Abstract
Background
Hepatoid adenocarcinoma (HAC) of the lung (HAL) is a rare and aggressive extrahepatic adenocarcinoma with an unknown etiology and unfavorable prognosis, which is similar to the pathophysiological characteristics of hepatocellular carcinoma (HCC).
Methods
We first presented a 67-year-old patient diagnosed with HAC in the right middle lobe of the lung. Then, a systematic literature search was performed for HAL cases recorded between 1990 and 2020 based on three databases. The clinicopathological features, therapeutic method, and prognosis of this rare disease were reviewed, and corresponding prognostic factors were explored using Kaplan–Meier (K-M) curve and Cox proportional hazards regression model. Additionally, the potential biological mechanisms of HAL were further explored and compared with HCC and lung adenocarcinoma (LUAD) based on online databases.
Results
In the present study, we reported an HAL patient who underwent surgical resection combined with chemotherapy and succumbed to disease 13 months after surgery. Additionally, a total of 43 experimental studies with 49 HAL patients, including the present case, met the inclusion criteria and were included in the present review. We found that HAL is characterized by a male-dominated incidence and is more common in the right lung. Patients in the surgical subgroup have a better prognosis than those in the non-surgical subgroup (p = 0.034). Moreover, the Cox proportional hazards regression model demonstrated that surgical resection can significantly improve the prognosis of HAL patients (p = 0.016). HAL is a rare disease associated with gene mutations that has a distinctive cause and unique pathogenesis. Additionally, Afatinib and Gefitinib may be new effective agents to better combat HAL.
Conclusion
In conclusion, males may exhibit an increased risk of developing HAL and poorer prognosis than females. Surgical resection combined with chemotherapy may prolong the survival of patients with HAL. HAL has its unique clinicopathological characteristics and biological mechanisms.
Keywords: hepatoid adenocarcinoma, HAL, prognosis, biological mechanisms, review
Introduction
Hepatoid adenocarcinoma (HAC) is a relatively rare extrahepatic adenocarcinoma with microscopic features resembling those of hepatocellular carcinoma (HCC). HAC often expresses α-fetoprotein (AFP) in the tumor cells and has an unfavorable prognosis and unknown pathogenesis with an occurrence rate of less than 1% worldwide.1,2 Histopathological characteristics of HACs have shown that the tissue of this tumor contains an intermingling of neuroendocrine carcinoma, signet-ring cells, papillary adenocarcinoma, typical acinar, or pure HAC tissues,3,4 which play a significant role in the diagnosis of HAC. HAC can occur almost everywhere in extrahepatic organs, including the ovary,5 endometrium,6,7 bladder,8 pancreas,9 and stomach.10 However, HAC arising from the lung is extremely rare, with an incidence of less than 5% among cases of malignant lung tumor.11
The oncogenic mechanisms and biological significance of the hepatoid adenocarcinoma of the lung (HAL) remain unclear, and clinical presentations are atypical depending on the anatomic location.2 Although elevated AFP and imaging findings may provide some indications for the clinical diagnosis of HAL, morphological and immunohistochemical examinations are required for the accurate diagnosis of most lesions.1,3 To date, a standardized consensus regarding HAL management has not been reached due to limited experience with this rare disease. Surgical resection may be the only potentially curative treatment for HAL, but the effect of surgical treatment remains inadequate for advanced patients. In addition, HAL has a high mortality rate and a poor prognosis due to its extensive vascular, lymphatic invasions, and frequent metastasis to other organs.
Previous studies have shown that HAL tends to mimic HCC in terms of histopathology and immunohistochemistry (IHC). Additionally, many researchers have explored the clinicopathological features, treatment, and prognosis of this unusual disease and made some progress.2,3,12,13 However, these efforts are insufficient. The molecular alterations, functional information, and enrichment pathway of HAL-related oncogenes have not been investigated. In this study, an unusual HAC in the right middle lobe of the lung in a 67-year-old Chinese male patient was presented. Previous cases of HACs in the lung were reviewed from the English literature to provide centralized clinicopathological information and identify potential biological mechanisms for patients with HAL.
Materials and Methods
Study Design
To our knowledge, there remains no standardized consensus regarding the treatment of HAC in the lung due to limited experience with this rare disease. Previous studies have shown that clinicopathological characteristics, including age, gender, tumor location, tumor size, T classification, and the serum AFP level, may be related to the prognosis of patients with HAL. We conducted a systematic review of the literature to assess the optimal therapeutic model and explore potential prognostic predictors for HAL patients. In this study, English literature was selected from PubMed, MEDLINE, and Web of Science databases from 1990 to December 2020. According to the inclusion and exclusion criteria, we selected 42 articles to read full-texts. Then, the clinicopathological characteristics and therapeutic model of patients were obtained for literature records. The study design is outlined below. Ethical approval is not required because this study is based on aggregate data and does not involve experiments on humans or animals.
Data Sources and Search Strategy
This review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.14 Several electronic databases, including PubMed, MEDLINE, and Web of Science, were searched systematically using predetermined terms “Hepatoid adenocarcinoma”, “HAC”, “HAL”, “lung”, and “pulmonary” between January 1, 1990 and December 31, 2020 (This work was accomplished by two independent reviewers: Zhitao Chen and Chenchen Ding). In this study, 1990 was selected as the starting year for search because the conception of HAC was first introduced in this year.15 In addition, the electronic search strategy was supplemented by hand-searching reference lists of key articles.
Study Selection Criteria
Two independent reviewers (Zhitao Chen and Chenchen Ding) chose eligible articles by examining titles, abstracts, and the full text. The literature will be included if it meets the following criteria: (i) The diagnosis of HAL is confirmed by histopathological inspection; (ii) There are clear therapies. The literature will be excluded if it meets the following criteria: (i) The case is not histopathologically diagnosed as primary HAL; (ii) Combined with other tumors; (iii) Lack of available full text in the literature.
Data Extraction
Two independent reviewers (Zhitao Chen and Chenchen Ding) extracted and evaluated the data from all included studies. Basic information, such as the name of the first author, year of publication, country of study, age of patients, gender, and smoking status, was collected. Clinicopathological features including symptoms, tumor location, tumor sizes, serum AFP levels, and TNM classification were collected. Additionally, therapies and prognosis data were also collected. The overall survival (OS) period was defined as the period from surgery to death (patients alive were censored at the last follow-up). The median survival time (MST) was calculated for all-cause mortality, defined as the time from HAL diagnosis until the cumulative survival proportion reached 50%.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics version 19.0 software (IBM, Armonk, NY, USA), with p < 0.05 as a threshold of statistical significance. Basic information and clinicopathological features of patients, including country of study, gender of patients, the proportion of smoker cases, symptoms, tumor location, and proportion of serum AFP-positive cases, were expressed in percentage. Furthermore, the Kolmogorov–Smirnov test was used to determine whether the metrological data followed the normal distribution. Normally distributed data were reported using the mean, and non-normally distributed data were reported using the median with corresponding 25th and 75th percentiles. Survival curves were calculated by the Kaplan–Meier (K-M) method, and independent prognostic factors were analyzed by the Cox proportional hazard regression model.
Results
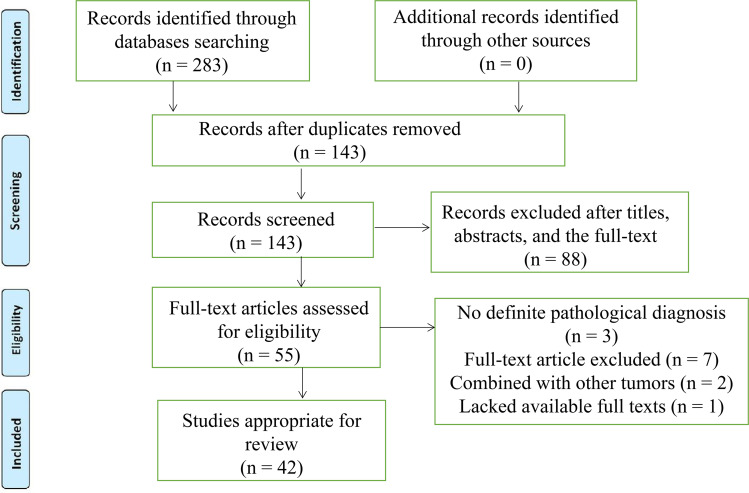
After the selection procedure in Figure 1, the search revealed 48 reported cases with primary HAL since 1990. Eventually, a total of 49 cases, including the present case, were summarized and conducted to better understand primary HAL1–3,13,16–53 (Table 1). The geographical distribution of reported cases was explored, with the majority of reported cases in Asia (30.6% in China and 22.4% in Japan).
Figure 1.
The flowchart of literature selection.
Table 1.
Reported Cases of Hepatoid Adenocarcinoma of the Lung (HAL) in the English Literature
| First Author, Year | Country | Age (Years)/ Sex | Location | Symptom | Smoker | Tumor Marker | Size (cm) | TNM | Treatment | Survival/Fustat |
|---|---|---|---|---|---|---|---|---|---|---|
| Okunaka et al 199219 | Japan | 49/male | RUL | Cough, bloody sputum | UN | AFP | 6 | eT3 | RS | 11 months/alive |
| Yoshino et al 199629 | Japan | 54/male | RUL | None | UN | AFP+CEA | 2 | pT1bN0M0 | RS | 24 months/alive |
| Carlnfante et al 200030 | Italy | 82/male | LLL | Cough, dyspnea | Yes | Normal | 3.5 | cT2aN0M0 | RS | 84 months/alive |
| Hayashi et al 200231 | Japan | 55/male | RUL | UN | Yes | CEA | 6.5 | pT3N0M0 | RS | 32 months/alive |
| Arnould et al 199725 | France | 36/male | LUL | Weight loss, asthenia, anorexia | Yes | AFP | 11 | pT4N2 | C + RS | 7 months/dead |
| Hiroshima et al 200226 | Japan | 71/male | RLL | Common cold | Yes | AFP+CA19-9+CA125 | 10.5 | pT3N1M0 | RS | 12 months/dead |
| Japan | 70/male | RUL | UN | Yes | AFP | 6 | pT4N2M0 | RS | 5 months/dead | |
| Japan | 64/female | RLL | UN | Yes | AFP | 3.3 | T4N2M0 | RS + C+ R | 24 months/dead | |
| Terracciano et al 200332 | Switzerland | 49/male | LLL | Cough, dyspnea | UN | AFP | 5 | pT2bN0M0 | RS | 2 months/dead |
| Oshiro et al 200433 | Japan | 77/male | RLL | UN | UN | AFP+PIVKA- II | 18 | cT2N0M0 | RS | 18 months/dead |
| Wu et al 200734 | China | 50/male | RUL | Weight loss | Yes | Normal | 6 | cT2N1M0 | RS | 45 months/alive |
| Kishimoto et al 200835 | Japan | 64/male | LLL | UN | UN | AFP | 7.5 | cT3N0M0 | RS | UN |
| Fornasa et al 201021 | Italy | 68/female | LUL | Shoulder pain | No | Normal | 4.5 | pT2bN0M1 | C | 15 months/alive |
| Papatsimpas et al 201122 | Greece | 48/male | RUL | Shoulder pain | UN | AFP | 20 | cT4 | C+ R | 6 months/dead |
| Kitada et al 201136 | Japan | 69/male | RLL | Epigastric pain | Yes | CA19-9+AFP+CEA | 6.5 | pT3N2M0 | C+ RS | 12 months/alive |
| Valentino et al 201220 | Italy | 71/male | RLL | Chest pain | No | CA19-9+AFP | 1.8 | pT1N0M1 | C+ R+ RS | 4 months/dead |
| Mokrim et al 201237 | Morocco | 52/male | LUL | Dyspnea, weight loss, chest pain | Yes | AFP | 12 | cT4N1M0 | C | 7 months/alive |
| Khozin et al 201238 | America | 56/female | RML | Chest pain, cough, night sweats | Yes | Normal | 5.5 | cT4 | C | 6 months/alive |
| Lin et al 201339 | China | 66/male | RUL | Cough, dyspnea | Yes | AFP | 7.4 | cT3N2M0 | RS + C | 15 months/alive |
| Cavalcante et al 201340 | Brazil | 66/male | RLL | Cough, dyspnea, weight loss | Yes | CEA | 5 | pT2bN0M0 | None | 0.4 months/dead |
| Che et al 201441 | China | 66/male | LUL | Back pain | Yes | AFP | 10 | pT4N1M0 | C+ R | 36 months/dead |
| Shaib et al 201442 | America | 53/female | RUL | Chest pain | Yes | AFP | 9.5 | pT3N0M0 | RS + C | 48 months/alive |
| Haninger et al 201417 | America | 51/male | RUL | Cough | Yes | UN | 4.2 | cT2bN3M0 | C+ R+ RS | 14 months/dead |
| America | 52/male | RUL | None | Yes | UN | 2.5 | pT1bN0M1b | C+ R+ RS | 37 months/alive | |
| America | 64/male | LUL | None | Yes | UN | 3.2 | cT2aN0M1b | C+ R+ RS | 10 months/alive | |
| America | 54/female | LUL | Chest pain | Yes | UN | 1 | cT1aN0M1b | C+ R+ RS | 108 months/alive | |
| America | 60/male | RUL | Cough, Weight loss | Yes | AFP | 11.2 | cT3N2M1b | C+ R | 1 months/alive | |
| Gavrancic et al 201527 | America | 64/male | RUL | Cough, hemoptysis | UN | AFP | 3.8 | cT2aN2M1 | C+ R+ T | 11 months/dead |
| Grossman et al 20161 | America | 54/male | RUL | Cough, dyspnea, hemoptysis weight loss | Yes | Normal | 5.1 | pT4N0M1b | C+ R | 4 months/dead |
| Motooka et al 201618 | Japan | 69/male | LUL | UN | Yes | AFP | 4.3 | cT2aN0M0 | RS + C | 51 months/alive |
| Sun et al 201643 | China | 59/male | RUL | Cough | Yes | Normal | 4.5 | pT2aN0M0 | RS | 23 months/alive |
| Qian et al 201644 | China | 79/male | RUL | Cough, sputum | Yes | CEA +AFP | 2.7 | cT1cN0M0 | T | 0.83 months/dead |
| Wang et al 201645 | China | 56/male | RUL | None | UN | UN | 4.8 | cT2N1M0 | UN | UN |
| Valle et al 201746 | America | 61/male | Left lung | Chest pain | UN | UN | UN | cT4NxM1 | C+ R | 55 months/dead |
| Basse et al 201828 | France | 43/male | UN | None | Yes | UN | UN | cTxN3M1 | C + T + I | UN |
| Yang et al 20193 | China | 70/male | LLL | Cough, hemoptysis, sputum | Yes | UN | 6 | pT3N1M0 | RS | 18 months/dead |
| Ayub et al 201947 | America | 61/male | RUL | None | Yes | UN | 2.3 | pT1N0M0 | RS +R | 6 months/dead |
| Kuan et al 201948 | America | 47/male | RUL | Chest pain, fatigue, edema | Yes | UN | 14 | cT4N0M0 | RS | 4 months/dead |
| Chen et al 20192 | China | 53/male | RUL | Cough, sputum, fever | No | AFP | 5.3 | pT3N0M0 | RS +C+T | 36 months/alive |
| Li et al 201924 | China | 71/male | RLL | Stomachache, fatigue | No | AFP | 7 | T4N3M1 | R | 5 months/dead |
| Wang et al 201949 | China | 70/male | RUL | None | Yes | UN | 6 | cT3N2M1 | C + R + T | 9 months/dead |
| Shi et al 201950 | China | 60/male | RUL | Cough, sputum | Unknown | AFP | 7 | pT3N2M0 | RS + C | 15 months/dead |
| Miyama et al 202023 | Japan | 58/male | RLL | Cough, dyspnea | Unknown | CEA | 6 | pT3NxM1 | C | 6 months/dead |
| Wang et al 202051 | China | 41/male | RUL | Fever, chest pain | Yes | NSE+SCC | 5.8 | pT3N3M0 | R | 12 months/dead |
| Chen et al 202016 | China | 65/female | RLL+LLL | None | No | AFP | 9.2 | pT4NxM1 | C+ T+ I | 52 months/dead |
| Chen et al 202013 | China | 63/male | RUL+LLL | Lumbar soreness | Unknown | CEA+CA19-9+CA72-4+CYFPA21-1 | UN | pT4N3M1 | Palliative surgery | 4 months/dead |
| Muroyama et al 202052 | America | 66/male | LUL | Alexia, fatigue | Yes | AFP | 8 | T4N3M1b | C+R | 19 months/dead |
| Tonyali et al 202053 | Turkey | 62/female | LUL | Back pain | Yes | AFP | 8 | T4N1M0 | RS +C+I+R | 14 months/dead |
| Current case | China | 67/male | RML | Cough | Yes | CA19-9, CA125, CA72-4, SCC CYFRA21-1, | 7.1 | T4N1M0 | C+ RS | 13 months/dead |
Abbreviations: LUL, left upper lobe; RUL, right upper lobe; RLL, right lower lobe; LLL, left lower lobe; RML, Right middle lobe; UN, unknown; RS, radical surgery; C, chemotherapy; I, Immunotherapy; R, Radiotherapy; T, Targeted therapy; AFP, Alpha fetoprotein; CA19-9, Carbohydrate antigen 19-9; CA72-4, Carbohydrate antigen 72-4; CA125, Carbohydrate antigen 125; SCC, Squamous cell carcinoma antigen; CYFRA21-1, Cytokeratin fragment 21-1; CEA, Carcinoembryonic antigen; NSE, neuron-specific enolase; PIVKA-, II Protein Induced by Vitamin K Absence or Antagonist-II.
Case Report
A 67-year-old Chinese male with a 30-year history of smoking 60 cigarettes per day was admitted to Shulan (Hangzhou) Hospital on October 27, 2020. The patient complained of a 2-month history of cough but no fever, jaundice, or weight loss. A week ago, the patient presented to a local hospital for a consultation, and the chest X-ray examination showed a mass in the right middle lobe of the lung located near the hilar area. Subsequently, the patient was admitted to our hospital for further treatment. Laboratory work-ups of the patient on admission showed a slightly elevated leukocyte count (13.2×109/L, normal 3.5–9.5×109/L) and a significantly elevated c-reactive protein (131.0 mg/dL, normal 0–10.0 mg/L). Systemic tumor marker tests showed a significantly elevated carbohydrate antigen (CA) 19–9 (623.2 U/mL, normal 0–24.0 U/mL), CA 125 (67.3 U/mL, normal 0–43.0 U/mL), CA 72–4 (57 IU/mL, normal 0–7.0 IU/mL), cytokeratin-19-fragment (CYFRA21-1) (8.2 ng/mL, normal 0–3.3 ng/mL), and squamous cell carcinoma antigen (9.2 ng/mL, normal 0–1.8 ng/mL). Carcinoembryonic antigen (CEA) and AFP levels were within normal limits.
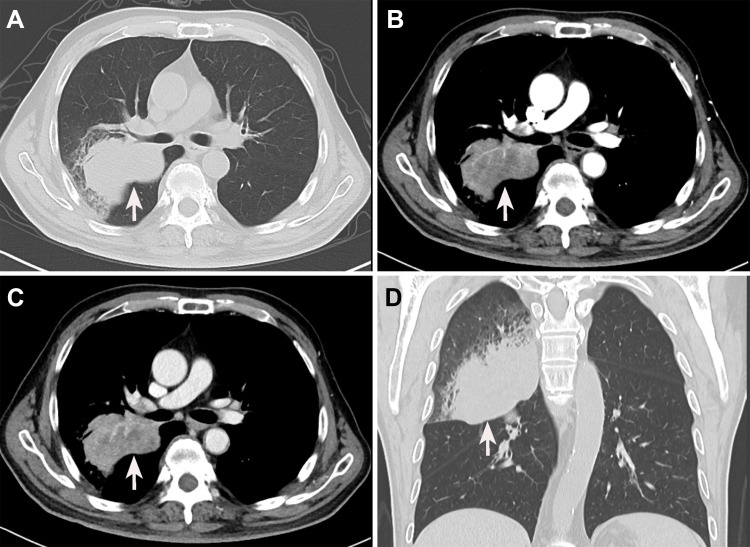
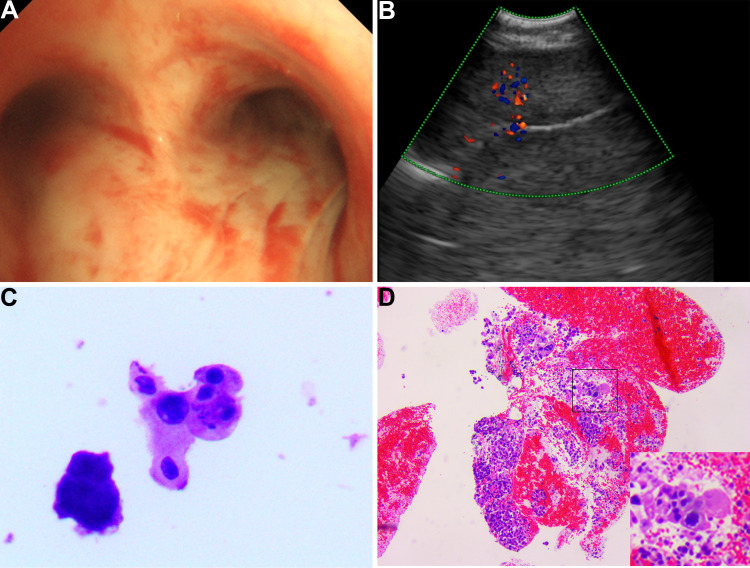
The thoracic computed tomography (CT) showed a 7.1×5.3 cm ill-defined mass located in the right middle lobe of the lung, and an enhanced CT scan revealed markedly inhomogeneous enhancement (Figure 2A–D). In addition, multiple enlarged lymph nodes were found in the right hilar area and mediastinum. Fiberbronchoscopy was unremarkable, except for a focal mucosal thickening in the right tracheal wall with multiple lymphadenectasis in stations 4R and 7 (Figure 3A). Preoperational imaging studies of systemic organs excluded the presence of metastatic disease and other primary tumors. Subsequently, the patient underwent endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) under local anesthesia (Figure 3B). After identification and analysis of the irregular mass in the right middle lobe of the lung, endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) (OLYMPUS NA-201SX-4021 Single Use Aspiration Needle) was performed on suspected lesions. Moreover, bronchoalveolar lavage fluid was collected. Microscopy showed that the bronchoalveolar lavage fluid is composed of inflammatory cells containing few heteromorphic cells (Figure 3C). Staining of the suspected mass with hematoxylin and eosin revealed poorly differentiated carcinoma with tumor cell morphology resembling the HCC (Figure 3D). Additionally, in combination with immunohistochemical reactions, the mass in the lung can be diagnosed as HAL (T4N1M0, Stage IIIB based on the TNM Classification of lung cancers, 8th edition).
Figure 2.
Preoperative enhanced computed tomography images of the chest. (A) A plain chest CT scan reveals a mass-like lesion in the right upper lung with an irregular margin (white arrow). (B) The mass is markedly enhanced in the arterial phase (white arrow). (C) The enhancement of the venous phase is slightly weaker than that of the venous phase (white arrow). (D) Coronal sections reveal an irregular lesion in the right upper lung.
Figure 3.
The image of fiber bronchoscopy, endobronchial ultrasonography (EBUS), and pathology. (A) Fibronchofibroscope examination reveals a focal mucosal thickening of the right tracheal wall. (B) EBUS shows right pulmonary neoplasm. (C) The bronchoalveolar lavage fluid is composed of inflammatory cells with few heteromorphic cells. (D) The pathologic evaluation suggests hepatoid adenocarcinoma of the lung.
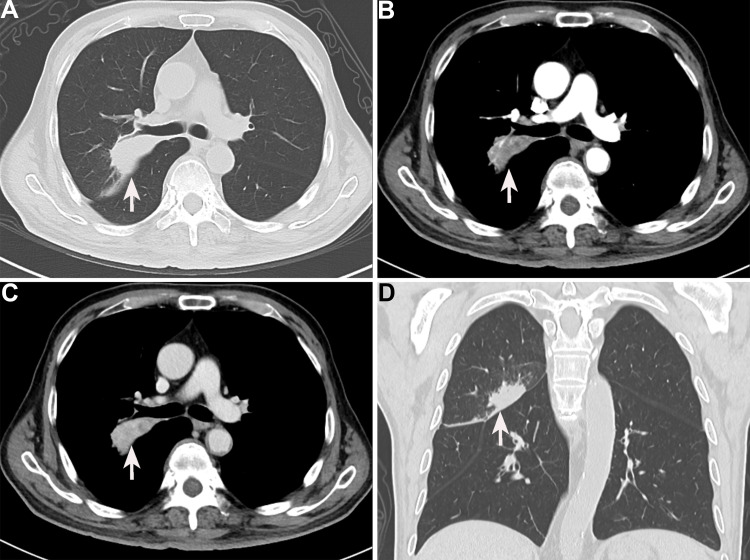
After a multidisciplinary team (MDT) discussion, complete resection (R0 resection) of the tumor was difficult considering that the tumor was too close to the hilar area. Additionally, previous cases of HAL have proved that neoadjuvant chemotherapy may be effective. To date, no standard neoadjuvant chemotherapy regimen has been established for HAL, considering that albumin-bound paclitaxel combined with cisplatin is recommended as first-line regimens for non-small cell lung carcinomas. Subsequently, the patient received systemic chemotherapy with albumin-bound paclitaxel 125 mg/m2 on days 1 and 8 and cisplatin 25 mg/m2 on days 1 to 3. The neoadjuvant chemotherapy cycles were repeated every 3 weeks. After three cycles of chemotherapy, a chest CT was performed to further evaluate the therapeutic response. The results presented that the tumor shrinks to 5.3 cm, and the distance of the tumor from the hilar area is increased (Figure 4A–D). Additionally, the patient underwent the left upper lobectomy after the multidisciplinary team discussion. The entire tumor was solid with a grey-white cross-section surface. An intraoperative frozen section was assessed. There was suspected HAC in the lung, and all margins were reported negative. Detailed postoperative histopathological examination demonstrated mass lesions in the lung with abundant HAC tumor cells accompanied by partial acinar adenocarcinoma tissues (approximately 30%) and 7 of 27 lymph nodes positive for metastatic disease (Figure 5A–D). IHC showed that HAC in the lung is positive for CEA, CK7, E-Cad, NapsinA, SP-B, TTF1, Hepatocyte, D-PAS, PAS, P53, and β-catenin and negative for P40, P63, PD-1, AFP, and Glypican-3 (Figure 6A–E). Additionally, the proliferation index by Ki-67 staining is 70%, which may indicate a poor prognosis in patients with HAL (Figure 6F). Finally, the clinicopathological diagnosis showed that the mass in the right lung is HAC. The postoperative course was uncomplicated, and the patient was discharged on the 8th postoperative day.
Figure 4.
Enhanced computed tomography findings of the chest after chemotherapy (white arrow). A significant decrease in the size of the lesions in plain chest CT (A), arterial phase (B), venous phase (C), and coronal sections (D).
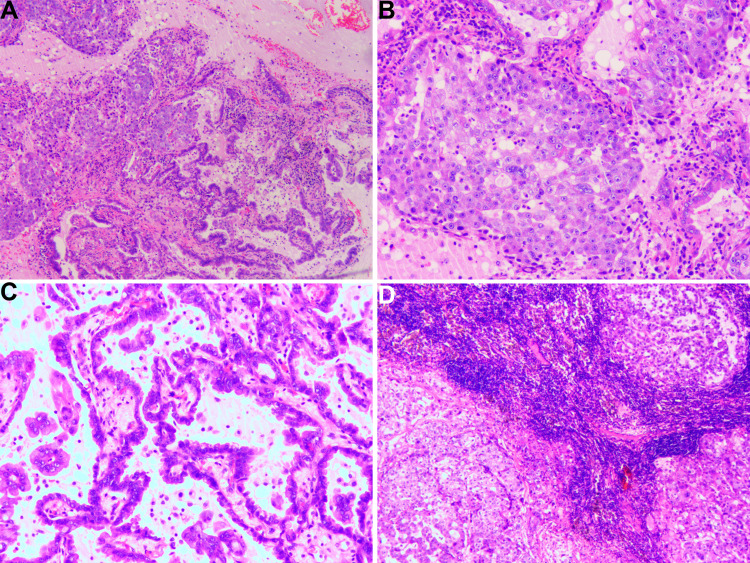
Figure 5.
The pathological findings of the resected pulmonary mass. (A) The mass combines two components, including hepatoid adenocarcinoma (HAC) of the lung and lung adenocarcinoma. (B) The pathological results of pulmonary hepatoid adenocarcinoma. The cytoplasm of tumor cells is abundant, and nuclei are markedly enlarged with prominent eosinophilic nucleoli. The individual neoplastic cells resemble hepatocytes. (C) The pathological results of lung adenocarcinoma. The tumor cells show acinar, micropapillary, and papillary with significant atypia. (D) The lymph nodes show HAC cells metastasis.
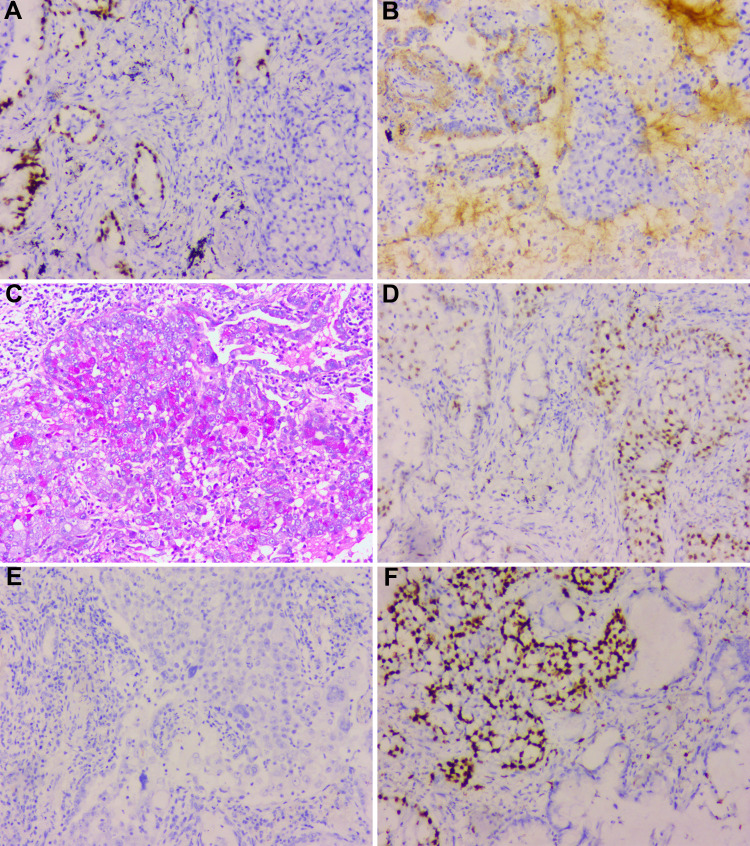
Figure 6.
The immunohistochemistry results of hepatoid adenocarcinoma. The hepatoid adenocarcinoma of the lung of our patient is positive for TTF1 (A), SP-B (B), PAS (C), and P53 (D) and negative for AFP (E). (F) The proliferation index by Ki-67 staining is above 70%.
The neoadjuvant chemotherapy has improved the progression of HAL, but the tumor was accompanied by lymph node metastasis. Postoperative chemotherapy is recommended to the patient as systemic therapy by oncologists and thoracic surgeons. Twenty-eight days after the surgery, the patient accepted regular adjuvant chemotherapy with albumin-bound paclitaxel plus cisplatin for four cycles. The tumor biomarkers dropped dramatically after the surgery and gradually decreased to the normal range after the postoperative chemotherapy. The patient succumbed to disease 13 months after the surgery.
Discussion
Lung cancer is one of the most common malignant tumors worldwide, and its incidence is increasing.54,55 Adenocarcinoma is the most frequent histological subtype in primary lung cancers.55 HAC, a kind of extrahepatic adenocarcinoma with microscopic features remarkably similar to HCC, was first reported by Corlin et al56 in 1972 and first concepted by Ishikura et al15 in 1990. HAC occurs almost anywhere in extrahepatic organs, and the most common location is the gastrointestinal tract.10,57 However, HAC arising from the lung is an extremely rare histological subtype, with an incidence of 2.3% of all HACs.2 Hence, there is a need to better understand the potential prognostic indicator and molecular pathogenesis of this disease based on limited case reports.
Clinicopathologic Part
The 49 cases of HAL comprise 42 males (42/49, 85.7%) and 7 females (6/47, 14.3%), with a male-to-female ratio of 7:1, which is similar to squamous cell carcinoma of the lung with an obvious male predilection. Additionally, HAL is usually diagnosed in middle to old age, with all reported cases ranging from 36 to 82 years (median age: 61 years, interquartile range: 53–67). The tumor size in all reported cases ranges between 1.0 to 20.0 cm, with a median of 6.0 cm (interquartile range: 4.4–7.9). The tumor is commonly located in the right lung (32/49, 65.3%), including 8 cases in the right lower lobe, 2 cases in the right middle lobe, and 22 cases in the right upper lobe, followed by the left lung (14/49, 28.6%), including 4 cases in the left lower lobe, 7 cases in the left upper lobe, and 1 case unknown. These data indicate that HAL may be more common in the right lung. HAL is usually solitary and unilateral; however, multifocal and bilateral involvement have also been reported.13,16 According to the reports of HAL, the prognosis of HAL is dismal, with a median OS of 16.0 months.58
The clinical symptoms and imaging findings of HAL are often non-specific and similar to common lung cancer, which is associated with the primary site and size of the mass. As previously reported, most patients with HAL have a history of smoking (32/49, 65.3%), and the most common symptom of HAL is cough (17/49, 34.7%), followed by chest pain (8/49, 16.3%), dyspnea (7/49, 14.3%), and weight loss (6/49, 12.2%). The characteristics of physical examination and imaging are unspecific and similar to other lung cancers. In laboratory examinations, elevated AFP may provide some indications for the clinical diagnosis of HAL. However, of the 49 reported cases, only 26 (26/49, 53.1%) display elevated AFP levels, and 23 cases show a normal or undetectable AFP level. These findings are compatible with the opinions of Pasricha et al12 and Haninger et al17 that elevated AFP is not necessary for the diagnosis of HAL. There is no doubt that the high serum AFP levels are related to tumor cell activity and often significantly decline after effective treatment of the primary tumor.18–20
Without distinct features, HAL is always confused with other types of lung cancers, such as lung adenocarcinoma, pulmonary squamous cell carcinoma, and small-cell lung carcinoma. Therefore, the accurate diagnosis of HAL is difficult and often depends on histopathological examination and immunohistochemical features. HAL, a rare subtype of non-small cell lung carcinomas, possesses unique histological and morphological features.59 HAL can masquerade as a metastatic lung tumor from primary HCC in morphological and functional features, as shown in the present case. Haninger et al17 proposed two criterions for diagnosing HAL: (1) The tumor tissues can contain an intermingling of neuroendocrine carcinoma, signet-ring cells, papillary adenocarcinoma, typical acinar, or pure HAC tissues. (2) AFP expression is not mandatory for diagnosis as long as other markers of hepatic differentiation are expressed.
Although IHC plays a significant role in distinguishing HAL from metastatic HCC, no consensus exists on the immunostaining patterns for diagnosing HAL. Haninger et al17 have reported 5 HAL cases and demonstrated that CK8, HEA 125, and MOC31 are always positive in HAL (5/5 patients, 100%) and negative in HCC; CK18, HepPar1, and TTF-1 are always positive in HAL (5/5 patients, 100%) and HCC; CK7 and CEA are often positive in HAL (3/5 patients, 60%) and negative in HCC. These findings are in accordance with immunostaining patterns reported by Pasricha et al12 who have revealed that TTF-1 and Hep Par 1 immunoexpression are found in both HCC and HAL tissues, but CK7 and EMA are usually positive in HALs and negative in HCC. However, for our patient, HAC in the lung is positive for CEA, CK7, E-Cad, NapsinA, SP-B, TTF1, Hepatocyte, D-PAS, PAS, P53, and β-catenin and negative for P40, P63, PD-1, AFP, and Glypican-3.
Currently, the standardized treatment guidelines for HAL have not been well established due to limited treatment experience. Additionally, most treatment options for this malignant tumor follow the management protocols used for treating classical non-small cell lung carcinomas. Radical surgery of the tumor and lymph nodes is recommended for all HAL cases if imaging findings confirm that R0 resection (complete resection) of the tumor is feasible. Subsequent chemoradiotherapy and surgery are considered necessary for advanced tumors.17,18 In the present literature review, more than half of HAL cases underwent radical surgery of the mass and lymph nodes (29/49, 59.2%) and achieved a favorable prognosis. However, surgical treatment is not always available due to its extensive vascular and lymphatic invasions and frequent metastasis to other organs.21–23 Li et al24 have reported a novel therapeutic method for advanced unresectable HAL by radiofrequency ablation and also achieved a favorable prognosis. Additionally, whether neoadjuvant chemotherapy should be a significant part of the primary treatment of primary HAL is unclear, but it may increase the possibility of R0 resection and contribute to long-term survival,25 which is also evident in our patient.
Predictors of Survival
The prognosis of HAL is generally worse than that of other malignant tumors in the lung. As previously reported, a total of 46 HAL patients with complete survival information were included in the K-M analysis. The survival time of HAL varies from 0.4 to 108 months, with a median survival time of 12.5 months (interquartile range: 6.0–24.0 months), and 1- and 3- year OS rates are 50.0% and 17.4% (Figure 7A). The MST of patients with early (T1+T2, n = 17) and advanced HAL (T3+T4, n = 28) are 15.0 and 12.0 months, respectively, without significant differences (p = 0.173, Figure 7B). Similarly, there are no significant difference in OS rates between young (age ≤ 61, n = 23) and older (age > 61, n = 23) HAL patients (p = 0.188, Figure 7C). Previous literature has reported that female patients not only have a low incidence rate but also seem to have a better prognosis.17 Estrogen protection may contribute to a better prognosis for female patients, but the exact mechanism is still unknown. In the present review, it is worth noting that the MST of male (n= 39) and female (n = 7) patients are 12.0 and 24.0 months, respectively. Unfortunately, the better prognosis of female HLA patients has not been confirmed in the present review (p = 0.116, Figure 7D), which may be due to an insufficient sample size. Thus, more cases and clinical evidence are required to confirm these findings. Research has revealed that elevated AFP level, vascular and lymphatic invasions and metastasis to other organs in HAL patients predict poor prognosis.26 However, our results showed that the serum AFP level before treatment has no significant effect on the prognosis (p = 0.909, Figure 7E). Compared with the tumor sizes of ≤6 cm subgroup, there are no significant differences in the prognosis of the tumor sizes > 6 cm group (p = 0.621, Figure 7F). Although statistically insignificant (p = 0.170), the HAL located in the right lung may have a better prognosis than HAL located in the left lung, with MST of 18 months and 12 months, respectively (Figure 7G). Moreover, results suggest that radical surgery could significantly improve the survival of HAL patients (p = 0.034, Figure 7H). Although not statistically significant (p = 0.483), combined chemotherapy can improve survival compared to single radical surgery (Figure 7I).
Figure 7.
Kaplan-Meier survival curves of patients with hepatoid adenocarcinoma of the lung in different clinicopathological subgroups. (A) Overall survival of all patients with HAL. (B) Overall survival according to the T classification (T1+T2 vs T3+T4). (C) Overall survival according to the age of patients (≤ 61 years vs > 61 years). (D) Overall survival according to the gender of patients (male vs female). (E) Overall survival according to the serum AFP levels (increase vs normal). (F) Overall survival according to the maximal tumor size (≤ 6.0 cm vs > 6.0 cm). (G) Overall survival according to the tumor localization (left lung vs right lung). (H) Overall survival according to the therapeutic method (surgery vs non-surgery). (I) Overall survival according to the therapeutic method (surgery vs surgery + chemotherapy).
In order to gain potential prognostic indicators for HAL patients, a Cox proportional hazards regression analysis was conducted for 47 HAL patients with effective survival data (Table 2). The results showed that surgical resection (HR 0.29, 95% CI: 0.10–0.79, p = 0.016) could significantly improve the prognosis of HAL patients. However, the age (HR 1.82, 95% CI: 0.68–4.91, p = 0.23) and gender (HR 2.15, 95% CI: 0.48–9.73, p = 0.32) of patients, tumor location (HR 3.48, 95% CI: 0.98–12.31, p = 0.05), tumor sizes (HR 1.00, 95% CI: 0.33–3.06, p = 0.99), serum AFP levels (HR 1.08, 95% CI: 0.40–2.92, p = 0.89), and T classification (HR 1.06, 95% CI: 0.36–3.18, p = 0.91) have no significant effect on their prognosis.
Table 2.
The Cox Proportional Hazards Regression Analysis for Various Clinicopathological Parameters
| Clinicopathological Parameters | HR | 95% CI | p-value |
|---|---|---|---|
| Surgical resection | 0.29 | 0.10–0.79 | 0.016 |
| Age | 1.82 | 0.68–4.91 | 0.230 |
| Gender | 2.15 | 0.48–9.73 | 0.320 |
| Tumor location | 3.48 | 0.98–12.31 | 0.050 |
| Tumor sizes | 1.00 | 0.33–3.06 | 0.990 |
| Serum AFP levels | 1.08 | 0.40–2.92 | 0.890 |
| T classification | 1.06 | 0.36–3.18 | 0.910 |
Abbreviations: HR, Hazard Ratio; AFP, Alpha fetoprotein; CI, Confidence interval.
Exploration of Potential Mechanism
Primary HAL is an extremely rare tumor whose molecular mechanisms and genetic characteristics remain unclear. Although HAL has been reported for more than 50 years and concepted for 31 years, mainly single cases are reported, and research on the molecular mechanism and genomic profiling of HAL is scarce. Ishikura et al15 argued that the aberrant differentiation process may lead to the differentiation of certain adenocarcinomas tissues in the lung into hepatocytes because the respiratory system below the throat is similar to the liver and gastrointestinal tract and develops in the anterior intestine of the embryonic primitive digestive tract. Over time, a small amount of HACs was also found in organs that did not develop from the original digestive tract, such as endometrium60 and adrenal glands.61,62
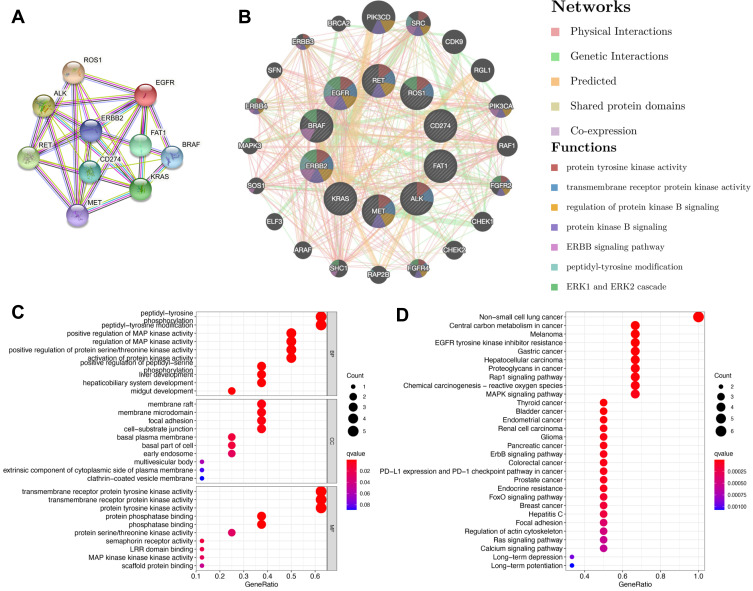
Recently, further understanding of the molecular mechanism of tumor biology has led to the development of tumor molecular targeted therapy, which exhibits improved target selectivity and decreased toxicity. A previous publication has reviewed that EGFR, ALK, ROS1, PD-L1, BRAF, HER2, KRAS, MET, RET, and FAT1 may be associated with the occurrence of HAL based on whole-genome sequencing analysis24 (Table 3). Furthermore, in addition to FAT1, other driver genes were detected as wild type.24 To better understand the biologic properties and potential therapeutic targets of this rare tumor, we utilized these potential oncogenes to further explore the biologic mechanisms of tumorigenesis based on several bioinformatics databases. Firstly, a protein-protein interaction (PPI) network was constructed with the STRING63 (https://string-db.org/) database to analyze the potential interactions among these oncogenes. The constructed PPI network includes a total of 10 nodes and 32 edges with a local clustering coefficient of 0.901 (Figure 8A). According to the results of GeneMania64 (http://genemania.org/), the oncogenes in the major classified functions of HAL are protein tyrosine kinase activity, transmembrane receptor protein kinase activity, regulation of protein kinase B signaling, protein kinase B signaling, ERBB signaling pathway, peptidyl-tyrosine modification, and ERK1 and ERK2 cascades (Figure 8B). Subsequently, TRRUST65 (version 2, https://www.grnpedia.org/trrust/) was used to investigate the potential transcription factor targets of these oncogenes. Transcription factors, including YBX1, TFAP2C, PGR, VDR, TFAP2A, ESR1, YY1, AR, SP1, and TP53, were predicted to be the potential targets of these oncogenes (Table 4). In order to better understand the functions of these potential oncogenes, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) distribution analyses were performed using the R Software. The results showed that the functional ontologies of these potential oncogenes in biological processes are mainly enriched in peptidyl-tyrosine phosphorylation, peptidyl-tyrosine modification, and positive regulation of MAP kinase activity (Figure 8C). For the category of cellular component, these potential oncogenes primarily cluster in membrane rafts, membrane microdomain, and focal adhesions. The functional ontologies of these potential oncogenes in molecular function are mainly enriched in transmembrane receptor protein tyrosine kinase activity, transmembrane receptor protein kinase activity, and protein tyrosine kinase activity (Figure 8C). In addition, the KEGG pathway enrichment analysis also demonstrated that these potential oncogenes are significantly enriched in the typical pathways of non-small cell lung cancer, central carbon metabolism in cancer, and HCC (Figure 8D). These results suggested that top significantly clustered GO function enrichment and KEGG pathways may serve important biological roles in the initiation and progression of HLA, providing new insights into the progression and potential immunotherapeutic pathways for HLA patients.
Table 3.
The Information of Hepatoid Adenocarcinoma of the Lung (HAL)-Related Oncogene from Previous Articles
| Genes | Original Name | Cytoband | Exon Count |
|---|---|---|---|
| EGFR | Epidermal growth factor receptor | 7p11.2 | 31 |
| ALK | ALK receptor tyrosine kinase | 2p23.2-p23.1 | 29 |
| ROS1 | ROS proto-oncogene 1, receptor tyrosine kinase | 6q22.1 | 46 |
| PD-L1 | CD274 molecule | 9p24.1 | 7 |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase | 7q34 | 23 |
| HER2 (ERBB2) | Human epithelial growth factor receptor 2 | 17q12 | 35 |
| KRAS | KRAS proto-oncogene, GTPase | 12p12.1 | 6 |
| MET | MET proto-oncogene, receptor tyrosine kinase | 7q31.2 | 24 |
| RET | Ret proto-oncogene | 10q11.21 | 20 |
| FAT1 | FAT atypical cadherin 1 | 4q35.2 | 29 |
Figure 8.
Potential biological functions of genes related to hepatoid adenocarcinoma of the lung. (A) Protein-protein interaction network for HLA-related genes using STRING. (B) Protein-function interaction network for HLA-related genes using GeneMANIA. (C) Gene Ontology functional enrichment for HLA-related genes. (D) Kyoto Encyclopedia of Genes and Genomes pathway for HLA-related genes.
Table 4.
Key Regulated Factor of Hepatoid Adenocarcinoma of the Lung (HAL)-Related Genes
| Key TF | Description | Overlapped Genes | P-value | FDR | |
|---|---|---|---|---|---|
| 1 | YBX1 | Y box binding protein 1 | EGFR, ERBB2, MET | 4.32E-07 | 4.32E-06 |
| 2 | TFAP2C | Transcription factor AP-2 gamma (activating enhancer binding protein 2 gamma) | ERBB2, RET | 1.14E-05 | 5.68E-05 |
| 3 | PGR | Progesterone receptor | EGFR, ERBB2 | 7.53E-05 | 2.51E-04 |
| 4 | VDR | Vitamin D (1,25- dihydroxyvitamin D3) receptor | EGFR, ERBB2 | 2.15E-04 | 5.38E-04 |
| 5 | TFAP2A | Transcription factor AP-2 alpha (activating enhancer binding protein 2 alpha) | EGFR, ERBB2 | 6.16E-04 | 1.18E-03 |
| 6 | ESR1 | Estrogen receptor 1 | EGFR, RET | 7.06E-04 | 1.18E-03 |
| 7 | YY1 | YY1 transcription factor | EGFR, ERBB2 | 1.01E-03 | 1.32E-03 |
| 8 | AR | Androgen receptor | EGFR, ERBB2 | 1.05E-03 | 1.32E-03 |
| 9 | SP1 | Sp1 transcription factor | EGFR, ERBB2, MET | 1.64E-03 | 1.82E-03 |
| 10 | TP53 | Tumor protein p53 | EGFR, MET | 3.23E-03 | 3.23E-03 |
Abbreviations: TF, transcription factor; EGFR, Epidermal growth factor receptor; ERBB2, Erb-b2 receptor tyrosine kinase 2; MET, MET proto-oncogene, receptor tyrosine kinase; RET, ret proto-oncogene; FDR, False discovery rate.
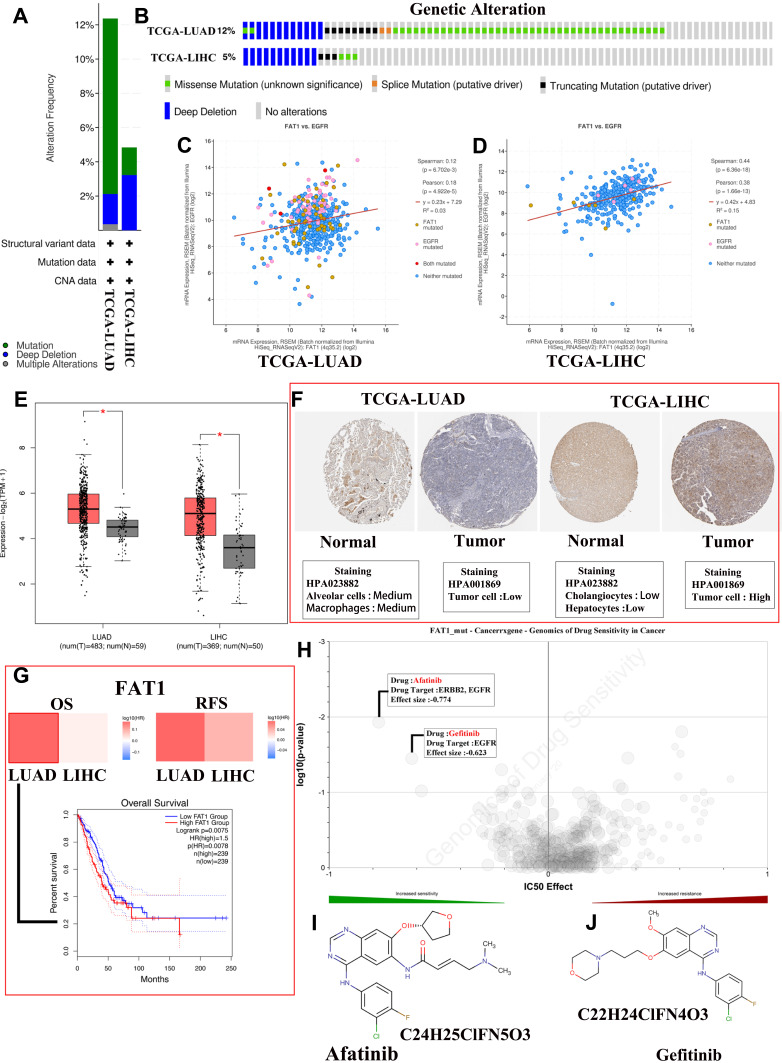
Literature review suggests that many gene mutations are implicated in the pathogenesis of neoplasms.66 Li et al24 found that the FAT1 gene is mutated in HAL tissues, with the mutation forms at c.3940T (Thymine)>A (Adenine). Because of the similarities of the tissue characteristics and pathologies of HLA, HCC, and lung adenocarcinoma (LUAD), the potential biological mechanisms of liver hepatocellular carcinoma (LIHC) and LUAD were discussed and compared together in this study. The cBioPortal database was used to explore the gene mutation information of FAT1 in LIHC (data from TCGA, PanCancer Atlas with 372 patients) and LUAD (data from TCGA, PanCancer Atlas with 566 patients). The FAT1 is altered in 12.37% of 566 cases and 4.84% of 372 cases in the sequencing data from TCGA-LUAD and TCGA-LIHC samples, respectively (Figure 9A). In addition, we found that missense mutation is the main type of mutated FAT1 in TCGA-LUAD, while deep deletion is the main type of mutated FAT1 in TCGA-LIHC (Figure 9B). EGFR is a potential cancer therapy target. Methods to inhibit EGFR activity and associated signal transduction have been intensively studied in several human malignant tumors, including HCC and LUAD. Thus, we explored the correlation between mutated FAT1 and EGFR expression in HCC and LUAD tissues through the cBioPortal database (Figure 9C and D). Furthermore, the expression levels of FAT1 were explored using GEPIA267 (http://gepia2.cancer-pku.cn/#index). Results showed that FAT1 is significantly up-expressed in LIHC and LUAD tissues with the p < 0.05 and log FC (fold change) = 0.585 as the significance threshold. (Figure 9E). Additionally, immunohistochemical staining was used to assess FAT1 expression level in normal liver and liver tumor tissues as well as normal lung and lung tumor tissues using Human Protein Atlas database (Figure 9F). Furthermore, we assessed the relationship of FAT1 expression with OS and relapse-free survival of HCC or LUAD patients. K-M curves display that high expression of FAT1 is related to shorter OS (p = 0.0078, HR = 1.5) of lung cancer patients (Figure 9G).
Figure 9.
Comprehensive analysis of mutated FAT1. (A) The alteration frequency with mutation type in liver hepatocellular carcinoma (LIHC) and lung adenocarcinoma (LUAD). (B) Summary of alterations in FAT1 expression in LIHC and LUAD. (C and D) Correlation between FAT1 mutation and EGFR mutation in LUAD (C) and LIHC (D). (E) Differential expression levels of FAT1 in LIHC and LUAD, *p < 0.05. (F) Immunohistochemical staining for FAT1 in normal liver and lung tissues as well as LIHC and LUAD tissues using Human Protein Atlas database. (G) The prognostic value of FAT1 in LIHC and LUAD patients. (H) Genomics of cancer drug sensitivity. (I and J) The chemical formula and structural formula of Afatinib (I) and Gefitinib (J).
Genetic mutations influence the clinical and drug responses to chemotherapy treatment, targeted therapy treatment, and immunotherapy. It is necessary to investigate the potential therapeutic agents and explore the underlying molecular mechanisms for HAL patients. Therefore, we evaluated the role of mutated FAT1 on chemotherapy or targeted therapy drug sensitivity and drug resistance of cancer cell lines from the GDSC68 (https://www.cancerrxgene.org/) dataset based on pan-cancer datasets. The results showed drug sensitivity toward Afatinib, Gefitinib, and MCT4_1422 (negative correlation with IC50) (Figure 9H) and drug resistance toward A-770041, NVP-TAE684, and Dasatinib (positive correlation with IC50) (Figure 7H). The chemical formulas of Afatinib and Gefitinib are C24H25ClFN5O3 and C22H24ClFN4O3, respectively, and their structural formulas are shown in Figure 9I and J. These results indicated that the mutated FAT1 may represent a potential therapeutic target and mediate the sensitivity to chemotherapy for patients with HAL.
Targeted therapy and immunotherapy are potential emerging therapeutic approaches for the treatment of several malignant tumors in recent years. Mounting evidence from studies has demonstrated that EGFR is expressed in most non-small cell lung carcinomas.69,70 Importantly, tyrosine kinase inhibitors (TKIs) targeting the kinase domain of EGFR have shown significant therapeutic efficacy in patients with EGFR mutations. Interestingly, EGFR mutations are also discovered in patients with HAL and respond to TKI therapy.2 Furthermore, our findings also suggested that HLA may be sensitive to the therapeutic effects of EGFR target-related drugs, such as Afatinib and Gefitinib. Platinum-based doublet chemotherapy and Sorafenib have also been used in HAL patients with wild-type EGFR and have shown a favorable prognosis.27 More molecular profiling of HAL is required to explore the therapeutic efficacy of targeted therapy with a TKI in HAL patients. KRAS mutation is another driver mutation found in HAL patients.16 KRAS has been considered undruggable tumor-related driver mutation in the past three decades,71 despite its well-recognized significance in the initiation and progression of lung cancer and continuous efforts to discover RAS-targeted anticancer therapies.
Programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) is a significant immune checkpoint modulatory signaling pathway that inhibits T cell activation by regulating Ras-Raf-MEK-ERK and PI3K-AKT axis.72 Tumor-associated immune cells and tumor microenvironment play a crucial role in the initiation and progression of various tumors. Immunotherapy based on the altered status of immune surveillance is emerging as the most promising cancer treatment strategy. Chen et al16 have reported that anti-PD-1 therapy is well-tolerated in HAL patients harboring a KRAS mutation. As a result, the patient with an unresectable HAL achieves a long-term survival benefit (OS: 52 months). Additionally, according to studies by Pasricha et al12 most HAL cases (4/6 patients) are positive for PD-L1 in immunohistochemical stains, and 2 cases are confirmed to have tumor proportion score of >50%. Interestingly, though the expression of PD-L1 is negative in IHC, anti-PD-L1 therapy may also show a promising response in clinical treatment.28 These results indicated that HAL patients may present a potential sensitivity to immunotherapy. However, more cases and clinical evidence are required to confirm these findings.
There are some limitations of the study. Firstly, the studies included were retrospective small-size studies, which means the argumentation intensity may not be strong enough, and only a hypothesis can be generated. We hope that there will be more large-scale RCTs in the future. Secondly, molecular mechanism and functions of potential oncogenes were under-researched.
Conclusions
In conclusion, HAC arising from the lung is a rare and aggressive extrahepatic adenocarcinoma that is extremely challenging to accurately diagnose preoperatively due to its histological features resemblance to metastatic HCC. Chemotherapy combined with surgical resection may prolong the survival of patients with HAL. We discussed the potential biological mechanisms based on the previously published studies and bioinformatics databases. These findings may raise some critical points worthy of further exploration.
Acknowledgments
The authors acknowledge the support of the Department of Radiology at Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College.
Funding Statement
There was no funding for this paper.
Abbreviations
HAL, Hepatoid adenocarcinoma of lung; HAC, Hepatoid adenocarcinoma; HCC, hepatocellular carcinoma; K-M, Kaplan-Meier; LUAD, lung adenocarcinoma; AFP, α-fetoprotein; PRISMA, Preferred Reporting Guidelines for Systematic Reviews and Meta-Analysis; OS, overall survival; CA, Carbohydrate antigen; CYFRA21-1, Cytokeratin-19-fragment; SCC, squamous cell carcinoma; CEA, Carcinoembryonic antigen; CT, computed tomography; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; FNA, fine-needle aspiration; MDT, multidisciplinary team; IHC, immunohistochemistry; MST, median survival time; BP, biological processes; CC, cellular component; MF, molecular function; LIHC, liver hepatocellular carcinoma; FC, fold change; RFS, relapse free survival; EGFR, Epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors; PD-1/PD-L1, Programmed cell death protein 1/programmed cell death ligand 1; TPS, Tumor proportion score; GO, Gene Ontology, KEGG, Kyoto Encyclopedia of Genes and Genomes.
Consent
Written permission to publish this case report was obtained from the patient.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Grossman K, Beasley MB, Braman SS. Hepatoid adenocarcinoma of the lung: review of a rare form of lung cancer. Respir Med. 2016;119:175–179. doi: 10.1016/j.rmed.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Chen HF, Wang WX, Li XL, et al. Hepatoid adenocarcinoma of the lung with EGFR mutation and the response to tyrosine kinase inhibitors. J Thorac Oncol. 2019;14(10):e217–e219. doi: 10.1016/j.jtho.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 3.Yang K, Jiang H, Li Q. Primary pulmonary hepatoid adenocarcinoma: a case report and review of the literature. Medicine. 2019;98(14):e15053. doi: 10.1097/md.0000000000015053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19(2):498–507. doi: 10.1007/s10120-015-0497-9 [DOI] [PubMed] [Google Scholar]
- 5.Ishikura H, Scully RE. Hepatoid carcinoma of the ovary. A newly described tumor. Cancer. 1987;60(11):2775–2784. doi: [DOI] [PubMed] [Google Scholar]
- 6.Hoshida Y, Nagakawa T, Mano S, Taguchi K, Aozasa K. Hepatoid adenocarcinoma of the endometrium associated with alpha-fetoprotein production. Int J Gynecol Pathol. 1996;15(3):266–269. doi: 10.1097/00004347-199607000-00012 [DOI] [PubMed] [Google Scholar]
- 7.Hwang JH, Song SH, Kim YH, et al. Primary hepatoid adenocarcinoma of the endometrium with a high alphafetoprotein level. Scott Med J. 2011;56(2):120. doi: 10.1258/smj.2011.011100 [DOI] [PubMed] [Google Scholar]
- 8.Friedman P, Lai JP. Liver metastasis of urothelial carcinoma with hepatoid features: an unusual morphological finding. Anticancer Res. 2017;37(2):801–804. doi: 10.21873/anticanres.11380 [DOI] [PubMed] [Google Scholar]
- 9.Yang C, Sun L, Lai JZ, et al. Primary hepatoid carcinoma of the pancreas: a clinicopathological study of 3 cases with review of additional 31 cases in the literature. Int J Surg Pathol. 2019;27(1):28–42. doi: 10.1177/1066896918783468 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Sun L, Li Z, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22(6):1183–1192. doi: 10.1007/s10120-019-00965-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33(5):263–269. doi: 10.1159/000305717 [DOI] [PubMed] [Google Scholar]
- 12.Pasricha S, Grover S, Kamboj M, et al. Hepatoid adenocarcinoma of lung: a diagnostic challenge - series of six cases with histopathological, predictive molecular and PD.L1 assessment. Indian J Pathol Microbiol. 2021;64(1):128–131. doi: 10.4103/ijpm.Ijpm_334_20 [DOI] [PubMed] [Google Scholar]
- 13.Chen JX, Lyu LL, Zhu WH, Chen XY, Zheng L. Hepatoid adenocarcinoma of the lung accompanied with multiple systemic metastases. Chin Med J. 2020;134(2):237–238. doi: 10.1097/cm9.0000000000000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990;417(1):73–80. doi: 10.1007/bf01600112 [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Han X, Gao Y, et al. Anti-PD-1 therapy achieved disease control after multiline chemotherapy in unresectable KRAS-positive hepatoid lung adenocarcinoma: a case report and literature review. Onco Targets Ther. 2020;13:4359–4364. doi: 10.2147/ott.S248226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haninger DM, Kloecker GH, Bousamra IM, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27(4):535–542. doi: 10.1038/modpathol.2013.170 [DOI] [PubMed] [Google Scholar]
- 18.Motooka Y, Yoshimoto K, Semba T, et al. Pulmonary hepatoid adenocarcinoma: report of a case. Surg Case Rep. 2016;2(1):1. doi: 10.1186/s40792-016-0129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okunaka T, Kato H, Konaka C, Yamamoto H, Furukawa K. Primary lung cancer producing alpha-fetoprotein. Ann Thorac Surg. 1992;53(1):151–152. doi: 10.1016/0003-4975(92)90778-3 [DOI] [PubMed] [Google Scholar]
- 20.Valentino F, Torchio M, Morbini P, Danova M. Synchronous presentation of hepatoid alpha-fetoprotein-producing lung cancer and colorectal adenocarcinoma. Tumori. 2012;98(5):130e–134e. doi: 10.1700/1190.13214 [DOI] [PubMed] [Google Scholar]
- 21.Fornasa F. Soft-tissue localization of hepatoid adenocarcinoma: first case report. Case Rep Oncol. 2010;3(2):212–217. doi: 10.1159/000317419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papatsimpas G, Kamposioras K, Goula K, et al. Hepatoid pancoast tumor. A case report and review of the literature. Lung Cancer. 2012;77(2):239–245. doi: 10.1016/j.lungcan.2012.05.102 [DOI] [PubMed] [Google Scholar]
- 23.Miyama Y, Fujii T, Murase K, Takaya H, Kondo F. Hepatoid adenocarcinoma of the lung mimicking metastatic hepatocellular carcinoma. Autops Case Rep. 2020;10(2):e2020162. doi: 10.4322/acr.2020.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Qi H, Xu B, et al. Genomic profiles of a patient of pulmonary hepatoid adenocarcinoma with high AFP level: a case report. Front Oncol. 2019;9:1360. doi: 10.3389/fonc.2019.01360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnould L, Drouot F, Fargeot P, et al. Hepatoid adenocarcinoma of the lung: report of a case of an unusual alpha-fetoprotein-producing lung tumor. Am J Surg Pathol. 1997;21(9):1113–1118. doi: 10.1097/00000478-199709000-00018 [DOI] [PubMed] [Google Scholar]
- 26.Hiroshima K, Iyoda A, Toyozaki T, et al. Alpha-fetoprotein-producing lung carcinoma: report of three cases. Pathol Int. 2002;52(1):46–53. doi: 10.1046/j.1440-1827.2002.01311.x [DOI] [PubMed] [Google Scholar]
- 27.Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. 2015;13(4):387–91;quiz 391. doi: 10.6004/jnccn.2015.0054 [DOI] [PubMed] [Google Scholar]
- 28.Basse V, Schick U, Guéguen P, et al. A mismatch repair-deficient hepatoid adenocarcinoma of the lung responding to anti-PD-L1 durvalumab therapy despite no PD-L1 expression. J Thorac Oncol. 2018;13(7):e120–e122. doi: 10.1016/j.jtho.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Yoshino I, Hayashi I, Yano T, Takai E, Mizutani K, Ichinose Y. Alpha-fetoprotein-producing adenocarcinoma of the lung. Lung Cancer. 1996;15(1):125–130. doi: 10.1016/0169-5002(96)00577-6 [DOI] [PubMed] [Google Scholar]
- 30.Carlinfante G, Foschini MP, Pasquinelli G, Scotti R, Cavazza A. Hepatoid carcinoma of the lung: a case report with immunohistochemical, ultrastructural and in-situ hybridization findings. Histopathology. 2000;37(1):88–89. doi: 10.1046/j.1365-2559.2000.00955-5.x [DOI] [PubMed] [Google Scholar]
- 31.Hayashi Y, Takanashi Y, Ohsawa H, Ishii H, Nakatani Y. Hepatoid adenocarcinoma in the lung. Lung Cancer. 2002;38(2):211–214. doi: 10.1016/s0169-5002(02)00214-3 [DOI] [PubMed] [Google Scholar]
- 32.Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27(10):1302–1312. doi: 10.1097/00000478-200310000-00002 [DOI] [PubMed] [Google Scholar]
- 33.Oshiro Y, Takada Y, Enomoto T, Fukao K, Ishikawa S, Iijima T. A resected case of metachronous liver metastasis from lung cancer producing alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II). Hepatogastroenterology. 2004;51(58):1144–1147. [PubMed] [Google Scholar]
- 34.Wu Z, Upadhyaya M, Zhu H, Qiao Z, Chen K, Miao F. Hepatoid adenocarcinoma: computed tomographic imaging findings with histopathologic correlation in 6 cases. J Comput Assist Tomogr. 2007;31(6):846–852. doi: 10.1097/RCT.0b013e318038f6dd [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto T, Yano T, Hiroshima K, Inayama Y, Kawachi K, Nakatani Y. A case of *-fetoprotein-producing pulmonary carcinoma with restricted expression of hepatocyte nuclear factor-4* in hepatoid foci: a case report with studies of previous cases. Hum Pathol. 2008;39(7):1115–1120. doi: 10.1016/j.humpath.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 36.Kitada M, Ozawa K, Sato K, et al. Alpha-fetoprotein-producing primary lung carcinoma: a case report. World J Surg Oncol. 2011;9:47. doi: 10.1186/1477-7819-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokrim M, Belbaraka R, Allaoui M, et al. Hepatoid adenocarcinoma of the lung: a case report and literature review. J Gastrointest Cancer. 2012;43(Suppl 1):S125–7. doi: 10.1007/s12029-011-9318-5 [DOI] [PubMed] [Google Scholar]
- 38.Khozin S, Roth MJ, Rajan A, et al. Hepatoid carcinoma of the lung with anaplastic lymphoma kinase gene rearrangement. J Thorac Oncol. 2012;7(11):e29–31. doi: 10.1097/JTO.0b013e3182697a23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin SF, Hsu WH, Chou TY. Primary pulmonary hepatoid carcinoma: report of a case and review of the literature. Kaohsiung J Med Sci. 2013;29(9):512–516. doi: 10.1016/j.kjms.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 40.Cavalcante LB, Felipe-Silva A, de Campos FPF, Martines J. Hepatoid adenocarcinoma of the lung. Autops Case Rep. 2013;3(1):5–14. doi: 10.4322/acr.2013.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Che YQ, Wang S, Luo Y, Wang JB, Wang LH. Hepatoid adenocarcinoma of the lung: presenting mediastinal metastasis without transfer to the liver. Oncol Lett. 2014;8(1):105–110. doi: 10.3892/ol.2014.2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaib W, Sharma R, Mosunjac M, Farris AB, El Rayes B. Hepatoid adenocarcinoma of the lung: a case report and review of the literature. J Gastrointest Cancer. 2014;45(Suppl 1):99–102. doi: 10.1007/s12029-013-9558-7 [DOI] [PubMed] [Google Scholar]
- 43.Sun JN, Zhang BL, Li LK, Yu HY, Wang B. Hepatoid adenocarcinoma of the lung without production of α-fetoprotein: a case report and review of the literature. Oncol Lett. 2016;12(1):189–194. doi: 10.3892/ol.2016.4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian GQ, Yin FY, Li GX, Chu JG. Hepatoid adenocarcinoma of the lung. Qjm. 2016;109(9):619–620. doi: 10.1093/qjmed/hcw107 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Li M, Chen H, Li J, Zeng Q. FDG PET/CT in hepatoid adenocarcinoma of the lung. Clin Nucl Med. 2016;41(7):e340–1. doi: 10.1097/rlu.0000000000001231 [DOI] [PubMed] [Google Scholar]
- 46.Valle L, Thomas J, Kim C, et al. Hepatoid adenocarcinoma of the lung metastasizing to the tonsil. Mol Clin Oncol. 2017;6(5):705–707. doi: 10.3892/mco.2017.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayub A, Nunez Lopez O, Booth A, Okereke I. Pulmonary hepatoid adenocarcinoma. J Thorac Cardiovasc Surg. 2019;158(4):e139–e140. doi: 10.1016/j.jtcvs.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 48.Kuan K, Khader SN, El Hussein S. Hepatoid adenocarcinoma of the lung. Diagn Cytopathol. 2019;47(8):831–833. doi: 10.1002/dc.24195 [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Xu G, Wu G, et al. Hepatoid adenocarcinoma of the lung metastasizing to the gingiva. Onco Targets Ther. 2019;12:8765–8768. doi: 10.2147/ott.S222974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi YF, Lu JG, Yang QM, et al. Primary hepatoid adenocarcinoma of the lung in Yungui Plateau, China: a case report. World J Clin Cases. 2019;7(13):1711–1716. doi: 10.12998/wjcc.v7.i13.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, Li G. Radiotherapy of pulmonary hepatoid adenocarcinoma with intrahepatic hemangioma: a case report. Onco Targets Ther. 2020;13:11947–11955. doi: 10.2147/ott.S275340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muroyama Y, Tamiya H, Tanaka G, et al. Alpha-fetoprotein-producing lung hepatoid adenocarcinoma with brain metastasis treated with S-1. Case Rep Oncol. 2020;13(3):1552–1559. doi: 10.1159/000511763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tonyali O, Gonullu O, Ozturk MA, Kosif A, Civi OG. Hepatoid adenocarcinoma of the lung and the review of the literature. J Oncol Pharm Pract. 2020;26(6):1505–1510. doi: 10.1177/1078155220903360 [DOI] [PubMed] [Google Scholar]
- 54.Schabath MB, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1563–1579. doi: 10.1158/1055-9965.Epi-19-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 56.Corlin R, Tompkins R. Serum alpha-fetoglobulin in a patient with hepatic metastasis from brobchogenic carcinoma. Am J Dig Dis. 1972;17:533–535. doi: 10.1007/BF02231210 [DOI] [PubMed] [Google Scholar]
- 57.Zhou K, Wang A, Ao S, et al. The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis. BMC Cancer. 2020;20(1):671. doi: 10.1186/s12885-020-07031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou Z, Xie J, Zhang L, Dai G, Chen Y, He L. Hepatoid adenocarcinoma of the lung: a systematic review of the literature from 1981 to 2020. Front Oncol. 2021;11:702216. doi: 10.3389/fonc.2021.702216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun H, Li X, Zhang J, Liu Y. Clinicopathological features and genomic profiles of hepatoid adenocarcinoma of the lung: report of four cases. Pathol Res Pract. 2022;229:153652. doi: 10.1016/j.prp.2021.153652 [DOI] [PubMed] [Google Scholar]
- 60.Yin H, Hu Y, Xiu Y, Luo R, Shi H. A rare case of primary endometrial hepatoid adenocarcinoma on 18F-FDG PET/CT. Clin Nucl Med. 2019;44(7):e425–e427. doi: 10.1097/rlu.0000000000002538 [DOI] [PubMed] [Google Scholar]
- 61.Deng X, Jin Y, Yang W, et al. An adrenal hepatoid adenocarcinoma with left renal vein thrombosis extending into the inferior vena cava. Urol J. 2019;16(5):511–514. doi: 10.22037/uj.v0i0.5250 [DOI] [PubMed] [Google Scholar]
- 62.Lin J, Cao Y, Yu L, Lin L. Non-α-fetoprotein-producing adrenal hepatoid adenocarcinoma: a case report and literature review. Medicine. 2018;97(39):e12336. doi: 10.1097/md.0000000000012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–d613. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franz M, Rodriguez H, Lopes C, et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46(W1):W60–w64. doi: 10.1093/nar/gky311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46(D1):D380–d386. doi: 10.1093/nar/gkx1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z, Zhang L, Huang J, et al. Targeted-gene sequencing and bioinformatics analysis of patients with pancreatic mucoepidermoid carcinoma: a case report and literature review. Onco Targets Ther. 2021;14:3567–3581. doi: 10.2147/ott.S305248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–w560. doi: 10.1093/nar/gkz430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Databaseissue):D955–61. doi: 10.1093/nar/gks1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye L, Mesbah Ardakani N, Thomas C, et al. Detection of low-level EGFR c.2369 C > T (p.Thr790Met) resistance mutation in pre-treatment non-small cell lung carcinomas harboring activating EGFR mutations and correlation with clinical outcomes. Pathol Oncol Res. 2020;26(4):2371–2379. doi: 10.1007/s12253-020-00833-z [DOI] [PubMed] [Google Scholar]
- 70.Simone G, Mangia A, Malfettone A, et al. Chromogenic in situ hybridization to detect EGFR gene copy number in cell blocks from fine-needle aspirates of non small cell lung carcinomas and lung metastases from colo-rectal cancer. J Exp Clin Cancer Res. 2010;29(1):125. doi: 10.1186/1756-9966-29-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z, Liang SQ, Saliakoura M, et al. Synergistic effects of FGFR1 and PLK1 inhibitors target a metabolic liability in KRAS-mutant cancer. EMBO Mol Med. 2021;e13193. doi: 10.15252/emmm.202013193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796 [DOI] [PMC free article] [PubMed] [Google Scholar]