INTRODUCTION
Intra-articular injection of high molecule weight hyaluronan (HMWH), used extensively in the treatment of patients with osteoarthritis [3; 33; 41; 54; 94], thought to attenuate pain by its viscoelastic/cushioning properties [35], has also been shown to have anti-inflammatory and immunosuppressant effects [36; 49; 57; 68; 100]. HMWH binds to and signals via plasma membrane receptors, best characterized for cluster of differentiation 44 (CD44), considered to be the cognate hyaluronan receptor [93; 97; 98], which is present on nociceptors [9; 43; 85]. We have previously demonstrated that attenuation of nociceptor CD44 expression on, by intrathecal administration of an oligodeoxynucleotide antisense to CD44 mRNA, or inhibition by intradermal administration of a CD44 receptor antagonist, decreases HMWH-induced anti-hyperalgesia [13; 43].
Neuropathic pain is a well-described side effect of several forms of cancer chemotherapy [102], chemotherapy-induced peripheral neuropathy (CIPN), for which there are currently few therapeutic options. The prevalence of CIPN is chemotherapy agent-dependent, being particularly high for platinum-based chemotherapy (70%-100%) [10; 64; 102]. Oxaliplatin, a third-generation platinum-based chemotherapy drug used to treat solid tumors [56], produces neuropathic pain in approximately 90% of patients [20]. And, paclitaxel, a first-line taxane chemotherapy widely used for the treatment of ovarian, breast, lung, cervical, pancreatic, and other solid tumors [17; 27; 32; 77; 88; 99] also induces cytotoxicity, by promoting stabilization of tubulin polymers, resulting in microtubule dysfunction [8; 24; 25; 79] producing neuropathic pain [39; 47; 78; 83] that can persist for months after completion of chemotherapy [59].
The response to hyaluronic acid (HA), mediated by CD44, has been shown to be related to its molecular weight [61; 62; 70; 80; 91; 95] with LMWH producing hyperalgesia while HMWH produces anti-hyperalgesia [14; 43]. We previously demonstrated that HMWH markedly attenuates mechanical hyperalgesia induced by LMWH [43], and chemotherapy-induced neuropathic pain (CIPN) produced by paclitaxel [45]. And, we have recently shown that blocking signaling pathways downstream of CD44, including PI3K, attenuates HMWH-induced anti-hyperalgesia in preclinical models of inflammatory pain [13; 43]. Elucidating the mechanism by which HMWH attenuates nociceptor sensitization could help identify novel therapeutic targets.
In the present experiments we focus on signaling pathways downstream of CD44 in HMWH-induced anti-hyperalgesia, in preclinical models of oxaliplatin- and paclitaxel-induced painful peripheral neuropathy, to evaluate the second messengers mediating HMWH induced anti-hyperalgesia.
METHODS
Animals
Experiments were performed on 220-400 g female and male Sprague-Dawley rats (Charles River Laboratories, Hollister, CA, USA). Animals were housed three per cage, under a 12-hour light/dark cycle, in a temperature- and humidity-controlled room in the animal care facility at the University of California, San Francisco. Food and water were available ad libitum. Experimental protocols were approved by the University of California, San Francisco, Institutional Animal Care and Use Committee, and adhered to the National Institutes of Health Guidelines for the care and use of laboratory animals.
Measuring nociceptive threshold
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter (Stoelting, Wood Dale, IL, USA), to perform the Randall-Selitto paw-withdrawal test [76; 89; 90]. This device applies a linearly increasing mechanical force to the dorsum of the rat's hindpaw. Rats were placed in cylindrical acrylic restrainers with lateral ports to allow access to the hind paw, as described previously [6], to acclimatize them to the testing procedure.
Mechanical nociceptive threshold is defined as the force in grams at which a rat withdraws its paw. Baseline threshold is defined as the mean of three readings taken before injection of test agents. To minimize experimenter bias, individuals conducting the behavioral experiments were blinded to experimental treatments; each experiment was performed on a different group of rats. Data are presented as mechanical nociceptive threshold in grams (g).
Drugs
The following drugs were used in this study: high molecular weight hyaluronan (HMWH) [hyaluronic acid sodium salt from Streptococcus pyogenes], and AS605240 (a PI3Kγ inhibitor) from Tocris (Minneapolis, MN, USA), and U73122 (a phospholipase C inhibitor), Y27632 (a ROK inhibitor) and the cancer chemotherapeutic agents paclitaxel and oxaliplatin from Sigma-Aldrich (St. Louis, MO).
Aliquots of HMWH, dissolved in distilled water to a concentration of 1 μg/μL, were further diluted in saline to the concentration used in each experiment. Stock solutions containing 1 μg/μL of AS605240, U73122 and Y27632 were made in 100% dimethyl sulfoxide (DMSO), and further diluted in 0.9% NaCl containing 1% DMSO to their final concentration.
Oxaliplatin and paclitaxel chemotherapy induced neuropathy: Oxaliplatin was freshly dissolved in normal saline at a concentration of 2 mg/mL just prior to intravenous (i.v.) administration (1 mL/kg), via a tail vein, in rats briefly anesthetized with isoflurane (2.5% in O2). Paclitaxel was dissolved in absolute ethanol and polyethoxylated castor oil (Cremophor EL; 1:1; Sigma-Aldrich) [1; 5; 26; 31] and diluted in saline, to a concentration of 1 mg/mL, just prior to intraperitoneal (i.p.) injection [24; 53]. Paclitaxel (1 mg/kg, i.p.) was administered, every other day for a total of 4 doses, in rats anesthetized with isoflurane (2.5% in O2).
Drugs were administered intradermally, in a volume of 5 μL (when injected alone) or 3 μL each (when two or more drugs were injected), on the dorsum of the hind paw, using a 30-gauge hypodermic needle attached to a 50 μL Hamilton syringe by a segment of PE-10 polyethylene tubing (Becton Dickinson - Franklin Lakes, NJ, USA). The administration of AS605240 was preceded by a hypotonic shock (1 μL of distilled water, separated by an air bubble, to avoid mixing in the segment of PE-10 tubing), to transiently enhance cell permeability, to get these reagents inside the nerve terminal [16; 22].
Oligodeoxynucleotide (ODN) antisense.
The role of PI3Kγ, CD44 and GPR30 was assessed by intrathecal treatement with ODN antisense against a unique region of the rat mRNA sequence.
Antisense (AS) ODN sequence:
PI3Kγ ODN antisense: 5’-AAA AGT TGC AGT CCA GGA GTT-3’ (GenBank accession number NM_133399.3)
CD44 ODN antisense: 5’-GAA AAG GGT CGC GGG GG-3’ (GenBank accession number NM_012924.2)
GPR30 ODN antisense 5′-ATG TTC AGA GAG GTC CCC AG-3′ (GenBank accession number NM_133573)
Mismatch ODN sequences corresponding to the antisense sequence with some bases mismatched (denoted by bold letters), had no sequence homology in the rat gene database.
Mismatch (MM) ODN sequences:
PI3Kγ ODN mismatch: 5’-AAA CGT AGC ATT CCT CGA GAT-3’
CD44 ODN mismatch: 5’-CCC CCG CGA CCC TTT TC-3’
GPR30 ODN mismatch 5′-AGG TCC AGA AAG ATG CCA AG-3′
These ODN antisense sequences, synthesized by Life Technologies (Carlsbad, CA, USA), have previously been shown to produce a decrease in PI3Kγ [37], CD44 [13] and GPR30 [4] protein in rat DRG. Before use, ODNs are reconstituted in nuclease-free 0.9% NaCl and then administered intrathecally. As described previously [2], rats were anesthetized with isoflurane (2.5% in O2) and 120 μg of ODN, in a volume of 20 μL, injected intrathecally (i.t.) using a syringe (300 units/μL) attached to a 29-gauge hypodermic needle, inserted into the subarachnoid space between the L4 and L5 vertebrae. The intrathecal site of injection was confirmed by a sudden flick of the rat’s tail, a reflex that is evoked by subarachnoid space access and bolus intrathecal injection [65]. Animals regained consciousness approximately 2 minutes after i.t. injections. The use of antisense ODN administered intrathecally, to attenuate the expression of proteins essential for their role in nociceptor sensitization, is well supported by previous studies by others [69; 75; 82; 86; 87], as well as our group [6; 7; 11; 43-45; 71].
Gonadectomy
Ovariectomy was performed on female rats at 3 weeks of age (i.e., prepubertal), and animals were used for behavioral experiments 3 weeks later (i.e., as adults) [50]. For surgery, animals were anesthetized with isoflurane (3% in oxygen) and received preoperative meloxicam (~5 mg/kg, s.c.) and bupivacaine (~0.1 mg/kg s.c. injected at the incision site) for pain control. Briefly, ovaries were accessed by means of bilateral cutaneous and peritoneal incisions. Once located, their vascular bundles were ligatured with 4-0 silk suture (Perma-Hand Silk® Ethicon, Johnson & Johnson, Somerville, NJ). Ovaries were then excised, and the peritoneal and cutaneous incisions closed with 5-0 silk suture (Perma-Hand Silk® Ethicon, Johnson & Johnson, Somerville, NJ).
Statistical analysis
We used 90 male and 36 female rats. In each rat only one hind paw was used. In behavioral experiments, data are presented as mechanical nociceptive threshold. The behavioral experiments were performed with the experimenter blinded to experimental group. Repeated-measures one-way ANOVA followed by Bonferroni’s post hoc multiple comparisons test or Student's t-test was used to analyze data. Prism 8.0 (GraphPad Software) was used for the graphics and to perform statistical analyses; P<0.05 was considered statistically significant. Data are presented as mean ± SEM.
RESULTS
HMWH-induces anti-hyperalgesia in male rats with CIPN
We have previously shown that HMWH induces anti-hypealgesia in models of inflammatory pain [43] and CIPN [45]. Here we evaluate HMWH anti-hyperalgesia in male and female rats (FIG. 1) with paclitaxel and oxaliplatin CIPN. For oxaliplatin CIPN, male (FIG. 1A) and female (FIG. 1B) rats received an intravenous injection of oxaliplatin (1 mL/kg; i.v.). Seven days later, they received HMWH (1 μg) or vehicle, injected intradermally (i.d.) on the dorsum of the hind paw, at the site of nociceptive testing. In male rats with oxaliplatin CIPN, but not females, HMWH induces anti-hyperalgesia. Separate groups of male (FIG. 1C) and female (FIG. 1D) rats were treated with paclitaxel, every other day for 4 days. Seven days after the first dose of paclitaxel, rats received HMWH (1 μg, i.d.) or vehicle (i.d.). The anti-hyperalgesia induced by administration of HMWH in paclitaxel-induced CIPN was only observed in male rats.
Figure 1. HMWH induces anti-hyperalgesia in male rats with oxaliplatin- and paclitaxel-induced CIPN.

A. Male rats received oxaliplatin (2 mg/kg, i.v.) on day 0. On day 7 after oxaliplatin administration, HMWH was injected (1 μg/ 5 μL, i.d.) on the dorsum of the hind paw at the site of nociceptive threshold testing. Mechanical nociceptive threshold was evaluated before oxaliplatin and 30 min, and 7 days after its administration, and 30 min after HMWH. Oxaliplatin decreases mechanical nociceptive threshold (i.e., produces hyperalgesia) in male rats. Intradermal administration of HMWH attenuates the hyperalgesia induced by oxaliplatin, in male rats (F(3,30)= 18.37, ****p<0.0001, when CIPN, HMWH was compared to CIPN, vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test).
B. Male rats received paclitaxel (1 mg/kg, i.p.), every other day for a total of 4 doses (days 0, 2, 4 and 6). On day 7, approximately 24 h after the last dose of paclitaxel, HMWH (1 μg/ 5 μL) was injected intradermally (i.d.) on the dorsum of one hind paw. Mechanical nociceptive threshold was evaluated before the 1st paclitaxel injection and 1, 3, 5 and 7 days after, and then 30 min after HMWH. Paclitaxel decreases mechanical nociceptive threshold (i.e., produces hyperalgesia) in male rats. Intradermal administration of HMWH attenuates the hyperalgesia induced by paclitaxel (F(5,50)= 8.60, ****p<0.0001, when CIPN, HMWH-treated group was compared to the CIPN, vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test).
C. Female rats received oxaliplatin (2 mg/kg, i.v.) on day 0. On day 7 after oxaliplatin administration, HMWH was injected (1 μg/ 5 μL, i.d.) on the dorsum of the hind paw, at the site of nociceptive threshold testing. Mechanical nociceptive threshold was evaluated before oxaliplatin and 30 min, and 7 days after its administration, and then 30 min after HMWH. Oxaliplatin decreases mechanical nociceptive threshold (i.e., produces hyperalgesia) in male rats. Intradermal administration of HMWH attenuates the hyperalgesia induced by oxaliplatin, in male rats. In female rats, oxaliplatin also decreases mechanical nociceptive threshold. However, in females HMWH does not attenuate oxaliplatin-induced hyperalgesia (F(3,30)= 0.06, p=0.98, when CIPN treated with HMWH was compared to the CIPN vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n=6 per group.
D. Female rats received paclitaxel (1 mg/kg, i.p.), every other day for a total of 4 doses (days 0, 2, 4 and 6). On day 7, approximately 24 h after the last dose of paclitaxel, HMWH (1 μg/ 5 μL) was injected intradermally (i.d.) on the dorsum of one hind paw. Mechanical nociceptive threshold was evaluated before the 1st paclitaxel injection and 1, 3, 5 and 7 days after, and then 30 min after HMWH. Administration of paclitaxel decreases mechanical nociceptive threshold in female rats. However, HMWH does not attenuate the hyperalgesia induced by paclitaxel in females (F(5,50)= 0.80, p=0.56, when CIPN, HMWH-treated group was compared to CIPN, vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Results in all figures are presented as mechanical nociceptive threshold in grams. n=6 per group.
To evaluate the role of sex hormones in sexual dimorphism in the inhibition of CIPN hyperalgesia by HMWH, the experiment was repeated in a group of female rats ovariectomized 2 weeks prior, and then treated with oxaliplatin (FIG. 2A) or paclitaxel (FIG. 2B). In ovariectomized female rats HMWH now induced anti-hyperalgesia, of similar magnitude to the response observed in gonad intact male rats. To further explore the role of sex hormones in the sexually dimorphic effect of HMWH in rats treated with oxaliplatin (FIG. 2C) and paclitaxel (FIG. 2D) CIPN, female rats were treated intrathecally with ODN antisense to GPR30 mRNA for 3 consecutive days. Female rats that received GPR30 antisense also demonstrated HMWH-induced anti-hyperalgesia.
Figure 2. HMWH-induced anti-hyperalgesia in females is sex hormone dependent.
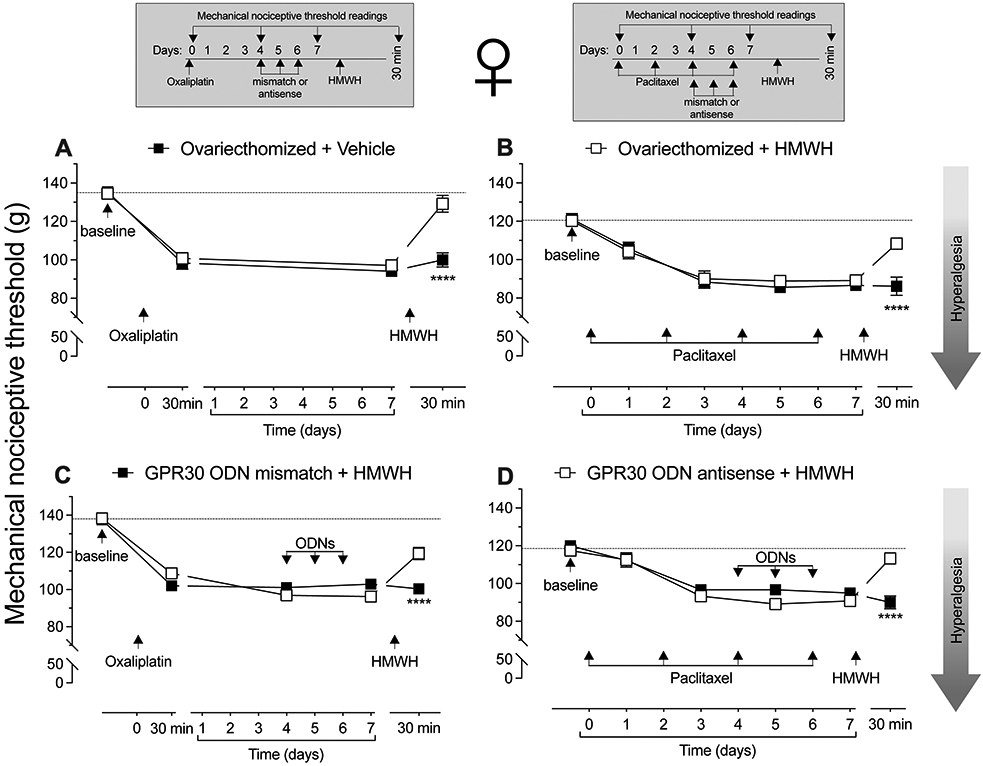
A. Female rats underwent ovariectomy 14 days prior to receiving oxaliplatin (2 mg/kg, i.v.), on day 0. On day 7, HMWH (1 μg/ 5 μL, i.d.) was injected on the dorsum of the hind paw. Mechanical nociceptive threshold was evaluated before oxaliplatin and 30 min, and 7 days after, and again 30 min after HMWH. Intradermal administration of HMWH attenuates the hyperalgesia induced by oxaliplatin in ovariectomized female rats (F(3,30)= 17.04, ****p<0.0001, when CIPN treated with HMWH was compared to the CIPN vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
B. Another group of female rats underwent ovariectomy 14 days prior to receiving paclitaxel (1 mg/kg, i.p.), every other day for a total of 4 doses (days 0, 2, 4 and 6). Seven days later, rats were treated with HMWH (1 μg/ 5 μL, i.d.) on the dorsum of the hind paw. Mechanical nociceptive threshold was evaluated before paclitaxel, and on days 1, 3, 5 and 7 after, and then 30 min after HMWH. Intradermal administration of HMWH attenuates the hyperalgesia induced by paclitaxel in ovariectomized rats (F(5,50)= 9.61, ****p<0.0001, when CIPN treated with HMWH was compared to the CIPN vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
C. Female rats received oxaliplatin (2 mg/kg, i.v.) on day 0. Four days later, they were treated with an oligodeoxynucleotide (ODN) antisense or mismatch (120 μg/ 20 μL, i.t.) for GPR30 mRNA, daily for 3 consecutive days. On day 7, approximately 24 h after the last dose of ODN, HMWH (1 μg/ 5 μL, i.d.) was injected on the dorsum of the hind paw. Mechanical nociceptive threshold was evaluated before oxaliplatin and 30 min, 4 and 7 days after its administration, and then 30 min after HMWH. Oxaliplatin similarly decreases mechanical nociceptive threshold in both GPR30 antisense- and mismatch-treated rats. Intradermal administration of HMWH attenuates the hyperalgesia induced by oxaliplatin in the GPR30 antisense-treated group (F(4,40)= 15.99, ****p<0.0001, when CIPN treated with HMWH was compared to the CIPN vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
D. Female rats received paclitaxel, administered intraperitoneally (1 mg/kg, i.p.) every other day for a total of 4 doses (days 0, 2, 4 and 6). Four days after the 1st paclitaxel injection, rats were treated with antisense or mismatch ODN antisense for GPR30 mRNA (120 μg/ 20 μL, i.t.), daily for 3 consecutive days. On day 7, approximately 24 h after the last ODN dose and the last dose of paclitaxel, HMWH (1 μg/ 5 μL, i.d.) was injected on the dorsum of the hind paw. Mechanical nociceptive threshold was evaluated before paclitaxel, and on day 1, 3, 5 and 7 after its administration, and then 30 min after HMWH. In both GPR30 antisense- and mismatch-treated groups, paclitaxel decreases mechanical nociceptive threshold. In the group treated with ODN antisense to GPR30 mRNA, intradermal administration of HMWH attenuates the hyperalgesia induced by paclitaxel (F(5,50)= 8.75, ****p<0.0001, when CIPN treated with HMWH was compared to the CIPN vehicle-treated group; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
CD44 antisense attenuates HMWH-induced anti-hyperalgesia
We have previously shown that HMWH-induced anti-hyperalgesia in models of inflammatory pain is CD44 dependent [12-14; 43; 45]. In the present experiments we tested whether HMWH-induced anti-hyperalgesia is CD44-dependent in CIPN. Male rats received a single intravenous injection of oxaliplatin (FIG. 3A) or an intraperitoneal injection of paclitaxel every other day for 4 doses (FIG. 3B). Four days after oxaliplatin or after the first paclitaxel injection, rats received intrathecal injections of ODN antisense or mismatch to CD44 mRNA, for 3 consecutive days. On the fourth day, approximately 24 h after the last administration of ODN, HMWH (1 μg, i.d.) was injected intradermally, on the dorsum of the hind paw. HMWH induces anti-hyperalgesia in both oxaliplatin- and paclitaxel-treated male rats that is attenuated by CD44 antisense (FIG. 3A and 3B).
Figure 3. HMWH-induced anti-hyperalgesia is attenuated by ODN antisense to CD44 mRNA.
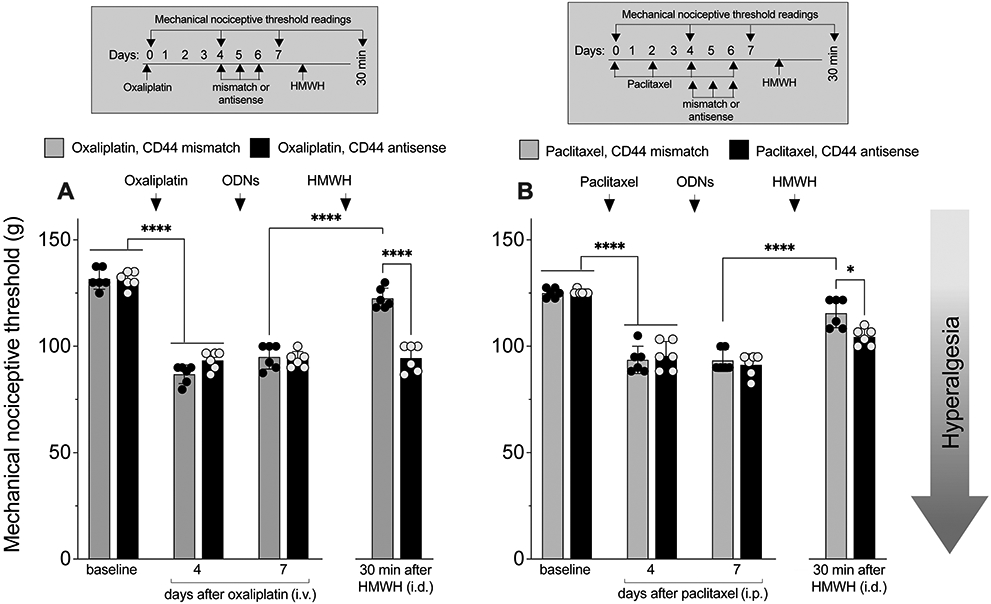
A. Male rats received oxaliplatin (2 mg/kg, i.v.) or saline (i.v.) on day 0. Four days later, they were treated with an ODN antisense or mismatch for CD44 mRNA (120 μg/ 20 μL, i.t.), daily for 3 days. On day 7, approximately 24 h after the last intrathecal administration of ODN, HMWH was injected (1 μg/ 5 μL, i.d.). Mechanical nociceptive threshold was evaluated on day 0, and on day 4 and 7 after administration of oxaliplatin, and then 30 min after HMWH. Results are presented as mechanical nociceptive threshold in grams. Oxaliplatin decreased mechanical nociceptive threshold (i.e., produced hyperalgesia), measured 4 days after intravenous injection (F(7,35)= 97.99, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test), in both CD44 antisense- and mismatch-treated groups. HMWH attenuates the hyperalgesia induced by oxaliplatin (F(7,35)= 97.99, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test) only in the CD44 mismatch-treated group. n= 6 per group.
B. Male rats received paclitaxel (1 mg/kg, i.p.), every other day for a total of 4 doses (days 0, 2, 4 and 6). Four days after the 1st paclitaxel injection, rats were treated with an oligodeoxynucleotide (ODN) antisense or mismatch (120 μg/ 20 μL, i.t.) for CD44 mRNA, daily for 3 consecutive days. On day 7, approximately 24 h after the last dose of ODN, and the last intraperitoneal injection of paclitaxel, HMWH was injected (1 μg/ 5 μL, i.d.). Mechanical nociceptive threshold was evaluated on day 0, and 4 and 7 days after oxaliplatin, and then 30 min after HMWH. Paclitaxel decreased mechanical nociceptive threshold (i.e., produced hyperalgesia), measured 4 days (F(7,35)= 52.53, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test) after its first dose, in both CD44 antisense- and mismatch-treated groups. HMWH attenuates the hyperalgesia induced by paclitaxel in the CD44 mismatch-treated group (F(7,35)= 52.53, *p=0.0107; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
Second messengers mediating HMWH-induced anti-hyperalgesia
We have previously demonstrated involvement of second messengers downstream of CD44 by which HWMH signals to inhibit PGE2 hyperalgesia, involving ROK, PLC and PI3Kγ [13; 15]. To test the hypothesis that HMWH also signals through this pathway to induce anti-hyperalgesia for CIPN, we first treated male rats with oxaliplatin or paclitaxel CIPN with a ROK inhibitor (Y27632, 1 μg, i.d.) and then, 10 min later HMWH (1 μg, i.d.), at the same site on the dorsum of the hind paw. Rats treated with the ROK inhibitor demonstrated attenuation of HMWH-induced anti-hyperalgesia (FIG. 4).
Figure 4. HMWH anti-hyperalgesia is attenuated by a RhoA inhibitor.

A. Male rats received oxaliplatin (2 mg/kg, i.v.) or saline (i.v.) on day 0. On day 7, a ROK inhibitor (a component of RhoA signaling pathway) (Y27632, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.) and mechanical nociceptive threshold was evaluated on day 0 and 7 days after administration of oxaliplatin, and then again 30 min after intradermal HMWH or vehicle. Results are presented as mechanical nociceptive threshold, in grams. Oxaliplatin decreased mechanical nociceptive threshold measured 7 days after its administration (F(8,40)= 106.85, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Anti-hyperalgesia induced by HMWH was attenuated by the ROK inhibitor (F(8,40)= 106.85, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
B. Paclitaxel was administered intraperitoneally (1 mg/kg, i.p.), in male rats, every other day for a total of 4 doses (days 0, 2, 4 and 6). On day 7, approximately 24 h after the last dose of paclitaxel, a RhoA inhibitor (Y27632, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.) and mechanical nociceptive threshold evaluated on days 0 and 7 after the 1st paclitaxel injection, and then again 30 min after HMWH or vehicle. Paclitaxel decreases mechanical nociceptive threshold on day 7 after its first dose (F(8,40)= 118.45, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Intradermal administration of RhoA the inhibitor attenuates HMWH-induced anti-hyperalgesia (F(8,40)= 118.45, ****p=0.0010; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
RhoA activates ROK which, in turn, phosphorylates PLCε and PLCγ1 [18]. To determine if PLC is also involved in HMWH-induced anti-hyperalgesia for CIPN, we treated groups of male rats with oxaliplatin and paclitaxel, and then a PLC inhibitor (U73122, 1 μg, i.d.), followed 10 min later by HMWH, at the same site. In both the oxaliplatin and paclitaxel CIPN rats treated with the PLC inhibitor, HMWH-induced anti-hyperalgesia was attenuated (FIG. 5).
Figure 5. HMWH anti-hyperalgesia is attenuated by a PLC inhibitor.
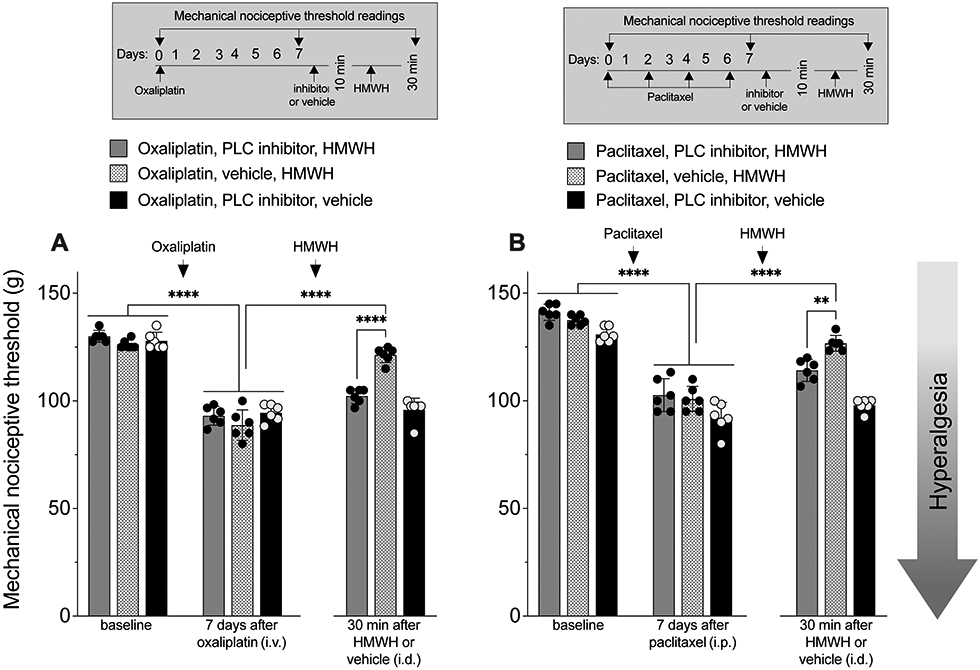
A. Male rats received oxaliplatin (2 mg/kg, i.v.) or saline (i.v.) on day 0. On day 8, a PLC inhibitor (U73122, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.) and mechanical nociceptive threshold evaluated on day 0, and 7 days after oxaliplatin, and then again 30 min after HMWH or vehicle. Oxaliplatin decreased mechanical nociceptive threshold after administration on day 7 (F(8,40)= 104.79, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Anti-hyperalgesia induced by HMWH was attenuated by the PLC inhibitor (F(8,40)= 104.79, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
B. Male rats received paclitaxel (1 mg/kg, i.p.), administered every other day for a total of 4 doses (on days 0, 2, 4 and 6). On day 7, approximately 24 h after the last dose of paclitaxel, a PLC inhibitor (U73122, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.). Mechanical nociceptive threshold was evaluated on day 0 and on day 7 after the 1st dose of paclitaxel, and then again 30 min after HMWH or vehicle. Paclitaxel induced mechanical hyperalgesia on day 7 (F(8,40)= 84.20, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Intradermal administration of the PLC inhibitor also attenuated HMWH-induced anti-hyperalgesia (F(8,40)= 84.20, **p=0.0028; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
Finally, to determine if HMWH anti-hyperalgesia for CIPN is PI3Kγ dependent, groups of rats treated with oxaliplatin or paclitaxel received intrathecal injection of ODN antisense or mismatch to PI3Kγ mRNA, for 3 consecutive days. On the fourth day, approximately 24 h after the last administration of ODN, HMWH was injected intradermally, on the dorsum of the hind paw. Attenuation of HMWH-induced anti-hyperalgesia was observed in both oxaliplatin- and paclitaxel-treated rats that received ODN antisense to PI3Kγ mRNA (FIG. 6A and 6B). Additional groups of male rats treated with oxaliplatin or paclitaxel received an intradermal injection of a PI3Kγ inhibitor (AS605240, 3 μg, i.d.), and then 10 min later HMWH was injected at the same site on the dorsum of the hindpaw. Rats receiving the PI3Kγ inhibitor also showed attenuation of HMWH-induced anti-hyperalgesia, of similar magnitude to that produced by PI3Kγ antisense (FIG. 7A and 7B).
Figure 6. HMWH anti-hyperalgesia is attenuated by ODN antisense to PI3Kγ mRNA.

A. Male rats received oxaliplatin (2 mg/kg, i.v.) or saline (i.v.) on day 0. Four days later they were treated with an oligodeoxynucleotide (ODN) antisense or mismatch (120 μg/ 20 μL, i.t.) for PI3Kγ mRNA, daily for 3 consecutive days. On day 7, approximately 24 h after the last dose of ODN, HMWH (1 μg/ 5 μL, i.d.) was injected. Mechanical nociceptive threshold was evaluated on days 0, 4 and 7 after administration of oxaliplatin and 30 min after HMWH. Oxaliplatin decreased mechanical nociceptive threshold (i.e., produced hyperalgesia), observed 4 days after intravenous injection in both PI3Kγ antisense- and mismatch-treated groups (F(7,35)= 103.47, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). Intradermal administration of HMWH does not attenuate the hyperalgesia induced by oxaliplatin in PI3Kγ antisense-treated rats (F(7,35)= 103.47, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
B. Male rats received paclitaxel (1 mg/kg, i.p.) every other day for a total of 4 doses (days 0, 2, 4 and 6). Four days after the 1st dose of paclitaxel, rats were treated with an oligodeoxynucleotide (ODN) antisense or mismatch (120 μg/ 20 μL, i.t.) against PI3Kγ mRNA, daily for 3 consecutive days. On day 7, approximately 24 h after the last injection of ODN, and paclitaxel, HMWH (1 μg/ 5 μL, i.d.) was injected. Mechanical nociceptive threshold was evaluated on day 0 and days 4 and 7 after administration of oxaliplatin, and again 30 min after HMWH. Paclitaxel decreases mechanical nociceptive threshold (i.e., produces hyperalgesia), measured on day 4 (F(7,35)= 54.74, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test), in both PI3Kγ antisense- and mismatch-treated groups. However, HMWH does not attenuate the hyperalgesia induced by paclitaxel in PI3Kγ antisense-treated rats (F(7,35)= 54.74, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
Figure 7. HMWH anti-hyperalgesia is attenuated by a PI3Kγ inhibitor.
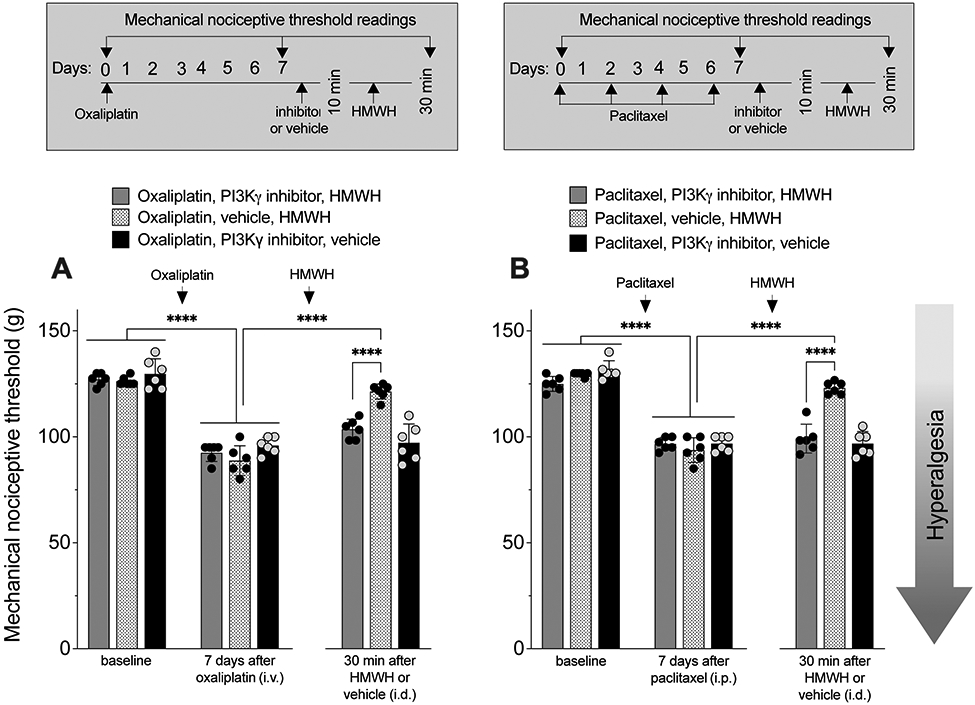
A. Male rats received oxaliplatin (2 mg/kg, i.v.) or saline (i.v.) on day 0. On day 7, a PI3Kγ inhibitor (AS605240, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.); mechanical nociceptive threshold was evaluated on days 0 and 7 after administration of oxaliplatin, and again 30 min after intradermal HMWH or vehicle. Oxaliplatin decreased mechanical nociceptive threshold on day 7 (F(8,40)= 61.33, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). HMWH-induced anti-hyperalgesia was attenuated in the rats treated with the PI3Kγ inhibitor (F(8,40)= 61.33, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
B. Male rats received paclitaxel (1 mg/kg, i.p.) every other day for a total of 4 doses (days 0, 2, 4 and 6). On day 7, approximately 24 h after the last dose of paclitaxel, a PI3Kγ inhibitor (AS605240, 1 μg/ 5 μL, i.d.) or vehicle (5 μL, i.d.) was injected. Ten minutes later, rats received an injection of HMWH (1 μg/5 μL, i.d.) or vehicle (5 μL, i.d.) and mechanical nociceptive threshold was evaluated on day 0 and day 7 after the 1st dose of paclitaxel, and then again 30 min after HMWH or vehicle. The PI3Kγ inhibitor attenuates HMWH anti-hyperalgesia (F(8,40)= 79.37, ****p<0.0001; two-way ANOVA followed by Bonferroni’s post hoc comparisons test). n= 6 per group.
DISCUSSION
CIPN occurs in over 38% [66; 73] of the 16.9 million cancer survivors in the United States [67], bringing an urgency to understanding its mechanisms and developing effective treatments. We have previously demonstrated that HMWH attenuates hyperalgesia induced by PGE2, in female and male rats. This anti-hyperalgesia is dependent on the action of HMWH at CD44, the cognate hyaluronan receptor. In contrast, a contribution of toll-like receptor 4 (TLR4) to HMWH-induced anti-hyperalgesia was only observed in male rats [12]. And while we previously found that HMWH attenuates CIPN [45], currently little is known about underlying mechanisms.
To elucidate the mechanism underlying HMWH-induced anti-hyperalgesia in CIPN, we first evaluated the effect of HMWH in rats treated with oxaliplatin and paclitaxel, which are thought to induce CIPN by different mechanisms [21; 34; 46; 92]. Intradermal administration of HMWH attenuated oxaliplatin and paclitaxel CIPN in male rats, but not in females. We next evaluated the role of sex hormones in the failure of HMWH to induce anti-hyperalgesia for CIPN in females. In ovariectomized female rats and rats treated with antisense ODN against GPR30, a G-protein coupled estrogen receptor [48] found in DRG neurons [42], HMWH now attenuates paclitaxel and oxaliplatin CIPN. Of note, since many female oncology patients are post-menopausal, with low estrogen levels, treatment with HMWH may be effective in these patients. Deletion of estrogen receptor α (ERα), but not ERβ, blocks a rapid modulation of P2X receptor in small-diameter DRG neurons by estrogen [28], and deletion of ERα in TRPV1+ nociceptors abolishes pain in females [63], demonstrating a role of ERα in primary afferent neurons. Whether ERα and/or ERβ signaling also contributes to sex hormone regulation of HMWH effects in nociceptors remains to be examined.
We have previously demonstrated that intrathecal administration of TLR4 antisense attenuates HMWH-induced anti PGE2-hyperalgesia, in male but no in female rats [12]. Spinal TLR4 mediates inflammatory and neuropathic hyperalgesia in male, but not in female mice, and while expression levels of TLR4 are not different in male and female mice, testosterone induces a switch to TLR4-dependence in female mice [84]. Since estrogen can act at GPR30 to reduce TLR4 mRNA and protein [104], these observations support the suggestion that the response of CIPN to HMWH in female rats may be, at least in part, TLR4-dependent, an effect that is suppressed by female sex hormones.
Treatment with CD44 antisense attenuates HMWH-induced anti-hyperalgesia in rats with both oxaliplatin and paclitaxel CIPN, supporting the suggestion that HMWH acts at CD44, the cognate HMWH receptor, which is present on nociceptors [9; 43; 85], to produce anti-hyperalgesia [12; 13; 45]. Importantly, since HMWH does not change mechanical nociceptive threshold in naïve control rats, it is acting specifically to reverse nociceptor sensitization [13; 15]. CD44 signals via RhoA and Rac1 [18], and HMWH-induced anti-PGE2 hyperalgesia is RhoA and Rac1 dependent [13]. RhoA and Rac1 can, in turn, phosphorylate PLCε and PLCγ1 [18], to activate PI3K [18]. Different PI3K isoforms can execute distinct, and sometimes opposing functions [96]; and multiple PI3K isoforms are present in DRG neurons [29; 60]. We have previously demonstrated that HMWH-induced anti-hyperalgesia is reversed by inhibition of the gamma isoform of PI3K, PI3Kγ [15]. PI3Kγ is expressed in small- and medium-diameter sensory neurons, which are predominantly C- and Aδ-fibers [37; 58; 60; 74] and it has been suggested that PI3Kγ is involved in the peripheral action of opioids on nociceptors, to induce peripheral antinociception [37; 38]. In the current study, we evaluated the role of PI3Kγ in HMWH-induced anti-hyperalgesia in rats with CIPN. We found that intrathecal administration of ODN antisense to PI3Kγ and intradermal administration of a PI3Kγ selective inhibitor (AS605240), administered adjacent to the nociceptor peripheral terminal, both markedly attenuate HMWH-induced anti-hyperalgesia. The anti-hyperalgesia effect of HMWH is mediated by its action at CD44 receptors and downstream signaling via PI3Kγ in nociceptors [12; 13; 23; 40; 45]; while signaling pathways in nociceptors activated by hyaluronan had not been evaluated, in other cell types (e.g. fibroblasts, chondrocytes, tumor cell lines, immune cells) hyaluronan is well-established to signal via RhoA, PLC and PI3Kγ [19; 55; 81; 103]. Thus, PI3Kγ in nociceptors produces pro- and anti-hyperalgesic effects [15; 52]. whether this is due the subcellular compartmentalization or presence of PI3Kγ in different nociceptor populations remains to be elucidated.
HMWH has been shown to reduce the excitability of the transient receptor potential vanilloid subtype 1 (TRPV1) ion channel, by stabilizing its closed state [23; 40]. PI3K might also induce heat hyperalgesia by ERK dependent regulation of TRPV1 activity [106], and capsaicin responses were greatly reduced in neurons from p85α (regulatory subunit of PI3K) null mice, suggesting that PI3K and MAPK but not the PLC, pathways underlie the acute sensitization of TRPV1 [105]. While recent studies have also increased our knowledge of how HMWH signals via other plasma membrane receptors such as the receptor for HA-mediated motility (RHAMM), and toll-like receptor 4 (TLR4) [12; 13; 45; 93; 97; 98] the contribution of these receptors to the sexually dimorphic effect of MHWH remains to be explored. In the central and peripheral nervous system, PI3K signaling can mediate mechanical and thermal hyperalgesia induced by nerve injury, incision, or inflammation [30; 74; 101]. The specific isoform involved is unknown, as only a non-selective PI3K inhibitor was used to attenuate mechanical allodynia [51; 72]. Our results, showing an attenuation in HMWH-induced anti-hyperalgesia by inhibiting PI3Kγ signaling, is in agreement with the prior demonstration that PI3Kγ, in sensory neurons, can mediate μ-opioid receptor agonist-induced anti-hyperalgesia [37; 38; 58]. While the intradermal injection of the selective PI3Kγ inhibitor at the peripheral terminal of the nociceptor did not alone affect CIPN, ODN antisense for PI3Kγ, which impacts PI3Kγ at the central as well as the peripheral terminal of the nociceptor, affected mechanical nociceptive threshold in CIPN and decreased HMWH-induced anti-hyperalgesia.
In the present study we demonstrate sexual dimorphism in HMWH-induced anti-hyperalgesia in rats with CIPN induced by two neurotoxic chemotherapeutic drugs that are thought to induce CIPN by different mechanisms, with a sex hormone-dependent lack of HMWH-induced anti-hyperalgesia in female rats. In rats with CIPN HMWH acts at CD44 on the nociceptor plasma membrane to induce anti-hyperalgesia, mediated by a RhoA, PLC and PI3Kγ (FIG. 8), opening a novel line of research into molecular targets for the treatment of chronic neuropathic pain produced by chemotherapy agents.
Figure 8. HMWH-induced anti-hyperalgesia signaling pathway.
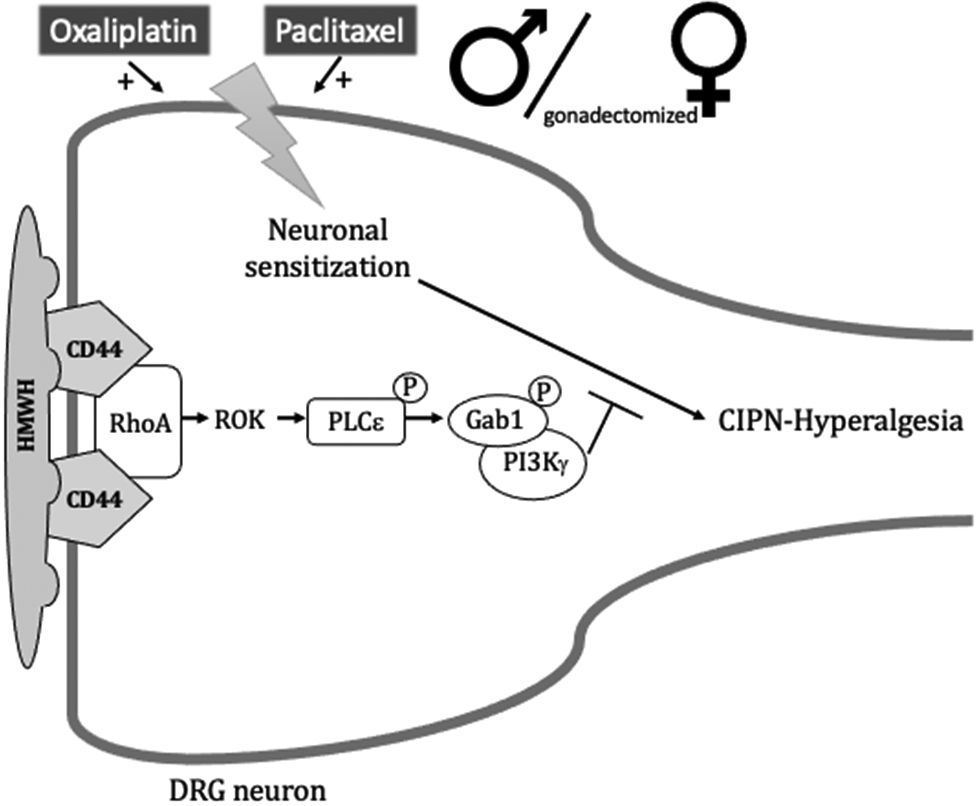
In male and gonadectomized female rats, oxaliplatin and paclitaxel induces CIPN-hyperalgesia that is reversed by HMWH, which binds to CD44 to induce its clustering in cell membrane lipid rafts and initiate signaling in downstream second messenger pathways. After binding to CD44, HMWH can signal via RhoA and Rac1, which in turn, activate ROK and PKN, respectively, leading to phosphorylation of PLCε and PLCγ1, respectively. Binding of HMWH to CD44 also stimulates RhoA, which activates ROK to phosphorylate PLCε, increasing serine/threonine phosphorylation of the adaptor protein, Gab-1 and leading to activation of PI3Kγ. Abbreviations: CD44, cluster of differentiation 44 (hyaluronan receptor); Gab1, scaffold protein; HMWH, high molecular weight hyaluronan; PI3Kγ, phosphatidylinositol (PI) 3-kinase gamma; PKN, fatty acid-activated serine/threonine kinase; PLCε, phospholipase C epsilon; PLCγ1, phospholipase Cγ1; Rac1, Rho family of GTPases; RhoA, Rho family of GTPases; ROK, Rho-associated kinase.
Acknowledgements:
The authors would like to thank Niloufar Mansooralavi for technical assistance. This study was funded by National Institutes of Health (NIH) grants AR075334 and CA250017.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES
- [1].Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr 1993(15):141–147. [PubMed] [Google Scholar]
- [2].Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003;39(3):497–511. [DOI] [PubMed] [Google Scholar]
- [3].Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol 1998;25(11):2203–2212. [PubMed] [Google Scholar]
- [4].Alvarez P, Bogen O, Levine JD. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain 2014;155(12):2680–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Apfel SC, Lipton RB, Arezzo JC, Kessler JA. Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol 1991;29(1):87–90. [DOI] [PubMed] [Google Scholar]
- [6].Araldi D, Bogen O, Green PG, Levine JD. Role of Nociceptor Toll-like Receptor 4 (TLR4) in Opioid-Induced Hyperalgesia and Hyperalgesic Priming. J Neurosci 2019;39(33):6414–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Araldi D, Ferrari LF, Levine JD. Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain 2017;158(7):1204–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arnal I, Wade RH. How does taxol stabilize microtubules? Curr Biol 1995;5(8):900–908. [DOI] [PubMed] [Google Scholar]
- [9].Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen 2014;22(5):579–593. [DOI] [PubMed] [Google Scholar]
- [10].Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 2017;7(1):e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci 2012;32(6):2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bonet IJM, Araldi D, Green PG, Levine JD. Sexually Dimorphic Role of Toll-like Receptor 4 (TLR4) in High Molecular Weight Hyaluronan (HMWH)-induced Anti-hyperalgesia. J Pain 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bonet IJM, Araldi D, Khomula EV, Bogen O, Green PG, Levine JD. Mechanisms Mediating High-Molecular-Weight Hyaluronan-Induced Antihyperalgesia. J Neurosci 2020;40(34):6477–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bonet IJM, Green PG, Levine JD. Sexual dimorphism in the nociceptive effects of hyaluronan. Pain 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bonet IJM, Khomula EV, Araldi D, Green PG, Levine JD. PI3Kgamma/AKT signaling in high molecular weight hyaluronan (HMWH)-induced anti-hyperalgesia and reversal of nociceptor sensitization. J Neurosci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science 1982;217(4556):252–254. [DOI] [PubMed] [Google Scholar]
- [17].Bossi P, Miceli R, Locati LD, Ferrari D, Vecchio S, Moretti G, Denaro N, Caponigro F, Airoldi M, Moro C, Vaccher E, Sponghini A, Caldara A, Rinaldi G, Ferrau F, Nole F, Lo Vullo S, Tettamanzi F, Hollander L, Licitra L. A randomized, phase 2 study of cetuximab plus cisplatin with or without paclitaxel for the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2017;28(11):2820–2826. [DOI] [PubMed] [Google Scholar]
- [18].Bourguignon LY, Shiina M, Li JJ. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv Cancer Res 2014;123:255–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bourguignon LY, Singleton PA, Zhu H, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem 2003;278(32):29420–29434. [DOI] [PubMed] [Google Scholar]
- [20].Branca JJV, Carrino D, Gulisano M, Ghelardini C, Di Cesare Mannelli L, Pacini A. Oxaliplatin-Induced Neuropathy: Genetic and Epigenetic Profile to Better Understand How to Ameliorate This Side Effect. Front Mol Biosci 2021;8:643824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, Pritchard JR, Pommier Y, Lippard SJ, Hemann MT. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 2017;23(4):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A 1987;84(18):6374–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caires R, Luis E, Taberner FJ, Fernandez-Ballester G, Ferrer-Montiel A, Balazs EA, Gomis A, Belmonte C, de la Pena E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat Commun 2015;6:8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cavaletti G, Bogliun G, Crespi V, Marzorati L, Zincone A, Marzola M, Rota S, Galli A, Tredici P, Tredici G. Neurotoxicity and ototoxicity of cisplatin plus paclitaxel in comparison to cisplatin plus cyclophosphamide in patients with epithelial ovarian cancer. J Clin Oncol 1997;15(1):199–206. [DOI] [PubMed] [Google Scholar]
- [25].Cavaletti G, Bogliun G, Marzorati L, Zincone A, Marzola M, Colombo N, Tredici G. Peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer 1995;75(5):1141–1150. [DOI] [PubMed] [Google Scholar]
- [26].Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol 1995;133(1):64–72. [DOI] [PubMed] [Google Scholar]
- [27].Cejalvo JM, Jacob W, Fleitas Kanonnikoff T, Felip E, Navarro Mendivil A, Martinez Garcia M, Taus Garcia A, Leighl N, Lassen U, Mau-Soerensen M, Adessi C, Michielin F, James I, Ceppi M, Hasmann M, Weisser M, Cervantes A. A phase Ib/II study of HER3-targeting lumretuzumab in combination with carboplatin and paclitaxel as first-line treatment in patients with advanced or metastatic squamous non-small cell lung cancer. ESMO Open 2019;4(4):e000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res 2005;81(1):31–37. [DOI] [PubMed] [Google Scholar]
- [29].Chen SP, Zhou YQ, Liu DQ, Zhang W, Manyande A, Guan XH, Tian YK, Ye DW, Omar DM. PI3K/Akt Pathway: A Potential Therapeutic Target for Chronic Pain. Curr Pharm Des 2017;23(12):1860–1868. [DOI] [PubMed] [Google Scholar]
- [30].Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 2010;149(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM, DiStefano PS. Physiological characterization of Taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol 1998;43(1):46–55. [DOI] [PubMed] [Google Scholar]
- [32].Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist 2007;12(6):713–718. [DOI] [PubMed] [Google Scholar]
- [33].Cohen MM, Altman RD, Hollstrom R, Hollstrom C, Sun C, Gipson B. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int 2008;29(7):657–663. [DOI] [PubMed] [Google Scholar]
- [34].Colvin LA. Chemotherapy-induced peripheral neuropathy: where are we now? Pain 2019;160 Suppl 1:S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Res 2015;4:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest 2001;108(7):1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Funez MI, Dias QM, Schivo IR, Domingues AC, Sachs D, Chiavegatto S, Teixeira MM, Hothersall JS, Cruz JS, Cunha FQ, Ferreira SH. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci U S A 2010;107(9):4442–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cunha TM, Souza GR, Domingues AC, Carreira EU, Lotufo CM, Funez MI, Verri WA Jr., Cunha FQ, Ferreira SH. Stimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kgamma/AKT/nNOS/NO signaling pathway. Mol Pain 2012;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Iuliis F, Taglieri L, Salerno G, Lanza R, Scarpa S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit Rev Oncol Hematol 2015;96(1):34–45. [DOI] [PubMed] [Google Scholar]
- [40].de la Pena E, Gomis A, Ferrer-Montiel A, Belmonte C. TRPV1 channel modulation by hyaluronan reduces pain. Channels (Austin) 2016;10(2):81–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage 1993;1(2):97–103. [DOI] [PubMed] [Google Scholar]
- [42].Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res 2009;87(7):1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferrari LF, Araldi D, Bogen O, Levine JD. Extracellular matrix hyaluronan signals via its CD44 receptor in the increased responsiveness to mechanical stimulation. Neuroscience 2016;324:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci Rep 2016;6:31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ferrari LF, Khomula EV, Araldi D, Levine JD. CD44 Signaling Mediates High Molecular Weight Hyaluronan-Induced Antihyperalgesia. J Neurosci 2018;38(2):308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006;122(3):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth 2017;119(4):737–749. [DOI] [PubMed] [Google Scholar]
- [48].Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 2006;346(3):904–910. [DOI] [PubMed] [Google Scholar]
- [49].Furuta J, Ariyoshi W, Okinaga T, Takeuchi J, Mitsugi S, Tominaga K, Nishihara T. High molecular weight hyaluronic acid regulates MMP13 expression in chondrocytes via DUSP10/MKP5. J Orthop Res 2017;35(2):331–339. [DOI] [PubMed] [Google Scholar]
- [50].Green PG, Dahlqvist SR, Isenberg WM, Miao FJ, Levine JD. Role of adrenal medulla in development of sexual dimorphism in inflammation. Eur J Neurosci 2001;14(9):1436–1444. [DOI] [PubMed] [Google Scholar]
- [51].Guan XH, Fu QC, Shi D, Bu HL, Song ZP, Xiong BR, Shu B, Xiang HB, Xu B, Manyande A, Cao F, Tian YK. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol 2015;263:39–49. [DOI] [PubMed] [Google Scholar]
- [52].Guan XH, Lu XF, Zhang HX, Wu JR, Yuan Y, Bao Q, Ling DY, Cao JL. Phosphatidylinositol 3-kinase mediates pain behaviors induced by activation of peripheral ephrinBs/EphBs signaling in mice. Pharmacol Biochem Behav 2010;95(3):315–324. [DOI] [PubMed] [Google Scholar]
- [53].Hamers FP, Pette C, Neijt JP, Gispen WH. The ACTH-(4-9) analog, ORG 2766, prevents taxol-induced neuropathy in rats. Eur J Pharmacol 1993;233(1):177–178. [DOI] [PubMed] [Google Scholar]
- [54].Huang TL, Chang CC, Lee CH, Chen SC, Lai CH, Tsai CL. Intra-articular injections of sodium hyaluronate (Hyalgan(R)) in osteoarthritis of the knee. a randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord 2011;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Imeri F, Blanchard O, Jenni A, Schwalm S, Wunsche C, Zivkovic A, Stark H, Pfeilschifter J, Huwiler A. FTY720 and two novel butterfly derivatives exert a general anti-inflammatory potential by reducing immune cell adhesion to endothelial cells through activation of S1P(3) and phosphoinositide 3-kinase. Naunyn Schmiedebergs Arch Pharmacol 2015;388(12):1283–1292. [DOI] [PubMed] [Google Scholar]
- [56].Johnstone TC, Suntharalingam K, Lippard SJ. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem Rev 2016;116(5):3436–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kataoka Y, Ariyoshi W, Okinaga T, Kaneuji T, Mitsugi S, Takahashi T, Nishihara T. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem Biophys Res Commun 2013;432(4):580–585. [DOI] [PubMed] [Google Scholar]
- [58].Konig C, Gavrilova-Ruch O, von Banchet GS, Bauer R, Grun M, Hirsch E, Rubio I, Schulz S, Heinemann SH, Schaible HG, Wetzker R. Modulation of mu opioid receptor desensitization in peripheral sensory neurons by phosphoinositide 3-kinase gamma. Neuroscience 2010;169(1):449–454. [DOI] [PubMed] [Google Scholar]
- [59].Kunitoh H, Saijo N, Furuse K, Noda K, Ogawa M. Neuromuscular toxicities of paclitaxel 210 mg m(−2) by 3-hour infusion. Br J Cancer 1998;77(10):1686–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Leinders M, Koehrn FJ, Bartok B, Boyle DL, Shubayev V, Kalcheva I, Yu NK, Park J, Kaang BK, Hefferan MP, Firestein GS, Sorkin LS. Differential distribution of PI3K isoforms in spinal cord and dorsal root ganglia: potential roles in acute inflammatory pain. Pain 2014;155(6):1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem 2000;275(35):26967–26975. [DOI] [PubMed] [Google Scholar]
- [62].Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res 2011;9(12):1573–1586. [DOI] [PubMed] [Google Scholar]
- [63].Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021;109(17):2691–2706 e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009;8(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 1994;32(4):197–200. [DOI] [PubMed] [Google Scholar]
- [66].Miaskowski C, Paul SM, Mastick J, Abrams G, Topp K, Smoot B, Kober KM, Chesney M, Mazor M, Mausisa G, Schumacher M, Conley YP, Sabes JH, Cheung S, Wallhagen M, Levine JD. Associations Between Perceived Stress and Chemotherapy-Induced Peripheral Neuropathy and Otoxicity in Adult Cancer Survivors. J Pain Symptom Manage 2018;56(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- [68].Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG, Moghimi SM, Peer D. Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release 2011;156(2):231–238. [DOI] [PubMed] [Google Scholar]
- [69].Oliveira-Fusaro MCG, Zanoni CIS, Dos Santos GG, Manzo LP, Araldi D, Bonet IJM, Tambeli CH, Dias EV, Parada CA. Antihyperalgesic effect of CB1 receptor activation involves the modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol 2017;798:113–121. [DOI] [PubMed] [Google Scholar]
- [70].Padmanabhan J, Gonzalez AL. The effects of extracellular matrix proteins on neutrophil-endothelial interaction--a roadway to multiple therapeutic opportunities. Yale J Biol Med 2012;85(2):167–185. [PMC free article] [PubMed] [Google Scholar]
- [71].Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience 2003;120(1):219–226. [DOI] [PubMed] [Google Scholar]
- [72].Pereira PJS, Lazarotto LF, Leal PC, Lopes TG, Morrone FB, Campos MM. Inhibition of phosphatidylinositol-3 kinase gamma reduces pruriceptive, inflammatory, and nociceptive responses induced by trypsin in mice. Pain 2011;152(12):2861–2869. [DOI] [PubMed] [Google Scholar]
- [73].Petrovchich I, Kober KM, Wagner L, Paul SM, Abrams G, Chesney MA, Topp K, Smoot B, Schumacher M, Conley YP, Hammer M, Levine JD, Miaskowski C. Deleterious Effects of Higher Body Mass Index on Subjective and Objective Measures of Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors. J Pain Symptom Manage 2019;58(2):252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pezet S, Marchand F, D'Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci 2008;28(16):4261–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J. Intrathecal PLC(beta3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res 2012;1472:38–44. [DOI] [PubMed] [Google Scholar]
- [76].Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 1957;111(4):409–419. [PubMed] [Google Scholar]
- [77].Safra T, Waissengrin B, Levy T, Leidner E, Merose R, Matceyevsky D, Grisaru D, Laskov I, Mishaan N, Shayzaf R, Wolf I. Weekly Carboplatin and Paclitaxel: A Retrospective Comparison with the Three-Weekly Schedule in First-Line Treatment of Ovarian Cancer. Oncologist 2021;26(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Salat K. Chemotherapy-induced peripheral neuropathy: part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep 2020;72(3):486–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980;77(3):1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Senbanjo LT, Chellaiah MA. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol 2017;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shimabukuro Y, Terashima H, Takedachi M, Maeda K, Nakamura T, Sawada K, Kobashi M, Awata T, Oohara H, Kawahara T, Iwayama T, Hashikawa T, Yanagita M, Yamada S, Murakami S. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J Cell Physiol 2011;226(3):809–821. [DOI] [PubMed] [Google Scholar]
- [82].Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull 2009;78(6):335–341. [DOI] [PubMed] [Google Scholar]
- [83].Song SJ, Min J, Suh SY, Jung SH, Hahn HJ, Im SA, Lee JY. Incidence of taxane-induced peripheral neuropathy receiving treatment and prescription patterns in patients with breast cancer. Support Care Cancer 2017;25(7):2241–2248. [DOI] [PubMed] [Google Scholar]
- [84].Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011;31(43):15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 2006;85(8):699–715. [DOI] [PubMed] [Google Scholar]
- [86].Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 2011;12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res 2013;91(4):545–553. [DOI] [PubMed] [Google Scholar]
- [88].Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S, Onoe T, Homma A, Taguchi J, Suzuki M, Minato K, Yane K, Ueda S, Hara H, Saijo K, Yamanaka T. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol 2018;29(4):1004–1009. [DOI] [PubMed] [Google Scholar]
- [89].Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res 1989;487(1):148–151. [DOI] [PubMed] [Google Scholar]
- [90].Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res 1989;492(1-2):397–399. [DOI] [PubMed] [Google Scholar]
- [91].Tammi R, MacCallum D, Hascall VC, Pienimaki JP, Hyttinen M, Tammi M. Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J Biol Chem 1998;273(44):28878–28888. [DOI] [PubMed] [Google Scholar]
- [92].Tanishima H, Tominaga T, Kimura M, Maeda T, Shirai Y, Horiuchi T. Hyperacute peripheral neuropathy is a predictor of oxaliplatin-induced persistent peripheral neuropathy. Support Care Cancer 2017;25(5):1383–1389. [DOI] [PubMed] [Google Scholar]
- [93].Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J 2019;286(15):2883–2908. [DOI] [PubMed] [Google Scholar]
- [94].Triantaffilidou K, Venetis G, Bika O. Efficacy of hyaluronic acid injections in patients with osteoarthritis of the temporomandibular joint. A comparative study. J Craniofac Surg 2013;24(6):2006–2009. [DOI] [PubMed] [Google Scholar]
- [95].Underhill C. CD44: the hyaluronan receptor. J Cell Sci 1992;103 ( Pt 2):293–298. [DOI] [PubMed] [Google Scholar]
- [96].Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010;11(5):329–341. [DOI] [PubMed] [Google Scholar]
- [97].Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta 2014;1840(8):2452–2459. [DOI] [PubMed] [Google Scholar]
- [98].Vigetti D, Viola M, Karousou E, De Luca G, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol 2014;35:8–13. [DOI] [PubMed] [Google Scholar]
- [99].Villaruz LC, Cobo M, Syrigos K, Mavroudis D, Zhang W, Kim JS, Socinski MA. A phase II study of nab-paclitaxel and carboplatin chemotherapy plus necitumumab in the first-line treatment of patients with stage IV squamous non-small cell lung cancer. Lung Cancer 2019;136:52–56. [DOI] [PubMed] [Google Scholar]
- [100].Wu PT, Kuo LC, Su FC, Chen SY, Hsu TI, Li CY, Tsai KJ, Jou IM. High-molecular-weight hyaluronic acid attenuated matrix metalloproteinase-1 and −3 expression via CD44 in tendinopathy. Sci Rep 2017;7:40840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Xu B, Guan XH, Yu JX, Lv J, Zhang HX, Fu QC, Xiang HB, Bu HL, Shi D, Shu B, Qin LS, Manyande A, Tian YK. Activation of spinal phosphatidylinositol 3-kinase/protein kinase B mediates pain behavior induced by plantar incision in mice. Exp Neurol 2014;255:71–82. [DOI] [PubMed] [Google Scholar]
- [102].Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci 2019;20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang Y, Thant AA, Machida K, Ichigotani Y, Naito Y, Hiraiwa Y, Senga T, Sohara Y, Matsuda S, Hamaguchi M. Hyaluronan-CD44s signaling regulates matrix metalloproteinase-2 secretion in a human lung carcinoma cell line QG90. Cancer Res 2002;62(14):3962–3965. [PubMed] [Google Scholar]
- [104].Zhang Z, Qin P, Deng Y, Ma Z, Guo H, Guo H, Hou Y, Wang S, Zou W, Sun Y, Ma Y, Hou W. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J Neuroinflammation 2018;15(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci 2007;34(4):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 2004;24(38):8300–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]


