Abstract
Background:
Type 2 diabetes mellitus (T2DM) afflicts about six percent of the global population, and these patients suffer from a two-fold increased fracture risk. Thiazolidinediones (TZDs), including rosiglitazone, are commonly used medications in T2DM because they have a low incidence of monotherapy failure. It is known that rosiglitazone is associated with secondary osteoporosis, further increasing the fracture risk in an already susceptible population. However, it is not yet understood how rosiglitazone impacts endochondral bone healing after fracture. The aim of this study is to elucidate how rosiglitazone treatment impacts endochondral fracture healing, and how rosiglitazone influences the differentiation of skeletal stem and progenitor cells from the bone marrow and the periosteum.
Methods:
An in-vivo mouse femur fracture model was employed to evaluate differences in fracture healing between mice treated with and without rosiglitazone chow. Fracture healing was assessed with histology and micro computed tomography (μCT). In-vitro assays utilized isolated mouse bone marrow stromal cells and periosteal cells to investigate how rosiglitazone impacts the osteogenic capability and adipogenicity of these cells.
Results:
The in-vivo mouse femur fracture model showed that fracture callus in mice treated with rosiglitazone had significantly more adipose content than those of control mice that did not receive rosiglitazone. In addition, μCT analysis showed that rosiglitazone treated mice had significantly greater bone volume, but overall greater porosity when compared to control mice. In-vitro experimentation showed significantly less osteogenesis and more adipogenesis in bone marrow derived progenitor cells that were cultured in osteogenic media. In addition, rosiglitazone treatment alone caused significant increases in adipogenesis in both bone marrow and periosteum derived cells.
Conclusion:
Rosiglitazone impairs endochondral fracture healing in mice by increasing adipogenesis and decreasing osteogenesis of both bone marrow and periosteum derived skeletal progenitor cells.
Introduction:
An estimated 6% of the global population is afflicted by type 2 diabetes mellitus (T2DM).[1] These patients suffer from a two-fold increased fracture risk, despite having increased bone mineral density. Factors such as increased falls, renal disease, and metabolic dysregulation contribute to this proclivity.[2] T2DM has been observed to alter the fate of multipotent progenitor mesenchymal stem cells (MSCs), which can differentiate into both osteoblasts and adipocytes.[3] The balance between these two cell lineages dictates the overall quality of resultant bone. In T2DM, hyperglycemia promotes adipogenesis in known osteo-primed cell types, increases osteoblast apoptosis, and inhibits osteoclastogenesis.[4–6] Conversely, insulin signaling stimulates osteoblast production and differentiation.[7]
Understanding how medications used to treat T2DM impact bone remodeling is a topic of ongoing research. In the United States, about 20% of patients with prediabetes and T2DM are treated with thiazolidinediones (TZDs).[8] This medication class has a lower cumulative incidence of monotherapy failure compared to other glucose-lowering drugs such as metformin and glyburide in T2DM.[9] TZDs, such as the widely used rosiglitazone, regulate glucose and lipid metabolism by signaling through peroxisome proliferator-activated receptor γ (PPAR-γ). In bone, PPAR-γ modulates the differentiation of mesenchymal progenitor cells toward bone forming osteoblasts and hematopoietic progenitor cells toward bone resorbing osteoclasts.[7, 10–12] Animal studies have demonstrated that bony changes following rosiglitazone treatment include decreased trabecular bone volume, decreased number of osteogenic cells, and decreased bone strength.[12–14] Therefore, it is unsurprising that rosiglitazone is associated with secondary osteoporosis and a two-fold increase in fracture risk in the already fracture prone diabetic population.[15]
As the diabetic population grows, significantly more patients taking rosiglitazone will suffer from fractures. Yet, limited evidence exists describing how rosiglitazone impacts endochondral fracture healing, an understanding vital to the practicing orthopaedic surgeon. In endochondral fracture healing, cells from both the marrow space and periosteum are vital for bony regeneration. Although it is known that rosiglitazone can increase adipogenicity in the marrow space, it is unknown how this medication influences the differentiation capacity of stem and progenitor cells (SSPCs) specifically from the periosteum.[6] Therefore, the aim of this study is to utilize an in vivo mouse femur fracture model to elucidate how endochondral fracture healing is influenced by rosiglitazone treatment. In addition, via in vitro assays, we aim to better characterize how the differentiation profiles of SSPCs from the marrow space and the periosteum are individually impacted by rosiglitazone.
Materials and Methods
Mice
This research was approved by the institutional committee on animal research of the authors’ affiliated institution. The studies were conducted on C57BL/6J mice purchased from Jackson Laboratories (Farmington, CT, USA). Food for the mice was either standard pellets or pellets supplemented with rosiglitazone (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 0.14 mg of rosiglitazone per 1 gram of pellets as previously described.[6]
Femur Defect Model
We utilized a mouse femoral shaft fracture model to study rosiglitazone’s effects on endochondral bone formation. The surgery and post-operative care were as previously described.[16] Briefly, mice were anesthetized with 1–5% isoflurane inhalation and an incision was made along the anterolateral femur. A 27-gauge needle was inserted retrograde in the femur via a small incision medial to the patellar tendon. The needle was partially withdrawn, and the mid-shaft of the femur was transected with surgical scissors. The needle was then re-inserted into the femur to stabilize the fracture. The soft tissues were closed. Mice were allowed to ambulate freely after the procedure. Pain was controlled with buprenorphine 1 mg/kg twice daily for 3 days. The mice were euthanized by CO2 asphyxiation followed by cervical dislocation at post-operative days (POD) 14 and 28 and the femurs were harvested for analysis. Mice exposed to rosiglitazone were fed food pellets supplemented with rosiglitazone as above for two weeks prior to the surgery and during the entire post-surgical period.
Histology Staining
Histology slides were prepared as previously described.[17] After careful dissection, femurs were fixed in 4% paraformaldehyde overnight at 4°C and then decalcified in 19% EDTA at 4°C. Femurs were paraffin embedded at cut into 10μm thick sections. Pentachrome staining was performed to identify osseous tissue as previously described.[18] Sections were photographed using a Leica digital imaging system (Wetzlar, Germany).
μCT Analyses
Analyses were completed as previously described.[17] In brief, samples were scanned using a high-resolution SkyScan μCT system (Bruker, Billerica, MA, USA). Images were acquired at 9μm isotropic resolution using a 10MP digital detector, 10W energy (100 kV and 100A), and a 0.5mm aluminum filter with 9.7 μm image voxel size. A fixed global threshold method was used based on the manufacturer’s recommendations and preliminary studies, which showed that mineral variation between groups was not high enough to warrant adaptive thresholding. The following parameters were analyzed: total bone volume (BV), total tissue volume (TV), respective mineralized volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.S), total porosity (%), and polar moment of inertia following described guidelines.[19] The volume of interest included a region of the femur with the defect centered including bone distal and proximal to the injury site in order to capture periosteal callus formation outside of the defect. The volume of interest was contoured to capture the entire callus region and total volume represents the entire callus volume within the mentioned volume of interest.
Bone Marrow Stromal Cell Isolation
Bone marrow stromal cells (BMSCs) were obtained from the femurs of the above-described mice as previously described.[20] The proximal and distal ends of the bones were removed with dissection scissors. The bones were subsequently centrifuged (1,400 rpm) into 500μL tubes containing 100μL of growth media consisting of DMEM (Thermo Fisher Scientific, Waltham, MA, USA), 10% fetal bovine serum (FBS) (Thermo Fisher Scientific), and 1% penicillin/streptomycin (Thermo Fisher Scientific). The growth media and cells were transferred to T150 culture flasks (USA Scientific, Ocala, FL, USA) at 37°C and 5% CO2.
Periosteal Cell Isolation
Periosteal cell isolation was conducted as previously described from the femurs and tibias of the above mice.[21] Cells were plated in growth media DMEM (Thermo Fisher Scientific), 10% FBS (Thermo Fisher Scientific), and 1% penicillin/streptomycin (Thermo Fisher Scientific) at 37°C and 5% CO2.
Osteogenic Differentiation and Alizarin Red Staining
Bone marrow stromal cells from mice femurs and tibias were isolated and submitted to osteogenic differentiation, alizarin red staining, and in vitro mineralization quantification as previously described.[21] Media was supplemented with either DMSO or rosiglitazone at the listed concentrations, determined from prior work.[8] Assays were run in triplicate in 6-well culture plates (USA Scientific) with cells from three animals.
Oil-Red-O Staining
Bone marrow stromal cells and periosteal progenitor cells from mice femurs and tibias were isolated as described above and cultured in growth media consisting of DMEM containing 10% FBS and 1% penicillin/streptomycin supplemented with either DMSO or rosiglitazone at the listed concentrations, determined from prior work.[8] The media was replaced every 4 days. After 10–14 days, cultured were stained with Oil-Red-O (Sigma-Aldrich). The cells were washed twice with distilled water after staining. Pictures were obtained on a Leica DMi1 microscope at 20x magnification. Oil-Red-O was subsequently eluted from the cells in 100% isopropanol and quantified on a Flexstation 3 Multi-mode Microplate Reader with SoftMax Pro software at 540nm (Molecular Devices, San Jose, CA, USA). Assays were run in triplicate in 6-well culture plates with cells from three animals.
Quantitative RT-PCR
Total RNA was isolated from either BMSCs or periosteal cells using an RNeasy mini kit (Qiagen, Germantown, MD, USA). The RNA was reverse transcribed into cDNA with the iScript™ cDNA Synthesis Kit (Biorad, Hercules, CA, USA). The cDNA was amplified for specific targets using specific primers listed below (Integrated DNA Technologies, Coralville, IA, USA) and RT SYBR Green Rox PCR Master Mix (Qiagen) in a QuantStudio3 Real-Time PCR System (Thermo Fisher Scientific) as previously described.[17] Results are presented as 2−ΔΔCt values normalized to the expression of 18S. All reactions were performed in triplicate.
M. musculus Osterix Forward GGAGACCTTGCTCGTAGATTTC
M. musculus Osterix Reverse GGGATCTTAGTGACTGCCTAAC
M. musculus PPARɣ Forward ATAGGTGTGATCTTAACTGCCG
M. musculus PPARɣ Reverse CCAACAGCTTCTCCTTCTCG
M. musculus FABP4 Forward AAGAAGTGGGAGTGGGCTTT
M. musculus FABP4 Reverse AATCCCCATTTACGCTGATG
M. musculus 18S Forward ACGAGACTCTGGCATGCTAACTAGT
M. musculus 18S Reverse CGCCACTTGTCCCTCTAAGAA
Statistical Analysis
Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical computations. A Student’s t test was used for all comparisons in which there were two groups. Error bars represent standard deviation. Results were considered to be statistically significant at a p value of less than 0.05. A single asterisk symbol (*) denotes a p value of less than 0.05, two ** denotes a p value of less than 0.01, and three *** denotes a p value of less than 0.001.
Results
Rosiglitazone causes weight gain in mice
We utilized a femur defect model in 12-week-old mice to investigate the effects of rosiglitazone administration on fracture healing. One group of mice was given a standard diet while the other group was given a rosiglitazone-supplemented diet starting two weeks prior to the planned the femur defect surgery. There were 12 mice in each cohort. As expected from prior studies, the mice that were given a rosiglitazone diet gained significantly more weight than mice provided with the standard diet (Figure 1).[13] After 13 days of treatment, the weight of the control mice averaged 27.00 ± 2.28 g while the rosiglitazone treated mice weighed an average of 29.75 ± 3.17g (p=0.0086). A significant difference in weight persisted between the two cohorts until the end of experimentation at 34 days where 6 mice in each cohort remained. At this time, the control mice weighed an average of 27.00 ± 2.90 g while the rosiglitazone mice weighed 30.83 ± 0.75 g (p=0.0106).
Figure 1:
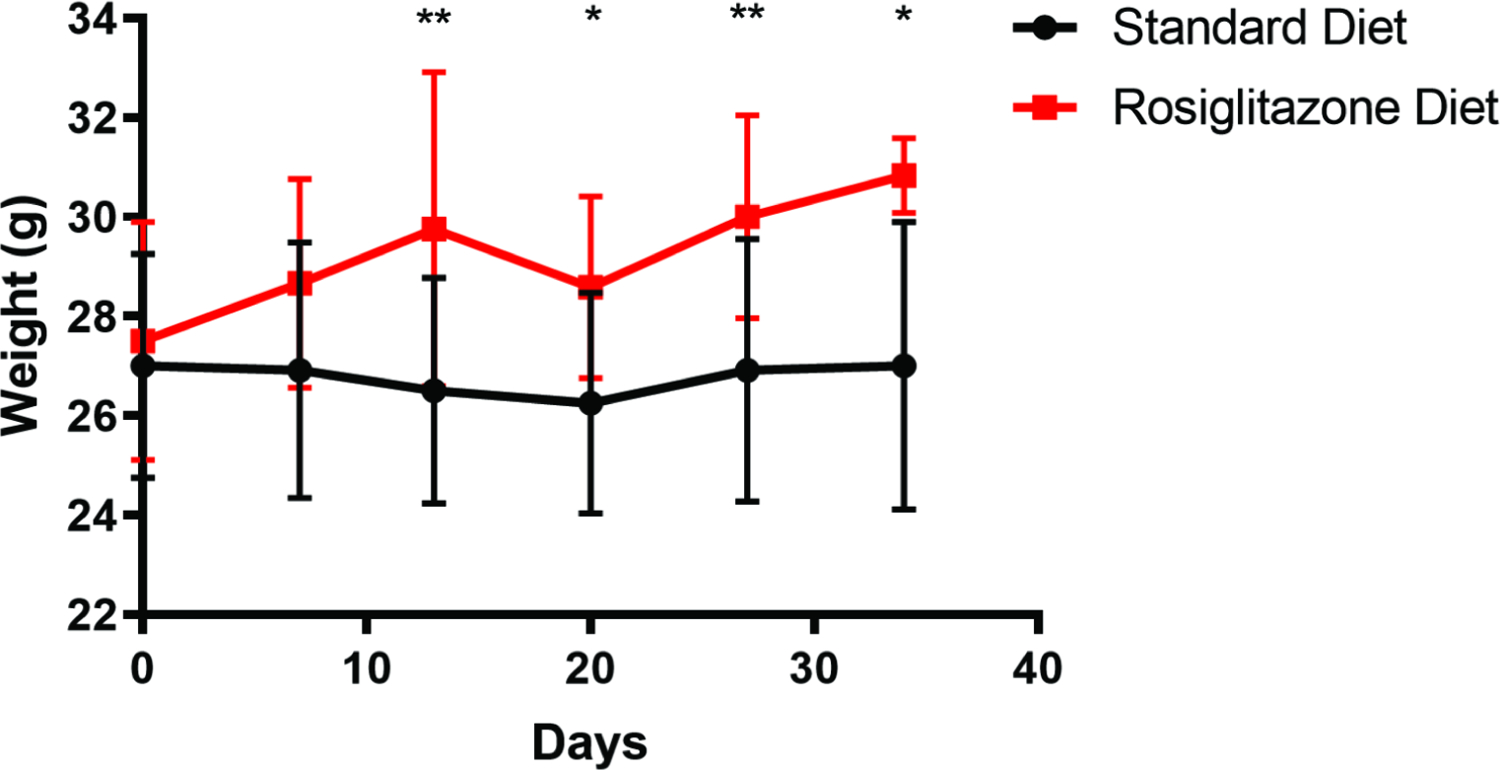
Weights (grams) of mice provided with a standard diet or standard diet supplemented with rosiglitazone (N=12 in each group). Recording of weights began on the day the mice were provided their respective diets. *, p<0.05; **, p<0.01.
Rosiglitazone disrupts fracture callus structure in mice
POD28 after femoral defects were made, femurs were harvested from 3 control mice and from 3 rosiglitazone treated mice. Qualitative histology utilizing Movat’s pentachrome staining was used to evaluate for any differences in structural properties of the callus at the fracture sites (Figure 2). It was observed that the fracture callus was more heterogenous in the rosiglitazone group when compared to the control group. The control group had visible, organized cortical bone regenerate, while the rosiglitazone regenerate was disordered and often not continuous. Interestingly, the callus in the rosiglitazone group was larger and filled with significantly more adipose content. This fat was not only in the marrow space as previously described, but also in the periphery of the callus normally generated by the periosteum.
Figure 2:
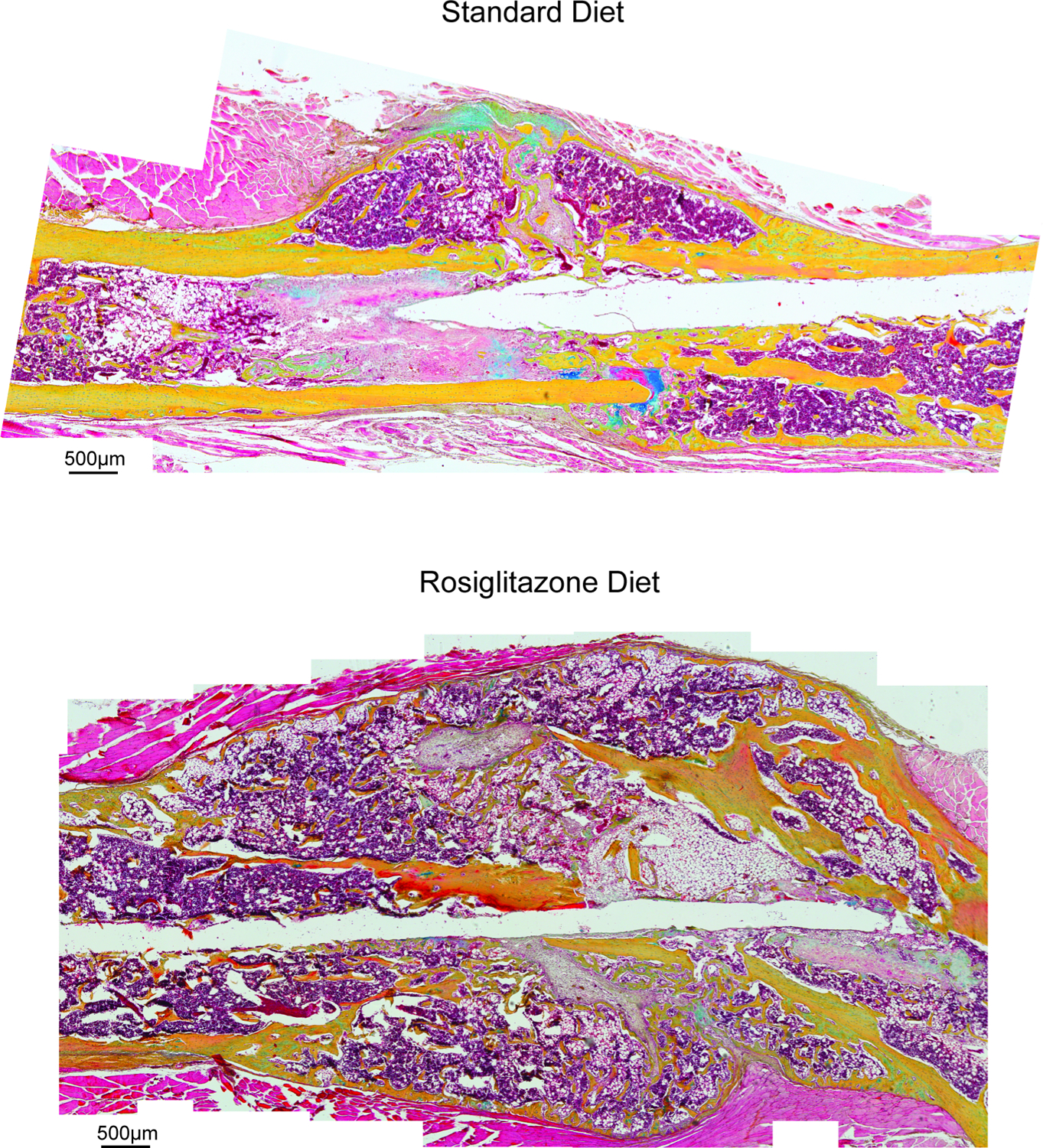
Stitched images demonstrating pentachrome staining of histological slices sampled from the fracture callus present POD28 after a femoral defect in a mouse provided with either a standard diet or rosiglitazone supplemented diet. Scale = 500μm.
For better examination of the quality of bone healing, μCT analyses were completed for harvested femurs at POD14 and POD28 (Figure 3). At each time point, 3 femurs were harvested from both the control and the rosiglitazone groups. Histomorphometry confirmed the findings shown in the pentachrome histology. The rosiglitazone treated mice had significantly higher bone volume and total volume at POD28. At POD14, bone volume/total volume [BV/TV] was significantly reduced in the rosiglitazone mice, but not at POD28. There was no significant difference in trabecular thickness, but interestingly, trabecular number was reduced in the rosiglitazone bone mice at both time points. At POD14 and 28, trabecular spacing tended to be higher in the rosiglitazone mice, but this did not reach significance. In terms of total bone porosity, the rosiglitazone group had more porous bone at POD14, but this difference was not seen at POD28. These findings support the formation of a weaker, more disorganized callus structure and bone regenerate in the rosiglitazone treated mice compared to the control group.
Figure 3:
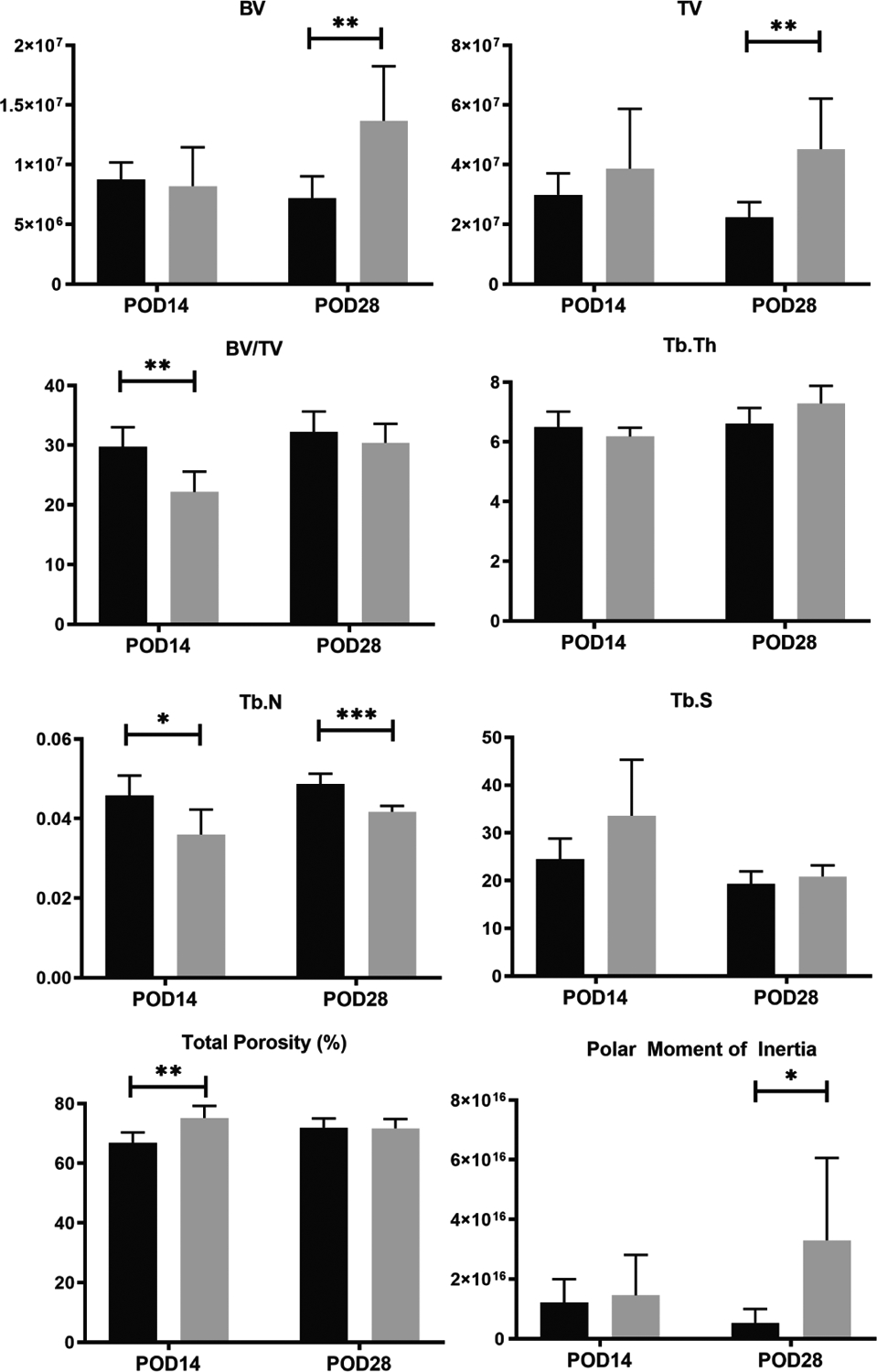
Fracture callus μCT analyses in mice provided with a standard diet (black) or rosiglitazone supplemented diet (grey). *, p<0.05; **, p<0.01; ***, P<0.001. BV, bone volume; TV, trabeculae volume; TB.Th, trabeculae thickness; Tb.N, trabeculae number; Tb.S, trabeculae spacing.
Rosiglitazone negatively regulates osteogenic differentiation in vitro
After significant differences were seen in the femur fracture calluses of mice treated with rosiglitazone, attention was turned to understanding how rosiglitazone impacts osteogenic differentiation. Bone marrow stromal cells were isolated from 10-week old mouse femurs. These BMSCs were treated with standard growth media, osteogenic media, or osteogenic media with 1μM or 10μM of rosiglitazone. After 10 days in culture, alizarin red staining was performed and quantified to evaluate differences in osteoid production (Figure 4A). When compared to the cells treated in only osteogenic media, cells in osteogenic media treated with 1μM or 10μM rosiglitazone showed significantly reduced osteogenesis (p=0.0110 and p=0.0049, respectively).
Figure 4A and 4B:
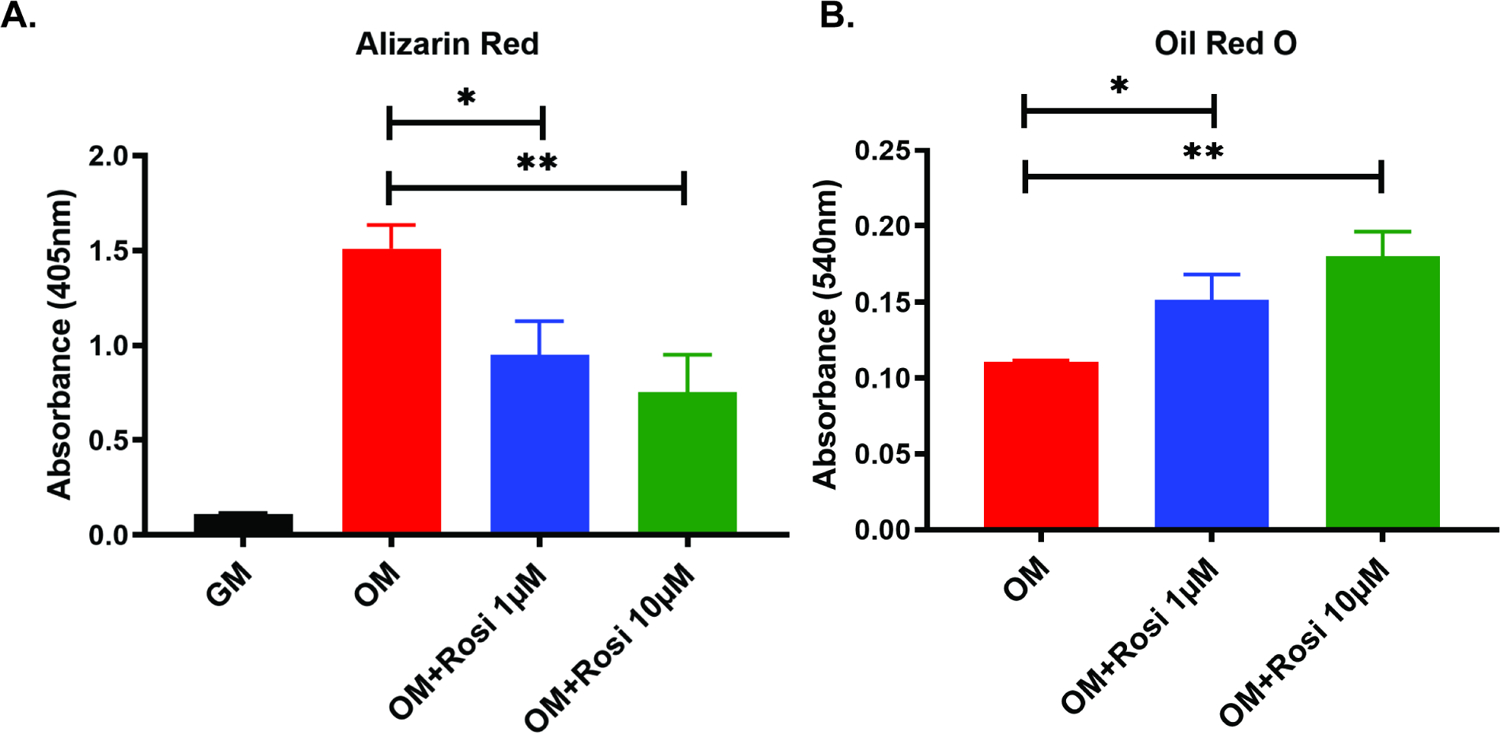
Quantification via absorbance of alizarin red staining (4A) and Oil-Red-O staining (4B) of mouse BMSCs cultured in osteogenic differentiation media with or without rosiglitazone. *, p<0.05; **, p<0.01. GM, growth media; OM, osteogenic media; Rosi, rosiglitazone.
In addition, Oil-Red-O staining was performed to determine if there was an increase in adipogenic differentiation in cells treated with rosiglitazone despite an osteogenic culture environment (Figure 4B). The cells treated with 1μM and 10μM rosiglitazone in osteogenic media had significantly more fat content than cells treated with only osteogenic media (p=0.0131 and p=0.0017, respectively), suggesting that in addition to reducing osteogenic differentiation, progenitor cells are pushed toward adipogenesis.
Osterix (Osx) is a transcription factor upregulated during osteogenesis. Quantitative PCR data (Figure 5) showed that although Osx increased 18-fold in cells treated with osteogenic media when compared to growth media (p= 0.0001), this expression fell more than 4-fold when 10μM rosiglitazone was added to the osteogenic media (p= 0.0004).
Figure 5:
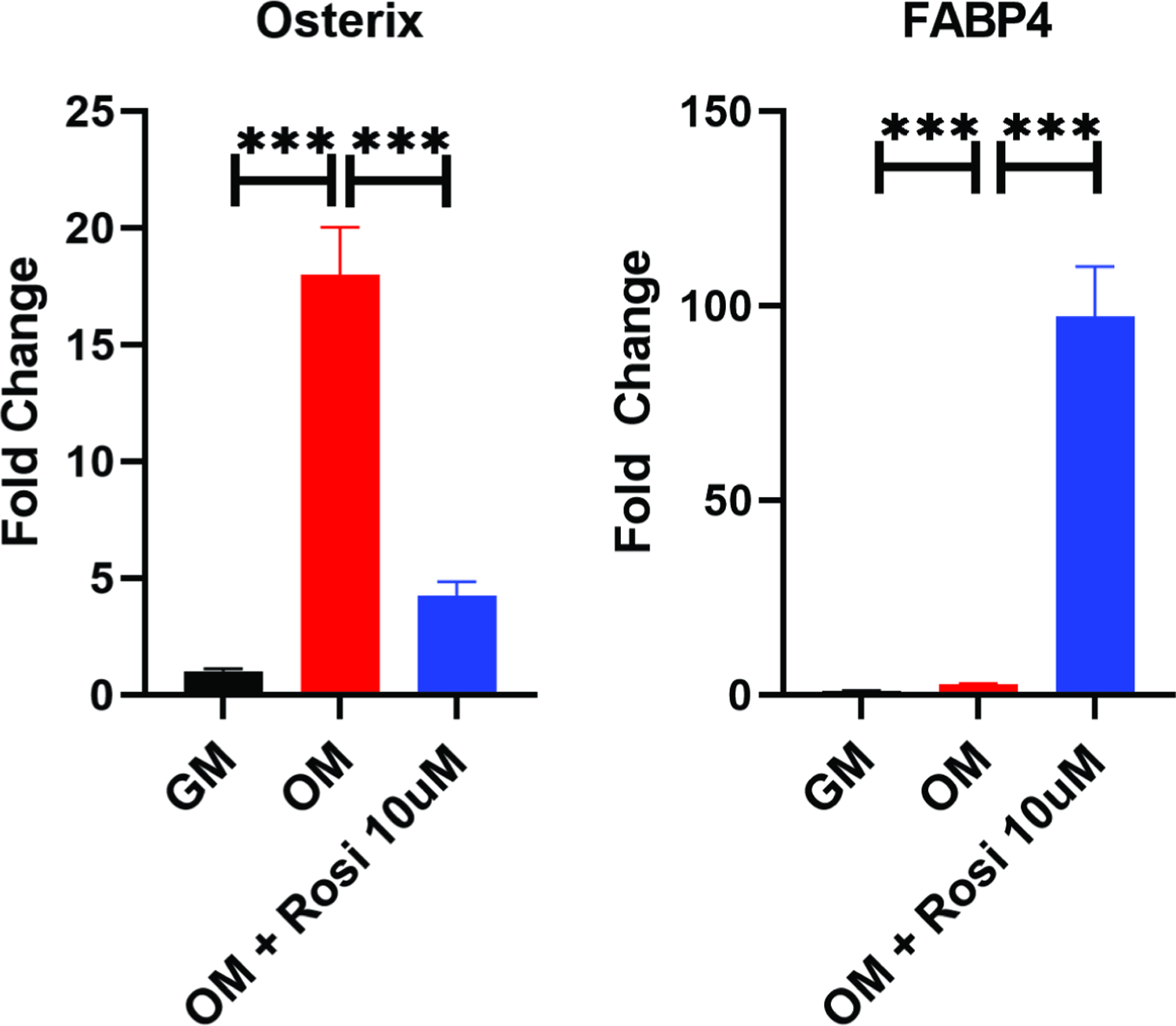
Quantitative PCR of mouse BMSCs cultured in OM with or without rosiglitazone. Example experiments completed in technical triplicates are shown.; ***, P<0.001. GM, growth media; OM, osteogenic media; Rosi, rosiglitazone.
Fatty Acid Binding Protein-4 (FABP4), which is expressed by adipocytes and therefore an appropriate marker of adipogenic differentiation, increased 8-fold in cells in osteogenic media compared to those in growth media (p=0.0002). However, there was over a 300-fold increase in the cells in osteogenic media supplemented with rosiglitazone (p=0.0002). Altogether, these findings demonstrate that the supplementation of osteogenic media with rosiglitazone inhibits osteogenic differentiation in favor or adipogenesis.
Rosiglitazone leads to increased adipogenesis of both mouse BMSCs and periosteal cells
We next performed in vitro assays of both BMSCs and periosteal cells to determine if the observed adipogenic effects from rosiglitazone supplementation were specific to one or both cell types. BMSCs and periosteal cells were isolated from 10-week-old mice and cultured for 14 days in either standard growth media with vehicle control or growth media supplemented with 10μM rosiglitazone.
In BMSCs, Oil-Red-O staining (Figure 6A and 6B) showed rosiglitazone treatment significantly increased adipogenesis (p=0.0167). FABP4 showed a 40-fold increase in the rosiglitazone treated cells when compared to cells in standard growth media (p=0.0012), but PPAR-γ showed no significant change (Figure 7).
Figure 6A and 6B:
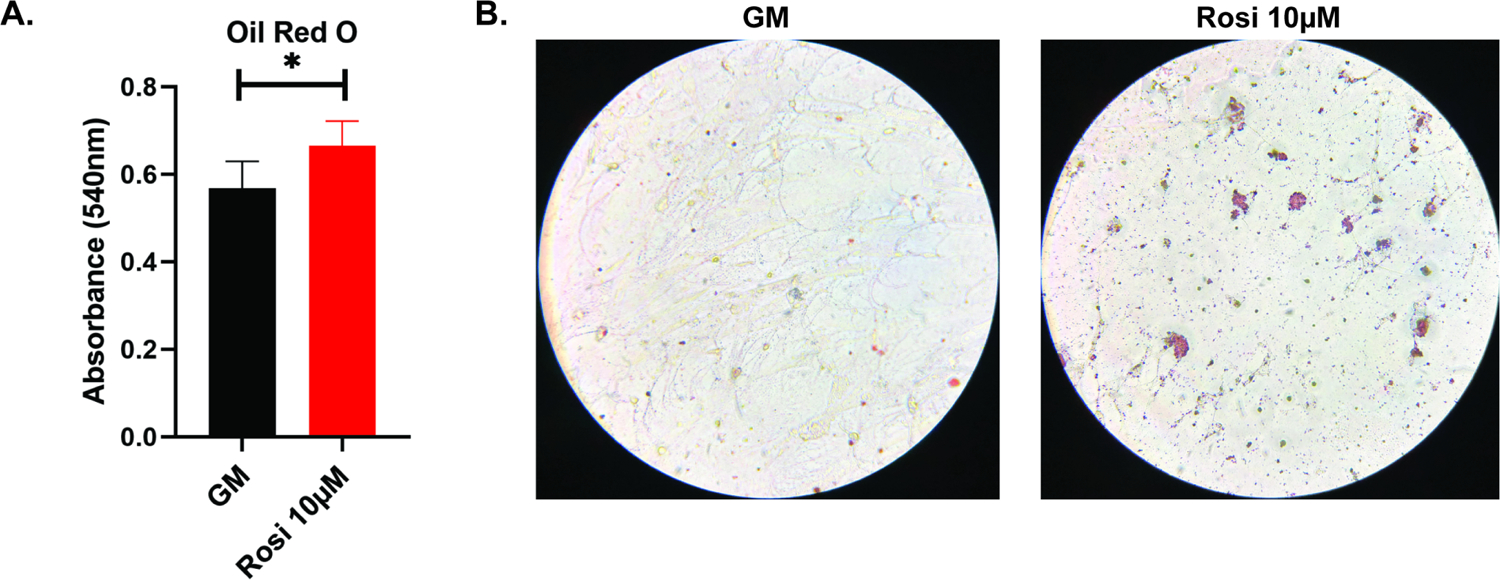
Quantification (6A) and imaging (6B) of Oil-Red-O staining of mouse BMSCs cultured with or without rosiglitazone. *, p<0.05. GM, growth media; Rosi, rosiglitazone.
Figure 7:
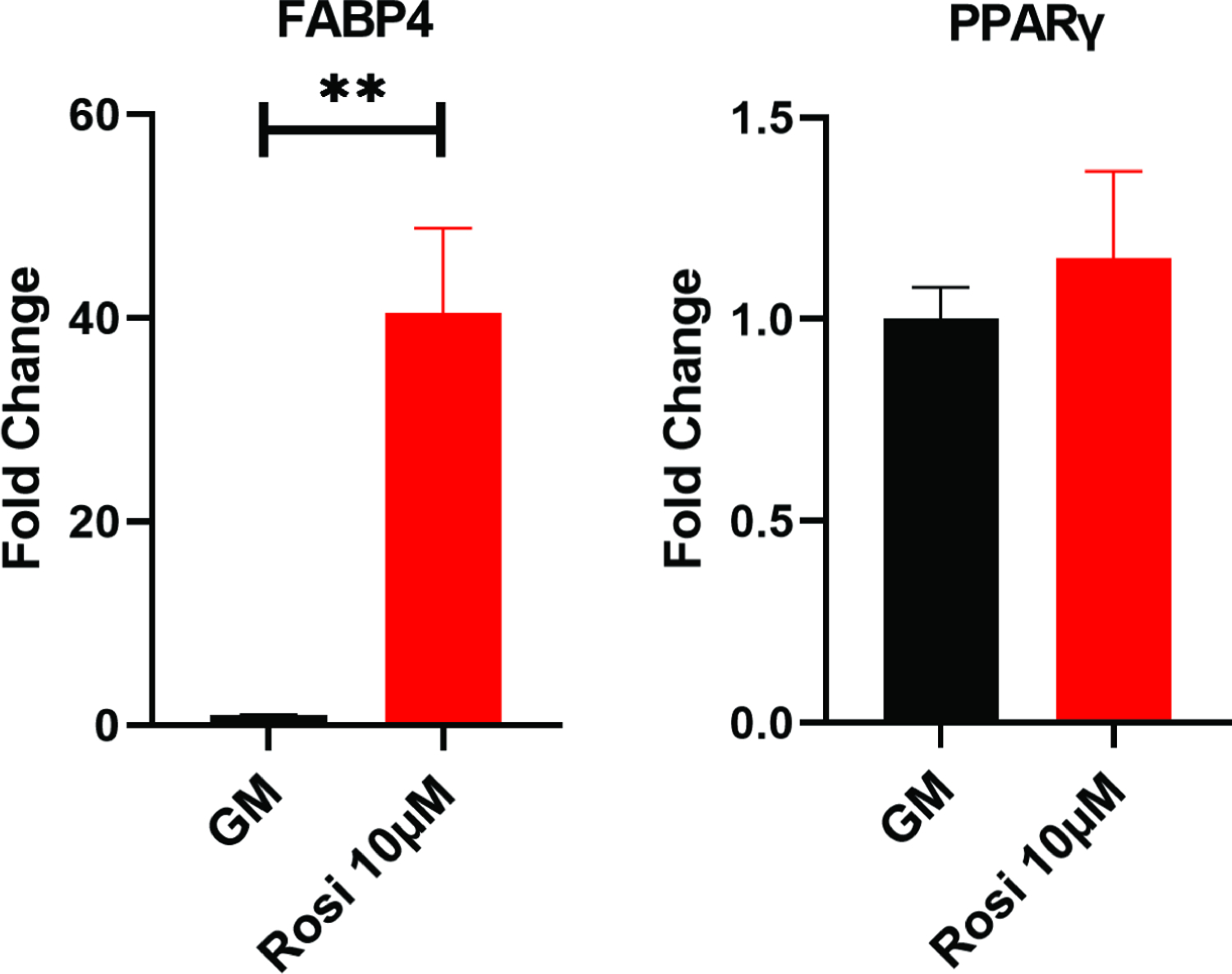
Quantitative PCR of mouse BMSCs cultured with or without rosiglitazone. Example experiments completed in technical triplicates are shown. **, p<0.01. GM, growth media; Rosi, rosiglitazone.
In periosteal cells, Oil-Red-O staining (Figure 8A and 8B) showed rosiglitazone treatment significantly increased adipogenesis (p=0.0028). FABP4 showed a 28-fold increase in the rosiglitazone treated cells (p=0.0004), and PPAR-γ showed a 1.5-fold increase (p=0.038) (Figure 9). These results support rosiglitazone as being capable of driving adipogenesis in both BMSCs and periosteal cells.
Figure 8A and 8B:
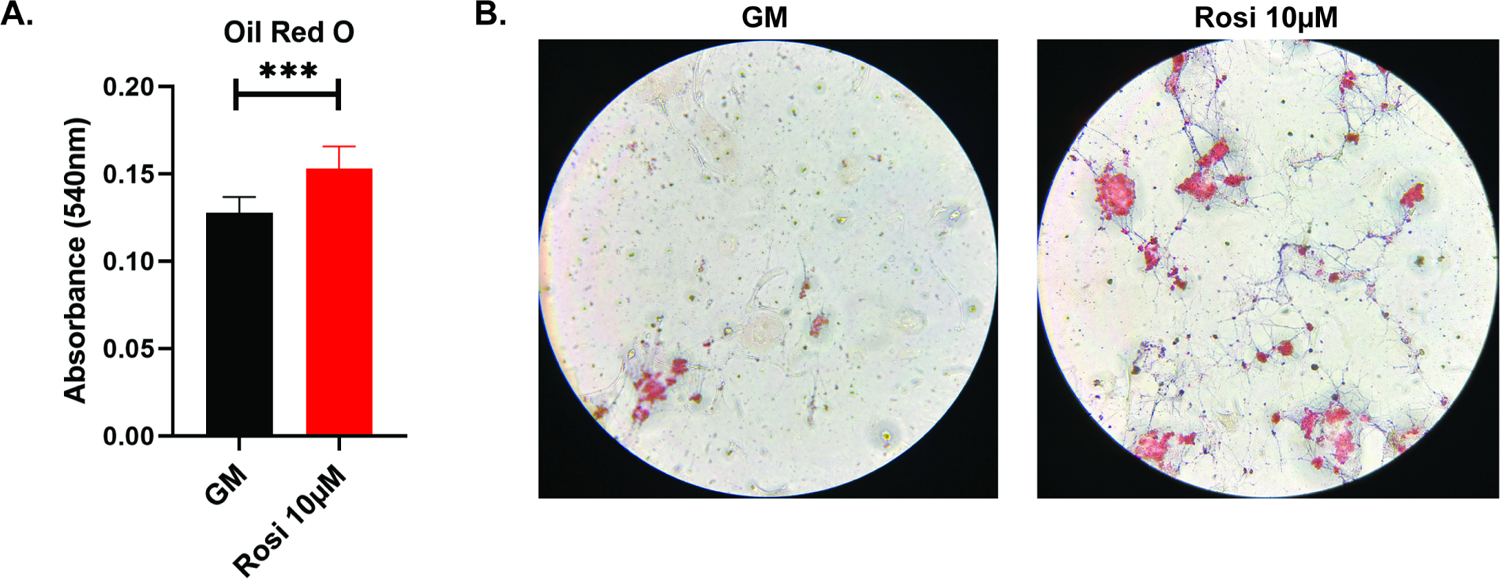
Quantification (8A) and imaging (8B) of Oil-Red-O staining of mouse periosteal cells cultured with or without rosiglitazone. ***, p<0.001. GM, growth media; Rosi, rosiglitazone.
Figure 9:
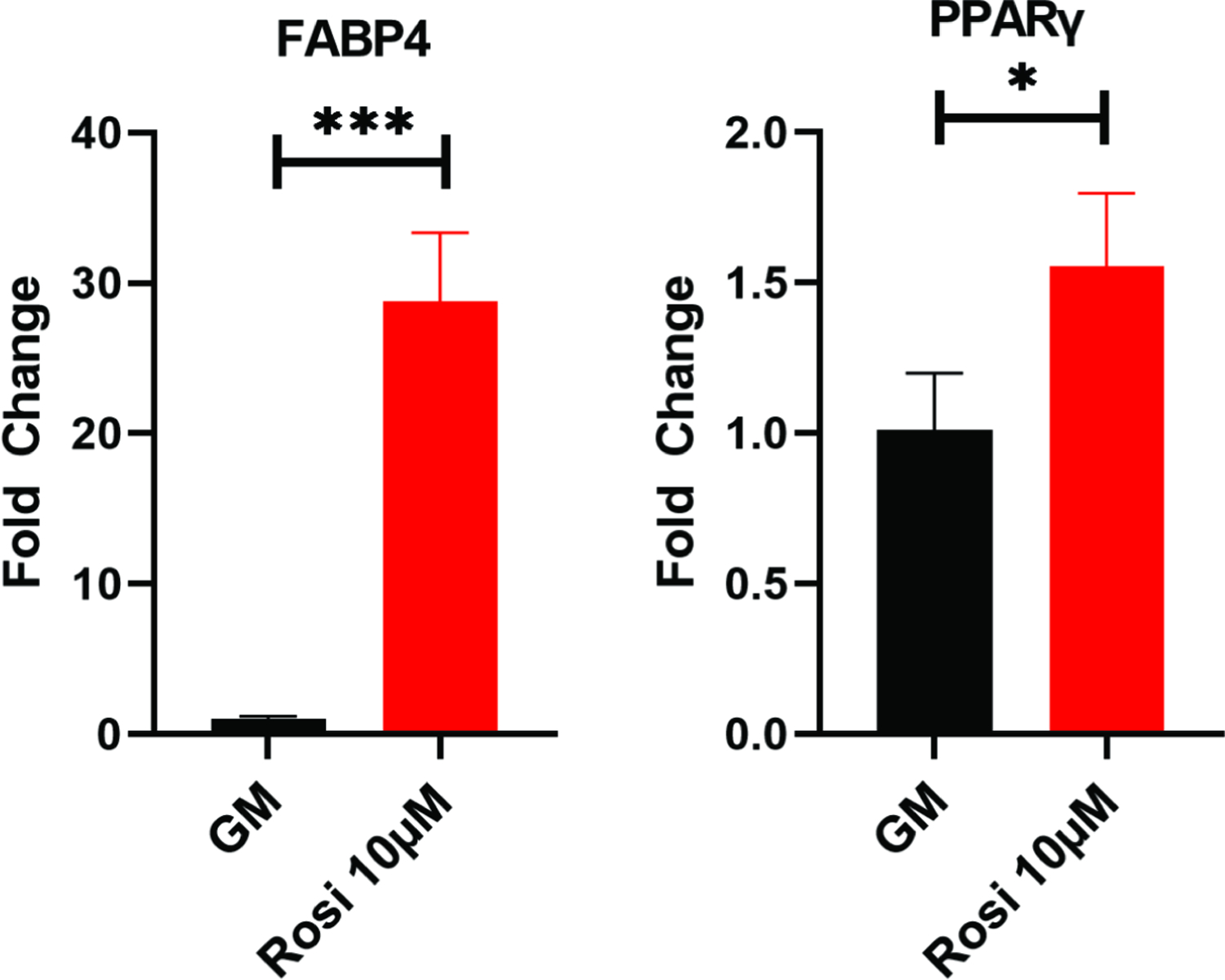
Quantitative PCR of mouse periosteal cells cultured with or without rosiglitazone. Example experiments completed in technical triplicates are shown. *, p<0.05; ***, p<0.001.
Discussion:
This study confirms that rosiglitazone treatment in mice leads to impaired endochondral fracture healing. Our in vivo femur fracture model shows that rosiglitazone treated mice tend to have weaker, more disorganized fracture calluses than untreated mice. This callus change is defined by less bone formation and significantly more accumulation of adipose in and around the fracture site. We also confirm, via in vitro assays, that rosiglitazone decreases osteogenic capacity, and increases adipogenesis, of stem and progenitor cells from both the marrow and the periosteum. This alteration is differentiation capability may be an important contributor to the observed disrupted fracture healing.
Our findings certainly support previous clinical studies that suggest altered bone metabolism in patients with chronic rosiglitazone use. An analysis from A Diabetes Outcome Progression Trial (ADOPT) in 2008 noted that long term treatment with rosiglitazone approximately doubled the fracture risk in females with type 2 diabetes.[15] Another study found that rosiglitazone treatment significantly increased bone turnover markers in post-menopausal women and was associated with lower bone mineral density of the femoral neck, total hip, and lumbar spine.[22] Additionally. Aubert et al. noted a 39% increase in fracture rate in both men and women treated with TZDs.[4] Through our current study, we can reasonably suggest that this increased fracture risk and weaker bone in patients utilizing rosiglitazone is due to a tendency of SSPCs toward adipogenesis within the bone.
Several prior studies have shown that rosiglitazone promotes adipogenesis of MSCs.[7, 13, 14] Yet, these studies have all focused on the cells only from the bone marrow space.[23] In bone healing, however, it is well known that SSPCs arise from both the marrow space and the periosteum, and therefore, no study of fracture healing is complete without analysis of both categories of cells. To our knowledge this is the first study that specifically evaluates the effect of rosiglitazone treatment on isolated periosteal cells, in addition to bone marrow cells. The significant increase in adiposity in our periosteal assays suggest that rosiglitazone treatment halts osteoblast differentiation of both marrow and periosteal derived SSPCs cells and drives these populations toward the adipocyte lineage.
In the marrow, a few mechanisms have been suggested to explain this change in SSPC fate. TZDs are known activate PPAR-γ, which in turn promotes adipogenesis. This switch is thought to reduce the supply of progenitor cells available for osteogenic differentiation. In addition, TZDs shift bone metabolism toward resorption over bone formation via an increase in leptin, a decrease in amylin, and a reduction in estrogen production.[7, 9, 10] It is likely that this shift toward resorption may result in blocked osteogenic differentiation of SSPCs. Whether or not these pathways are maintained in the periosteal environment is certainly a topic for further study.
This study surely has limitations that would make interesting topics of further study. First, in order to limit the negative pan-inflammatory effect of diabetes on fracture healing, we did not utilize a diabetic mouse model in this study. However, this would indeed better mimic the clinical patient population that uses rosiglitazone. In addition, we did not analyze histological and mechanical trends in the fracture callus over the course of endochondral fracture healing. From a clinical perspective, understanding how this callus evolves may influence patient outcomes such as time to weightbearing and tendency toward nonunion in patients treated with rosiglitazone. Furthermore, pioglitazone, the other major utilized TZD, has been shown to clinically increase fracture risk.[24] It would be interesting to see if the mechanisms by which rosiglitazone disrupts endochondral fracture healing hold true for pioglitazone.
Overall, our findings prompt important mechanistic questions for future research. For example, is there a reduction in the supply of osteogenic cells when they are preferentially driven toward adipogenesis? Or does rosiglitazone cause the majority of osteogenic-primed cells to remain senescent, preferentially upregulating the differentiation program in an already adipose-committed cell pool? Answers to these questions would certainly elucidate the true effects of rosiglitazone on stem and progenitor cells and pave the way for better targeted therapeutics for patients recovering from fractures and those with osteoporosis.
Conclusion:
This is the first study to confirm that rosiglitazone treatment disrupts endochondral fracture healing due to decreased osteogenesis and increased adipogenesis of SSPCs from both the bone marrow and periosteum. Orthopaedic surgeons and other providers should be aware that rosiglitazone can impair the healing potential of fractures in their patients.
Acknowledgements:
This work was supported by an OREF Resident Research Grant (#17-048) to NY. We thank Ripa Chowdhury (NYU College of Dentistry) for assistance with the μCT imaging, funded through the NIH grant S10 OD010751.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: No conflicts.
Data and materials availability:
Requests for data and materials should be addressed to P.L.
References
- 1.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020. Mar;10(1):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: The women’s health initiative observational study. J Clin Endocrinol Metab. 2006. Sep;91(9):3404–10. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016. Jul;23(7):1128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010. Aug;12(8):716–21. [DOI] [PubMed] [Google Scholar]
- 5.Piccinin MA, Khan ZA. Pathophysiological role of enhanced bone marrow adipogenesis in diabetic complications. Adipocyte. 2014. Oct-Dec;3(4):263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Aronson J, Huang S, Lu Y, Czernik P, Rahman S, Kolli V, Suva LJ, Lecka-Czernik B. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif Tissue Int. 2012. Aug;91(2):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on bmd and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008. Sep;4(9):507–13. [DOI] [PubMed] [Google Scholar]
- 8.Beck GR Jr., Khazai NB, Bouloux GF, Camalier CE, Lin Y, Garneys LM, Siqueira J, Peng L, Pasquel F, Umpierrez D, Smiley D, Umpierrez GE. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl Res. 2013. Mar;161(3):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy CE, Rodgers PT. Effects of thiazolidinediones on bone loss and fracture. Ann Pharmacother. 2007. Dec;41(12):2014–8. [DOI] [PubMed] [Google Scholar]
- 10.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: A randomized, controlled trial. J Clin Endocrinol Metab. 2007. Apr;92(4):1305–10. [DOI] [PubMed] [Google Scholar]
- 11.Cho ES, Kim MK, Son YO, Lee KS, Park SM, Lee JC. The effects of rosiglitazone on osteoblastic differentiation, osteoclast formation and bone resorption. Mol Cells. 2012. Feb;33(2):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007. Jun;148(6):2669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardone LD, Renlund R, Willett TL, Fantus IG, Grynpas MD. Effect of rosiglitazone on bone quality in a rat model of insulin resistance and osteoporosis. Diabetes. 2011. Dec;60(12):3271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu W, Wang W, Wang S, Feng Y, Liu K. Rosiglitazone promotes bone marrow adipogenesis to impair myelopoiesis under stress. PLoS One. 2016;11(2):e0149543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G, Diabetes Outcome Progression Trial Study G. Rosiglitazone-associated fractures in type 2 diabetes: An analysis from a diabetes outcome progression trial (adopt). Diabetes Care. 2008. May;31(5):845–51. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Remark LH, Buchalter DB, Josephson AM, Wong MZ, Litwa HP, Ihejirika R, Leclerc K, Markus D, Yim NL, Tejwani R, Bradaschia-Correa V, Leucht P. Propranolol reverses impaired fracture healing response observed with selective serotonin reuptake inhibitor treatment. J Bone Miner Res. 2020. May;35(5):932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephson AM, Bradaschia-Correa V, Lee S, Leclerc K, Patel KS, Muinos Lopez E, Litwa HP, Neibart SS, Kadiyala M, Wong MZ, Mizrahi MM, Yim NL, Ramme AJ, Egol KA, Leucht P. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019. Apr 2;116(14):6995–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leucht P, Kim JB, Wazen R, Currey JA, Nanci A, Brunski JB, Helms JA. Effect of mechanical stimuli on skeletal regeneration around implants. Bone. 2007. Apr;40(4):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010. Jul;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 20.Kelly NH, Schimenti JC, Patrick Ross F, Van Der Meulen MCH. A method for isolating high quality rna from mouse cortical and cancellous bone. Bone. 2014. 2014-11-01;68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradaschia-Correa V, Leclerc K, Josephson AM, Lee S, Palma L, Litwa HP, Neibart SS, Huo JC, Leucht P. Hox gene expression determines cell fate of adult periosteal stem/progenitor cells. Sci Rep. 2019. Mar 25;9(1):5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilezikian JP, Josse RG, Eastell R, Lewiecki EM, Miller CG, Wooddell M, Northcutt AR, Kravitz BG, Paul G, Cobitz AR, Nino AJ, Fitzpatrick LA. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013. Apr;98(4):1519–28. [DOI] [PubMed] [Google Scholar]
- 23.Knight MN, Hankenson KD. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle). 2013. Jul;2(6):306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, Gorman M, Kelly MA, Lovejoy AM, Kernan WN, Investigators IT. Pioglitazone and risk for bone fracture: Safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017. Mar 1;102(3):914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for data and materials should be addressed to P.L.


