Summary
Background
Ebola virus disease (EVD) outbreaks in West Africa (2013-2016) and the Democratic Republic of Congo (2018-2020) have resulted in thousands of EVD survivors who remain at-risk for survivor sequelae. While EVD survivorship has been broadly reported in adult populations, pediatric EVD survivors are under-represented. In this cross-sectional study, we investigated the prevalence of eye disease, health-related quality-of-life, vision-related quality-of-life, and the burden of mental illness among pediatric EVD survivors in Sierra Leone.
Methods
Twenty-three pediatric EVD survivors and 58 EVD close contacts were enrolled. Participants underwent a comprehensive ophthalmic examination and completed the following surveys: Pediatric Quality of Life Inventory Version 4.0, Effect of Youngsters Eyesight on Quality-of-Life, and the Revised Child Anxiety and Depression Scale.
Findings
A higher prevalence of uveitis was observed in EVD survivor eyes (10·8%) cohort compared to close contacts eyes (1·7%, p=0·03). Overall, 47·8% of EVD survivor eyes and 31·9% of close contact eyes presented with an eye disease at the time of our study (p=0·25). Individuals diagnosed with an ocular complication had poorer vision-related quality-of-life (p=0·02).
Interpretation
Both health related quality-of-life and vision-related quality-of-life were poor among EVD survivors and close contacts. The high prevalence of eye disease associated with reduced vision health, suggests that cross-disciplinary approaches are needed to address the unmet needs of EVD survivors.
Funding
National Institutes of Health R01 EY029594, K23 EY030158; National Eye Institute; Research to Prevent Blindness (Emory Eye Center); Marcus Foundation Combating Childhood Illness; Emory Global Health Institute; Stanley M. Truhlsen Family Foundation.
Keywords: Ebola virus disease, Ebola, Emerging infectious diseases, Mental health, Psychosocial stressors, Uveitis, Post-Ebola virus disease syndrome
Research in context.
Evidence before the study
We searched for studies that had investigated the ocular complications, quality of life impact of Ebola virus disease (EVD) sequalae among pediatric (<18 years old) survivors. We searched for PubMed indexed articles that were published by January 2021. The search algorithm utilized terms related to pediatric Ebola virus disease survivorship (“Ebola” or “Ebola virus disease”) and (“survivors” or “Pediatric survivors” or “child survivors”); Ebola sequalae (“sequelae” or “complication” or “Post-Ebola Syndrome”); and our specific sequalae of interest (“Eye disease” or “Uveitis” or “Quality-of-life” or “mental health” or “Psychosocial Health”); Study setting (“Sierra Leone”, Democratic Republic of the Congo”, “Liberia”, “Guinea”). To date, The Prevail III study group has enrolled the largest cohort of EVD survivors of the 2013-2016 West African Ebola outbreak (966 EDV survivor and 2350 EVD negative close contacts). They found an initial prevalence of uveitis at enrollment, 26·4% among EVD Survivors vs. 12·1% of close contacts; at year 1, 33·3% vs. 15·4%. However, the PREVAIL III study group did not specifically report the vision-related quality of life or health-related quality of life related to eye disease. Furthermore, pediatric populations were included in the study, but rates of uveitis exclusive to pediatric survivors was not presented. A recent study of EVD child survivors(n=159) and their close contacts(n=303) were enrolled in Western and Eastern Sierra Leone. While they found a statistically significant increased odds of “mood changes”, “photophobia”, “eye pain”, and “eye redness” among pediatric EVD survivors compared to close contacts, there was no ophthalmic exam performed making it difficult to assess rates of eye complication.
Added value of this study
Based upon our literature review, this is the first study that focuses on the ophthalmic manifestations of pediatric EVD patients in association with the multifaceted needs of pediatric EVD patients. As such, we assessed the spectrum of eye disease including uveitis and present the novel finding of vernal keratoconjunctivitis associated with EVD. We also report the relationship ocular disease on vision-related quality-of-life, overall health-related quality-of-life, and assess mental health measures including anxiety and depression in child and parent reports.
Implications of all the available evidence
The available evidence suggests that are many pediatric EVD survivors are either already suffering or at-risk of potentially vision-threatening eye disease that contributes to vision disability and reduced quality of life. These findings highlight the long-term challenges of Ebola virus disease, findings that extend to relationships with other emerging infectious diseases that are relevant long past survival from acute infectious disease illness. Our study findings suggest the need for systematic, multifaceted approach to provide long-term eye care and mental health services following an EVD outbreak. Given the magnitude of the EVD outbreak in West Africa and the Democratic Republic of the Congo (DRC) previously, as well as recent sporadic outbreaks in West Africa and the DRC, this study highlights the ongoing need to continue gathering evidence for clinical service, education, and a comprehensive understanding of eye disease burden.
Alt-text: Unlabelled box
Introduction
Ebola virus disease (EVD), COVID-19 and other emerging infectious diseases continue to threaten our global health security and require a multifaceted approach to address the array of patient needs during survivorship. Following the largest EVD outbreak in history within West Africa (2013-2016), which resulted in over 28,600 cases and 11,300 deaths,1 the Democratic Republic of Congo (2018-2020) confronted the second largest outbreak with 3,814 cases and 2,299 deaths.2 The unprecedented scale of recent EVD outbreaks has resulted in a large cohort of pediatric EVD survivors that requires greater understanding of clinical care needs for EVD sequelae.
EVD survivors have presented with a wide variety of post Ebola sequelae with symptoms including arthralgia, uveitis, mental health, and psychosocial stressors.3, 4, 5, 6, 7, 8 The diversity of symptoms reported by EVD survivors has been termed post-Ebola virus disease syndrome (PEVDS). Uveitis, a potentially blinding eye disease, is one of the most common ocular manifestations of PEVDS, affecting 13-34% of EVD survivors.9, 10, 11, 12
Mental health disorders including anxiety and depression are commonly reported comorbidities during acute EVD infection and during convalescence13,14 PEVDS can be particularly debilitating to children who have faced a daunting illness, isolation from Ebola treatment units (ETU), and the potential loss of guardians.15 The combination of psychosocial stressors in pediatric-aged patients and high rate of uveitis observed in adult EVD survivor cohorts raise questions about not only eye disease, but also their interplay in child EVD survivors.
In this cross-sectional study of pediatric subjects in Sierra Leone, we assessed the prevalence of eye disease in EVD survivors and close contacts. We further evaluated potential relationships of eye disease with vision health related quality-of-life and the prevalence of mental illness.
Methods
A cross-sectional observational study was performed to assess pediatric EVD survivors and close contacts of EVD patients at the Lowell and Ruth Gess Kissy Eye Hospital in Freetown, Sierra Leone in April 2018. Institutional Review Board approval was obtained from Emory University and the Office of Ethics and Scientific Review Committee, Sierra Leone Ministry of Health and Sanitation. Human subject research was conducted according to the Tenets of the Declaration of Helsinki, and informed consent was obtained with the assistance of Sierra Leonean interpreters. Patients enrolled in this study underwent a medical history, a complete ocular examination, and completion of multiple surveys in child and parent/ guardian: Pediatric Quality of Life Inventory Version 4·0 (PedsQL), Effect of Youngsters Eyesight on Quality-of-Life (Eye-Q) and the Revised Child Anxiety and Depression Scale (RCADS).
Patient recruitment and evaluation
Pediatric patients were recruited from an EVD survivor network in partnership with the Sierra Leone Association of Ebola Survivors (SLAES) and Survivor Health Advocates (SHA), comprised largely of EVD survivors who advocated for the health care for EVD survivors and in communication with the Ministry of Health, and World Health Organization representatives
A cohort of EVD survivors and pediatric close contact controls were invited to participate from Western Area Urban, Western Area Rural and Port Loko districts in Sierra Leone. These districts were selected given their proximity to Freetown. In both the pediatric EVD survivor group and close contact group, inclusion criteria included: 1) Parent or guardian for informed consent; 2) Age less than 18 years-old at study recruitment. EVD survivor status was confirmed with survivor certificates, Ebola serum IgG, and a history of EVD requiring ETU admission.16,17
Data collection
Data collected included past medical and ocular history, ETU admission and discharge dates, ocular and systemic symptoms during acute EVD. An ophthalmic examination consisting of visual acuity (i.e., Snellen VA or tumbling “E” chart depending on age and literacy), pupil examination, extraocular motility, visual fields, intraocular pressure measurement (Reichert Technologies, Depew, NY), slit lamp examination, and dilated fundus examination with indirect ophthalmoscopy, were performed.
Questionnaires
Health related quality of life (HRQL) was evaluated with the Pediatric Quality of Life Inventory Version 4·0 (PedsQL).17 Children aged 5-18 independently completed the questionnaire. A parent proxy report was also completed for children aged 2-18. PedsQL is a 23-item questionnaire that assessed quality of life in 4 domains: 1) Physical function; 2) Emotional function; 3) Social function; 4) School function. Scores range from 0-100 with higher scores indicating better HRQL. Impaired levels are defined by each domain: Physical Functioning (HRQL score ≤ 72·98), Emotional Functioning (HRQL score ≤ 59·57), Social Functioning (HRQL score ≤ 66·61, School Functioning (HRQL score ≤ 62·99), and Total Score (HRQL score ≤ 69·71) on the PedsQL™ Generic Core Scales.18, 19, 20
The Revised Child Anxiety and Depression Scale (RCADS) is a 25-item questionnaire developed to assess symptoms of anxiety disorders and depression in children aged 6-18.21 T scores ≥ 65 were considered at risk for a mental health disorder and needed further evaluation by a mental health provider. A T-score ≥ 70 was indicative of a mental health disorder and required prompt treatment. Individuals with potential mental health disorders were referred to the appropriate mental health counselors and care providers.22
Effect of Youngsters Eyesight on Quality-of-Life (EYE-Q) was utilized to evaluate vision related quality of life (VRQOL) and vision-related functioning (VRF). EYE-Q is composed of parent reports and patient self-reports for children ≥8 years of age. Children and parent proxies completed a 23-item questionnaire that asked parents to reflect upon their child's ability to carry out a variety of visual tasks including: 1) Distance vision; 2) Near vision; 3) Color vision; 4) Night vision; and 5) Photosensitivity. This tool is scored on a scale of 0-100, with higher scores corresponding to better VRQOL or VRF.23, 24, 25, 26
Questionnaires were administered to children in the EVD survivor and close contact groups who underwent ophthalmic exam. The questionaries were verbally read by a study team member with interpretation by the EVD SHA in Krio or dialect as appropriate and the respondent would reply verbally. A similar process occurred for the corresponding parent/guardian. In instances where there was a group home or multiple children cared for by one guardian, one guardian may have completed surveys for multiple children. Administration of questionaries were overseen by a health care provider with mental health counseling training.
Data analysis
Data was displayed as frequencies or means with standard deviations (SD) for continuous variables. The level of visual acuity in EVD survivors and close contacts were divided into normal or mild vision impairment (20/40 or better), moderate vision impairment (20/50 to 20/200) and blindness (20/400 or worse).27 Visual acuities were then converted to logarithm of the minimum angle of resolution (LogMAR) for continuous data analysis. The following logMAR visual acuities without a decimal visual acuity notated were converted as follows: Counting fingers – logMAR 2·3; Hand motions – LogMAR 3·0. Light perception and no light perception were excluded from logMAR analysis given that a geometric visual angle cannot be attributed to these measures. For patients unable to undergo standard visual acuity, the central, steady, and maintained (CSM) notation was utilized but were excluded from continuous analysis measures.
The prevalence of ocular disease in pediatric EVD survivors was compared to close contacts using a Fisher's exact test. We utilized an unpaired t-test to compare means for PedsQL, RCADS, and EYE-Q questionnaires between EVD survivors and close contacts. Descriptive and univariate analyses were carried out using Microsoft Excel (v2013, Redmond, WA), SPSS (v24, Chicago, IL), and SAS (v9·4, Cary, NC). All tests were two-sided and a p-value < 0·05 was considered statistically significant for all comparisons.
Role of funding source: Our funding sources played no role in the study design, data collection, data analysis, nor the writing of this paper. All study authors had access to the data set and concurred with the decision to submit for publication
Results
Patient demographic and cohort characteristics
Eighty-one pediatric patients were enrolled at the Lowell and Ruth Gess Eye Hospital. Twenty-three pediatric EVD survivors with a documented history of clinical EVD and prior Ebola virus exposure was assessed with Ebola IgG serology. Fifty-eight close contact controls who were first-degree relatives of EVD patients and/or household contacts of EVD patients were recruited. All close contact controls were confirmed to be Ebola virus IgG seronegative. Among EVD survivors, 13 had confirmed positive EVD IgG serology, 1 was unable to be tested, and the remaining 9 tested negative for EVD serology. In patients with a negative Ebola IgG serology, survivor certificates were used to verify EVD survivor status. In the EVD survivor cohort, 14 patients (60·9%) were male and comparable to the close-contact cohort in which 26 patients (44·8%) were male. The mean age was younger in the close contact population with ages of 11·5 and 9·0 years in EVD survivors and close contacts, respectively (p=0·03, Table 1).
Table 2.
Summary table - patient reported outcome measures in an ebolavirus disease pediatric cohort.
| Parent - Report |
Child- Report |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EYE-Q Parent, mean (SD) |
RCADS Total, mean (SD) | PedsQL Total, mean (SD) | EYE-Q Child, mean (SD) |
RCADS Total, mean (SD) | PedsQL Total, mean (SD) | |||||
| EYE-Q Total | Visual Function | Uveitis-related QOL | EYE-Q Total | Visual Function | Uveitis-related QOL | |||||
| Cohort | ||||||||||
| N=10 | N=10 | N=10 | N=11 | N=11 | N=16 | N=16 | N=16 | N=18 | N=16 | |
| EVD Survivor | 49·14 (25·10)a | 48·27 (28·15)b | 45·78 (23·93)c | 62·00 (8·92)d | 60·73 (14·40)e | 60·10 (26·21)f | 59·33 (27·31)g | 54·98 (28·20)h | 49·36 (8·61)i | 67·32 (13·37) j |
| N=27 | N=27 | N=27 | N=32 | N=32 | N=33 | N=33 | N=33 | N=46 | N=42 | |
| Close Contact | 58·56 (21·12) | 57·56 (23·25) | 55·32 (24·94) | 58·63 (9·92) | 66·18 (16·97) | 63·21 (20·0) | 63·80 (21·30) | 58·61 (24·75) | 45·15 (6·74) | 71·61 (13·56) |
NOTE:
Group Differences (T Score = 1·06 p = 0·15);
Group Differences (T Score= 0·93 p= 0·18);
Group Differences (T score= 1·06 p= 0·15);
Group Differences (T Score = -0·99, p = 0·33);
Group Differences (T Score = 0·974, p = 0·17);
Group Differences (T Score 0·419 p = 0·34);
Group Differences (T score= 0·487 p:0·58);
Group Differences (T score=0·414 p=0·34)
Group Differences (T Score = -2·05, p = 0·045);
Differences between emotional functioning between groups (T Score = 1·09, p = 0·14).
Table 1.
Demographic and ophthalmic clinical features of pediatric EVD survivors and close contacts.
| Characteristics, N (%) | EVD Survivor(n = 23)a | Close Contact(n = 58)b | P-value (Fisher's exact unless notedd) |
|---|---|---|---|
| Age, Mean (SD) | 11·5 (3·8) | 9·1 (4·3) | 2·01 (0·03)d |
| Male (%) | 14·0 (60·9) | 26·0 (44·8) | 0·53 |
| Sierra Leonean (%) | 23·0 (100·0) | 58 (100·0) | 1·0 |
| Ocular Characteristics | |||
|---|---|---|---|
| Worst Eye LogMar VA, Median (IQR) | 0·0 (0·3) | 0·30 (0·18) | 2·0 (0·36)d |
| Best Eye LogMar VA, Mean (IQR) | 0·0 (0·2) | 0·11 (0·18) | 1·8 (0·48)d |
| Mild vision loss (%)c | 1 (10·9) | 3 (17·2) | 1·0 |
| Moderate vision loss (%)c | 5 (10·9) | 12 (10·3) | 0·44 |
| Legal Blindness (%)c | 3 (6·5) | 6 (5·2) | 0·48 |
| Abnormal Eye Motility (%) | 3 (6·5) | 5 (4·3) | 0·69 |
| Ocular Complication (%) | 22 (47·8) | 37 (31·9) | 0·25 |
| Vernal Keratoconjunctivitis (%) | 10 (21·7) | 11 (9·4) | 0·08 |
| Corneal Scar (%) | 5 (10·9) | 7 (6·0) | 0·33 |
| Glaucoma or Glaucoma Suspect (%) | 3 (6·5) | 3 (2·6) | 0·36 |
| Uveitis (%) | 5 (10·8) | 2 (1·72) | 0·03 |
| Synechiae (%) | 2 (4·3) | 1 (0·86) | 0·20 |
| Cataracts (%) | 1 (2·2) | 1 (0·86) | 0·50 |
| Corneal Edema (%) | 1 (2·2) | 1 (0·86) | 0·50 |
| Embyrotoxon (%) | 1 (2·2) | 2 (1·72) | 1·0 |
| Other (%) | 3 (6·5) | 9 (7·8) | 1·0 |
Ocular complications are reported by eyes
46 total eyes examined among EVD survivors;
116 eyes examined among EVD close contacts;
The International Classification of Diseases 11 (2018);
t-Test: unpaired Two-Sample Assuming Unequal Variances.
Ophthalmic manifestations and visual acuity
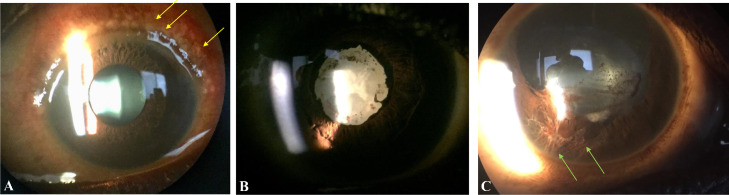
Ophthalmic diagnosis of any type was observed in 47·8% and 31·9 % (p=0·25) of EVD survivor eyes and close contact eyes, respectively. A higher prevalence of uveitis was observed in EVD survivors (5 eyes, 10·8%) compared to close contacts (2 eyes, 1·72%, p=0·03). Interestingly, vernal keratoconjunctivitis was observed at a greater rate in survivors (10 of 46 eyes, 21·7%) compared to close contacts (11 of 116 eyes, 9·4%, p=0·08). When assessing the entire cohort, anterior segment disease was particularly notable with vernal keratoconjunctivitis being the most common diagnosis (n=21 eyes, 13·0%) followed by corneal scarring (n=12 eyes, 7·4%) and glaucoma (n=6 eyes, 3·7%). Patients with vernal keratoconjunctivitis showed moderate to severe conjunctival injection with Horner-Trantas dots, inflammatory aggregates circumscribing the cornea, which can be associated with corneal scarring (Figure 1). Patients with uveitis showed posterior synechiae, lens pigment, iris atrophic change, and associated cataract (Figure 1).
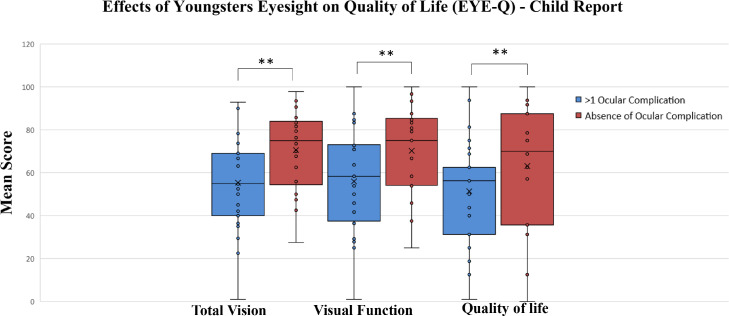
Figure 2.
Relationship of ocular complications (presence or absence) to total vision score, visual function score, and vision related quality of life score. Box and whisker plots denote median with interquartile range (Q1, Q3; Box) and minimum and maximum values (Whiskers) for total vision score, visual function score, and vision related quality of life scores. EVD survivors and close contacts who showed at least one ocular complication showed reduced total vision score, visual function score, and vision related quality of score compared to individuals without an ocular complication. Note: ** indicates statistically significant difference between mean scores (p<0·05).
Figure 1.
Representative slit lamp photos of EVD survivors. (A) EVD survivor with vernal keratoconjunctivitis with diffuse conjunctival injection and characteristic Horner-Trantas dots, inflammatory aggregates in circumlimbal distribution (yellow arrows). (B) EVD survivor with dense white cataractous lens with surrounding posterior synechiae form the iris to the lens and pigment on the lens capsule. The patient is legally blind from the cataract associated with uveitis. (C) EVD close contact with a history of severe ocular trauma, dense adhesions from the iris to the cornea (green arrows), posterior synechiae and pigment on the lens capsule.
VA were compared between cohorts by better seeing and worse seeing eyes to document the more functional eye and less functional, diseased eye, respectively. Within the EVD survivor cohort, the mean VA in the better seeing eye was LogMAR 0·0 (SD: 0·2, Snellen visual acuity 20/20). Five eyes (10·9%) showed moderate vision loss visual acuity equal to or poorer than 20/50 to 20/200 and 3 eyes (6·5%) demonstrated visual acuity worse than 20/200, meeting the case definition for legal blindness based on WHO criteria.26 There were 3 eyes with particularly poor vision at HM, LP and NLP vision in one eye of each of 3 EVD survivors, but normal vision in the contralateral eye. Diagnoses included uveitis in two and corneal scar in one patient.
Within the close-contact population, the mean vision in the better seeing eye was LogMAR 0·11 (SD: 0·18, Snellen equivalent 20/25). No difference was observed when comparing VA in the better seeing eye of EVD survivors versus close contacts (p=0·48). Twelve eyes (10·3%) demonstrated moderate visual acuity loss within the 20/50 to 20/200 range and 6 eyes (5·2%) showed 20/400 or poorer vision.
Pediatric quality of life inventory version 4.0 (PedsQL)
The PedsQL survey was completed by 16 EVD survivors (70%) and 42 EVD close contacts (72%) with corresponding parent surveys for 11 EVD survivors (48%) and 32 close contacts (55%), respectively (Table 3). The average total PedsQL score for pediatric EVD survivors was 67.32 (SD: 13.37) compared to close contact score of 71·61 (SD: 13·56, p=0.14 for comparison between EVD survivors and close contacts). For parent reported scores, averages were 60·73 (SD: 14·40) in EVD survivors and 66·18 (SD: 16·97) in close contacts, respectively (p=0·17). In the PedsQL report in children, EVD survivors demonstrated lower scores across all domains of physical, emotional, social and school functioning, and psychosocial health. The differences between pediatric EVD survivors and close contacts approached statistical significance in emotional functioning (T-score 1·61, p=0·06) and school functioning (T-score 1·52, p=0·07).
Table 3.
PedsQL scores in EVD survivors and close contacts.
| PedsQL – Children Report | EVD Survivor(n =16) Mean, (SD) | Close Contact (n = 42)Mean, (SD) | T-Score (p-value) |
|---|---|---|---|
| Physical Functioning | 73·54 (17·67) | 75·96 (18·85) | 0·595 (0·28) |
| Emotional Functioning | 63·20 (11·62) | 69·14 (14·49) | 1·61 (0·06) |
| Social Functioning | 69·38 (16·32) | 73·75 (18·3) | 0·88 (0·19) |
| School Functioning | 57·97 (17·16) | 65·36 (13·15) | 1·52 (0·07) |
| Psychosocial Health | 63·96 (13·31) | 69·35 (13·15) | 1·31 (0·10) |
| Total Score | 67·32 (13·37) | 71·61 (13·56) | 1·09 (0·14) |
| PedsQL- Parent Report | EVD Survivor (n =11)Mean, (SD) | Close Contact (n = 32)Mean, (SD) | T-Score (p-value) |
|---|---|---|---|
| Physical Functioning | 63·83 (20·72) | 71·58 (20·52) | 1·12 (0·14) |
| Emotional Functioning | 57·68 (11·63) | 68·40 (17·39) | 2·24 (0·02) |
| Social Functioning | 60·63 (23·06) | 64·63 (21·96) | 0·398 (0·35) |
| School Functioning | 60·0 (15·81) | 59·68 (17·05) | -0·276 (0·39) |
| Psychosocial Health | 59·64 (13·48) | 63·15 (16·58) | 0·681 (0·25) |
| Total Score | 60·73 (14·40) | 66·18 (16·97) | 0·974 (0·17) |
Further relationships were observed in the emotional functioning domain of the child and parent report. The pediatric EVD survivor and parent surveys showed a lower level of emotional functioning compared to close contacts. Specifically, in the child report, the mean emotional functioning was 63·20 (SD:11·62) in EVD survivors and 69·14 (SD: 14·49) in the close contacts (Table 3, p=0·05). In the child report, one specific question contributing to this difference in emotional function was the question “I feel sad or blue”, with EVD survivors scores being significantly lower than close contacts (51·56 vs 63·69 p=0·02). In addition, “I have trouble sleeping” was significantly lower in EVD survivor children compared to close contacts (70·31 vs 85·12, p=0·04). The parent report mean score in emotional functioning within EVD survivors was 56·79 (SD: 9·33) and lower than the score of 68·00 (SD: 19·45), (p=0·03) within the close contacts. The primary driver of this difference in the parent report was the statement, “I feel sad or blue”, which was substantially lower in EVD survivor compared to close contact parents (48·21 vs 65·70, p=0·04).
Effect of youngsters eyesight on quality-of-life (EYE-Q)
The EYE-Q was completed by 49 children including 16 Ebola survivors (70%) and 33 close contacts (57%). The parent survey was completed by 37 parents total including 10 parents of EVD survivors and 27 parents of close contacts. Total vision related quality of life (VRQOL) scores were 60·10 (SD: 26·21) and 63·21 (SD:21·30) for child EVD survivors and close contacts, respectively (p=0·34). Assessment of parent EYE-Q showed a lower score in EVD survivor parents with a mean of 49·14 (SD: 25·10) compared to 58·56 (SD: 21·12) in the parent close contact cohort (p=0·15 for comparison, Supplemental Table 4).
We also evaluated VRQOL in all pediatric patients enrolled (EVD survivors and close contacts), stratified by those patients with and without an ocular complication and observed significantly lower EYE-Q/VRQOL scores in patients with an ocular complication in the child report 55·35 (SD: 22·44) compared to those without an ocular complication with a mean score of 70·58 (SD: 18·65, p=0·01, Supplemental Table 1). Vision function scores were also significantly lower in subjects with an ocular complication group (55·94, SD: 23·72 in patients with an ocular complication compared to 70·20, SD: 20·45 in patients without an ocular finding, p=0·01). A lower VRQOL was observed in children with an ocular complication with a score of 51·39 (SD: 23·78) compared to 66·62 (SD: 24·62, p=0·02). No differences were observed for EYE-Q scores in the parent reports when comparing pediatric EVD survivors with close contacts for total vision and visual function scores (p>0·05 for all comparisons).
The revised child anxiety and depression scale (RCADS)
The RCADS tool was completed by 64 children which included 18 EVD survivors (78%) and 46 close contacts (79%). Parent reports were completed by 43 parents of whom 11 were parents of EVD survivors and 32 were parents of close contacts. In the entire child cohort (i.e., EVD survivors and close contacts), the mean total score for children was 46·37 with a mean anxiety subscale of 48·97 and a mean depression subscale of 43·72. In EVD survivor children, RCADs scores were higher with a mean total score, mean anxiety sub-scale, and mean depression subscale measuring 49·36, 51·62, and 46·62, respectively (Supplemental Table 1). In pediatric close contacts, the mean scores were lower with a mean total score, mean anxiety sub-scale, and mean depression subscale of 45·15, 47·85, and 42·53, respectively. There was a significant difference between the mean total score (p=0·04) and the mean depression subscale (p=0·03) with the EVD survivor population having higher scores, but the increased scores in EVD survivor populations did not meet criteria for a mental health disorder or diagnosis.
In the parent surveys for the entire cohort, the mean scores were higher than the children with an overall cohort mean score of 59·49, with the anxiety subscale mean of 57·82 and the depression sub scale mean of 59·44. In the parent EVD survivor cohort, the mean total score, mean anxiety sub-scale, and mean depression subscale measured 62·00, 60·12, and 61·30 respectively (Supplemental Table 2). These values in the parents of EVD survivors were greater than the RCADS scores in the parents of close contacts with a mean total score, mean anxiety subscale, and mean depression subscale score of 58·63, 57·03, and 58·80 respectively.
Discussion
Following recent EVD outbreaks of unparalleled magnitude in West and Central Africa, thousands of EVD survivors remain at-risk for survivor sequelae including ophthalmic disease, psychosocial stressors, systemic sequelae, and the risk of viral persistence.28, 29, 30 While EVD survivor care needs have been broadly reported in adult populations, sequelae of pediatric EVD survivorship are under-represented in the literature. Our study presents a multifaceted approach to these urgent needs via characterization of ophthalmic findings in a pediatric EVD survivor and close contact population, in addition to visual function, vision-related quality-of-life and mental health assessments.
We observed that ophthalmic complications were common in both the EVD survivor and close contact cohorts. Uveitis, the most common ophthalmic complication observed in adult EVD survivor populations, was observed with a greater prevalence in the EVD survivor group compared to the control population. However, the rate of uveitis observed in the pediatric EVD survivor population of 10% was lower than the prevalence of 13-34% described in primarily adult populations.9, 10, 11
While these findings impacted the vision of the affected eye, we also observed that the mean vision in the better seeing eye in both the EVD survivor group and close contact groups was good with the predominance of patients in the normal or mild visual impairment category of visual acuity (i.e. Vision better than or equal to 20/40). Yet, because of our inability to quantify LP or NLP vision in EVD survivors, there is a slight bias to better vision among the EVD survivors. The percentage of patients that had poorer vision was higher in the EVD survivor group, likely skewed by patients with uveitis who demonstrated poorer vision on presentation.
Another notable diagnosis observed in over 20% of EVD survivors and over 10% of close contacts was vernal keratoconjunctivitis, often with corneal scarring. The severely symptomatic conjunctival changes and associated corneal changes highlight the need for ophthalmic subspecialty care and services for children in Sierra Leone. Prior studies have not specifically assessed the impact of EVD on ophthalmic disease in pediatric patients and while uveitis was also observed with a higher prevalence in EVD survivors in this study, larger cohorts are needed to fully elucidate the ophthalmic manifestations in pediatric cohorts.
Besides these ophthalmic findings, we also observed that EYE-Q and PedsQL scores were reduced overall in the EVD survivor and close contact population. Patients with ocular complications reported worse VRQOL via the EYE-Q instrument when compared to patients without a documented ocular complication. EYE-Q also captures the perceived difficulty in the performance of activities of daily living and the impact vision impairment has on tasks performed by children.24, 25, 26 The parent report for the EYE-Q was comparable between parents and guardians of children with and without an ocular complication. Potential reasons for discrepancies between parent and children report include limited parental awareness of the impact of ocular conditions on activities, and underreporting of vision challenges by children, if the unaffected eye maintains functional vision.31
We also sought to further characterize the impact of depression and anxiety in this unique patient population with RCADs. In the child report, the mean total RCADS, anxiety and depression subscale scores of the EVD survivors exceeded the close contact cohort, particularly for depressive symptoms. Interestingly, a greater prevalence of reported anxiety and depression symptoms was observed in the parent reports for both pediatric cohorts. Prior reports have documented discordant results related to parental insight from the child's psychosocial state.32 However, parent scores did suggest a larger number of children who required further mental health evaluation to determine if patients met clinical criteria for anxiety and depression. Prior research has documented clinically significant mental health challenges despite metrics that do specifically reach diagnostic criteria.33, 34, 35
Limitations of this study include the small sample size of pediatric-aged EVD survivors in this cohort relative to the total population of survivors. Selection bias for symptomatic children could also bias the results to a higher prevalence of ophthalmic disease compared to the true EVD pediatric survivor population. One guardian also cared for multiple children, which could artificially select for a guardian with greater stressors from multiple children requiring care. Future studies with a larger sample size and intra-cluster correlation assessments could help to refine our understanding of relationship between vision health, vision-related quality-of-life and psychosocial stressors in children and their guardians. Of note, some study participants had significant time constraints including travel from more distant locations that prevented individuals from completing all study questionnaires. Lastly, the questionnaires were not originally developed in Krio, the lingua franca of Sierra Leone, which may have affected interpretation of specific questions.
Despite the limitations, we observed a higher frequency of uveitis in eyes of pediatric EVD survivors, as well as vernal keratoconjunctivitis in a substantial proportion of patients evaluated. The presence of ocular complications was notably associated with reduced vision-related quality-of-life and vision function. Larger studies in pediatric-aged patients are needed to determine the true prevalence of EVD-associated ophthalmic disease and the potential sight-threatening inflammatory and cicatricial corneal disease observed. These findings raise questions related to differential ophthalmic findings in pediatric EVD survivors compared to adults, as well as the need for expanded capacity for subspecialty eye care in resource-limited settings. Moreover, the mental health measures including anxiety and depression from this cohort will better inform health professionals and increase preparedness measures after an EVD outbreak. Recent EVD outbreaks and the ongoing COVID-19 pandemic have brought to light the many medical care and mental health challenges that may require attention during a public health emergency - these challenges remind us of the importance of cross-disciplinary research to address challenges associated with EIDs and future pandemic outbreak response.36
Contributors
Conceptualization Ideas; formulation or evolution of overarching research goals and aims: (Jessica G. Shantha & Steven Yeh)
Methodology Development or design of methodology; creation of models: (Jessica G. Shantha, Steven Yeh, Gilberte Bastien, Sheila T. Angeles-Han, Natalie Weil)
Software Programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components: (Dominick Canady, Caleb Hartley, Amy Cassedy)
Validation Verification, whether as a part of the activity or separate, of the overall replication/ reproducibility of results/experiments and other research outputs: (Dominick Canady, Caleb Hartley, Amy Cassedy, Steven Yeh, Jessica Shantha)
Formal analysis Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data:(Dominick Canady, Caleb Hartley, Amy Cassedy, Jessica Shantha, Steven Yeh)
Investigation Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection: (Jessica G. Shantha, Steven Yeh, Gilberte Bastien, Chris Miller, Lloyd Harrison-Willliams, Matthew Vandy)
Resources Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools: (Jessica G. Shantha, Steven Yeh, Chris Miller, Lloyd Harrison-Willliams, Matthew Vandy)
Data Curation Management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later reuse: (Dominick Canady, Caleb Hartley, Amy Cassedy, Steven Yeh, Jessica Shantha)
Verification and accessing the data (Dominick Canady, Jessica Shantha, Steven Yeh)
Writing - Original Draft Preparation, creation and/or presentation of the work, specifically writing the initial draft (including substantive translation): (Jessica G. Shantha, Steven Yeh, Dominick Canady, Sheila-Angeles-Han)
Writing - Review & Editing Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre-or postpublication stages (Jessica G. Shantha, Steven Yeh, Dominick Canady, Sheila T. Angeles-Han, Chris Miller, Lloyd Harrison-Williams, Matthew Vandy, Natalie Weil)
Visualization Preparation, creation and/or presentation of the published work, specifically visualization/ data presentation: (Jessica G. Shantha, Steven Yeh, Dominick Canady)
Supervision Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team: (Jessica G. Shantha,Steven Yeh, Matthew Vandy, Lloyd Harrison-Williams, Gilberte Bastien, Sheila Angeles-Han, Amy Cassedy)
Project administration Management and coordination responsibility for the research activity planning and execution: (Jessica G. Shantha, Steven Yeh, Dominick Canady, Matthew Vandy, Lloyd Harrison-Williams)
Funding acquisition: Acquisition of the financial support for the project leading to this publication: (Jessica G. Shantha & Steven Yeh)
Decision to submit the manuscript: Dominick Canady, Sheila Angeles-Han, Jessica G. Shantha, Steven Yeh
Declaration of interests
The funding organizations highlighted above had no role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors on this manuscript declare no financial conflicts of interest in relationship to the work presented.
Data sharing statement
Data requests can be made to the corresponding author with a supplemental proposal outlining the proposed analysis. If permission is granted, deidentified study participant data in an excel file format will be made available. Data requests will be reviewed pending publication.
Funding
This project was supported by the National Eye Institute, National Institutes of Health R01 EY029594 (Yeh) and K23 EY030158 (Shantha). Funding support was also provided via an Unrestricted Grant from Research to Prevent Blindness (Emory Eye Center, Emory University School of Medicine), the Marcus Foundation Combating Childhood Illness Seed Grant, Emory Global Health Institute, and the Stanley M. Truhlsen Family Foundation, Inc. The funding organization had no role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101483.
Appendix. Supplementary materials
References
- 1.2014-2016 Ebola Outbreak in West Africa [Internet]. Cdc.gov. 2020 [cited 2021 Dec 5]. Available from: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html
- 2.Ebola virus disease – Democratic Republic of the Congo [Internet]. Who.int. [cited 2021 Dec 5]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/ebola-virus-disease-democratic-republic-of-the-congo_1
- 3.Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis. 2016;16(3):331–338. doi: 10.1016/S1473-3099(15)00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15(8):905–912. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 5.Etard J-F, Sow MS, Leroy S, et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis. 2017;17(5):545–552. doi: 10.1016/S1473-3099(16)30516-3. [DOI] [PubMed] [Google Scholar]
- 6.Shantha JG, Crozier I, Hayek BR, et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia. Liberia. Ophthalmology. 2017;124(2):170–177. doi: 10.1016/j.ophtha.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola syndrome. Sierra Leone. Emerg Infect Dis. 2016;22(4):641–646. doi: 10.3201/eid2204.151302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetter P, Kaiser L, Schibler M, Ciglenecki I, Bausch DG. Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis. 2016;16(6):e82–e91. doi: 10.1016/S1473-3099(16)00077-3. [DOI] [PubMed] [Google Scholar]
- 9.Hereth-Hebert E, Bah MO, Etard JF, et al. Ocular complications in survivors of the Ebola outbreak in Guinea. Am J Ophthalmol. 2017;175:114–121. doi: 10.1016/j.ajo.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372(25):2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis. 2016;62(11):1360–1366. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lötsch F, Schnyder J, Goorhuis A, Grobusch MP. Neuropsychological long-term sequelae of Ebola virus disease survivors - a systematic review. Travel Med Infect Dis. 2017;18:18–23. doi: 10.1016/j.tmaid.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 13.James PB, Wardle J, Steel A, Adams J. Post-Ebola psychosocial experiences and coping mechanisms among Ebola survivors: a systematic review. Trop Med Int Health. 2019;24(6):671–691. doi: 10.1111/tmi.13226. [DOI] [PubMed] [Google Scholar]
- 14.Tucci V, Moukaddam N, Meadows J. The forgotten plague: Psychiatric manifestations of ebola, zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151. doi: 10.4103/jgid.jgid_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans DK, Popova A. West African Ebola crisis and orphans. Lancet. 2015;385(9972):945–946. doi: 10.1016/S0140-6736(15)60179-9. [DOI] [PubMed] [Google Scholar]
- 16.Shantha JG, Mattia JG, Goba A, et al. Ebola virus persistence in ocular tissues and fluids (EVICT) study: reverse transcription-polymerase chain reaction and cataract surgery outcomes of Ebola survivors in Sierra Leone. EBioMedicine. 2018;30:217–224. doi: 10.1016/j.ebiom.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wauquier N, Becquart P, Gasquet C, Leroy EM. Immunoglobulin G in Ebola outbreak survivors. Gabon. Emerg Infect Dis. 2009;15(7):1136–1137. doi: 10.3201/eid1507.090402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Huang I-C, Thompson LA, Chi Y-Y, et al. The linkage between pediatric quality of life and health conditions: establishing clinically meaningful cutoff scores for the PedsQL. Value Health. 2009;12(5):773–781. doi: 10.1111/j.1524-4733.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther. 2000;38(8):835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Silverman W., Albano A. Psychological Corporation; San Antonio, TX: 1996. The Anxiety Disorders Interview Schedule for Children–IV (Child and parent versions) [Google Scholar]
- 23.Piqueras JA, Martín-Vivar M, Sandin B, San Luis C, Pineda D. The revised child anxiety and depression scale: a systematic review and reliability generalization meta-analysis. J Affect Disord. 2017;218:153–169. doi: 10.1016/j.jad.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Cassedy A, Altaye M, Andringa J, et al. Assessing the validity and reliability of the effects of youngsters’ eyesight on quality of life (EYE-Q) questionnaire among children with uveitis. Arthritis Care Res (Hoboken) [Internet] 2020 doi: 10.1002/acr.24491. (acr.24491) [DOI] [PubMed] [Google Scholar]
- 25.McDonald J, Cassedy A, Altaye M, et al. Comprehensive assessment of quality of life, functioning and mental health in children with juvenile idiopathic arthritis and non-infectious uveitis. Arthritis Care Res (Hoboken) [Internet] 2021 doi: 10.1002/acr.24551. (acr.24551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeles-Han ST, Yeh S, McCracken C, et al. Using the effects of youngsters’ eyesight on quality of life questionnaire to measure visual outcomes in children with uveitis: EYE-Q measures vision in children with uveitis. Arthritis Care Res (Hoboken) 2015;67(11):1513–1520. doi: 10.1002/acr.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Definitions - the international agency for the prevention of blindness [Internet]. Iapb.org. 2020 [cited 2021 Dec 5]. Available from: https://www.iapb.org/learn/vision-atlas/about/definitions/
- 28.Jalloh MF, Li W, Bunnell RE, et al. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Glob Health. 2018;3(2) doi: 10.1136/bmjgh-2017-000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canady D, Weil NC, Miller C, Shantha JG, Bastien G, Yeh S. Ophthalmic and psychosocial sequelae in Ebola virus disease survivors: ongoing need for health systems strengthening across disciplines. Expert Rev Anti Infect Ther. 2021;19(2):117–119. doi: 10.1080/14787210.2020.1808461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PREVAIL III Study Group, Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, et al. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380(10):924–934. doi: 10.1056/NEJMoa1805435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143(4):647–655. doi: 10.1016/j.ajo.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammed A, Sheikh TL, Gidado S, et al. An evaluation of psychological distress and social support of survivors and contacts of Ebola virus disease infection and their relatives in Lagos, Nigeria: a cross sectional study–2014. BMC Public Health. 2015;15(1):824. doi: 10.1186/s12889-015-2167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weems CF, Zakem AH, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: association with symptoms of anxiety disorders and cognitive bias. J Clin Child Adolesc Psychol. 2005;34(4):712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- 34.Kaputu-Kalala-Malu C, Musalu EM, Walker T, Ntumba-Tshitenge O, Ahuka-Mundeke S. PTSD, depression and anxiety in Ebola virus disease in Beni town, democratic republic of the Congo. BMC Psychiatry. 2021 Jul 8;21(1):342. doi: 10.1186/s12888-021-03343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly JD, Hoff NA, Spencer D, et al. Neurological, cognitive, and psychological findings among survivors of ebola virus disease from the 1995 ebola outbreak in Kikwit, democratic republic of congo: a cross-sectional study. Clin Infect Dis. 2019 Apr 8;68(8):1388–1393. doi: 10.1093/cid/ciy677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.