Abstract
Microglial homeostasis has emerged as a critical mediator of health and disease in the central nervous system. In their neuroprotective role as the predominant immune cells of the brain, microglia surveil the microenvironment for debris and pathogens, while also promoting neurogenesis and performing maintenance on synapses. Chronological aging, disease onset, or traumatic injury promotes irreparable damage or deregulated signaling to reinforce neurotoxic phenotypes in microglia. These insults may include cellular senescence, a stable growth arrest often accompanied by the production of a distinctive pro-inflammatory secretory phenotype, which may contribute to age- or disease-driven decline in neuronal health and cognition and is a potential novel therapeutic target. Despite this increased scrutiny, unanswered questions remain about what distinguishes senescent microglia and non-senescent microglia reacting to insults occurring in aging, disease, and injury, and how central the development of senescence is in their pivot from guardian to assailant. To intelligently design future studies to untangle senescent microglia from other primed and reactionary states, specific criteria must be developed that define this population and allow for comparisons between different model systems. Comparing microglial activity seen in homeostasis, aging, disease, and injury allows for a more coherent understanding of when and how senescent and other harmful microglial subpopulations should be targeted.
Keywords: senescence, microglia, aging, TBI, neurodegenerative disease
Introduction
Globally, the number of people aged 65+ years will likely be greater than 1.5 billion by 2050; comprising ~16% of the world’s population [1]. With this aging population comes a predicted increase in various age-related diseases, including many neurodegenerative diseases (ND) like Alzheimer’s disease (AD) and Parkinson’s disease. Exploring the link between increased age and increased risk of ND has been an attractive area of research, especially given the high costs involved with the treatment and care of the afflicted. For example, the cost of AD and other dementias on the United States healthcare system alone is estimated to reach $355 billion in 2021, without even considering informal costs to caregivers [2]. Unfortunately, there is currently no cure for most age-related NDs, and treatment strategies usually center around simply managing the symptoms of a disease. This deficiency highlights the need for the development of new targets and therapeutic strategies to combat these ailments.
Another area of recent concern in neurological health has been the implication of traumatic brain injury in contact sport athletes and soldiers. Kinetic insults to the central nervous system (CNS) may not only be acutely disabling, but can promote persistent changes in the cellular populations and microenvironment of the CNS for years post-injury [3]. Compelling similarities exist between the post-injury phenotype seen in traumatic brain injury (TBI) and the disease phenotype observed in the brains of patients with AD and other dementias, including dysregulation of amyloid and tau proteins and a sustained alteration of glial phenotype [4–8]. Recent meta-analyses have even found potential connections between TBI occurring early in life and the risk of developing ND with age in humans [9,10], although issues with self-reporting and grading of TBI severity complicate the correlation [11,12]. Similar to geriatric NDs, no therapies exist to relieve the long-term effects of TBI beyond preventative measures, which is problematic as approximately 69 million individuals worldwide are estimated to sustain a TBI every year [13].
An attractive emerging therapeutic target in many maladies of aging is cellular senescence, which could provide a possible link between a dysfunctional CNS microenvironment and increased risk of ND. Senescence describes a cellular state where an irreversible cell-cycle arrest is induced by the expression of cyclin-dependent kinase inhibitors Cdkn1a (p21CIP1, hereafter p21) and/or Cdkn2a (p16INK4a, hereafter p16) [14–16]. Senescent cells produce a distinct inflammatory secretome and have been found to accumulate with age and in many diseases; potentially contributing to pathology [17–21]. A challenge in targeting senescence is the apparent heterogeneity of this cellular state, wherein a variety of insults may incite senescence, the inflammatory secretory phenotype is modified depending on cell type, and the senescent phenotype may even change based on the stage of senescence [22–24]. Even defining senescence is difficult, with different groups using different criteria to define a senescent cell. To address this, the International Cell Senescence Association recently put forth a recommendation for determining the presence of senescence, describing a combination of cell-cycle arrest, macromolecular damage, deregulated metabolism, and the production of a senescent-associated secretory phenotype (SASP) [25]. Another group has also proposed a two-step algorithm to assess senescence [24]. These features of senescence in the context of microglia will be explored later in this review.
If senescent cells are active drivers of neurodegeneration, then identifying the cellular population responsible becomes critical. Putative senescent states have been suggested in a variety of neural subpopulations with aging and in various disease contexts, including microglia [26–30], neurons [31–33], astrocytes [26,34,35], and oligodendrocyte progenitor cells [36]. Vascular and endothelial cells of the blood-brain barrier have also demonstrated senescence properties [37,38]. It is also likely that multiple cell types become senescent in complex diseases, which further complicates the cause-effect relationship between specific populations of senescent cells and disease processes.
Microglia, the resident mononuclear phagocytes of the CNS, play key roles in immune surveillance and defense [39], synaptic remodeling [40], and homeostasis [41]. They have also been identified as a putative senescent population in natural aging in both humans [42] and mice [27], mouse models of AD pathology [26], and in TBI [28,29]. However, in a reactionary or dystrophic state, microglia also contribute to the pathogenesis of various NDs and post-TBI syndromes [43–45]. The overlapping phenotypes of reactive and senescent microglia together with the heterogeneity of microglial phenotypes described in aging and disease [45,46] present a nuanced challenge to defining senescent microglia and parsing out their contribution to dysfunction.
In this review, we discuss the features of microglia phenotypes associated with aging, disease, and injury, and propose how senescent microglia may contribute to these conditions. With the known phenotypical overlaps between microglia across these contexts, we will also suggest ways to distinguish senescent microglia from other microglial populations.
Phenotypic complexity of microglia in aging, neurodegenerative disease, and injury
Microglial states are usually defined in relation to the conditions present at homeostasis (Figure 1). Although the term ‘homeostasis’ can be over-simplified – indeed the exact nature of these homeostatic microglia may differ based on many factors including brain region and temporal heterogeneity [47] – we will refer to the state of microglia in non-pathological contexts as ‘homeostatic’ for the purposes of this review. In this state, microglia are associated with immune protection, the support of neuron health, and synaptic remodeling [40,47].
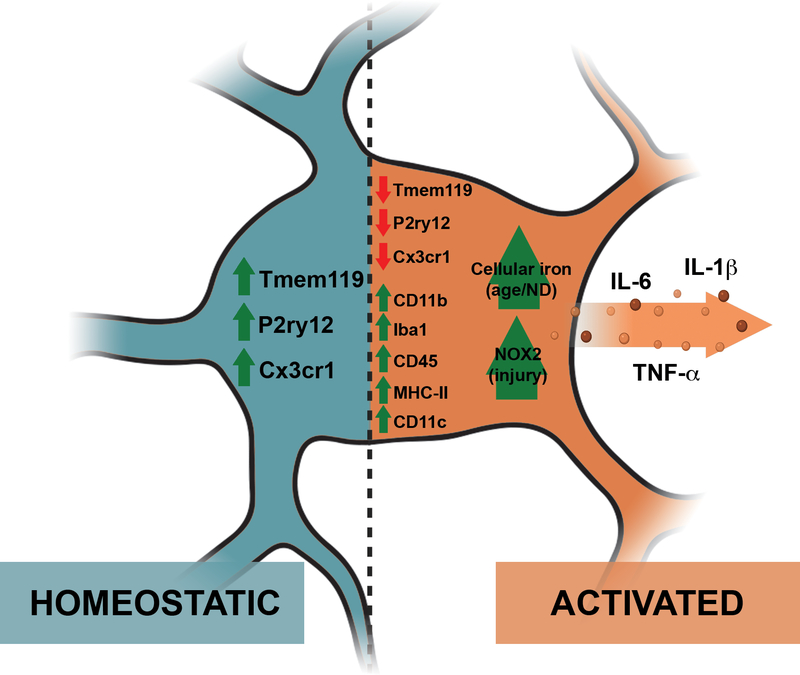
Figure 1. Contrasting features of microglia in homeostatic and inflammatory ‘activated’ states.
The ‘activated’ inflammatory microglial state differs from its resting homeostatic state in many ways, such as the downregulation of homeostatic markers (Tmem119, P2ry12, Cx3cr1) and the upregulation of other markers like CD11b, Iba1, CD45, MHC-II, and Cd11c. ‘Activated’ microglia also have a pro-inflammatory secretome, which includes IL-6, IL-1β, and TNF-α. They also differ in morphology, with homeostatic microglia having highly ramified processes and ‘activated’ inflammatory microglia having shorter processes with a larger cell body. Some putative markers proposed for ‘activated’ inflammatory microglia in specific contexts include an increase in cellular iron with age and neurodegenerative disease (ND) and an upregulation of NOX2 in injury. Figure created in BioRender.
Dysfunctional microglia are distinguished by a loss of homeostatic function, including impaired phagocytic capacity and the acquisition of a pro-inflammatory profile [48]. For the purposes of this review, these pro-inflammatory (yet non-senescent) microglia associated with pathological conditions will be referred to as ‘activated’ microglia (Figure 1). While we acknowledge that the use of the label ‘activated’ can be vague, it is outside the scope of this review to dissect the various states of microglia that have been described in aging, neurodegenerative disease and injury. Other reviews [47,49,50] have more closely examined the nuanced heterogeneity of microglia in those states.
Senescent and dysfunctional microglia, under various names, are often referenced interchangeably in studies of aging, ND, and TBI. We postulate that senescence is a distinct cellular state which can be distinguished from otherwise non-senescent inflammatory microglia (Figure 2). Given the putative differentiating features between microglial phenotypes outlined below, unique roles played by senescent microglia may be delineated and not attributed to non-senescent dysfunctional microglia.
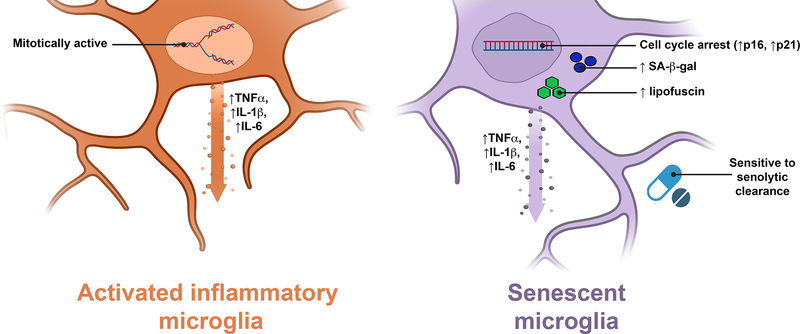
Figure 2. Similarities and differences between ‘activated’ inflammatory and senescent microglia.
Senescent microglia can be difficult to differentiate from an ‘activated’ inflammatory state as they share many characteristics, such as an inflammatory secretome which may include TNF-α, IL-1β, and IL-6. Some features we propose that can differentiate senescent microglia include cell cycle arrest (especially with an upregulation in p16 and p21), an increase in SA-β-gal staining, an increase in lipofuscin staining, as well as sensitivity to senolytic clearance. Figure created in BioRender.
Aging
Several changes are associated with the microglial populations in the aged brain. An accumulation of microglia with a ‘dystrophic’ appearance or de-ramified microglia with cytoplasmic fragmentations have been noted in aged human brains [42,51]. Aged microglia also tend to accumulate lipofuscin [52–54], aggregations of highly oxidized proteins cross-linked with sugars and lipids [55], which have been associated with dysfunction and inflammation [56,57]. Additionally, microglia in naturally aged mice appear to have lower phagocytic activity compared to young mice [48]. This, combined with an apparent decrease in motility [58,59], has been suggested to contribute to dysfunctional synaptic pruning and potentially to the cognitive deficits associated with age [60,61].
In both humans [62] and mice [52,63,64], aged microglia adopt a pro-inflammatory profile including higher expression of genes including TNFα, IL-6, and IL-1β, as well as markers of ‘priming’ such as CD11b and MHC-II (Figure 1). This state of ‘priming’ is thought to make aged microglia disproportionately reactive to immunological challenges, leading to prolonged neuroinflammation and the cognitive deficits associated with age [65,66]. The exact cause for the shifting of microglia to this pro-inflammatory state with age is unknown, although several theories such as the increased permeability of the blood-brain barrier introducing more ‘priming’ signals [67,68] and/or monocyte-derived cells [69], or age-related myelin fragment accumulation [70] have been proposed. It is also interesting to note that while the existing body of literature strongly indicates systemic organismal aging influences microglia function, the reverse is also true – that a change in microglial function could also result in age-related cognitive changes. For example, microglial repopulation in naturally aged mice led to a rescue in age-related cognitive, synaptic and neuronal deficits [71].
The concept of increased microglial senescence in aged brains has gained traction in recent years. An accumulation of senescent microglia with age has been shown in rodents both in vivo [72] and ex vivo [51,72], although the evidence of senescent microglia accumulation in aged human brains is limited [42]. Further developing the concept of microglial senescence remains challenging though, given the lack of consensus on the hallmarks needed to define its presence. In particular, better resolution of the expression patterns and composition of the SASP is needed, especially in comparison to a normal responsive state.
Neurodegenerative disease
The loss of homeostatic function and an ‘activated’ phenotype in microglia has also been associated with ND. Previously, disease-relevant microglia were often compared to M1 pro-inflammatory ‘activated’ macrophages [73], but increasingly other labels like disease-associated microglia (DAMs) [44], ‘dark’ microglia [74], ‘primed’ microglia [65,66], or more nuanced references to the exact cell markers expressed by specific microglial populations, have grown in popularity to better reflect the heterogeneous nature of microglial biology [75]. Morphologically speaking, microglia with a dystrophic appearance are also increased in several NDs [42,76].
It remains to be seen if there is a common microglial phenotype that is associated with ND. The downregulation of genes associated with microglial homeostasis, Tmem119, P2ry12, and Cx3cr1, have been identified in many murine models representing AD [44,77–80], amyotrophic lateral sclerosis (ALS) [44,77,79], and multiple sclerosis [79]; suggesting similar signaling pathways are present. It has also been suggested that microglia could mechanistically contribute to ND through aberrant synaptic pruning via the complement system [81]. These losses of homeostatic function are also not unique to microglia in ND and are seen in the context of aging [44,60,61,77,82]. This concurrence could explain the strong link between increased age and ND [83].
There is of yet no consensus on the identifying markers or genes for the ‘activated’ inflammatory microglia associated with disease [47]. It’s likely that different populations of microglia can play variable roles depending on the disease, stage of the disease, or brain region affected, making the comparison between studies especially challenging. Some markers commonly identified as upregulated in microglia associated with ND are Cd11c [44,84–86], Iba1 [74,87,88], Trem2 variants [89,90], and MHC-II [91,92]. Pro-inflammatory molecules like IL-1β, IL-6, TNFα, and MCP-1 are also associated with ‘activated’ microglia in disease [93].
Senescent microglia have also been identified as contributing to the pathogenesis of NDs. Whole-body clearance of p16-expressing cells in a mouse model of tauopathy led to an amelioration of disease, with microglia identified as a senescent cell population in this disease model [26]. Another study in a mouse model of AD found that replicative senescence in microglia was associated with early pathology [30]. A mouse model of ALS also found gliosis and motor neuron loss was associated with increased senescence indicators [94]. However, it is likely microglia are but one neural subpopulation that becomes senescent in age and disease. Recent studies have produced evidence for senescence in neurons and oligodendrocyte progenitors in other mouse models of tauopathy and AD [33,36]. Regardless of the hypothesized identity of the senescent population, each of these studies demonstrated pathology mitigation following pharmacological removal of senescent cells [26,33,36].
The relationship between senescent and ‘activated’ microglia in the context of disease remains unclear – one state could promote the other, or they may be overlapping populations. This is especially challenging since the SASP, a key feature of senescent cells, commonly encompasses pro-inflammatory molecules also associated with ‘activated’ microglia like TNFα, IL-1β, and IL-6 [95]. It’s also unclear whether senescent microglia upregulate markers associated with ‘activated’ microglia or downregulate microglial homeostatic genes.
Injury
Traumatic brain injury (TBI) differs from aging and disease in that the inciting factor for its associated pathology is external and more acute. A variety of mechanical insults including contusion, closed-head impact, and blast injury can produce mild, moderate, or severe TBI depending on individual circumstances [96–99]. TBI is a biphasic injury: after the mechanical primary injury, a secondary dystrophic state produces long-term dysfunction in the brain that includes elevated neuroinflammation as a result of blood-brain barrier disruption, increased oxidative stress, and persistent changes in the microglial population [100–106].
In the minutes following TBI, microglia are recruited via purinergic signaling to clear necrotic debris and adopt a phagocytic state [100,107,108]. The mode of activation following TBI is somewhat age-dependent and may be less neuroprotective and more toxic in older animals [109]. In the initial week following injury, the microglia population adopts a balanced distribution between pro- and anti-inflammatory states to promote neurogenesis and enhance the immune response to injury [89,110,111]. However, as time passes following the primary injury the neuroprotective functions are lost, and microglia become the prime motivators of neuroinflammatory gene expression in the post-TBI brain [112]. In vitro, conditioned media derived from microglia in an induced pro-inflammatory ‘M1-like’ state reduces survival of oligodendrocytes in vitro, whereas conditioned media from microglia in an induced anti-inflammatory ‘M2-like’ state promotes remyelination [111,113]. In human patients, increased microglial activation is even detectable by PET scan 17 years after the primary injury [114].
Microglia associated with the long-term inflammatory phenotype following TBI are commonly defined as MHC-II/CD86/Cd11b positive, with upregulated expression of NOX24, Tlr4, Trem2, CD68, Clec7a, and Stat1, and higher expression of interferon- and immune-associated signaling genes [100,112,115,116]. This resembles the ‘primed’ state microglia appear to enter with aging and is also determined both by cell-intrinsic changes and external influences from the altered microenvironment [105]. Of recent interest is how post-injury microglia promote increased oxidative damage leading to neurodegeneration through a NOX2-dependent mechanism [100,117,118]. The activity of NOX2 in glia may in fact track with the severity of TBI in patients [119]. While microglial NOX2 is chronically active in neurodegenerative contexts, its rapid increase in activity in microglia following TBI is relevant to their role in driving pathology and an important distinguishing feature [120,121]. Importantly, a recent study in mice demonstrated pharmacological clearance of microglia post-injury is neuroprotective and reduces cognitive impairment [112].
Inquiry into pro-inflammatory microglia as a therapeutic target in TBI secondary injury has coincided with increasing interest in microglial senescence. Elderly individuals are at greater risk to suffer significant cognitive impairment and early morbidity following TBI, potentially due to an already present dysfunction in the microglial compartment [109,122,123]. Due to the difficulty of distinguishing senescent microglia from other primed or dystrophic states, the evidence for these cells becoming senescent following TBI remains preliminary. There is evidence of reductions in telomere length and deficiencies in DNA repair following TBI which could predispose neural cells to senescence [124–126]. Two recent publications identified a possible senescence signature following TBI in adult mice [28,29]. Although their ultimate role in the secondary injury cascade is still under investigation, this emerging evidence points to senescent microglia as contributors to the post-TBI phenotype.
Distinguishing microglia in aging, neurodegenerative disease, and injury
In the past decade, the study of microglial biology has evolved beyond the M1 vs. M2 paradigm, acknowledging the broad spectrum of intermediate states these cells exist in depending on context. There is a large overlap between microglial phenotypes in aging, neurodegenerative disease, and injury – mainly a loss of homeostasis and a pro-inflammatory phenotype. For now, there is no cellular marker or component of the secretome that is specific for microglia found in aging, disease, or injury.
Attempting to distinguish microglia found in the aging and neurodegenerative contexts implies that it’s possible to have an aged ‘healthy’ brain without ND. Some may argue that aging itself is a pathology – neuropathological features like neurofibrillary tangles and amyloid plaques have been identified post-mortem in individuals who otherwise did not demonstrate overt cognitive impairment [127–130]. It is unknown if these pathological features indicate that these individuals were in the pre-clinical stages of a disease or if they would continue to be nondemented despite their neuropathology [131].
Regardless, there are some studies that have offered putative ways to distinguish these closely related microglial populations. An interesting example is iron accumulation and metabolism. One study by Shahidehpour et al. suggests that there is increased ferritin accumulation in human microglia in ND but not old age, and proposed altered iron homeostasis as a distinguishing factor between aged ‘healthy’ microglia and those associated with neurodegeneration [132]. Indeed, elevated iron in the CNS is associated with ND (reviewed in [133] and [134]), possibly at levels higher than with normal aging [133]. Given that increased iron levels are also associated with advanced age [133–135] more studies are needed to ascertain if altered iron homeostasis is in fact specific to ND-associated microglia. Additionally, increased cellular iron accumulation has been posited as a feature of senescence, although it’s not critical to maintaining this fate [136,137]. Whether changes in iron homeostasis reflect senescent cell accumulation with CNS aging or is in fact relevant to the emergence of disease remains to be seen.
Some investigators have turned to the resolution provided by single-cell sequencing technology to clarify any differing roles played by microglia. Several single-cell studies in murine microglia have been carried out to delineate the distinct roles microglia could play in these closely related contexts. For example, a study by Hammond et al. compared microglia from developing, aged, and mice injured from a focal white matter injury and identified two populations highly concentrated in aged mice – one defined by Ccl4, and another enriched in several interferon-response genes [45]. In addition, a cluster predominantly in injured mice characterized by Ifi27l2a was also identified [45]. Other studies have delineated specific populations of microglia associated with disease [44,138–140], however, the specific characteristics of the microglia of interest in each study differed. This potentially highlights a general difficulty in comparing single-cell studies – that the high resolution provided is extremely sensitive to the exact model used and even the isolation and preparation method of the microglia. Another difficulty in interpreting the results from single-cell studies is the lack of validating studies showing functional implications of the unique microglial populations identified. Regardless, single-cell profiling of microglia remains an important tool in parsing apart the differences between the different microglial states.
Several of the aforementioned studies incompletely investigate the role of senescent microglia in the various microglia phenotypes described. This could be because the common features seen in senescent cells and ‘activated’ inflammatory microglia make it difficult to distinguish how these populations contribute to pathology.
Distinguishing between senescent microglia and ‘activated’ microglia
The potential overlap between senescent microglia and ‘activated’ microglia remains an underexplored area in microglial research (Figure 2). Further investigation into this relationship could help explain neurodegeneration in age, disease, and injury and provide novel treatment options in the removal of senescent cells with pharmaceutical interventions known as senolytic drugs.
Perhaps one reason why studies on senescent microglia have been inconsistent is due to a lack of stringent criteria in labeling a cellular state as ‘senescent’. In immunological terms, senescence is often used loosely or interchangeably with immunosenescence, to describe a general decline in immune function with age [141]. However, cellular senescence as described here refers to a specific cellular state, often defined by a combination of cell-cycle arrest, macromolecular damage, deregulated metabolism, and a SASP [25]. Even with the latter definition in mind, many papers have not investigated all of these aspects of the cellular state when describing senescence [22,25], and the ‘senescent’ microglia previously reported may in fact be referring to different groups of ‘activated’ microglia.
Another roadblock in trying to define senescence in microglia is the overlap with ‘activated’ microglia. For example, the presence of a SASP is often used to show that a cellular population is senescent [25]. However, a SASP factor specific to senescent microglia has not been identified, and several components of the SASP can be attributed to the secreted inflammatory milieu of non-senescent ‘activated’ microglia [93]. To add extra confusion, the SASP can also differ based on cell type, cause of senescence, or even stage of senescence [37,95]. This makes defining microglial senescence by this one aspect difficult.
A common marker used to define senescent cells, senescence-associated β-galactosidase (SA-β-gal), may not be specific to senescence in microglia. SA-β-gal is a colorimetric assay to detect senescent cells through histochemical means and is thought to rely on the increased lysosomal mass [142,143]. However, it is becoming increasingly evident that SA-β-gal may not be a marker of senescence in all contexts [144]. In particular, non-senescent macrophages may exhibit SA-β-gal positivity [145,146], and microglia can also stain positive in non-senescent contexts due to their phagocytic function and the possible associated change in lysosomal numbers [147]. Still, the SA-β-gal assay could potentially be used with the right controls and assessed in terms of an increase in SA-β-gal activity rather than a presence/absence assay [30,148]. To validate this method, the specificity of SA-β-gal in microglia needs to be formally assessed.
Another possible alternative to the SA-β-gal assay particularly in the context of microglia is the use of Sudan Black B, a stain for lipofuscin [149]. Lipofuscin has been shown to accumulate in aged microglia [52–54], and is generally considered a hallmark of aging [150]. It has been suggested that Sudan Black B has a high overlap with SA-β-gal staining, which is especially relevant in contexts where the latter is not suitable such as in formalin-fixed, paraffin-embedded tissues [151,152], or in microglia where SA-β-gal is likely to stain false positive. However, whether the Sudan Black B assay is specific to senescence, its rate of false positivity, and its overlap with SA-β-gal in microglia remains to be thoroughly investigated.
A possible differentiating factor between ‘activated’ and senescent microglia is cell cycle arrest. A senescent cell, by definition, is in a state of cell cycle arrest [25]. In contrast, increased proliferation has been associated with ‘activated’ pro-inflammatory microglia in animal models [84,153–155]. This makes the assessment of cell cycle arrest, whether it be through the upregulation of certain senescence-associated cell cycle inhibitors like p16 and p21 [25] and/or through directly assessing proliferation by incorporation of EdU or cell tracking, a possible avenue to differentiate senescent cells from ‘activated’ cells. However, using the expression of cell cycle inhibitors as a surrogate for cell cycle arrest comes with its own limitations. For example, p21 may have roles outside of cell cycle inhibition [156] and its upregulation is not specific to senescence [157]. p21 levels may also decrease in ‘late’ senescence or when senescence has been achieved [115,158], whereas high levels of p16 are thought to be maintained throughout senescence [158,159]. Although p16 is often considered a more reliable marker of senescence, it can also be upregulated in non-senescent contexts [18,48,72]. The uncertain specificity of p16 and p21 in microglia should be taken into consideration, re-enforcing the need for a combinatorial approach to senescence markers.
The increasing adoption of single-cell RNA sequencing has also led some to use the technology to define senescent populations. However, the considerable variability of SASP expression [22,37] and non-specificity to senescence in microglia [93] still applies. A gene specific to cellular senescence also has not been identified, and different papers will use different marker genes to describe their senescent cell population [37,160]. Regardless, single-cell RNA sequencing could be used to support the presence of a senescent cell population if multiple aspects of senescence are addressed – including that of cell cycle arrest (especially with an upregulation of the senescence-associated Cdkn2a and Cdkn1a), a pro-inflammatory secretome, and possible other context-specific senescent markers. Pathway analyses may also be useful, with cellular senescence available as a gene ontology term that has been used to define senescence in the brain [161]. This should be done with caution, as several genes in that pathway are not specific to senescence, and using pathway analyses should be a supportive tool and not used alone to define senescence.
Another possible way to support the presence of senescence in a microglia population is to probe their response to senolytics. Senolytics are pharmacological interventions that selectively clear senescent cells, and mostly work by exploiting senescent cell reliance on anti-apoptotic and pro-survival pathways [162], which have been termed the Senescent Cell Anti-Apoptotic Pathways (SCAPs) [163]. The targets of commonly used senolytic agents can vary. Navitoclax inhibits the B-cell lymphoma 2 (BCL-2) family of anti-apoptotic factors [164,165], and while fisetin inhibits some of these and other SCAP network components it may also exert its senolytic effect through other activities like the inhibition of the PI3K/AKT/mTOR pathway [166–168]. The combination of dasatinib and quercetin also inhibits SCAP network components and multiple tyrosine kinases [169]. When SCAPs are targeted, there is theoretically preferential death of senescent cells by senolytics at doses that will not affect non-senescent cells [170]. Perhaps an increased reliance on pro-survival pathways and susceptibility to senolytics could help differentiate senescent microglia from non-senescent inflammatory microglia. The first-generation senolytics currently employed have the potential for off-target effects and are effective in different cell types, and the correct dose and class of senolytics necessary for the selective clearance of senescent microglia are still undetermined. Nevertheless, some senolytics have been effectively used in mouse models of neurological disease to alleviate pathology including dasatinib and quercetin [36] and navitoclax [26], which has led to senolytic therapies entering clinical trials for the modulation of AD [171]. Given the global effects of all current senolytics, a major consideration for future studies should be differentiating CNS-specific vs. systemic results of senescent cell clearance.
It is critical that studies aimed at rigorously defining microglial senescence in aging, disease, and injury must demonstrate a clear senescent signature. This is particularly important due to the absence of a single, unambiguous senescence biomarker, which complicates definitive claims. Techniques such as bulk or single-cell RNA sequencing on sorted microglia can be expensive and time-consuming, but such methods provide a solid foundation from which to form hypotheses about senescence involvement in an experimental system. Due to the highly situational nature of the senescence program, gene-expression profiling is essential to determine a particular microglia subpopulation to assay for further senescence markers (cell-cycle arrest, p16/p21 upregulation, macromolecular damage, etc.). Transgenic mouse lines where senescent cells can be cleared by a genetic construct and commercially available senolytic drugs should also be considered as sensitivity to senolytic methods is one of the strongest lines of evidence currently used in the literature [167,172–174]. Finally, although published evidence for microglial senescence is highly variable it can still be valuable as a reference point when designing a study and should be expanded upon to provide a comprehensive picture of how senescent microglia drive neurodegeneration. Distinguishing senescent microglia and defining how they drive neurodegeneration will have major implications in developing new treatment strategies, given the unique and exploitable features of senescent cells.
Acknowledgments
The authors would like to thank T. Bussian for helpful discussions and feedback.
This work was supported by the Ellison Medical Foundation, the Glenn Foundation for Medical Research, the Cure Alzheimer’s Fund, the National Institutes of Health (R01 AG053229 and R01 AG068076), the Mayo Clinic Children’s Research Center and the Alzheimer’s Disease Research Center of Mayo Clinic (all to D.J.B.).
Conflicts of Interest
D.J.B. is a shareholder and co-inventor on patent applications licensed to or filed by Unity Biotechnology, a company developing senolytic medicines, including small molecules that selectively eliminate senescent cells. Research in his lab has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies. The funders had no role in the writing of the manuscript or in the decision to publish.
Abbreviations:
- AD
Alzheimer’s disease
- BCL-2
B-cell lymphoma 2
- CNS
central nervous system
- ND
neurodegenerative disease
- SA-β-gal
senescent-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
- SCAP
Senescent Cell Anti-Apoptotic Pathway
- TBI
traumatic brain injury
References
- 1.United Nations Department of Economic and Social Affairs (2020) World Population Ageing 2020 Highlights. In Choice Reviews Online. [Google Scholar]
- 2.Alzheimer’s Association (2021) 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 17. [DOI] [PubMed] [Google Scholar]
- 3.Hiploylee C, Dufort PA, Davis HS, Wennberg RA, Tartaglia MC, Mikulis D, Hazrati L-N & Tator CH (2017) Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J Neurotrauma 34, 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshino S, Tamaoka A, Takahashi M, Kobayashi S, Furukawa T, Oaki Y, Mori O, Matsuno S, Shoji S, Inomata M & Teramoto A (1998) Emergence of immunoreactivities for phosphorylated tau and amyloid-beta protein in chronic stage of fluid percussion injury in rat brain. Neuroreport 9, 1879–1883. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt ML, Zhukareva V, Newell KL, Lee VM & Trojanowski JQ (2001) Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol 101, 518–524. [DOI] [PubMed] [Google Scholar]
- 6.Saha P & Sen N (2019) Tauopathy: A common mechanism for neurodegeneration and brain aging. Mech Ageing Dev 178, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokiko-Cochran ON & Godbout JP (2018) The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Wang Z-H, Liu X, Zhang Z, Gu X, Yu SP, Keene CD, Cheng L & Ye K (2020) Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog Neurobiol 185, 101730. [DOI] [PubMed] [Google Scholar]
- 9.Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Reagan A, Salat DH, Wolf EJ, McGlinchey RE, Milberg WP, Stone A, Schichman SA & Miller MW (2017) Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 140, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fann JR, Ribe AR, Pedersen HS, Fenger-Grøn M, Christensen J, Benros ME & Vestergaard M (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 5, 424–431. [DOI] [PubMed] [Google Scholar]
- 11.Grasset L, Glymour MM, Yaffe K, Swift SL, Gianattasio KZ, Power MC & Hazzouri AZA (2020) Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimer’s & Dementia 16, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LoBue C, Woon FL, Rossetti HC, Hynan LS, Hart J & Cullum CM (2018) Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology 32, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV & Park KB (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg, 1–18. [DOI] [PubMed] [Google Scholar]
- 14.Stein GH, Drullinger LF, Soulard A & Dulić V (1999) Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 19, 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbig U, Jobling WA, Chen BPC, Chen DJ & Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14, 501–513. [DOI] [PubMed] [Google Scholar]
- 16.Serrano M, Lin AW, McCurrach ME, Beach D & Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602. [DOI] [PubMed] [Google Scholar]
- 17.Childs BG, Gluscevic M, Baker DJ, Laberge R-M, Marquess D, Dananberg J & van Deursen JM (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16, 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpless NE & Sherr CJ (2015) Forging a signature of in vivo senescence. Nature Reviews Cancer 15, 397–408. [DOI] [PubMed] [Google Scholar]
- 19.Campisi J & Robert L (2014) Cell senescence: Role in aging and age-related diseases. In Aging: Facts and Theories pp. 45–61. S. Karger AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker DJ & Petersen RC (2018) Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest 128, 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrero A, De Strooper B & Arancibia-Cárcamo IL (2021) Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci, S0166–2236(21)00119–3. [DOI] [PubMed] [Google Scholar]
- 22.Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S & Campisi J (2017) Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Childs BG, Durik M, Baker DJ & Van Deursen JM (2015) Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nature Medicine 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli J, Wang B, Brandenburg SM, Basisty N, Evangelou K, Varela-Eirin M, Campisi J, Schilling B, Gorgoulis V & Demaria M (2021) Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc 16, 2471–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M & Demaria M (2019) Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- 26.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM & Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL & Jurk D (2021) Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 20, e13296–e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab N, Ju Y & Hazrati LN (2021) Early onset senescence and cognitive impairment in a murine model of repeated mTBI. Acta Neuropathologica Communications, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA & Loane DJ (2019) Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol Aging 77, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Fryatt GL, Ghorbani M, Obst J, Menassa DA, Martin-Estebane M, Muntslag TAO, Olmos-Alonso A, Guerrero-Carrasco M, Thomas D, Cragg MS & Gomez-Nicola D (2021) Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep 35, 109228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K & von Zglinicki T (2012) Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng Y-Q, Guan J-T, Xu X-H & Fu Y-C (2010) Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem Biophys Res Commun 396, 866–869. [DOI] [PubMed] [Google Scholar]
- 33.Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q & Orr ME (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, 12840–12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C & Torres C (2012) Astrocyte senescence as a component of Alzheimer’s disease. PLoS One 7, e45069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J & Andersen JK (2018) Cellular Senescence Is Induced by the Environmental Neurotoxin Paraquat and Contributes to Neuropathology Linked to Parkinson’s Disease. Cell Rep 22, 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P, Kishimoto Y, Grammatikakis loannis, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Sen JM, Gorospe M & Mattson MP (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiss T, Nyúl-Tóth Á, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A & Ungvari Z (2020) Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojas-Vázquez S, Blasco-Chamarro L, López-Fabuel I, Martínez-Máñez R & Fariñas I (2021) Vascular Senescence: A Potential Bridge Between Physiological Aging and Neurogenic Decline. Front Neurosci 15, 666881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang I, Han SJ, Kaur G, Crane C & Parsa AT (2010) The Role of Microglia in Central Nervous System Immunity and Glioma Immunology. J Clin Neurosci 17, 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA & Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginhoux F & Prinz M (2015) Origin of microglia: Current concepts and past controversies. Cold Spring Harbor Perspectives in Biology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streit WJ, Sammons NW, Kuhns AJ & Sparks DL (2004) Dystrophic Microglia in the Aging Human Brain. GLIA 45, 208–212. [DOI] [PubMed] [Google Scholar]
- 43.Hansen DV, Hanson JE & Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M & Amit I (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e17. [DOI] [PubMed] [Google Scholar]
- 45.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA & Stevens B (2019) Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood EK & Brown DR (2021) Senescent microglia: The key to the ageing brain? International Journal of Molecular Sciences 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvin A & Ginhoux F (2018) Microglia heterogeneity along a spatio–temporal axis: More questions than answers. Glia 66, 2045–2057. [DOI] [PubMed] [Google Scholar]
- 48.Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E & McCullough LD (2015) Age- and location-related changes in microglial function. Neurobiology of Aging 36, 2153–2163. [DOI] [PubMed] [Google Scholar]
- 49.Spittau B (2017) Aging Microglia—Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Frontiers in Aging Neuroscience 9, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donat CK, Scott G, Gentleman SM & Sastre M (2017) Microglial Activation in Traumatic Brain Injury. Front Aging Neurosci 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flanary BE, Sammons NW, Nguyen C, Walker D & Streit WJ (2007) Evidence That Aging And Amyloid Promote Microglial Cell Senescence. Rejuvenation Research 10, 61–74. [DOI] [PubMed] [Google Scholar]
- 52.Sierra A, Gottfried-Blackmore AC, McEwen BS & Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. [DOI] [PubMed] [Google Scholar]
- 53.Tremblay M-È, Zettel ML, Ison JR, Allen PD & Majewska AK (2012) Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia 60, 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Chen M, Manivannan A, Lois N & Forrester JV (2008) Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell 7, 58–68. [DOI] [PubMed] [Google Scholar]
- 55.Jung T, Bader N & Grune T (2007) Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 1119, 97–111. [DOI] [PubMed] [Google Scholar]
- 56.Ma W, Coon S, Zhao L, Fariss RN & Wong WT (2013) A2E accumulation influences retinal microglial activation and complement regulation. Neurobiology of Aging 34, 943–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh Kushwaha S, Patro N & Kumar Patro I (2018) A Sequential Study of Age-Related Lipofuscin Accumulation in Hippocampus and Striate Cortex of Rats. AON 25, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN & Wong WT (2011) Age-related alterations in the dynamic behavior of microglia. Aging Cell 10, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A & Jucker M (2014) Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell 13, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Triviño JJ & von Bernhardi R (2021) The effect of aged microglia on synaptic impairment and its relevance in neurodegenerative diseases. Neurochem Int 144, 104982. [DOI] [PubMed] [Google Scholar]
- 61.Kettenmann H, Kirchhoff F & Verkhratsky A (2013) Microglia: New Roles for the Synaptic Stripper. Neuron 77, 10–18. [DOI] [PubMed] [Google Scholar]
- 62.Primiani CT, Ryan VH, Rao JS, Cam MC, Ahn K, Modi HR & Rapoport SI (2014) Coordinated gene expression of neuroinflammatory and cell signaling markers in dorsolateral prefrontal cortex during human brain development and aging. PLoS One 9, e110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henry CJ, Huang Y, Wynne AM & Godbout JP (2009) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain, Behavior, and Immunity 23, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hart AD, Wyttenbach A, Perry VH & Teeling JL (2012) Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences. Brain Behav Immun 26, 754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norden DM & Godbout JP (2013) Microglia of the Aged Brain: Primed to be Activated and Resistant to Regulation. Neuropathol Appl Neurobiol 39, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perry VH & Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10, 217–224. [DOI] [PubMed] [Google Scholar]
- 67.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ & Rubin LL (2014) Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM & Wyss-Coray T (2014) Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou A, Krukowski K, Morganti JM, Riparip L-K & Rosi S (2018) Persistent Infiltration and Impaired Response of Peripherally-Derived Monocytes after Traumatic Brain Injury in the Aged Brain. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ & Simons M (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elmore MRP, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, Najafi AR, Spangenberg EE, Wood MA, West BL & Green KN (2018) Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17, e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stojiljkovic MR, Ain Q, Bondeva T, Heller R, Schmeer C & Witte OW (2019) Phenotypic and functional differences between senescent and aged murine microglia. Neurobiology of Aging 74, 56–69. [DOI] [PubMed] [Google Scholar]
- 73.Tang Y & Le W (2016) Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol 53, 1181–1194. [DOI] [PubMed] [Google Scholar]
- 74.Bisht K, Sharma KP, Lecours C, Sánchez MG, Hajj HE, Milior G, Olmos-Alonso A, Gómez-Nicola D, Luheshi G, Vallières L, Branchi I, Maggi L, Limatola C, Butovsky O & Tremblay M-È (2016) Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19, 987–991. [DOI] [PubMed] [Google Scholar]
- 76.Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ & Nelson PT (2015) Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, Wes PD, Möller T, Orre M, Kamphuis W, Hol EM, Boddeke EW & Eggen BJ (2015) Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta neuropathologica communications 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, Cao Y, Lee S-H, Haddick PCG, Ngu H, Modrusan Z, Larson JL, Kaminker JS, van der Brug MP & Hansen DV (2018) Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Reports 22, 832–847. [DOI] [PubMed] [Google Scholar]
- 79.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C & Butovsky O (2017) The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sobue A, Komine O, Hara Y, Endo F, Mizoguchi H, Watanabe S, Murayama S, Saito T, Saido TC, Sahara N, Higuchi M, Ogi T & Yamanaka K (2021) Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathologica Communications 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ & Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M & Becher B (2018) High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48, 380–395.e6. [DOI] [PubMed] [Google Scholar]
- 83.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL & Bohr VA (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15, 565–581. [DOI] [PubMed] [Google Scholar]
- 84.Kamphuis W, Kooijman L, Schetters S, Orre M & Hol EM (2016) Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1862, 1847–1860. [DOI] [PubMed] [Google Scholar]
- 85.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS & Flavell RA (2008) Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med 14, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vom Berg J, Prokop S, Miller KR, Obst J, Kälin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, Winter Y, Becher B & Heppner FL (2012) Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med 18, 1812–1819. [DOI] [PubMed] [Google Scholar]
- 87.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP & Weiner HL (2014) Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y & Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57, 1–9. [DOI] [PubMed] [Google Scholar]
- 89.Song W, Hooli B, Mullin K, Jin SC, Cella M, Ulland TK, Wang Y, Tanzi RE & Colonna M (2017) Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement 13, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colonna M & Wang Y (2016) TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci 17, 201–207. [DOI] [PubMed] [Google Scholar]
- 91.Perlmutter LS, Scott SA, Barrón E & Chui HC (1992) MHC class II-positive microglia in human brain: association with Alzheimer lesions. J Neurosci Res 33, 549–558. [DOI] [PubMed] [Google Scholar]
- 92.Hopperton KE, Mohammad D, Trépanier MO, Giuliano V & Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry 23, 177–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wyss-Coray T & Rogers J (2012) Inflammation in Alzheimer Disease—A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harb Perspect Med 2, a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trias E, Beilby PR, Kovacs M, Ibarburu S, Varela V, Barreto-Núñez R, Bradford SC, Beckman JS & Barbeito L (2019) Emergence of Microglia Bearing Senescence Markers During Paralysis Progression in a Rat Model of Inherited ALS. Front Aging Neurosci 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coppé J-P, Desprez P-Y, Krtolica A & Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maas AIR, Stocchetti N & Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7, 728–741. [DOI] [PubMed] [Google Scholar]
- 97.Goldstein LE, Fisher AM, Tagge CA, Zhang X-L, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK & McKee AC (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang X-L, Wojnarowicz MW, Casey N, Lu H, Kokiko-Cochran ON, Saman S, Ericsson M, Onos KD, Veksler R, Senatorov VV, Kondo A, Zhou XZ, Miry O, Vose LR, Gopaul KR, Upreti C, Nowinski CJ, Cantu RC, Alvarez VE, Hildebrandt AM, Franz ES, Konrad J, Hamilton JA, Hua N, Tripodis Y, Anderson AT, Howell GR, Kaufer D, Hall GF, Lu KP, Ransohoff RM, Cleveland RO, Kowall NW, Stein TD, Lamb BT, Huber BR, Moss WC, Friedman A, Stanton PK, McKee AC & Goldstein LE (2018) Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langlois JA, Rutland-Brown W & Wald MM (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21, 375–378. [DOI] [PubMed] [Google Scholar]
- 100.Loane DJ, Kumar A, Stoica BA, Cabatbat R & Faden AI (2014) Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol 73, 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WST & Nicoll JAR (2004) Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Science International 146, 97–104. [DOI] [PubMed] [Google Scholar]
- 102.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH & Stewart W (2013) Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soares HD, Hicks RR, Smith D & McIntosh TK (1995) Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci 15, 8223–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nonaka M, Chen XH, Pierce JE, Leoni MJ, McIntosh TK, Wolf JA & Smith DH (1999) Prolonged activation of NF-kappaB following traumatic brain injury in rats. J Neurotrauma 16, 1023–1034. [DOI] [PubMed] [Google Scholar]
- 105.Nagamoto-Combs K, McNeal DW, Morecraft RJ & Combs CK (2007) Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma 24, 1719–1742. [DOI] [PubMed] [Google Scholar]
- 106.Clarke GJB, Skandsen T, Zetterberg H, Einarsen CE, Feyling C, Follestad T, Vik A, Blennow K & Håberg AK (2021) One-Year Prospective Study of Plasma Biomarkers From CNS in Patients With Mild Traumatic Brain Injury. Front Neurol 12, 643743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL & McGavern DB (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML & Gan W-B (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- 109.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI & Loane DJ (2013) Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34, 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD & Frank JA (2014) Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation 11, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK-F, Leak RK, Gao Y & Chen J (2013) Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 33, 1864–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Witcher KG, Bray CE, Chunchai T, Zhao F, O’Neil SM, Gordillo AJ, Campbell WA, McKim DB, Liu X, Dziabis JE, Quan N, Eiferman DS, Fischer AJ, Kokiko-Cochran ON, Askwith C & Godbout JP (2021) Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J Neurosci 41, 1597–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM & Ffrench-Constant C (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G & Sharp DJ (2011) Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 70, 374–383. [DOI] [PubMed] [Google Scholar]
- 115.Izzy S, Liu Q, Fang Z, Lule S, Wu L, Chung JY, Sarro-Schwartz A, Brown-Whalen A, Perner C, Hickman SE, Kaplan DL, Patsopoulos NA, El Khoury J & Whalen MJ (2019) Time-Dependent Changes in Microglia Transcriptional Networks Following Traumatic Brain Injury. Front Cell Neurosci 13, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin X, Ishii H, Bai Z, Itokazu T & Yamashita T (2012) Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One 7, e41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar A, Alvarez-Croda D-M, Stoica BA, Faden AI & Loane DJ (2016) Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma 33, 1732–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S & Aruga T (2010) Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, Tian F, Shao Z, Shen X, Qi X, Li H, Wang Z & Chen G (2015) Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol Sci 36, 61–71. [DOI] [PubMed] [Google Scholar]
- 120.Wilkinson BL, Cramer PE, Varvel NH, Reed-Geaghan E, Jiang Q, Szabo A, Herrup K, Lamb BT & Landreth GE (2012) Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s Disease. Neurobiol Aging 33, 197.e21–197.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA & Fujimoto S (2000) Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun 273, 5–9. [DOI] [PubMed] [Google Scholar]
- 122.Hukkelhoven CWPM, Steyerberg EW, Rampen AJJ, Farace E, Habbema JDF, Marshall LF, Murray GD & Maas AIR (2003) Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 99, 666–673. [DOI] [PubMed] [Google Scholar]
- 123.Mak CHK, Wong SKH, Wong GK, Ng S, Wang KKW, Lam PK & Poon WS (2012) Traumatic Brain Injury in the Elderly: Is it as Bad as we Think? Curr Transl Geriatr Exp Gerontol Rep 1, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wright DK, O’Brien TJ, Mychasiuk R & Shultz SR (2018) Telomere length and advanced diffusion MRI as biomarkers for repetitive mild traumatic brain injury in adolescent rats. Neuroimage Clin 18, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hehar H & Mychasiuk R (2016) The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats following a concussion/mild traumatic brain injury. Neurobiol Dis 87, 11–18. [DOI] [PubMed] [Google Scholar]
- 126.Schwab N, Grenier K & Hazrati L-N (2019) DNA repair deficiency and senescence in concussed professional athletes involved in contact sports. Acta Neuropathol Commun 7, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braak H & Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- 128.Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, Kruchowsky JC, Woltjer R, Kaye J, Castaño EM, Sabbagh MN, Beach TG & Roher AE (2011) Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One 6, e27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH & Aronson MK (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13, 179–189. [DOI] [PubMed] [Google Scholar]
- 130.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW & Markesbery WR (2000) “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 55, 370–376. [DOI] [PubMed] [Google Scholar]
- 131.Murray ME & Dickson DW (2014) Is pathological aging a successful resistance against amyloid-beta or preclinical Alzheimer’s disease? Alzheimer’s Research & Therapy 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shahidehpour RK, Higdon RE, Crawford NG, Neltner JH, Ighodaro ET, Patel E, Price D, Nelson PT & Bachstetter AD (2021) Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol Aging 99, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andersen HH, Johnsen KB & Moos T (2014) Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci 71, 1607–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L & Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155, 96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopes KO, Sparks DL & Streit WJ (2008) Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia 56, 1048–1060. [DOI] [PubMed] [Google Scholar]
- 136.Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos A-T, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI & Cater MA (2017) Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol 14, 100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Killilea DW, Wong SL, Cahaya HS, Atamna H & Ames BN (2004) Iron accumulation during cellular senescence. Ann N Y Acad Sci 1019, 365–367. [DOI] [PubMed] [Google Scholar]
- 138.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, Reinacher PC, Michel A, Sure U, Gold R, Grün D, Priller J, Stadelmann C & Prinz M (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. [DOI] [PubMed] [Google Scholar]
- 139.Sousa C, Golebiewska A, Poovathingal SK, Kaoma T, Pires-Afonso Y, Martina S, Coowar D, Azuaje F, Skupin A, Balling R, Biber K, Niclou SP & Michelucci A (2018) Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Reports 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen W-T, Woodbury ME, Srivastava G, Möller T, Hudry E, Das S, Saido T, Karran E, Hyman B, Perry VH, Fiers M & De Strooper B (2019) The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Reports 27, 1293–1306.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aw D, Silva AB & Palmer DB (2007) Immunosenescence: emerging challenges for an ageing population. Immunology 120, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D & Hwang ES (2006) Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195. [DOI] [PubMed] [Google Scholar]
- 143.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I & Pereira-Smith O (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hernandez-Segura A, Nehme J & Demaria M (2018) Hallmarks of Cellular Senescence. Trends in Cell Biology 28, 436–453. [DOI] [PubMed] [Google Scholar]
- 145.Bursuker I, Rhodes JM & Goldman R (1982) Beta-galactosidase--an indicator of the maturational stage of mouse and human mononuclear phagocytes. J Cell Physiol 112, 385–390. [DOI] [PubMed] [Google Scholar]
- 146.Frescas D, Hall BM, Strom E, Virtuoso LP, Gupta M, Gleiberman AS, Rydkina E, Balan V, Vujcic S, Chernova OB & Gudkov AV (2017) Murine mesenchymal cells that express elevated levels of the CDK inhibitor p16(Ink4a) in vivo are not necessarily senescent. Cell Cycle 16, 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yegorov YE, Akimov SS, Hass R, Zelenin AV & Prudovsky IA (1998) Endogenous β-Galactosidase Activity in Continuously Nonproliferating Cells. Experimental Cell Research 243, 207–211. [DOI] [PubMed] [Google Scholar]
- 148.Tominaga T, Shimada R, Okada Y, Kawamata T & Kibayashi K (2019) Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLOS ONE 14, e0213673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jung T, Höhn A & Grune T (2010) Lipofuscin: detection and quantification by microscopic techniques. Methods Mol Biol 594, 173–193. [DOI] [PubMed] [Google Scholar]
- 150.Terman A & Brunk UT (2004) Lipofuscin. Int J Biochem Cell Biol 36, 1400–1404. [DOI] [PubMed] [Google Scholar]
- 151.Georgakopoulou E, Tsimaratou K, Evangelou K, Fernandez M-P, Zoumpourlis V, Trougakos I, Kletsas D, Bartek J, Serrano M & Gorgoulis V (2012) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, Kouloukoussa M, Kittas C, Georgakilas AG & Gorgoulis VG (2019) Senescence and senotherapeutics: a new field in cancer therapy. Pharmacology & Therapeutics 193, 31–49. [DOI] [PubMed] [Google Scholar]
- 153.Olmos-Alonso A, Schetters STT, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH & Gomez-Nicola D (2016) Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 139, 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simon E, Obst J & Gomez-Nicola D (2019) The Evolving Dialogue of Microglia and Neurons in Alzheimer’s Disease: Microglia as Necessary Transducers of Pathology. Neuroscience 405, 24–34. [DOI] [PubMed] [Google Scholar]
- 155.Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A & Tsai L-H (2017) Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep 21, 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Daszkiewicz L, Vázquez-Mateo C, Rackov G, Ballesteros-Tato A, Weber K, Madrigal-Avilés A, Di Pilato M, Fotedar A, Fotedar R, Flores JM, Esteban M, Martínez-A C & Balomenos D (2015) Distinct p21 requirements for regulating normal and self-reactive T cells through IFN-γ production. Sci Rep 5, 7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Mera-Rodríguez JA, Álvarez-Hernán G, Gañán Y, Martín-Partido G, Rodríguez-León J & Francisco-Morcillo J (2021) Is Senescence-Associated β-Galactosidase a Reliable in vivo Marker of Cellular Senescence During Embryonic Development? Frontiers in Cell and Developmental Biology 9, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cecco MD, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD, Le O, Beauséjour C, Ambati J, Ambati K, Simon M, Seluanov A, Gorbunova V, Slagboom PE, Helfand SL, Neretti N & Sedivy JM (2019) LINE-1 derepression in senescent cells triggers interferon and inflammaging. Nature 566, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johmura Y, Shimada M, Misaki T, Naiki-Ito A, Miyoshi H, Motoyama N, Ohtani N, Hara E, Nakamura M, Morita A, Takahashi S & Nakanishi M (2014) Necessary and Sufficient Role for a Mitosis Skip in Senescence Induction. Molecular Cell 55, 73–84. [DOI] [PubMed] [Google Scholar]
- 160.Chen W, Wang X, Wei G, Huang Y, Shi Y, Li D, Qiu S, Zhou B, Cao J, Chen M, Qin P, Jin W & Ni T (2020) Single-Cell Transcriptome Analysis Reveals Six Subpopulations Reflecting Distinct Cellular Fates in Senescent Mouse Embryonic Fibroblasts. Frontiers in Genetics 11, 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, Mayweather BA, Nguyen L, Niziolek Z, Ozek C, Butty VL, Isserlin R, Buchanan SM, Levine SS, Regev A, Bader GD, Levin JZ & Rubin LL (2019) Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci 22, 1696–1708. [DOI] [PubMed] [Google Scholar]
- 162.Wang E (1995) Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 55, 2284–2292. [PubMed] [Google Scholar]
- 163.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ & Kirkland JL (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD & Kirkland JL (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A & Zhou D (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD & Niedernhofer LJ (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T & Kirkland JL (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Syed DN, Adhami VM, Khan MI & Mukhtar H (2013) Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem 13, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kirkland JL & Tchkonia T (2020) Senolytic drugs: from discovery to translation. Journal of Internal Medicine 288, 518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirkland JL & Tchkonia T (2017) Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wake Forest University Health Sciences (2021) Phase II Clinical Trial to Evaluate the Safety and Feasibility of Senolytic Therapy in Alzheimer’s Disease clinicaltrials.gov. [Google Scholar]
- 172.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL & van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD & van Deursen JM (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge R-M, Vijg J, Van Steeg H, Dollé MET, Hoeijmakers JHJ, de Bruin A, Hara E & Campisi J (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]