uninhibited cells, while those with 21 h of prior exposure had a rate that was only ∼12% that of the uninhibited cells. When the experiment was repeated with only 20 μg of chloramphenicol per ml, the short preexposure time (0.2 h) had no effect, while a partial inhibition occurred with the longer (17 h) preexposure (data not shown). Cumulatively, these results suggest that cells contain a constitutive MeBr oxidase which will degrade low levels (e.g., 18 nM) of this substance but that the enzyme responsible for significant consumption of >100 μM MeBr requires induction.
FIG. 3.
Consumption of nanomolar levels of MeBr by glucose-grown cell suspensions of strain IMB-1. Symbols: ●, no additions; □, exposed to chloramphenicol (250 μg/ml) for 2 h; ▴, exposed to chloramphenicol (250 μg/ml) for 21 h; ◊, sterile medium. Cell densities were 1.2 × 109 cells/ml. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation. Absence of bars indicates that the error was smaller than the symbols.
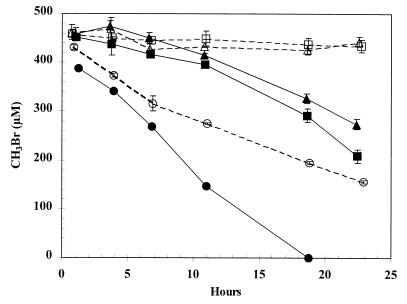
To test this hypothesis, we conducted an experiment with glucose-grown cells which had been exposed to either 20 nM MeBr or 100 μM MeBr. After consumption of the MeBr, cells were washed and divided into two fractions. One fraction contained chloramphenicol (250 μg/ml), and the other did not. The results are shown in Fig. 4. No consumption of ∼450 μM MeBr occurred in chloramphenicol-inhibited cells that had either no prior exposure to MeBr or ∼1 h prior exposure to 20 nM MeBr. Equivalent MeBr consumption rates were displayed by the uninhibited cells. However, cells which had been exposed to ∼100 μM MeBr demonstrated immediate uptake of the 450 μM MeBr and faster consumption rates as compared with the conditions described above. Chloramphenicol-inhibited controls in the latter case demonstrated an initial uptake rate which was comparable to the uninhibited rate, but after 6 h of incubation the rate declined, probably due to the lack of replacement of the enzyme.
FIG. 4.
Consumption of micromolar concentrations of MeBr by strain IMB-1 exposed to either nanomolar or micromolar concentrations of MeBr. Symbols: ▵ and ▴, not exposed to MeBr; □ and ■, exposed to 20 nM MeBr; ○ and ●, exposed to 100 μM MeBr. Open symbols indicate cells incubated with chloramphenicol (250 μg/ml). Cell densities were 1.1 × 109 cells/ml in both experiments. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation. Absence of bars indicates that the error was smaller than the symbols.
Neither MeF nor DFM proved inhibitory to MeBr consumption, even when it was added at high dissolved concentrations (Fig. 5). DBM and DCM had no effect on MeBr consumption (data not shown). The mean consumption rate in cells without DBM or DCM was 203.4 ± 9.4 pmol of MeBr · 106 cells−1 · h−1 (n = 3; ± 1 standard error), and no significant differences were observed in the presence of 106, 377, and 957 μM DCM or with 85, 376, and 930 μM DBM. Similarly, PpyI did not inhibit consumption of MeBr by washed cell suspensions of MeBr-grown cells, and no significant rate differences (range, 220 to 250 pmol of MeBr · 106 cells−1 · h−1) were observed in samples incubated with 0, 1, 5, or 20 μM PpyI (data not shown). No consumption of PpyI, MeF, DFM, DBM, or DCM occurred at any of the applied concentrations, even after all the MeBr had been consumed (data not shown).
FIG. 5.
Effect of MeF (A) and DFM (B) on the consumption of MeBr by washed suspensions of glucose-grown cells. (A) Symbols: ●, without MeF; ■, 6,000 μM MeF; ◊, sterile medium. (B) Symbols: ●, without DFM; ■, 220 μM DFM; ⧫, 470 μM DFM; ▴, 610 μM DFM; ◊, sterile medium. Cell densities were 3.4 × 108 cells/ml in both experiments. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation. Absence of bars indicates that the error was smaller than the symbols.
In contrast to the results described above, MeI inhibited MeBr consumption (Fig. 6). Partial inhibition of MeBr consumption was initially observed at 270 μM MeI, equivalent to a rate of ∼17 pmol of MeBr · 106 cells−1 · h−1 (Fig. 6A). MeI and MeBr consumption occurred simultaneously (Fig. 6B). After the supply of MeI was exhausted, the rate of MeBr consumption increased by 3.2-fold, but it still was not as rapid as in the uninhibited samples (106 pmol of MeBr · 106 cells−1 · h−1). MeBr and MeI consumption were completely inhibited at higher concentrations of MeI (>600 μM).
FIG. 6.
Consumption of MeBr (A) and MeI (B) by MeBr-grown washed cell suspensions incubated with 400 μM MeBr and various concentrations of MeI. Symbols: ●, without MeI; ■, 270 μM MeI; ○, 600 μM MeI; ▴, 1,200 μM MeI; ⧫, 3,000 μM MeI; ◊, sterile medium. The cell density was 3.3 × 108 cells/ml. Symbols represent the mean of three suspensions, and bars indicate ±1 standard deviation. Absence of bars indicates that the error was smaller than the symbols.
No inhibition of 400 μM MeBr uptake was observed at applied concentrations of 130, 350, and 750 μM MeCl, and only partial inhibition (19 to 30%) of MeBr consumption occurred at higher MeCl concentrations (>3.3 mM [data not shown]). We then examined the inhibition of the consumption of 400 μM MeCl by 120 or 430 μM MeBr. For the first 8 h of incubation, no MeCl consumption occurred at these concentrations of MeBr, during which time the cells preferentially consumed the MeBr. Controls incubated without MeBr immediately consumed MeCl (data not shown). After all the MeBr was consumed in the inhibited samples, rates of MeCl consumption were comparable to those of the controls.
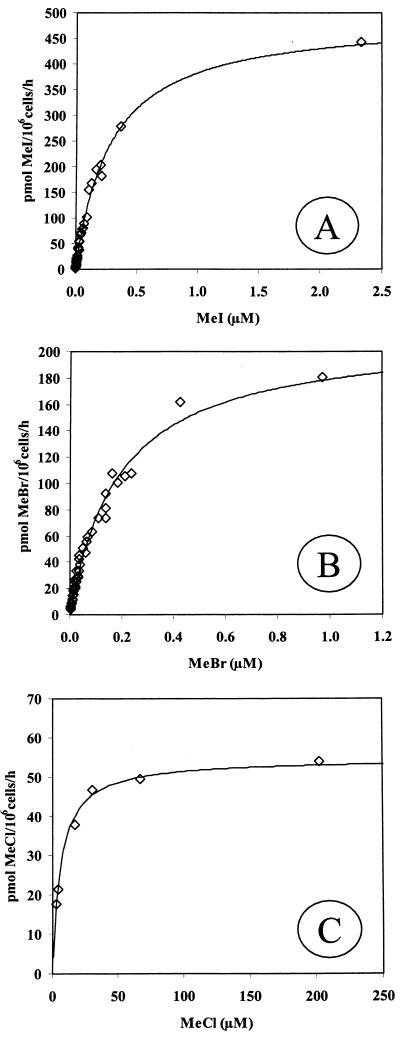
The results of the kinetic experiments with MeI, MeBr, and MeCl are shown in Fig. 7A, B, and C, respectively. Strain IMB-1 displayed Michaelis-Menten kinetics for all of these methyl halides, indicating Vmax values of 490, 210, and 55 pmol/106 cells/h and apparent Ks values of 280, 190, and 6,100 nM for MeI, MeBr, and MeCl, respectively. The calculated apparent first order rate constants (k) for a cell density of 106 were 0.087, 0.056, and 0.0004 h−1 for MeI, MeBr, and MeCl, respectively.
FIG. 7.
Results of methyl halide Michaelis-Menten kinetic experiments for consumption of MeI (A) (average cell density = 5.3 × 106 cells/ml; n = 3 experiments), MeBr (B) (average cell density = 9.6 × 105 cells/ml; n = 3 experiments), and MeCl (C) (6.0 × 107 cells/ml; n = 1 experiment) by cell suspensions of strain IMB-1.
Glucose-grown cells oxidized [14C]MeBr, [14C]methanol, and [14C]formaldehyde to 14CO2 (Table 1). Oxidation of [14C]MeBr was about 3.5-fold greater for glucose-grown cells which had been exposed to MeBr than for those which had not. However, pre-exposure to MeBr did not affect oxidation of either [14C]methanol or [14C]formaldehyde. About half of the added formaldehyde was oxidized within 15 min, as compared with only 5% for methanol.
TABLE 1.
Oxidation of [14C]MeBr, [14C]methanol, or [14C]formaldehyde by glucose-grown strain IMB-1 cells in 15 min with or without preexposure to 18 μM MeBr for 6 h
| Substratea | Exposed to MeBr
|
Without Preexposure
|
||
|---|---|---|---|---|
| pmol/106 cells/hb | % Oxidized | pmol/106 cells/hb | % Oxidized | |
| MeBr | 1.04 (0.16) | 32 | 0.29 (0.05) | 9 |
| Methanol | 0.10 (0.03) | 6 | 0.09 (0.02) | 5 |
| Formaldehyde | 0.80 (0.18) | 45 | 0.76 (0.26) | 46 |
Substrate concentrations were all ∼2.7 (±0.1) μM.
Values represent the average of two cell suspensions, and values in parentheses indicate the range; cell densities were equal to 5.8 × 109 cells/ml; cells were taken through five transfers on glucose-based medium prior to exposure to MeBr.
A balance was achieved between the amount of bromide (11.4 μmol/bottle) or iodide (7.7 μmol/bottle) recovered and the amount of MeBr (11.9 μmol/bottle) or MeI (7.8 μmol/bottle) consumed by the cells. Connell Hancock et al. (5) reported recovery of only ∼33% of the MeI consumed as iodide accumulated in the growth medium of IMB-1. We attribute this disparity to the types of standards used to calibrate MeI by gas chromatography with syringe injection. Connell Hancock et al. (5) assumed that 100% volatilization of MeI occurred in sealed serum bottles, which in retrospect, did not occur. In this study, we injected MeI into sealed serum bottles which contained sterile aqueous medium, followed by injection of vapor-phase samples into the gas chromatograph. Because we now accounted for the partitioning between the gas and liquid phases by using Henry’s Law, we achieved ∼98% recovery of iodide from MeI.
DISCUSSION
The global atmospheric budget for methyl halides is complicated by the fact that there exist both natural and anthropogenic sources for their supply to the troposphere, as well as several identified sinks for their removal from that reservoir. In the case of MeBr, an important tropospheric sink is its biological degradation in the oceans and in soils (20, 25, 28). The most recent estimate for the tropospheric residence time of MeBr is ∼0.8 year, based on calculation of its annual degradation rate in the oceans and soils plus its reaction with hydroxyl radicals (47). With regard to possible microbiological sinks for MeBr oxidation, attention was drawn to methanotrophs and ammonium-oxidizing nitrifiers because of the well-established capacities of their monooxygenases to oxidize analogs of methane, including MeBr (16, 32, 34).
Soil experiments, however, have indicated that microorganisms other than methanotrophs are responsible for part (32) or all (16, 28) of the observed MeBr degradation occurring therein. The ability to grow on MeCl displayed by aerobic, non-methane-utilizing, facultative methylotrophs of the genera Hyphomicrobium and Methylobacterium (9) suggested the possibility that these or similar organisms may oxidize MeBr as well. The bacterium investigated here, strain IMB-1, was isolated from fumigated agricultural soil by using MeBr as a sole source of carbon and energy (28). This bacterium can grow on MeI, MeCl, and methylamines. It does not grow on formaldehyde or methanol (5), but it can oxidize these compounds (Table 1). Metabolism of MeBr and MeI was reported for the facultative methylotroph Methylobacterium sp. strain CM4 (42), as well as for the recently isolated strain CC 495, which is closely related to strain IMB-1 (7). In addition, the methanesulfonate-degrading methylotrophs Methylosulfomonas methylovora and Marinosulfonomonas methylotropha (18) can also oxidize MeBr (30), as can other marine methylotrophs (12). It appears that various facultative methylotrophs from diverse clades within the alpha subgroup of the class Proteobacteria have the ability to oxidize MeBr and may therefore be important agents in the global biogeochemical cycling of methyl halides.
Consumption of high levels of MeBr (>100 μM) required induction in cells grown on glucose, methylamine, or acetate (Fig. 1 and 2), which suggests that strain IMB-1 survives by metabolizing substrates other than methyl halides. However, we also noted that at low MeBr concentrations (∼20 nM) there was constitutive consumption of MeBr (Fig. 3 and 4). A preliminary communication establishes that strain IMB-1 can readily consume as little as 12 parts of MeBr per trillion by volume, which is comparable to its tropospheric mixing ratio (11). Clearly, IMB-1 has a constitutive ability to consume nanomolar levels of MeBr, but the consumption of micromolar levels requires the induction of new enzyme. Induction of MeBr degradation at high MeBr concentrations appears to be slow (∼8 h), but this may be due in part to its toxic effects. In the radioassay, which is done at intermediate MeBr concentrations, we observed a small amount of [14C]MeBr oxidation in nonpreconditioned cells which were incubated for only 15 min with 2.7 μM MeBr that was probably carried out by the constitutive enzyme (Table 1). Previously, we had reported that the enzyme(s) for MeBr oxidation appeared to be constitutive based on the observation of oxidation of [14C]MeBr to 14CO2 over a >2-h incubation period (5). In retrospect, this incubation time was probably sufficient to allow for induction of the enzyme(s) responsible for oxidation of micromolar amounts of MeBr. These results suggest that this organism can rapidly exploit MeBr as a carbon and energy source either when it is released at low concentrations into the local soil environment by fungi (15) or plants (10) or when it is injected at high concentrations into soils as a fumigant (28).
Bacterial populations in soils can consume MeBr at its ambient tropospheric mixing level of ∼10 parts per trillion (16, 38). It is unlikely that microbes can achieve any physiological benefit in terms of its energy balance or growth capacity by metabolizing such low concentrations (6), but nonetheless these observations have significance to the global cycling of MeBr. Very recent results with strain IMB-1 have revealed that it is capable of rapid consumption of ambient levels of MeBr (∼12 parts per trillion), thereby making it a good model to explain the consumption phenomenon observed for soils (11).
Consumption of MeBr by cell suspensions of strain IMB-1 was not inhibited by MeF or DFM (Fig. 5). These fluorinated compounds are employed as inhibitors of methane-oxidizing bacteria (24, 28, 31), so these results underscore specificity with regard to that target group. The methane monooxygenase of Methylococcus capsulatus can oxidize MeF when it is supplied at less than fully inhibitory levels (24, 31). However, MeF was neither inhibited nor consumed by strain IMB-1, which illustrates the enzymatic differences between IMB-1 and methanotrophs with regard to methyl halide degradation. Furthermore, MeI, MeCl, and MeBr serve as sources of energy and carbon for strain IMB-1 to achieve growth, but methyl halides are cometabolized by methanotrophs. In addition, strain IMB-1 can oxidize MeI, MeCl, and MeBr but not MeF, whereas M. capsulatus can oxidize MeF, MeCl, and MeBr but not MeI (4, 26, 39). Finally, methane monooxygenases are nonspecific dehalogenators (14). However, the enzyme system of strain IMB-1 is specific for methyl halides because DBM and DCM were neither inhibitory nor consumed.
Certain marine methylotrophs have also demonstrated growth on specific brominated methanes. For example, one culture oxidized MeBr but not DBM, while a second culture oxidized DBM but not MeBr or DCM (12). This suggests that the enzymes responsible for the metabolism of brominated methanes by marine methylotrophs are highly specific. Chloropicrin (trichloronitromethane [“tear gas”]) is used in conjunction with MeBr to fumigate agricultural fields. Although chloropicrin is a C1 compound which inhibits MeBr oxidation by strain IMB-1 (5), its mode of inhibition probably does not involve the enzyme(s) used for methyl halide oxidation because it also inhibits growth on glucose, acetate, and monomethylamine (5).
The only tested substances that inhibited MeBr consumption were MeI (Fig. 6) and MeCl. Because strain IMB-1 grows on all three methyl halides (5), their metabolism is likely served by a common enzyme system and therefore the observed inhibition was of a competitive nature. The fact that chloramphenicol was unable to block MeCl consumption by MeBr-grown cells (and vice versa) gives further evidence for this interpretation. However, MeI was a far more potent inhibitor of MeBr consumption than was MeCl. This result follows the order of chemical reactivity: MeI > MeBr > MeCl. It also indicates that the enzyme(s) involved in the methyl halide oxidation has different affinities for MeI, MeBr, and MeCl. Indeed, this supposition is borne out by the results of our kinetic studies for whole cells (Fig. 7). It is curious, however, that the lowest Ks value was for MeBr, while MeI exhibited the highest Vmax. It is not clear whether these results can be attributed entirely to the higher chemical reactivity of MeI or to the fact that we selected for an organism with a high affinity for MeBr because we used MeBr as a sole source of carbon and energy to achieve isolation of strain IMB-1 (28), whereas isolation of other methylotrophs employed MeCl (7, 9). It would therefore be of interest to examine the kinetic parameters of these methylotrophs regarding MeBr and MeI as well as MeCl.
The fact that no inhibition of MeBr consumption was achieved during dark incubation with PypI indicates the lack of involvement of cobalamin compounds (23). This rules out the possibility of a B12-mediated methyltransferase reaction occurring in the oxidation pathway, although methyl transfers can still be achieved by other means (e.g., glutathione-S-transferase) and followed by a dehydrogenase reaction (43). Vannelli et al. (42) found a pathway for aerobic MeCl oxidation in Methylobacterium sp. strain CM4 that involves a corrinoid-dependent methyltransferase system, which also was discovered independently in strain CC 495 (7). However, PpyI does not necessarily inhibit all cobalamin-mediated reactions, including the methyltransferase of strain CC 495 (7), which leaves the possibility open for a cobamide-mediated reaction in strain IMB-1. The fact that we observed generally low levels of [14C]methanol oxidation in MeBr-induced cells (Table 1) argues against the presence of a hydrolase pathway that converts MeBr to methanol plus HBr. Thus, either a monooxygenase or a combination of methyltransferase and dehydrogenase reactions is still a possible pathway for strain IMB-1 (43).
Application of suspensions of strain IMB-1 to the surface of soils has been proposed as a means to reduce outward emissions of MeBr to the atmosphere from fumigated fields (5). However, use of MeBr as a growth substrate for mass culture of IMB-1 is impractical because of the possibility of exposure of workers to this gas, as well as the poor growth yields achieved on MeBr (5). Although the enzyme system for oxidation of fumigation levels (i.e., micromolar) of methyl halides requires induction, this can be readily achieved by exposure of harvested cell suspensions to micromolar concentrations of MeBr for several hours. Hence, cells can be grown to high densities on substrates like glucose, harvested, and exposed to MeBr for a relatively short time period in order to induce the methyl halide oxidation enzyme(s) prior to spreading the suspensions on soils. This strategy circumvents growing IMB-1 on methyl halides in order to achieve high levels of MeBr oxidation. It remains to be determined whether this strategy will prove effective in reducing MeBr emissions from fumigated fields.
ACKNOWLEDGMENTS
This work was supported by the U.S. Geological Survey New Technologies Program and by NASA (5188-AU-0080). We thank K. Goodwin and C. Murrell for their constructive comments on an earlier draft of the manuscript.
REFERENCES
- 1.Braker W, Mossman A L. Matheson gas data bank. 6th ed. Lindhurst, N.J: Matheson Co.; 1980. [Google Scholar]
- 2.Butler J H, Rodriguez J M. Methyl bromide in the atmosphere. In: Bell C H, Price N, Chakrabarti B, editors. The methyl bromide issue. J. New York, N.Y: Wiley & Sons; 1996. pp. 28–90. [Google Scholar]
- 3.Cicerone R J. Halogens in the atmosphere. Rev Geophys Space Phys. 1981;19:123–139. [Google Scholar]
- 4.Colby J, Stirling D I, Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus Bath. Biochem J. 1977;165:395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell Hancock T L, Costello A M, Lidstrom M E, Oremland R S. Strain IMB-1, a novel bacterium for the removal of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1998;64:2899–2905. doi: 10.1128/aem.64.8.2899-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulter, C., J. T. G. Hamilton, W. C. McRoberts, L. Kulakov, M. J. Larkin, and D. B. Harper. 1999. Halomethane: bisulfide/halide ion methyltransferase, an unusual corrinoid enzyme of environmental significance isolated from an aerobic methylotroph using chloromethane as sole carbon source. Appl. Environ. Microbiol. (in press). [DOI] [PMC free article] [PubMed]
- 8.Culbertson C W, Zehnder A J B, Oremland R S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981;41:396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doronina N V, Sokolov A P, Trotsenko Y A. Isolation and initial characterization of aerobic chloromethane-utilizing bacteria. FEMS Microbiol Lett. 1996;142:179–183. [Google Scholar]
- 10.Gan J, Yates S R, Ohr H D, Sims J J. Production of methyl bromide by terrestrial higher plants. Geophys Res Lett. 1998;25:3595–3598. [Google Scholar]
- 11.Goodwin K D, Varner R A, Crill P M, Oremland R S. Uptake of near-ambient levels of methyl bromide by strain IMB-1, a facultative methylotrophic bacterium. Eos Trans Am Geophys Union. 1999;80:S64. [Google Scholar]
- 12.Goodwin K D, Schaefer J K, Oremland R S. Bacterial oxidation of dibromomethane and methyl bromide in natural waters and enrichment cultures. Appl Environ Microbiol. 1998;64:4629–4636. doi: 10.1128/aem.64.12.4629-4636.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossett J. Measurement of Henry’s Law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 14.Green J, Dalton H. Substrate specificity of soluble methane monooxygenase. J Biol Chem. 1989;264:17698–17703. [PubMed] [Google Scholar]
- 15.Harper D B. Biosynthesis of halogenated methanes. Biochem Soc Trans. 1994;22:1007–1011. doi: 10.1042/bst0221007. [DOI] [PubMed] [Google Scholar]
- 16.Hines M E, Crill P M, Varner R K, Talbot R W, Shorter J H, Kolb C E, Harriss R C. Rapid consumption of low concentrations of methyl bromide by soil bacteria. Appl Environ Microbiol. 1998;64:1864–1870. doi: 10.1128/aem.64.5.1864-1870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A J, Kelly D P, Baker S C, Thompson A S, De Marco P, Kenna E M, Murrell J C. Methylosulfomonas methylovora gen. nov., sp. nov., and Marinosulfonomonas methylotropha gen. nov., sp. nov.: novel methylotrophs able to grow on methanesulfonic acid. Arch Microbiol. 1997;167:46–53. doi: 10.1007/s002030050415. [DOI] [PubMed] [Google Scholar]
- 19.Hunter-Smith R J, Balls P W, Liss P S. Henry’s Law constants and the air-sea exchange of various low molecular weight halocarbon gases. Tellus. 1983;35B:170–176. [Google Scholar]
- 20.Lobert J M, Yvon-Lewis S A, Butler J H, Montzka S A, Meyers R C. Undersaturation of CH3Br in the southern ocean. Geophys Res Lett. 1997;24:171–172. [Google Scholar]
- 21.Lobert J M, Butler J H, Montzka S A, Geller L S, Meyers R C, Elkins J W. A net sink for atmospheric CH3Br in the East Pacific Ocean. Science. 1995;267:1002–1005. doi: 10.1126/science.267.5200.1002. [DOI] [PubMed] [Google Scholar]
- 22.Lovelock J E. Natural halocarbons in air and sea. Nature. 1975;256:193–195. doi: 10.1038/256193a0. [DOI] [PubMed] [Google Scholar]
- 23.Mägli A, Messmer M, Leisinger T. Metabolism of dichloromethane by the strict anaerobe Dehalobacterium formicoaceticum. Appl Environ Microbiol. 1998;64:646–650. doi: 10.1128/aem.64.2.646-650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheson L J, Jahnke L L, Oremland R S. Inhibition of methane oxidation by Methylococcus capsulatus with hydrochlorofluorocarbons and fluorinated methanes. Appl Environ Microbiol. 1997;63:2952–2956. doi: 10.1128/aem.63.7.2952-2956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellouki A, Taludar R K, Schmoltner A, Gierczak T, Mills M J, Solomon S, Ravishankara A R. Atmospheric lifetimes and ozone depletion potentials of methyl bromide (CH3Br) and dibromomethane (CH2Br2) Geophys Res Lett. 1992;19:2059–2062. [Google Scholar]
- 26.Meyers A J. Evaluation of the dimethyl ether pathway for methane metabolism. Ph.D. Dissertation. Miami, Fla: University of Miami; 1978. [Google Scholar]
- 27.Miller L G, Sasson C, Oremland R S. Difluoromethane, a new and improved inhibitor of methanotrophy. Appl Environ Microbiol. 1998;64:4357–4362. doi: 10.1128/aem.64.11.4357-4362.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller L G, Connell T L, Guidetti J R, Oremland R S. Bacterial oxidation of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1997;63:4346–4354. doi: 10.1128/aem.63.11.4346-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina M, Rowland F S. Stratospheric sink for chlorofluoromethanes: chlorine atom catalyzed destruction of ozone. Nature. 1974;249:810–812. [Google Scholar]
- 30.Murrell, J. C. Personal communication.
- 31.Oremland R S, Culbertson C W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992;58:2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oremland R S, Miller L G, Culbertson C W, Connell T L, Jahnke L L. Degradation of methyl bromide by methanotrophic bacteria in cell suspensions and soils. Appl Environ Microbiol. 1994;60:3640–3646. doi: 10.1128/aem.60.10.3640-3646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oremland R S, Miller L G, Oremland R S. Degradation of methyl bromide in anaerobic sediments. Environ Sci Technol. 1994;28:514–520. doi: 10.1021/es00052a026. [DOI] [PubMed] [Google Scholar]
- 34.Ou L-T, Joy P J, Thomas J E, Hornsby A G. Stimulation of degradation of methyl bromide in soil during oxidation of an ammonia fertilizer by nitrifiers. Environ Sci Technol. 1997;31:717–722. [Google Scholar]
- 35.Prather M J. Timescales in atmospheric chemistry: CH3Br, the ocean, and ozone depletion potentials. Global Biogeochem Cycles. 1997;11:393–400. [Google Scholar]
- 36.Rasmussen R A, Khalil M A K, Gunawardena R, Hoyt S D. Atmospheric methyl iodide (CH3I) J Geophys Res. 1982;87:3086–3090. [Google Scholar]
- 37.Serça D, Guenther A, Klinger L, Helmig D, Hereid D, Zimmerman P. Methyl bromide deposition to soils. Atmos Environ. 1998;32:1581–1586. [Google Scholar]
- 38.Shorter J H, Kolb C E, Crill P M, Kerwin R A, Talbot R W, Hines M E, Harriss R C. Rapid degradation of atmospheric methyl bromide in soils. Nature. 1995;377:717–719. [Google Scholar]
- 39.Sterling D I, Dalton H. The fortuitous oxidation and co-metabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1979;5:315–318. [Google Scholar]
- 40.Tse G, Orbey H, Sandler S I. Infinite dilution activity coefficients and Henry’s Law coefficients of some priority water pollutants determined by a relative gas chromatographic method. Environ Sci Technol. 1992;26:2017–2022. [Google Scholar]
- 41.United Nations Environmental Programme. Report of the methyl bromide technical options committee. Nairobi, Kenya: United Nations Environmental Programme; 1994. [Google Scholar]
- 42.Vannelli T, Messmer M, Struder A, Vuilleumier S, Leisinger T. A corrinoid-dependent catabolic pathway for growth of a Methylobacterium strain with chloromethane. Proc Natl Acad Sci USA. 1999;96:4615–4620. doi: 10.1073/pnas.96.8.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vannelli T, Struder A, Kertesz M, Leisinger T. Chloromethane metabolism by Methylobacterium sp. strain CM4. Appl Environ Microbiol. 1998;64:1933–1936. doi: 10.1128/aem.64.5.1933-1936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Yates S R, Ennst F F, Gan J, Jury W A. Reducing methyl bromide emissions with a high barrier plastic film and reduced dosage. Environ Sci Technol. 1997;31:3686–3691. [Google Scholar]
- 45.Widdel F, Kohring G-W, Mayer F. Studies on the dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol. 1983;134:286–294. [Google Scholar]
- 46.Wilhelm E, Battino R, Wilcock R J. Low-pressure solubility of gases in liquid water. Chem Rev. 1977;77:219–262. [Google Scholar]
- 47.Yvon S A, Butler J H. An improved estimate of the oceanic lifetime of atmospheric CH3Br. Geophys Res Lett. 1996;23:53–56. [Google Scholar]
- 48.Yvon-Lewis S A, Butler J H. The potential effect of oceanic biological degradation on the lifetime of atmospheric CH3Br. Geophys Res Lett. 1997;24:1227–1230. [Google Scholar]