Abstract
Aortic dissection is a rare but serious condition. Its association with pulmonary embolism is exceptional and produces a real therapeutic dilemma. We are discussing the case of a 67-year-old male patient who presented with paraplegia with infectious syndrome. The chest X-ray performed to screen for an infectious site led to the suspicion of an aortic aneurysm and the CT angiography showed Stanford type B aortic dissection associated with bilateral proximal pulmonary embolism. The treatment was symptomatic and resulted in the patient's death 48 hours after diagnosis. Management of this pathological association is not standardized between establishing anticoagulant therapy and therapeutic abstention. This management depends on the teams and has a very cautious prognosis.
Keywords: Aortic dissection, Pulmonary embolism, Therapeutic dilemma
Introduction
Aortic dissection is a tear in the aortic wall causing blood to surge between the tunica intima and tunica adventitia [1,2]. There are several classifications, but the most commonly used are Stanford and DeBakey because of their therapeutic application. Stanford's classification distinguishes 2 types depending on the location of the entry point: type A, when the entry point is located on the ascending aorta, constitutes a cardiovascular surgical emergency; type B, when the entry point is located on the descending aorta, is usually medically treated. DeBakey's classification distinguishes 3 types: type I when dissection involves the entire aorta, type II when it involves only the ascending aorta, and type III when dissection involves only the descending aorta. DeBakey types I and II correspond to Stanford type A, and type III to Stanford type B [1,2]. It is a rare condition that causes high mortality [1,2]. Pulmonary embolism is a common cardiovascular emergency, the third most common after acute coronary syndrome and stroke. The combination of these 2 conditions is exceptional and forms a true therapeutic dilemma [3,6,7], between the anticoagulant treatment for pulmonary embolism that would negatively affect the dissection and the harmful therapeutic abstention due to the embolism [3–5]. We are discussing a case to describe the radiological aspects and our therapeutic conduct.
Comments
Mr. NA, 67 years old, a retired security officer residing in Ouagadougou, consulted the Bogodogo CHU medical emergency room for a motor deficit of the lower limbs progressing for two 2 months with prolonged bed rest, gluteal bedsores and fever. He had a history of recently discovered high blood pressure, not monitored and not treated, and spinal trauma since childhood. On physical examination there was a preserved general condition, WHO stage II, clear consciousness with a Glasgow score of 15/15, blood pressure of 170/130 mmHg, heart rate of 117 bpm, temperature of 38.5°C, proportional paraplegia with motor strength of 1/5 and gluteal bedsores. Peripheral pulses were perceived and symmetrical. Oxygen saturation was normal at 98%. Regarding laboratory results, hyperleukocytosis was recorded at 17,390 /mL, predominantly neutrophilic, as well as normal hemoglobin and platelets at 399,000 /mL. CRP was high at 9.27 and serum creatinine was 165 μmol/L with a glomerular filtration rate of 46.50 mL/min. The electrocardiogram was normal with sinus rhythm. The CT scan of the lumbar spine had led to a conclusion of staged spondylarthrosis with severe discopathy L1-L2, L2-L3, and L3-L4 and compression of vertebrae L3 and L4 by more than 50% (Fig. 1). The patient was hospitalized in the Rheumatology Department. In view of the infectious and inflammatory syndrome, an infectious origin of the discovertebral lesions was suspected and a chest X-ray was performed to look for an infectious site. It showed enlargement of the middle and lower mediastinum, suggesting a thoracic aortic aneurysm (Fig. 2). A CT angiography was indicated to support this diagnosis. The aortic CT angiography performed showed Stanford type B and DeBakey type III aortic dissection associated with massive bilateral proximal pulmonary embolism (Fig. 3a). The entry point was in the descending thoracic aorta (Fig. 3b). An aneurysmal dilation of the thoracic-abdominal aorta was noted measuring 76 mm in maximum diameter at the thoracic level and 56 mm at the abdominal level, depending on the false lumen (Fig. 3b and d). The dissection extended to the left renal artery. The right renal artery emerged from the false lumen with right renal infarction (Fig. 3c). The exit point was located in the right common iliac artery and the left common femoral artery (Fig. 3d, e). The false lumen was circulating and partially thrombosed. The patient was therefore transferred to the cardiovascular resuscitation unit. After consultation with the healthcare team, the decision was made to abstain from anticoagulant therapy. Treatment was symptomatic with antihypertensive, analgesic, cough suppressant and laxative drugs. The clinical course was marked by cardiovascular arrest 48 hours after diagnosis, probably from rupture of the aorta.
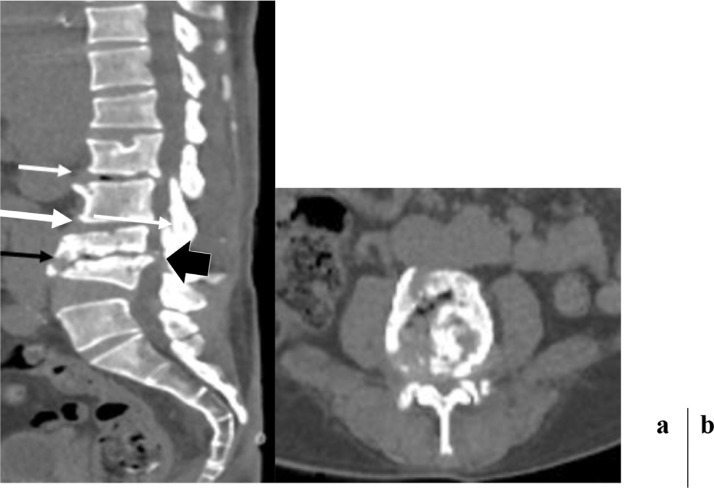
Fig. 1.
CT scan of the lumbar spine in sagittal reformation (a) and axial slice passing through L5 (b) pancake-like compression of L3 and cuneiform of L4 of at least 50% of the height of the above and underlying vertebrae. Severe narrowing of the L3-L4 interbody space with [disk void] and condensation of the adjacent vertebral endplates (black arrow). Receding of the posterior wall of L4 encroaching on the thecal sac (black arrow head). Anterior marginal [osteophytes] of L1 and L2, narrowing of the interbody spaces L1-L2 and L2-L3, and [disk void] in L1-L2 (white arrow). Fragmented, puzzle-like appearance of L4, no thickening of the perivertebral soft tissues.
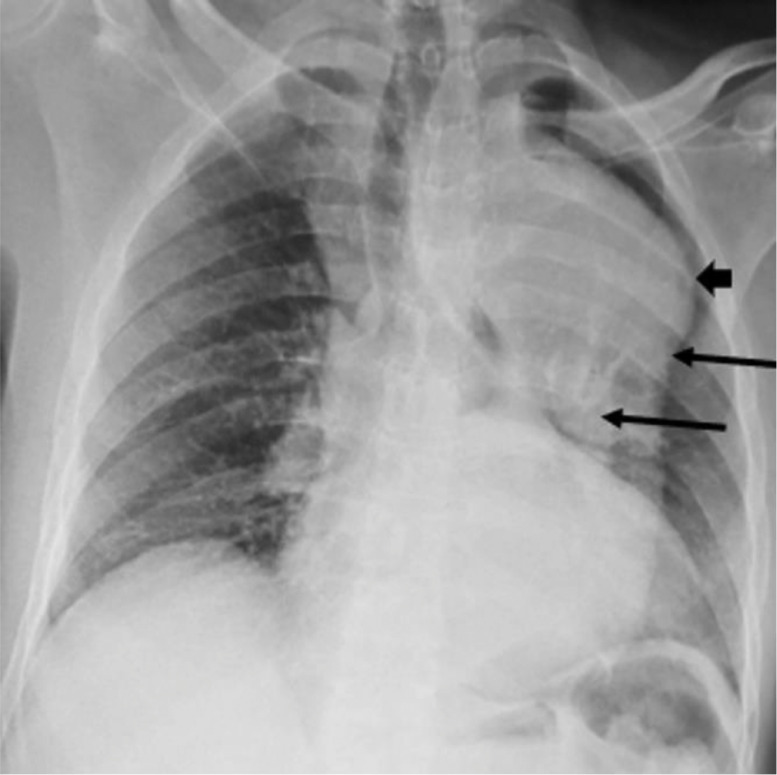
Fig. 2.
Frontal chest X-ray. Enlargement of the mediastinum and division of the edges of the aorta (arrow): a pattern suggestive of aneurysm and dissection of the aorta.
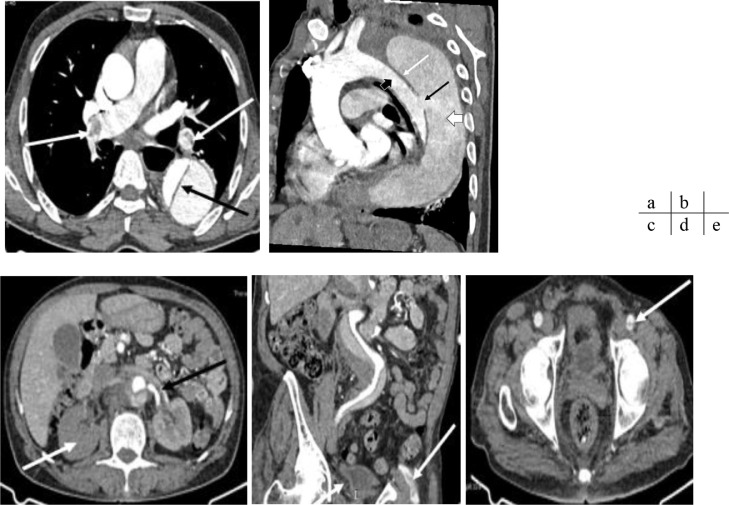
Fig. 3.
CT angiography of the chest and abdomen, axial slice (a, c, and e); sagittal (b) and coronal (d) reformation. Massive bilateral proximal pulmonary embolism (white arrows), aortic dissection (black arrow). Stanford type B aortic dissection with an entry point in the descending thoracic aorta (black arrow); the intimal flap (white arrow) separates the true lumen (black arrow head) from the false lumen (white arrow head). The false lumen is circulating and partially thrombosed. The dissection extends to the left renal artery, which is dissected (black arrow) the right renal artery emerges from the false lumen with right renal infarction (white arrow). The exit points are located in the right common iliac artery and left common femoral artery (white arrows). Aneurysmal dilation of the thoracic-abdominal aorta measuring 76 mm in maximum diameter at the thoracic level and 56 mm at the abdominal level, depending on the false lumen, extending from the aortic arch to the suprarenal aorta.
Discussion
Aortic dissection and pulmonary embolism are both potentially life-threatening cardiovascular emergencies. Their association is rare occurrence that is barely discussed. Since the first observation, reported by Leu in Taiwan in 2005, we are now presenting the 8th case in literature, to our knowledge, in a 67-year-old man from Burkina Faso [3], [4], [5], [8], [9], [10], [11]. Although aortic dissection is rare, type A is the most common. Indeed, 5 of the 7 cases described in literature were type A dissections associated with pulmonary embolism and only 2 were type B [1], [2], [3], [4]. Our patient is the third type B reported. The typical patient profile appears to be the elderly male subject. Chaulagai in the United States, Ng in Australia, Ramponi in Australia, and Tudoran in Romania reported cases of male subjects aged between 61 and 75, much like our patient who was 67 years old and male [3], [4], [5], [8]. This could be explained by the fact that the most common risk factor is high blood pressure which occurs in this patient profile. Indeed, like our patient, 6 of the 7 cases reported had high blood pressure [1]. However, the rarity of the observations means that conclusions cannot be drawn. Among the acquired risk factors, tobacco and drug use have been reported [1], [3]. None of these factors were found in our patient. Apart from these acquired factors, we have hereditary factors like Marfan syndrome, Ehlers-Danlos syndrome, and familial aortic dissection which occur in younger patients, as illustrated by the case reported by Herrera et al. In Argentina in a 47-year-old female patient with Marfan syndrome [1], [2], [9]. The common clinical circumstances leading to discovery are respiratory symptoms such as chest pain, dyspnea and desaturation. These symptoms may be related to both the embolism and dissection [1], [2], [6]. But the clinical circumstances may be minimally suggestive, as in our case where the patient had no respiratory symptoms. A similar situation was described by Chaulagai in the United States where the patient complained of nausea, vomiting and dizziness with an almost incidental finding of pulmonary embolism and aortic dissection [3]. The electrocardiogram may show signs of pulmonary embolism with an S1Q3 appearance as described by Rokotoniaina in Madagascar [10]. However, the normality of the ECG does not rule out the diagnosis. Regarding laboratory results, increased D-dimers are almost always observed in relation to pulmonary embolism. It should be noted that while this examination has an excellent negative predictive value, its specificity remains very low because it is often high in various situations [6], [7]. The key test for the diagnosis of these 2 conditions remains a CT angiography of the chest and abdomen. This scan is both very sensitive and very specific for the diagnosis of proximal and distal pulmonary embolism up to the subsegmental branches of the pulmonary artery [1], [2], [6], [7]. In most cases reported, pulmonary embolism is proximal and often bilateral. It is rarely distal and unilateral. The CT angiography enables the positive diagnosis of dissection by showing the entry point, the intimal flap, the true and false lumen, as well as the exit point and possible re-entry points. It also specifies whether or not the false lumen is circulating and determines the extent of the dissection to the visceral artery branches. It can also screen for signs of aortic prerupture [1], [2]. If the diagnosis poses less concern, the challenge of this morbid association remains the treatment. Indeed, the anticoagulant treatment indicated for pulmonary embolism is contraindicated for dissection. Given the rarity of the case, no protocol exists for therapeutic management. In type A dissection, treatment is firstly surgical with the ascending aorta being replaced by a Dacron tube [1], [2], [6], [7]. Some teams have taken advantage of this surgery time to successfully perform a thrombectomy, then give anticoagulation treatment to prevent recurrence followed by close monitoring [5]. In type B, treatment is usually medical, and surgery is performed if there is evidence of prerupture. Given the association with embolisms, some teams opted for surgery with anticoagulation treatment and a vena cava filter with close multidisciplinary monitoring [4]. Finally, other teams, such as Chaulagai's teams in the United States and our team, opted for symptomatic treatment and close monitoring [3]. The short-term prognosis is often good at the expense of close, multidisciplinary management [3], [4], [5], [9]. However, cases of death on the operating table or in postoperative recovery have been reported [8].
Conclusion
The association of type B aortic dissection and pulmonary embolism is a morbid combination that renders the caregiver powerless, between harmful therapeutic abstention due to the embolism and anticoagulant treatment which negatively affects the dissection. In the absence of a clear protocol, patient management is left to the care of multidisciplinary teams that adjust management with more or less significant outcomes.
Patient consent
The authors declare that the work described did not involve experiments on the patient. The authors declare that the article does not contain any personal data that can allow identifying the patient. As the patient died, we got the authorization from his close relative to publish this observation.
Footnotes
Competing Interests: The authors declare that there is no conflict of interest.
Contributor Information
Bénilde Marie-Ange Tiemtoré-Kambou, Email: kbenildema@yahoo.fr.
Adjirata Koama, Email: adjikoama@gmail.com.
Solange Kontogom, Email: kontsolo@yahoo.fr.
Joelle Zabsonré/Tiendrébéogo, Email: t_joelle@hotmail.com.
Donald Bayala, Email: macdonald81@yahoo.fr.
Nina Astrid Ndé/Ouédraogo, Email: ninawed@hotmail.com.
Moussa Zanga, Email: zazomo@yahoo.fr.
Aischa Madina Napon, Email: napon.madina@gmail.com.
Ousséini Diallo, Email: odiallo75@yahoo.fr.
Claudine Lougue-Sorgho, Email: lougueclaudine@gmail.com.
Rabiou Cissé, Email: ciss.rabi@gmail.com.
References
- 1.Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628–635. doi: 10.1161/01.CIR.0000087009.16755.E4. [DOI] [PubMed] [Google Scholar]
- 2.Badidi M, Benyass A, Lakhal Z, Chaib A, Raissouni M, et al. Conduite à tenir devant une dissection aortique aigue. Maroc Méd. 2009;31(3):213–218. [Google Scholar]
- 3.Chaulagai B, Acharya D, Poudel S, Puri P. Simultaneous aortic dissection and pulmonary embolism: a therapeutic dilemma. Cureus. 2021;13(1):e12952. doi: 10.7759/cureus.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng E, Ekladious A, Wheeler LP. Thrombus risk versus bleeding risk: a clinical conundrum. BMJ Case Rep. 2019;12(3) doi: 10.1136/bcr-2018-228344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramponi F, Papps T, Edwards J. Successful repair of concomitant acute type A aortic dissection and saddle pulmonary embolism. Aorta (Stamford) 2018;6(1):34–36. doi: 10.1055/s-0038-1639345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Righini M, Robert-Ebadi H, Le Gal G. Diagnosis of acute pulmonary embolism. J Thromb Haemost. 2017;15(7):1251–1261. doi: 10.1111/jth.13694. [DOI] [PubMed] [Google Scholar]
- 7.Doherty S. Pulmonary embolism: an update. Aust Fam Physician. 2017;46(11):816–882. [PubMed] [Google Scholar]
- 8.Tudoran M, Tudoran C. High-risk pulmonary embolism in a patient with acute dissecting aortic aneurysm. Niger J Clin Pract. 2016;19(6):831–833. doi: 10.4103/1119-3077.181355. [DOI] [PubMed] [Google Scholar]
- 9.Herrera RN, Miotti JA, Pereyra AS, Lobo MV, Ibarra MT, Tomé Guzmán AF. Marfan syndrome associated with aortic dissection, venous thromboembolism and hyperhomocysteinemia. Medicina (B Aires) 2012;72(06):478–480. [in Spanish] [PubMed] [Google Scholar]
- 10.Masinarivo DR, Rakotomanana JL. Aspect clinico-électrocardiographique d'embolie pulmonaire masquant une dissection aortique révélé par l'angioscanner thoracique [Clinical and electrocardiographic aspect of pulmonary embolism masking aortic dissection revealed by thoracic CT angiography] Pan Afr Med J. 2017;28:3. doi: 10.11604/pamj.2017.28.3.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leu HB, Yu WC. Images in cardiology: massive pulmonary embolism in a patient with type A aortic dissection. Clin Cardiol. 2005;28(01):53–57. doi: 10.1002/clc.4960280113. [DOI] [PMC free article] [PubMed] [Google Scholar]