Abstract
Most-probable-number (liquid serial dilution culture) counts were obtained for polysaccharolytic and saccharolytic fermenting bacteria in the anoxic bulk soil of flooded microcosms containing rice plants. The highest viable counts (up to 2.5 × 108 cells per g [dry weight] of soil) were obtained by using xylan, pectin, or a mixture of seven mono- and disaccharides as the growth substrate. The total cell count for the soil, as determined by using 4′,6-diamidino-2-phenylindole staining, was 4.8 × 108 cells per g (dry weight) of soil. The nine strains isolated from the terminal positive tubes in counting experiments which yielded culturable populations that were equivalent to about 5% or more of the total microscopic count population belonged to the division Verrucomicrobia, the Cytophaga-Flavobacterium-Bacteroides division, clostridial cluster XIVa, clostridial cluster IX, Bacillus spp., and the class Actinobacteria. Isolates originating from the terminal positive tubes of liquid dilution series can be expected to be representatives of species whose populations in the soil are large. None of the isolates had 16S rRNA gene sequences identical to 16S rRNA gene sequences of previously described species for which data are available. Eight of the nine strains isolated fermented sugars to acetate and propionate (and some also fermented sugars to succinate). The closest relatives of these strains (except for the two strains of actinobacteria) were as-yet-uncultivated bacteria detected in the same soil sample by cloning PCR-amplified 16S rRNA genes (U. Hengstmann, K.-J. Chin, P. H. Janssen, and W. Liesack, Appl. Environ. Microbiol. 65:5050–5058, 1999). Twelve other isolates, which originated from most-probable-number counting series indicating that the culturable populations were smaller, were less closely related to cloned 16S rRNA genes.
Rice paddy soil is a system in which processes leading to methane emission have been quantified (12, 36), and the importance of these processes to world climate has been recognized (14). Since rice paddy soils are flooded, they are largely anoxic and typically (but not exclusively) methanogenic. Plant polymers and root exudates are important sources of carbon and energy for microbial activity, and methane is formed by a trophic web of largely uncharacterized microbial populations. The carbon and electron fluxes through this system are similar to those in other methanogenic habitats, such as sediments and anaerobic waste treatment systems (8, 12, 29), but the identities of the microorganisms that degrade organic matter are not known. Soils, with their matrices of inorganic components, gradients of oxygen and other electron acceptors, seasonal changes, high levels of heterogeneity, and plant and meofauna influences, are complex systems, and studying microbial community structure in such systems is thus very difficult.
Studies of microbial communities in which bacteria are isolated in pure cultures yield very important data, because the organisms can be characterized phenotypically and their roles in the soil can be deduced. However, it is known that enrichment cultures select for fast-growing bacteria with high growth yields and for the bacteria that are best adapted to the growth medium used for cultivation (33). This means that microorganisms isolated from a system are not necessarily significant numerically in the habitat being studied. In addition, since the growth conditions favor microorganisms that are best adapted to the medium, there is also no guarantee that the bacteria obtained have significant roles in the biogeochemical processes in the environment being studied. These problems, together with the general labor-intensiveness of many cultivation methods, have resulted in an increase in the popularity of molecular approaches for studying microbial populations. Generally, the 16S rRNA gene has been used as a molecular marker which allows identification (or at least phylogenetic assignment) of the organism from which it originated without cultivation and isolation of the organism. However, it has been shown that some bias may be introduced into the data obtained due to differential amplification of different sequence types in one sample (23, 33, 51). One of the limitations of the purely molecular approach is the difficulty of assigning phenotypes to the microorganisms detected. This problem becomes increasingly difficult as the relationship to the closest known cultivated relative increases or if the inferred phylogenetic position indicates that the organism belongs to a taxon containing organisms with very diverse phenotypes.
In an attempt to address this problem, we used both molecular and cultivation techniques in a combined approach to investigate the numerically dominant members of the bacterial community in anoxic rice paddy soil. We used serial liquid dilution cultures to determine population sizes and isolated, characterized, and identified microorganisms obtained from the terminal and subterminal positive dilution steps (i.e., preparations receiving dilute inocula and presumably containing species that were present in high numbers in the soil sample). In a parallel study, the bacterial community of the same soil was investigated by constructing a clone library of PCR-amplified 16S rRNA genes of the bacterial fraction of the total community DNA (27).
MATERIALS AND METHODS
Medium preparation.
Sulfide-reduced, bicarbonate-buffered mineral medium SM supplemented with vitamins was described by Chin et al. (9) and was used for characterization studies. The mineral salts solution of medium SdM contained (per liter of H2O) 0.1 g of MgCl2 · 6H2O, 0.1 g of NH4Cl, 0.07 g of KH2PO4, and 0.1 g of CaCl2 · 2H2O and was prepared and supplemented in the same way as medium SM, except that 1 ml of a riboflavin solution (50 mg in 1 liter of H2O; filter sterilized) was added per liter. Medium SdM was used for viable counting experiments.
Substrates and other supplements were prepared as neutralized (with NaOH or HCl, as required) 200 mM to 2 M or 5% (wt/vol) stock solutions and were sterilized by autoclaving or, in the case of heat-labile compounds and sugars, by filtration (pore size, 0.2 μm). Substrates were added to sterile media just before inoculation. l Isomers of organic and amino acids and d isomers of sugars were used. Amorphous cellulose and pectin (28) and filter paper cellulose and microcrystalline cellulose (9) were prepared as described previously.
Viable numbers of bacteria.
Rice (Oryza sativa var. Roma, type japonica) was grown in plastic containers in the laboratory, as described by Frenzel et al. (18), in flooded soil obtained from rice fields of the Italian Rice Research Institute in Vercelli, Italy. Three-tube most-probable-number (MPN) counts were obtained for soil cores from such laboratory rice cultures in which the plants were 45 or 90 days old. First, the shoots of the plants were cut off, and weeds and water were removed from the surfaces of the microcosms. Cores were then obtained from the bulk soil between the plants by pressing a plastic corer into the soil to a depth of about 15 cm. Only the lower 10-cm portions of the cores were used. The soil was mixed with sterile, deionized water (1:1, wt/wt) which had previously been degassed with a vacuum pump. All visible roots were removed from each suspension. The MPN counts were obtained as described previously (28) by using medium SdM, which was incubated in the dark at 25°C. Tubes were considered positive if fermentation products (>1 mM acetate, propionate, lactate, etc.) were produced. Tubes without added substrates did not produce fermentation products (<0.05 mM). The MPN was calculated from the dry weight of the soil (part of the soil suspension was dried to a constant weight at 105°C), the dilution factor, and tables for three parallel dilution series based on a statistical treatment of the counting methods (4). The significance of the difference between two estimated population sizes was tested as described by Cochran (10). DNA was extracted from part of the soil suspension obtained from the microcosm containing 90-day-old plants and was used to generate a clone library of PCR-amplified 16S rRNA genes, and the results are described in detail in the accompanying paper (27).
Phenotypic and phylogenetic characterization.
The methods used for isolating pure cultures, for checking culture purity, and for phenotypic characterization of the isolates have been described previously (9, 28). Strains were identified and their phylogenetic positions were elucidated by performing a comparative analysis of the sequences of their 16S rRNA genes (27).
Microscopic cell counts.
To determine total numbers of bacteria, soil samples from a microcosm containing 90-day-old rice plants were fixed in 4% (wt/vol) paraformaldehyde (22). Subsamples were dispersed in 0.1% (wt/vol) sodium pyrophosphate in distilled water by mild sonication, spotted onto gelatin-coated slides (55), and stained with the DNA intercalating dye 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, Basel, Switzerland) (22). The number of members of the Bacteria was determined by in situ hybridization with Cy3-labeled oligonucleotide Eub338 (1) by using the protocol described by Zarda et al. (55). Preparations were mounted with Citifluor solution (Citifluor, Canterbury, United Kingdom) and were examined with an Axiophot microscope (Zeiss, Oberkochen, Germany) fitted for epifluorescence detection with a high-pressure mercury bulb (50 W) and filter sets 02 (G 365, FT 395, LP 420; Zeiss) and HQ-Cy3 (G 535/50, FT 565, BP 610/75; AHF Analysentechnik, Tübingen, Germany). Microorganisms were counted at a magnification of ×1,000 by using six samples distributed over a 53-mm2 circular area; for each sample at least 20 fields, each covering an area of 0.01 mm2, were used.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA genes used in this study have been deposited in the EMBL and GenBank databases under accession no. AJ229234 to AJ229252.
RESULTS AND DISCUSSION
Total and culturable community sizes.
We defined the bulk soil as the soil fraction that was below the surface root layer and and largely free of roots of the rice plants growing in the microcosms. The total concentration of cells in the bulk soil that could be stained with DAPI was 4.8 × 108 ± 2.3 × 108 cells per g (dry weight) of soil (mean ± standard deviation). Approximately 60% of the DAPI-stained cells (2.8 × 108 ± 1.5 × 108 cells per g [dry weight] of soil) were detected in bulk soil by in situ hybridization with oligonucleotide probe Eub338, which targets the 16S rRNA of members of the Bacteria. Bulk soil is considered a nutritionally poor environment to which some bacteria may adapt by forming resting or dormant cells, such as dwarf cells, cysts, or spores. The signal intensity obtained for an individual cell after hybridization with rRNA-targeted probes depends on the ribosome content (16, 42). The large percentage of cells detectable by in situ hybridization with a probe for members of the Bacteria in rice paddy soil, therefore, meant that cells containing sufficient amounts of rRNA for detection were present and that there was sufficient permeability or permeabilization of the cells. The cells may have been metabolically active (22, 52) or may have belonged to species which do not degrade most of their rRNA under starvation conditions (17). The cells that were not detected may not have contained sufficient ribosomes, may not have been permeable to the labeled probe, or may have been members of the Archaea, organisms which are also present in large numbers in this soil (21).
MPN counting experiments performed with a variety of substrates (Table 1) revealed that there were culturable populations of saccharolytic bacteria in the bulk soil of the microcosms consisting of up to 2.5 × 108 cells per g (dry weight) of soil, as determined with the substrates pectin and xylan. The viable counts obtained for microcosms with 45-day-old rice plants and microcosms with 90-day-old rice plants were not significantly different (P < 0.05). Cell counts obtained with xylan and pectin were not significantly higher (P < 0.05) than cell counts obtained with a mixture of seven sugars but were significantly higher (P < 0.05) than cell counts obtained with glucose, cellobiose, amorphous cellulose, filter paper cellulose, or microcrystalline cellulose as the growth substrate.
TABLE 1.
MPN counts for saccharolytic populations in anoxic soil of flooded rice microcosms containing 45-day-old or 90-day-old rice plants
| Substrate (concn) | MPN count (no. of cells per g [dry wt] of soil)
|
|
|---|---|---|
| 45-Day-old plants | 90-Day-old plantsa | |
| Amorphous cellulose (0.1% [wt/vol]) | 2.3 × 106 (0.4 × 106–8.5 × 106)b | 5.5 × 106 (0.9 × 106–27 × 106) |
| Filter paper cellulose (0.1% [wt/vol]) | 2.3 × 106 (0.4 × 106–8.5 × 106) | 2.5 × 106 (0.4 × 106–9.4 × 106) |
| Microcrystalline cellulose (0.1% [wt/vol]) | 3.1 × 105 (0.7 × 105–9.4 × 105) | 2.5 × 106 (0.4 × 106–9.4 × 106) |
| Xylan (0.1% [wt/vol]) | 2.3 × 108 (0.4 × 108–8.5 × 108) | 2.5 × 108 (0.4 × 108–9.4 × 108) |
| Pectin (0.1% [wt/vol]) | 4.9 × 107 (0.8 × 107–24 × 107) | 2.5 × 108 (0.4 × 108–9.4 × 108) |
| Sugar mixturec | 9.4 × 107 (1.8 × 107–13 × 107) | 3.4 × 107 (0.8 × 107–10 × 107) |
| Glucose (4 mM) | 7.3 × 105 (1.4 × 105–31 × 105) | 5.9 × 106 (1.1 × 106–25 × 106) |
| Cellobiose (2 mM) | 4.1 × 106 (0.7 × 106–20 × 106) | 5.9 × 105 (1.1 × 105–25 × 105) |
DNA extracted from the same soil sample was used to generate the clone library used in a parallel study (27).
The values in parentheses are 95% confidence intervals.
The mixture contained 1 mM arabinose, 1 mM cellobiose, 1 mM fructose, 1 mM galactose, 1 mM glucose, 1 mM maltose, and 1 mM xylose.
The highest viable cell count, 2.5 × 108 cells per g (dry weight) of soil, represented 52% of the total DAPI-stained cell count. This efficiency of cultivation is higher than the efficiencies generally reported for soils, from which viable counting procedures generally recover only a small percentage of the total population (perhaps up to 5% and often less than 1%) (2, 33, 40, 55). Obviously, not all of the microorganisms in the rice paddy soil were saccharolytic. Other studies revealed that there were large culturable populations of methanogens (21), homoacetogenic bacteria (45), sulfate-reducing bacteria (45), and Fe(III)-reducing bacteria (19) in the bulk soil of similar rice microcosms. Using a sugar monomer (glucose or cellobiose) in the counting medium resulted in lower culturable cell counts than using the polysaccharides xylan and pectin resulted in. However, all of the isolates obtained with xylan or pectin were later shown to be able to grow with glucose and cellobiose (see below). Thus, the populations of glucose- and cellobiose-utilizing bacteria present in the soil were much larger than the populations that could be cultured when these substrates were used. It is thought that bacteria growing under substrate-limitating conditions in a natural environment are subject to “substrate-accelerated death” when they are transferred to high-substrate-concentration conditions (41, 50). Polymers may be better substrates for determining cell numbers because they first have to be hydrolyzed, and this may eliminate sudden exposure of the soil bacteria to high concentrations of growth substrates.
The large proportion of culturable microorganisms points to (i) the efficiency of the liquid serial dilution culture procedure, as observed previously for a marine system (7), and (ii) the numerical abundance of polysaccharolytic bacteria in anoxic rice paddy soil microcosms. Each counting experiment can result in the growth of phenotypically similar bacteria which may be phylogenetically very diverse. The theory of the liquid dilution procedure predicts that the terminal positive tubes should contain an inoculum consisting of the numerically significant organisms present in the sample (7). Thus, isolates that originate from the terminal positive tubes (i.e., the tubes exhibiting growth which received the most dilute inoculum) should be representatives of species whose populations in the soil are large. This should be true only if within each group of phenotypically similar organisms there are a few species to which the majority of the individuals belong. We hypothesized that the organisms which we isolated are representatives of numerically significant microbial populations in the anoxic rice paddy soil microcosms. We tested this hypothesis by performing a parallel, cultivation-independent investigation of the bacterial communities in the microcosms (27).
Pure cultures were isolated from the terminal positive tubes of an MPN counting series, and the isolates were identified by performing a comparative sequence analysis of their 16S rRNA genes (Table 2) (see reference 27 for details) and were characterized phenotypically (see below). Additional phenotypic information for the strains not included in Table 3 is available from P. H. Janssen.
TABLE 2.
Strains isolated in this investigation
| No. of cells per g (dry wt) of soil | Strain | Taxona | Substrateb | Age of plants (days) |
|---|---|---|---|---|
| 108 to 109 | PB90-1 | Verrucomicrobia | Pectin | 90c |
| PB90-2 | CFB group | Pectin | 90c | |
| XB45 | CFB group | Xylan | 45 | |
| XB90 | Clostridial cluster XIVa | Xylan | 90c | |
| 107 to 108 | PB90-3 | Verrucomicrobia | Pectin | 90c |
| PB90-4 | Actinobacteria | Pectin | 90c | |
| PB90-5 | Actinobacteria | Pectin | 90c | |
| SB45 | Bacillus sp. | Sugar mixture | 45 | |
| SB90 | Clostridial cluster IX | Sugar mixture | 90c | |
| Less than 107 | VeSm15 | Actinobacteria | Sugar mixture | 90 |
| VeCb6 | Actinobacteria | Cellobiose | 90 | |
| VeCb10 | Clostridial cluster I | Cellobiose | 90 | |
| VeGlc14 | Actinobacteriad | Glucose | 90 | |
| ACB45 | Actinobacteria | Amorphous cellulose | 45 | |
| ACB90 | Verrucomicrobia | Amorphous cellulose | 90c | |
| KCB45 | Actinobacteria | Microcrystalline cellulose | 90c | |
| KCB90 | α-Proteobacteria | Microcrystalline cellulose | 90c | |
| FCB45 | Clostridial cluster III | Filter paper cellulose | 45 | |
| FCB90-1 | Clostridial cluster III | Filter paper cellulose | 90c | |
| FCB90-2 | Clostridial cluster III | Filter paper cellulose | 90c | |
| FCB90-3 | Clostridium-like species | Filter paper cellulose | 90c |
The designations of the phylogenetic groups are the designations used by Collins et al. (11), Hedlund et al. (26), Olsen et al. (39), and Stackebrandt et al. (48, 49).
The strains were isolated in pure culture by using the substrates used in the MPN counting experiments in which they were originally obtained.
Isolated from the soil sample used in a parallel clone library study (27).
The taxon was identified only on the basis of phenotypic characteristics; no comparative 16S rRNA gene sequence analysis was performed with this strain.
TABLE 3.
Phenotypic characteristics of isolated strains indicative of large culturable populationsa
| Characteristic | Strain PB90-1 | Strain PB90-3 | Strain PB90-2 | Strain XB45 | Strain XB90 | Strain SB90 | Strain SB45 | Strain PB90-4 | Strain PB90-5 |
|---|---|---|---|---|---|---|---|---|---|
| Cell form | Oval, rod | Oval | Oval, rod | Oval | Rod | Curved rod | Rod | Rod | Oval |
| Cell length (μm) | 1–3 | 1–2 | 0.8–2.5 | 0.8–2.5 | 2–16 | 2–5 | 2.5–14 | 2–4 | 2–3 |
| Cell width (μm) | 0.6–0.8 | 0.9 | 0.5–0.8 | 0.7–0.8 | 0.7–1.4 | 0.5–0.6 | 0.5–0.6 | 0.9 | 0.9 |
| Aggregate formation | − | − | − | − | Chains | − | − | Irregular clumps | − |
| Motility | + | − | + | + | + | + | + | + | − |
| Gram stain reaction | − | − | − | − | ± | + | − | + | ND |
| Lysis in 3% (wt/vol) KOH | + | + | + | + | + | − | − | − | − |
| G+C content (mol%) | 73.7 | ND | 40.2 | 41.2 | 40.8 | 35.5 | 45.6 | 70.7 | 72.9 |
| Product from NO3− | NO2− | NU | NO2− | NU | NO2− | NU | NU | NU | NU |
| Aerobic growth | − | − | − | − | − | − | + | + | + |
| Urease activity | − | − | − | − | − | − | − | + | − |
| Esculin hydrolysis | + | + | + | + | + | − | − | + | + |
| Indole production | + | − | − | − | − | − | − | + | + |
| Major products from glucoseb | A,P | S,A,P | A,P | S,A,P | A,P | A,P | A,P | A,P | L,F,A,E |
| Growth with: | |||||||||
| Maltose | + | + | + | + | + | + | − | − | + |
| Cellobiose, glucose, galactose | + | + | + | + | + | + | + | + | + |
| Fructose | + | − | + | + | + | + | − | + | + |
| Xylose | − | + | + | + | + | + | − | + | + |
| Arabinose, starch | + | + | + | + | + | + | − | + | + |
| Galacturonic acid | + | + | + | + | − | − | − | − | − |
| Cellulose, lactate | − | − | − | − | − | − | − | − | − |
| Xylan | + | + | + | + | + | − | − | − | + |
| Pectin | + | + | + | + | + | − | − | + | + |
| Arabinogalactan | − | − | + | + | − | − | − | − | − |
| Pyruvate | − | + | + | − | + | − | − | + | + |
| Malate | − | − | + | − | + | + | − | − | − |
| Taxonc | Verrucomicrobia | Verrucomicrobia | CFB group | CFB group | Clostridial cluster XIVa | Clostridial cluster IX | Bacillus spp. | Actinobacteria | Actinobacteria |
All nine strains gave negative results in catalase and oxidase activity tests and in gelatin hydrolysis tests. +, positive, −, negative; ±, variable; ND, not determined; NU, nitrate not utilized.
The mean final concentrations (as determined in triplicate experiments) were >1.5 mM. Abbreviations: A, acetate; E, ethanol; F, formate; L, lactate; P, propionate; S, succinate.
Taxon designations as in Table 2.
The identities of the strains isolated from the terminal positive tubes in counting experiments which yielded culturable populations that accounted for about 5% or more of the total microscopic counts (i.e., strains PB90-1, PB90-2, PB90-3, PB90-4, PB90-5, XB45, XB90, SB45, and SB90) suggested that the dominant culturable saccharolytic bacteria in the rice paddy soil belonged to the following groups: the division Verrucomicrobia, the Cytophaga-Flavobacterium-Bacteroides (CFB) division, clostridial cluster XIVa, clostridial cluster IX, Bacillus spp., and the class Actinobacteria.
Isolation from terminal (strains PB90-1 and PB90-2) and subterminal (strains PB90-3, PB90-4, and PB90-5) positive steps in the dilution culture counting experiments in which we used pectin and soil from a microcosm containing 90-day-old rice plants yielded organisms which were phenotypically similar (Tables 2 and 3) but phylogenetically diverse (27) and belonged to different divisions. Two of the strains were members of the division Verrucomicrobia, one strain was a member of the CFB division, and the other two strains belonged to the class Actinobacteria. These organisms represented numerically significant populations in the soil, since the counts obtained with pectin yielded 2.5 × 108 culturable cells per g (dry weight) of soil. It seems from these data that there was not a single dominant culturable species that accounted for a large part of the total (poly)saccharolytic microbial community but there were a number of (completely unrelated) species whose populations were relatively large.
Members of the division Verrucomicrobia.
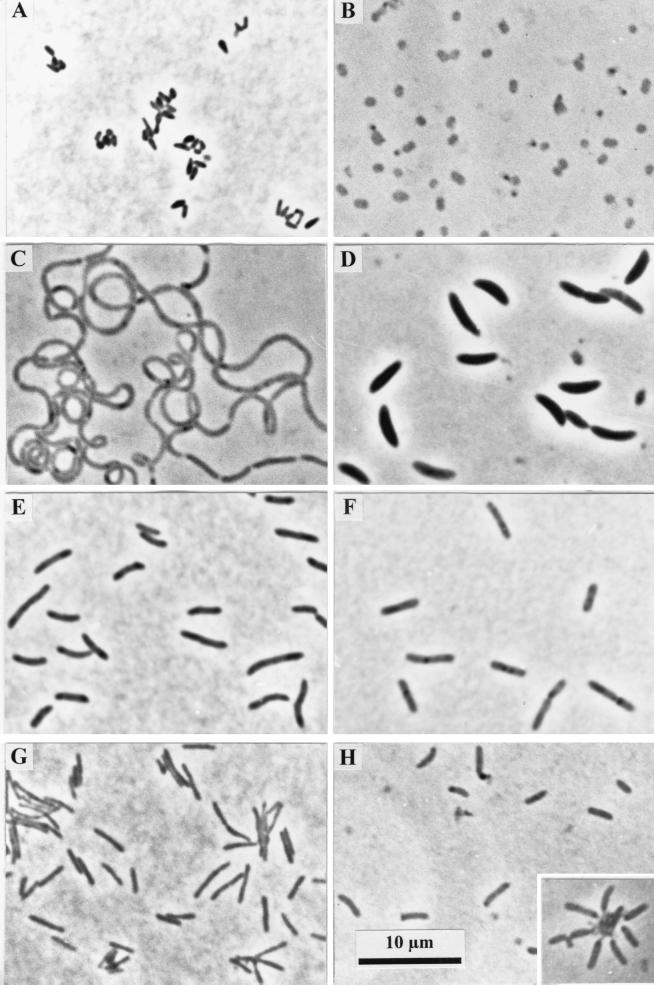
The following three strains belonging to the division Verrucomicrobia were isolated: strains PB90-1, PB90-3, and ACB90 (Tables 2 and 3 and Fig. 1A). These organisms were characterized by their rod-shaped to oval cells and high genomic DNA G+C contents (69 to 74 mol%). Spores were not formed. All three strains were strict anaerobes and produced acetate and propionate as fermentation end products. Strain ACB90 was cellulolytic.
FIG. 1.
Phase-contrast photomicrographs of strains isolated from rice paddy soil. (A) Strain PB90-1. (B) Strain XB45. (C) Strain XB90. (D) Strain FCB90-1. (E) Strain SB90. (F) Strain SB45. (G) Strain KCB45. (H) Strain KCB90. The insert in panel H shows cells of strain KCB90 surrounding an aggregate of amorphous cellulose. All panels are at the same magnification.
The division Verrucomicrobia (26) contains only a few organisms, including Verrucomicrobium spinosum (46) and Prosthecobacter spp. (25, 26). Most of our information concerning the diversity of this group came from molecular ecological studies based on the recovery of 16S rRNA genes from soils and other habitats (5, 31, 32, 54, 56). Recently, Janssen et al. (28) described three new anaerobic strains belonging to this group, which were also isolated from microcosms containing Italian rice paddy soil. The closest known relative of these three strains, based on a comparative 16S rRNA gene sequence analysis, is an as-yet-uncultured member of the microbial community in a Japanese rice paddy soil (28). The three strains isolated in our study are closely related to the three strains described previously and are most closely related to two different cloned 16S rRNA gene sequences obtained from the same soil sample; all of these organisms have levels of 16S rRNA gene sequence similarity of >98% (27). Our findings confirm the apparent widespread distribution of members of the division Verrucomicrobia in soils and extend the range of phenotypes in this lineage of bacterial descent to include cellulolytic bacteria.
Members of the CFB lineage.
The following two strains belonging to the CFB lineage (39) were isolated: strains PB90-2 and XB45 (Tables 2 and 3 and Fig. 1B). These organisms were gram-negative, motile, non-spore-forming bacteria. The cells were oval, and the G+C contents of the genomic DNA were about 40 mol%. The organisms were strict anaerobes which produced acetate and propionate as the end products of glucose fermentation. Strains PB90-2 and XB45 were most closely related to three cloned 16S rRNA gene sequences obtained from the same microcosms and to “Anaeroflexus maritimus” (27). Members of the CFB lineage have been detected, on the basis of their 16S rRNA genes, in a number of soils (5, 6, 31), but it was found that these organisms accounted for only a small proportion of the bacterial community in a forest soil when oligonucleotide probes targeting the rRNA of members of the group were used (55). Many members of the CFB group are polysaccharolytic and are often isolated from soils, sediments, and anaerobic systems.
Members of the Clostridium complex.
The following seven strains belonging to the genus Clostridium and related genera (11) were isolated: strains XB90, SB90, VeCb10, FCB45, FCB90-1, FCB90-2, and FCB90-3 (Tables 2 and 3 and Fig. 1C through E). These organisms were typified by the low G+C contents of their genomic DNA (28 to 51 mol%) and by generally positive Gram stain reactions. Strains FCB90-1, FCB90-2, and FCB90-3 produced endospores. All of the strains were able to grow fermentatively, and different end products were formed depending on the strain. Strains XB90 and SB90 fermented glucose to acetate and propionate (Table 3), while the other strains produced mainly lactate, formate, acetate, ethanol, and butyrate in different combinations. Only the four strains isolated on cellulosic substrates (FCB45, FCB90-1, FCB90-2, and FCB90-3) were able to grow with cellulose.
Saccharolytic Clostridium spp. are widely regarded as typical fermenting microorganisms in anoxic habitats, such as sediments and sludge digesters, and have been reported to occur in rice paddy soils (9, 38, 53). Strain XB90, which was isolated in counting experiments which indicated that the populations were very large, belongs phylogenetically to cluster XIVa of Collins et al. (11) and is closely related to the cultivated species Clostridium aminovalericum and Clostridium populeti. Its closest known relatives are uncultivated strains that were detected as three cloned 16S rRNA gene sequences in the same rice paddy soil microcosm; all of the organisms exhibited levels of 16S rRNA gene sequence similarity of >95% (27).
Members of clostridial cluster IX of Collins et al. (11) are strict anaerobes that have been isolated from a number of sediment and digester habitats. They ferment sugars and some organic acids (typically lactate), generally to acetate and propionate. One strain belonging to this group, strain SB90, was isolated from the terminal positive tube of a dilution series in which a mixture of sugars was used as the growth substrate. Strain SB90 forms a lineage of descent with Clostridium quercicolum, which is not a true Clostridium sp. However, the closest known relative of strain SB90 is an as-yet-uncultured strain that was detected as a 16S rRNA gene in the same rice paddy soil microcosm (27).
We isolated a range of Clostridium spp. that originated from counting experiments in which we recovered only small portions of the total microbial community (i.e., <0.5% of the total microscopic cell counts). These organisms included cellulolytic strains. Cellulolytic and polysaccharolytic Clostridium spp. have been recovered from rice paddy soil (9), and our strains are very similar to the strains isolated previously. Strain FCB90-2 is very similar to strain RCel1, which was isolated in an enrichment culture experiment during a previous study (9), and both of these organisms are closely related to Clostridium papyrosolvens in clostridial cluster III. Strain VeCb10 is related to Clostridium puniceum (27) in clostridial cluster I. None of these organisms was closely related to bacteria detected as 16S rRNA genes in the same soil (27).
Bacillus sp. strain SB45.
Strain SB45 (Tables 2 and 3 and Fig. 1F), which was identified as a member of the genus Bacillus, was able to grow aerobically, but spore formation was never observed under any of the growth conditions used. Rice paddy soils are typified by anaerobic degradation processes (8, 18, 19, 29, 30), and we therefore carried out our isolation studies on this basis. Isolation of a member of the genus Bacillus with these techniques was unexpected, although Bacillus spp. have been reported to occur in rice paddy soils (38, 53). Bacillus sp. strain SB45 was capable of fermentative growth on a number of sugars, like many species of the genus Bacillus (47). The closest known cultivated relative of strain SB45 is Bacillus pseudomegaterium. Strain SB45 was closely related to nine cloned 16S rRNA gene sequences recovered from the same rice paddy soil microcosms (27). Of the 57 cloned 16S rRNA gene sequences in this sample, 12 belonged to Bacillus spp. or their relatives. This suggests that Bacillus spp. are a numerically important part of the soil microbial community. Nothing is known about the activities of these organisms, and it is not known if they occur largely as spores.
Members of the class Actinobacteria.
Seven strains belonging to the class Actinobacteria were isolated (Tables 2 and 3 and Fig. 1G). These organisms were characterized by their rod-shaped or oval cells and by the high G+C contents of their genomic DNA (68 to 73 mol%). Some of the strains were able to grow under atmospheric O2 tensions. All of the strains were able to grow fermentatively, and the following two groups were identified: strains PB90-4, VeGlc14, and VeSm15 formed acetate and propionate (and sometimes lactate); and the other strains produced combinations of lactate, formate, acetate, and ethanol as end products. Only the two strains isolated by using cellulosic substrates, strains ACB45 and KCB45, were able to grow with cellulose. Members of the class Actinobacteria (49) have been isolated from and detected in a wide range of soils, and actinobacteria are generally considered numerically important members of the rice paddy soil microbial community (24, 38, 53). Many of our strains originated from counting experiments in which we recovered only small portions of the total community. In addition, the strains that were isolated in counting experiments which indicated that the population sizes were larger (strains PB90-4, PB90-5, and VeSm15) originated from subterminal positive steps of the dilution series, not from the positive steps receiving the most dilute inoculum. Organisms belonging to other phylogenetic groups were isolated from the terminal positive tubes (strains PB90-1 and PB90-2). These findings suggest that smaller populations of various actinobacteria were present in the soil. These populations were apparently able to outcompete more numerous organisms when they were introduced together into liquid growth medium. Only 1 of the 57 cloned 16S rRNA genes obtained from the same soil sample indicated that a member of the class Actinobacteria was present (27). There may be a number of explanations for this, including (i) the presence of a truly small population of actinobacteria compared to the more abundant groups, such as the Verrucomicrobia or clostridial cluster XIVa, (ii) bias in the PCR against 16S rRNA genes originating from actinobacteria, or (iii) failure of the lysis methods used to extract DNA from actinobacterial cells in the soil (35, 51).
Strain KCB90.
Strain KCB90 was identified as a member of the α subgroup of the division Proteobacteria (α-Proteobacteria) (Table 2 and Fig. 1H) and represented a new lineage in this radiation (27). The rod-shaped cells were motile and gram negative. The G+C content of the genomic DNA was 64.2 mol%. This strain grew aerobically and also by mixed acid fermentation, producing acetate and ethanol from glucose. Fermentative growth occurred with a range of sugars and sugar polymers, including cellulose. The ability to utilize cellulose is not common among members of the division Proteobacteria. Some Pseudomonas strains are able to degrade cellulose, and strains of Rhizobium, Azoarcus, and Erwinia spp. possess endoglucanases (13, 37, 43). Strain KCB90 originated from a counting experiment which suggested that the population size was small. Three of 57 cloned 16S rRNA genes from the same rice paddy soil microcosm belonged to the α-Proteobacteria, although they were not closely related to strain KCB90 (27). In contrast, 19 of 110 cloned 16S rRNA genes from the rhizoplane of a rice paddy soil microcosm belonged to the α-Proteobacteria (44). It is possible that facultatively anaerobic strain KCB90 is normally found in the rhizosphere and is associated with living or senescent plant tissues, like its cellulose-degrading relatives. Indeed, many members of the α-Proteobacteria, equivalent to De Ley’s rRNA superfamily IV, are associated with plants (15).
General conclusions.
In flooded anoxic rice paddy soil, cellulose represents one of the major polymeric inputs of carbon into the system and is thus an important substrate for the anaerobic microbial trophic web in this methanogenic soil. However, the number of culturable cellulolytic bacteria indicated that such organisms account for only a small percentage (0.06 to 1.1%) of the total microbial community. This may be due to the poor culturability of the bacteria, the presence of cellulolytic eukaryotes (3) as the major cellulolytic organisms, the presence of the organisms in the soil as microcolonies on cellulose-containing plant residues (resulting in underestimates of their numbers by MPN counting techniques), or truly low numbers. Use of different forms of cellulose did not result in greatly different culturable numbers but did yield strains belonging to very different phylogenetic groups. Using filter paper cellulose resulted in isolation of members of clostridial cluster III, while using amorphous and crystalline cellulose resulted in isolation of members of the class Actinobacteria, the division Verrucomicrobia, and the α-Proteobacteria. Thus, while we cannot comment on the true sizes of the populations in the cellulolytic bacterial community, it is clear that the phylogenetic diversity of cellulolytic bacteria extends to groups other than Clostridium spp. and their relatives.
The strains isolated in this study were generally able to grow on a wide range of sugars. Eight of the nine strains that originated from counting experiments which yielded culturable cell counts accounting for about 5% or more of the total microscopic counts fermented sugars to acetate and propionate (and some strains also produced succinate). Isolation of propionate-producing bacteria may be favored by the bicarbonate-containing growth medium used, since succinate- and propionate-producing pathways can utilize carboxylation reactions (20, 34) and thus may require a high pCO2. However, the reason for the dominance of propionate-forming bacteria over bacteria that ferment sugars via pathways leading to other products (e.g., lactate, butyrate, or ethanol) is not known. Propionate is known to be an intermediate in the rice paddy soil system which we studied (8, 29) and has been shown to be a significant precursor of methane (30). Our identification of isolates which indicate that the numerically dominant populations are propionate-producing bacterial populations highlights a limitation of the purely molecular approach of surveying 16S rRNA genes, which would not have identified this common feature.
Bacterial strains isolated by using liquid dilution series and thought to be representatives of large populations (accounting for >5% of the total microscopic cell counts) in the rice paddy soil belonged to six different lineages of bacterial descent. The members of the division Verrucomicrobia, the CFB division, clostridial cluster XIVa, clostridial cluster IX, and the genus Bacillus were in all cases most closely related to cloned 16S rRNA genes in the same rice paddy soil microcosm (27). Members of the class Actinobacteria are exceptions, as discussed above. We suggest that the close but not absolute correlations which we found indicate that multiple strains of closely related bacteria form dominant populations in the rice paddy soil. It seems unlikely that these organisms would be detected in both cultivation-based and cultivation-independent studies unless cells of a number of closely related strains were particularly abundant. Relationships between isolates and cloned 16S rRNA gene fragments that were less close were observed with strains originating from MPN counting series that indicated that the culturable populations were smaller (<1.2% of the total microscopic cell counts). One explanation for this is that at the dilution levels used, a larger number of different populations were present, and random selection of a small number of isolates and clones (relative to the total number of different 16S rRNA species present in the soil) did not allow us to obtain similar sequence types.
ACKNOWLEDGMENT
We thank Alexandra Schuhmann-Pidun for excellent technical assistance.
REFERENCES
- 1.Amann R I, Krumholz L, Stahl D A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken L R, Olsen R A. The relationship between cell size and viability of soil bacteria. Microb Ecol. 1987;13:103–114. doi: 10.1007/BF02011247. [DOI] [PubMed] [Google Scholar]
- 3.Bauchop T. Biology of gut anaerobic fungi. BioSystems. 1989;23:53–64. doi: 10.1016/0303-2647(89)90008-7. [DOI] [PubMed] [Google Scholar]
- 4.Beliaeff B, Mary J Y. The “most probable number” estimate and its confidence limits. Water Res. 1993;27:799–805. [Google Scholar]
- 5.Borneman J, Triplett E W. Molecular microbial diversity in soils from eastern Amazonia: evidence for unusual microorganisms and microbial population shifts associated with deforestation. Appl Environ Microbiol. 1997;63:2647–2653. doi: 10.1128/aem.63.7.2647-2653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borneman J, Skroch P W, O’Sullivan K M, Palus J A, Rumjamek N G, Jansen J L, Nienhuis J, Triplett E W. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl Environ Microbiol. 1996;62:1935–1943. doi: 10.1128/aem.62.6.1935-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Button D K, Schut F, Quang P, Martin R, Robertson B R. Viability and isolation of typical marine oligotrophic bacteria by dilution culture: theory, procedures, and initial results. Appl Environ Microbiol. 1993;59:881–891. doi: 10.1128/aem.59.3.881-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin K-J, Conrad R. Intermediary metabolism in methanogenic paddy soil and the influence of temperature. FEMS Microbiol Ecol. 1995;18:85–102. [Google Scholar]
- 9.Chin K-J, Rainey F A, Janssen P H, Conrad R. Methanogenic degradation of polysaccharides and characterization of polysaccharolytic clostridia from anoxic rice field soil. Syst Appl Microbiol. 1998;21:185–200. [Google Scholar]
- 10.Cochran W G. Estimation of bacterial densities by means of the “most probable number.”. Biometrics. 1950;6:105–116. [PubMed] [Google Scholar]
- 11.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 12.Conrad R. Mechanisms controlling methane emission from wetland rice fields. In: Oremland R S, editor. Biogeochemistry of global change: radiatively active trace gases. New York, N.Y: Chapman and Hall; 1993. pp. 317–355. [Google Scholar]
- 13.Coughlan M P, Mayer F. The cellulose-decomposing bacteria and their enzyme systems. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer Verlag; 1992. pp. 460–516. [Google Scholar]
- 14.Crutzen P J. On the role of CH4 in atmospheric chemistry: sources, sinks and possible reductions in anthropogenic sources. Ambio. 1995;24:52–55. [Google Scholar]
- 15.De Ley J. The proteobacteria: ribosomal RNA cistron similarities and bacterial taxonomy. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer Verlag; 1992. pp. 2111–2140. [Google Scholar]
- 16.DeLong E F, Wickham G S, Pace N R. Phylogenetic strains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 17.Flärdh K, Cohen P S, Kjelleberg S. Ribosomes exist in large excess over the apparent demand for protein synthesis during carbon starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1992;174:6780–6788. doi: 10.1128/jb.174.21.6780-6788.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenzel P, Rothfuss F, Conrad R. Oxygen profiles and methane turnover in a flooded rice microcosm. Biol Fert Soils. 1992;14:84–89. [Google Scholar]
- 19.Frenzel P, Bosse U, Janssen P H. Rice roots and methanogenesis in a paddy soil: ferric iron as an alternative electron acceptor in the rooted soil. Soil Biol Biochem. 1999;31:421–430. [Google Scholar]
- 20.Galivan J H, Allen S H G. Methylmalonyl coenzyme A decarboxylase. Its role in succinate decarboxylation by Micrococcus lactilyticus. J Biol Chem. 1968;243:1253–1261. [PubMed] [Google Scholar]
- 21.Großkopf R, Janssen P H, Liesack W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol. 1998;64:960–969. doi: 10.1128/aem.64.3.960-969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn D, Amann R I, Ludwig W, Akkermans P D L, Schleifer K-H. Detection of microorganisms in soil after in situ hybridization with rRNA-targeted, fluorescently labelled oligonucleotides. J Gen Microbiol. 1992;138:879–887. doi: 10.1099/00221287-138-5-879. [DOI] [PubMed] [Google Scholar]
- 23.Hansen M C, Tolker-Nielsen T, Givskov M, Molin S. Biased 16S rDNA PCR amplification caused by interference from DNA flanking the template region. FEMS Microbiol Ecol. 1998;26:141–149. [Google Scholar]
- 24.Hayashi S, Furusaka C. Studies on Propionibacterium isolated from paddy soils. Antonie Leeuwenhoek. 1979;45:565–574. doi: 10.1007/BF00403656. [DOI] [PubMed] [Google Scholar]
- 25.Hedlund B P, Gosink J J, Staley J T. Phylogeny of Prosthecobacter, the fusiform caulobacters: members of a recently discovered division of the Bacteria. Int J Syst Bacteriol. 1996;46:960–966. doi: 10.1099/00207713-46-4-960. [DOI] [PubMed] [Google Scholar]
- 26.Hedlund B P, Gosink J J, Staley J T. Verrucomicrobia div. nov., a new division of the Bacteria containing three new species of Prosthecobacter. Antonie Leeuwenhoek. 1997;72:29–38. doi: 10.1023/a:1000348616863. [DOI] [PubMed] [Google Scholar]
- 27.Hengstmann U, Chin K-J, Janssen P H, Liesack W. Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil. Appl Environ Microbiol. 1999;65:5050–5058. doi: 10.1128/aem.65.11.5050-5058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen P H, Schuhmann A, Mörschel E, Rainey F A. Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol. 1997;63:1382–1388. doi: 10.1128/aem.63.4.1382-1388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumböck M, Conrad R. Metabolism of position-labeled glucose in anoxic methanogenic paddy soil and lake sediment. FEMS Microbiol Ecol. 1991;85:247–256. [Google Scholar]
- 30.Krylova N I, Janssen P H, Conrad R. Turnover of propionate in methanogenic paddy soil. FEMS Microbiol Ecol. 1997;23:107–117. [Google Scholar]
- 31.Kuske C R, Barns S M, Busch J D. Diverse uncultivated bacterial groups from soils of the arid southwestern United States that are present in many geographic regions. Appl Environ Microbiol. 1997;63:3614–3621. doi: 10.1128/aem.63.9.3614-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liesack W, Janssen P H, Rainey F A, Ward-Rainey N L, Stackebrandt E. Microbial diversity in soil: the need for a combined approach using molecular and cultivation techniques. In: van Elsas J D, Trevors J T, Wellington E M H, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker; 1997. pp. 375–439. [Google Scholar]
- 34.Macy J M, Ljungdahl L G, Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978;134:84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVeigh H P, Munro J, Embley T M. Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J Ind Microbiol. 1996;17:197–204. [Google Scholar]
- 36.Minami K, Neue H-U. Rice paddies as a methane source. Clim Change. 1994;27:13–26. [Google Scholar]
- 37.Morales V, Martinez-Molina E, Hubbell D. Cellulase production by Rhizobium. Plant Soil. 1984;80:407–415. [Google Scholar]
- 38.Nelidov S N. Microbiology of the flooded soils of rice paddies. Eurasian Soil Sci. 1994;26(8):41–56. [Google Scholar]
- 39.Olsen G J, Woese C R, Overbeek R. The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol. 1994;176:1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsen R A, Bakken L R. Viability of soil bacteria: optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb Ecol. 1987;13:59–74. doi: 10.1007/BF02014963. [DOI] [PubMed] [Google Scholar]
- 41.Postgate J R, Hunter J R. Accelerated death of Aerobacter aerogenes starved in the presence of growth limiting substrates. J Gen Microbiol. 1964;34:459–473. doi: 10.1099/00221287-34-3-459. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhold-Hurek B, Hurek T, Claeyssens M, van Montagu M. Cloning, expression in Escherichia coli, and characterization of cellulolytic enzymes of Azoarcus sp., a root-invading diazotroph. J Bacteriol. 1993;175:7056–7065. doi: 10.1128/jb.175.21.7056-7065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosencrantz, D., and W. Liesack. Unpublished data.
- 45.Rosencrantz D, Rainey F A, Janssen P H. Culturable populations of Sporomusa spp. and Desulfovibrio spp. in the anoxic bulk soil of flooded rice microcosms. Appl Environ Microbiol. 1999;65:3526–3533. doi: 10.1128/aem.65.8.3526-3533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesner H. Verrucomicrobium spinosum gen. nov., sp. nov.: a fimbriated prosthecate bacterium. Syst Appl Microbiol. 1987;10:54–56. [Google Scholar]
- 47.Slepecky R A, Hemphill H E. The genus Bacillus—nonmedical. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer Verlag; 1992. pp. 1663–1696. [Google Scholar]
- 48.Stackebrandt E, Murray R G E, Trüper H G. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the “purple bacteria and their relatives.”. Int J Syst Bacteriol. 1988;38:321–325. [Google Scholar]
- 49.Stackebrandt E, Rainey F A, Ward-Rainey N L. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol. 1997;47:479–491. [Google Scholar]
- 50.Straskrabová V. The effect of substrate shock on populations of starving aquatic bacteria. J Appl Bacteriol. 1983;54:217–224. [Google Scholar]
- 51.von Wintzingerode F, Göbel U B, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 52.Wagner R. The regulation of ribosomal RNA synthesis and bacterial cell growth. Arch Microbiol. 1994;161:100–106. doi: 10.1007/BF00276469. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe I, Furusaka C. Microbial ecology of flooded rice soils. Adv Microb Ecol. 1980;4:125–168. [Google Scholar]
- 54.Wise M G, McArthur J V, Shimkets L J. Bacterial diversity of a Carolina bay as determined by 16S rRNA gene analysis: confirmation of novel taxa. Appl Environ Microbiol. 1997;63:1505–1514. doi: 10.1128/aem.63.4.1505-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarda B, Hahn D, Chatzinotas A, Schönhuber W, Neef A, Amann R I, Zeyer J. Analysis of bacterial community structure in bulk soil by in situ hybridization. Arch Microbiol. 1997;168:185–192. [Google Scholar]
- 56.Zwart G, Huismans R, van Agterveld M P, Van de Peer Y, De Rijk P, Eenhoorn H, Muyzer G, van Hannen E J, Gons H J, Laanbroek H J. Divergent members of the bacterial division Verrucomicrobiales in a temperate freshwater lake. FEMS Microbiol Ecol. 1998;25:159–169. [Google Scholar]