Abstract
BACKGROUND:
Women experience major depression and post-traumatic stress disorder (PTSD) approximately twice as often as men. Estrogen is thought to contribute to sex differences in these disorders, and reduced estrogen is also known to be a key driver of menopause symptoms such as hot flashes. Moreover, estrogen is used to treat menopause symptoms. In order to test for potential shared genetic influences between menopause symptoms and psychiatric disorders, we conducted a GWAS of estrogen medication use (as a proxy for menopause symptoms) in the UK Biobank.
METHODS:
The analysis included 232,993 women aged 39–71 in the UK Biobank. The outcome variable for genetic analyses was estrogen medication use, excluding women using hormonal contraceptives. Trans-ancestry GWAS meta-analyses were conducted along with genetic correlation analyses on the European ancestry GWAS results. Hormone usage was also tested for association with depression and PTSD.
RESULTS:
GWAS of estrogen medication use (compared to non-use) identified a locus in the TACR3 gene, which was previously linked to hot flashes in menopause (top rs77322567, OR=.78, p=7.7×10−15). Genetic correlation analyses revealed shared genetic influences on menopause symptoms and depression (rg=0.231, s.e.=0.055, p=2.8×10−5). Non-genetic analyses revealed higher psychiatric symptoms scores among women using estrogen medications.
CONCLUSIONS:
These results suggest that menopause symptoms have a complex genetic etiology which is partially shared with genetic influences on depression. Moreover, the TACR3 gene identified here has direct clinical relevance; antagonists for the neurokinin 3 receptor (coded for by TACR3) are effective treatments for hot flashes.
Keywords: Depression, PTSD, GWAS, genetic correlation, menopause, vasomotor symptoms, hormone therapy, estrogen, progestogen, hormone replacement therapy
BACKGROUND
Depression and post-traumatic stress disorder (PTSD) are common and etiologically complex psychiatric conditions (Kessler et al., 2005; Kilpatrick et al., 2013). The individual and global impact of these disorders is substantial, with high rates of suicide, hospital admissions, substance abuse, and millions of working days lost (Davidson, 2000; Greenberg et al., 2003). One crucial consideration is that women are approximately two times more likely to develop depression or PTSD as compared to men (depression: 26% versus 15%; PTSD: 12% versus 5%) (Hasin et al., 2018; Kessler et al., 2005). Sex hormones are known to be major determinants of sex differences and could contribute to the higher rates of depression and PTSD among women (Altemus, Sarvaiya, & Neill Epperson, 2014; Angold & Worthman, 1993; Nolen-Hoeksema & Girgus, 1994). Both endogenous variation in hormone levels and exogenous sex hormone medications may influence risk for psychiatric disorders.
Focus on exogenous hormone medications and potential effects on mental health is critical because over 80% of American women have taken hormone medications at least once (Centers for Disease Control, 2019). Estrogen-containing medications are most frequently taken as contraceptives, but they are also frequently used as hormone therapy around the time of menopause. Note that “hormone therapy” for menopause-related symptoms was previously referred to as “hormone replacement therapy” HRT. The median age of menopause in the US is 51 years, and it is clinically defined as the lack of a period for one year (Bromberger et al., 1997; Cooper & Sandler, 1998; Gottschalk, Eskild, Hofvind, Gran, & Bjelland, 2020; McKinlay, Brambilla, & Posner, 1992). Most women experience menopause symptoms such as hot flashes, oftentimes for years at a time, surrounding the menopause transition (perimenopause) (“ACOG Practice Bulletin No. 141,” 2014). While many symptoms may occur, vasomotor (i.e. hot flashes and night sweats) and vaginal symptoms are the most common. Importantly, sleep is disrupted for many women (“ACOG Practice Bulletin No. 141,” 2014), and depression and anxiety occur at higher rates among women experiencing hot flashes during the menopause transition (Freeman, Sammel, & Lin, 2009; Freeman et al., 2005; Joffe et al., 2016). In sum, menopause symptoms are common, last for years, and can cause serious life impairment.
Defining a proxy variable for menopause symptoms using medication data in the UK Biobank
With the dual goals of better understanding the genetic basis of menopause symptoms and determining the degree to which genetic influences on menopause symptoms might be shared with genetic influences on psychiatric disorders, we sought to use the UK Biobank to perform a GWAS of menopause symptoms. While the age range (39–71 years old) and size of the UK Biobank (232,993 women) are well suited for performing a GWAS of menopause symptoms, such symptoms were not assessed directly. Consequently, we used a proxy variable for menopause symptoms: estrogen medication use, not as contraceptive. The strategy of analyzing medication use was implemented previously in the UK Biobank for many non-hormonal medication classes. It successfully identified known genetic risk factors for the target conditions of various medications (Wu et al., 2019). For example, there was a high genetic correlation between antidepressant use and depression, and a high genetic correlation between vasodilators used in cardiac disease and coronary artery disease (Wu et al., 2019). Thus, prior work demonstrated successful use of medication proxies in GWAS. The medications used most frequently to treat menopause symptoms are estrogens and estrogens and progestogens in combination (“ACOG Practice Bulletin No. 141,” 2014; Skouby et al., 2005). The other primary indication for estrogens is as contraceptive, thus we excluded women using hormonal contraceptives. Progestogens alone are typically used for contraception, as part of fertility treatments, or for pregnancy maintenance (Carp, 2018, p. 2018; Sitruk-Ware, 2018).
To summarize, we built upon the work of Wu et al (2019) which classified medications in the UK Biobank according to the Anatomical Therapeutic Chemical (ATC) classification system. We identified the medication classes most likely to be used to treat menopause symptoms including hot flashes and night sweats: G03C (estrogens) and G03F (estrogens and progestogens in combination). We hypothesized that estrogen medication use – excluding use as a contraceptive, and in an appropriately aged sample – would be a useful (albeit imperfect) proxy for menopause symptoms.
Synopsis of present study goals
Here we examined the phenotypic and genetic correlates of estrogens use (and estrogens combined with progestogens) by women in the UK Biobank (age 39–71) (Bycroft et al., 2018). We first conducted non-genetic analyses to test for associations between estrogen use and the psychiatric phenotypes of lifetime depression and future PTSD. We then conducted genome-wide association studies (GWAS) comparing women taking estrogen to women not taking estrogen in order to characterize the genetic architecture of estrogen medication use not as a contraceptive, which we hypothesize to be a proxy for menopause symptoms. Consistent with current best practices, GWAS within ancestry groups was followed by trans-ancestry GWAS meta-analysis. Using the results from the European ancestry GWAS, we then conducted genetic correlation analyses to identify shared genetic influences between estrogen use and psychiatric phenotypes.
METHODS
(see supplementary text for more complete descriptions of these topics)
UK Biobank Participants
The UK Biobank is a well-documented, population-based study of 502,492 individuals, sampled from 23 different locations in Great-Britain (Bycroft et al., 2018; Collins, 2012; Sudlow et al., 2015). It is a prospective study of predictors (e.g., clinical, demographic, and genetic) of diseases and traits more commonly observed in middle and older age. Only women were included in our analysis, and they were between the ages of 39 and 71. Between 2006 and 2010, participants were recruited (see Figure 1). At the initial recruitment visit they completed questionnaires, underwent physical measurements, performed cognitive tests, and gave biological samples (blood, urine, and saliva). Given the focus of this investigation on menopause symptoms, women were excluded for hormonal contraceptive use. This resulted in exclusion of only 1.9% of women (consistent with the older age of this sample). Women were also excluded if specific variables were missing (menopause status, socioeconomic status, and self-reported ancestry), and if they were related to other individuals in the UK Biobank. The final dataset included 232,993 women (9.6% of women were removed based on these exclusion criteria).
Figure 1. Timeline of assessments in the UK Biobank.
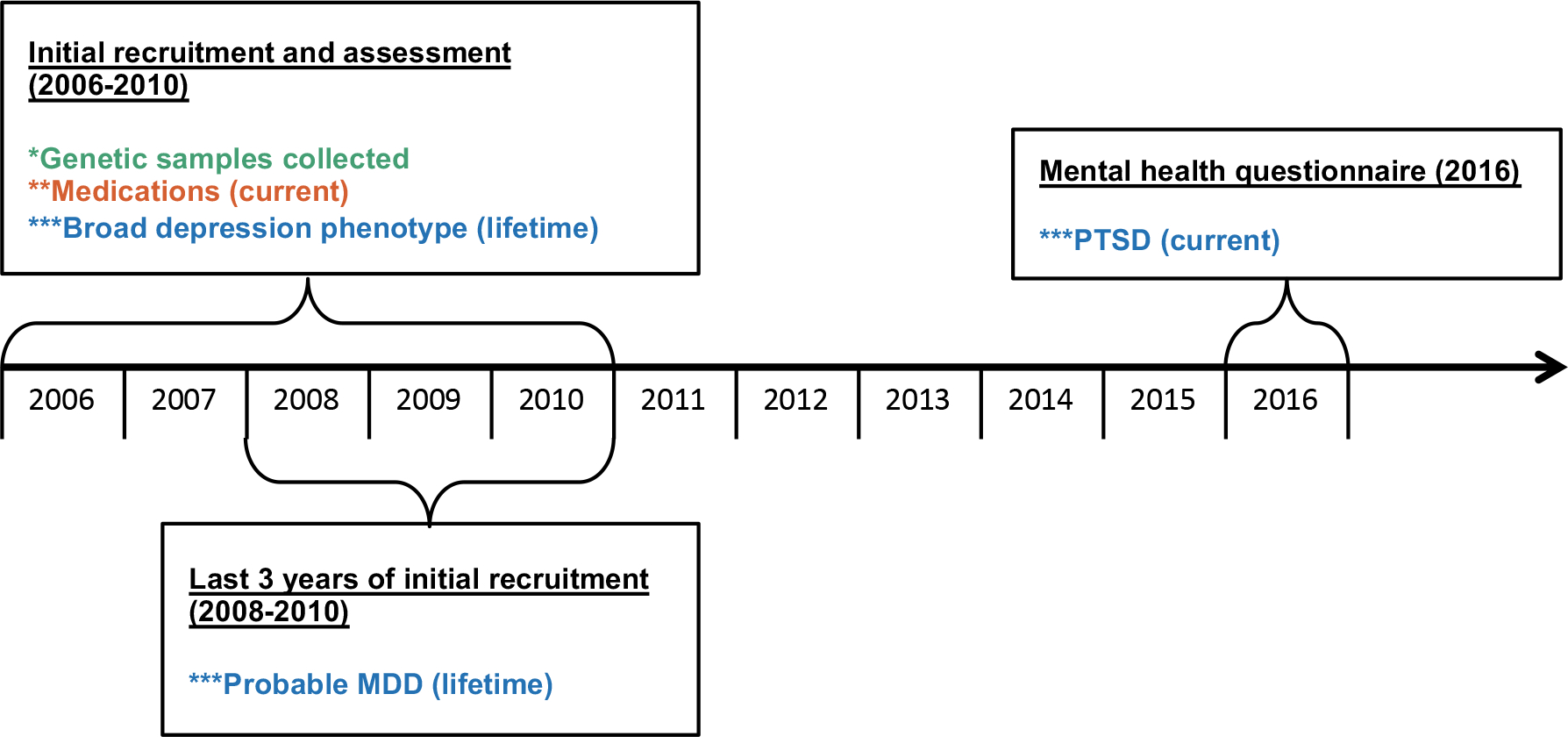
*Genetic samples are in green, **medication usage variables are in red, and ***psychiatric phenotypes are in blue. Medication usage was assessed concurrently with the depression phenotypes and 6–10 years prior to the assessment of PTSD.
Estrogen and Progestogen medication-taking variables
During the initial UK Biobank assessment interviews (2006–2010), participants were asked to register, on a touch screen, the current use of prescription and over-the-counter medications. Using published protocols, all medications were mapped to their corresponding active ingredients (e.g., estradiol) and then grouped according to the Anatomical Therapeutic Chemical (ATC) Classification System (Santos et al., 2017; Wu et al., 2019). We then used three subgroups from the G03 Sex hormones and modulators of the genital system: G03C (estrogens), G03D (progestogens), and G03F (estrogens and progestogens in combination) to define our hormone use variables.
UK Biobank phenotypes of depression and PTSD
We used UK Biobank data to construct three psychiatric phenotypes for analysis: broad depression, probable major depressive disorder, and PTSD. Criteria used to define case-control status for these three phenotypes were taken from previously published papers (Howard et al., 2019; Nievergelt et al., 2019; Smith et al., 2013). We also used available data to make continuous variable versions of these three phenotypes to be tested along with binary case-control status (neuroticism symptoms score for depression phenotypes and total PTSD symptom score for PTSD; see below).
Phenotypic analysis
We assessed potential differences in age, socioeconomic status (Townsend Index), assessment center, ethnic background, and menopause status (pre/post-menopause, or had a hysterectomy) between women taking estrogen medications to those not taking them. We also compared depression and PTSD cases and controls with respect to the previously mentioned variables. Case and control definitions for depression and PTSD were defined according to previously published procedures which utilized self-report of symptoms and medical record diagnoses for depression and self-report symptoms for PTSD (Howard et al., 2019; Nievergelt et al., 2019). We also used a continuous score for PTSD symptoms (scores from 0–29) and, as a related variable to depression symptoms: neuroticism symptom count (scores from 0–12). We used this surrogate variable for depression symptoms because 1) it was available on nearly all women and 2) because neuroticism has been shown to be highly genetically and phenotypically correlated with depression and depression symptoms (Kendler, Gatz, Gardner, & Pedersen, 2006; Luciano et al., 2018).
Using the R Statistical Computing Environment 3.5.1 (R Core Team, 2005), baseline comparisons of phenotypic differences between classes of women taking and not taking hormone medications were tested using t-tests (for continuous variables) and chi-square tests (for categorical variables). Regression analyses were used to test for associations between medication use and psychiatric phenotypes while controlling for the above-mentioned covariates. Logistic regression was used for binary psychiatric phenotypes and linear regression for continuous phenotypes.
Genome-Wide Association Study (GWAS) of hormone medication use
Imputed genotypes from the UK Biobank were cleaned according to published procedures (Duncan et al., 2018). Consistent with current best practices for the analysis of multi-ancestry data(Peterson et al., 2019), we then extracted unrelated individuals of European (n=187,465), South-Asian (n=3,713), African (n=3,582), and East-Asian (n=1,190) ancestry for ancestry-specific GWAS. We performed GWAS comparing women taking estrogen medications to women that did not take them: G03C=estrogens (n=10,103 medication positive / 222,890 medication negative) and G03C+F=estrogens and estrogens and progestogens in combination (n=15,305 medication positive / 217,688 medication negative). Age, Townsend index, menopause information, and 20 genetic principal components (PCs, to control for population stratification) were added as covariates to each GWAS. Eight individual GWAS were performed (4 ancestry groups × 2 medication categories) using PLINK v1.90b5.3 (Chang et al., 2015; Purcell et al., 2007). Trans-ancestry meta-analyses were performed for each medication group using GWAMA version 2.2.2 (Mägi & Morris, 2010). The standard genome-wide significance threshold of p<5×10−8 was used; note that results also exceeded Bonferroni correction for 8 GWAS (p<6.3×10−9). Multi-marker Analysis of GenoMic Annotation (MAGMA) (de Leeuw, Mooij, Heskes, & Posthuma, 2015), incorporated into the FUMA-GWAS webtool(Watanabe, Taskesen, Bochoven, & Posthuma, 2017), was used to perform gene and gene-set analyses. SNP heritability (h2SNP) of estrogen medication use and genetic correlations (rg) with psychiatric phenotypes were estimated using linkage-disequilibrium score regression (LDSC) (B. Bulik-Sullivan et al., 2015; B. K. Bulik-Sullivan et al., 2015). We restricted to use of the European ancestry only summary statistics for LDSC analyses because LDSC can only be applied to groups with more homogenous ancestry and because power was too low for the non-European ancestry groups.
RESULTS
Descriptive statistics
We observed numerous phenotypic differences between women taking estrogen medication and those who do not (Table 1). First, women taking estrogen medication (either estrogen alone or estrogens and progestogens in combination; G03C+F) were more likely to be white (97% versus 94%, p=9.9×10−45), older (57.4 versus 56.7, p=4.05×10−37), and from areas of higher socioeconomic status (lower scores correspond to higher SES on Townsend Index: −1.45 versus −1.34, p=1.19×10−5). Furthermore, women taking these medications were more likely to have had a hysterectomy (32% versus 11%, p<2.2×10−16); among women who had not had a hysterectomy, women taking estrogen medication were more likely to be post-menopausal (92% versus 72%, p<2.2×10−16). Women taking estrogen medication had higher rates of lifetime broad depression (50% versus 40%, p=1.26×10−94), lifetime probable major depressive disorder (40% versus 31%, p=7.73×10−22), and future PTSD (11% versus 10%, p=2.22×10−4) as compared to controls.
Table 1. Demographic characteristics by medication group.
Results for the most inclusive estrogen medication group are presented here, which corresponds to women taking estrogen-only medications and estrogens and progestogens in combination (G03C+F), excluding use as contraceptives. T-tests were used for comparisons of continuous variables (age, Townsend, and continuous measures of psychiatric symptoms) and chi-square tests for categorical measures (ethnic background, binary psychiatric symptoms, and menopause symptoms). “Ethnic background” categories are those provided by the UK Biobank. Note that we added “Chinese” to “Asian or Asian British”.
| Demographic Characteristics | Medication Group: G03C+F (n=232,993) |
||||
|---|---|---|---|---|---|
| Medication positive (n=15,305) |
Medication negative (n=217,688) |
p-value | |||
| n/mean | (%/sd) | n/mean | (%/sd) | ||
|
| |||||
| Age | 57.43 | (6.43) | 56.73 | (8.00) | 4.0E-37 |
| Townsend index | −1.45 | (3.01) | −1.34 | (3.04) | 1.2E-05 |
| Female | 15,305 | (100.00%) | 2,17,688 | (100.00%) | NA |
| Ethnic background | 2.1E-46 | ||||
| White | 14,843 | (96.98%) | 2,05,272 | (94.30%) | |
| Black or Black British | 116 | (0.76%) | 3,926 | (1.80%) | |
| Asian or Asian British | 153 | (1.00%) | 4,813 | (2.21%) | |
| Mixed and other ethnicities | 193 | (1.26%) | 3,677 | (1.69%) | |
| Menopause status* | <2.2E-308 | ||||
| Pre-Menopause | 841 | (5.49%) | 53,793 | (24.71%) | |
| Post-Menopause | 9,541 | (62.34%) | 1,40,698 | (64.63%) | |
| Hysterectomy | 4,923 | (32.17%) | 23,197 | (10.66%) | |
| Probable MDD binary measure | 4.1E-24 | ||||
| Yes | 1,188 | (40.20%) | 13,446 | (31.22%) | |
| No | 1,767 | (59.80%) | 29,629 | (68.78%) | |
| Surrogate depression continuous measure^ | 4.65 | (3.28) | 4.33 | (3.19) | 3.0E-07 |
| Broad depression binary measure | 6.0E-99 | ||||
| Yes | 6,151 | (49.93%) | 68,922 | (40.24%) | |
| No | 6,168 | (50.07%) | 1,02,338 | (59.76%) | |
| Surrogate depression continuous measure^ | 4.80 | (3.31) | 4.52 | (3.24) | 3.3E-19 |
| PTSD binary measure | 9.8E-05 | ||||
| Yes | 614 | (11.54%) | 6,708 | (9.87%) | |
| No | 4,707 | (88.46%) | 61,280 | (90.13%) | |
| PTSD continuous measure | 7.45 | (4.10) | 7.08 | (3.91) | 2.3E-10 |
|
| |||||
| Medication positive (n=10,382) | Medication negative (n=194,483) | p-value | |||
|
| |||||
| Menopause status (no hysterectomy) | <2.2E-308 | ||||
| Pre-Menopause | 841 | (8.10%) | 53,793 | (27.66%) | |
| Post-Menopause | 9,541 | (91.90%) | 1,40,698 | (72.35%) | |
For menopause status among the subsample of women without a hysterectomy; see the bottom section of this table.
As a surrogate continuous depression variable, neuroticism scores were used; see text.
Demographic characteristics and medication status by disorder status (of broad depression, probable MDD, and PTSD) are given in Supplementary Table S2. Women with each of these disorders, on average, were younger, came from areas of lower socioeconomic status, and were more likely to have had a hysterectomy. Cases also had higher rates of prescribed hormone medications.
Phenotypic association analysis
Using regression analyses that controlled for the covariates of age, socioeconomic status, ethnic background, and menopause status, we observed significant positive associations between the use of estrogen medications and all three psychiatric phenotypes. As shown in Figure 2, women using estrogen-only medications (medication group: G03C) were more likely to have lifetime broad depression (OR=1.36, s.e.=0.02, p=7.7×10−39), lifetime probable major depressive disorder (OR=1.41, s.e.=0.05, p=3.0×10−12), and to develop PTSD 6–10 years later (OR=1.21, s.e.=0.06, p=8.1×10−4). These odds ratios were slightly attenuated, but more statistically significant, when women taking combined estrogen and progestogen medications were added to the group of women taking estrogen only medications (i.e. medication group: G03C+F). The continuous variable versions of these psychiatric variables yielded similar results (see Supplementary Table S3 for full results). In contrast we did not observe any significant associations between the use of progestogen only medication (G03D) and psychiatric phenotypes: lifetime broad depression (OR=0.99, s.e.=0.08, p=0.94), lifetime probable major depressive disorder (OR=1.10, s.e.=0.18, p=0.60), and future PTSD (OR=1.00, s.e.=0.18, p=0.99) (see Supplementary Table S3 for all results and sample sizes for each comparison).
Figure 2. Density plots of age for women taking (and not taking) each of three classes of medications.
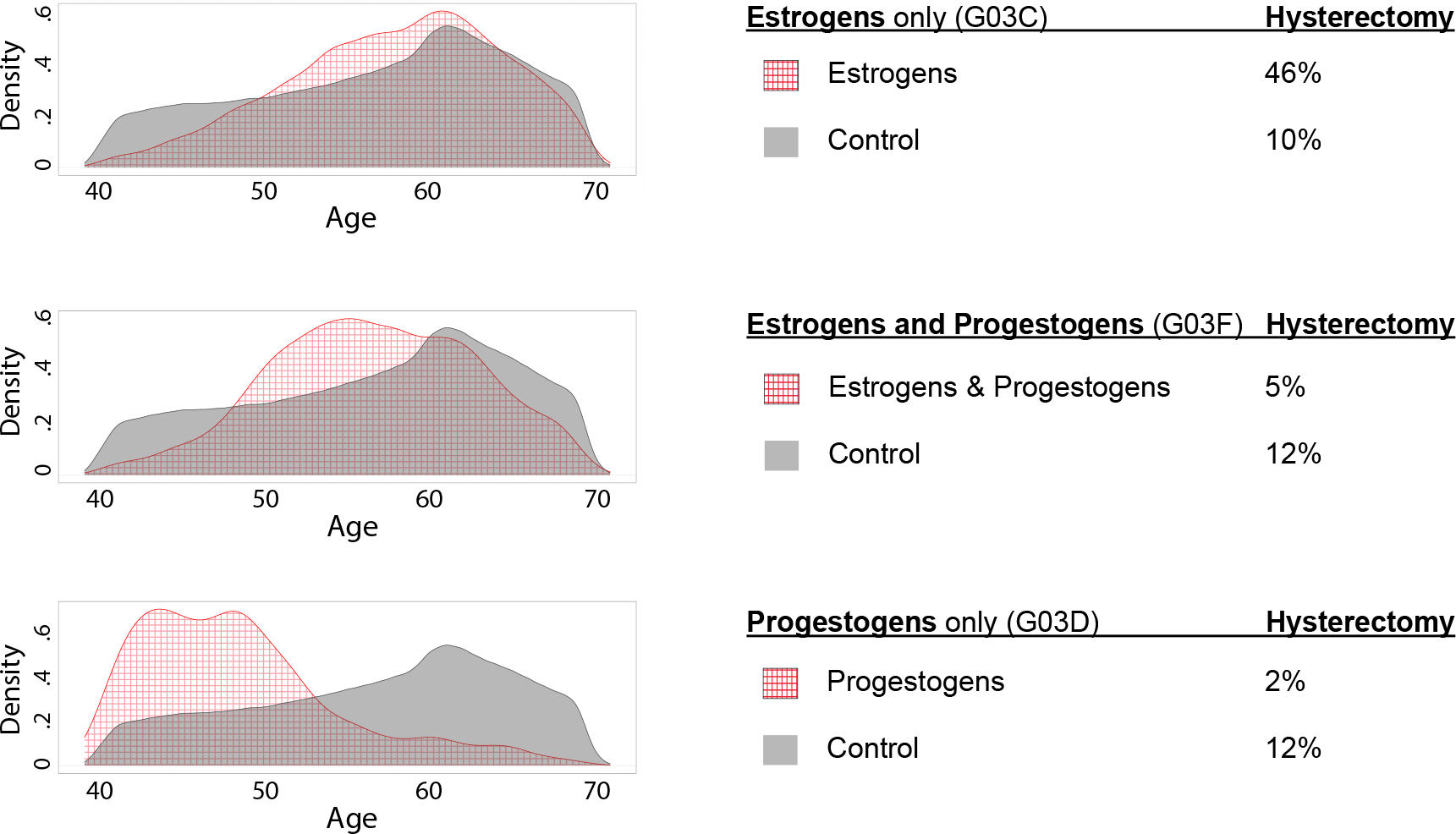
G03C=estrogens only, G03F=estrogens and progestogens in combination, and G03D=progestogens only. The red grid pattern depicts ages for women using each medication group and the grey shading depicts controls, meaning women who took none of these medications. Women using contraceptive medications were excluded. Percentages of women who had a hysterectomy are also given for each group. Consistent with indications for prescription, estrogens and estrogens and progestogens in combination were taken by women around the time of menopause, whereas progestogens only tended to be taken by younger women.
Estrogen medications can also be categorized into different subtypes, and we provide results for two sub-categories of estrogen medications: conjugated versus non-conjugated and oral versus non-oral administration. As shown in Figure 2 and in Supplementary Table S3, the odds ratios for non-conjugated estrogens were larger than conjugated estrogens, but confidence intervals usually overlapped.
Age distribution and hysterectomy prevalence of women in medication groups
Given our use of a proxy phenotype for menopause symptoms, we sought to understand more about the women that used particular hormone medication classes (not as contraceptives). To this end, we examined age distributions for three groups of women (estrogens alone=G03C, estrogens and progestogens in combination=G03F, and progestogens alone=G03D). We also compared the frequency of hysterectomy for women in these groups; see Figure 3. As expected based on the indicated use of estrogens and estrogens and progestogens in combination as hormone (replacement) therapy, women taking these two classes of medications tended to be around the average age of menopause, which is commonly reported as 51 years (Bromberger et al., 1997; Cooper & Sandler, 1998; Gottschalk et al., 2020; McKinlay et al., 1992). In contrast, progestogens alone are used for indications more common among younger women (amenorrhea, preterm delivery); accordingly, the women taking progestogens only (G03D) tended to be younger. Hysterectomy rates also conformed to expectations, namely, that estrogen plus progestogen is recommended for women with a uterus (because progestogens reduce the risk of endometrial cancer), but estrogen alone is suitable for women who have had a hysterectomy (and therefore no longer need progestogen to prevent endometrial cancer). Note that only 5% of women taking estrogens and progestogens in combination had a hysterectomy as compared to 46% hysterectomy among women taking estrogens only.
Figure 3. Forest plot of medication associations with depression and PTSD.
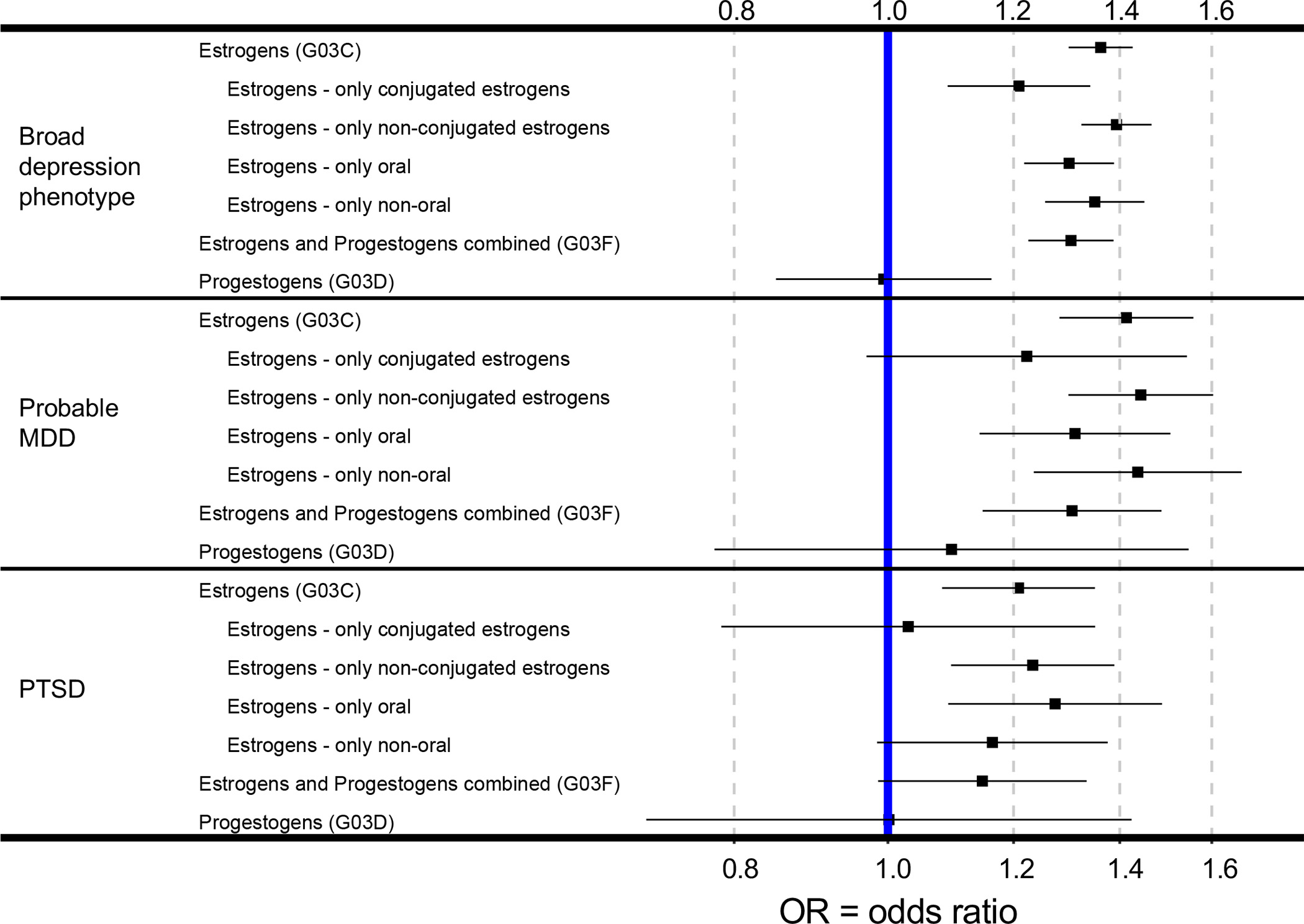
The vertical blue bar denotes OR=1, which is the null hypothesis. Error bars denote 95% confidence intervals. Note wide confidence intervals for progestogen only medication analyses (G03D); wider confidence intervals are consistent with the smaller sample sizes of these analyses.
Genome-Wide Association Study (GWAS) of estrogen medication use
We observed genome-wide significant associations with estrogen medication use in participants of European ancestry (n=187,465, which is 94% of the sample) and in the trans-ancestry meta-analysis (described below). All genome-wide significant variants were on chromosome 4 in the tachykinin receptor 3 gene (TACR3) (see Figure 4), with top SNP rs77322567 (G03C+F OR=.78, p=7.7×10−15). Furthermore, gene-level significant associations (p<2.27×10−6) were observed for TACR3 (p=4.7×10−13) and for two genes located close together on chromosome 9 PTGES2 (p=1.9×10−6) and LCN2 (p=1.9×10−6). Full genome results for each ancestry group are provided separately along with trans-ancestry meta-analytic results (Psychiatric Genomics Consortium, downloads page and available from authors), and see Supplementary Table S4 for all genome-wide significant SNPs from the trans-ancestry meta-analysis (G03C+F; the broadest category of estrogen medication use). All ancestry groups other than European ancestry were underpowered for single variant and gene-level analyses but will be useful for future meta-analyses and fine-mapping efforts.
Figure 4. SNP-based Manhattan plots for genome-wide association studies (GWAS) of estrogen medication use.
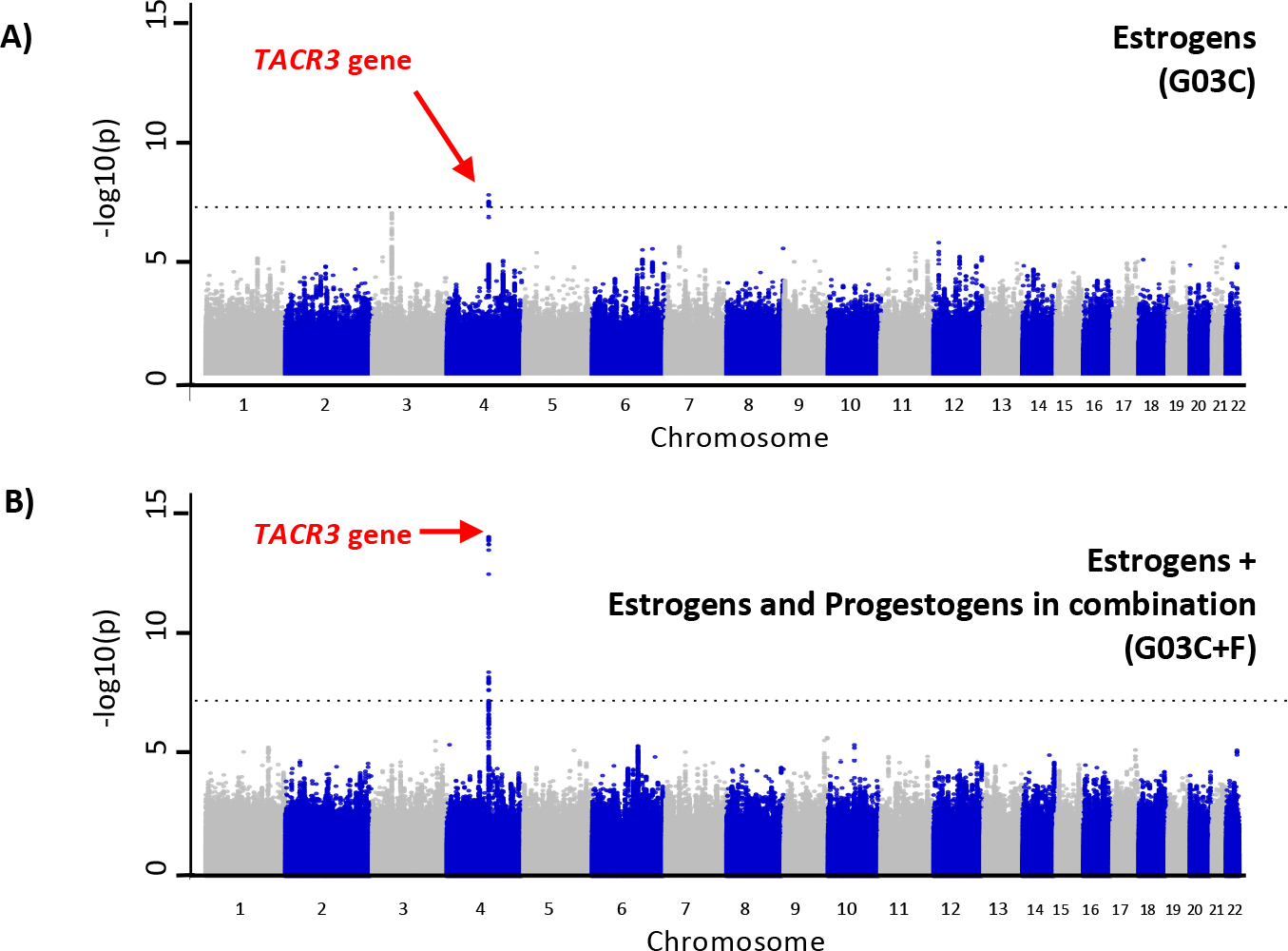
A) estrogen medications only (G03C) and B) estrogens + estrogens and progestogens in combination (G03C+F). The dotted line represents genome-wide statistical significance (p=5×10−8). The chromosome 4 locus, in the TACR3 gene, is a known locus for hot flashes and night sweats (i.e. vasomotor symptoms). The chromosome 4 locus observed in the estrogen medication only GWAS (A) is more statistically significant in the larger sample (B). Results for European ancestry participants are depicted here but are almost identical to trans-ancestry results.
Gene set analyses
No gene sets from the default list analyzed with the FUMA webtool (using MAGMA gene set analysis) were significant after correcting for multiple testing. However, it is notable that three of the top twenty gene sets pertained to estrogen biosynthesis. Specifically, “estrogen biosynthesis” was the second most strongly associated gene set (p=.0001; from Reactome), “estradiol-17beta-dehydrogenase activity” was the eighth (p=.0007; from Gene Ontology, GO), and “estrogen biosynthetic process” was the thirteenth (p=.0009; from Gene Ontology, GO).
Heritability and genetic correlation estimation with linkage-disequilibrium score regression (LDSC)
Using LDSC as applied to the European ancestry summary statistics, SNP heritability (h2SNP) estimates were small in magnitude but significant. Note that estimates were similar for the most inclusive estrogen use analysis (G03C+F h2SNP=0.015, se=0.003, p=1.5×10−8) and the estrogen only analysis (G03C h2SNP=0.0092, se=0.003, p=.0015). Significant positive genetic correlations were found between genetic influences on the use of estrogen medication (medication group: G03C+F) and depression (rg=0.231, s.e.=0.06, p=2.81×10−5) and neuroticism (rg=0.231, s.e.=0.08, p=0.0024). The genetic correlation between estrogen medication use and PTSD was not significant, but this could be due to low power in the PTSD GWAS. See Supplementary Table S5 for full genetic correlation results.
CONCLUSION
Hormone Medication Associations with Depression and PTSD
In this study, we investigated associations between estrogen medication use by women age 39–71 and the psychiatric phenotypes of depression and PTSD in the UK Biobank (N=232,993). In this sample, women using estrogen medications were more likely to have lifetime broad depression, lifetime probable major depressive disorder, and to develop PTSD 6–10 years later. These results held after accounting for socioeconomic status, age, menopause status, and self-reported ethnic background. The exclusive use of progestogens was not associated with differential risk for these psychiatric phenotypes, but statistical power may have been too low. Alternatively, baseline difference between these two medication-defined groups of women or effects of these medications could have contributed to the observed higher rates of depression and PTSD among women taking estrogen medications – but not progestogens alone – in this UK Biobank sample.
Regarding the possible public health significance of these findings and the need for additional studies, we note that all large observational studies (Gerber et al., 2015; Wassertheil-Smoller et al., 2004) reveal higher levels of depression and PTSD among older women taking hormone medications. Among postmenopausal women in the Women’s Health Initiative (WHI) study, the current use of estrogen was associated with a greater risk for current depression (OR=1.25; 95% CI, 1.18–1.32; N=93,676) (Wassertheil-Smoller et al., 2004). This finding is similar to our finding that current use of estrogen medication was associated with higher lifetime depression (OR=1.36, s.e.=0.02, p=7.7×10−39), though it must be noted that the timing of lifetime depression was not available in this study and depression undoubtedly preceded estrogen medication use for many women. The relationship between hormone therapy and PTSD was previously assessed in women veterans using a cross-sectional design (Gerber et al., 2015). Gerber et al. found that women with PTSD were more likely to use hormone therapy (OR=1.27; 95% CI = 1.20–1.34). Similarly, Lawn et al found that, compared to women that experienced no trauma, the hazard ratio for initiating menopausal hormone therapy was higher among women with PTSD (e.g. HR=1.31, 95% CI =1.25–1.36 for women in the high PTSD severity category) (Lawn et al., 2020). Thus, hormone therapy among older women appears to be positively correlated with both depression and PTSD.
Why is the prevalence of depression and PTSD higher among women taking hormone therapy? Hormone therapy could increase psychiatric symptoms or vice versa; psychiatric symptoms could make it more likely that women use hormone therapy. A third possibility is that both variables – hormone therapy and depression (and/or PTSD) – are influenced by an unmeasured variable, such as shared genetic influences. Our results, described below, suggest that shared genetic factors are likely to be at least part of the explanation for higher depression prevalence among women taking hormone medications for menopause symptoms.
Genetic Effects on Menopause Symptoms (via estrogen medication use proxy)
The GWAS results were consistent with our premise that estrogen medication use would serve as a useful (albeit imperfect) proxy for menopause symptoms including hot flashes. Specifically, the TACR3 locus reported here was previously identified in a GWAS of hot flashes and night sweats (Crandall et al., 2017). Further, the protein coded for by TACR3 (neurokinin B / neurokinin 3) plays a critical role in hot flashes, as demonstrated in experimental studies in animals (Padilla, Johnson, Barker, Patterson, & Palmiter, 2018; Rance, Dacks, Mittelman-Smith, Romanovsky, & Krajewski-Hall, 2013) and clinical trials in humans (Fraser et al., 2020; Jayasena et al., 2015; Prague et al., 2018). In addition, neurokinin 3 receptor antagonists have recently been demonstrated to be effective treatments for hot flashes (Fraser et al., 2020; Prague et al., 2018; Santoro et al., 2020). Taken together, these findings suggest that genetic variability in the neurokinin B receptor is one factor relevant to women’s different experiences of hot flashes. Intriguingly, the neurokinin B signaling system has also been the focus of numerous investigations of psychiatric symptoms (Al Abed, Reynolds, & Dehorter, 2021; Zhang, Wang, & Chu, 2020).
Finally, we found positive genetic correlations between our estrogen use phenotypes and both depression and neuroticism. These findings are consistent with previous observations that depression- and anxiety-related phenotypes are more common among women that experience hot flashes (Deecher, Andree, Sloan, & Schechter, 2008; Freeman et al., 2009, 2005; Joffe et al., 2016). These findings are also interesting because treatments for depression (e.g. selective serotonin reuptake inhibitors, SSRIs) are sometimes used for vasomotor symptoms (Ruddy & Loprinzi, 2015) and vice versa (i.e. estrogen for depression). Thus, partially shared etiology may explain the overlap between medications used to treat depression and vasomotor symptoms.
Future directions: extension to other hormone medications and Mendelian Randomization
Interestingly, other forms of hormone medications commonly prescribed to women – contraceptive medications – have also been associated with higher rates of depression in large population samples (N=1,061,997 and N=815,662) (Skovlund, Mørch, Kessing, & Lidegaard, 2016; Zettermark, Vicente, & Merlo, 2018). Importantly, these studies employed prospective study designs, controlled for many confounding variables, and excluded women that developed psychiatric conditions prior to contraceptive use. Putative mental health effects of contraceptives could be very different from effects of hormone therapy for menopause (given differences in age, formulations, dose, and endogenous hormone levels), and yet, the direction of results is the same in large observational studies: higher depression has been consistently observed among women using estrogen medications. Future studies should test for shared genetic influences between depression and contraceptive use. Another important step will be to use Mendelian Randomization to better understand putatively causal pathways involving genetics, hormone medications, and psychiatric phenotypes.
Finally, the relationship of hormones and mood (or affect) may be more complex than can be fully explained by the presence/absence of exogenous hormones. For example, the prevailing understanding of the etiology of Premenstrual Dysphoric Disorder is that a subset of women may be particularly susceptible to affective dysregulation caused by changes in endogenous hormone levels rather than absolute levels (Reid & Soares, 2018). Differential sensitivity to hormone fluctuation has also been implicated in the pathophysiology of postpartum depression (Bloch et al., 2000). Likewise, perimenopausal depression is hypothesized to be related to sensitivity in some women to endogenous hormonal fluctuations (Freeman et al., 2004; Gordon et al., 2015) or withdrawal (Peter J. Schmidt et al., 2015). Therefore, it is possible that a subset of women may also be more susceptible to deleterious mood symptoms or mood benefits brought about by exogenous hormone treatments. Additionally, such sensitivity may be related to either temporary changes in hormone levels or to longer-term effects of hormone use.
Limitations
This study has a number of limitations related to data availability in the UK Biobank. We used estrogen medication use as a proxy for menopause symptoms because the UK Biobank does not currently contain information about presence or absence of specific menopause symptoms. Estrogen medications are also used for hypogonadism, moderate acne vulgaris, prevention of osteoporosis, primary ovarian insufficiency, and oral contraception (the latter of which we excluded) (Delgado & Lopez-Ojeda, 2021; Levine, 2003). Note that depression is not an FDA approved indication for estrogen medication use, nor is it a standard treatment in the UK (Craig, 2013). Nevertheless, there is some evidence that estrogen therapy enhances mood and has antidepressant effects, particularly in perimenopausal women (de Novaes Soares, Almeida, Joffe, & Cohen, 2001; Gordon et al., 2018; Rasgon et al., 2002; P. J. Schmidt et al., 2000). Consensus guidelines suggest estrogen therapies for the treatment of depression should be used with caution, preferably when also indicated for other menopausal symptoms (Maki et al., 2019). Given these other indications for estrogen medications, some mis-classification of women must have occurred, and the effects of this misclassification in our GWAS could be either reduced power due to greater heterogeneity in phenotypes or false positives (in either the TACR3 locus and/or the polygenic signal captured in SNP heritability and SNP genetic correlations). Arguably most impactful for our analysis was the likelihood that some women who had previously experienced menopausal symptoms were not taking hormone medication at the time of assessment. Thus, our proxy variable for menopause symptoms almost certainly had many false negatives, and this likely contributed to the low SNP heritability estimates for estrogen medication use. It is notable that our GWAS nevertheless identified a locus for menopause symptoms and further implicated estrogen biosynthesis pathways. Thus, we report these results with the caveat that it is also possible that genetic findings reported here could be linked to any of the other conditions treated with estrogen in this population (e.g. hypogonadism, osteoporosis, acne).
The psychiatric phenotypes used in this analysis are also sub-optimal. Both the depression and PTSD phenotypes were based on abbreviated self-report measures rather than standardized clinician diagnoses. We also had limited temporal information about diagnoses. Thus, we cannot draw conclusions about whether hormone medication use preceded or started after development of particular episodes of depression or PTSD. Finally, the non-random sampling of participants in the UK Biobank (e.g. participants are older and of higher socioeconomic status than average UK residents) means that selection bias could have attenuated or inflated estimates of observed relationships.
Supplementary Material
Synopsis.
The UK Biobank and other observational studies are frequently used to test for relationships between putative risk factors and medical outcomes, but genetic confounders are not typically considered. The present results demonstrate that genetic factors very likely influence estrogen medication use. Specifically, the present GWAS of estrogen medication use identified a genetic locus previously linked to hot flashes, suggesting that genetic factors influence menopause symptoms and therefore indirectly influence women’s decisions to take hormone medications. Further, evidence suggests that genetic influences on depression overlap with genetic influences on hot flashes. Taking these genetic factors into account, it is clear that higher depression among older women taking estrogen medications is likely caused, in part, by shared genetic influences on psychiatric and menopause symptoms. This work also demonstrated a polygenic etiology for menopause symptoms and suggested that estrogen signaling genes likely contribute to menopause symptoms, consistent with a priori expectations about the central role of decreased estrogen in menopause symptoms. Finally, the TACR3 gene identified here implicates a known pharmacological target of certain medications used to treat hot flashes (neurokinin 3 receptor antagonists). Thus, this work confirmed – with genetic methods – known mechanisms contributing to hot flashes (Fraser et al., 2020; Jayasena et al., 2015; Prague et al., 2018; Rance et al., 2013), and it yielded evidence that shared genetic factors likely contribute to increased rates of anxiety and depression around the time of menopause.
Acknowledgments
This research has been conducted using the UK Biobank Resource under application 52498. We are grateful to all the individuals who have volunteered and the whole UK Biobank team, including the interviewers, technicians, research scientists, healthcare professionals, clerical workers, and receptionists. We thank the PGC teams that conducted the PTSD and depression GWAS meta-analyses for providing the summary statistics necessary for this research.
Funding
This work was supported by the National Institute of Mental Health (LD, R01 MH123486 & R21MH125358) and the Stanford WHSDM Center (LD, seed grant from Women’s Health & Sex Differences in Medicine).
Footnotes
Conflicts of Interest
Conflicts of Interest: None
REFERENCES
- ACOG Practice Bulletin No. 141: Management of menopausal symptoms. (2014). Obstetrics and Gynecology, 123(1), 202–216. 10.1097/01.AOG.0000441353.20693.78 [DOI] [PubMed] [Google Scholar]
- Al Abed AS, Reynolds NJ, & Dehorter N (2021). A Second Wave for the Neurokinin Tac2 Pathway in Brain Research. Biological Psychiatry, 90(3), 156–164. 10.1016/j.biopsych.2021.02.016 [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, & Neill Epperson C (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology, 35(3), 320–330. 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, & Worthman CW (1993). Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders, 29(2–3), 145–158. 10.1016/0165-0327(93)90029-J [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, & Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. The American Journal of Psychiatry, 157(6), 924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, & Plantinga P (1997). Prospective study of the determinants of age at menopause. American Journal of Epidemiology, 145(2), 124–133. 10.1093/oxfordjournals.aje.a009083 [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, … Neale BM (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47(11), 1236–1241. 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium, … Neale BM (2015). LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics, 47(3), 291–295. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, … Marchini J (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature, 562(7726), 203. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp HJA (2018). Progestogens and pregnancy loss. Climacteric: The Journal of the International Menopause Society, 21(4), 380–384. 10.1080/13697137.2018.1436166 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. (2019, November 6). CDC website Key Statistics from the National Survey of Family Growth—Listing C. Retrieved October 8, 2020, from CDC website Key Statistics from the National Survey of Family Growth—Listing C website: https://www.cdc.gov/nchs/nsfg/key_statistics/c.htm [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, & Lee JJ (2015). Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience, 4(1), 7. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R (2012). What makes UK Biobank special? Lancet (London, England), 379(9822), 1173–1174. 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- Cooper GS, & Sandler DP (1998). Age at Natural Menopause and Mortality. Annals of Epidemiology, 8(4), 229–235. 10.1016/S1047-2797(97)00207-X [DOI] [PubMed] [Google Scholar]
- Craig MC (2013). Should psychiatrists be prescribing oestrogen therapy to their female patients? The British Journal of Psychiatry, 202(1), 9–13. 10.1192/bjp.bp.111.102855 [DOI] [PubMed] [Google Scholar]
- Crandall CJ, Manson JE, Hohensee C, Horvath S, Wactawski-Wende J, LeBlanc ES, … Sinsheimer JS (2017). Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause (New York, N.Y.), 24(3), 252–261. 10.1097/GME.0000000000000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR (2000). Trauma: The impact of post-traumatic stress disorder. Journal of Psychopharmacology (Oxford, England), 14(2 Suppl 1), S5–12. [DOI] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, & Posthuma D (2015). MAGMA: Generalized gene-set analysis of GWAS data. PLoS Computational Biology, 11(4), e1004219. 10.1371/journal.pcbi.1004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Novaes Soares C, Almeida OP, Joffe H, & Cohen LS (2001). Efficacy of Estradiol for the Treatment of Depressive Disorders in Perimenopausal Women: A Double-blind, Randomized, Placebo-Controlled Trial. Archives of General Psychiatry, 58(6), 529–534. 10.1001/archpsyc.58.6.529 [DOI] [PubMed] [Google Scholar]
- Deecher D, Andree TH, Sloan D, & Schechter LE (2008). From menarche to menopause: Exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology, 33(1), 3–17. 10.1016/j.psyneuen.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Delgado BJ, & Lopez-Ojeda W (2021). Estrogen. In StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK538260/ [Google Scholar]
- Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, … Koenen KC (2018). Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular Psychiatry, 23(3), 666–673. 10.1038/mp.2017.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Lederman S, Waldbaum A, Kroll R, Santoro N, Lee M, … Ramael S (2020). A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause (New York, N.Y.), 27(4), 382–392. 10.1097/GME.0000000000001510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, & Lin H (2009). TEMPORAL ASSOCIATIONS OF HOT FLASHES AND DEPRESSION IN THE TRANSITION TO MENOPAUSE. Menopause (New York, N.Y.), 16(4), 728–734. 10.1097/gme.0b013e3181967e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, & Ferdousi T (2005). The role of anxiety and hormonal changes in menopausal hot flashes. Menopause, 12(3), 258–266. 10.1097/01.GME.0000142440.49698.B7 [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, & Hollander L (2004). Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry, 61(1), 62–70. 10.1001/archpsyc.61.1.62 [DOI] [PubMed] [Google Scholar]
- Gerber MR, King MW, Pineles SL, Wiltsey-Stirman S, Bean-Mayberry B, Japuntich SJ, & Haskell SG (2015). Hormone Therapy Use in Women Veterans Accessing Veterans Health Administration Care: A National Cross-Sectional Study. Journal of General Internal Medicine, 30(2), 169–175. 10.1007/s11606-014-3073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, … Wisner KL (2015). Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. The American Journal of Psychiatry, 172(3), 227–236. 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, & Girdler SS (2018). Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA Psychiatry, 75(2), 149–157. 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk MS, Eskild A, Hofvind S, Gran JM, & Bjelland EK (2020). Temporal trends in age at menarche and age at menopause: A population study of 312 656 women in Norway. Human Reproduction, 35(2), 464–471. 10.1093/humrep/dez288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, & Corey-Lisle PK (2003). The economic burden of depression in the United States: How did it change between 1990 and 2000? The Journal of Clinical Psychiatry, 64(12), 1465–1475. 10.4088/jcp.v64n1211 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, & Grant BF (2018). Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry, 75(4), 336–346. 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, … McIntosh AM (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience, 22(3), 343–352. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C, … Dhillo WS (2015). Neurokinin B Administration Induces Hot Flushes in Women. Scientific Reports, 5(1), 8466. 10.1038/srep08466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Crawford SL, Freeman MP, White DP, Bianchi MT, Kim S, … Cohen LS (2016). Independent Contributions of Nocturnal Hot Flashes and Sleep Disturbance to Depression in Estrogen-Deprived Women. The Journal of Clinical Endocrinology & Metabolism, 101(10), 3847–3855. 10.1210/jc.2016-2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, & Pedersen NL (2006). Personality and major depression: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry, 63(10), 1113–1120. 10.1001/archpsyc.63.10.1113 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, … Zaslavsky AM (2005). Prevalence and Treatment of Mental Disorders, 1990 to 2003. New England Journal of Medicine, 352(24), 2515–2523. 10.1056/NEJMsa043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria. Journal of Traumatic Stress, 26(5), 537–547. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn RB, Nishimi KM, Kim Y, Jung SJ, Roberts AL, Sumner JA, … Kubzansky LD (2020). Posttraumatic Stress Disorder and Likelihood of Hormone Therapy Use among Women in the Nurses’ Health Study II: A 26-Year Prospective Analysis. Cancer Epidemiology and Prevention Biomarkers. 10.1158/1055-9965.EPI-20-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JP (2003). Long-term estrogen and hormone replacement therapy for the prevention and treatment of osteoporosis. Current Women’s Health Reports, 3(3), 181–186. [PubMed] [Google Scholar]
- Luciano M, Hagenaars SP, Davies G, Hill WD, Clarke T-K, Shirali M, … Deary IJ (2018). Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nature Genetics, 50(1), 6–11. 10.1038/s41588-017-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mägi R, & Morris AP (2010). GWAMA: Software for genome-wide association meta-analysis. BMC Bioinformatics, 11(1), 288. 10.1186/1471-2105-11-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, … Soares CN (2019). Guidelines for the Evaluation and Treatment of Perimenopausal Depression: Summary and Recommendations. Journal of Women’s Health (2002), 28(2), 117–134. 10.1089/jwh.2018.27099.mensocrec [DOI] [PubMed] [Google Scholar]
- McKinlay SM, Brambilla DJ, & Posner JG (1992). The normal menopause transition. Maturitas, 14(2), 103–115. 10.1016/0378-5122(92)90003-M [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, … Koenen KC (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nature Communications, 10(1), 1–16. 10.1038/s41467-019-12576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Girgus JS (1994). The emergence of gender differences in depression during adolescence. Psychological Bulletin, 115(3), 424–443. 10.1037/0033-2909.115.3.424 [DOI] [PubMed] [Google Scholar]
- Padilla SL, Johnson CW, Barker FD, Patterson MA, & Palmiter RD (2018). A Neural Circuit Underlying the Generation of Hot Flushes. Cell Reports, 24(2), 271–277. 10.1016/j.celrep.2018.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Kuchenbaecker K, Walters RK, Chen C-Y, Popejoy AB, Periyasamy S, … Duncan LE (2019). Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell, 179(3), 589–603. 10.1016/j.cell.2019.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Mohideen P, … Dhillo WS (2018). Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action. Menopause (New York, N.y.), 25(8), 862–869. 10.1097/GME.0000000000001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, … Sham PC (2007). PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. American Journal of Human Genetics, 81(3), 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2005). Development Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051-07–0. Retrieved from http://www.R-project.org [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, & Krajewski-Hall SJ (2013). Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flushes. Frontiers in Neuroendocrinology, 34(3), 211–227. 10.1016/j.yfrne.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, & Rapkin AJ (2002). Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. The Journal of Clinical Psychiatry, 63 Suppl 7, 45–48. [PubMed] [Google Scholar]
- Reid RL, & Soares CN (2018). Premenstrual Dysphoric Disorder: Contemporary Diagnosis and Management. Journal of Obstetrics and Gynaecology Canada: JOGC = Journal d’obstetrique et Gynecologie Du Canada: JOGC, 40(2), 215–223. 10.1016/j.jogc.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Ruddy KJ, & Loprinzi CL (2015). Antidepressants decrease hot flashes and improve life quality. Menopause, 22(6), 587–588. 10.1097/GME.0000000000000449 [DOI] [PubMed] [Google Scholar]
- Santoro N, Waldbaum A, Lederman S, Kroll R, Fraser GL, Lademacher C, … Ramael S (2020). Effect of the neurokinin 3 receptor antagonist fezolinetant on patient-reported outcomes in postmenopausal women with vasomotor symptoms: Results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause (New York, N.Y.). 10.1097/GME.0000000000001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, … Overington JP (2017). A comprehensive map of molecular drug targets. Nature Reviews. Drug Discovery, 16(1), 19–34. 10.1038/nrd.2016.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, & Rubinow DR (2000). Estrogen replacement in perimenopause-related depression: A preliminary report. American Journal of Obstetrics and Gynecology, 183(2), 414–420. 10.1067/mob.2000.106004 [DOI] [PubMed] [Google Scholar]
- Schmidt Peter J., Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, … Rubinow DR (2015). Effects of Estradiol Withdrawal on Mood in Women With Past Perimenopausal Depression: A Randomized Clinical Trial. JAMA Psychiatry, 72(7), 714–726. 10.1001/jamapsychiatry.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R (2018). Non-clinical studies of progesterone. Climacteric, 21(4), 315–320. 10.1080/13697137.2018.1463982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouby SO, Al-Azzawi F, Barlow D, Calaf-Alsina Erdogan Ertüngealp J, Gompel A, Graziottin A, … Stevenson JC (2005). Climacteric medicine: European Menopause and Andropause Society (EMAS) 2004/2005 position statements on peri- and postmenopausal hormone replacement therapy. Maturitas, 51(1), 8–14. 10.1016/j.maturitas.2005.02.019 [DOI] [PubMed] [Google Scholar]
- Skovlund CW, Mørch LS, Kessing LV, & Lidegaard Ø (2016). Association of Hormonal Contraception With Depression. JAMA Psychiatry, 73(11), 1154–1162. 10.1001/jamapsychiatry.2016.2387 [DOI] [PubMed] [Google Scholar]
- Smith DJ, Nicholl BI, Cullen B, Martin D, Ul-Haq Z, Evans J, … Pell JP (2013). Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: Cross-sectional study of 172,751 participants. PloS One, 8(11), e75362. 10.1371/journal.pone.0075362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, … Collins R (2015). UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Medicine, 12(3), e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, … Dunbar-Jacob J (2004). Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI). Archives of Internal Medicine, 164(3), 289–298. 10.1001/archinte.164.3.289 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Taskesen E, Bochoven A, & Posthuma D (2017). Functional mapping and annotation of genetic associations with FUMA. Nature Communications, 8(1), 1826. 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Byrne EM, Zheng Z, Kemper KE, Yengo L, Mallett AJ, … Wray NR (2019). Genome-wide association study of medication-use and associated disease in the UK Biobank. Nature Communications, 10(1), 1891. 10.1038/s41467-019-09572-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettermark S, Vicente RP, & Merlo J (2018). Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: A pharmacoepidemiological study on 800 000 Swedish women. PLOS ONE, 13(3), e0194773. 10.1371/journal.pone.0194773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang Y, & Chu Y-X (2020). Tacr3/NK3R: Beyond Their Roles in Reproduction. ACS Chemical Neuroscience. (world). 10.1021/acschemneuro.0c00421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


