Summary
Background
The forecast accuracy of the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) and Asian Pacific Association for the Study of the Liver (APASL) acute-on-chronic liver failure (ACLF) criteria in assessing long-term outcomes after liver transplantation (LT) is still unclear, especially when the staging of the two standards is inconsistent.
Methods
A retrospective cohort (NCT05036031) including 565 patients from January 2015 to June 2021 was conducted. The 28 and 90 days, 1- and 3-years overall survival (OS) after LT were compared between different grades.
Findings
Total of 162 (28.7%) and 230 (40.7%) patients met the ACLF standards. In the EASL-CLIF criteria, the 3-year OS rates were 83·0%, 80·3%, and 69·8% for ACLF1-3, respectively. In the APASL criteria, the 3-year OS rates were 85·7% for APASL ACLF Research Consortium (AARC)-1, similar to ACLF-1. The 3-year OS rates were 84·5% for AARC-2, which were slightly better than ACLF-2. Regarding AARC-3, the 3-year OS rate was 5·8% higher than ACLF-3. For patients who met neither set of criteria for ACLF, the 3-year OS rates were 89·8%. The multivariate analysis showed that alanine aminotransferase >100 U/L, respiration failure, and cerebral failure were independent risk factors for post-LT death.
Interpretation
This study provides the first large-scale long-term follow-up data in Asia. Both criteria showed favorable distinguishing ability for post-LT survival. Patients with ACLF had a higher post-LT mortality risk, and ACLF-3 and AARC-3 correlated with significantly greater mortality.
Funding
National Natural Science Foundation of China and Science and Technology Commission of Shanghai Municipality
Keywords: Acute-on-chronic liver failure, Liver transplantation, Overall survival, Liver, Decompensation
Abbreviation: ACLF, acute-on-chronic liver failure; LT, liver transplantation; EASL-CLIF, European Association for the Study of the Liver-Chronic Liver Failure; APASL, Asian Pacific Association for the Study of the Liver; OS, overall survival; AD, acute decompensation; INR, international normalized ratio; HE, hepatic encephalopathy; AARC, APASL ACLF Research Consortium; TB, total bilirubin; CLIF-C OFs, CLIF-C organ failure score; TEA, Transplantation for EASL-CLIF and APASL acute-on-chronic liver failure (ACLF) patients; LDLT, living donor liver transplantation; OLT, orthotopic liver transplantation; SLT, split liver transplantation; CsA, cyclosporine; MMF, mycophenolate mofetil; WBC, white blood count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MELD, Model for End-Stage Liver Disease score; IQR, interquartile range; HBV, Hepatitis B virus; AIH, autoimmune hepatitis; ECLIS, ELITA/EF-CLIF collaborative study; ICU, Intensive care unit; SBP, spontaneous bacterial peritonitis; MDRO, multidrug-resistant organism
Research in context.
Evidence before this study
To appraise the current evidence, we searched PubMed for research articles published up to May 7, 2022, using the terms “European Association for the Study of the Liver - Chronic Liver Failure (EASL-CLIF)”, “Asian Pacific Association for the Study of the Liver (APASL)”, “acute on chronic liver failure” (ACLF), and “liver transplantation (LT)”. We found eighteen peer-reviewed scientific publications and were unable to identify any studies comparing these two criteria in the prediction of post-transplant prognosis in ACLF.
Added value of this study
Existing high-quality data regarding liver transplantation for ACLF mainly came from the UNOS and ECLIS studies with a one-year follow-up. Our study provides the first large-scale long-term follow-up data in Asia. In this retrospective study of 565 cirrhosis patients who underwent LT, both EASL-CLIF and APASL-ACLF criteria showed favorable ability to distinguish post-LT survival, with a similar 3-year overall survival (OS) between Grade 1 and non-ACLF patients, and a significantly lower OS in Grade 3. In addition, except for ACLF-3, for the consistently graded ACLF, the survival rate of the same grade in the EASL-CLIF criteria could be a reference point, with a 3-year OS of 76% for the ACLF2/ APASL ACLF Research Consortium (AARC)2 group and 61% for the ACLF3/AARC3 group. When the two grades were inconsistent, the OS was close to that predicted by the lower grade. Multivariate analysis showed that alanine aminotransferase >100 U/L, respiration failure, and cerebral failure were independent risk factors for post-LT death.
Implications of all the available evidence
The present study might provide more insight into the impact of severity of illness, multiple dimensional evaluation, and clinician decision-making in ACLF patients.
Alt-text: Unlabelled box
Introduction
Acute-on-chronic liver failure (ACLF) is a clinical syndrome characterized by liver decompensation caused by an acute liver insult in patients with chronic liver disease and is associated with high short-term mortality rates.1,2 The etiology of acute insults includes bacterial infection, viral infection, hepatotoxic drugs, and hepatic ischemia. Liver cirrhosis presents progressively in patients. Unless effective treatment is provided, the destruction of liver cells inevitably leads to decompensation.3
Although their clinical presentation is similar, patients with acute decompensation and ACLF have different clinical phenotypes and baseline inflammatory profiles. In addition, patients with ACLF have intense systemic inflammation and alterations in major metabolic pathways compared with patients with cirrhosis without ACLF, leading to organ failure.4, 5, 6, 7
Currently, several organizations have suggested different definitions of ACLF.8, 9, 10, 11 At present, the most widely used standard is the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) ACLF standard, which is based on a large-scale prospective study (CANONIC study) focused on organ failure that was published in 2013. It evaluated whether patients had ACLF and determined severity by scoring six organs or systems.9,12 An alternative definition proposed by the Asian Pacific Association for the Study of the Liver (APASL) focuses mainly on liver dysfunction, jaundice (serum bilirubin ≥5 mg/dL), coagulopathy (international normalized ratio [INR] ≥1·5 or prothrombin activity <40%), ascites, and/or hepatic encephalopathy (HE) for diagnosis. A liver failure score (APASL ACLF Research Consortium, AARC score) calculated using total bilirubin level (TB), INR, HE grades, and plasma lactate and serum creatinine levels was proposed to distinguish disease severity and predict outcomes. ACLF patients can be divided into three groups based on their score. Considering the high 28-day mortality rate (85·9%) in grade 3 patients (AARC score >10), immediate interventions and liver transplantation (LT) are recommended to improve clinical outcomes.8 Regardless of the absence of a unified standard to assess whether patients with ACLF could achieve maximum benefit through LT, it is the only treatment for patients with severe ACLF that effectively increases survival, especially if surgery is offered early after diagnosis.13 However, the upper limits of recipient severity of illness should be considered to avoid futile LT in the current era of organ shortage.
Because of the different predominant etiologies of underlying liver disease, the nature of precipitating events in different regions and liver disease's complicated pathological mechanism, observations in patients in large-scale clinical trials in the East and West differed, resulting in discrepancies in the definition and diagnostic criteria for ACLF. In the Asia-Pacific region, the underlying lesions and exacerbations of ACLF strongly correlated with hepatitis B virus (HBV) infection, while in Western countries, the cause was mainly non-viral liver damage. A study by Li H et al. suggested that in patients with HBV-related cirrhosis, the CLIF-C organ failure score standard proposed in the CANONIC study could also be applied to predict short-term mortality.14 At present, the therapeutic effect of LT in patients with liver cirrhosis still needs a sensitive standard to predict and evaluate outcomes.
In this study, we collected the clinical data of patients who underwent LT for ACLF at our center to evaluate the etiology of ACLF, characteristics of patients with disease of different severity levels, and impact on complication and survival rates after LT.
Methods
Study cohort
In this retrospective study, the Transplantation for EASL-CLIF and APASL ACLF cohort, including 565 patients who underwent LT for cirrhosis at Renji Hospital, affiliated with School of Medicine Shanghai Jiao Tong University, between January 1st 2015 and June 30th 2021, were considered for inclusion (NCT05036031). Patients with malignant tumor or who underwent living donor LT were excluded. No organs from executed prisoners were used in the present study. ACLF diagnosis followed the APASL and EASL-CLIF definitions. The non-ACLF cohort here included patients with progressive chronic liver disease who underwent transplant electively. In patients with cirrhosis undergoing LT at our institution, patients who did not fulfill the diagnostic ACLF criteria before LT were included in the non-ACLF cohort. The median time from listing to LT was 7 days.
Urgent LT for ACLF is an evolving therapeutic option. The selection criteria for urgent LT in our center are based on the “Basic principles and core policies on allocation and Sharing of Human Organs.” Medical urgency scores for patients waiting for LT are shown in Supplementary Table 1-2. The allocation of grafts was basing on COTRS (China Organ Transplant Response System, https://www.cot.org.cn/). The study was reviewed and approved by the Ethical Committee of Renji Hospital. Written informed consent on study aims, participation requirements and the right to refuse was obtained from all participants, and the trial conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All procedures were in accordance with the STROBE guidelines.
Surgical procedure and postoperative immunosuppression
All surgical procedures at our institution were performed by surgeons specialized in LT. Most patients underwent classic orthotopic LT, while 25 underwent split LT.
Patients received our center's standard immunosuppressive program post-LT. To prevent acute rejection, methylprednisolone was used during surgery at a dose of 500 mg.15 The postoperative immunosuppression protocol included basiliximab, a tapered dose of methylprednisolone, and a drug regimen of calcineurin inhibitor (tacrolimus or cyclosporine) and/or mycophenolate mofetil.
Data collection and follow-up
Clinical data of patients from both perioperative and long-term follow-up was obtained from our institutional database. Characteristics listed in the patient chart included basic demographics (age, sex), etiology, preoperative clinical parameters (white blood cell count [WBC], hemoglobin, platelets, albumin, alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin, creatinine, INR, lactate), and clinical manifestations (ascites, hepatic encephalopathy, and gastrointestinal hemorrhage). Preoperative severity was classified using the Child–Pugh score and Model for End-Stage Liver Disease (MELD) score. Surgical variables included waiting time and intraoperative factors (duration of operation, estimated bleeding, transfusion, and anhepatic period). Outcome variables included length of hospital stay and short-term and long-term survival rates. The median follow-up time was 33 months.
Statistical analysis
Categorical variables distributions were compared using the chi-squared or Fisher's exact test and are presented as n (percentage). Continuous variables were compared using the t-test, Mann-Whitney U test or the Kruskall-Wallis test, when appropriate and are expressed as mean ± standard deviation or median value (interquartile range [IQR]). Survival of different patient groups was estimated using Kaplan–Meier survival analysis and compared using a log-rank test. Multivariable analysis was conducted by logistic regression to identify the risk factors for post-LT death. All statistical tests were performed using IBM SPSS Statistics (version 26; SPSS, Inc. Chicago, IL) and R (version 4.0.3). All tests were two-tailed; p < 0·05 was considered statistically significant.
Role of the funding source
The study was funded by the National Natural Science Foundation of China (92059205, 81902388), Shanghai Natural Science Foundation (18ZR1424200), and Shanghai Medical Innovation Program (20Y11908900). The funding source did not have any involvement in study design, data collection and management, data analysis or interpretation, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication. Hao Feng, Qiang Xia, Lei Xia, and Zi-yun Qiao accessed and verified the data and responsible for the manuscript submission.
Results
Baseline characteristics and pre-LT assessment
There were 565 patients enrolled in this study. The basic characteristics of the recipients and donors are summarized in Table 1. HBV infection was the most common etiology of ACLF (53·3% in non-ACLF and 66·8% in ACLF patients). Autoimmune hepatitis (ACLF-AIH) was found in 113 (20%) patients, and 31 (5·5%) had alcoholic liver failure. Fourteen patients had more than one etiology, and 11 of those had hepatitis B combined with another etiology.
Table 1.
Baseline clinical characteristics of patients.
| Characteristic | Non-ACLF(n=330) | EASL-CLIF ACLF(n=162) | APASL ACLF(n=230) | p |
|---|---|---|---|---|
| Age, years | 50 (41-57) | 47 (38-55) | 47 (39-55) | 0.036 |
| Sex, male | 206 (62.4%) | 129 (79.6%) | 183 (79.6%) | <0.001 |
| Etiology | <0.001 | |||
| Hepatitis B | 176 (53.3%) | 115 (71.0%) | 155 (67.4%) | |
| Hepatitis C | 10 (3.0%) | 1 (0.6%) | 1 (0.4%) | |
| Alcohol | 19 (5.8%) | 6 (3.7%) | 11 (4.8%) | |
| Autoimmune | 86 (26.1%) | 15 (9.3%) | 26 (11.3%) | |
| Drug | 4 (1.2%) | 4 (2.5%) | 8 (3.5%) | |
| Wilson's disease | 4 (1.2%) | 5 (3.0%) | 9 (3.9%) | |
| Unknown | 20 (6.1%) | 7 (4.3%) | 10 (4.3%) | |
| Multiple etiologya | 6 (1.8%) | 6 (3.7%) | 8 (3.5%) | |
| Othersb | 5 (1.5%) | 3 (1.9%) | 2 (0.9%) | |
| Clinical parameters | ||||
| White blood count, *109/L | 3.5 (2.4-5.0) | 7.8 (5.6-11.1) | 6.9 (4.7-10.3) | <0.001 |
| Hemoglobin, g/dL | 101 (81-118) | 107 (84-127) | 105 (86-125) | 0.043 |
| Platelets, *109/L | 73 (46-124) | 62 (40-102) | 60 (40-93) | 0.020 |
| Albumin, g/dL | 34.2 ± 6.0 | 33.6 ± 5.7 | 33.2 ± 5.7 | 0.178 |
| Alanine aminotransferase, U/L | 29 (19-50) | 101 (50-242) | 82 (41-212) | <0.001 |
| Aspartate aminotransferase, U/L | 43 (29-78) | 110 (72-201) | 108 (68-201) | <0.001 |
| Total bilirubin, mg/dL | 2.2 (1.2-4.3) | 25.1 (17.6-32.1) | 23.5 (15.7-30.6) | <0.001 |
| Creatinine, mg/dL | 0.8 (0.7-1.0) | 1.0 (0.7-1.6) | 0.9 (0.7-1.3) | <0.001 |
| INR | 1.38 (1.22-1.55) | 2.73 (2.29-3.27) | 2.38 (1.81-2.97) | <0.001 |
| Lactate, mmol/L | 1.3 (1.1-1.6) | 2.5 (1.7-3.4) | 2.2 (1.6-3.2) | <0.001 |
| Decompensating events | ||||
| Ascites | 232 (70.3%) | 150 (92.6%) | 215 (93.5%) | <0.001 |
| Hepatic Encephalopathy | <0.001 | |||
| I-II | 7 (2.1%) | 35 (21.6%) | 38 (16.5%) | |
| III-IV | 4 (1.2%) | 23 (14.2%) | 25 (10.9%) | |
| Gastrointestinal hemorrhage | 133 (40.3%) | 13 (8.0%) | 24 (10.4%) | <0.001 |
| Bacterial infection | 36 (10.9%) | 49 (30.2%) | 62 (30.0%) | <0.001 |
| MELD score | 11 (7-16) | 32 (27-38) | 29 (23-35) | <0.001 |
| Child-Pugh score | 8 (7-10) | 12 (11-13) | 12 (11-12) | <0.001 |
Results are expressed as mean±standard deviation, median value (interquartile range) or n (percentage). BMI, body mass index; INR, international normalized ratio. a Hepatitis B + drug (n = 6, 1.1%), hepatitis B + alcohol (n = 3, 0.5%), hepatitis B + AIH (n = 1, 0.2%), hepatitis B + hepatitis C (n = 1, 0.2%), alcohol + drug (n = 1, 0.2%), alcohol + hepatic venule occlusion (n = 1, 0.2%), AIH + drug (n = 1, 0.2%). b Polycystic liver disease (n=4, 0.7%), schistosomiasis cirrhosis (n=3, 0.5%), Langerhans cell hyperplasia (n = 1, 0.2%).
Among the clinical manifestations of decompensated liver cirrhosis, 220 (93·6%) patients with ACLF and 232 (70·3%) without ACLF developed ascites. Significantly more patients with ACLF (63, 26·4%) than patients without ACLF (11, 3·3%) had HE (p<0·001). In patients without ACLF, the reason for admission was significantly more likely to be gastrointestinal hemorrhage (p<0·001). Compared with patients without ACLF, the majority of patients who developed ACLF were male (p<0·001) and had a lower PLT (p=0.020), a higher WBC (p<0·001), hemoglobin (p=0·043), ALT (p<0·001), AST (p<0·001), TB (p<0·001), creatinine (p<0·001), INR (p<0·001), and lactate (p<0·001). Among decompensating events secondary to cirrhosis, gastrointestinal hemorrhage happened more constantly in non-ACLF patients (p<0.001), constituting a crucial reason for LT. Pre-LT infection developed more frequently in ACLF patients (p<0.001), and mainly appeared as spontaneous bacterial peritonitis (SBP) and pneumonia. They also had a worse status in various scores related to liver function, with a MELD score of 29 (23–35) vs. 11 (7–16) (p<0·001), and a Child–Pugh score of 12 (11–12) vs. 8 (7–10) (p<0·001). Based on the preoperative temperature, infectious index, and bacterial hemoculture data, 63 infections (26.8%) in ACLF patients and 36 (10.9%) in the non-ACLF group were found.
Patient distribution in EASL-ACLF and APASL criteria (AARC grade)
According to the EASL-CLIF criteria, 162 patients were diagnosed with ACLF, of whom, 18, 97, and 47 had ACLF-1, ACLF-2, or ACLF-3, respectively. Using the APASL criteria, there were 230 patients diagnosed with ACLF, of whom 45, 118, and 67 had AARC-1, AARC-2, or AARC-3, respectively (Table 2).
Table 2.
Classification and survival of all patients underwent LT. COD, cause of death; MODS, multiple organ dysfunction syndrome.
 |
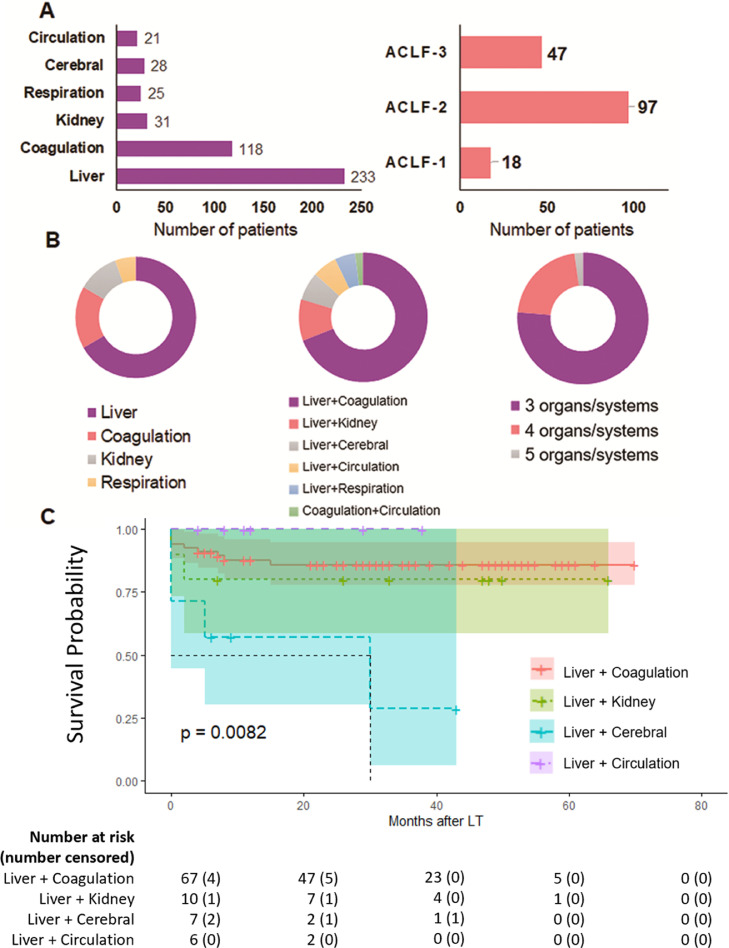
Liver failure was the most common type of organ failure (Figure 1A). According to the organ failure standard of EASL-CLIF, among all 565 patients, liver failure was the most common occurrence (233 patients, 41·2%), followed by coagulation failure (118, 20·9%). There were 31 (5·5%), 28 (5·0%), 25 (4·4%), and 21 (3·7%) with renal, cerebral, respiratory, and circulatory failure, respectively. In the 18 patients with ACLF-1, 12 had liver failure combined with renal insufficiency and/or grade 1–2 HE. Among patients with ACLF-2, liver and coagulation failure was the most common combination of organ failure (67/97, 69·1%), and 21 (28·9%) patients had liver failure combined with another organ failure. There were only two patients who had coagulation and circulation failure without liver failure. Among patients with ACLF-3, three, four, and five organ failures occurred in 36, 10, and 1 patients, respectively (Figure 1B).
Figure 1.
Distribution of organ/system failure according to CLIF-OF criteria. A. Organ/system failure found in all 565 patients. B. Number of patients diagnosed in ACLF-1, -2, and -3. C. Types of organ/system failure in EASL-CLIF ACLF patients of all levels. D. OS in EASL-CLIF ACLF-2 patients with different types of organ/system failure.
Outcomes of patients with different types of organ/system failure
In 97 patients with EASL-CLIF ACLF-2, patients with liver and coagulation failure or liver and kidney failure before LT had no significant difference in OS. Patients with liver circulatory failure (n=6) had the highest OS, which may be related to discrepancies in clinical use of vasopressors. Patients with liver and cerebral failure had a significantly poorer prognosis (p=0·0082, Figure 1C).
Comorbidity, mortality, and hospital stay
Table 3 shows the operative variables and clinical outcomes of all patients. Patients with a higher grade according to either APASL or EASL-CLIF criteria were considered more urgently in need of LT and had a shorter waiting time (p<0·001). Although there was no significant difference in the surgery duration or anhepatic period in these patients, they had more intraoperative bleeding (p<0·001) and underwent more blood transfusions (p<0·001), potentially because of the more severe coagulation disorder. There was no significant difference in hospital stay length between patients with all grades of APASL ACLF (p=0·056). However, according to the EASL-CLIF criteria, patients with ACLF had longer hospital stays (p=0·003). ACLF-1 and ACLF-3 patients had relatively longer hospital stays of 26 (IQR: 15–44) and 26 (IQR: 17–33) days, respectively, which were longer than those of ACLF-2 and non-ACLF patients.
Table 3.
Operative variables and clinical outcomes.
| Characteristic | AARC-I(n=45) | AARC-II(n=118) | AARC-III(n=67) | AARC non-ACLF(n=335) | p | ACLF-1(n=18) | ACLF-2(n=97) | ACLF-3(n=47) | EASL-CLIF non-ACLF (n=403) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Waiting time, days | 9 (5-22) | 5 (2-13) | 4 (2-9) | 28 (10-68) | <0.001 | 4 (1-15) | 5 (2-9) | 4 (2-9) | 24 (8-55) | <0.001 |
| Intraoperative factors | ||||||||||
| Duration, h | 6.8 (6.3-7.6) | 7.1 (6.5-8.1) | 7.0 (6.4-7.6) | 6.9 (6.2-7.8) | 0.092 | 7.2 (6.4-8.2) |
7.1 (6.5-7.8) |
7.2 (6.7-7.7) |
6.9 (6.2-7.8) |
0.704 |
| Bleeding, mL | 800 (500-1200) |
800 (500-1200) |
800 (600-1500) |
600 (400-1000) |
<0.001 | 900 (500-2000) |
800 (500-1200) |
1000 (600-1600) |
600 (400-1000) |
<0.001 |
| Transfusion, mL | 800 (800-1600) |
1200 (800-1700) |
1400 (800-2000) |
800 (400-1600) |
<0.001 | 1600 (800-2500) |
1200 (800-2000) |
1400 (800-2000) |
800 (400-1600) |
<0.001 |
| Anhepatic period, min | 44 (39-50) | 46 (39-51) | 42 (38-49) | 45 (38-51) | 0.625 | 43 (37-49) | 43 (38-50) | 45 (40-50) | 45 (38-51) | 0.851 |
| Hospital stays, days | 18 (14-24) | 21 (16-31) | 21 (16-32) | 18 (15-25) | 0.056 | 26 (15-44) | 20 (17-29) | 26 (17-33) | 18 (15-24) | 0.003 |
| 28-day survival | 95.6% | 94.1% | 85.1% | 96.7% | <0.001 | 94.4% | 89.7% | 85.1% | 97.0% | <0.001 |
| 90-day survival | 93.3% | 91.5% | 79.1% | 92.8% | <0.001 | 88.9% | 87.6% | 76.6% | 93.5% | <0.001 |
| 1-year survival | 91.0% | 85.3% | 75.6% | 91.3% | <0.001 | 83.0% | 83.2% | 69.8% | 91.8% | <0.001 |
| 3-year survival | 85.7% | 84.5% | 75.6% | 89.7% | <0.001 | 83.0% | 80.3% | 69.8% | 90.0% | <0.001 |
| 3-year graft survival | 85.7% | 84.5% | 74.6% | 89.7% | <0.001 | 83.0% | 79.4% | 69.8% | 90.0% | <0.001 |
Results are expressed as mean±standard deviation, median value (interquartile range) or n (percentage).
The survival rate and specific causes of death after LT are listed in Table 2 and Figure 1. Three hundred thirty patients who did not have ACLF according to either set of criteria had the best prognosis, with 28-day and 90-day survival rates of 96·7% and 93·0%, respectively. The major causes of early postoperative death were severe infection in nine patients, multiple organ dysfunction syndrome in six patients, and graft versus host disease in four patients. One-year and 3-year survival rates were 91·5% and 89·8%, respectively, and the major causes of death were biliary complications in four patients and liver failure in two patients.
Post-LT survival of patients with ACLF1–3 and AARC1–3
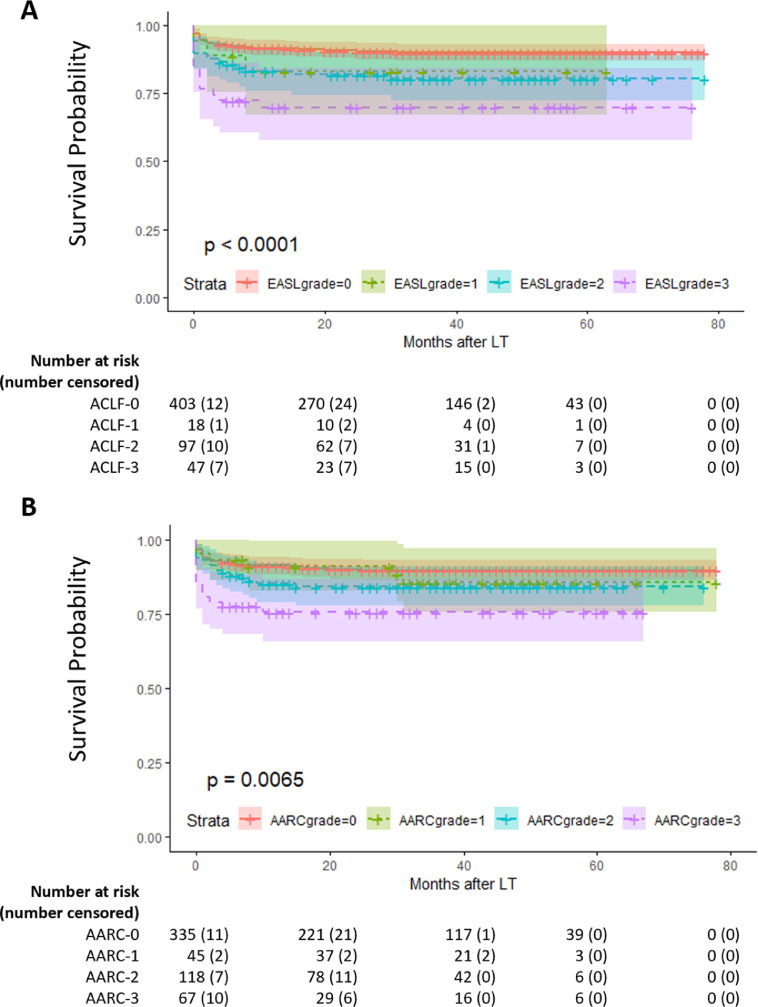
In the two diagnostic criteria, the short-term and long-term survival rates of ACLF patients decreased as the grade increased. The survival rate of AARC-1 patients was closest to that of non-ACLF patients, with 90-day and 3-year survival rates of 93·3% and 85·7%, respectively. A horizontal comparison of the two diagnostic criteria showed that 47 patients with ACLF-3 and 67 patients with AARC3 had relatively similar prognoses, which were significantly worse than that of the other two grades. The 90-day survival rates were 76·6% and 79·1%, respectively, and the 3-year survival rates were 69·8% and 75·6%. Figure 2A-B shows patients’ survival curve. Both APASL and EASL-CLIF criteria had good ability to distinguish patients (p=0·0065 and p<0·0001, respectively). Among patients classified according to the APASL, AARC-1 patients had a survival rate close to that of AARC-0 patients for nearly 2 years after LT; however, long-term survival after 2 years had a downward trend and was closer to that of AARC-2 patients. The EASL-CLIF criteria showed better discrimination between ACLF-1 and non-ACLF patients. The survival rate of ACLF-1 and -2 patients were similar, and both were significantly higher than that of ACLF-3 patients.
Figure 2.
A. Overall survival (OS) in AARC no ACLF and I, II, and III groups (P = 0·0065). B. OS in EASL-CLIF non-ACLF and ACLF-1, -2, and -3 groups (P < 0·0001).
Predictors for post-LT mortality
In univariate analysis, risk factors that were independently associated with post-LT death were bacterial infection, ALT>100U/L, AST>80U/L, TB>15mg/dL, creatinine>1.0 mg/dL, INR>2.0, lactate>2.0 mmol/L, liver failure, coagulation failure, kidney failure, respiration failure, cerebral failure, and circulation failure. A multivariate analysis was also utilized, the results showed that ALT>100 U/L (HR: 2.030, 95%CI: 1.157-3.561, p=0.014), respiration failure (HR: 3.516, 95%CI: 1.420-8.706, p=0.007), and cerebral failure (HR: 0.009, 95%CI: 1.338-7.572, p=0.009) were independent risk factors for post-LT death (Table 4).
Table 4.
Univariable and multivariable analyses of predictors for post-LT death.
| Predictors | Univariable analysisHR (95% CI) | p | Multivariable analysisHR (95% CI) | p |
|---|---|---|---|---|
| Age > 60 years | 1.186 (0.632-2.226) | 0.594 | ||
| Gender, male | 1.392 (0.791-2.450) | 0.251 | ||
| Ascites | 1.313 (0.681-2.532) | 0.416 | ||
| Gastrointestinal hemorrhage | 0.978 (0.564-1.696) | 0.936 | ||
| Bacterial infection | 1.973 (1.118-3.481) | 0.019 | - | 0.276 |
| WBC > 10 *109/L | 2.607 (1.475-4.606) | 0.001 | - | 0.364 |
| Hemoglobin < 80 g/dL | 1.228 (0.661-2.282) | 0.516 | ||
| Platelets < 100 *109/L | 0.946 (0.549-1.630) | 0.841 | ||
| Albumin < 28 g/dL | 1.587 (0.876-2.874) | 0.127 | ||
| ALT > 100 U/L | 2.805 (1.674-4.699) | <0.001 | 2.030 (1.157-3.561) | 0.014 |
| AST > 80 U/L | 1.756 (1.071-2.877) | 0.026 | - | 0.962 |
| TB > 15 mg/dL | 2.573 (1.559-4.249) | <0.001 | - | 0.095 |
| Creatinine > 1.0 mg/dL | 1.943 (1.170-3.227) | 0.010 | - | 0.094 |
| INR > 2.0 | 1.761 (1.058-2.930) | 0.029 | - | 0.734 |
| Lactate > 2.0 mmol/L | 1.894 (1.137-3.156) | 0.014 | - | 0.870 |
| Liver failure | 2.432 (1.471-4.021) | 0.001 | - | 0.151 |
| Coagulation failure | 2.074 (1.211-3.550) | 0.008 | - | 0.471 |
| Kidney failure | 3.004 (1.325-6.810) | 0.008 | - | 0.109 |
| Respiration failure | 6.058 (2.634-13.931) | <0.001 | 3.516 (1.420-8.706) | 0.007 |
| Cerebral failure | 4.957 (2.220-11.069) | <0.001 | 3.183 (1.338-7.572) | 0.009 |
| Circulation failure | 1.674 (1.011-2.770) | 0.045 | - | 0.841 |
HR, hazard ratio; CI, confidence interval; WBC, White blood count; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; TB, Total bilirubin; INR, international normalized ratio.
Prognosis prediction for consistent and discrepant grades in the EASL and APASL criteria
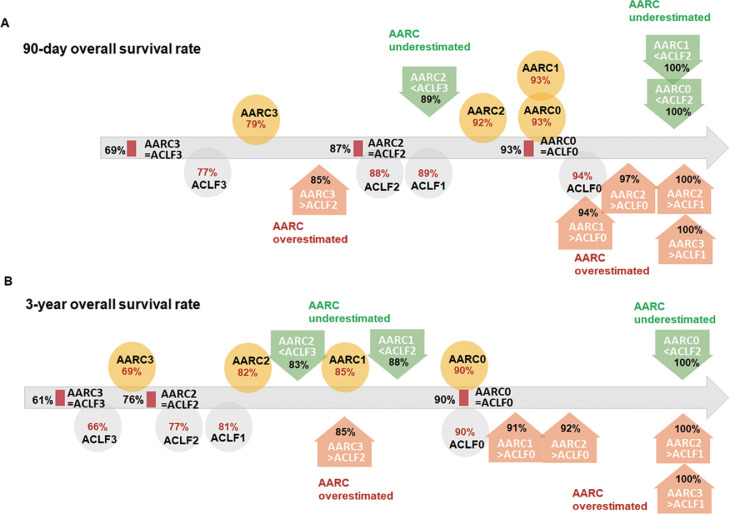
For consistently graded ACLF according to both criteria, the 3-year OS was 76% for the ACLF2/AARC2 group and 61% for the ACLF3/AARC3 group. For inconsistent classifications, using EASL-CLIF classification as a reference, the 3-year OS rates of APASL criteria overestimated patients (e.g., AARC3 but ACLF2) proximity to the related EASL-CLIF standards (e.g., ACLF2). The 3-year OS rates of the APASL criteria underestimated patients (e.g., AARC2 but ACLF3) relative to the related APASL criteria (e.g., AARC2). The major cause of 28-day postoperative mortality was severe infection, and that of long-term mortality was biliary complications (Figure 3).
Figure 3.
Comparison of the predictive value of APASL and EASL-CLIF criteria for the survival of cirrhosis patients after LT. Patients were divided into three groups. When the both criteria provided the same grade, patients were included in the ACLF-AARC consistent group. “AARC overestimated” (orange circles) represented patients in whom the APASL grade was higher than the EASL-CLIF grade, and “AARC underestimated” represented patients in whom the APASL grade was lower than the EASL-CLIF grade (green circles). A. 90-day overall survival rate after LT. B. 3-year overall survival rate after LT. For patients with consistent ACLF grading according to both criteria, the 3-year OS was 76% for the ACLF2/AARC2 group and 61% for the ACLF3/AARC3 group. For patients with inconsistent classifications, using the EASL-CLIF classification as a reference, the 3-year OS rates of the APASL criteria overestimated patients (e.g., AARC3 but ACLF2) relative to the related EASL-CLIF standards (e.g., ACLF2). In contrast, the 3-year OS rates of the APASL criteria underestimated patients (e.g., AARC2 but ACLF3) relative to the related APASL criteria (AARC2).
Discussion
This study evaluated the value of two diagnostic criteria, EASL-CLIF ACLF and APASL, in determining the prognosis following LT in patients with ACLF. EASL-CLIF obtained the definition of ACLF based on the CANONIC study, requiring 3 major characteristics of the syndrome: Acute decompensation (AD) defined by the acute development of one or more major complications of liver disease (i.e., ascites, encephalopathy, gastrointestinal hemorrhage, bacterial infection), organ failure, and high 28-day mortality rate.9,12 We strictly followed this protocol when evaluating whether patients meet the CLIF criteria. APASL ACLF is defined as an acute hepatic insult manifesting as jaundice and coagulopathy complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis and is associated with a high 28-day mortality. The EASL-CLIF ACLF criteria have relatively stricter standards that comprehensively evaluate the failure of six organs or systems to assess the presence and grade of ACLF. The APASL criteria are more convenient to implement and require a few routine tests, and the patient's medical history and clinical characteristics to make a diagnosis. There are similarities between the two. APASL criteria uses TB and INR as the main clinical indicators for judging liver decompensation, while the EASL-CLIF ACLF criteria uses these two indicators as standards for liver and coagulation failure, to predict the prognosis of patients with cirrhosis. Regarding differences between the two, the APASL criteria focus on patients with HBV infection and pay more attention to clinical changes brought about by the decline of synthesis and metabolism after liver failure, such as ascites and HE. The EASL-CLIF ACLF criteria attach great importance to extrahepatic organ/system failure, especially renal and cerebral failure. It defines renal insufficiency or stage I–II HE combined with other organ/system failure, or single renal failure as ACLF-1, suggesting that patients with these two conditions had higher severity and lower survival expectations than those with other organ/system failures.
To clarify which criteria should be used to make a preliminary prediction of survival after LT when the ACLF grades assessed according to the two criteria are inconsistent, we conducted a grouping analysis. When focusing on the 3-year survival rate, it was found out that survival in the AARC overestimated group was closer to that predicted by EASL-CLIF, but the result of the AARC underestimated group was the opposite, and the survival rate of most subgroups was closer to that of their original APASL grade. In this analysis, patients with AARC-2 and ACLF-3, as well as those with AARC-3 and ACLF-2, theoretically should have a worse clinical status and prognosis than patients with grade 2 according to both criteria; however, their actual 3-year survival rates were even higher. After comparing the 90-day survival in Figure 3A with the 3-year survival in Figure 3B, we found that the deaths that led to this contradictory result mainly occurred within 90 days to 3 years after LT. There were five deaths in patients with AARC-2 and ACLF-2 (n=52) during this period, two of which were caused by chronic rejection and postoperative infection, while the other three cases were not related to the primary disease and LT surgery (cardiovascular diseases, renal failure, and new bile duct cancer). One death occurred in patients with AARC-2 and EASL-3 (n=18) due to biliary complications, while no deaths happened from 90 days to 3 years after LT in patients with AARC-3 and EASL-2 (n=34). Subsequent large-scale clinical trials may strengthen the results of this study.
Previous studies had initially demonstrated that pre-existing organ failure was related to higher waiting list and post-LT mortality; however, in the process of organ allocation, the existence of extrahepatic organ failure has not received sufficient attention.16 Sundaram V. et al. compared the 1-year survival rate of more than 3600 patients diagnosed with ACLF-3 when entering the waiting list based on whether there was a specific organ failure at the time of transplantation.17 They found that the presence of liver or renal failure did not increase mortality in that particular patient group, but patients with respiratory (p<0·001), circulatory (p<0·001), and cerebral failure (p=0·002) had a higher risk than patients without these organ failures. A recent study from the ELITA/EF-CLIF collaborative study (ECLIS) evaluated the survival of over 2000 LT patients, of whom 234 were diagnosed with EASL-CLIF ACLF.18 One-year post-LT survival varied between 78·9% for ACLF-3 and 88·6% for ACLF-1 (log-rank p=0·38). Patients with ACLF-3 had an increased risk of complications and longer intensive care unit and hospital stay. In most countries, the MELD score is the most commonly used standard to assess the severity of a patient's condition.19 However, the MELD score mainly focuses on abnormalities in liver, kidney, and coagulation function, but omits the negative effects of other organ failures on overall survival. With deepening research on ACLF, there may be insufficient model complexity, as some patients with more urgent clinical conditions can be overlooked during selection.
The overall 90-day transplant-free survival of ACLF patients was approximately 50%, with rates of 35%–59%, 33%–53%, and 0%–37% among ACLF-1, ACLF-2, and ACLF-3 patients, respectively, compared with 5%–10% in non-ACLF patients.3,14,20 In our study, patients with higher AARC or EASL-CLIF grades were in an urgent clinical state, and their waiting times were shorter. The 90-day survival rate of patients after transplantation was 79·1% in AARC-3 patients and 76·6% in ACLF-3 patients. The 3-year survival rate was 75·6% and 69·8%, respectively. Compared with patients without LT, the survival rate was significantly better, and a relatively stable status could be maintained during long-term postoperative follow-up.
Regardless of their ACLF status, the majority of deaths occur in the early postoperative period, and serious uncontrollable infection is an important cause of early death. The APASL definition does not include bacterial infection or sepsis as a primary cause for liver failure, but in the EASL definition, bacterial infection or sepsis is considered as the most common precipitant. Bacterial infection or sepsis was also not included in the exclusion criteria of APASL. On the other hand, APASL highlights the role of bacterial infection in the progression of ACLF, which is only not part of the diagnostic criteria. Patients with cirrhosis are at increased risk of bacterial infection, which increases the mortality rate by 3·75 times.21 Patients with cirrhosis who develop ACLF usually have a significantly higher level of systemic inflammation upon admission, and the levels of inflammatory markers are higher when accompanied by ACLF progression, leading to a higher prevalence of liver failure, renal dysfunction, ascites, HE, and bacterial infections.22 Fernandez J et al. reported that infection was observed in approximately 36% of patients among over 2000 cases, with spontaneous bacterial peritonitis (SBP), urinary tract infections, and pneumonia the most frequent infection types, in which 23%–29% were caused by multidrug-resistant organisms (MDROs).23 Bacterial infections occurring within 1 month before surgery are related to infection after LT.24 Our research also proved that infection has an important link with the disease process and survival in patients with liver cirrhosis who undergo LT. For ACLF patients, it is necessary to closely examine patients for the presence of infection, pay attention to patients’ body temperature and changes in symptoms, and regularly check blood or body fluids for early diagnosis and treatment. Once a bacterial infection is diagnosed or suspected, broad-spectrum antibacterial agents or a combination of antibiotics are preferred, and the treatment is then adjusted according to the results of sensitivity tests.
In the long-term follow-up, LT has outstanding efficacy in improving the long-term prognosis of patients with cirrhosis. The major cause of death related to the patients’ primary disease and operation were biliary complications, which led to eight deaths (4/235, 1·7% in ACLF patients; 4/330, 1·2% in non-ACLF patients). Previous studies indicated that the overall death and graft loss rate caused by biliary complications was approximately 1·5%–2·0% in post-LT patients.25,26 After comparison, the appearance of ACLF did not increase the risk of biliary complications in this study.
Our study had several limitations. As a single-center retrospective study, selection bias inevitably appeared. In addition, the etiology of patients is restricted by region, mainly composed of patients with HBV infection, and might not accurately represent the prognosis of global ACLF patients. When discussing the impact of organ failure types on survival prognosis, the number of patients in some categories was small, such as liver and brain failure, and liver and circulatory failure, leading to errors in statistical analysis results. Multi-center prospective studies are needed during the follow-up to reduce this bias.
In conclusion, our study provides the first large-scale long-term follow-up data in Asia for ACLF treatment with LT. Although the EASL-CLIF and APASL ACLF criteria had their own different key tendencies, they could both be used in the diagnosis and classification of patients with cirrhosis mainly caused by hepatitis B, and they were also of good value in assessing post-LT survival. The two could be combined in diagnosis based on the medical environment. Patients diagnosed with ACLF-3 or AARC-3 had very low transplant-free survival; although LT could significantly improve prognosis, it was still significantly worse than that of non-ACLF and lower-grade ACLF patients. Therefore, it is necessary to strengthen supportive treatment for ACLF patients and perform LT as soon as possible before disease progression.
Contributors
Conceptualization: QZ, FH, XQ, ZJ
Methodology: FH, QZ, TY, XL, WT
Investigation: XL, QZ, ZZ,FH,LZ,TH,CX,SH
Visualization: XL, QZ, WH
Funding acquisition: XQ, FH
Project administration: XQ, FH
Supervision: XQ, FH, ZJ
Writing – original draft: QZ, XL, ZZ, WH
Writing – review & editing: FH
Declaration of interests
Authors declare that they have no competing interests.
Acknowledgments
Data sharing statement
All data are available in the main text or the supplementary materials.
Acknowledgments
We thank John Holmes, MSc, from Edanz for editing the English text of a draft of this manuscript.
Funding
Major research projects of National Natural Science Foundation, China (XQ, 92059205), National Natural Science Foundation, China (HF, 81902388), Shanghai Natural Science Foundation (HF, 18ZR1424200), Shanghai Medical Innovation Program (HF, 20Y11908900).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101476.
Contributor Information
Jian-jun Zhang, Email: zhangjianjun0221@126.com.
Hao Feng, Email: surgeonfeng@live.com.
Qiang Xia, Email: xiaqiang@medmail.com.cn.
Appendix. Supplementary materials
References
- 1.Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869–881. doi: 10.1016/S0140-6736(19)31894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernaez R, Sola E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut. 2017;66(3):541–552. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016:2. doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Claria J, Aguilar F, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020;72(4):688–701. doi: 10.1016/j.jhep.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Trebicka J, Amoros A, Pitarch C, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019:10. doi: 10.3389/fimmu.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claria J, Moreau R, Fenaille F, et al. Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology. 2019;69(4):1686–1701. doi: 10.1002/hep.30363. [DOI] [PubMed] [Google Scholar]
- 7.Sole C, Sola E, Morales-Ruiz M, et al. Characterization of inflammatory response in acute-on-chronic liver failure and relationship with prognosis. Sci Rep. 2016:6. doi: 10.1038/srep32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update (vol 13, pg 353, 2019) Hepatol Int. 2019;13(6):826–828. doi: 10.1007/s12072-019-09980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147(1):4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, O'leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60(1):250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau R, Jalan R, Gines P, et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology. 2013;144(7):1426–U189. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram V, Jalan R, Wu T, et al. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology. 2019;156(5):1381. doi: 10.1053/j.gastro.2018.12.007. -+ [DOI] [PubMed] [Google Scholar]
- 14.Li H, Chen L-Y, Zhang N-N, et al. Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci Rep. 2016:6. doi: 10.1038/srep25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams DH, Sanchez-Fueyo A, Samuel D. From immunosuppression to tolerance. J Hepatol. 2015;62:S170–S185. doi: 10.1016/j.jhep.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Karvellas C J, Francoz C, Weiss E. Liver transplantation in acute-on-chronic liver failure. Transplantation. 2021;105(7):1471–1481. doi: 10.1097/TP.0000000000003550. [DOI] [PubMed] [Google Scholar]
- 17.Sundaram V, Kogachi S, Wong RJ, et al. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72(3):481–488. doi: 10.1016/j.jhep.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belli LS, Duvoux C, Artzner T, et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: results of the ELITA/EF-CLIF collaborative study (ECLIS) J Hepatol. 2021;75(3):610–622. doi: 10.1016/j.jhep.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Kamath P S, Kim W R. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Liu Y, Cai M, et al. The Use of NACSELD and EASL-CLIF classification systems of ACLF in the prediction of prognosis in hospitalized patients with cirrhosis. Am J Gastroenterol. 2020;115(12):2026–2035. doi: 10.14309/ajg.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 21.Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL special conference 2013. J Hepatol. 2014;60(6):1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Trebicka J, Fernandez J, Papp M, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez J, Prado V, Trebicka J, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70(3):398–411. doi: 10.1016/j.jhep.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Bertuzzo V R, Giannella M, Cucchetti A, et al. Impact of preoperative infection on outcome after liver transplantation. Br J Surg. 2017;104(2):e172–e181. doi: 10.1002/bjs.10449. [DOI] [PubMed] [Google Scholar]
- 25.Wan P, Zhang J J, Li Q G, et al. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: a case-matched comparison. World J Gastroenterol. 2014;20(15):4393–4400. doi: 10.3748/wjg.v20.i15.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley D P, Fernandez L A, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.