Abstract
Coronavirus disease 2019 (COVID-19) is an acute respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The prevention of SARS-CoV-2 transmission has become a global priority. Previously, we showed that a protein subunit vaccine that was developed based on the fusion of the SARS-CoV-2 receptor-binding domain (RBD) to the Fc portion of human IgG1 (RBD-Fc), produced in Nicotiana benthamiana, and adjuvanted with alum, namely, Baiya SARS-CoV-2 Vax 1, induced potent immunological responses in both mice and cynomolgus monkeys. Hence, this study evaluated the protective efficacy, safety, and toxicity of Baiya SARS-CoV-2 Vax 1 in K18-hACE2 mice, monkeys and Wistar rats. Two doses of vaccine were administered three weeks apart on Days 0 and 21. The administration of the vaccine to K18-hACE2 mice reduced viral loads in the lungs and brains of the vaccinated animals and protected the mice against challenge with SARS-CoV-2. In monkeys, the results of safety pharmacology tests, general clinical observations, and a core battery of studies of three vital systems, namely, the central nervous, cardiovascular, and respiratory systems, did not reveal any safety concerns. The toxicology study of the vaccine in rats showed no vaccine-related pathological changes, and all the animals remained healthy under the conditions of this study. Furthermore, the vaccine did not cause any abnormal toxicity in rats and was clinically tolerated even at the highest tested concentration. In addition, general health status, body temperature, local toxicity at the administration site, hematology, and blood chemistry parameters were also monitored. Overall, this work presents the results of the first systematic study of the safety profile of a plant-derived vaccine, Baiya SARS-CoV-2 Vax 1; this approach can be considered a viable strategy for the development of vaccines against COVID-19.
Keywords: COVID-19, SARS-CoV-2, Plant-produced subunit vaccine, Receptor binding domain, Protective immunity, Toxicology
1. Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory infection caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, initially referred to as nCoV-2019), which was first identified in Wuhan, Hubei Province, China, in December 2019. SARS-CoV-2 is the third pathogenic Betacoronavirus known to infect humans after Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV) [1], [2], [3], [4], [5]. Within three months of its outbreak, the World Health Organization (WHO) declared COVID-19 as a global pandemic in March 2020. COVID-19 has become a serious public health threat worldwide. SARS-CoV-2 is an enveloped virus consisting of a positive sense single-stranded RNA genome that belongs to the family Coronaviridae and subfamily Coronavirinae. The clinical symptoms of COVID-19 range from mild to severe and are characterized by fever, cough, fatigue, headache, sore throat, nausea, diarrhea pneumonia and multiorgan system failure [6], [7]. According to the WHO, a total of >485 million cases have been confirmed, and >6 million SARS-CoV-2 infection-related deaths have occurred in 220 countries as of April 2022 [8]. The high mortality rate and lack of an effective strategy for prevention or treatment highlight the importance and need for developing an effective vaccine to control viral spread.
In addition to the SARS-CoV-2 variant that was initially identified in Wuhan, the recent emergence of variants of concern (VOCs), such as Alpha, Beta, Gamma, Delta and Omicron [9], has had a catastrophic impact worldwide, and these VOCs have exhibited high transmissibility and mortality rates [10]. Even a relatively small number of mutations in VOCs promote immune escape and resistance to neutralizing antibodies [11], [12]. Earlier reports have demonstrated that sera from convalescent and vaccinated individuals cross-neutralize SARS-CoV-2 variants with slightly decreased potency [13], [14]. The recently emerged Omicron variant harbors a large number of mutations (>30) in the spike protein, which is the major target of neutralizing antibodies. Evidence suggested that a booster dose of a vaccine is crucial for providing protection against VOCs, including Omicron [15], [16]. Hence, booster doses have now been administered in several countries worldwide.
Vaccination is a vital approach for combatting the pandemic. The sudden onset and frequent emergence of SARS-CoV-2 variants have urged the global scientific community to develop crucial highly protective vaccines. An ideal vaccine candidate should be safe and effective in eliciting both humoral and cellular immune responses. Furthermore, the protective immune response induced by a vaccine should be maintained in order to prevent recurrent infection. Hence, long-lasting and broadly neutralizing antibody and T cell-mediated immune responses are critical for preventing and controlling the transmission of SARS-CoV-2 [17], [18].
Since the outbreak of COVID-19, several vaccine candidates have been developed with multiple platforms, including inactivated, viral vector, nucleic acid and virus-like particle vaccines; these vaccines are being examined and tested in various animal models. A number of vaccine candidates are currently in different stages of clinical trials, and several candidates have been approved in multiple countries [19]. Subunit vaccines based on the full-length Spike (S) protein or the receptor-binding domain (RBD) located in the S protein induce significant neutralizing antibody responses [20], [21]. Of note, anti-S or anti-RBD neutralizing antibodies have been associated with protection against coronavirus infection. Indeed, a recombinant subunit vaccine based on a dimeric form of the RBD (Anhui Zhifei Longcom) of SARS-CoV-2 was evaluated in a phase I/II trial in China, and this trial showed the vaccine to be well tolerated, immunogenic and efficacious in protecting against COVID-19 [22]. In addition, a subunit vaccine (NVX-CoV2373) developed by Novavax based on the full length S protein along with Matrix-M adjuvant induced a robust immune response and was found to be safe and effective in protecting against COVID-19 [23]. Furthermore, previous research on similar coronaviruses, such as SARS and MERS, also suggested that the S protein or RBD contains a critical neutralizing domain, emphasizing the importance of this region for subunit vaccine development. Nevertheless, proper adjuvants are crucial for boosting the immune response, as subunit vaccines tend to elicit poor immune responses [24], [25], [26].
In this context, an investigational subunit vaccine, Baiya SARS-CoV-2 Vax 1, which is based on the RBD of SARS-CoV-2, was produced as a fusion protein with the Fc region of human IgG1 (RBD-Fc) using N. benthamiana as an expression host. The expression level of the RBD-Fc fusion protein in plants was found to be 25 µg/g fresh weight. Furthermore, this plant-produced vaccine was effective in eliciting an immune response after two doses of intramuscular immunization on Days 0 and 21 (3-week interval) in Institute of Cancer Research (ICR) mice and cynomolgus monkeys (Macaca fascicularis) [27]. Hence, the present study examined the protective efficacy, preclinical safety and toxicity of this plant-produced subunit vaccine candidate, Baiya SARS-CoV-2 Vax 1, in K18-hACE2 mice, cynomolgus macaques and Wistar rats; this study was conducted with good laboratory practice in compliance with OECD principles (OECD-GLP). The potential effects of this vaccine candidate were addressed, and this study allows better risk assessment in early-stage clinical trials to evaluate the safety and efficacy of this vaccine in humans. Taken together, these nonclinical results are an important basis for a clinical trial of a plant-produced subunit vaccine, Baiya SARS-CoV-2 Vax 1.
2. Materials and methods
2.1. Ethical statement for laboratory animal care and use
The challenge study using K18–hACE2 mice was performed following the Institutional Animal Care and Use Committee (IACUC) protocol approved by the IACUC and Biosafety Review Committee at the Armed Forces Research Institute of Medical Sciences (AFRIMS), Thailand, which is an AAALAC International-accredited facility. The IACUC protocol number was PN21-04. The animal research was conducted in compliance with Thai laws, the Animal Welfare Act, and all applicable U.S. Department of Agriculture, Office of Laboratory Animal Welfare and U.S. Department of Defense guidelines.
The experiments involving cynomolgus monkeys were approved by the IACUC of the National Primate Research Center of Thailand-Chulalongkorn University (NPRCT-CU) (Protocol review no. 2075015). The animals were housed in individual cages and maintained in a temperature-controlled environment at 25 ± 2 °C with 60 ± 10% humidity and a 12 h:12 h light–dark cycle (lights on at 06:00 am). The animals were fed standard monkey chow (Perfect Companion Group Co., Ltd. Bangkok, Thailand) in the morning (09:00–10:00 am) and their diet was supplemented with fresh fruits in the afternoon (02:30–03:30 pm). The animals were provided with water ad libitum.
The experiments involving Wistar rats were approved by the Institutional Animal Care and Use Committee of the National Laboratory Animal Center Animal Care and Use Committee (NLAC-ACUC), Mahidol University; Thailand (Protocol review no. RA2020-36). Six-week-old pathogen-free Wistar rats (Rattus norvegicus) were maintained at 22 ± 3 °C with 30–70% relative humidity and a 12 h light–dark cycle. The animals were given a standard diet (Perfect Companion Group Co., Ltd. Bangkok, Thailand) and water ad libitum. The animals were monitored at least once a day.
Toxicology studies in Wistar rats and safety pharmacology studies in cynomolgus monkeys were performed in compliance with the OECD-GLP principles (GLP2020-25 and GLP-20-03, respectively). The protocol for the toxicology study in Wistar rats was designed in accordance with WHO Technical Report Series No. 927 and the WHO guidelines on the nonclinical evaluation of vaccines, and the protocol for the safety pharmacology studies in cynomolgus monkeys followed the ICH S7A: Safety Pharmacology Studies for Human Pharmaceuticals (ICH, 2000) published by the European Medicines Agency.
All the animal facilities are AAALAC International Accredited (AAALAC 1752 for NPRCT-CU). Thus, the experiments strictly adhered to the principles stated in the “Guide for the Care and Use of Laboratory Animals [28]”.
2.2. Adjuvants and excipients
Alhydrogel® adjuvant (2%; catalog: vac-alu-250, CAS number: 21645-51-2) containing an aqueous suspension of aluminum hydroxide gel (alum) was procured from InvivoGen, USA. Vaccine excipients sucrose (catalog: 107651, CAS number: 57-50-1) was purchased from Merck, Germany, and glycine (catalog: PR0608, CAS number: 56-40-6) was acquired from Vivantis Technologies, Malaysia.
2.3. Vaccine formulation
The subunit vaccine Baiya SARS-CoV-2 Vax 1 was developed based on the recombinant SARS-CoV-2 RBD-Fc protein produced in plants. Briefly, the RBD of SARS-CoV-2 was fused with the Fc region of human immunoglobulin G1 (IgG1), cloned into a plant expression geminiviral vector and expressed in N. benthamiana via., transient expression (agroinfiltration). The recombinant RBD-Fc protein was extracted from the plants and purified through affinity column chromatography using protein-A beads (Expedeon, Cambridge, United Kingdom) as described previously [27]. The candidate vaccine is formulated to the required doses with aluminum hydroxide as an adjuvant plus sucrose and glycine as excipients.
2.4. Challenge studies in K18-hACE2 mice
Female K18-hACE2 mice (6 weeks old) were purchased from the Jackson laboratory (Bar Harbor, ME, USA; stock No. 034860) and maintained in microisolator cages at the BSL-2 facility prior to SARS-CoV-2 challenge or at the BSL-3 facility after the challenge. The mice were given nestlets as part of their cage environment and ad libitum access to commercial pelleted diet and chlorinated water. Seventeen K18-hACE2 mice were randomly assigned to three groups (n = 5/group in the control group and n = 6/group in the challenged groups). The groups of mice were intramuscularly administered with two doses of 5 µg Baiya SARS-CoV-2 Vax 1 (n = 6), 10 µg Baiya SARS-CoV-2 Vax 1 (n = 6) or alum control (n = 5) at a three-week interval (Days 0 and 21) via., the quadriceps. The mice were bled on Days 0, 14, 21, and 35 prior to challenge. On Day 35, the mice were intranasally inoculated with 2 × 104 PFU of SARS-CoV-2 virus (50 µl), Wuhan lineage, isolate Hong Kong/VM20001061/2020, NR-52282 (stock titer of 1 × 107 PFU/ml). The mice were observed daily for clinical signs of disease, including changes in body weight, inappetence, and behaviors, and were humanely euthanized when they met a euthanasia criterion or at the end point (Day 41) by qualified technicians using CO2 inhalation, in accordance with institutional and AVMA guidelines. Blood and tissues were collected to determine virus titers in different tissues and for histopathology.
2.5. SARS-CoV-2 cytopathic effect (CPE)-based microneutralization assay
A microneutralization (MN) assay was used to measure levels of neutralizing (NT) antibodies against the SARS-CoV-2 Wuhan strain. All the procedures were performed in a BSL-3 laboratory at Armed Force Research Institute of Medical Sciences (AFRIMS) following a standard neutralization assay procedure and using a CPE-based colorimetric read-out and AFRIMS standard operating procedure as described elsewhere [29]. The MN50 titers were calculated as the reciprocal serum dilution that neutralized 50% of virus observed in virus control wells using probit analysis, SPSS program.
2.6. Cell line and SARS-CoV-2 virus
The Vero E6 green monkey kidney epithelial cell line was obtained from ATCC. The cells were grown in Eagle’s minimum essential medium (EMEM, Invitrogen, US) supplemented with 5% heat-inactivated fetal bovine serum (HIFBS, Invitrogen, USA), 1% L-glutamine, 1% P&S, 40 µg/ml gentamicin and 0.25 µg/ml fungizone at 35 ± 2 °C in a 5% CO2 incubator. The SARS-CoV-2 virus, Wuhan strain lineage, isolate Hong Kong/VM20001061/2020, NR-52282, was obtained through the Biodefense and Emerging Infections Research Resources Repository (BEI Resources, NIAID, USA), and its titers were quantitated in Vero E6 cells by calculating the tissue culture infectious dose 50 (TCID50) using the Reed-Muench method based on eight replicates per titration. Then, the virus was propagated to generate sufficient titers (100TCID50) for the microneutralization assay.
2.7. SARS-CoV-2 viral RNA extraction and quantitative RT–PCR
The SARS-CoV-2 viral loads in serum and tissue samples were measured using quantitative RT–PCR. Viral RNA was extracted from serum and tissue samples using the QIAamp viral RNA mini kit (QIAGEN, Germany) and RNeasy Mini Kit (QIAGEN, Germany), respectively, by following the manufacturer’s instructions. A total volume of 50 µl of viral RNA was obtained from each sample. Five microliters of each RNA sample was used for quantitative RT–PCR, which was performed using the CDC procedure [30] and in vitro SARS-CoV-2 RNA transcripts (IVTs). Three controls, including a no-template control (NTC), a negative extraction control (NEC) and a positive extraction control (PEC), were run along with the test samples in each experiment. The number of copies of viral RNA per sample was determined based on standard curves of serial dilutions of IVTs (5, 50, 5x102, 5x103, 5x104, 5x105 RNA copy number or genomic equivalent (GE)/reaction). The GE per ml of virus in a serum sample was calculated by multiplying the number of copies/reaction by 10,000 and by dividing the volume of a serum sample used for extraction (µl). The viral GE per gram of tissue sample was calculated by multiplying the number of copies/reaction by 10,000 and by dividing the weight of a tissue sample used for extraction (mg).
2.8. Histopathological analysis
The mice were euthanized and necropsied in a BSL-3 laboratory. Major organs, including the lungs, lymphoid tissues (lymph nodes, spleen and thymus), brains, nasal turbinates and adrenal glands, were collected for histological observation. All the tissues were immediately fixed in 10% neutral buffered formalin, paraffin embedded and sectioned at a thickness of 4 µm. Then, the tissue sections were deparaffinized and rehydrated through xylene substitutes and a series of isopropanol solutions. To identify histopathological changes, the tissue sections were stained with hematoxylin-eosin and analyzed by a board-certified veterinary pathologist. All the slides were initially reviewed in a blinded manner. All lesions were identified and scored from 0 to 4 according to severity (minimal to severe).
2.9. In situ hybridization
To detect SARS-CoV-2 RNA in mouse tissues, formalin-fixed, paraffin-embedded (FFPE) lung and nasal cavity tissues were analyzed using an RNAscope® in situ hybridization (ISH) assay. A SARS-CoV-2 probe (RNAscope® Probe, V-nCoV2019-S) developed by a commercial company (Advanced Cell Diagnostics ACD, Newark, CA (ACD, 848561)) was used. The probe is specific for the SARS-CoV-2 S gene that encodes the spike protein based on the complete genome of the Wuhan seafood market pneumonia virus isolate Wuhan-Hu-1, GenBank accession number NC_045512.2 (targeting region 21631-23303). The RNAscope® ISH assay was performed according to the RNAscope 2.5 HD Red Detection Kit (ACD, 322360). The FFPE tissue slides were deparaffinized with Xylene substitute (Thermo Scientific, USA), rehydrated through graded concentrations of ethanol in water, and treated with hydrogen peroxide (10 min at room temperature) followed by antigen retrieval in 1X target retrieval solution in a steamer at 99 °C for 15 min. The slides were then incubated with protease plus for 30 min at 40 °C in a HybEZ™ oven (ACD) and subsequently incubated with the SARS-CoV-2 specific probe for 2 h at 40 °C in the HybEZ™ oven. The signal was amplified using a specific set of amplifiers (AMP1-6) as recommended by the manufacturer, and the amplified signal was visualized by incubation with Fast Red solution for 5 min at room temperature. The slides were counterstained with 50% Gill hematoxylin III (Sigma Aldrich, St Louis, USA) for 2 min and extensively washed with tap water. The slides were dehydrated in a 60 °C dry oven until completely dry and then immersed in xylene (JT Baker, Avantor, USA) before mounting with mounting medium. The slides were scored from 1 to 5 based on the number of positive cells (minimal to severe).
2.10. Safety pharmacology and immunogenicity studies in cynomolgus macaques
Ten juvenile/subadult female monkeys were randomly assigned to 2 groups (n = 5 monkeys in each group). The first group was intramuscularly injected with 0.5 ml of 0.5 mg Alhydrogel adjuvant containing excipients, and the second group received 10 µg of Baiya SARS-CoV-2 Vax 1 mixed with 0.5 mg Alhydrogel adjuvant containing excipients. The immunizations were administered on Days 0 and 21 by intramuscular injection into the quadriceps femoris muscle. The local toxicity at the injection site was monitored by assessing erythema and edema at 1, 4, 24, 48 and 72 h after immunization. The grading scales for local reactions followed the OECD Test guideline 404: 0, None/absent; 1, very slight; 2, slight; 3, moderate; 4, severe.
The animals’ health, behaviors (aggression, stereotypies, and alertness deficit), appetite and excretion (diarrhea) were monitored and recorded daily without anesthetization. Blood samples were collected from the femoral vein from each monkey prior to the first immunization (Day 0) and 14 days after immunization (Days 14 and 35), chilled on ice and processed for hematological and blood biochemistry and immunogenicity analyses. Their body weights, rectal temperatures and the functions of three vital organ systems, namely, the respiratory, cardiovascular and central nervous systems, were assessed on Days 0, 14 and 35 before blood collection.
2.11. Hematological and blood biochemical evaluations in cynomolgus monkeys
A 0.5 ml blood sample was transferred into an EDTA tube, mixed well and assessed to quantify the levels of red blood cells (RBCs), white blood cells (WBCs), hemoglobin (HGB), hematocrit (HCT), platelets (PLTs), plateletcrit (PCT), nucleated red blood cells (NRBCs), mean platelet volume (MPV), neutrophils (NEUTs), lymphocytes (LYMPs), monocytes (MONOs), eosinophils (EOs), and basophils (BASOs). The hematological assays were performed using an Automated Hematology Analyzer (Sysmex, Japan).
A 1.0 ml blood sample was transferred into a heparinized tube. The blood plasma was separated at 4 °C and 1,000xg and assayed to quantify the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), alkaline phosphatase (ALP), total bilirubin (TBIL), cholesterol (CHOL), glucose (GLU), triglycerides (TRIG), uric acid (UA), globulin (GLOBU) and creatinine phosphate (CREA-P) using a Clinical Chemistry Analyzer (Sysmex, Japan).
2.12. Physiological functions of three vital organ systems in cynomolgus monkeys
The physiological functions of three vital organ systems, namely, the central nervous system (CNS), cardiovascular system (CVS) and respiratory system (RS), were evaluated in a safety pharmacology study after Baiya SARS-CoV-2 Vax 1 immunization. The safety pharmacology endpoints of the CNS monitored in this study were motor activity (paresis and posture), sensory function (visual field and auditory response), and sensory/motor reflex response (pinch test). The endpoints of the CVS included heart rate, systolic and diastolic blood pressure, mean arterial blood pressure and electrocardiogram (ECG). RS endpoints were oxygen saturation (SpO2), respiratory rate and lung sound. The physiological functions of the CNS, CVS, and RS were diagnosed, scored, and recorded by a veterinarian based on the standard operating procedure of the NPRCT-CU following approved guidelines.
2.13. Immunological analysis in cynomolgus monkeys: SARS-CoV-2 RBD-Fc-specific antibodies
SARS-CoV-2 RBD-specific antibody titers in serum samples were measured by ELISA. Briefly, 2 µg of the SARS-CoV-2 spike protein RBD expressed in Sf9 insect cells (GenScript, USA) was used to coat 96-well microtiter plates (Greiner Bio-One GmbH, Germany) and incubated at 4 °C overnight. After blocking with 5% w/v skim milk (BD Difco, USA) in 1x PBS for 2 h at 37 °C, the plates were washed three times with 0.05% PBST (Tween 20 in PBS). Sera from immunized monkeys were two-fold serially diluted in 1xPBS, added to the plates and incubated for 2 h at 37 °C. The plates were again washed with 0.05% PBST thrice and then incubated with goat anti-monkey IgG HRP conjugated antibodies (Abcam, UK) at a dilution of 1:2000 for 1 h at 37 °C. The binding of the secondary antibodies was visualized by adding TMB substrate solution (SurModics, USA). The enzymatic reaction was terminated by adding 1 M H2SO4. The absorbance at 450 nm (A450) was read on a microplate reader (BMG Labtech, Germany). The endpoint titer was determined by a previously described method [31].
2.14. Immunological analysis in cynomolgus monkeys: SARS-CoV-2 microneutralization assay
A microneutralization (MN) assay was performed in a certified biosafety level (BSL) 3 facility at the Department of Microbiology, Faculty of Science, Mahidol University, Thailand, as described previously [27]. The experimental protocol was approved by Mahidol University, and all the experiments were performed by following standard protocols approved by the institutional review committee.
2.15. Toxicity studies in Wistar rats
A total of 120 rats (60 males and 60 females) were randomly divided into different groups, and each group included an equal number of males and females. The animals were assigned to the inactive control group, active control group, and three main groups (low, medium and high), each of which included 10 animals per sex, and two recovery groups (vehicle control and high dose group), each which included 5 animals per sex. The rats were injected intramuscularly according to the intended dosing schedule, namely, on Days 0 and 21 (3-week interval), with 0.1 ml of a low dose (25 µg), medium dose (50 µg) or high dose (100 µg) of Baiya SARS-CoV-2 Vax 1; the inactive control group was administered phosphate-buffered saline (PBS), and the active control group was administered PBS with alum-containing adjuvants to ensure that there were no negative effects associated with the adjuvant and excipients. The animals were sacrificed after a recovery period of 3 days or 14 days (recovery groups alone) after the second injection. The animals were euthanized using CO2 inhalation. To analyze the immune response in the rats (Day 24 for main study and Day 35 for recovery), the same protocol was followed as described above, except that goat anti-rat IgG HRP conjugate antibodies (Abcam, UK) were used for detection.
2.16. Clinical observations in rats
The overall health status of the animals was monitored throughout the course of the study. Body weight, food and water consumption and results of ophthalmological exams were monitored before immunization (pre-dose), daily during the first week of administration and once a week thereafter. The animals were observed at least once a day for clinical signs and twice per day for evidence of mortality and moribundity. The immunization sites were examined to assess systemic adverse effects at 4, 24, 48 and 72 h after each injection by monitoring the immunization sites for signs of erythema and edema. The effect of the vaccine on rat muscular strength was determined by the grip strength test, and the maximal grip strength value (in newtons (N)) of the animal and motor activity (number of steps) were recorded. The tests were performed on all the animals at least once a week.
2.17. Hematological and serum biochemistry parameters of rats
Hematological parameters, such as RBC and WBC counts, hemoglobin levels, were measured with an automated analyzer Procyte Dx (IDEXX Laboratories, Westbrook, MA, USA). Routine clinical biochemical parameters, including sodium, potassium, chlorine, glucose, cholesterol, triglycerides, uric acid, blood urea nitrogen, creatinine, total protein, albumin, globulin, low-density lipoprotein, high-density lipoprotein, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase levels, were measured on a Cobas c311 automated blood analyzer (Roche, Switzerland).
2.18. Gross pathology, organ weight and histopathology of rats
The animals were euthanized using CO2 inhalation. For gross pathology, the external surface of the body, injection site and all the internal organs in the abdominal and thoracic cavities were grossly examined. The selected organs were weighed (paired organs were weighed separately) at the time of scheduled necropsy, and the organ weights were converted to relative organ weights based on the organ-to-body weight ratio. The following organs were weighed: liver, kidney, heart, lung, spleen, brain, adrenals, testes, epididymides, ovaries, uterus, thymus, small intestine, large intestine, thyroid, eyes, urinary bladder, and lymph nodes. Histopathological examination was performed on selected organs that were harvested from all the animals. For histopathological analysis, tissues were embedded in paraffin wax, and 4- to 5-μm-thick sections were generated, and the sections were stained with hematoxylin and eosin.
2.19. Statistical analysis
The results of the challenge study in hACE2 mice and safety pharmacology study in monkeys were statistically analyzed with GraphPad Prism 9.0 (GraphPad Software Inc., USA). The differences were considered statistically significant at p value < 0.05 (*: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001). In the challenge study, the percent weight change and MN50 titer results of the control group were compared with those of the vaccinated groups, and the differences were analyzed by two-way analysis of variance (ANOVA) and Dunnett and Tukey multiple comparisons tests, respectively. The viral load in the challenged mice was compared with that in the control mice, and the differences were statistically analyzed by one-way ANOVA and Dunnett’s multiple comparisons test. Differences in the immunogenicity data and total IgG and MN50 titers in monkeys were statistically analyzed by two-way ANOVA and the Šídák test. The hematological and blood chemical parameters and cardiovascular and respiratory system observations were compared with the values on Day 0, and differences were analyzed by Dunnett’s multiple comparisons test. In the toxicity study, statistical analyses were performed with SPSS® Statistics software 18.0.0. Differences were considered significant at the 0.05 level, p < 0.05. The obtained data were statistically analyzed by Kolmogorov–Smirnova and Levene’s tests, which were used to compare the inactive control (PBS) group and treatment groups and the inactive control-recovery (Re-PBS) group and high dose-recovery (Re-100 μg) group.
3. Results
3.1. Baiya SARS-CoV-2 Vax 1 protects K18-hACE2 mice against lethal viral challenge
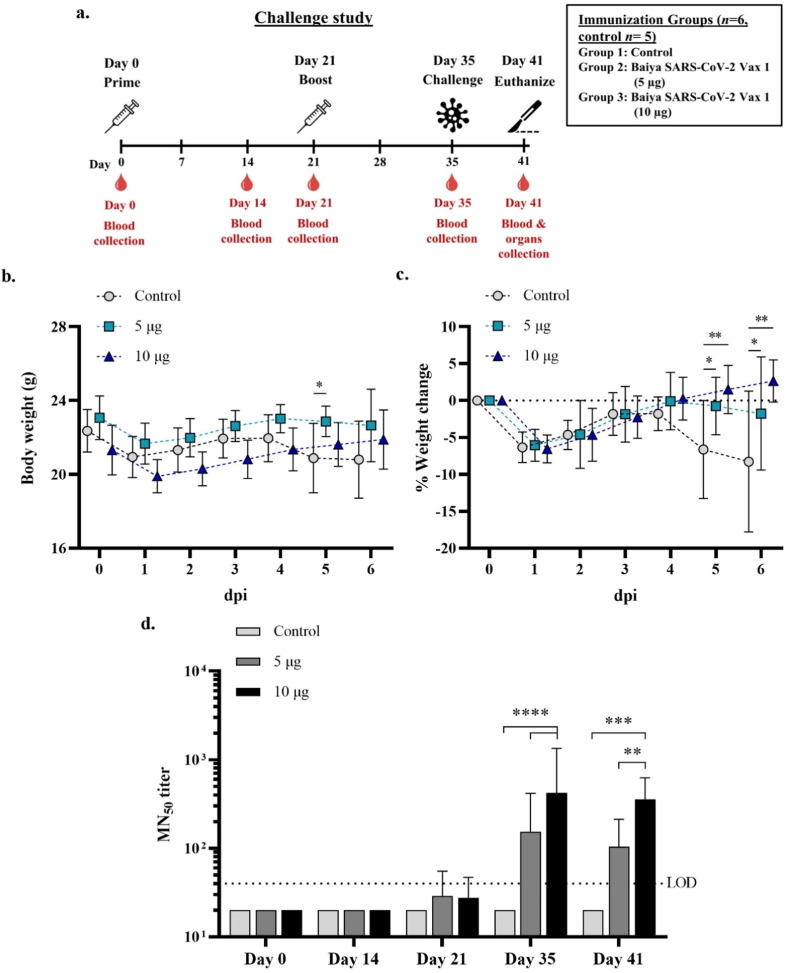
To evaluate the protective efficacy of Baiya SARS-CoV-2 Vax 1 in K18-hACE2 mice, mice were immunized with 5 and 10 µg of vaccine and challenged with SARS-CoV-2 virus (Wuhan lineage) two weeks after the second immunization (Fig. 1 a). Five days post-infection (dpi), 2 out of 5 control mice abruptly experienced the onset of symptoms, including anorexia, lethargy, rough fur, immobility, and labored respiration; they were euthanized on Day 5. The rest of the control mice succumbed to infection at 6 dpi. No mortality was observed in either group of immunized mice. On 6 dpi, 2 out of 6 immunized mice in Group 2 (5 µg of Baiya SARS-CoV-2 Vax 1) exhibited anorexia and lethargy with mildly rough fur (Supplementary Table S1). Changes in body weight were monitored daily, and a significant percentage of weight change among the groups was observed at 5 and 6 dpi (Fig. 1b and c). All the mice lost body weight on Day 1 after infection. The mice immunized with 10 µg of Baiya SARS-CoV-2 Vax 1 gained more weight over time until the end of the study; however, the mice in Groups 1 and 2 started to lose body weight at 5 dpi.
Fig. 1.
Schematic representation of immunization and blood collection schedule in the hACE2 mouse challenge study. Mice were divided into 3 groups (n = 6, control n = 5), i.e., the 5 µg Baiya SARS-CoV-2 Vax 1, 10 µg Baiya SARS-CoV-2 Vax 1 and control groups. The mice were immunized on Day 0 and 21 and bled on Day 0, 14, 21, 35 and 41. The mice were intranasally challenged with SARS-CoV-2 virus on Day 35 and were euthanized on Day 41 (6-day post-infection, dpi). Weight loss was monitored (a). Change in body weight after challenge (b) and percentage weight change (c) from 0 to 6 dpi in SARS-CoV-2 challenged hACE2 mice (n = 6, control n = 5). The data are shown as the mean ± SD. Two-way ANOVA, Dunnett test, was used (*: p < 0.05, **: p < 0.01). 50% microneutralizing (MN50) titers in immunized mice (d). The data are presented as GMT ± 95% CI of the endpoint titer in each group (n = 6, control n = 5). Values smaller than the limit of detection (LOD) are plotted as LOD. Two-way ANOVA, Tukey test, was used. (**: p < 0.01, ***: p < 0.001, ****: p < 0.001). n, number of animals per group.
3.2. Baiya SARS-CoV-2 Vax 1 induced a neutralizing antibody response in K18-hACE2 mice
Sera collected prior to/after immunization and virus challenge were used to measure the level of SARS-CoV-2 neutralizing antibodies (MN50 titer). The overall SARS-CoV-2-neutralizing activity of sera from vaccine-immunized mice was significantly different compared to that of the sera from control mice. Neutralizing antibodies were undetected in all the control mice, but 33.33% (n = 2/6) of the mice in each group that were immunized with 5 and 10 µg Baiya SARS-CoV-2 Vax 1 showed detectable neutralizing antibody levels beginning on Day 21 after the first immunization; the values were geometric mean titer (GMT) = 29 and 28 for the mice immunized with 5 and 10 µg Baiya vaccine, respectively. At Day 35 (7 days after the second immunization), the NT antibody levels peaked at GMT = 153 and 419 for the mice immunized with 5 and 10 µg Baiya SARS-CoV-2 Vax 1, respectively. Detectable levels of neutralizing antibodies were continuously observed until Day 41 (6 days post-inoculation); the values were GMT = 104 and 358 for the mice immunized with low and high doses of vaccine, respectively (Fig. 1d).
3.3. SARS-CoV-2 viral RNA levels in Baiya SARS-CoV-2 Vax 1 immunized mice
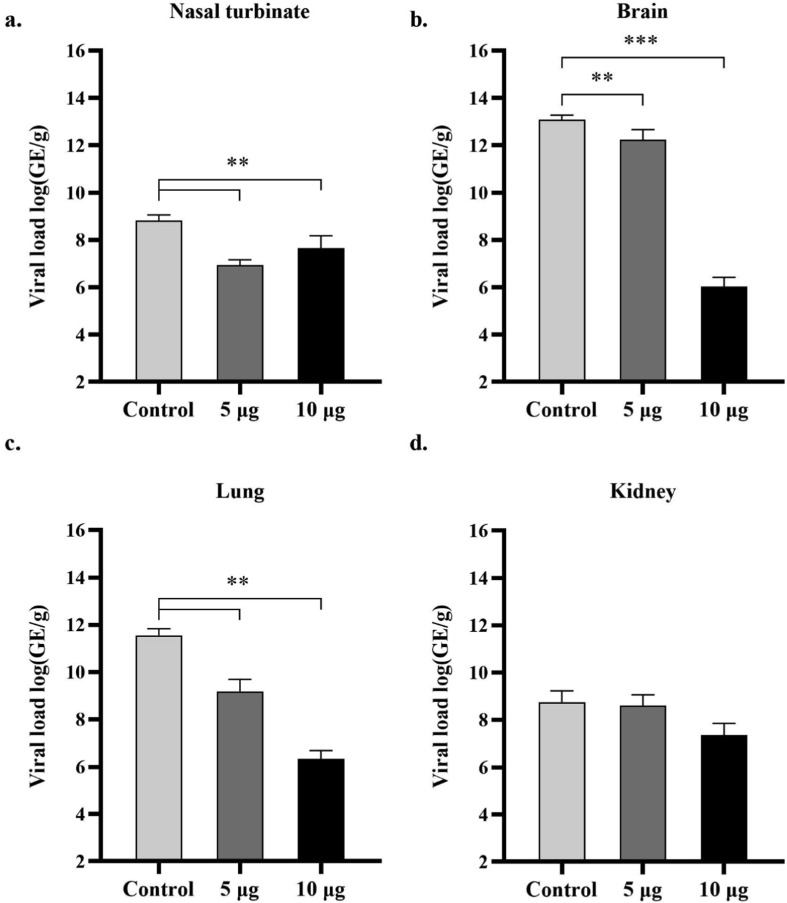
At 6 dpi, SARS-CoV-2 viral RNA was not detectable in the serum collected from the mice in either the low- or high-dose vaccine groups. However, 80% (n = 4/5) of the mice in the control group had detectable viremia. All the mice had detectable SARS-CoV-2 RNA levels in their nasal turbinate, brain, lung and kidney tissues (Fig. 2 ). The levels of viral RNA detected in the nasal turbinate, brain and lung tissues of vaccine-immunized mice were significantly lower than those in the tissues of control mice. Nevertheless, a partial reduction of the viral load in kidney tissues from the vaccine-immunized mice was observed. A dose-dependent effect of Baiya SARS-CoV-2 Vax 1 on reducing SARS-CoV-2 RNA levels was observed in brain and lung tissues.
Fig. 2.
Viral load in the indicated tissues (nasal turbinate, brain, lung and kidney) from mice vaccinated with Baiya SARS-CoV-2 Vax 1 and alum control at the termination end point of the study as assessed by qRT–PCR assay: nasal turbinate (a), brain (b), lung (c) and kidney (d) (n = 6, control n = 5). The data are expressed as the mean ± SD. One-way ANOVA and Dunnett’s test were used (**: p < 0.01; ***: p < 0.001). GE: Genomic equivalents. n, number of animals per group.
3.4. Baiya SARS-CoV-2 Vax 1 reduces SARS-CoV-2 induced pathology in mice
The histopathology studies related to SARS-CoV-2 infection were limited to the lung, brain and meninges, liver, adrenal gland, nasal turbinates and lymphoid tissues (Supplementary Fig. S1 and Supplementary Table S2). The control animals exhibited the highest number and most significant lesions, followed by the animals by 5 µg vaccinated animals. The animals vaccinated with 10 µg of Baiya SARS-CoV-2 Vax 1 were protected from significant histopathological changes, the disease-related findings were present only in the lung and nasal turbinates.
Evidence of systemic disease in this study includes pulmonary inflammation and vasculitis, adrenal necrosis, meningitis and encephalitis. The nasal epithelium, which was the site of challenge, was only impacted at the day of necropsy by the accumulation of fibrinous material. The lungs exhibited minimal or no inflammation in most of the cases. All control animals had at least minimal alveolar inflammation. Lung inflammation generally included mixed cell types, mostly including lymphocytes and plasma cells with fewer neutrophils, and centers on vessels and bronchioles; however, in the control group, inflammation was more diffusely spread throughout the alveoli. In the two outliers, where pulmonary pathology was more prominent, additional findings of vasculitis and type II pneumocyte hyperplasia were present along with the increased severity of inflammation. Lymphocyte apoptosis was observed in all the control group animals and included lymphoid atrophy in the thymus in four animals. Adrenal necrosis was only observed in the control animals.
The 10 µg vaccinated animals were protected from most pathological findings or less severe if present compared to the other groups. Of the six animals in this group, two had minimal to mild lung inflammation; one of these animals had focal vasculitis in the lung, and four had residual fibrinous material in their nasal turbinates. There was no evidence of epithelial damage to the airways in this group and no evidence of significant lymphocyte apoptosis or meningitis/encephalitis.
In situ hybridization was performed to measure viral RNA levels in the lungs and nasal turbinates. The lungs in the control group animals were positive (5/5), and one animal in 5 µg vaccinated group was positive. Positive staining was observed in the alveolar epithelium and alveolar macrophages and was regional; that is, all cells in a focal area were positive, and adjacent areas were completely negative. The nasal turbinates of the vaccine treated animals were negative, and only two minimally positive samples were observed in the control group. In those cases, individual nasal epithelial cells showed positive staining but retained normal architecture.
3.5. Baiya SARS-CoV-2 Vax 1 is safe in cynomolgus monkeys
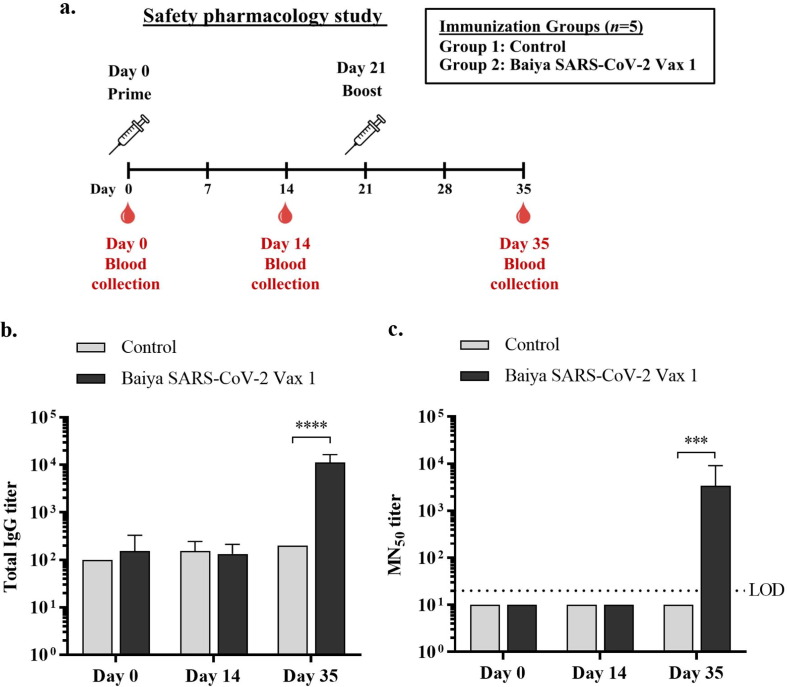
Safety pharmacology studies on Baiya SARS-CoV-2 Vax 1 were performed in cynomolgus monkeys administered two dose regimens on Days 0 and 21 (Fig. 3 a). The monkeys intramuscularly injected with 10 µg of Baiya SARS-CoV-2 Vax 1 were found to be healthy. There were no vaccine-related effects in terms of aggression, stereotypies, alertness deficit behaviors, food and water consumption, or diarrhea throughout the study. The average body weight and rectal temperatures (Supplementary Table S3) of the immunized animals were not significantly different from those of the control animals. No local toxicity at the immunization sites (erythema and edema) was observed after intramuscular injection on Days 0 and 21 (Supplementary Table S4).
Fig. 3.
Experimental design and timeline of vaccine immunizations, blood collection and safety pharmacology study in cynomolgus monkeys. Monkeys were divided into 2 groups (n = 5): the control and vaccine groups. The monkeys were immunized intramuscularly on Days 0 and 21, and blood was collected on Days 0, 14 and 35 (14 days after immunization) (a). Anti-RBD antibody responses were elicited by Baiya SARS-CoV-2 Vax 1 immunization. The total IgG levels in the immunized monkeys were measured by ELISA. The humoral immune responses (b and c) were analyzed in sera collected from immunized animals at different time points (Days 0, 14 and 35). The data are presented as geometric means with 95% confidence intervals. Two-way ANOVA and the Šídák test were used (****: p < 0.0001) (b). SARS-CoV-2 specific microneutralization (MN50) titers. The data are presented as geometric means with 95% confidence intervals. Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD. Two-way ANOVA and the Šídák test were used (***: p < 0.001) (c). n, number of animals per group.
Hematological and blood biochemical analyses were performed on Days 0, 14 and 35, and the results were within the normal range. No vaccine-related findings were observed in the hematological and plasma biochemistry data. No significant changes in the hematological values were observed, except that eosinophil (EO) numbers were significantly increased 14 days after Baiya SARS-CoV-2 Vax 1 vaccine boosting (Day 35). However, significant decrease in the levels of RBCs, WBCs, HGB, HCT, NEUTs and MONOs were detected after alum injection (control group). In addition, there were a few notable decrease in the levels of TP and BUN in the vaccine-treated group on Day 14, but these decreases were recovered later (Supplementary Table S5). However, these changes might have occurred due to the biological variability of the animals rather than due to the vaccine itself because fluctuations in the ALT and BUN levels at Day 14 and CREA-P levels at Day 35 were also observed in the control group. In addition, the values were maintained within the normal ranges of healthy animals.
Administration of Baiya SARS-CoV-2 Vax 1 did not cause effects on any parameters related to the CVS, CNS, and RS in cynomolgus monkeys (Supplementary Tables S6 and S7), except that the heart rates on Days 14 and 35 were higher than those on Day 0; however, the heart rate fluctuated within the normal range of healthy cynomolgus monkeys in the NPRCT-CU (81-142 bpm). Thus, these statistically significant differences were considered not to be associated with the vaccine. Overall, the results confirmed that there was no apparent safety concern for the use of this vaccine at a dose of 10 µg.
3.6. Vaccine elicited RBD-specific antibody responses in cynomolgus monkeys
In cynomolgus monkeys, the titers of antigen-specific (total IgG) antibodies were markedly increased 14 days after the second immunization (Day 35) with 10 µg of Baiya SARS-CoV-2 Vax 1 compared with those in the control group and those in the vaccine group on Day 0 and Day 14. This finding indicates that the Baiya SARS-CoV-2 Vax 1 vaccine adjuvanted with alum-containing excipients induced the production of SARS-CoV-2 RBD-specific IgG antibodies in monkeys after a two-dose immunization (Fig. 3b).
3.7. Neutralizing antibody responses after vaccine administration in cynomolgus monkeys
Similar to the SARS-CoV-2 RBD-specific IgG antibody response, the neutralizing antibody levels were significantly increased at Day 35 (14 days after the second immunization), with GMT = 3880 (Fig. 3c). No change in SARS-CoV-2-neutralizing activity was detected on Day 14 (14 days after the first immunization with 10 µg of Baiya SARS-CoV-2 Vax 1). As expected, in the control group, the SARS-CoV-2 neutralizing activity was maintained at low levels throughout the study period.
3.8. Baiya SARS-CoV-2 Vax 1 is well tolerated in Wistar rats
3.8.1. Clinical follow-up
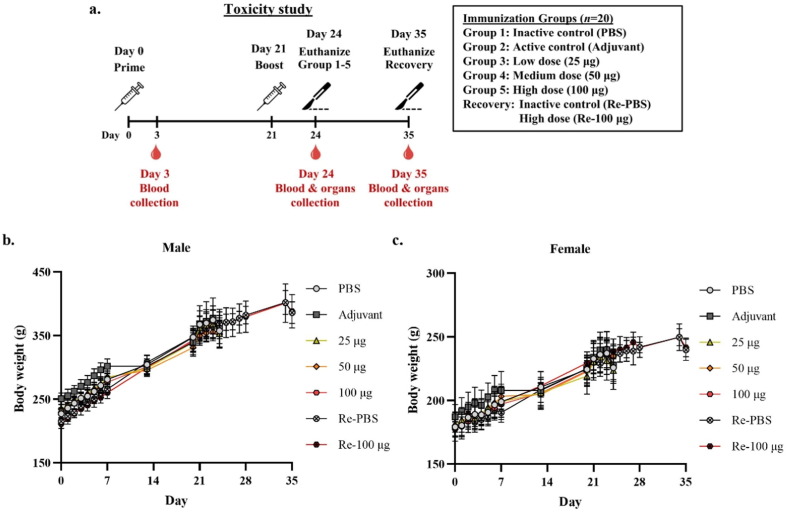
The potential toxicity of the plant-produced vaccine candidate Baiya SARS-CoV-2 Vax 1 was assessed in Wistar rats (Fig. 4 a). The animals were intramuscularly injected with low, medium and high doses of the vaccine and observed to monitor toxic effects related to the vaccine. The results showed that there was no obvious systemic toxicity in any of the animals administered 25 µg, 50 µg and 100 µg of Baiya SARS-CoV-2 during the immunization period or at the end of the 2-week recovery period under the conditions of this study. No abnormal changes were observed by clinical observation throughout the study period. The average animal body weights of the immunized rats are shown in Fig. 4b and c. The results of feed and water consumption are shown in the supplementary file (Figs. S2 and S3). No mortality or moribundity was reported (Supplementary Table S8). None of the animals exhibited adverse effects after vaccine administration. There were no clinical (cage side) or physical health examination findings (Supplementary Table S9). Observation of local tolerance at 2, 24, 48 and 72 h revealed no erythema, edema or inflammatory response at the administration site. The local tolerance scores are shown in Supplementary Table S10. Minor changes in motor activity (as measured by number of steps and forelimb and hindlimb grip strength) were not dose-dependent (Supplementary Tables S11, S12 and S13). There were no ophthalmological findings in the vaccine-treated group. Furthermore, the immune responses following the primary and booster vaccinations with Baiya SARS-CoV-2 Vax 1 in the rats were evaluated, and the results are shown in Supplementary Fig. S4.
Fig. 4.
Schematic representation of the immunization, blood collection and organ collection schedule for the toxicity study in Wistar rats. Rats were divided into 6 groups (n = 20), i.e., low, medium and high doses of Baiya SARS-CoV-2 Vax 1, inactive control (PBS alone), active control (PBS with adjuvant), and recovery (Re) groups. The rats were immunized on Days 0 and 21. The rats in Groups 1–5 were euthanized on Day 24. In the recovery group, the rats were euthanized on Day 35 (a). Changes in body weights of male (b) and female (c) Wistar rats at each time point are presented. The data are plotted as the mean ± SD.
3.8.2. Hematological and biochemical analyses
The rats in the immunized group showed significant differences in some of the hematological and serum biochemical parameters. At termination, there was a significant difference in the hematological parameters, such as increase in the levels of PLT, PCT, WBC, MONO, and WBC and decrease in the levels of RET, RET-He, LYMPH, and BASO in males; however, in females, increase in the levels of WBC and MONO and decrease in the levels of RBC, HGB, HCT, RET-He, and PLT were observed compared to the control group (Supplementary Table S14).
The following serum biochemical measurement results showed significant changes in the immunized groups compared to the control group: the sodium, TRIGL, UA2, and GLO levels were increased, while the SGLU3, U-BUN, ALB2, ALTL, and ALP2S levels were decreased in males; however, in females, the potassium, CREA2, GLO, ASTL, and ALP2S levels were increased and the SGLU3, UA2, U-BUN, and ALB2 levels were decreased (Supplementary Table S15).
No treatment-related effects on the hematology values or clinical chemistries were observed in either sex of the treated animals. Although statistically significant differences were observed in some of the values, the mean remained within the normal range for the species.
3.8.3. Organ weights, gross pathology and histopathological analysis
A marked increase or decrease in organ body weight was observed in the vaccine-treated animals compared to the control animals. The average animal organ weights per 100 g body weight are shown in Supplementary Table S16.
In males, significant increases in the weights of the left and right epididymides, left and right popliteal lymph nodes, thymus, right thyroid and parathyroid glands, right kidney, right and left eyes, spleen, and small intestine were observed in the immunized group compared to the control group. In females, the average weights of the left and right ovaries and oviduct, left popliteal lymph node, liver, small intestine, and spleen were significantly higher in the immunized group than in the control group. Increases in the organ weights of secondary lymphoid organs, such as lymph nodes and spleen, were observed in both males and females, and this result can be interpreted as an indication of immunological reaction after the administration of Baiya SARS-CoV-2 Vax 1 [32]. The organ weights of both sexes returned to normal after the recovery period. The changes in other organ weights were transient and not related to the vaccine, as they were not correlated with sex of the animals [33].
Gross necropsy findings showed white multifocal nodules at the site of intramuscular injection in all the animals administered either the control containing PBS with excipients/alum or the Baiya SARS-CoV-2 Vax 1 vaccine. As this was not observed after injection with PBS alone, the nodule appeared to be associated with the vehicle formulation and persisted in all the animals following a 2-week reversal phase. The nodules at the injection site were accompanied by histopathological observations at the injection site of granulomatous inflammation in skeletal muscle and the presence of neutrophils, lymphocytes and macrophages, myofiber degeneration and muscle necrosis.
Swelling in the left popliteal lymph node was observed in 3/20 (combined sex) animals administered the low dose, 3/20 animals administered the medium dose, and 5/20 animals administered the high dose and in 3/10 animals in the high dose-recovery group after a 2-week recovery period. Histopathological examination revealed diffuse lymphocyte hyperplasia in the left popliteal lymph node in 13/20 (combined sex) animals administered the low dose, 11/20 animals administered the medium dose and 18/20 animals administered the high dose. This could be due to reactogenicity, with 6/10 animals displaying these features after a 2–week recovery. Grossly observed mandibular lymph node swelling was present in 2/10 male animals administered the low dose and was accompanied by histopathological findings of slight diffuse lymphoid hyperplasia. Other gross or histopathological findings were either not dose-related or spontaneous incidental findings of no toxicological significance.
4. Discussion
SARS-CoV-2 remains a major global public health threat. Furthermore, Betacoronaviruses circulate among zoonotic hosts; thus, the human population is at risk of coronavirus pandemics at regular intervals [34]. The recently emerged Omicron variant is a heavily mutated VOC [35] that evades immune responses elicited by vaccination or infection. Hence, a safe, effective and affordable vaccine is a global priority for controlling the ongoing pandemic and preventing future infections. Currently, few vaccines are approved for human use, and several vaccine candidates are being validated in preclinical and clinical trials. Different strategies, including inactivated whole virus vaccines [36], [37], viral vector or adenovirus vaccines [38] and recombinant glycoprotein nanoparticle vaccines [39], have been examined. There are some limitations associated with the available vaccines, for instance, the requirements of biosafety level 3 facilities while handling live/attenuated viruses during the manufacturing of inactivated vaccines and the risk of adverse reactions in the case of viral vector vaccines [21], [40]. Recombinant subunit vaccines against SARS-CoV-2 lack many of these limitations and are easy and safe to produce [25], [41]; several subunit vaccines are already licensed, for example, Recombivax HB for hepatitis B, Gardasil 9 for human papillomavirus and Flublok for influenza [42]. The ability of plants to produce vaccines and therapeutics is well established [43], [44], [45], [46], [47]. Hence, we used plants for the production of the SARS-CoV-2 RBD protein due to the advantages of this approach, including cost-effectiveness, intrinsic safety, rapid scale-up potential, high yield and the potential for large-scale manufacturing in a short time [48]. Furthermore, proteins produced in plant expression systems undergo post-translational modification, which is essential for glycoprotein function [49], [50], [51], [52]. Glycosylation is intrinsically linked to protein folding, and some concerns remain about the presence of complex and truncated N-glycans carrying β1,2-xylose and core α1,3-fucose residues in the recombinant glycoproteins produced in plants. However, glycoengineering technologies can be used to regulate the function of glycoproteins by knocking down β1,2-xylosyltransferase (XT) and core α1,3-fucosyltransferase (FT) expression in N. benthamiana (ΔXT/FT) plants [53], [54]. Furthermore, the vaccine antigens produced in plants or insect cells are reported to be immunogenic and well tolerated, which shows that species-specific glycans do not appear to negatively regulate the protein folding or antigen immunogenicity [55], [56]. Recently, SARS-CoV-2 antigens and anti-SARS-CoV-2 monoclonal antibodies produced in plants have been shown to be functionally active [57], [58], [59], [60], [61], [62]. We generated a subunit-based COVID-19 vaccine that encodes the RBD protein of SARS-CoV-2 in a plant expression system. To increase the antigen expression level, the RBD gene was codon-optimized, fused with the Fc region, and expressed as a fusion protein in Nicotiana benthamiana; the purified protein was used for immunogenicity experiments in animals [27].
A key challenge in developing subunit vaccines is ensuring vaccine potency and stability. Although subunit vaccines are the safest type of vaccine [41], immunostimulatory agents, i.e., adjuvants, are often added to enhance the immune response of the vaccine antigens, thereby reducing the quantity of antigen required per dose [40], [63]. The most widely used adjuvant, alum, has been used in several licensed vaccines for a long time due to its safety record in clinical and post-marketing surveillance [64]. Further, consideration of optimal formulations is essential to facilitate the stability of vaccine antigens at least at 2–8 °C for widespread vaccination campaigns, especially in the developing world. As a step toward the development of a commercially viable subunit SARS-CoV-2 vaccine, we selected a candidate formulation that includes a combination of excipients, such as sucrose and glycine, to stabilize the Baiya SARS-CoV-2 Vax 1, and we adjuvanted it with alum [65] to induce a stronger immune response to the vaccine antigen. Furthermore, administration of 0.5 ml of vaccine via., the intramuscular route was selected for monkey studies, as this dose will be proposed for human clinical trials.
Safety, immunogenicity, and efficacy tests in animals are critical for vaccine development before it is applied to first-in-human clinical trials. The protective efficacy of our vaccine was evaluated using an established small animal model of SARS-CoV and SARS-CoV-2 infection, i.e., K18-hACE2 mice [66], [67]. We assessed mortality and clinical signs of disease upon virus challenge. The results related to clinical symptoms and body weight revealed reductions in body weight gain on Day 40, clinical symptoms of anorexia or reduced appetite, lethargy, movement with mild stimulation, and mildly rough hair in 2 of 6 animals in the 5 µg Baiya SARS-CoV-2 Vax 1-immunized group. The remaining 4 animals in the group appeared normal. On the other hand, the mice in the 10 µg immunized group maintained body weight gain from Day 36 and appeared normal, with no clinical symptoms. Furthermore, lower viral RNA levels were observed in the nasal turbinate, lung, and brain tissue samples from mice immunized with Baiya SARS-CoV-2 Vax 1 compared to the samples from the control mice. Although it is difficult to directly compare this plant-produced vaccine with the other currently approved vaccines due to the differences in the vaccine platforms/technologies used, the neutralization antibody titers and efficacy are comparable to those associated with the mRNA and inactivated vaccine tested in animal models. Preclinical studies with mRNA and inactivated vaccines have been shown to prevent viral replication in the respiratory tract in animal experiments [68], [69]. Similarly, the results showed that the two doses of Baiya SARS-CoV-2 Vax 1 conferred protection against SARS-CoV-2 challenge in K18-hACE2 transgenic mice. However, further studies are essential to evaluate the durability of the immune response and the protective effect of the vaccine against SARS-CoV-2 variants in vivo.
In preclinical vaccine trials, nonhuman primates, such as macaques, are suitable for assessing vaccine immunogenicity and safety, as they mimic their human counterparts in multiple ways [70], [71]. Hence, safety pharmacology and immunogenicity studies were performed in cynomolgus macaques after the administration of two doses of the vaccine on Days 0 and 21. The results showed that there were no vaccine-related effects on the CNS, respiration or cardiovascular systems. In addition, the humoral immune response induced by the Baiya SARS-CoV-2 Vax 1 vaccine was evaluated. The serum anti-RBD antibody titers elicited by Baiya SARS-CoV-2 Vax 1 were significantly higher after the second immunization than the titers observed in the control group. Our data demonstrated that Baiya SARS-CoV-2 Vax 1 can induce potent humoral immune responses in nonhuman primates after two immunizations. Recently, a plant-derived COVID-19 vaccine based on the VLP platform developed by Medicago has entered clinical trials. Pillet et al. reported the safety, immunogenicity and protection of a two-dose vaccination regimen of this plant-produced VLP vaccine (CoVLP) formulated with or without AS03 or CpG 1018 in nonhuman primates. After the second dose, the adjuvanted vaccine group demonstrated a higher immune response and a lower incidence of clinical signs symptoms, and the vaccine protected the animals by preventing virus replication [72]. Clinical trials have demonstrated that the vaccine is safe, well tolerated and immunogenic, and it does not cause adverse reactions [73].
Furthermore, the toxicity of the Baiya SARS-CoV-2 Vax 1 candidate vaccine was evaluated after two intramuscular injections in Wistar rats. Our results clearly indicated that the Baiya SARS-CoV-2 Vax 1 vaccine was safe and well tolerated and did not cause any clinical symptoms compared with the PBS control; no adverse clinical symptoms were observed in the immunized rats even at the highest tested dose. The general health status of the rats was monitored after immunization and throughout the study, and potential undesirable effects of the subunit vaccine on various tissues were assessed by histopathological analysis. Vaccine-related changes were observed, such as an increase in the incidence of granulomatous inflammation at the injection site, which was related to mandibular and left popliteal lymph node swelling that corresponded to diffuse lymphocyte hyperplasia, and an increase in the myeloid-to-erythroid precursor ratio. These transient responses occurred due to immunological and inflammatory reactions. Furthermore, lesions in the eyes, kidneys and testes were considered spontaneous/incidental findings due to their low incidence or low severity, lack of similar findings in both sexes, and presence of similar findings in the control group.
The significant differences observed in the hematological (RET-He and MONO) and clinical biochemical (ALB2 and GLO) analyses are related to dose but are within the normal ranges for Wistar rats. The WBC content in the male animals in the high-dose group was dose-dependent, but it may correlate with the immune response. These results revealed that the vaccine did not cause any adverse toxic side effects on internal organs and the biological functions of the rats. The safety, toxicity and efficacy results described in the present study are encouraging and represent an important step toward the use of the plant-produced subunit vaccine Baiya SARS-CoV-2 Vax 1 for further clinical trials.
In conclusion, our data suggested that two intramuscular immunizations with the plant-produced subunit vaccine candidate Baiya SARS-CoV-2 Vax 1, which was based on the RBD of SARS-CoV-2 and administered with proper adjuvant and excipient combinations, provided protection against SARS-CoV-2 infection in a mouse challenge model. The vaccine is safe and well tolerated, and it does not cause any adverse clinical symptoms or toxic effects on internal body organs and other physiological functions of animals under the conditions of this study. Furthermore, the vaccine formulation elicits the humoral immune response and production of potent neutralizing antibodies in cynomolgus macaques. Taken together, the results provided here demonstrate the potential of the plant-produced Baiya SARS-CoV-2 Vax 1 vaccine as a valuable antigen candidate for consideration for human clinical trials.
5. Data availability
All the data supporting the findings of this study are available within the paper and are also available from the corresponding author upon request.
Author contributions
ST and WP conceived the project. WP designed the plant experiments. BS, NK, CP, and AM performed the plant experiments and prepared the vaccine formulations. ST and WP designed the challenge experiment. RI, MI, PiS, TH, CK, and MR performed the challenge experiments. SM, ST, and WP designed the safety pharmacology experiments. TK, NS, and SM performed the safety pharmacology experiments. PaS and WP designed the toxicity experiments. PaS and AK performed the toxicity experiments. SM and AT performed the virus neutralization assay. BS drafted and revised the manuscript. All the authors analyzed the data and approved the submitted version.
Funding
This research was funded by the National Vaccine Institute, Thailand and Baiya Phytopharm Co., Ltd. Thailand.
Declaration of Competing Interest
ST and WP from Chulalongkorn University is a founder/shareholder of Baiya Phytopharm Co., Ltd., Thailand. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We appreciate the technical assistance provided by the technicians and staff at the experimental animal facility during the study. We would like to thank Bassam Hallis, Alex Sigal, and Tulio de Oliveira of BEI resources of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing the SARS-CoV-2, Wuhan strain lineage, isolate Hong Kong/VM20001061/2020, NR-52282.The author (NK) would like to thank The Second Century Fund (C2F), Chulalongkorn University for the financial support.
Disclaimer
The materials were reviewed by the Walter Reed Army Institute of Research. There is no objection to the presentation and/or publication of these materials. The opinions or assertions contained herein are solely those of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense. This research was conducted under an approved animal use protocol in an AAALACi accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations related to animals and experiments involving animal, and this research adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.087.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.-W., Yuan S., Kok K.-H., To K.-W., Chu H., Yang J., et al. novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F., Zhao S.u., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malla A., Shanmugaraj B., Ramalingam S. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an emerging zoonotic respiratory pathogen in humans. J Pure Appl Microbiol. 2020;14(suppl 1):931–936. [Google Scholar]
- 8.World Health Organization W. WHO Coronavirus Disease (COVID-19) Dashboard; 2021.
- 9.World Health Organization W. Tracking SARS-CoV-2 variants; 2021.
- 10.Shanmugaraj B., Khorattanakulchai N., Phoolcharoen W. SARS-CoV-2 variants: a continuing threat to global health. Asian Pac J Trop Med. 2022;15(1):1–3. [Google Scholar]
- 11.Noh J.Y., Jeong H.W., Shin E.-C. SARS-CoV-2 mutations, vaccines, and immunity: implication of variants of concern. Signal Transd Target Therapy. 2021;6:203. doi: 10.1038/s41392-021-00623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants — clinical, public health, and vaccine implications. N Engl J Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184(8):2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Beltran W.F., St. Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization W. COVID-19 vaccine tracker and landscape; 2021.
- 20.Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B., Prado E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vacc. 2021;6(1) doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Tenchov R., Smoot J., Liu C., Watkins S., Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021;7(4):512–533. doi: 10.1021/acscentsci.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S., Li Y., Dai L., Wang J., He P., Li C., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386(6):531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siriwattananon K., Manopwisedjaroen S., Shanmugaraj B., Prompetchara E., Ketloy C., Buranapraditkun S., et al. Immunogenicity studies of plant-produced SARS-CoV-2 receptor binding domain-based subunit vaccine candidate with different adjuvant formulations. Vaccines. 2021;9(7):744. doi: 10.3390/vaccines9070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siriwattananon K., Manopwisedjaroen S., Shanmugaraj B., Rattanapisit K., Phumiamorn S., Sapsutthipas S., et al. Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front Plant Sci. 2021;12 doi: 10.3389/fpls.2021.682953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Council N.R. Eighth Edition. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 29.Hunsawong T., Fernandez S., Buathong R., Khadthasrima N., Rungrojchareonkit K., Lohachanakul J., et al. Limited and short-lasting virus neutralizing titers induced by inactivated SARS-CoV-2 vaccine. Emerg Infect Dis. 2021;27(12):3178–3180. doi: 10.3201/eid2712.211772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. 2019-Novel Coronavirus (2019-nCoV) Real-time RT-PCR Primers and Probes; 2020.
- 31.Frey A., Di Canzio J., Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221(1-2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 32.Sheets RL, Stein J, Manetz TS, Andrews C, Bailer R, Rathmann J, et al. Toxicological safety evaluation of DNA plasmid vaccines against HIV-1, Ebola, Severe Acute Respiratory Syndrome, or West Nile virus is similar despite differing plasmid backbones or gene-inserts. Toxicological sciences : an official journal of the Society of Toxicology. 2006;91:620–30. [DOI] [PMC free article] [PubMed]
- 33.Planty C, Chevalier G, Duclos M-È, Chalmey C, Thirion-Delalande C, Sobry C, et al. Nonclinical safety assessment of repeated administration and biodistribution of ChAd3-EBO-Z Ebola candidate vaccine. 2020;40:748–62. [DOI] [PMC free article] [PubMed]
- 34.Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I., et al. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020;37:101830. doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanmugaraj B., Malla A., Khorattanakulchai N., Phoolcharoen W. SARS-CoV-2 omicron variant: Could it be another threat? J Med Virol. 2022;94:1284–1288. doi: 10.1002/jmv.27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang J.G., Su D., Song T.-Z., Zeng Y., Huang W., Wu J., et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat Commun. 2021;12(1):1346. doi: 10.1038/s41467-021-21634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hotez P.J., Bottazzi M.E., Gromowski G. Developing a low-cost and accessible COVID-19 vaccine for global health. PLoS Negl Trop Dis. 2020;14(7) doi: 10.1371/journal.pntd.0008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollet J., Chen W.-H., Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev. 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobato Gómez M., Huang X., Alvarez D., He W., Baysal C., Zhu C., et al. Contributions of the international plant science community to the fight against human infectious diseases - part 1: epidemic and pandemic diseases. Plant Biotechnol J. 2021;19(10):1901–1920. doi: 10.1111/pbi.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanmugaraj B., Siriwattananon K., Malla A., Phoolcharoen W. Potential for developing plant-derived candidate vaccines and biologics against emerging coronavirus infections. Pathogens. 2021;10(8) doi: 10.3390/pathogens10081051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniell H., Mangu V., Yakubov B., Park J., Habibi P., Shi Y., et al. Investigational new drug enabling angiotensin oral-delivery studies to attenuate pulmonary hypertension. Biomaterials. 2020;233 doi: 10.1016/j.biomaterials.2019.119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniell H, Rai V, Xiao Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. 2019;17:1357–68. [DOI] [PMC free article] [PubMed]
- 47.Shahid N., Daniell H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol J. 2016;14:2079–2099. doi: 10.1111/pbi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanmugaraj B., Khorattanakulchai N., Phoolcharoen W. In: Biomedical innovations to combat COVID-19. Rosales-Mendoza S., Comas-Garcia M., Gonzalez-Ortega O., editors. Academic Press; 2022. Chapter 12 - SARS-CoV-2 vaccines: current trends and prospects of developing plant-derived vaccines; pp. 213–229. [Google Scholar]
- 49.Shanmugaraj B., I. Bulaon C.J., Phoolcharoen W. Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants. 2020;9(7) doi: 10.3390/plants9070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanmugaraj B., Malla A., Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2) doi: 10.3390/pathogens9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang B., Shanmugaraj B., Daniell H. Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr Opin Chem Biol. 2017;38:17–23. doi: 10.1016/j.cbpa.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon K.-C., Daniell H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol J. 2015;13(8):1017–1022. doi: 10.1111/pbi.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strasser R., Stadlmann J., Schähs M., Stiegler G., Quendler H., Mach L., et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 54.Dicker M., Strasser R. Using glyco-engineering to produce therapeutic proteins. Expert Opin Biol Ther. 2015;15(10):1501–1516. doi: 10.1517/14712598.2015.1069271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle. Vaccine. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward B.J., Makarkov A., Séguin A., Pillet S., Trépanier S., Dhaliwall J., et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): two multicentre, randomised phase 3 trials. The Lancet. 2020;396(10261):1491–1503. doi: 10.1016/S0140-6736(20)32014-6. [DOI] [PubMed] [Google Scholar]
- 57.Rattanapisit K., Shanmugaraj B., Manopwisedjaroen S., Purwono P.B., Siriwattananon K., Khorattanakulchai N., et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-74904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanmugaraj B., Rattanapisit K., Manopwisedjaroen S., Thitithanyanont A., Phoolcharoen W. Monoclonal antibodies B38 and H4 produced in nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front Plant Sci. 2020;11 doi: 10.3389/fpls.2020.589995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diego-Martin B., González B., Vazquez-Vilar M., Selma S., Mateos-Fernández R., Gianoglio S., et al. Pilot production of SARS-CoV-2 related proteins in plants: a proof of concept for rapid repurposing of indoor farms into biomanufacturing facilities. Front Plant Sci. 2020;11 doi: 10.3389/fpls.2020.612781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makatsa M.S., Tincho M.B., Wendoh J.M., Ismail S.D., Nesamari R., Pera F., et al. SARS-CoV-2 antigens expressed in plants detect antibody responses in COVID-19 patients. Front Plant Sci. 2021;12 doi: 10.3389/fpls.2021.589940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rattanapisit K., Bulaon C.J.I., Khorattanakulchai N., Shanmugaraj B., Wangkanont K., Phoolcharoen W., et al. Plant-produced SARS-CoV-2 receptor binding domain (RBD) variants showed differential binding efficiency with anti-spike specific monoclonal antibodies. PLoS ONE. 2021;16(8):e0253574. doi: 10.1371/journal.pone.0253574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siriwattananon K., Manopwisedjaroen S., Kanjanasirirat P., Budi Purwono P., Rattanapisit K., Shanmugaraj B., et al. Development of plant-produced recombinant ACE2-Fc fusion protein as a potential therapeutic agent against SARS-CoV-2. Front Plant Sci. 2021;11:604663. doi: 10.3389/fpls.2020.604663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang Z., Zhu H., Wang X., Jing B., Li Z., Xia X., et al. Adjuvants for Coronavirus Vaccines. Front Immunol. 2020;11:589833. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kool M., Fierens K., Lambrecht B.N. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61:927–934. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 65.Hotez P.J., Corry D.B., Strych U., Bottazzi M.E. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol. 2020;20(7):399–400. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler E.S., Bailey A.L., Kafai N.M., Nair S., McCune B.T., Yu J., et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCray P.B., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogel A.B., Kanevsky I., Che Y.e., Swanson K.A., Muik A., Vormehr M., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 69.Yadav P.D., Ella R., Kumar S., Patil D.R., Mohandas S., Shete A.M., et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rivera-Hernandez T., Carnathan D.G., Moyle P.M., Toth I., West N.P., Young P.R., et al. The contribution of non-human primate models to the development of human vaccines. Discov Med. 2014;18:313–322. [PMC free article] [PubMed] [Google Scholar]
- 71.Grow D.A., McCarrey J.R., Navara C.S. Advantages of nonhuman primates as preclinical models for evaluating stem cell-based therapies for Parkinson's disease. Stem Cell Res. 2016;17(2):352–366. doi: 10.1016/j.scr.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 72.Pillet S., Arunachalam P.S., Andreani G., Golden N., Fontenot J., Aye P.P., et al. Safety, immunogenicity, and protection provided by unadjuvanted and adjuvanted formulations of a recombinant plant-derived virus-like particle vaccine candidate for COVID-19 in nonhuman primates. Cell Mol Immunol. 2022;19(2):222–233. doi: 10.1038/s41423-021-00809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.-Y., et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27(6):1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the paper and are also available from the corresponding author upon request.