Abstract
Background:
There is accumulating evidence of excess risk of cancer in various populations exposed at acute doses below several tens of mSv or doses received over a protracted period. There is also evidence that relative risks are generally higher after radiation exposures in utero or in childhood.
Methods and Findings:
We reviewed and summarised evidence from 89 studies of cancer following medical diagnostic exposure in utero or in childhood, in which no direct estimates of radiation dose are available. In all of the populations studied exposure was to sparsely ionising radiation (X-rays). Several of the early studies of in utero exposure exhibit modest but statistically significant excess risks of several types of childhood cancer. There is a highly significant (p<0.0005) negative trend of odds ratio with calendar period of study, so that more recent studies tend to exhibit reduced excess risk. There is no significant inter-study heterogeneity (p>0.3). In relation to postnatal exposure there are significant excess risks of leukaemia, brain and solid cancers, with indications of variations in risk by cancer type (p=0.07) and type of exposure (p=0.02), with fluoroscopy and computed tomography scans associated with the highest excess risk. However, there is highly significant inter-study heterogeneity (p<0.01) for all cancer endpoints and all but one type of exposure, although no significant risk trend with calendar period of study.
Conclusions:
Overall, this large body of data relating to medical diagnostic radiation exposure in utero provides support for an associated excess risk of childhood cancer. However, the pronounced heterogeneity in studies of postnatal diagnostic exposure, the implied uncertainty as to the meaning of summary measures, and the distinct possibilities of bias, substantially reduce the strength of the evidence from the associations we observe between radiation imaging in childhood and the subsequent risk of cancer being causally related to radiation exposure.
Keywords: radiation, childhood, in utero, cancer risk
Graphical Abstract
Figure 1. Meta-regression for studies of in utero exposure. Restricted maximum likelihood (REML) fits to odds ratio or relative risk by calendar year midpoint of study data ascertainment range (for <1950, 1950–1959, 1960–1969, 1970–1979, 1980–1989, 1990+). Plots are shown for (a) the four cancer endpoints analysis (leukaemia, lymphoma, brain/CNS cancer, other cancer) and (b) the any cancer endpoint analysis, for each in utero exposure study. Dashed red line is odds ratio/relative risk = 1.
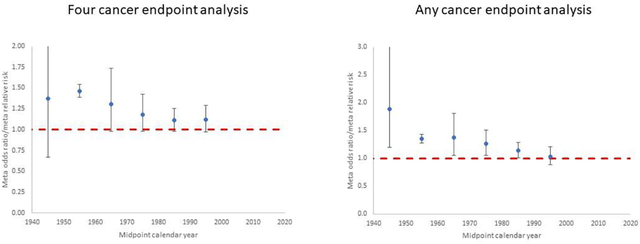
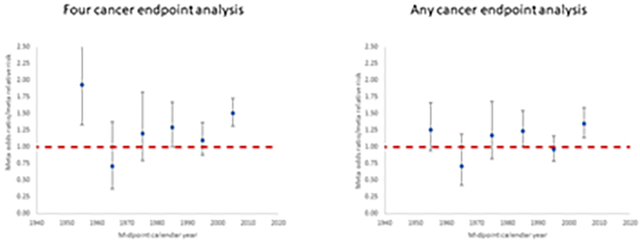
Figure 2. Meta-regression for studies of postnatal exposure. Restricted maximum likelihood (REML) fits to odds ratio or relative risk by calendar year midpoint of study data ascertainment range (for <1960, 1960–1969, 1970–1979, 1980–1989, 1990–1999, 2000+). Plots are shown for (a) the four cancer endpoints analysis (leukaemia, lymphoma, brain/CNS cancer, other cancer) and (b) the any cancer endpoint analysis, for each postnatal exposure study. Dashed red line is odds ratio/relative risk = 1.
1. Introduction
Quantitative estimates of the excess risk per unit dose of various types of cancer are the cornerstone of radiation protection (Armstrong et al., 2012; Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, 2006; International Commission on Radiological Protection (ICRP), 2007; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2008), with risk estimates at low doses supported by mechanistic information (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2021). From this it might be imagined that little could be learnt from studies which lack quantified dose estimates. However, this would be to over-state the case. One has only to consider, for example, the importance and impact of the British case-control study (which came to be known as the Oxford Survey of Childhood Cancers, OSCC) of childhood cancer mortality and antenatal radiography by Alice Stewart and her colleagues (Bithell et al., 2018; Bithell and Stewart, 1975; Stewart et al., 1956; Stewart et al., 1958). When first published, this study lacked dose information, although risk estimates based on fetal dose estimates have now been made (Bithell, 1993; Bithell and Stiller, 1988; Doll and Wakeford, 1997; Mole, 1990; Muirhead and Kneale, 1989; Wakeford and Little, 2003), and point to an excess relative risk (ERR) per unit fetal dose for childhood cancer of around 50% per 10 mGy, although there remains substantial uncertainty in this risk estimate (Wakeford and Little, 2003). Perhaps surprisingly, it would also appear that the risk associated with exposure in utero is proportionally raised to around the same extent for most of the cancers typical of childhood, with the possible exception of bone tumours (Bithell and Stewart, 1975; Wakeford and Bithell, 2021). There are many other studies of medical diagnostic radiation in utero, most without quantitative individual estimates of radiation dose, which also point to an associated increased risk of childhood cancer (Wakeford and Bithell, 2021). There is also growing evidence in a number of exposed groups (Grant et al., 2017; Little et al., 2020; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2013) that radiation exposure in childhood is generally associated with higher cancer risks compared with exposure later in life. However, in contrast to exposure in utero, evidence that low-level exposure to radiation in childhood is associated with an increased risk of subsequent cancer has been equivocal (Linet et al., 2012; Rajaraman et al., 2011; Wakeford, 2008), although the recent large studies of cancer following computed tomography (CT) scans at a young age have provided a stronger base of evidence (Gilbert et al., 2020). Again, in contrast to childhood cancers following exposure in utero, exposure in childhood is associated with subsequent increases in cancer risk that show a notable variation with cancer type. Although some of these studies have individual estimates of radiation dose (and therefore risk), this is not the case for all (Linet et al., 2012).
In the present paper we review studies of early life medical diagnostic exposures, both antenatal and postnatal, in which quantitative radiation dose estimates are lacking, though general indications of the magnitude of the doses are likely to be implicit. The present study complements a parallel and contemporary review that evaluated studies in which quantitative estimates of radiation risk with respect to doses are available (Little et al., 2022b).
2. Methods
2.1. Literature review
A literature search of PubMed was last performed on 16th May 2021 using the search terms given in the Supplementary Methods. Additionally, recent UNSCEAR reports (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2008; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2013; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2018) were scanned to assess additional literature, as well as recent review articles (Abalo et al., 2021; Han and Kim, 2018; Kendall et al., 2021; Linet et al., 2009; Linet et al., 2012; Memon et al., 2019; Wakeford and Bithell, 2021). We restricted attention to those studies of persons exposed in utero or postnatally at age 20 years or less to medical diagnostic radiographic procedures. There was no restriction on language or date of publication. Editorials, abstracts and reviews were excluded, except to identify potential additional studies.
A total of 3117 papers were returned. A PECO statement is given in Supplementary Table S3. The titles and abstracts of these papers were independently double scanned by MPL and GMK, and case reports, review papers and other clearly inapplicable results (e.g., relating to populations not exposed in childhood) were eliminated. Consistency was established via consensus. Additionally, recent UNSCEAR reports (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2008; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2013; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2018) were scanned to assess additional literature, as well as recent review articles (Abalo et al., 2021; Han and Kim, 2018; Kendall et al., 2021; Linet et al., 2009; Linet et al., 2012; Memon et al., 2019; Wakeford and Bithell, 2021). A total of 299 papers that were deemed applicable based on the title/abstract search, and the associated full publications were then obtained for more detailed review of these by MPL and GMK. Of the 299 consensus samples we restricted attention to those studies of persons exposed in utero or in childhood (age 20 years or less) to medical diagnostic radiographic procedures and in which quantitative estimates of radiation doses were not available. Again, consistency between the reviewers was established via consensus; all studies that had been superseded by others were eliminated. This yielded a total of 89 papers, 29 of which were derived from the PubMed search.
2.2. Meta-analysis
Meta-analysis was conducted of odds ratios (OR) or relative risks (RR), combining these equivalent measures. Wherever possible the maximally adjusted OR or RR were taken directly from the relevant publication; further details of the risk estimates for each study are given in Tables 1–2 and Supplementary Tables S1–S2. Further details of data exclusions and of how the data abstraction was performed for particular studies are given in the Supplementary Methods. The type of radiological procedure used within each study of postnatal exposure was classified as catheterisation, fluoroscopy, CT scan, X-ray, mixed or unknown; associated codes (C, F, CT, X, M, U, respectively) were given for each endpoint within studies in Table 2. For the purposes of the meta-analysis, to avoid double counting of cases, we concentrated on results in relation to procedures likely to result in the largest radiation dose, specifically catheterisation, fluoroscopy or CT scan.
Table 1.
Summary of case-control and cohort studies of childhood cancer and in utero exposure to medical diagnostic radiation that do not incorporate estimates of dose, with estimates of odds ratio (OR) or relative risk (RR)
| Case-control studies | ||||||
|---|---|---|---|---|---|---|
| Reference | Type of X-ray exposure, other featuresa | Description of study data | Study years | Cancer endpointb | Number of cases exposed / totalc | Odds Ratio, (95% CI) |
| (Kjeldsberg, 1957) | Fetal X-ray vs no X-ray | Oslo cohort, based on single hospital | 1946–1956 | Leukaemia | 5 / 55 | 0.56 (0.14, 2.22)d |
| Abdomen X-ray vs no abdomen X-ray | 5 / 55 | 0.69 (0.16, 2.72)d | ||||
| Abdomen X-ray vs no X-ray | 5 / 55 | 0.67 (0.16, 2.66)d | ||||
| (Kaplan, 1958) | Sibling control, maternal abdominal X-rays vs no abdominal | California mortality cohort | 1955–1956 | Acute leukaemia mortality | 40 / 150 | 1.91 (1.05, 3.53)d |
| Playmate control, maternal abdominal X-rays vs no abdominal | 34 / 125 | 1.35 (0.73, 2.53)d | ||||
| Sibling control, maternal abdominal X-rays vs no X-ray | 40 / 128 | 2.08 (1.13, 3.90)d | ||||
| Playmate control, maternal abdominal X-rays vs no X-ray | 34 / 106 | 1.29 (0.68, 2.47)d | ||||
| (Ford et al., 1959) | Abdominal or pelvic X-rays vs unexposed, medical-record based | Louisiana mortality cohort | 1951–1955 | All malignant tumour mortality | 42 / 152 | 1.77 (1.08, 2.87)d |
| Leukaemia mortality | 21 / 78 | 1.70 (0.90, 3.14)d | ||||
| Brain/CNS mortality | 6 / 16 | 2.77 (0.79, 8.83)d | ||||
| Kidney mortality | 4 / 14 | 1.85 (0.41, 6.71)d | ||||
| Neuroblastoma mortality | 2 / 11 | 1.03 (0.11, 5.17)d | ||||
| Lymphoma mortality | 4 / 14 | 1.85 (0.41, 6.71)d | ||||
| All solid tumour excluding brain/CNS mortality | 11 / 44 | 1.54 (0.66, 3.37)d | ||||
| (Murray et al., 1959) | Pelvimetry vs no pelvimetry, medical record based | Mortality cohort among patients in Monroe County, New York | 1930–1956 | Leukaemia mortality | 3 / 65 | 1.24 (0.21, 5.17)d |
| Other abdominal X-ray vs no other abdominal X-ray, medical record based | 0 / 65 | 0.00 (0.00, 8.99)d | ||||
| Pelvimetry + other abdominal X-ray vs no pelvimetry or other abdominal X-ray, medical record based | 3 / 65 | 0.92 (0.16, 3.56)d | ||||
| (Polhemus and Koch, 1959) | Pelvimetry vs no pelvimetry | Cohort based on Children’s Hospital of Los Angeles | 1950–1957 | Leukaemia | 66 / 251 | 1.19 (0.77, 1.82)d |
| Pelvimetry vs no exposure, excluding non-obstetric X-rays and maternal occupational radiation | 66 / 245 | 1.23 (0.80, 1.88)d | ||||
| (Wells and Steer, 1961) | X-ray pelvimetry vs unexposed (excluding dental), medical-record based | Columbia Presbyterian Medical Center, New York | <1961 | Leukaemia | 4 / 62 | 1.31 (0.27, 5.38)d |
| X-ray pelvimetry + other abdominal vs unexposed (excluding dental), medical-record based | 4 / 62 | 0.83 (0.19, 2.97)d | ||||
| X-ray pelvimetry vs unexposed (including dental), medical-record based | 4 / 67 | 1.22 (0.25, 5.02)d | ||||
| X-ray pelvimetry + other abdominal vs unexposed (including dental), medical-record based | 4 / 67 | 0.78 (0.17, 2.77)d | ||||
| (Gunz and Atkinson, 1964) | Abdominal X-ray vs unexposed | New Zealand national cohort | 1958–1961 | Leukaemia | 14 / 92 | 1.11 (0.43, 2.90)d |
| Any X-ray vs unexposed | 14 / 102 | 1.00 (0.48, 2.06)d | ||||
| (Ager et al., 1965) | Sibling controls, abdominal + pelvic X-ray vs not | Minnesota childhood leukaemia study, mortality | 1953–1957 | Leukaemia mortality | 20 / 107 | 1.21 (0.55, 2.67)d |
| Neighbourhood controls, abdominal + pelvic X-ray vs not | 20 / 107 | 1.35 (0.62, 2.98)d | ||||
| Sibling + neighbourhood controls, abdominal + pelvic X-ray vs not | 20 / 107 | 1.28 (0.65, 2.46)d | ||||
| Sibling controls, abdominal + pelvic X-ray vs no X-ray | 20 / 94 | 1.21 (0.55, 2.72)d | ||||
| Neighbourhood controls, abdominal + pelvic X-ray vs no X-ray | 20 / 94 | 1.32 (0.60, 2.94)d | ||||
| Sibling + neighbourhood controls, abdominal + pelvic X-ray vs no X-ray | 20 / 94 | 1.27 (0.64, 2.46)d | ||||
| (Graham et al., 1966) | Intrauterine abdominal radiation exposure vs no abdominal radiation exposure, medical-record based | USA Tri-state Study | 1959–1962 | Leukaemia | 27 / 313 | 1.40 (0.83, 2.31)d |
| Intrauterine abdominal radiation exposure vs no X-ray exposure, medical-record based | 27 / 244 | 1.54 (0.91, 2.56)d | ||||
| (Stewart, 1973) | Maternal X-ray, cancer mortality | Oxford Survey of Childhood Cancer Twin Study, deaths within 10 years of birth | 1943–1967 | Leukaemia mortality | 51 / 70 | 1.40 (0.84, 2.44)e |
| All tumour excluding leukaemia mortality | 60 / 91 | 1.05 (0.69, 1.64)e | ||||
| All cancer mortality | 111 / 161 | 1.18 (0.85, 1.66)e | ||||
| (Bithell and Stewart, 1975) | Maternal X-ray, cancer mortality | Oxford Survey of Childhood Cancer | 1953–1967 | Lymphatic leukaemia mortality | 290 / 2007 | 1.54 (1.34, 1.78) |
| Myeloid leukaemia mortality | 120 / 866 | 1.47 (1.20, 1.81) | ||||
| Other/unspecified leukaemia mortality | 159 / 1179 | 1.43 (1.19, 1.71) | ||||
| All leukaemia | 569 / 4052 | 1.47 (1.28, 1.69)d | ||||
| Lymphoma mortality | 92 / 719 | 1.35 (1.07, 1.69) | ||||
| All lymphatic/haemopoietic mortality | 661 / 4771 | 1.47 (1.32, 1.64) | ||||
| Wilms’ tumour | 87 / 590 | 1.59 (1.25, 2.01) | ||||
| CNS mortality | 179 / 1332 | 1.42 (1.20, 1.69) | ||||
| Neuroblastoma mortality | 99 / 720 | 1.46 (1.17, 1.83) | ||||
| Bone tumour mortality | 26 / 244 | 1.11 (0.74, 1.66) | ||||
| Other solid tumour (excluding Wilms’ tumour, CNS, neuroblastoma, bone tumour) mortality | 129 / 856 | 1.63 (1.33, 1.98) | ||||
| All solid tumour mortality | 520 / 3742 | 1.47 (1.31, 1.66) | ||||
| All malignant tumour mortality | 1181 / 8513 | 1.47 (1.34, 1.62) | ||||
| (Salonen, 1976) | Pelvic radiography, medical-record based | Finnish national cancer registry | 1959–1968 | Leukaemia | 15 / 300 | 1.10 (0.55, 2.10)d |
| Brain | 11 / 186 | 1.31 (0.59, 2.70)d | ||||
| Other tumour (excluding leukaemia, brain) | 15 / 278 | 1.19 (0.59, 2.27)d | ||||
| All tumour | 41 / 764 | 1.18 (0.72, 1.93)d | ||||
| All tumour excluding leukaemia | 26 / 464 | 1.24 (0.70, 2.15)d | ||||
| (Shiono et al., 1980) | “High” or “medium” doses of X-rays (barium enemas, pyelogram etc), medical-record based | Collaborative Perinatal Project | 1959–1965 | All malignant neoplasms | 7 / 40 | 1.09 (0.47, 2.40) |
| All benign neoplasms | 9 / 105 | 0.94 (0.46, 1.82) | ||||
| (Herrmann, 1980) | X-ray examination in pregnancy | German Democratic Republic leukaemia study | 1957–1973 | Leukaemia | 32 / 75 | 1.41 (0.70, 2.83)d |
| Abdominal X-ray examination in pregnancy vs unexposed | 3 / 46 | 1.23 (0.16, 9.66)d | ||||
| (Grufferman et al., 1982) | Radiographic examination during pregnancy | North Carolina statewide cohort | 1967–1976 | Rhabdomyosarcoma | 2 / 33 | 0.5 (0.1, 2.4) |
| (Preston-Martin et al., 1982) | Pelvic X-ray | Cancer Surveillance Program in Los Angeles county | 1972–1977 | Brain tumour | 38 / 209 | 1.28 (0.74, 2.22)d |
| (Monson and MacMahon, 1984) | Pelvimetry, flat plate of abdomen, upper or lower GI series, intravenous pyelogram or gallbladder series, medical-record based | 42 hospitals in New England and mid-Atlantic US states, mortality | 1947–1967 | Leukaemia mortality | 94 / 704 | 1.48 (1.17, 1.86)d |
| All cancer excluding leukaemia mortality | 68 / 638 | 1.15 (0.87, 1.49)d | ||||
| CNS mortality | 32 / 298 | 1.16 (0.77, 1.68)d | ||||
| All cancer excluding leukaemia and CNS mortality | 36 / 340 | 1.14 (0.78, 1.62)d | ||||
| All cancer mortality | 162 / 1342 | 1.32 (1.11, 1.58)d | ||||
| (van Steensel-Moll et al., 1985) | Prenatal radiation exposure | Netherlands national cohort | 1973–1980 | Acute lymphoblastic leukaemia | 41 / 519 | 2.2 (1.2, 3.8) |
| (Harvey et al., 1985) | Abdominal X-ray during pregnancy, medical-record based | Connecticut twin birth register | 1930–1969 | Leukaemia | 5 / 13 | 1.6 (0.4, 6.8) |
| All cancer excluding leukaemia | 7 / 18 | 3.2 (0.9, 10.7) | ||||
| All cancer | 12 / 31 | 2.4 (1.0, 5.9) | ||||
| Brain | 3 / 4 | 8.48 (0.65, 459.72)d | ||||
| Lymphoma | 1 / 3 | 1.44 (0.02, 28.69)d | ||||
| All solid excluding brain | 3 / 11 | 1.08 (0.17, 4.93)d | ||||
| (Hopton et al., 1985) | One or more pelvic X-rays, medical-record based | Inter-Regional Epidemiological Study of Childhood Cancer (N England) | 1980–1983 | Leukaemia + lymphoma | 37 / 245 | 1.33 (0.85, 2.08) |
| One or more pelvic X-rays, medical-record based | Solid tumour | 35 / 310 | 1.14 (0.73, 1.76) | |||
| One or more pelvic X-rays, medical-record based | All tumour | 72 / 555 | 1.23 (0.89, 1.70)d | |||
| Other X-rays, medical-record based | Leukaemia + lymphoma | 11 / 245 | 0.75 (0.37, 1.53) | |||
| Other X-rays, medical-record based | Solid tumour | 15 / 310 | 1.23 (0.63, 2.38) | |||
| Other X-rays, medical-record based | All tumour | 26 / 555 | 0.94 (0.56, 1.55)d | |||
| (Johnston et al., 1986) | X-rays during pregnancy, GP controls, medical-record based | Inter-Regional Epidemiological Study of Childhood Cancer | 1980–1983 | Germ cell tumour | 6 / 41 | 1.23 (0.28, 5.60)d |
| X-rays during pregnancy, hospital controls, medical-record based | 6 / 41 | 3.30 (0.54, 35.47)d | ||||
| X-rays during pregnancy, GP+hospital controls, medical-record based | 6 / 41 | 1.83 (0.47, 6.89)d | ||||
| (Kneale and Stewart, 1986) | X-rays during pregnancy | Oxford Survey of Childhood Cancer | 1953–1977 | Reticuloendothelial neoplasms | 1100 / 7347 | 1.39 (1.26, 1.54)d |
| Solid tumours | 1018 / 6582 | 1.32 (1.19, 1.46)d | ||||
| (Bunin et al., 1987) | Abdominal or pelvic X-ray | Based on three tertiary-care hospitals in Philadelphia-area, resident in New Jersey, Pennsylvania, Delaware, Maryland | 1970–1983 | Wilms’ tumour | ≥7 / 88 | 1.0 (0.3, 3.7) |
| (Operskalski et al., 1987) | Any X-ray during pregnancy | Los Angeles county | 1972–1982 | Osteosarcoma | 24 / 55 | 1.5 (0.8, 3.0) |
| Pelvic X-ray during pregnancy | 14 / 60 | 2.0 (0.9, 4.4) | ||||
| Other X-ray except dental during pregnancy | 9 / 60 | 1.8 (0.7, 4.8) | ||||
| (Shu et al., 1988) | Abdomen exposure | Shanghai Cancer Institute based cohort | 1974–1986 | Leukaemia | 8 / 307 | 1.5 (0.5, 4.1) |
| Acute lymphoblastic leukaemia | 6 / <307 | 2.0 (0.7, 5.9) | ||||
| Acute non-lymphoblastic leukaemia | 1 / <307 | 0.6 (0.1, 5.0) | ||||
| (Bunin et al., 1989) | Any X-ray during pregnancy, direct fetal exposure | Children’s Cancer Group (US+Canada hospitals) | 1982–1985 | Non-heritable retinoblastoma | 8 / 115 | 4.0 (0.8, 38.7) |
| Any X-ray during pregnancy, no direct fetal exposure | 10 / 115 | 1.7 (0.5, 5.6) | ||||
| Any abdominal/pelvic X-ray during pregnancy | 9 / 115 | 0.4 (0.2, 0.9) | ||||
| Any X-ray during pregnancy, direct fetal exposure | Sporadic-heritable retinoblastoma | 2 / 67 | 1.0 (0.07, 13.8) | |||
| Any X-ray during pregnancy, no direct fetal exposure | 5 / 67 | 1.3 (0.3, 6.3) | ||||
| Any abdominal/pelvic X-ray during pregnancy | 10 / 67 | 2.0 (0.6, 7.5) | ||||
| (Gilman et al., 1989) | Any pregnancy X-ray, partial medical-record based | Oxford Survey of Childhood Cancer | 1953–1981 | All cancer mortality | 2281 / 15,276 | 1.39 (1.30, 1.49)d |
| (Howe et al., 1989) | Abdominal X-ray | Southern Ontario cohort based on Princess Margaret Hospital, Toronto | 1977–1983 | Brain tumour | 7 / 74 | 0.896 (0.334, 2.41) |
| (Magnani et al., 1989) | Pelvic or abdominal X-ray | Cohort based in the pediatric hospitals of the universities of Turin and Padua | 1983–1984 | Soft tissue sarcoma | 4 / 52 | 1.9 (0.5, 6.5) |
| Rhabdomyosarcoma | 3 / 36 | 1.55 (0.28, 5.75)d | ||||
| (Gardner et al., 1990) | Area controls, using medical records for maternal abdominal X-ray exposure | West Cumbria (NW England) | 1950–1985 | Leukaemia | 3 / 20 | 1.15 (0.31, 4.28) |
| Local controls, using medical records for maternal abdominal X-ray exposure | Leukaemia | 3 / 20 | 1.21 (0.31, 4.66) | |||
| Area controls, using medical records for maternal abdominal X-ray exposure | Leukaemia + NHL | 5 / 28 | 1.19 (0.43, 3.32) | |||
| Local controls, using medical records for maternal abdominal X-ray exposure | Leukaemia + NHL | 5 / 28 | 1.34 (0.46, 3.88) | |||
| Local controls, using medical records for maternal abdominal X-ray exposure | NHL | 2 / 8 | 1.74 (0.14, 12.81)d | |||
| Pooled area and local controls, using medical records for maternal abdominal X-ray exposure | NHL | 2 / 8 | 1.52 (0.14, 9.52)d | |||
| (Golding et al., 1990) | Matched analysis of any X-ray exposure in pregnancy (including dental), medical-record based | UK cohort, based on 1 week of 1970 births | 1970–1980 | All cancer | 12 / 33 | 2.75 (1.22, 6.21) |
| Abdomen X-ray exposure vs X-ray unexposed, medical-record based | 4 / 25 | 3.16 (0.58, 16.14)d | ||||
| (Kuijten et al., 1990) | Abdominal or pelvic X-ray | Tumour registries of 8 hospitals in New Jersey, Pennsylvania and Delaware | 1980–1986 | Astrocytoma | 34 / 163 | 0.9 (0.5, 1.5) |
| (Rodvall et al., 1990) | All X-ray, medical-record based | Swedish Twin Register | 1936–1967 | All cancer | 39 / 95 | 1.2 (0.7, 2.1) |
| Abdominal X-ray, medical-record based | All cancer | 25 / 95 | 1.4 (0.8, 2.5) | |||
| All X-ray, medical-record based | Leukaemia | 12 / 29 | 1.0 (0.4, 2.6) | |||
| Abdominal X-ray, medical-record based | Leukaemia | 10 / 29 | 1.7 (0.7, 4.1) | |||
| All X-ray, medical-record based | CNS | 13 / 32 | 1.1 (0.4, 2.6) | |||
| Abdominal X-ray, medical-record based | CNS | 8 / 32 | 1.5 (0.5, 4.2) | |||
| All X-ray, medical-record based | All cancer except leukaemia and CNS | 14 / 34 | 1.7 (0.7, 4.2) | |||
| Abdominal X-ray, medical-record based | All cancer except leukaemia and CNS | 7 / 34 | 1.0 (0.3, 2.9) | |||
| All X-ray, medical-record based | All cancer except leukaemia | 27 / 66 | 1.34 (0.69, 2.56)d | |||
| Abdominal X-ray, medical-record based | All cancer except leukaemia | 15 / 66 | 1.20 (0.54, 2.59)d | |||
| (Magnani et al., 1990) | Pelvic + abdominal X-ray | Turin cohort | 1981–1984 | Acute lymphoblastic leukaemia | 8 / 142 | 1.1 (0.4, 2.8) |
| Abdominal + thoracic X-ray | Acute non-lymphoblastic leukaemia | 4 / 22 | 2.4 (0.8, 7.3) | |||
| (Golding et al., 1992) | X-ray of abdomen or pelvis, medical-record based | South West England cohort, with updated numbers from Wakeford and Bithell (Wakeford and Bithell, 2021) | 1971–1991 | All cancer | 37 / 185 | 1.78 (1.10, 2.82)d |
| Leukaemia | 14 / 63 | 2.03 (0.98, 3.99)d | ||||
| All cancer except leukaemia | 23 / 122 | 1.65 (0.93, 2.84)d | ||||
| (Holly et al., 1992) | Radiography during pregnancy | San Francisco 5 Bay area counties | 1978–1986 | Ewing’s sarcoma | 9 / 43 | 0.7 (0.3, 1.8) |
| (Stjernfeldt et al., 1992) | Abdomen/pelvis X-ray vs known unexposed | Swedish Child Leukaemia Group with numbers taken from Wakeford and Bithell (Wakeford and Bithell, 2021) | 1976–1981 | Solid tumour | 8 / 42 | 1.45 (0.50, 3.84)d |
| (Winn et al., 1992) | Diagnostic X-rays, using regional controls | Intergroup Ewing’s Sarcoma study | 1983–1985 | Ewing’s sarcoma | 44 / 204 | 0.8 (0.5, 1.2) |
| Diagnostic X-rays, using sibling controls | 41 / 191 | 1.5 (0.8, 3.2) | ||||
| (Fajardo-Gutierrez et al., 1993) | Any X-ray during pregnancy | Mexico City | <1993 | Leukaemia | 16 / 80 | 1.89 (0.84, 4.22) |
| (Roman et al., 1993) | Abdominal X-rays, using obstetric records | West Berkshire & North Hampshire | 1972–1989 | Leukaemia + NHL | 5 / 37 | 1.1 (0.3, 3.7) |
| (Sorahan and Stewart, 1993) | Maternal X-ray, cancer mortality, partially medical-record based | Oxford Survey of Childhood Cancer | <1993 | Retinoblastoma mortality | 17 / 86 | 1.95 (1.07, 3.36)d |
| (Bunin et al., 1994) | X-ray of lower abdomen | Children’s Cancer Group (US+Canada hospitals) | 1986–1989 | Astrocytoma | 6 / 155 | 1.1 (0.3, 3.9) |
| Primitive neuroectodermal tumour | 9 / 166 | 0.8 (0.3, 2.3) | ||||
| (McCredie et al., 1994a) | Diagnostic X-rays | Australian (New South Wales) registry | 1985–1989 | Brain tumour | 13 / 82 | 1.3 (0.6, 2.6) |
| (Shu et al., 1994b) | Any X-ray in pregnancy | Children’s Cancer Group (US+Canada hospitals) | 1983–1988 | All infant leukaemia | 59 / 302 | 1.12 (0.77, 1.63) |
| Lower abdomen X-ray and pelvimetry | All infant leukaemia | ≥7 / 302 | 1.26 (0.48, 3.29) | |||
| Any X-ray in pregnancy | Acute lymphoblastic leukaemia | NA / 203 | 0.84 (0.52, 1.35) | |||
| Lower abdomen X-ray and pelvimetry | Acute lymphoblastic leukaemia | 5 / 203 | 1.12 (0.36, 3.50) | |||
| Any X-ray in pregnancy | Acute myeloid leukaemia | NA / 88 | 1.58 (0.80, 3.12) | |||
| Lower abdomen X-ray and pelvimetry | Acute myeloid leukaemia | 2 / 88 | 1.48 (0.23, 9.52) | |||
| (Shu et al., 1994a) | Abdominal X-ray exposure | Shanghai Cancer Institute based cohort | 1981–1991, 1986–1991 for leukaemia | All cancer | 9 / 642 | 2.1 (0.7, 7.0) |
| Prenatal X-ray exposure | All cancer | 27 / 642 | 1.8 (0.9, 3.6) | |||
| Acute leukaemia | 7 / 166 | 2.4 (0.5, 10.6) | ||||
| Lymphoma | 6 / 87 | 3.6 (0.6, 21.6) | ||||
| Brain tumour | 3 / 107 | 1.3 (0.2, 9.0) | ||||
| (van Duijn et al., 1994) | Prenatal X-ray exposure | Dutch Childhood Leukemia Study Group | 1973–1979 | Acute non-lymphoblastic leukaemia | 6 / 80 | 1.7 (0.6, 5.3) |
| (Shu et al., 1995) | X-ray during pregnancy | Children’s Cancer Group | 1982–1989 | Germ cell tumour | 13 / 105 | 0.9 (0.5, 1.8) |
| (Roman et al., 1997) | Lower abdomen X-ray, medical-record based | South England study, based on three hospitals (Oxford, Cambridge, Reading) | 1962–1992 | Leukaemia | 16 / 143 | 0.7 (0.4, 1.3) |
| Pelvimetry, medical-record based | Leukaemia | 9 / 143 | 1.6 (0.6, 3.9) | |||
| Lower abdomen X-ray, medical-record based | Acute lymphoblastic leukaemia | 15 / 113 | 0.8 (0.4, 1.6) | |||
| Pelvimetry, medical-record based | Acute lymphoblastic leukaemia | 8 / 113 | 1.6 (0.6, 4.3) | |||
| Lower abdomen X-ray, medical-record based | Acute myeloid leukaemia | 0 / 15 | 0.0 (0.0, 2.9) | |||
| Pelvimetry, medical-record based | Acute myeloid leukaemia | 0 / 15 | 0.0 (0.0, 2.9) | |||
| Lower abdomen X-ray, medical-record based | NHL | 6 / 34 | 1.0 (0.3, 3.3) | |||
| Pelvimetry, medical-record based | NHL | 3 / 34 | 2.0 (0.4, 9.9) | |||
| (McKinney et al., 1999) | One or more abdominal X-rays vs none, medical-record based | Scotland UKCCS study | 1991–1994 | Leukaemia | 6 / 144 | 2.26 (0.69, 7.45) |
| Acute lymphoblastic leukaemia | 5 / 124 | 2.50 (0.67, 9.31) | ||||
| Lymphoma | 3 / 45 | 0.71 (0.17, 2.97) | ||||
| CNS tumours | 3 / 75 | 1.11 (0.24, 5.05) | ||||
| Other tumours (than leukaemia, lymphoma, CNS) | 3 / 26 | 1.20 (0.29, 5.02) | ||||
| All cancer | 15 / 290 | 1.80 (0.85, 3.73)d | ||||
| (Meinert et al., 1999) | Diagnostic X-ray in pregnancy | German Childhood Cancer registry | 1992–1994 | Leukaemia | 46 / 1184 | 0.94 (0.65, 1.36) |
| NHL | 12 / 234 | 1.22 (0.61, 2.44) | ||||
| Solid tumour | 40 / 940 | 0.92 (0.63, 1.35) | ||||
| Diagnostic X-ray of abdomen, pelvis, intestinal tract in pregnancy vs completely unexposed | Acute leukaemia | 3 / 1141 | 0.93 (0.16, 4.10)d | |||
| NHL | 2 / 224 | 3.19 (0.32, 16.87)d | ||||
| Solid tumour | 2 / 902 | 0.79 (0.08, 4.14)d | ||||
| All cancer | 7 / 2267 | 1.10 (0.33, 3.67)d | ||||
| (Fear et al., 2001) | Pelvimetry, medical-record based | Cohort assembled from birth records in three hospitals (Oxford, Cambridge, Reading) | 1956–1992 | Brain tumour | 7 / 83 | 0.9 (0.4, 2.4) |
| Abdominal X-ray, medical-record based | Brain tumour | 6 / 83 | 0.8 (0.3, 2.1) | |||
| (Naumburg et al., 2001) | Abdominal X-ray, adjusted for age at birth, gestational age, parity, smoking, cesarean section, birthweight, medical-record based | Sweden national cohort | 1973–1989 | Leukaemia | 68 / 624 | 1.14 (0.79, 1.65) |
| Lymphoblastic leukaemia | 55 / 552 | 1.01 (0.68, 1.51) | ||||
| Myeloid leukaemia | 13 / 72 | 1.74 (0.53, 5.74) | ||||
| (Schuz et al., 2001) | Diagnostic X-ray in pregnancy | German Childhood Cancer registry | 1993–1997 | CNS | 16 / 453 | 0.78 (0.44, 1.36) |
| (Shu et al., 2002) | Pelvimetric X-ray | Children’s Cancer Group (US+Canada hospitals) | 1989–1993 | Acute lymphoblastic leukaemia | 55 / 1842 | 1.2 (0.8, 1.7) |
| Any X-ray in pregnancy | 112 / 1842 | 1.0 (0.8, 1.7) | ||||
| (Infante-Rivard, 2003) | Pelvimetry | Quebec two-phase paediatric study cohort | 1980–1998 | Acute lymphoblastic leukaemia | 38 / 701 | 0.80 (0.50, 1.27)d |
| Abdominal X-ray | 4 / 701 | 2.00 (0.29, 22.20)d | ||||
| Pelvimetry + abdominal X-ray | 42 / 701 | 0.85 (0.54, 1.33)d | ||||
| (Patton et al., 2004) | Maternal gonadal X-ray exposure in pregnancy | Pediatric Oncology Group + Children’s Cancer Group | 1992–1994 | Neuroblastoma | 1 / 496 | 1.0 (0.1, 16.0) |
| Maternal X-ray exposure in 1st trimester | 7 / 496 | 0.7 (0.3, 1.8) | ||||
| Maternal X-ray exposure in 2nd trimester | 5 / 496 | 1.2 (0.3, 4.6) | ||||
| Maternal X-ray exposure in 3rd trimester | 6 / 496 | 1.2 (0.4, 3.9) | ||||
| (Stålberg et al., 2007) | Exposure to abdominal X-ray during pregnancy, medical-record based, adjusted for maternal age, parity, multiple birth, mother born in a Nordic | Sweden national cohort | 1975–1984 | Brain tumour | 55 / 503 | 1.02 (0.64, 1.62) |
| country, gestational age at birth, mode of delivery, breech position, birth weight, birth head circumference, level of hospital, hypertension during pregnancy | ||||||
| Exposure to non-abdominal X-ray during pregnancy, medical-record based, adjusted for maternal age, parity, multiple birth, mother born in a Nordic country, gestational age at birth, mode of delivery, breech position, birth weight, birth head circumference, level of hospital, hypertension during pregnancy | 53 / 503 | 0.78 (0.52, 1.17) | ||||
| Exposure to any abdominal X-ray during pregnancy compared with non-X-ray exposed, medical-record based | 55 / 459 | 1.17 (0.76, 1.81)d | ||||
| (Goel et al., 2009) | Maternal gonadal X-ray exposure in pregnancy, adjusted for income, maternal education and matched on age at diagnosis and geographic region of residence | Children’s Oncology Group | 1999–2002 | Wilms’ tumour | 1 / 506 | 1.0 (0.1, 15.5) |
| Maternal X-ray exposure in 1st trimester, adjusted for income, maternal education and matched on age at diagnosis and geographic region of residence | 9 / 506 | 0.8 (0.3, 2.1) | ||||
| Maternal X-ray exposure in 2nd trimester, adjusted for income, maternal education and matched on age at diagnosis and geographic region of residence | 8 / 506 | 0.7 (0.3, 1.8) | ||||
| Maternal X-ray exposure in 3rd trimester, adjusted for income, maternal education and matched on age at diagnosis and geographic region of residence | 8 / 506 | 0.9 (0.3, 2.4) | ||||
| (Grufferman et al., 2009) | Pelvis or abdomen X-ray exposure, matched on age, sex, race and adjusted for length of pregnancy, type of delivery, spotting/cramping/abnormal vaginal bleeding during pregnancy | Children’s Oncology Group | 1982–1988 | Rhabdomyosarcoma | 24 / 312 | 1.4 (0.7, 2.9) |
| (Spix et al., 2009) | Diagnostic X-ray exposure | German Childhood Cancer registry | 1993–2003 | Brain tumour | 2 / 88 | 0.31 (0.06, 1.68) |
| (Bailey et al., 2010) | Any plain abdominal X-ray or CT | Australia Study of Causes of Acute Lymphoblastic Leukaemia in Children <15 y age at diagnosis (Aus-ALL) | 2003–2006 | Acute lymphoblastic leukaemia | 4 / 388 | 0.73 (0.19, 2.84) |
| (Bartley et al., 2010) | Any X-ray in pregnancy | Northern California Childhood Leukemia Study | 1995–2008 | Acute lymphoblastic leukaemia | NA / 652 | 1.20 (0.71, 2.04) |
| Acute myeloid leukaemia | NA / 111 | 0.85 (0.26, 2.78) | ||||
| (Castro-Jimenez and Orozco-Vargas, 2011) | Any X-ray | Colombian 6-hospital neighbourhood-based study | 2000–2005 | Acute lymphoblastic leukaemia | 2f / 85 | 2.00 (0.18, 22.06) |
| (Rajaraman et al., 2011) | Any radiation exposure in utero, medical-record based | UKCCS study | 1976–1996 | All cancer | 120 / 2690 | 1.14 (0.90, 1.45) |
| Leukaemia | 48 / 1253 | 1.36 (0.91, 2.02) | ||||
| Acute lymphoblastic leukaemia | 36 / NA | 1.20 (0.76, 1.88) | ||||
| Acute myeloid leukaemia | 11 / NA | 2.44 (0.95, 6.33) | ||||
| Lymphoma | 16 / 231 | 1.06 (0.55, 2.06) | ||||
| NHL | 13 / NA | 1.48 (0.66, 3.32) | ||||
| Brain/CNS | 25 / 482 | 1.06 (0.64, 1.77) | ||||
| Sarcoma | 10 / NA | 1.13 (0.49, 2.61) | ||||
| Peripheral neural tumours | 7 / NA | 1.00 (0.37, 2.67) | ||||
| Renal | 5 / NA | 1.64 (0.48, 5.59) | ||||
| Abdominal radiation exposure in utero, medical-record based | All cancer | 90 / 2690 | 1.12 (0.85, 1.48) | |||
| Leukaemia | 37 / 1253 | 1.21 (0.78, 1.88) | ||||
| Acute myeloid leukaemia | 8 / NA | 1.76 (0.63, 4.90) | ||||
| (Hassanzadeh et al., 2011) | History of mother’s radiography | Southern Iran leukaemia cohort | 2005–2009 | Leukaemia | 6 / 163 | 3.00 (0.61, 14.86) |
| (Milne et al., 2014) | Any fetal X-ray exposure | Australian cohort via 10 paediatric oncology centres | 2005–2010 | Brain tumour | 8 / 293 | 1.71 (0.69, 4.23) |
| (Kumar et al., 2014a) | History of mother’s radiography | Sharma Institute, India cohort | 2008–2012 | Leukaemia | 32 / 132 | 0.79 (0.44, 1.42)d |
| (Tettamanti et al., 2017) | X-ray or other scan during pregnancy | CEFALO International multicentre study, diagnosed at age 7–19 y | 2004–2008 | Brain tumour | 31 / 337 | 0.96 (0.54, 1.68) |
| X-ray or other scan to the abdomen during pregnancy | 5 / 337 | 0.72 (0.17, 2.97) | ||||
| Cohort studies | ||||||
| Reference | Type of X-ray exposure, other featuresa | Description of study data | Study years | Cancer endpointb | Number of cases exposed / total | Relative risk, (95% CI) |
| (Diamond et al., 1973) | Abdominal X-rays, medical-record based | Cohort of mortality after births at nine hospitals in Baltimore | 1947–1959 | Leukaemia mortality | 6 / 13 | 1.62 (0.52,4.89)g |
| Lymphoma mortality | 2 / 5 | 1.30 (0.17, 7.88)g | ||||
| Brain/CNS mortality | 3 / 11 | 0.68 (0.15, 2.35)g | ||||
| All other malignant neoplasm mortality | 2 / 7 | 0.72 (0.10, 3.35)g | ||||
| (Ray et al., 2010) | Any diagnostic radiation exposure in utero, hazard ratio computed via Cox model, medical-record based | Ontario radiodiagnostic imaging infant birth cohort | 1992–2008 | All childhood malignancies | 4 / 2543 | 0.69 (0.26,1.82) |
questionnaire based, unless otherwise stated
incidence unless otherwise stated
the comparison “unexposed” group is generally given by the negation of the indicated exposed criteria, and so the total number of cases is therefore the combination of “exposed” + “unexposed”, unless otherwise stated.
based on odds ratio estimated via maximum likelihood from hypergeometric model conditional on marginal totals, with exact CI, estimated by fisher.test routine in R (R Project version 3.6.1, 2019).
via Poisson regression, using expected deaths as offset, with likelihood-based CI.
numbers of exposed cases (2) and controls (1) estimated via reverse engineering based on the OR and CI in (Castro-Jimenez and Orozco-Vargas, 2011).
via Poisson regression of sex-averaged data, with likelihood-based CI.
Table 2.
Summary of case-control and cohort studies of postnatal exposure to medical diagnostic radiation in childhood that do not incorporate estimates of dose, with estimates of odds ratio (OR) or relative risk (RR)
| Reference | Type of X-ray exposure, other featuresa | Description of study datab | Study years | Cancer endpointc | Number of cases exposed / total | Odds ratio/relative risk (95% CI) |
|---|---|---|---|---|---|---|
| (Stewart et al., 1958) | Diagnostic X-rays (X) | Oxford Survey of Childhood Cancers, with deaths before age 10 in 1953–1955 and birth-register controls, excluding therapeutically exposed | 1953–1955 | Leukaemia mortality | 90 / 614 | 1.15 (0.82, 1.62)d |
| Other cancer mortality | 88 / 677 | 0.89 (0.65, 1.24)d | ||||
| All cancer mortality | 178 / 1291 | 1.01 (0.80, 1.27)d | ||||
| (Polhemus and Koch, 1959) | Diagnostic X-rays (X) | Cohort based on Children’s Hospital of Los Angeles, excluding therapeutically exposed | 1950–1957 | Leukaemia | 135 / 214 | 2.13 (1.44, 3.18)d |
| Fluoroscopy (F) | 17 / 96 | 3.48 (1.35, 9.77)d | ||||
| (Ager et al., 1965) | Postnatal X-ray vs not, sibling controls; exposures within 1 y of death excluded (X) | Minnesota childhood leukaemia study, mortality, age < 5 y | 1953–1957 | Leukaemia mortality | 22 / 109 | 1.26 (0.59, 2.73)d |
| Postnatal X-ray vs not, neighbourhood controls; exposures within 1 y of death excluded (X) | 22 / 109 | 1.14 (0.55, 2.37)d | ||||
| (Graham et al., 1966) | Any postnatal radiation exposure vs none, excluding exposures 6 months before diagnosis, medical-record based (U) | USA Tri-state Study, diagnosed 0–14 y of age | 1959–1962 | Leukaemia | 93 / 319 | 0.73 (0.55, 0.97)d |
| Any postnatal radiation exposure vs none, excluding exposures 12 months before diagnosis, medical-record based (U) | 81 / 319 | 0.71 (0.53, 0.96)d | ||||
| (Preston-Martin et al., 1980) | First diagnostic medical X-ray exposure at age <20 y (X) | Cancer Surveillance Program in Los Angeles county study, women <65 y age at diagnosis | 1972–1975 | Intracranial meningiomas | 53 / 185 | 1.60 (0.97, 2.68)d |
| First diagnostic X-ray exposure (medical or dental) at age <20 y (X) | 99 / 185 | 1.51 (0.98, 2.32)d | ||||
| First full-mouth dental X-ray series at age <20 y (X) | 41 / 101 | 4.04 (2.07, 8.12)d | ||||
| (Preston-Martin et al., 1982) | Five or more full-mouth dental X-rays, starting at least 10 y before diagnosis (X) | Cancer Surveillance Program in Los Angeles county, aged 15–24 y at diagnosis | 1972–1977 | Brain tumour | 17 / 68 | 2.48 (0.92, 7.23)d |
| (Greenberg, 1983) | Chest radiograph, hospital non-cancer controls (X) | North Carolina paediatric (age < 15 y at diagnosis) neuroblastoma case-control study | 1972–1981 | Neuroblastoma or ganglioneuroblastom a | 16 / 104 | 0.29 (0.14, 0.61) |
| Chest radiograph, hospital Wilms’ tumour controls (X) | 16 / 104 | 1.95 (0.73, 5.19) | ||||
| Cranial radiograph, hospital non-cancer controls (X) | 2 / 104 | 0.30 (0.07, 1.36) | ||||
| Cranial radiograph, hospital Wilms’ tumour controls (X) | 2 / 104 | 1.57 (0.13, 19.13) | ||||
| Abdominal radiograph, hospital non-cancer controls (X) | 3 / 104 | 0.41 (0.12, 1.45) | ||||
| Abdominal radiograph, hospital Wilms’ tumour controls (X) | 3 / 104 | 0.81 (0.15, 4.34) | ||||
| (Spengler et al., 1983) | Mortality after cardiac catheterisation. Observed cases and expected, relative risk assessed via exact Poisson model (Garwood, 1936) (C) | Toronto Hospital for Sick Children cardiac catheterisation cohort study, medical record based; catheterisation at age <30 y (99.8% < age 20y), follow-up 1946–1975, age at death, 0–45 y. | 1946–1968 | All cancer mortality, male | 1 / 3.09e | 0.32 (0.01, 1.80)f |
| Leukaemia mortality, male | 1 / 1.22e | 0.82 (0.02, 4.57)f | ||||
| All cancer mortality, female | 4 / 1.78e | 2.25 (0.61, 5.75)f | ||||
| Leukaemia mortality, female | 2 / 0.66e | 3.03 (0.37, 10.95)f | ||||
| Kidney mortality, female | 1 / 0.09e | 11.11 (0.28, 61.91)f | ||||
| All cancer mortality | 5 / 4.87e | 1.03 (0.33, 2.40)f | ||||
| Leukaemia mortality | 3 / 1.88e | 1.60 (0.33, 4.66)f | ||||
| (Operskalski et al., 1987) | Any radiation exposure except dental X-ray (U) | Los Angeles county, diagnosed age <25 y | 1972–1982 | Osteosarcoma | 41 / 62 | 0.9 (0.4, 1.8) |
| (Hartley et al., 1988) | Neonatal X-ray (X) | Inter-Regional Epidemiological Study of Childhood Cancer, medical record based, diagnosed age <15 y | 1980–1983 | Any cancer incidence | 5 / 465 | 1.11 (0.32, 3.63) |
| (Shu et al., 1988) | Any X-ray exposure (X) | Shanghai Cancer Institute cancer registry, diagnosed age <15 y | 1974–1986 | Leukaemia | 79 / 309 | 0.91 (0.66, 1.26)d |
| 1–5 X-ray exposure vs none (X) | 71 / 301 | 0.8 (0.6, 1.1) | ||||
| 6+ X-ray exposure vs none (X) | 8 / 238 | 2.4 (0.6, 9.2) | ||||
| Any X-ray exposure (X) | Acute lymphoblastic leukaemia | 42 / 172 | 0.86 (0.57, 1.28)d | |||
| 1–5 X-ray exposure vs none (X) | 38 / 168 | 0.8 (0.5, 1.2) | ||||
| 6+ X-ray exposure vs none (X) | 4 / 134 | 3.3 (0.7, 15.9) | ||||
| Any X-ray exposure (X) | Acute non-lymphoblastic leukaemia | 26 / 94 | 1.02 (0.60, 1.68)d | |||
| 1–5 X-ray exposure vs none (X) | 25 / 93 | 0.9 (0.5, 1.5) | ||||
| 6+ X-ray exposure vs none (X) | 1 / 69 | 1.2 (0.1, 12.5) | ||||
| (Howe et al., 1989) | Chest X-rays, ever vs never (X) | Southern Ontario study, based on Princess Margaret Hospital, Toronto, diagnosed age <20 y, X-ray exposures within 5 y of diagnosis excluded | 1977–1983 | Brain tumour | 9 / 74 | 3.32 (1.17, 9.43) |
| Chest X-rays, per film (X) | 9 / 74 | 3.54 (1.61, 7.77) | ||||
| Skull X-rays, ever vs never (X) | 11 / 74 | 8.35 (2.13, 32.8) | ||||
| Skull X-rays, per film (X) | 11 / 74 | 2.67 (1.37, 5.19) | ||||
| Chest X-rays, ever vs never, adjusted for skull X-rays (X) | 9 / 74 | 2.07 (0.62, 6.95) | ||||
| Skull X-rays, ever vs never, adjusted for chest X-rays (X) | 11 / 74 | 6.71 (1.65, 27.3) | ||||
| (Magnani et al., 1989) | Any diagnostic X-ray exposure (X) | Paediatric hospital study of the universities of Turin and Padua, diagnosed in children | 1983–1984 | Rhabdomyosarcoma | 16 / 36 | 1.0 (0.5, 2.1) |
| Soft tissue sarcoma | 20 / 52 | 0.8 (0.4, 1.5) | ||||
| (Nishi and Miyake, 1989) | Dental X-ray film (X) | Hokkaido Prefecture study, diagnosed aged 0–14 y | 1981–1987 | Non T-cell acute lymphoblastic leukaemia | NA / 63 | 1.4 (1.0, 2.0) |
| Hip joint X-ray (X) | 49 / 63 | 1.1 (0.9, 1.3) | ||||
| (Kuijten et al., 1990) | Head or neck X-ray (X) | Tumour registries of 8 hospitals in New Jersey, Pennsylvania and Delaware, diagnosed aged <15 y | 1980–1986 | Astrocytoma | 18 / 163 | 1.0 (0.5, 2.1) |
| Dental X-ray (X) | 18 / 163 | 0.9 (0.4, 1.8) | ||||
| (Magnani et al., 1990) | Any diagnostic X-ray (X) | Turin study, diagnosed in childhood | 1974–1984 | Acute lymphoblastic leukaemia | 48 / 142 | 0.7 (0.5, 1.2) |
| Acute non-lymphoblastic leukaemia | 10 / 22 | 0.98 (0.37, 2.56)d | ||||
| NHL | 6 / 19 | 0.54 (0.17, 1.58)d | ||||
| (Fajardo - Gutierrez et al., 1993) | Any postnatal X-ray, hospital + community controls (X) | Mexico City study, diagnosed in childhood | <1993 | Leukaemia | 23 / 79 | 1.11 (0.57, 2.13) |
| Any postnatal X-ray, community controls (X) | 23 / 79 | 2.32 (0.97, 5.73) | ||||
| (McLaughlin et al., 1993) | Any catheterisation. Observed cases and expected, relative risk assessed via exact Poisson model (Garwood, 1936) (C) | Cohort study of cardiac catheterisation among Ontario residents at a major Toronto hospital, catheterised at age <19 y and followed to 1985 for incidence and mortality, medical record based | 1950–1965 | All cancer mortality | 7 / 5.70e | 1.23 (0.49, 2.53)f |
| All cancer incidence | 13 / 17.27e | 0.75 (0.40, 1.29)f | ||||
| Leukaemia incidence | 3 / 1.87e | 1.60 (0.33, 4.69)f | ||||
| (Bunin et al., 1994) | Dental X-ray (X) | Children’s Cancer Group (US+Canada hospitals), diagnosed at age 0–5 y | 1986–1989 | Astrocytoma | 14 / 155 | 1.0 (0.4, 2.7) |
| Dental X-ray (X) | Primitive neuroectodermal tumour (PNET) | 8 / 166 | 0.5 (0.1, 1.6) | |||
| Other head or neck X-ray (X) | Astrocytoma | 12 / 155 | 1.6 (0.6, 4.3) | |||
| Other head or neck X-ray (X) | Primitive neuroectodermal tumour (PNET) | 10 / 166 | 3.3 (0.7, 22.1) | |||
| Any head, neck, dental X-ray (X) | Astrocytoma | 24 / 155 | 1.2 (0.6, 2.4) | |||
| Any head, neck, dental X-ray (X) | Primitive neuroectodermal tumour (PNET) | 22 / 166 | 1.1 (0.5, 2.4) | |||
| (McCredie et al., 1994b) | X-rays of teeth (X) | Australian (New South Wales) registry, diagnosed at age 0–14 y | 1985–1989 | Brain tumour | 3 / 82 | 0.4 (0.1, 1.4) |
| X-rays of head (X) | 4 / 82 | 2.3 (0.5, 10.8) | ||||
| (Shu et al., 1994a) | Postnatal X-ray exposure (X) | Shanghai Cancer Institute based, diagnosed at age 0–14 y | 1981–1991 | All cancer | 223 / 642 | 1.3 (1.0, 1.7) |
| Acute leukaemia | 64 / 166 | 1.6 (1.0, 2.6) | ||||
| Lymphoma | 29 / 87 | 1.3 (0.6, 2.7) | ||||
| Brain tumour | 41 / 107 | 1.5 (0.8, 3.0) | ||||
| (Meinert et al., 1999) | Any diagnostic X-rays up to 1 year before diagnosis vs none (X) | German Childhood Cancer registry diagnosed at age <15 y, born after June 1975 | 1992–1994 (solid tumours) 1980–1994 (acute leukaemia+NH L) | Acute leukaemia | 328 / 1145 | 0.80 (0.68, 0.93)d |
| 1–4 diagnostic X-rays up to 1 year before diagnosis vs none (X) | Acute leukaemia | 289 / 1145 | 0.78 (0.65, 0.93) | |||
| 4+ diagnostic X-rays up to 1 year before diagnosis vs none (X) | Acute leukaemia | 39 / 1145 | 1.00 (0.65, 1.55) | |||
| Any diagnostic X-rays up to 1 year before diagnosis vs none (X) | NHL | 85 / 224 | 1.22 (0.91, 1.63)d | |||
| 1–4 diagnostic X-rays up to 1 year before diagnosis vs none (X) | NHL | 77 / 224 | 0.71 (0.51, 1.00) | |||
| 4+ diagnostic X-rays up to 1 year before diagnosis vs none (X) | NHL | 8 / 224 | 0.60 (0.27, 1.34) | |||
| Any diagnostic X-rays up to 1 year before diagnosis vs none (X) | Solid tumour | 261 / 922 | 0.79 (0.66, 0.93)d | |||
| 1–4 diagnostic X-rays up to 1 year before diagnosis vs none (X) | Solid tumour | 235 / 922 | 0.80 (0.55, 0.98) | |||
| 4+ diagnostic X-rays up to 1 year before diagnosis vs none (X) | Solid tumour | 26 / 922 | 0.78 (0.48, 1.27) | |||
| (Modan et al., 2000) | Cardiac catheterisationof children; observed cases and expected numbers based on Israeli national cancer incidence rates, follow-up starts 5 y after first catheterisation, relative risk assessed via exact Poisson model (Garwood, 1936) (C) | Israel national cardiac catheterisation due to congenital anomaly cohort, medical record based, follow-up to end-1996 | 1950–1970 | NHL, males | 3 / 0.45e | 6.7 (1.3, 19.5) |
| Hodgkin’s disease, males | 1 / 0.25e | 4.0 (0.05, 22.2) | ||||
| All lymphomas, males | 4 / 0.70e | 5.7 (1.5, 14.6) | ||||
| Melanoma, males | 3 / 0.62e | 4.87 (1.0, 14.2) | ||||
| Bladder, males | 1 / 1.86e | 0.54 (0.01, 3.0) | ||||
| Stomach, males | 1 / 0.13e | 7.8 (0.1, 43.6) | ||||
| Testis, males | 1 / 0.34e | 2.9 (0.04, 16.2) | ||||
| Prostate, males | 1 / 0.93e | 1.1 (0.01, 6.0) | ||||
| All sites, males | 11 / 4.75e | 2.3 (1.2, 4.1) | ||||
| All sites, females | 0 / 6.80e | 0.00 (0.00, 0.54)f | ||||
| All sites, males+females | 11 / 11.55e | 0.95 (0.48, 1.70)f | ||||
| (Schuz et al., 2001) | Any X-ray examination up to 1 y before diagnosis (X) | German national childhood cancer study diagnosed at age <15 y | 1993–1997 | All CNS | 142 / 458 | 0.73 (0.57, 0.94) |
| Astrocytoma | 42 / 118 | 0.78 (0.50, 1.23) | ||||
| Ependymoma | 10 / 49 | 0.57 (0.25, 1.31) | ||||
| Medulloblastoma | 32 / 110 | 0.78 (0.49, 1.23) | ||||
| (Shu et al., 2002) | Ever X-ray exposure, excluding dental X-rays (X) | Children’s Cancer Group (US+Canada hospitals) study | 1989–1993 | Acute lymphoblastic leukaemia | 939 / 1842 | 1.6 (1.4, 1.9) |
| Ever X-ray exposure, excluding exposures within 2 y of diagnosis (X) | Acute lymphoblastic leukaemia | NA / 1842 | 1.1 (0.9, 1.2) | |||
| T-cell acute lymphoblastic leukaemia | NA / 183 | 1.1 (0.7, 1.7) | ||||
| Early pre-B cell acute lymphoblastic leukaemia | NA / 893 | 1.1 (0.8, 1.3) | ||||
| Pre-B cell acute lymphoblastic leukaemia | NA / 233 | 1.7 (1.1, 2.7) | ||||
| (Infante-Rivard, 2003) | Single X-ray vs none (excluding dental) (X) | Quebec two-phase paediatric study, diagnosed at ages 0–14 y, males | 1980–1998 | Acute lymphoblastic leukaemia | 157 / 589 | 1.17 (0.79, 1.73) |
| ≥ 2 X-rays vs none (excluding dental) (X) | 196 / 628 | 1.41 (0.99, 2.01) | ||||
| Single X-ray vs none (excluding dental) (X) | 106 / 483 | 1.11 (0.78, 1.78) | ||||
| ≥ 2 X-rays vs none (excluding dental) (X) | 104 / 481 | 1.67 (1.01, 2.74) | ||||
| (Mellemkjaer et al., 2006) | Diagnostic X-rays, adjusted for gestational age (X) | Danish National Hospital Discharge Registry study, medical record based, diagnosed at ages 0–19 y | 1977–1989 | CNS tumours (excluding pituitary) | 11 / 25 | 2.20 (0.60, 8.80) |
| (Bailey et al., 2010) | Any diagnostic X-ray exposure more than 6 months before diagnosis (CT) | Australia Study of Causes of Acute Lymphoblastic Leukaemia in Children <15 y age at diagnosis (Aus-ALL) | 2003–2006 | Acute lymphoblastic leukaemia | 156 / 359 | 1.15 (0.88,1.51) |
| Any plain X-ray exposure more than 6 months before diagnosis (X) | 150 / 359 | 1.15 (0.88,1.52) | ||||
| Any CT exposure more than 6 months before diagnosis (CT) | 6 / 359 | 0.87 (0.32,2.34) | ||||
| (Bartley et al., 2010) | Any postnatal X-ray excluding dental X-rays and X-rays received within 1 y of diagnosis (X) | Northern California Childhood Leukemia Study | 1995–2008 | Acute lymphoblastic leukaemia | NA / 711 | 1.21 (0.96, 1.51) |
| B-cell acute lymphoblastic leukaemia | NA / 472 | 1.40 (1.06, 1.86) | ||||
| T-cell acute lymphoblastic leukaemia | NA / 52 | 0.54 (0.21, 1.35) | ||||
| Acute myeloid leukaemia | NA / 116 | 0.78 (0.38, 1.61) | ||||
| (Khan et al., 2010) | Head X-ray due to head injury (X) | Children’s Oncology Group Study, age <6 y at diagnosis | 1991–1997 | Medulloblastoma/pri mitive neuroectodermal tumours | 8 / 299 | 0.62 (0.21,1.9) |
| Head X-ray not due to head injury, with possibly tumour-related X-rays deemed unexposed (X) | 15 / 299 | 1.3 (0.49,3.7) | ||||
| Head X-ray any reason, with possibly tumour-related X-rays deemed unexposed (X) | 23 / 299 | 1.2 (0.54,2.5) | ||||
| Dental X-ray any reason (X) | 16 / 299 | 0.85 (0.37,1.90) | ||||
| (Rajaraman et al., 2011) | Any radiation exposure in early infancy (0–100 days), medical-record based, 2 y lag (U) | UK Childhood Cancer Study (UKCCS), diagnosed at ages 0–14 y | 1992–1996 | All cancer | 50 / 2690 | 1.19 (0.82, 1.74) |
| Leukaemia | 27 / 1253 | 1.35 (0.81, 2.27) | ||||
| Acute lymphoblastic leukaemia | 26 / NA | 1.55 (0.90, 2.67) | ||||
| Lymphoma | 7 / 231 | 5.14 (1.27, 20.80) | ||||
| NHL | 6 / NA | 6.85 (1.31, 35.70) | ||||
| Brain/CNS | 6 / 482 | 0.94 (0.31, 2.92) | ||||
| (Claus et al., 2012) | Bitewing X-ray at age < 10 y (X) | Five state (Connecticut, Massachusetts, North Carolina, California, Texas) meningioma case-control study | 2006–2011 | Meningioma | 239 / 1433 | 1.3 (1.0,1.7) |
| Bitewing X-ray at age 10–19 y (X) | 682 / 1433 | 1.4 (1.1,1.7) | ||||
| Full mouth X-ray at age < 10 y (X) | 100 / 1433 | 1.2 (0.8,1.7) | ||||
| Full mouth X-ray at age 10–19 y (X) | 371 / 1433 | 1.1 (0.9,1.4) | ||||
| (Huang et al., 2014) | Any CT examination, males, lag 2 y (CT) | Taiwan National Health Insurance research database (NHIRD), age < 18 y undergoing CT exams | 1996–2008 | Brain tumour overall | 11 / 28 | 2.62 (1.23,5.59) |
| Any CT examination, females, lag 2 y (CT) | Brain tumour overall | 8 / 21 | 2.48 (1.03,5.99) | |||
| Any CT examination, males, lag 2 y (CT) | Malignant brain tumour | 4 / 11 | 2.32 (0.68,7.92) | |||
| Any CT examination, females, lag 2 y (CT) | Malignant brain tumour | 1 / 5 | 1.00 (0.11,8.97) | |||
| Any CT examination, males, lag 2 y (CT) | Benign brain tumour | 7 / 17 | 2.82 (1.08,7.42) | |||
| Any CT examination, females, lag 2 y (CT) | Benign brain tumour | 7 / 16 | 3.15 (1.17,8.45) | |||
| Any CT examination, males, lag 2 y (CT) | Leukaemia | 6 / 18 | 2.02 (0.76,5.38) | |||
| Any CT examination, females, lag 2 y (CT) | Leukaemia | 2 / 7 | 1.62 (0.31,8.33) | |||
| Any CT examination, males, lag 2 y (CT) | Other cancers (than leukaemia, brain) | 7 / 52 | 0.62 (0.28,1.36) | |||
| Any CT examination, females, lag 2 y (CT) | Other cancers (than leukaemia, brain) | 5 / 34 | 0.69 (0.27,1.79) | |||
| (Liao et al., 2014) | Cystourethrography (X) | Taiwan National Health Insurance research database (NHIRD) case-control study, matched for sex, age (within 5 y), geographic region, parents occupation, aged 1–18, adjusted for age | 1997–2008 | All cancer | 52 / 151 | 1.92 (1.34,2.74) |
| Abdominal cancers excluding genitourinary cancers | 5 / 10 | 2.98 (0.77,11.60) | ||||
| Neuroendocrine cancers | 11 / 39 | 1.21 (0.57,2.59) | ||||
| Non-abdominal cancers | 1 / 11 | 0.49 (0.06,3.86) | ||||
| Genital cancers | 4 / 7 | 6.19 (1.37,28.00) | ||||
| Urinary system cancers | 7 / 11 | 5.80 (1.54,21.90) | ||||
| Hematologic cancers | 22 / 66 | 1.82 (1.05,3.13) | ||||
| All other cancers | 2 / 7 | 1.93 (0.37,9.97) | ||||
| (Milne et al., 2014) | Any diagnostic radiological procedure, lag 6 m (CT) | Australian cohort via 10 paediatric oncology centres | 2005–2010 | Brain tumour | 102 / 281 | 0.66 (0.48,0.90) |
| Any plain X-ray, lag 6 m (X) | 97 / 281 | 0.68 (0.49,0.93) | ||||
| Any CT scan, lag 6 m (CT) | 13 / 281 | 0.78 (0.38,1.59) | ||||
| Any diagnostic radiological procedure to the head (including dental), lag 6 m (CT) | 37 / 281 | 0.68 (0.42,1.08) | ||||
| Any plain X-ray to the head, lag 6 m (X) | 27 / 281 | 0.61 (0.36,1.03) | ||||
| Any CT scan to the head, lag 6 m (CT) | 12 / 281 | 0.83 (0.40,1.75) | ||||
| (Shih et al., 2014) | Any X-ray (X) | Taiwan National Health Insurance research database (NHIRD) based case-control study, ages 6–18, matched by age, sex, level of urbanisation, parental occupation, index year | 1998–2010 | Leukaemia | 34 / 58 | 2.14 (1.18,3.87) |
| (Tettamanti et al., 2017) | Exposure to any X-ray or scan (excluding dental X-rays) more than 2 y before diagnosis (CT) | CEFALO International multicentre study, diagnosed at age 7–19 y | 2004–2008 | Brain tumour | 159 / 350 | 0.76 (0.58,1.01) |
| Exposure to any X-ray or scan to head or body+head (excluding dental X-rays) more than 2 y before diagnosis (CT) | 41 / 350 | 1.09 (0.71,1.67) | ||||
| Exposure to CT scan to head or body+head vs no X-ray or scan to the head, more than 2 y before diagnosis (CT) | 10 / 350 | 1.86 (0.82,4.22) | ||||
| (Harbron et al., 2018) | X-ray guided cardiac catheterisation, including transplant recipients (C) | UK cardiac catheterisation study, exposed while ≤ 22 years of age (>90% aged <20 years at first exposure) | <1980–2014 | All malignancies | 36 / NA | 3.01 (2.09,4.19) |
| Leukaemia | 4 / NA | 1.73 (0.43,4.53) | ||||
| Lymphoma | 22 / NA | 9.15 (5.66,13.97) | ||||
| Hodgkin lymphoma | 4 / NA | 2.70 (0.68,7.07) | ||||
| NHL | 18 / NA | 19.49 (11.39,31.1 0) | ||||
| Central nervous system | 3 / NA | 1.57 (0.28,4.70) | ||||
| X-ray guided cardiac catheterisation, excluding post-transplant patients (C) | All malignancies | 11 / NA | 0.98 (0.48,1.77) | |||
| Leukaemia | 4 / NA | 1.80 (0.45,4.71) | ||||
| Central nervous system | 2 / NA | 1.10 (0.09,4.11) | ||||
| Lymphoma | 0 / NA | 0.00 (0.00, 1.64)f | ||||
| Other tumours (than leukaemia, lymphoma, brain/CNS) | 5 / NA | 1.01 (0.33, 2.35)f | ||||
| (Baaken et al., 2019) | Any diagnostic X-ray procedure during 1976–2003, exposure lag of 2 y (M) | Dr von Hauner Children’s Hospital cohort, age <14.5 y at first examination, without cancer at first examination, SIR analysis | 1980–2016 | All cancers | 71 / NA | 0.98 (0.76,1.23) |
| Leukaemia | 24 / NA | 0.99 (0.63,1.47) | ||||
| Lymphoblastic leukaemia | 16 / NA | 0.83 (0.48,1.36) | ||||
| Acute myeloid leukaemia | 4 / NA | 1.15 (0.31,2.94) | ||||
| Lymphomas | 7 / NA | 0.61 (0.25,1.26) | ||||
| Hodgkin lymphoma | 1 / NA | 0.21 (0.01,1.16) | ||||
| NHL | 5 / NA | 1.35 (0.44,3.16) | ||||
| CNS tumours | 8 / NA | 0.51 (0.22,1.00) | ||||
| Blastomas | 10 / NA | 1.89 (0.91,3.47) | ||||
| Sarcomas | 13 / NA | 1.54 (0.82,2.64) | ||||
| Other solid tumours | 9 /NA | 3.32 (1.52,6.31) | ||||
| (Hong et al., 2019) | Any diagnostic radiation exposure. exposure lag 1 y (U) | South Korean National Health Insurance System study, ages 0–19 y at exposure, 0–29 y at diagnosis | 2006–2015 | All cancer | 1921 / 21,912 | 1.72 (1.64, 1.80) |
| Any diagnostic radiation exposure. exposure lag 2 y (U) | All cancer | 1444 / 21,912 | 1.64 (1.56, 1.73) | |||
| Any diagnostic radiation exposure. exposure lag 5 y (U) | All cancer | 434 / 21,912 | 1.48 (1.35, 1.63) | |||
| Any diagnostic radiation exposure, exposure lag 2 y (U) | All solid | 987 / 15314 | 1.70 (1.59, 1.81) | |||
| Mouth/pharynx | 30 / 380 | 2.01 (1.38, 2.92) | ||||
| Digestive | 76 / 974 | 1.83 (1.22, 2.74) | ||||
| Respiratory | 35 / 431 | 1.95 (1.38, 2.75) | ||||
| Bone | 53 / 1123 | 1.05 (0.79, 1.38) | ||||
| Melanoma | 14 / 262 | 1.32 (0.77, 2.26) | ||||
| Soft tissue | 43 / 821 | 1.20 (0.88, 1.63) | ||||
| Breast | 14 / 239 | 2.32 (1.35, 3.99) | ||||
| Female genital | 76 / 1385 | 1.77 (1.41, 2.24) | ||||
| Male genital | 23 / 377 | 1.28 (0.84, 1.95) | ||||
| Urinary | 20 / 386 | 1.16 (0.74, 1.82) | ||||
| Brain | 183 / 2872 | 1.57 (1.38, 1.78) | ||||
| Thyroid | 363 / 5225 | 2.19 (1.97, 2.44) | ||||
| Unspecified solid | 57 / 839 | 1.68 (1.29, 2.20) | ||||
| Lymphoid & haemopoietic malignant neoplasms | 457 / 6598 | 1.53 (1.39, 1.69) | ||||
| Hodgkin lymphoma | 21 / 385 | 1.32 (0.85, 2.05) | ||||
| Other lymphoma | 47 / 599 | 1.73 (1.28, 2.32) | ||||
| Other lymphoid | 57 / 1024 | 1.27 (0.97, 1.66) | ||||
| Leukaemia & myeloid | 332 / 4590 | 1.58 (1.42, 1.77) | ||||
| Leukaemia | 294 / 4218 | 1.51 (1.34,1.71) | ||||
| Lymphoid leukaemia | 74 / 1879 | 0.81 (0.64,1.02) | ||||
| Other myeloid | 220 / 2339 | 2.14 (1.86,2.46) | ||||
| Myelodysplasia | 38 / 372 | 2.48 (1.77,2.47) | ||||
| Any computed tomography exposure, exposure lag 2 y (CT) | All solid | 840 / 15314 | 1.62 (1.51, 1.74) | |||
| Mouth/pharynx | 29 / 380 | 2.19 (1.50, 3.20) | ||||
| Digestive | 65 / 974 | 1.97 (1.53, 2.53) | ||||
| Respiratory | 34 / 431 | 2.01 (1.46, 2.78) | ||||
| Bone | 47 / 1123 | 1.03 (0.77, 1.38) | ||||
| Melanoma | 13 / 262 | 1.38 (0.79, 2.41) | ||||
| Soft tissue | 40 / 821 | 1.24 (0.90, 1.71) | ||||
| Breast | 13 / 239 | 2.53 (1.44, 4.43) | ||||
| Female genital | 71 / 1385 | 1.92 (1.51, 2.43) | ||||
| Male genital | 22 / 377 | 1.36 (0.88, 2.09) | ||||
| Urinary | 18 / 386 | 1.15 (0.72, 1.85) | ||||
| Brain | 166 / 2872 | 1.55 (1.36, 1.77) | ||||
| Thyroid | 273 / 5225 | 1.87 (1.65, 2.11) | ||||
| Unspecified solid | 49 / 839 | 1.61 (1.21, 2.15) | ||||
| Lymphoid & haemopoietic malignant neoplasms | 376 / 6598 | 1.38 (1.25, 1.54) | ||||
| Hodgkin lymphoma | 20 / 385 | 1.42 (0.90, 2.23) | ||||
| Other lymphoma | 41 / 599 | 1.66 (1.20, 2.27) | ||||
| Other lymphoid | 49 / 1024 | 1.22 (0.91, 1.62) | ||||
| Leukaemia & myeloid | 266 / 4590 | 1.38 (1.22, 1.57) | ||||
| Leukaemia | 233 / 4218 | 1.31 (1.15,1.49) | ||||
| Lymphoid leukaemia | 68 / 1879 | 0.82 (0.64,1.04) | ||||
| Other myeloid | 165 / 2339 | 1.73 (1.48,2.03) | ||||
| Myelodysplasia | 33 / 372 | 2.38 (1.66,3.40) | ||||
| (Li et al., 2020) | Computed tomography, 1 y exclusion (CT) | Taiwan National Health Insurance research database (NHIRD), age < 16 y at exposure and diagnosis | 1997–2013 | Leukaemia | NA / 1423 | 1.04 (0.72, 1.48) |
| Intracranial malignancy | NA / 838 | 1.95 (1.40, 2.71) | ||||
| Lymphoma | NA / 272 | 1.69 (1.34, 2.13) | ||||
| Computed tomography, 2 y exclusion (CT) | Leukaemia | NA / 1423 | 0.85 (0.54, 1.34) | |||
| Intracranial malignancy | NA / 838 | 1.56 (1.04, 2.33) | ||||
| Lymphoma | NA / 272 | 0.93 (0.42, 2.05) |
questionnaire based, unless otherwise stated. The coding of types of exposure is as follows: X=X-ray, F=fluoroscopy, C=catheterization, U=mixed diagnostic radiation/unknown, CT=computed tomography
case-control studies, unless otherwise mentioned
incidence unless otherwise stated
based on odds ratio estimated via maximum likelihood from hypergeometric model conditional on marginal totals, with exact CI, estimated by fisher.test routine in R (R Project version 3.6.1, 2019).
expected cases/deaths.
relative risk CI assessed via exact Poisson model (Garwood, 1936)
An aggregate estimate of meta OR (mOR) or meta RR (mRR) was computed across subsets of these studies (with non-overlapping endpoints within each study if more than one was available) using log-transformed risk estimates, random effects models and standard statistical methods (inverse variance weighted least squares) (see Supplementary Methods). Restricted maximum-likelihood fits were used by default to derive estimates of variation of risk over time; ordinary maximum likelihood fits were also used, as these facilitate comparison of nested models (in particular, to test against improvement over the null, i.e., lack of homogeneity of risk, where homogeneity of risk across categories is the assumed null hypothesis. Confidence intervals on mOR/mRR were derived using the method of Knapp and Hartung (Knapp and Hartung, 2003). The analysis was done in two ways. In the first, only those cancer sites within each study that contributed to one of the four endpoints (a) leukaemia, (b) lymphoma, (c) brain/central nervous system (CNS) tumours and (d) any other cancer (i.e., all solid cancers except brain/CNS tumours) were used; leukaemia, brain/CNS tumours and lymphoma are the commonest forms of cancer in childhood. In the second type of analysis, as far as possible the endpoint used was “any available cancer site” from each study.
In order to assess selection or publication bias, funnel plots were employed. Funnel plots are scatterplots of the central estimate of OR or RR against estimates of standard error, and as discussed by Egger et al (Egger et al., 1997; Sterne and Egger, 2001) are useful qualitative means of detecting various types of selection bias, in particular publication bias. If the funnel plot has the form of an inverted symmetric funnel, then selection bias is considered to be unlikely (Egger et al., 1997; Sterne and Egger, 2001). More formal tests of selection or publication bias were also conducted using the test statistic suggested by Egger et al (Egger et al., 1997). We also employed the trim-and-fill method of Duval and Tweedie (Duval and Tweedie, 2000) to assess the likely magnitude of the change in OR/RR that may result from selection bias.
All statistical models, including funnel plots, were fitted using the metafor package (Viechtbauer, 2010; Viechtbauer, 2020) in R (R Project version 3.6.1, 2019). Further details of the statistical methods are given in the Supplementary Methods.
3. Results
The literature review yielded 89 papers on cancer incidence/mortality following medical diagnostic radiation exposure antenatally or postnatally, principally of those diagnosed or dying while aged less than 21 years, in which quantitative dose estimates were not given. The papers were divided by whether they dealt with results of in utero exposure (70 papers) (Table 1, Figure 1) or were in relation to postnatal exposure (41 papers) (Table 2, Figure 2). There were 22 papers that contributed both to Tables 1 and 2. Overall, 29 of the 89 papers were derived from the PubMed database search. While for most of the studies in Table 1 follow-up is restricted to cancer incidence or death while ≤ 20 years of age, for eight studies this is not the case, specifically those of Gunz and Atkinson (Gunz and Atkinson, 1964), Preston-Martin et al (Preston-Martin et al., 1982), Operskalski et al (Operskalski et al., 1987), Bunin et al (Bunin et al., 1989), Gardner et al (Gardner et al., 1990), Holly et al (Holly et al., 1992), Winn et al (Winn et al., 1992) and Roman et al (Roman et al., 1997); only for Holly et al (Holly et al., 1992) and Roman et al (Roman et al., 1997) does the upper age limit exceed 25 years (at 31 and 29 years, respectively). Likewise four of the studies listed in Table 2, those of Preston-Martin et al (Preston-Martin et al., 1980) (age at diagnosis 18–64 years), McLaughlin et al (McLaughlin et al., 1993) (age at diagnosis/death 0->30 years), Modan et al (Modan et al., 2000) (age at diagnosis 15–49 years) and Hong et al (Hong et al., 2019) (age at diagnosis <30 years) relate to cancer occurring both in childhood and beyond. For all other studies in Table 2 exposure and follow-up both occurred at age ≤ 20 years.
Figure 1. In utero exposure, odds ratio/relative risk (+95% CI) by midpoint year of study data ascertainment for (a) any cancer, (b) leukaemia, (c) brain/CNS tumour and (d) lymphoma.
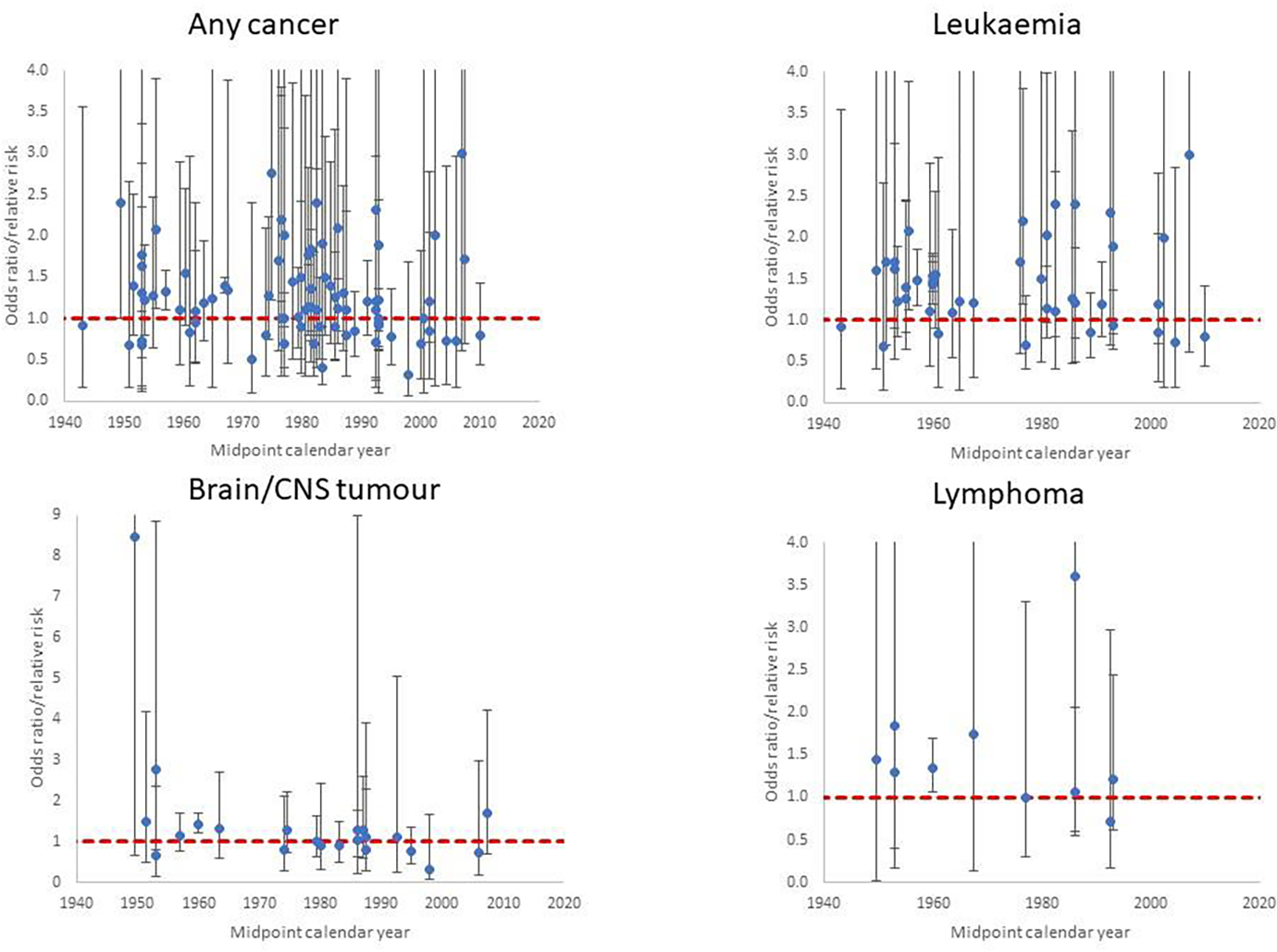
Each point corresponds to a single cancer endpoint (generally one per study), using all studies and endpoints in Table 1 (see Supplementary Table S1). Dashed red line is odds ratio/relative risk = 1
Figure 2. Postnatal exposure odds ratio/relative risk (+95% CI) for (a) leukaemia, (b) brain/CNS tumour and (c) lymphoma by midpoint year of study data ascertainment.
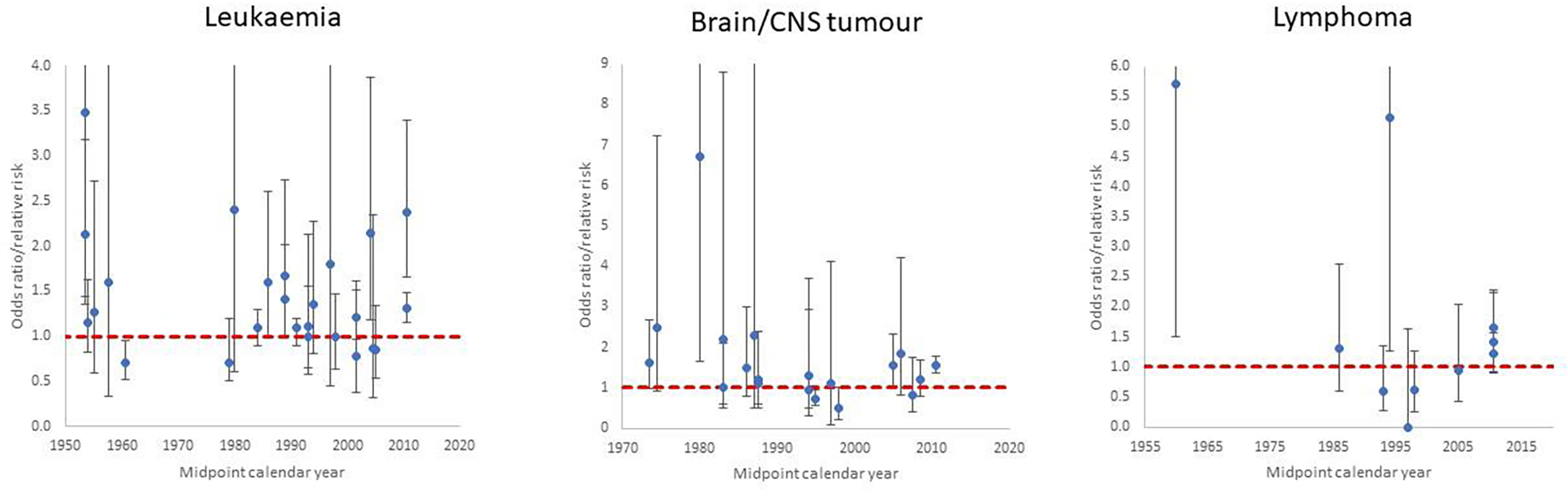
Each point corresponds to a single study and relevant endpoint in Table 2 (see Supplementary Table S2). Dashed red line is odds ratio/relative risk = 1
3.1. Risks of in utero exposure
Table 1 presents the data and risk estimates for malignant disease endpoints in a large number of studies of in utero exposure for medical diagnostic purposes; these are predominantly case-control studies, although there are two cohort studies of antenatal exposure (Diamond et al., 1973; Ray et al., 2010) (Table 1). The tendency for risks to be raised is apparent for the four cancer endpoints displayed in Figure 1 (any cancer, leukaemia, brain/CNS tumours, lymphoma), particularly for the earlier studies, and the large studies of Bithell and Stewart (Bithell and Stewart, 1975) and Monson and MacMahon (Monson and MacMahon, 1984) are notable in this respect; risks tend to reduce in the later studies.
3.2. Risks of radiation exposure in childhood
Table 2 presents the data and risk estimates for malignant disease endpoints in studies of postnatal exposure for medical diagnostic purposes. Again, the tendency for risks to be raised is apparent in Figure 2 showing risks from the studies for the three main cancer endpoints (leukaemia, brain/CNS tumours, lymphoma), and raised risks are more evident in earlier studies. Among the more striking of the reported risks are those for leukaemia in the study of patients exposed to diagnostic X-rays and fluoroscopy by Polhemus et al (Polhemus and Koch, 1959), for brain tumours and dental X-rays in the studies of Preston-Martin et al (Preston-Martin et al., 1980; Preston-Martin et al., 1982) for brain tumours and skull X-rays in the Canadian study of Howe et al (Howe et al., 1989), non-Hodgkin lymphoma (NHL) in the Israel cardiac catheterisation study of Modan et al (Modan et al., 2000), and in relation to various cancer endpoints and CT scans in the studies of Hong et al (Hong et al., 2019) and Li et al (Li et al., 2020).
It is notable that a large majority of the studies in Table 2 are from the period before 2010. The relative rarity of more recent studies without quantitative dose information is probably due to the greater availability of dose estimates with more modern radiography, particularly CT scans (Little et al., 2022b).
3.3. Meta-analysis of cancer risks associated with radiation exposure in early life
3.3.1. Exposure in utero
Significantly raised OR/RR estimates for in utero exposure are obtained from the meta-analysis for all four separate cancer endpoints and for any available cancer type (Table 3). There was little indication (p>0.3) of differences in mOR/mRR between different cancer endpoints, although there was perhaps a suggestion that the risk was somewhat lower (but still significantly greater than zero) for brain/CNS tumours (Table 3). There is a highly significant (p<0.0001) decreasing trend of OR/RR from studies of in utero exposure with calendar year (mid-point of the study period), by about 0.84% per year (Table 3, Figure 3). Little difference was made in central estimates or in width of CI by fitting using the 1-step method of DerSimonian and Laird (DerSimonian and Laird, 1986), restricted maximum likelihood (REML) or maximum likelihood random effects (results not shown). When analysis was done using, as far as possible, all cancers analysed together for each study rather than the four-endpoint analysis a slightly weaker temporal trend was found, of about 0.78% per year, although still highly significant (p=0.0002) (Table 3). The analyses do not suggest that notable heterogeneity is present, as indicated by the Q statistic (p>0.3), and values of the I2 statistic are generally small (mostly < 15% and all <25%) (Table 3).
Table 3.
Univariate meta-regression maximum likelihood fit and restricted maximum likelihood fits of random effects models to childhood cancer outcome data from studies of in utero exposure to medical diagnostic radiation (odds ratio (OR) and relative risk (RR) as in Table 1)
| Cancer endpoint (number of studies) | Meta odds ratio (mOR) / meta relative risk (mRR) (+95% CI)a | p-value improvement over null [model with constant risk]b | Residual heterogeneity p-valuec | I2 (%) (95% CI)d | Heterog eneity p-value |
|---|---|---|---|---|---|
|
| |||||
| Analysis by cancer endpoint (fitted via restricted maximum likelihood (REML)) | |||||
|
| |||||
| Leukaemia (38) | 1.35 (1.25,1.46) | <0.0001 | 0.6344 | 9.94 (0.00,34.86) | 0.3097 |
| Lymphoma (10) | 1.31 (1.15,1.49) | 0.0010 | 0.9674 | 0.00 (0.00,10.42) | |
| Brain/CNS (21) | 1.16 (1.02,1.32) | 0.0222 | 0.7094 | 10.56 (0.00,39.29) | |
| Other (19) | 1.34 (1.16,1.55) | 0.0004 | 0.3307 | 23.21 (0.00,62.30) | |
| All cancers [four cancer endpoints] (62) | 1.32 (1.25,1.40) | <0.0001 | 0.7629 | 10.19 (0.00,16.17) | |
| All cancers [any cancer] (66) | 1.21 (1.13,1.30) | <0.0001 | 0.4982 | 12.98 (0.00,28.16) | |
| Calendar year trend for any childhood cancer combined – four separate cancer endpoints analysis (fitted via REML) | |||||
| Trend in OR/RR, % per year | −0.84 (−1.16, −0.52) | <0.0001 | 0.9875 | 0.00 (NA) | - |
| Calendar year trend for any childhood canc er combined – any cancer analysis (fitted via REML) | |||||
| Trend in OR/RR, % per year | −0.78 (−1.17, −0.39) | 0.0002 | 0.8859 | 2.06 (0.00, 14.97) | - |
|
| |||||
| Analysis by cancer endpoint (fitted via maximum likelihood) | |||||
|
| |||||
| Leukaemia (38) | 1.36 (1.26,1.47) | <0.0001 | 0.6344 | 6.37 (0.00,34.86) | 0.3097 |
| Lymphoma (10) | 1.31 (1.15,1.49) | 0.0010 | 0.9674 | 0.00 (0.00,10.42) | |
| Brain/CNS (21) | 1.17 (1.04,1.33) | 0.0146 | 0.7094 | 7.96 (0.00,39.29) | |
| Other (19) | 1.38 (1.21,1.58) | <0.0001 | 0.3307 | 11.49 (0.00,62.30) | |
| All cancers [four cancer endpoints] (62) | 1.33 (1.26,1.40) | <0.0001 | 0.7629 | 8.73 (0.00,16.17) | |
| All cancers [any cancer] (66) | 1.21 (1.13,1.30) | <0.0001 | 0.7919 | 5.20 (0.00,13.95) | |
estimates and CI via restricted maximum likelihood.
significance evaluated via maximum likelihood fits.
significance of residual heterogeneity, assessed via Cochran’s Q statistic based on maximum likelihood fits.
contribution of inter-study heterogeneity to intra-study variance, via Higgins and Thompson (Higgins and Thompson, 2002) I2 statistic based on maximum likelihood fits.
Estimates and CI via maximum likelihood.
Figure 3. Meta-regression for studies of in utero exposure. Restricted maximum likelihood (REML) fits to odds ratio or relative risk by calendar year midpoint of study data ascertainment range (for <1950, 1950–1959, 1960–1969, 1970–1979, 1980–1989, 1990+).
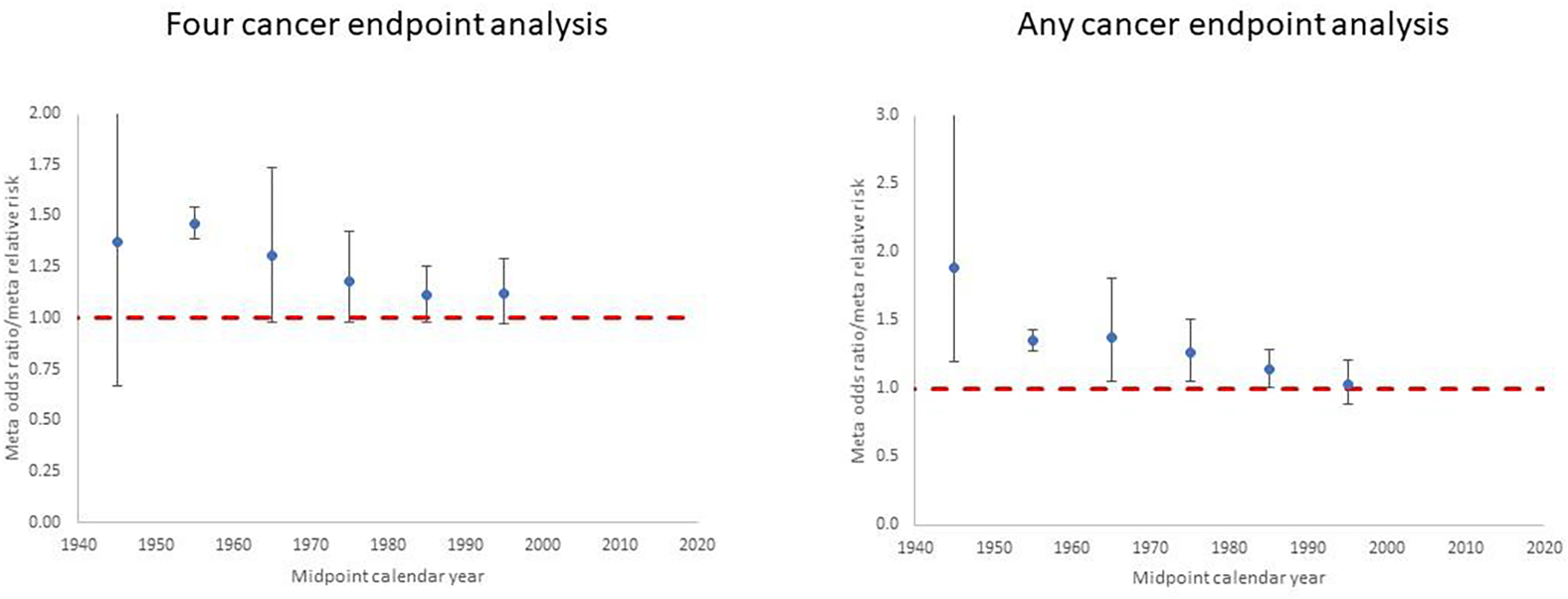
Plots are shown for (a) the four cancer endpoints analysis (leukaemia, lymphoma, brain/CNS cancer, other cancer) and (b) the any cancer endpoint analysis, for each in utero exposure study in Table 1 (see Supplementary Table S1). Dashed red line is odds ratio/relative risk = 1.
3.3.2. Postnatal exposure
The meta-analysis found statistically significant excess risks for leukaemia, brain/CNS tumours and the group of solid cancers other than brain/CNS tumours, but not for lymphoma; the excess risk was most pronounced for solid cancers other than brain/CNS tumours (Table 5). There were marginally significant variations in risk by the four cancer endpoints (Table 5, p=0.0663). As Table 2 shows, there are a variety of types of postnatal exposure included in studies, ranging from chest and dental X-rays to CT scans, and also in the cancer endpoints studied. The meta-analysis confirms that these types of exposure are associated with somewhat different risks (Table 5), although the precise level of statistical significance varies depending on whether analysis uses either four specific cancer endpoints (p=0.0242) or any cancer endpoint per study (p=0.0570). Risks tend to be higher for fluoroscopy (although based on a single study from 1959) and CT scans (five studies from 2010 or later), although the four-endpoint analysis of catheterisation (four studies) also indicates a high risk. There are no significant trends with calendar year (p>0.5), whichever type of analysis is performed (Table 5, Figure 4). Of note is that all analyses by cancer endpoint in the four-endpoint analysis yield highly significant heterogeneity, as indicated by the Q statistic (p<0.0005), and values of the I2 statistic are also uniformly high (>50%). For all types of exposure apart from fluoroscopy (for which there was only a single study) the heterogeneity was also highly significant (p<0.01) (Table 3). Analysis in which exposures due to CT scans, catheterisation or fluoroscopy were omitted yielded lower estimates of risks for all endpoints, so that only those for all cancers and leukaemia remained (marginally) statistically significant, with mOR/mRR = 1.21 (95% CI 1.04, 1.42) and mOR/mRR = 1.19 (95% CI 1.01, 1.39), respectively (results not shown), versus mOR/mRR = 1.37 (95% CI 1.23, 1.53) and mOR/mRR = 1.25 (95% CI 1.07, 1.46), respectively (Table 5), for the main analysis; the reduction was particularly pronounced in the risk for lymphoma, with a mOR/mRR = 1.10 (95% CI 0.26, 4.67) (results not shown) versus mOR/mRR = 1.30 (95% CI 0.66, 2.59) for the main analysis (Table 5).
Table 5.
Univariate meta-regression maximum likelihood fit and restricted maximum likelihood fits of random effects models to childhood cancer outcome data from studies of postnatal exposure to medical diagnostic radiation (odds ratio (OR) and relative risk (RR) as in Table 2)
| Cancer Endpoint / Type of Exposure (number of studies) | Meta odds ratio (mOR) / meta relative risk (mRR) (+95% CI)a | p-value improvement over null [model with constant risk]b | Residual heterog eneity p-valuec | I2 (%) (95% CI)d | Hetero geneity p-value |
|---|---|---|---|---|---|
|
| |||||
| Fits with four specific types of cancer considered separately | |||||
| Analysis by cancer endpoint | |||||
| Leukaemia (21) | 1.25 (1.07,1.46) | 0.0055 | <0.0001 | 71.53 (44.18,87.70) | 0.0663 |
| Lymphoma (8) | 1.30 (0.66,2.59) | 0.4065 | <0.0001 | 64.39 (<64.39,>99.91) | |
| Brain/CNS (18) | 1.26 (1.02,1.56) | 0.0339 | 0.0003 | 54.36 (14.88,85.36) | |
| Other(9) | 1.65 (1.37,1.97) | <0.0001 | 0.0004 | 56.51 (28.81,90.14) | |
| Any cancer (39) | 1.37 (1.23,1.53) | <0.0001 | <0.0001 | 68.45 (65.88,90.16) | |
|
| |||||
| Analysis by type of exposure | |||||
| X-ray (26) | 1.25 (1.08,1.45) | 0.0046 | 0.0002 | 53.04 (30.32,86.32) | 0.0242 |
| Fluoroscopy (1) | 3.48 (1.29,9.37) | 0.0136 | NA | NA | |
| Computed tomography (5) | 1.52 (1.34,1.72) | <0.0001 | <0.0001 | 69.23 (42.42,88.20) | |
| Catheterisation (4) | 2.14 (0.85,5.37) | 0.0962 | 0.0060 | 19.75 (<19.75,>99.26) | |
| Unknown/mixed (4) | 1.18 (0.77,1.80) | 0.4177 | 0.0003 | 71.19 (38.40,93.34) | |
| Calendar year trend for any cancer combined – unadjusted for type of exposure | |||||
| Trend in OR/RR, % per year | 0.15 (−0.51,0.81) | 0.6596 | <0.0001 | 67.57 (65.83,90.18) | - |
| Calendar year trend for any cancer combined – adjusted for type of exposure | |||||
| Trend in OR/RR, % per year | −0.14 (−0.86,0.59) | 0.7090 | <0.0001 | 62.89 (60.64,88.84) | - |
|
| |||||
| Fits using any type of cancer for each study | |||||
|
| |||||
| Analysis by type of exposure | |||||
| X-ray (27) | 1.19 (1.03,1.37) | 0.0167 | 0.0001 | 59.79 (33.07,85.36) | |
| Fluoroscopy (1) | 3.48 (1.29,9.37) | 0.0136 | NA | NA | |
| Computed tomography (5) | 1.32 (1.04,1.67) | 0.0272 | 0.0112 | 74.18 (5.78,96.75) | 0.0570 |
| Catheterisation (3) | 0.88 (0.61,1.27) | 0.2709 | 0.8030 | 0.00 (0.00,87.84) | |
| Unknown/mixed (4) | 0.92 (0.65,1.32) | 0.5315 | 0.1805 | 44.27 (0.00,94.96) | |
| Calendar year trend for any cancer combined – unadjusted for type of exposure | |||||
| Trend in OR/RR, % per year | 0.17 (−0.47,0.81) | 0.5921 | <0.0001 | 67.47 (47.23,86.14) | - |
| Calendar year trend for any cancer combined – adjusted for type of exposure | |||||
| Trend in OR/RR, % per year | 0.07 (−0.69,0.83) | 0.8563 | <0.0001 | 62.84 (36.70,83.87) | - |
estimates and CI via restricted maximum likelihood.
significance evaluated via maximum likelihood fits.
significance of residual heterogeneity, assessed via Cochran’s Q statistic based on maximum likelihood fits.
contribution of inter-study heterogeneity to intra-study variance, via Higgins and Thompson (Higgins and Thompson, 2002) I2 statistic based on maximum likelihood fits.
Figure 4. Meta-regression for studies of postnatal exposure. Restricted maximum likelihood (REML) fits to odds ratio or relative risk by calendar year midpoint of study data ascertainment range (for <1960, 1960–1969, 1970–1979, 1980–1989, 1990–1999, 2000+).
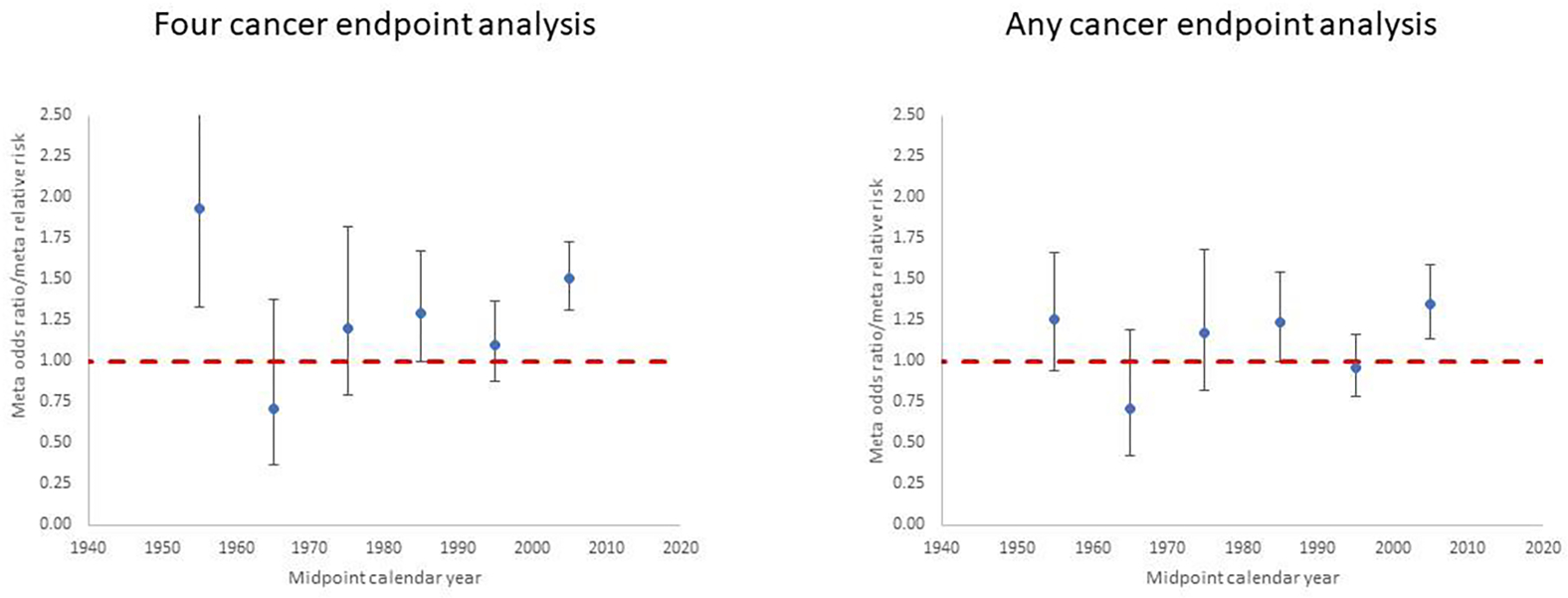
Plots are shown for (a) the four cancer endpoints analysis (leukaemia, lymphoma, brain/CNS cancer, other cancer) and (b) the any cancer endpoint analysis, for each postnatal exposure study in Table 2 (see Supplementary Table S2). Dashed red line is odds ratio/relative risk = 1.
3.3.3. Possible selection bias
The general symmetry of the funnel plots does not suggest any marked selection bias in relation to the in utero exposure studies (Figure 5), except, perhaps, for a few of the studies with largest standard error. However, the formal analysis of selection bias in Table 4 suggests that there are some indications of selection bias for these studies (p=0.0064), although only when analysed using the four cancer endpoints; when combining any cancer estimates per study there was little evidence of selection bias (p=0.1802). In any case, the analyses of Table 4 also demonstrate that adjusting for selection bias using the trim-and-fill method of Duval and Tweedie (Duval and Tweedie, 2000) does not in general lead to marked changes in the central estimate of mOR/mRR.
Figure 5.
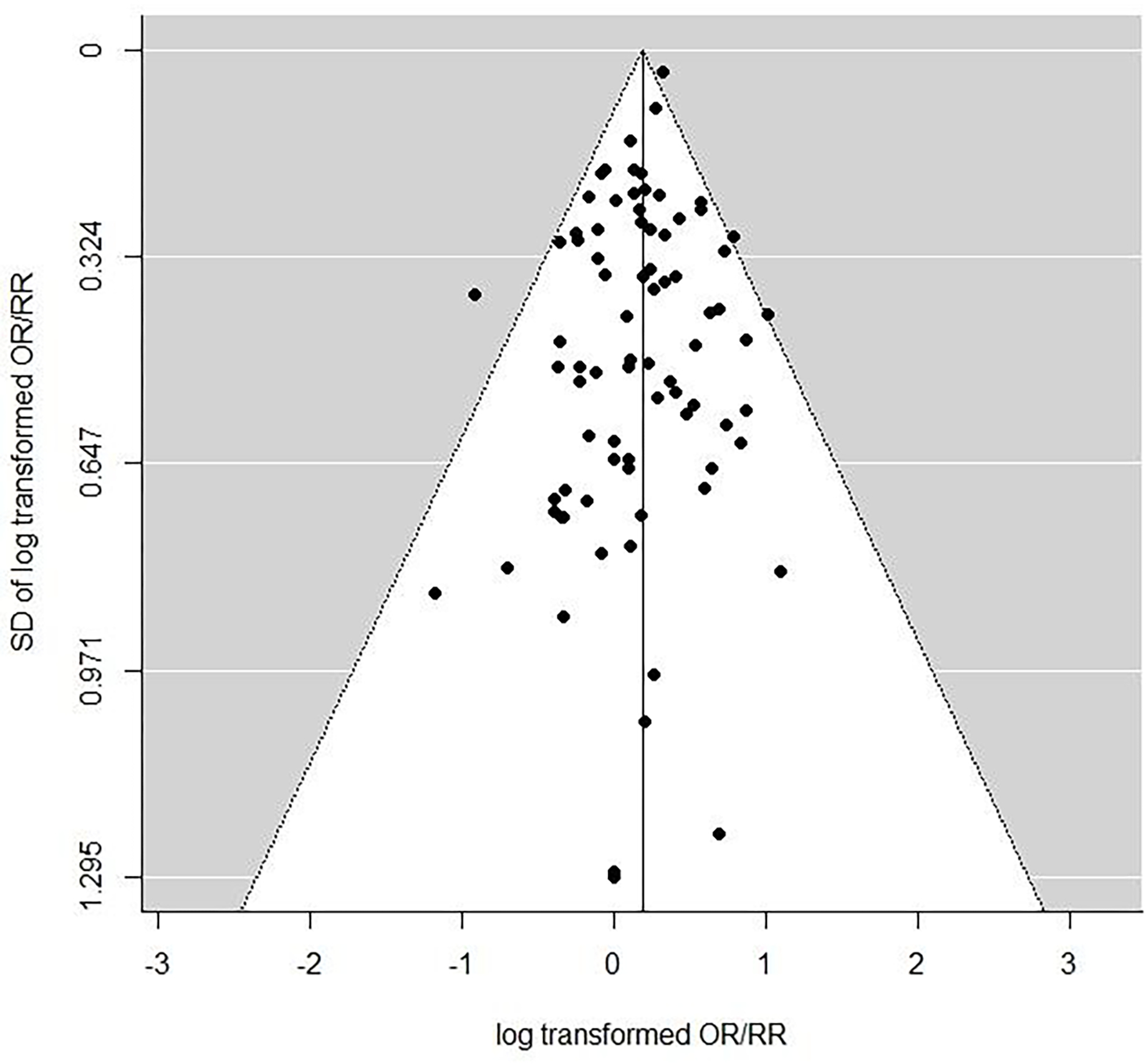
Funnel plot of in utero exposure study odds ratios/relative risks, using any cancer endpoints within all studies, as in Table 1.
Table 4.
Egger test (Egger et al., 1997) for selection bias for studies of in utero exposure risk, and magnitude of correction in raw meta odds ratio (mOR) or meta relative risk (mRR) suggested by trim-and-fill method of Duval and Tweedie (Duval and Tweedie, 2000)
| Egger test for selection bias, p-value | Meta odds ratio (mOR) / meta relative risk (mRR) (95% CI) (REML estimate) | Bias corrected meta odds ratio (mOR) / meta relative risk (mRR) (95% CI) (Duval & Tweedie trim-and-fill corrected REML estimate) |
|---|---|---|
|
| ||
| Four cancer endpoint analysis | ||
| 0.0064 | 1.32 (1.25,1.40) | 1.38 (1.30,1.47) |
|
| ||
| Any cancer analysis | ||
| 0.1802 | 1.21 (1.13,1.30) | 1.26 (1.17,1.35) |
There are at best weak suggestions of asymmetry in the funnel plot in relation to postnatal exposure (Figure 6), and this is confirmed by the Egger test, whether performed on the pooled four cancer endpoint data (p=0.3991) or using any cancer endpoint (p=0.5095); adjusting for selection bias using the trim-and-fill method of Duval and Tweedie (Duval and Tweedie, 2000) does not lead to marked changes in the central estimates of mOR/mRR.
Figure 6.
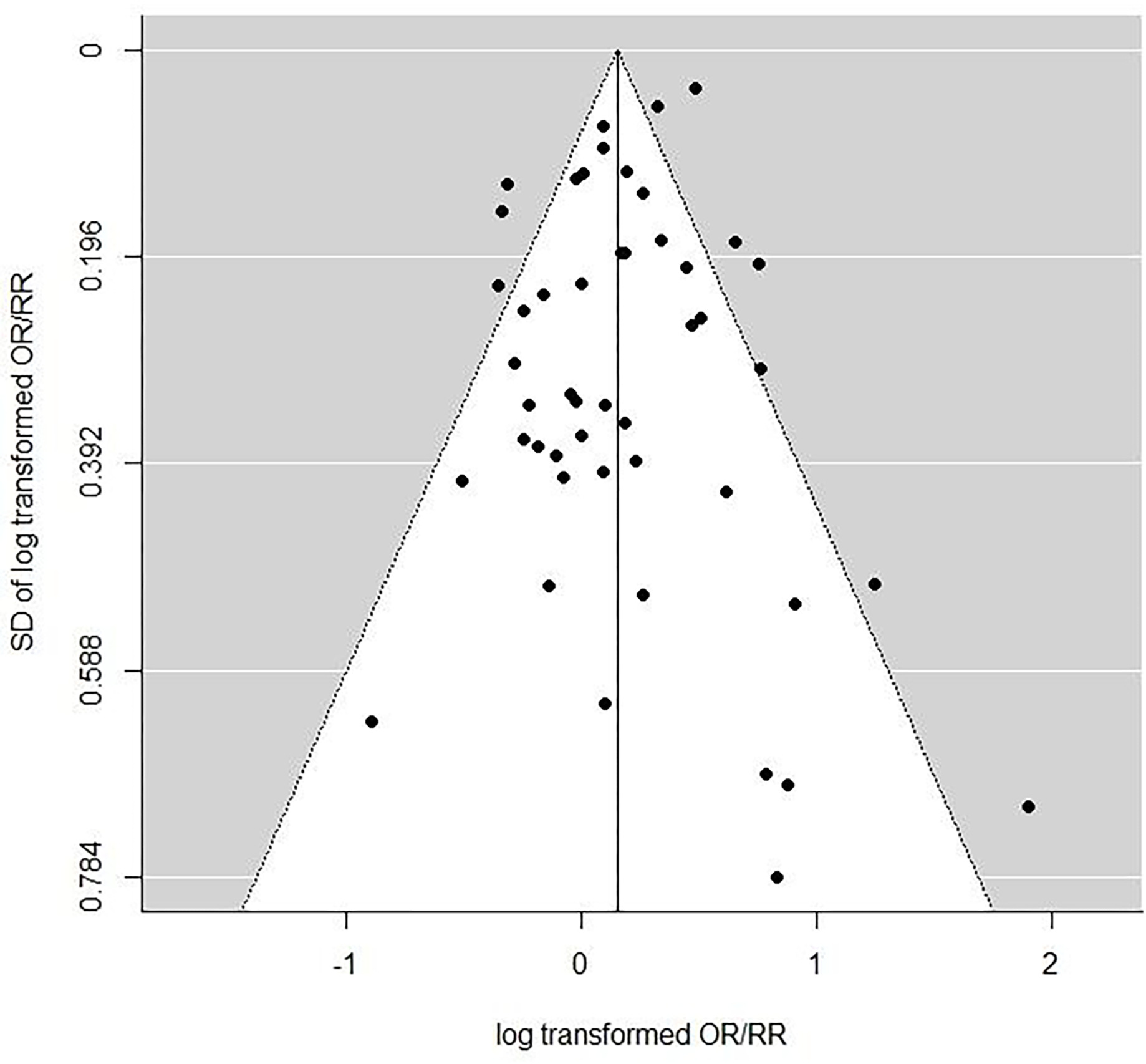
Funnel plot of postnatal exposure study odds ratios/relative risks, using any cancer endpoints within all studies, as in Table 2.
4. Discussion
Our review has highlighted that both in relation to in utero exposure and postnatal exposure to medical diagnostic radiation there are multiple studies that yield statistically significant excess cancer risks. For in utero exposure, modest but significant excess risks tend to be concentrated in the earlier studies (Table 1) while for postnatal exposure, significant excess risks are also apparent in later studies, in particular in recent CT scan studies (Table 2).
The meta-analysis of the in utero exposure data (Table 3) does not suggest that there are significant differences in mOR/mRR between the four cancer endpoints considered (p>0.3), although there is a highly significant decreasing trend (p<0.0005) with calendar year. This may well be due to a progressive decrease in fetal dose per X-ray examination (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 1972), reflecting advances in radiographic technology, and possibly also to the influence on medical practice of the early studies of Stewart et al (Stewart et al., 1956; Stewart et al., 1958). That the risks of childhood cancer associated with an antenatal X-ray examination are elevated to approximately the same extent for all the common types of cancer in childhood (with the possible exception of bone tumours) was reported in the mid-1970s by Bithell and Stewart (Bithell and Stewart, 1975) using data from the OSCC. This finding has provoked comment, such as that by Boice and Miller (Boice and Miller, 1999), who noted that this was “perplexing” and not the pattern of radiation-related cancer risks seen after postnatal exposure, and they suggested that this finding pointed away from a causal interpretation of the association. Nonetheless, recently Wakeford and Bithell (2021) reported that the results of all studies of antenatal radiography and childhood cancer other than the OSCC when appropriately combined in a meta-analysis produced a broadly similar pattern of raised relative risks as originally found by Bithell and Stewart (Bithell and Stewart, 1975), so the similarly increased risks for the typical cancers of childhood is not confined to the OSCC.
In contrast to the antenatal studies, a troubling feature of the meta-analysis of postnatal exposure is that for the four cancer outcomes analysed, highly significant heterogeneity (p<0.0005) was found, and the high values (generally >50%) of the I2 statistic imply that a material proportion of the variance is due to inter-study heterogeneity (Table 5). This makes interpretation of summary measures of risk especially problematic. Again, in contrast to the in utero data, there are no significant trends by calendar year (Table 5, Figure 4). Previous narrative reviews (e.g., Linet et al (Linet et al., 2012)) have concluded that studies of postnatal radiography and childhood cancer have produced ambivalent results, but these reviews have not included more recent studies including those of CT scans. The substantial heterogeneity of the risk estimates from the postnatal studies found here poses considerable difficulties to a reliable interpretation of the meta-analysis results – whether these results are indicative of underlying raised risks for the cancer endpoints is questionable under these circumstances.
This paper focuses exclusively on studies of persons receiving medical diagnostic exposures for which there are no individual estimates of radiation dose, and therefore of dose-related risk. In this respect it contrasts with the paper of Little et al (Little et al., 2022b) which included only those studies which had radiation dose estimates. Clearly, the latter analysis is more informative about the magnitude of risks in relation to the level of radiation exposure, but the large number of studies where quantified dose information is not available are still informative about the presence of radiation-related risk, if not its magnitude. We judge that the studies of in utero exposure are likely to be particularly informative in this respect because of the degree of homogeneity of the findings. However, owing to significant inter-study heterogeneity, the studies of postnatal exposure are more difficult to interpret. It is possible that some of the observed heterogeneity may result from disparities in the levels of radiation exposure in these postnatal studies. This is not implausible, as radiation doses from CT scans are likely to be considerably larger, by at least an order of magnitude, than those from many diagnostic X-ray procedures, in particular from dental X-rays (National Council on Radiation Protection and Measurements (NCRP), 2019). It is possible that other factors, such as the variety of populations under study and the types of exposure could contribute, and also methodological factors such as whether a cohort or case-control study; however, a case-control study nested within a cohort would be expected to yield the same relative risk as that in the underlying cohort (Breslow and Day, 1980), so this latter factor probably does not play a large role. There are indications that type of exposure accounts for borderline significant (p=0.02–0.06) variation in risk (Table 5), and it is therefore conceivable that uncontrolled variation in this factor could account for some of the inter-study heterogeneity.
The association between cancer in childhood and a prior radiographic examination of the abdomen of the pregnant mother identified by case-control studies such as those of the OSCC (Bithell and Stewart, 1975; Stewart et al., 1956) and Monson and MacMahon (Monson and MacMahon, 1984), and many others (Wakeford, 2008; Wakeford and Bithell, 2021) (see Table 1, Figure 1), provides persuasive epidemiological evidence that exposure in utero to external sources of penetrating radiation increases the risk of cancer in childhood. By definition, the exact level of dose incurred is not known for the studies considered here, but analogy with other studies (Little et al., 2022b) suggests that they would have been from several mGy to a few tens of mGy, values that are consistent with estimates of fetal doses made by UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 1972). This level of dose is much lower (by around an order of magnitude) than the lowest doses producing significantly increased risks of cancer in all other epidemiological studies except studies of natural background radiation (Little et al., 2022b; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2008), and indicates that cancer may be caused by low levels of exposure to radiation; it may be, however, that exposure in utero produces a higher risk per unit dose than postnatal exposure and that different types of cancer are affected (Doll and Wakeford, 1997).
Case-control studies have often been used in a medical setting, and can be subject to a number of biases, in particular selection, participation and recall biases. This can make them a poor choice for studies of medical exposure, but with care, large case-control studies have produced results without appreciable bias because they are entirely record-based (MacMahon, 1962). The interpretation of the associations found in studies of in utero exposure has long been controversial, see for example, the critical review of Boice and Miller (Boice and Miller, 1999). Some have concluded that the association is likely to have a causal explanation (Doll and Wakeford, 1997), although certain objections to this interpretation continue to prevent universal acceptance (International Commission on Radiological Protection (ICRP), 2003; National Council on Radiation Protection and Measurements (NCRP), 2013). Nonetheless, the concerns over a cause-and-effect interpretation have weakened in recent years (Armstrong et al., 2012; Wakeford and Bithell, 2021; Wakeford and Little, 2003) making this explanation more likely.
A potential problem in the analysis of the studies considered here is that there may have been an element of publication (sometimes called reporting) bias. By this we mean that studies (in particular small and underpowered studies) finding an apparent effect of radiation exposure were more likely to be published than similar studies that found no association, a bias that might affect smaller studies preferentially; large studies are more likely to be published whether positive or not (Hauptmann et al., 2020). There are indications of selection bias in the in utero exposure data, although only for the four cancer endpoint analysis (p=0.006); there is no significant indication of such bias when all cancers are analysed together (p>0.1) (Table 4), and nor is any bias suggested by the funnel plot (Figure 5). In any case, adjustment for such bias does not generally change the mOR/mRR estimate (Table 4). Examination of the funnel plot (Figure 6) for the studies of postnatal exposure, as well as more formal tests of selection bias (Table 6), do not suggest any such bias. It is a priori more plausible that selection bias may preferentially affect older studies, particularly in the period before 1970. One disconcerting aspect of our analysis is the small proportion, ~33% (29/89), of papers that were ascertained in our PubMed search. Part of the reason for this is undoubtedly that the older papers tend not to be indexed in PubMed; the percentage of papers covered by the PubMed search was markedly lower among papers published before 1970, 10% (1/10), increasing to 28% (7/25) for papers published in 1970–1989 and to ~39% (21/54) among papers published in 1990 or later.
Table 6.
Egger test (Egger et al., 1997) for selection bias for studies of postnatal exposure risk, and magnitude of correction in raw meta odds ratio (mOR) or meta relative risk (mRR) suggested by trim-and-fill method of Duval and Tweedie (Duval and Tweedie, 2000)
| Egger test for selection bias, p-value | Meta odds ratio (mOR) / meta relative risk (mRR) (95% CI) (REML estimate) | Bias corrected meta odds ratio (mOR) / meta relative risk (mRR) (95% CI) (Duval & Tweedie trim-and-fill corrected REML estimate) |
|---|---|---|
|
| ||
| Four cancer endpoint analysis | ||
| 0.3991 | 1.37 (1.23, 1.53) | 1.34 (1.22, 1.48) |
|
| ||
| Any cancer combined analysis | ||
| 0.5095 | 1.17 (1.05, 1.30) | 1.16 (1.04, 1.28) |
The presence of bias resulting from confounding by indication, in other words the possibility that conditions predisposing to cancer also lead to an increase in prevalence of radiation imaging, must always be considered in studies of medical diagnostic exposure. This is particularly so in relation to the finding of large excess risk associated with catheterisation in the four-cancer endpoint analysis (Table 5). It is known that many chromosomal congenital abnormalities are associated with heart defects and increased risk of cancer (Johnson et al., 1997; Ko, 2015; Lupo et al., 2019), and catheterisation is commonly used as part of the diagnosis and treatment of cardiac anomalies (Kumar et al., 2014b). Further, in their cohort study of cardiac catheterisations, the striking findings of Harbron et al (Harbron et al., 2018) for a markedly raised risk of lymphoma (in particular, NHL) in relation to organ transplantation and associated radiation exposure demonstrate the importance of confounding as a potential explanation for radiation exposures in a medical context because the excess of lymphoma disappeared completely when the transplant patients were excluded from the study, and the raised risk was very likely to be attributable to the immunosuppressive drugs used after transplantation.
There have been a variety of studies evaluating risks of cancer after a childhood CT scan, some indicating excess risk (Berrington de Gonzalez et al., 2016; Journy et al., 2015; Journy et al., 2016; Kojimahara et al., 2020; Krille et al., 2015; Mathews et al., 2013; Meulepas et al., 2019; Pearce et al., 2012). However, the interpretation of these findings is not straightforward (Boice, 2015; Walsh et al., 2014). Reverse causation, that is the possibility that the CT scan might have been taken because of early symptoms from pre-existing (latent) disease and was therefore not a cause of the disease, is a source of concern in these studies, and as noted above, confounding by indication is also a source of potential bias (Boice, 2015; Walsh et al., 2014). Both issues are usually dealt with in the analysis by employing lag and exclusion periods, and a simulation study suggests that this should be enough to eliminate bias from this cause (Little et al., 2022a). However, for solid cancers it is common to use larger values of lag and exclusion than the period of two years used in the studies of Tettamanti et al (Tettamanti et al., 2017), Hong et al (Hong et al., 2019) and Li et al (Li et al., 2020), or the period of 6 months employed in the studies of Bailey et al (Bailey et al., 2010) and Milne et al (Milne et al., 2014), that are considered here.
Tettamanti et al (Tettamanti et al., 2017) and Hong et al (Hong et al., 2019) employed a variety of different lags between 1 year and 5 years in analysis of all cancers, and both studies observed a modest diminution in RR when longer lag periods were used. Milne et al (Milne et al., 2014) also evaluated lag periods between 6 months and 5 years in relation to all radiological procedures, but little difference in brain tumour risk was observed. Reverse causation and confounding by indication are general problems in studies of diagnostic radiation exposure; only a few of the studies of other types of postnatal exposure assembled here deal with this by use of lag periods (Ager et al., 1965; Baaken et al., 2019; Bartley et al., 2010; Graham et al., 1966; Howe et al., 1989; Meinert et al., 1999; Preston-Martin et al., 1982; Rajaraman et al., 2011; Schuz et al., 2001; Shu et al., 2002), even to the limited extent that is attempted by Tettamanti et al (Tettamanti et al., 2017), Hong et al (Hong et al., 2019), Li et al (Li et al., 2020), Bailey et al (Bailey et al., 2010) and Milne et al (Milne et al., 2014) (see Table 2). Hence, one cannot discount the possibility that the relatively modest increases we have seen for postnatal exposures may largely result from such biases, and in this respect it is of interest that in the CT scan study of Hong et al (Hong et al., 2019), of the many different types of cancer investigated, the RR (with a lag of 2 years) was <1.0 only for lymphoid leukaemia while the RR estimates were >1.0 for all other types of cancer, some significantly so (e.g., digestive and respiratory cancers and NHL) and others not.
The meta-analysis for antenatal radiography reported here differs somewhat from that conducted by Wakeford and Bithell (Wakeford and Bithell, 2021), the principal objective of which was to compare the results of the OSCC studies for different childhood cancers with those produced by all other case-control/case-cohort studies appropriately combined in a meta-analysis. However, the broad compatibility of these findings of the two sets of studies (Wakeford and Bithell, 2021) invites the pooling of the results of all studies, including the OSCC, that has been conducted here. We have confined attention to four main cancer endpoint groups (leukaemia, lymphoma, brain/CNS tumours, other solid cancers), which inter alia were also employed by Wakeford and Bithell (Wakeford and Bithell, 2021), but we also considered the composite of any type of cancer, which was not examined by Wakeford and Bithell (Wakeford and Bithell, 2021). Wakeford and Bithell (Wakeford and Bithell, 2021) also conducted a separate analysis of the (admittedly few) cohort studies, whereas in the present paper both types of study were analysed together.
There are other methodological differences, specifically the use by Wakeford and Bithell (Wakeford and Bithell, 2021) of unadjusted OR estimates derived from the exposed and unexposed totals of cases and controls in each study, as opposed to our use, wherever possible, of OR (wherever possible the maximally adjusted ones) as given in the various publications. In those cases where an OR or CI were not given in a study we employed a hypergeometric model to derive an estimate of the OR, fitted by maximum likelihood, with Fisher exact CI (see footnotes to Table 1), rather than the crude OR and asymptotic CI (Breslow and Day, 1980) employed by Wakeford and Bithell (Wakeford and Bithell, 2021) for all their individual estimates. It is to be expected that the crude OR and asymptotic CI will be nearly the same as the hypergeometric maximum likelihood estimates (and exact CI) when numbers of cases and controls are large in all four parts of the associated 2 × 2 table ([case, controls] × [exposed, unexposed]), but this will not be the case when numbers are small (< 10) in any component cell, as is the case for a few studies in our analysis (Table 1); however, these small studies would not be expected to be of much consequence in the meta-analysis. Table 7 compares results of the meta-analysis of Wakeford and Bithell (Wakeford and Bithell, 2021) with those of the present paper taken from Table 3. The Mantel-Haenszel random-effects meta-analysis of Wakeford and Bithell (Wakeford and Bithell, 2021) yields a mOR for all cancers for the OSCC of 1.39 (95% CI 1.30, 1.49), and for all other antenatal case-control and cohort studies of 1.30 (95% CI 1.18, 1.43), both of which are comparable with both the maximum likelihood and REML mOR from our own analysis (Table 3, Table 7) of 1.33 (95% CI 1.26, 1.40) and 1.32 (95% CI 1.25, 1.40), respectively. Wakeford and Bithell (Wakeford and Bithell, 2021) do not incorporate the results of antenatal cohort studies in their meta-analysis, and the cohort studies were considered separately from the case-control studies, but as these are mostly small (Table 1) they would be expected to have little weight in the analysis.
Table 7.
Comparison of meta odds ratios (mOR)/meta relative risks (mRR) obtained by Wakeford and Bithell (Wakeford and Bithell, 2021) from the data of the Oxford Survey of Childhood Cancers (OSCC) and a meta-analysis of results from all other case-control/case-cohort studies (using a Mantel-Haenszel random-effects model) with those obtained from the meta-analysis of antenatal exposure in the present study (taken from Table 3).
| Endpoint | Wakeford and Bithell (Wakeford and Bithell, 2021) OSCC mOR (+95% CI) | Wakeford and Bithell (Wakeford and Bithell, 2021) non-OSCC case-control and case-cohort studies mOR (+95% CI) | Present analysis (maximum likelihood) mOR/mRR (+95% CI) | Present analysis (restricted maximum likelihood) mOR/mRR (+95% CI) |
|---|---|---|---|---|
|
| ||||
| Leukaemia | 1.51 (1.35, 1.69) | 1.28 (1.16, 1.41) | 1.36 (1.26,1.47) | 1.35 (1.25,1.46) |
| Lymphoma | 1.34 (1.06, 1.69) | 1.75 (1.08, 2.84) | 1.31 (1.15,1.49) | 1.31 (1.15,1.49) |
| Brain/CNS tumours | 1.42 (1.19, 1.69) | 1.13 (0.97, 1.31) | 1.17 (1.04,1.33) | 1.16 (1.02,1.32) |
| All solid cancer except brain/CNS tumours | 1.51 (1.32, 1.72) | 1.28 (0.89, 1.85) | 1.38 (1.21,1.58) | 1.34 (1.16,1.55) |
| All cancers | 1.39 (1.30, 1.49) | 1.30 (1.18, 1.43) | 1.33 (1.26,1.40)a | 1.32 (1.25,1.40)a |
four separate cancers
Inevitably, meta-analysis using the results reported in published papers (as opposed to pooled analysis using individual data from each study) presents problems in that, for example, different approaches to adjustments have been employed to obtain ORs or the actual numbers of cases and controls used in deriving ORs are not presented. This leads to difficulties in interpreting results, particularly when significant heterogeneity between studies is present (see, for example, (Blettner et al., 2014; Blettner et al., 1999)). However, the consistency of the findings for studies of antenatal radiography and childhood cancer found using the approach of Bithell and Wakeford (Wakeford and Bithell, 2021) and that of the present study encourages confidence in the reliability of the results of the meta-analysis of in utero exposure studies reported here.
As well as the medical diagnostic exposure studies without assessed doses, there is information in a large number of studies of various exposed groups in which individual dose estimates are available, which are reviewed elsewhere (Little et al., 2022b) with the conclusion that they offer support for low doses (<0.1 Gy) received in early life increasing the subsequent risk of cancer.
5. Conclusions
We have considered the relationship between low-level exposure to radiation in utero and in childhood and the consequent risk of cancer, principally at a young age, in medical diagnostic studies which did not include estimates of radiation dose. A large body of data relates to children exposed in utero, which suggests a radiation-related cancer risk that has attenuated over time, most likely due to reductions in the doses received from antenatal radiography. Taken together with findings of studies based upon quantitative dose data in a parallel review of radiation risk (Little et al., 2022b) this strengthens the evidence for a carcinogenic effect of low doses of radiation with respect to exposure in utero. However, the pronounced heterogeneity of findings from studies of medical diagnostic exposure after birth, and the real possibilities of bias due to reverse causation or confounding by indication, substantially reduces the strength of a causal interpretation of the association we have found between postnatal radiation exposure and the subsequent risk of cancer.
Supplementary Material
Highlights for “Cancer risks among studies of medical diagnostic radiation exposure in early life without quantitative estimates of dose”.
There is mounting evidence of cancer risk from low dose radiation in childhood.
A review was conducted of cancer after exposure in utero or childhood among studies without individual quantitative radiation dose estimates.
There were excess cancer risks associated with radiation exposure in utero.
There were excess cancer risks associated with radiation exposure in childhood, but there was also substantial heterogeneity in effect.
Overall, the totality of this large body of data relating to medical diagnostic radiation exposure in utero provides support for the existence of a consequent excess risk of childhood cancer; the pronounced heterogeneity in studies of postnatal diagnostic exposure, the implied uncertainty as to the meaning of summary measures, and the distinct possibilities of bias, substantially reduce the likelihood of the risk of cancer being causally related to postnatal radiation exposure for these studies.
6. Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors are grateful for the detailed and helpful comments of the two referees, and for those of Dr Jay Lubin on an early version of the manuscript.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Richard Wakeford receives a consultancy fee as a member of the Technical Working Party of the Compensation Scheme for Radiation-linked Diseases (http://www.csrld.org.uk). No other authors report conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abalo KD, Rage E, Leuraud K, Richardson DB, Le Pointe HD, Laurier D, et al. Early life ionizing radiation exposure and cancer risks: systematic review and meta-analysis. Pediatr Radiol 2021; 51: 45–56. [DOI] [PubMed] [Google Scholar]
- Ager EA, Schuman LM, Wallace HM, Rosenfield AB, Gullen WH. An epidemiological study of childhood leukemia. J. Chronic Dis 1965; 18: 113–32. [DOI] [PubMed] [Google Scholar]
- Armstrong B, Brenner DJ, Baverstock K, Cardis E, Green A, Guilmette RA, et al. Radiation. Volume 100D. A review of human carcinogens. Lyon, France: International Agency for Research on Cancer, 2012. [Google Scholar]
- Baaken D, Hammer GP, Seidenbusch MC, Schneider K, Spix C, Blettner M, et al. Second follow-up of a German cohort on childhood cancer incidence after exposure to postnatal diagnostic x-ray. J Radiol Prot 2019; 39: 1074–1091. [DOI] [PubMed] [Google Scholar]
- Bailey HD, Armstrong BK, De Klerk NH, Fritschi L, Attia J, Lockwood L, et al. Exposure to diagnostic radiological procedures and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiology Biomarkers and Prevention 2010; 19: 2897–2909. [DOI] [PubMed] [Google Scholar]
- Bartley K, Metayer C, Selvin S, Ducore J, Buffler P. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol 2010; 39: 1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer 2016; 114: 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell JF. Statistical issues in assessing the evidence associating obstetric irradiation and childhood malignancy. In: Lengfelder E, Wendhausen H, editors. Neue Bewertung des Strahlenrisikos: Niedrigdosis-Strahlung und Gesundheit. MMV Medizin Verlag, Munich, 1993, pp. 53–60. [Google Scholar]
- Bithell JF, Draper GJ, Sorahan T, Stiller CA. Childhood cancer research in Oxford I: the Oxford Survey of Childhood Cancers. Br J Cancer 2018; 119: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell JF, Stewart AM. Pre-natal irradiation and childhood malignancy: a review of British data from the Oxford Survey. Br.J.Cancer 1975; 31: 271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell JF, Stiller CA. A new calculation of the carcinogenic risk of obstetric X-raying. Statistics in Medicine 1988; 7: 857–864. [DOI] [PubMed] [Google Scholar]
- Blettner M, Krahn U, Schlattmann P. Meta-analysis in epidemiology. In: Ahrens WPI, editor. Handbook of Epidemiology. Springer, New York, NY, 2014, pp. 1377–1411. [Google Scholar]
- Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int.J.Epidemiol 1999; 28: 1–9. [DOI] [PubMed] [Google Scholar]
- Boice JD Jr. Radiation epidemiology and recent paediatric computed tomography studies. Annals ICRP 2015; 44: 236–248. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr., Miller RW. Childhood and adult cancer after intrauterine exposure to ionizing radiation. Teratology 1999; 59: 227–233. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci. Publ 1980: 1–350. [PubMed] [Google Scholar]
- Bunin GR, Buckley JD, Boesel CP, Rorke LB, Meadows AT. Risk factors for astrocytic glioma and primitive neuroectodermal tumor of the brain in young children: a report from the Children’s Cancer Group. Cancer Epidemiol Biomarkers Prev 1994; 3: 197–204. [PubMed] [Google Scholar]
- Bunin GR, Kramer S, Marrero O, Meadows AT. Gestational risk factors for Wilms’ tumor: results of a case-control study. Cancer Res 1987; 47: 2972–7. [PubMed] [Google Scholar]
- Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res 1989; 49: 5730–5. [PubMed] [Google Scholar]
- Castro-Jimenez MA, Orozco-Vargas LC. Parental exposure to carcinogens and risk for childhood acute lymphoblastic leukemia, Colombia, 2000–2005. Prev Chronic Dis 2011; 8: A106. [PMC free article] [PubMed] [Google Scholar]
- Claus EB, Calvocoressi L, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M. Dental x-rays and risk of meningioma. Cancer 2012; 118: 4530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation NRC. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII - Phase 2. Washington, DC, USA: National Academy Press, 2006. [PubMed] [Google Scholar]
- Court Brown WM, Doll R, Bradford Hill A. Incidence of leukaemia after exposure to diagnostic radiation in utero. Br Med J 1960; 2: 1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin.Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- Diamond EL, Schmerler H, Lilienfeld AM. The relationship of intra-uterine radiation to subsequent mortality and development of leukemia in children. A prospective study. Am J Epidemiol 1973; 97: 283–313. [DOI] [PubMed] [Google Scholar]
- Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br.J.Radiol 1997; 70: 130–139. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association 2000; 95: 89–98. [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo-Gutierrez A, Garduno-Espinosa J, Yamamoto-Kimura L, Hernandez-Hernandez DM, Mejia-Arangure M, Gomez-Delgado A, et al. [Risk factors associated with the development of leukemia in children]. Bol Med Hosp Infant Mex 1993; 50: 248–57. [PubMed] [Google Scholar]
- Fear NT, Roman E, Ansell P, Bull D. Malignant neoplasms of the brain during childhood: the role of prenatal and neonatal factors (United Kingdom). Cancer Causes Control 2001; 12: 443–449. [DOI] [PubMed] [Google Scholar]
- Ford DD, Paterson JCS, Treuting WL. Fetal exposure to diagnostic X rays, and leukemia and other malignant diseases in childhood. J. Natl Cancer Inst 1959; 22: 1093–1104. [PubMed] [Google Scholar]
- Gardner MJ, Snee MP, Hall AJ, Powell CA, Downes S, Terrell JD. Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ 1990; 300: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood F Fiducial limits for the Poisson distribution. Biometrika 1936; 28: 437–442. [Google Scholar]
- Gilbert ES, Little MP, Preston DL, Stram DO. Issues in Interpreting Epidemiologic Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. J Natl Cancer Inst Monogr 2020; 2020: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman EA, Stewart AM, Knox EG, Kneale GW. Trends in obstetric radiography, 1939–81. Journal of Radiological Protection 1989; 9: 93–101. [Google Scholar]
- Goel R, Olshan AF, Ross JA, Breslow NE, Pollock BH. Maternal exposure to medical radiation and Wilms tumor in the offspring: a report from the Children’s Oncology Group. Cancer Causes Control 2009; 20: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ 1992; 305: 341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Paterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. Br.J.Cancer 1990; 62: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Levin ML, Lilienfeld AM, Schuman LM, Gibson R, Dowd JE, et al. Preconception, intrauterine, and postnatal irradiation as related to leukemia. Natl Cancer Inst Monogr 1966; 19: 347–71. [PubMed] [Google Scholar]
- Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat Res 2017; 187: 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RS. The population distribution and possible determinants of neuroblastoma in children. Department of Epidemiology. PhD. The University of North Carolina at Chapel Hill, University of North Carolina at Chapel Hill, 1983, pp. i-xvii + 1–278. [Google Scholar]
- Grufferman S, Ruymann F, Ognjanovic S, Erhardt EB, Maurer HM. Prenatal X-ray exposure and rhabdomyosarcoma in children: A report from the children’s oncology group. Cancer Epidemiology Biomarkers and Prevention 2009; 18: 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grufferman S, Wang HH, Delong ER, Kimm SYS, Delzell ES, Falletta JM. Environmental factors in the etiology of rhabdomysarcoma in childhood. Jnci-Journal of the National Cancer Institute 1982; 68: 107–113. [PubMed] [Google Scholar]
- Gunz FW, Atkinson HR. Medical radiations and leukaemia: a retrospective survey. Br Med J 1964; 1: 389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MA, Kim JH. Diagnostic X-Ray Exposure and Thyroid Cancer Risk: Systematic Review and Meta-Analysis. Thyroid 2018; 28: 220–228. [DOI] [PubMed] [Google Scholar]
- Harbron RW, Chapple CL, O’Sullivan JJ, Lee C, McHugh K, Higueras M, et al. Cancer incidence among children and young adults who have undergone x-ray guided cardiac catheterization procedures. Eur J Epidemiol 2018; 33: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AL, Birch JM, McKinney PA, Blair V, Teare MD, Carrette J, et al. The Inter-Regional Epidemiological Study of Childhood Cancer (IRESCC): past medical history in children with cancer. J.Epidemiol.Community Health 1988; 42: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EB, Boice JD Jr., Honeyman M, Flannery JT. Prenatal x-ray exposure and childhood cancer in twins. N.Engl.J.Med 1985; 312: 541–545. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh J, Mohammadi R, Rajaeefard AR, Bordbar MR, Karimi M. Maternal and prenatal risk factors for childhood leukemia in southern of iran. Iran Red Crescent Med J 2011; 13: 398–403. [PMC free article] [PubMed] [Google Scholar]
- Hauptmann M, Daniels RD, Cardis E, Cullings HM, Kendall G, Laurier D, et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J Natl Cancer Inst Monogr 2020; 2020: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T [Retrospective analysis of intrauterine radiation exposures in children with leukemia]. Radiobiol Radiother (Berl) 1980; 21: 501–6. [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- Hirayama T. Descriptive and analytical epidemiology of childhood malignancy in Japan. . Recent Advances in Managements of Children with Cancer. Commemorating the 10th Anniversary of the Children’s Cancer Association of Japan and International Year of the Child, Tokyo, 12–14 December 1979. The Children’s Cancer Association of Japan, Tokyo, 12–14 December 1979, 1979, pp. 27–43. [Google Scholar]
- Holly EA, Aston DA, Ahn DK, Kristiansen JJ. Ewing’s bone sarcoma, paternal occupational exposure, and other factors. Am J Epidemiol 1992; 135: 122–9. [DOI] [PubMed] [Google Scholar]
- Hong JY, Han K, Jung JH, Kim JS. Association of Exposure to Diagnostic Low-Dose Ionizing Radiation With Risk of Cancer Among Youths in South Korea. JAMA Netw Open 2019; 2: e1910584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopton PA, McKinney PA, Cartwright RA, Mann JR, Birch JM, Hartley AL, et al. X-rays in pregnancy and the risk of childhood cancer. Lancet 1985; 2: 773. [DOI] [PubMed] [Google Scholar]
- Howe GR, Burch JD, Chiarelli AM, Risch HA, Choi BC. An exploratory case-control study of brain tumors in children. Cancer Res 1989; 49: 4349–52. [PubMed] [Google Scholar]
- Huang WY, Muo CH, Lin CY, Jen YM, Yang MH, Lin JC, et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: A nation-wide population-based cohort study. British Journal of Cancer 2014; 110: 2354–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Rivard C Diagnostic x rays, DNA repair genes and childhood acute lymphoblastic leukemia. Health Phys 2003; 85: 60–4. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). Biological effects after prenatal irradiation (embryo and fetus). ICRP publication 90. Ann. ICRP 2003; 33: 1–206. [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007; 37: 1–332. [DOI] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MC, Hing A, Wood MK, Watson MS. Chromosome abnormalities in congenital heart disease. Am J Med Genet 1997; 70: 292–8. [DOI] [PubMed] [Google Scholar]
- Johnston HE, Mann JR, Williams J, Waterhouse JA, Birch JM, Cartwright RA, et al. The Inter-Regional, Epidemiological Study of Childhood Cancer (IRESCC): case-control study in children with germ cell tumours. Carcinogenesis 1986; 7: 717–722. [DOI] [PubMed] [Google Scholar]
- Journy N, Rehel JL, Ducou Le Pointe H, Lee C, Brisse H, Chateil JF, et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer 2015; 112: 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journy N, Roué T, Cardis E, Ducou Le Pointe H, Brisse H, Chateil J-F, et al. Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot 2016; 36: N1–7. [DOI] [PubMed] [Google Scholar]
- Kaplan HS. An evaluation of the somatic and genetic hazards of the medical uses of radiation. Am J Roentgenol Radium Ther Nucl Med 1958; 80: 696–706. [PubMed] [Google Scholar]
- Kendall GM, Little MP, Wakeford R. A review of studies of childhood cancer and natural background radiation. Int J Radiat Biol 2021; 97: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Evans AA, Rorke-Adams L, Orjuela MA, Shiminski-Maher T, Bunin GR. Head injury, diagnostic X-rays, and risk of medulloblastoma and primitive neuroectodermal tumor: a Children’s Oncology Group study. Cancer Causes Control 2010; 21: 1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsberg H [Radiotherapy of leukemia in children]. Tidsskr Nor Laegeforen 1957; 77: 1052–3. [PubMed] [Google Scholar]
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22: 2693–710. [DOI] [PubMed] [Google Scholar]
- Kneale GW, Stewart AM. Prenatal x rays and cancers: further tests of data from the Oxford Survey of Childhood Cancers. Health Phys. 1986; 51: 369–376. [PubMed] [Google Scholar]
- Ko JM. Genetic Syndromes associated with Congenital Heart Disease. Korean Circ J 2015; 45: 357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojimahara N, Yoshitake T, Ono K, Kai M, Bynes G, Schuz J, et al. Computed tomography of the head and the risk of brain tumours during childhood and adolescence: results from a case-control study in Japan. J Radiol Prot 2020; 40: 1010–1023. [DOI] [PubMed] [Google Scholar]
- Krille L, Dreger S, Schindel R, Albrecht T, Asmussen M, Barkhausen J, et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys 2015; 54: 1–12. [DOI] [PubMed] [Google Scholar]
- Kuijten RR, Bunin GR, Nass CC, Meadows AT. Gestational and familial risk factors for childhood astrocytoma: results of a case-control study. Cancer Res 1990; 50: 2608–12. [PubMed] [Google Scholar]
- Kumar A, Vashist M, Rathee R. Maternal factors and risk of childhood leukemia. Asian Pac J Cancer Prev 2014a; 15: 781–4. [DOI] [PubMed] [Google Scholar]
- Kumar P, Joshi VS, Madhu PV. Diagnostic pediatric cardiac catheterization: Experience of a tertiary care pediatric cardiac centre. Medical journal, Armed Forces India 2014b; 70: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li IG, Yang YH, Li YT, Tsai YH. Paediatric computed tomography and subsequent risk of leukaemia, intracranial malignancy and lymphoma: a nationwide population-based cohort study. Sci Rep 2020; 10: 7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YH, Lin CL, Wei CC, Tsai PP, Shen WC, Sung FC, et al. Subsequent cancer risk of children receiving post voiding cystourethrography: A nationwide population-based retrospective cohort study. Pediatric Nephrology 2014; 29: 885–891. [DOI] [PubMed] [Google Scholar]
- Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 2009; 39 Suppl 1: S4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linet MS, Slovis TL, Miller DL, Kleinerman R, Lee C, Rajaraman P, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Patel A, Lee C, Hauptmann M, Berrington de Gonzalez A, Albert P. Impact of Reverse Causation on Estimates of Cancer Risk Associated With Radiation Exposure From Computerized Tomography: A Simulation Study Modeled on Brain Cancer. Am J Epidemiol 2022a; 191: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Pawel D, Misumi M, Hamada N, Cullings HM, Wakeford R, et al. Lifetime mortality risk from cancer and circulatory disease predicted from the Japanese atomic bomb survivor Life Span Study data taking account of dose measurement error. Radiat Res 2020; 194: 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Bouffler SD, Abalo K, Hauptmann M, Hamada N, et al. Review of the risk of cancer following low and moderate doses of sparsely ionising radiation received in early life in groups with individually estimated doses. Environ Int 2022b; 159: 106983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo PJ, Schraw JM, Desrosiers TA, Nembhard WN, Langlois PH, Canfield MA, et al. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncol 2019; 5: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B Prenatal x-ray exposure and childhood cancer. J.Natl.Cancer Inst 1962; 28: 1173–1191. [PubMed] [Google Scholar]
- Magnani C, Pastore G, Luzzatto L, Carli M, Lubrano P, Terracini B. Risk factors for soft tissue sarcomas in childhood: a case-control study. Tumori 1989; 75: 396–400. [DOI] [PubMed] [Google Scholar]
- Magnani C, Pastore G, Luzzatto L, Terracini B. Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkin’s lymphomas in childhood: a case-control study. Tumori 1990; 76: 413–9. [DOI] [PubMed] [Google Scholar]
- Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346: f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCredie M, Maisonneuve P, Boyle P. Antenatal risk factors for malignant brain tumours in New South Wales children. Int J Cancer 1994a; 56: 6–10. [DOI] [PubMed] [Google Scholar]
- McCredie M, Maisonneuve P, Boyle P. Perinatal and early postnatal risk factors for malignant brain tumours in New South Wales children. Int J Cancer 1994b; 56: 11–5. [DOI] [PubMed] [Google Scholar]
- McKinney PA, Juszczak E, Findlay E, Smith K, Thomson CS. Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study. Br J Cancer 1999; 80: 1844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JR, Kreiger N, Sloan MP, Benson LN, Hilditch S, Clarke EA. An historical cohort study of cardiac catheterization during childhood and the risk of cancer. Int. J. Epidemiol 1993; 22: 584–591. [DOI] [PubMed] [Google Scholar]
- Meinert R, Kaletsch U, Kaatsch P, Schüz J, Michaelis J. Associations between childhood cancer and ionizing radiation: Results of a population-based case-control study in Germany. Cancer Epidemiology Biomarkers and Prevention 1999; 8: 793–799. [PubMed] [Google Scholar]
- Mellemkjaer L, Hasle H, Gridley G, Johansen C, Kjaer SK, Frederiksen K, et al. Risk of cancer in children with the diagnosis immaturity at birth. Paediatr Perinat Epidemiol 2006; 20: 231–7. [DOI] [PubMed] [Google Scholar]
- Memon A, Rogers I, Paudyal P, Sundin J. Dental X-Rays and the Risk of Thyroid Cancer and Meningioma: A Systematic Review and Meta-Analysis of Current Epidemiological Evidence. Thyroid 2019; 29: 1572–1593. [DOI] [PubMed] [Google Scholar]
- Meulepas JM, Ronckers CM, Smets A, Nievelstein RAJ, Gradowska P, Lee C, et al. Radiation Exposure From Pediatric CT Scans and Subsequent Cancer Risk in the Netherlands. J Natl Cancer Inst 2019; 111: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Greenop KR, Fritschi L, Attia J, Bailey HD, Scott RJ, et al. Childhood and parental diagnostic radiological procedures and risk of childhood brain tumors. Cancer Causes and Control 2014; 25: 375–383. [DOI] [PubMed] [Google Scholar]
- Modan B, Keinan L, Blumstein T, Sadetzki S. Cancer following cardiac catheterization in childhood. Int.J.Epidemiol 2000; 29: 424–428. [PubMed] [Google Scholar]
- Mole RH. Childhood cancer after prenatal exposure to diagnostic X-ray examinations in Britain. Br.J.Cancer 1990; 62: 152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RR, MacMahon B. Prenatal x-ray exposure and cancer in children. In: Boice JD Jr, Fraumeni JF Jr, editors. Radiation carcinogenesis: epidemiology and biological significance. Raven Press, New York, NY, 1984, pp. 97–105. [Google Scholar]
- Muirhead CR, Kneale GW. Prenatal irradiation and childhood cancer. J. Radiol. Prot 1989; 9: 209–212. [Google Scholar]
- Murray R, Heckel P, Hempelmann LH. Leukemia in children exposed to ionizing radiation. New Engl. J. Med 1959; 261: 585–589. [DOI] [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Report No. 174. Preconception and prenatal radiation exposure: health effects and protective guidance. 174. National Council on Radiation Protection and Measurements (NCRP), 7910 Woodmont Avenue, Suite 400 / Bethesda, MD 20814–3095, USA, 2013, pp. 1–418. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Report No. 184. Medical radiation exposure of patients in the United States. National Council on Radiation Protection and Measurements (NCRP), 7910 Woodmont Avenue, Suite 400 / Bethesda, MD 20814–3095, USA, 2019, pp. 1–298. [Google Scholar]
- Naumburg E, Bellocco R, Cnattingius S, Hall P, Boice JD, Jr., Ekbom A. Intrauterine exposure to diagnostic X rays and risk of childhood leukemia subtypes. Radiat.Res 2001; 156: 718–723. [DOI] [PubMed] [Google Scholar]
- Nishi M, Miyake H. A case-control study of non-T cell acute lymphoblastic leukaemia of children in Hokkaido, Japan. J Epidemiol Community Health 1989; 43: 352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operskalski EA, Preston-Martin S, Henderson BE, Visscher BR. A case-control study of osteosarcoma in young persons. Am J Epidemiol 1987; 126: 118–26. [DOI] [PubMed] [Google Scholar]
- Patton T, Olshan AF, Neglia JP, Castleberry RP, Smith J. Parental exposure to medical radiation and neuroblastoma in offspring. Paediatr Perinat Epidemiol 2004; 18: 178–85. [DOI] [PubMed] [Google Scholar]
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus DW, Koch R. Leukemia and medical radiation. Pediatrics 1959; 23: 453–61. [PubMed] [Google Scholar]
- Preston-Martin S, Paganini-Hill A, Henderson BE, Pike MC, Wood C. Case-control study of intracranial meningiomas in women in Los Angeles County, California. J Natl Cancer Inst 1980; 65: 67–73. [PubMed] [Google Scholar]
- Preston-Martin S, Yu MC, Benton B, Henderson BE. N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer Res 1982; 42: 5240–5. [PubMed] [Google Scholar]
- R Project version 3.6.1. R: A language and environment for statistical computing. version 3.6.1 https://www.r-project.org. R Foundation for Statistical Computing, Vienna, Austria, 2019. [Google Scholar]
- Rajaraman P, Simpson J, Neta G, Berrington de GA, Ansell P, Linet MS, et al. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ 2011; 342: d472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Schull MJ, Urquia ML, You JJ, Guttmann A, Vermeulen MJ. Major radiodiagnostic imaging in pregnancy and the risk of childhood malignancy: a population-based cohort study in Ontario. PLoS Med 2010; 7: e1000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvall Y, Pershagen G, Hrubec Z, Ahlbom A, Pedersen NL, Boice JD. Prenatal X-ray exposure and childhood cancer in Swedish twins. Int.J.Cancer 1990; 46: 362–365. [DOI] [PubMed] [Google Scholar]
- Roman E, Ansell P, Bull D. Leukaemia and non-Hodgkin’s lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br.J.Cancer 1997; 76: 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Watson A, Beral V, Buckle S, Bull D, Baker K, et al. Case-control study of leukaemia and non-Hodgkin’s lymphoma among children aged 0–4 years living in west Berkshire and north Hampshire health districts. BMJ 1993; 306: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen T. Prenatal and perinatal factors in childhood cancer. Ann Clin Res 1976; 8: 27–42. [PubMed] [Google Scholar]
- Schuz J, Kaletsch U, Kaatsch P, Meinert R, Michaelis J. Risk factors for pediatric tumors of the central nervous system: results from a German population-based case-control study. Med.Pediatr.Oncol 2001; 36: 274–282. [DOI] [PubMed] [Google Scholar]
- Shih TY, Wu J, Muo CS, Kao CH. Association between leukaemia and X-ray in children: A nationwide study. Journal of Paediatrics and Child Health 2014; 50: 615–618. [DOI] [PubMed] [Google Scholar]
- Shiono PH, Chung CS, Myrianthopoulos NC. Preconception radiation, intrauterine diagnostic radiation, and childhood neoplasia. J Natl Cancer Inst 1980; 65: 681–6. [DOI] [PubMed] [Google Scholar]
- Shu XO, Gao YT, Brinton LA, Linet MS, Tu JT, Zheng W, et al. A population-based case-control study of childhood leukemia in Shanghai. Cancer 1988; 62: 635–44. [DOI] [PubMed] [Google Scholar]
- Shu XO, Jin F, Linet MS, Zheng W, Clemens J, Mills J, et al. Diagnostic X-ray and ultrasound exposure and risk of childhood cancer. Br J Cancer 1994a; 70: 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XO, Nesbit ME, Buckley JD, Krailo MD, Robinson LL. An exploratory analysis of risk factors for childhood malignant germ-cell tumors: report from the Childrens Cancer Group (Canada, United States). Cancer Causes Control 1995; 6: 187–98. [DOI] [PubMed] [Google Scholar]
- Shu XO, Potter JD, Linet MS, Severson RK, Han D, Kersey JH, et al. Diagnostic X-rays and ultrasound exposure and risk of childhood acute lymphoblastic leukemia by immunophenotype. Cancer Epidemiol Biomarkers Prev 2002; 11: 177–85. [PubMed] [Google Scholar]
- Shu XO, Reaman GH, Lampkin B, Sather HN, Pendergrass TW, Robison LL. Association of paternal diagnostic X-ray exposure with risk of infant leukemia. Investigators of the Childrens Cancer Group. Cancer Epidemiol Biomarkers Prev 1994b; 3: 645–53. [PubMed] [Google Scholar]
- Sorahan T, Stewart AM. Retinoblastoma and fetal irradiation. BMJ 1993; 307: 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler RF, Cook DH, Clarke EA, Olley PM, Newman AM. Cancer mortality following cardiac catheterization: a preliminary follow-up study on 4,891 irradiated children. Pediatrics 1983; 71: 235–239. [PubMed] [Google Scholar]
- Spix C, Schulze-Rath R, Kaatsch P, Blettner M. Case-control study on risk factors for leukaemia and brain tumours in children under 5 years in Germany. Klin.Padiatr 2009; 221: 362–368. [DOI] [PubMed] [Google Scholar]
- Stålberg K, Haglund B, Axelsson O, Cnattingius S, Pfeifer S, Kieler H. Prenatal X-ray exposure and childhood brain tumours: A population-based case-control study on tumour subtypes. British Journal of Cancer 2007; 97: 1583–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology 2001; 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- Stewart A, Webb J, Giles D, Hewitt D. Malignant disease in childhood and diagnostic irradiation in utero. Lancet 1956; 268: 447. [DOI] [PubMed] [Google Scholar]
- Stewart A, Webb J, Hewitt D. A survey of childhood malignancies. Br.Med.J 1958; 1: 1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM. Cancer as a cause of abortions and stillbirths: the effect of these early deaths on the recognition of radiogenic leukaemias. Br.J.Cancer 1973; 27: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernfeldt M, Berglund K, Lindsten J, Ludvigsson J. Maternal smoking and irradiation during pregnancy as risk factors for child leukemia. Cancer Detect Prev 1992; 16: 129–35. [PubMed] [Google Scholar]
- Tettamanti G, Shu X, Adel Fahmideh M, Schuz J, Roosli M, Tynes T, et al. Prenatal and Postnatal Medical Conditions and the Risk of Brain Tumors in Children and Adolescents: An International Multicenter Case-Control Study. Cancer Epidemiol Biomarkers Prev 2017; 26: 110–115. [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). A report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly, with annexes. Levels and Effects. United Nations, New York, 1972, pp. 1–447. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. New York: United Nations, 2008. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Volume II. Scientific Annex B: Effects of radiation exposure of children. E.14.IX.2, New York, 2013, pp. 1–269. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources, effects and risks of ionizing radiation. UNSCEAR 2017 report to the General Assembly. Scientific annexes A and B. E.18.IX.1. United Nations, New York, 2018, pp. 1–194. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2020/2021 Report. Volume III. Annex C. Biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation. New York: United Nations, 2021. [Google Scholar]
- van Duijn CM, van Steensel-Moll HA, Coebergh JW, van Zanen GE. Risk factors for childhood acute non-lymphocytic leukemia: an association with maternal alcohol consumption during pregnancy? Cancer Epidemiol Biomarkers Prev 1994; 3: 457–60. [PubMed] [Google Scholar]
- van Steensel-Moll HA, Valkenburg HA, Vandenbroucke JP, van Zanen GE. Are maternal fertility problems related to childhood leukaemia? Int J Epidemiol 1985; 14: 555–9. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W Conducting meta-analyses in R with the metafor package. J. Statist. Software 2010; 36: 1–48. [Google Scholar]
- Viechtbauer W metafor. Version 2.4–0. CRAN - The Comprehensive R Archive Network; 2020. [Google Scholar]
- Wakeford R Childhood leukaemia following medical diagnostic exposure to ionizing radiation in utero or after birth. Radiat. Prot. Dosimetry 2008; 132: 166–174. [DOI] [PubMed] [Google Scholar]
- Wakeford R, Bithell JF. A review of the types of childhood cancer associated with a medical X-ray examination of the pregnant mother. Int J Radiat Biol 2021; 97: 571–592. [DOI] [PubMed] [Google Scholar]
- Wakeford R, Little MP. Risk coefficients for childhood cancer after intrauterine irradiation: a review. Int. J. Radiat. Biol 2003; 79: 293–309. [DOI] [PubMed] [Google Scholar]
- Walsh L, Shore R, Auvinen A, Jung T, Wakeford R. Risks from CT scans - what do recent studies tell us? J Radiol Prot 2014; 34: E1–E5. [DOI] [PubMed] [Google Scholar]
- Wells J, Steer CM. Relationship of leukemia in children to abdominal irradiation of mothers during pregnancy. Am. J. Obstet. Gynecol 1961; 81: 1059–1063. [DOI] [PubMed] [Google Scholar]
- Winn DM, Li FP, Robison LL, Mulvihill JJ, Daigle AE, Fraumeni JF Jr. A case-control study of the etiology of Ewing’s sarcoma. Cancer Epidemiol Biomarkers Prev 1992; 1: 525–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


