Abstract
Background
Postoperative surgical adhesions constitute a major health burden internationally. A wide range of materials have been evaluated, but despite constructive efforts and the obvious necessity, there remains no specific barrier widely utilized to prevent postoperative adhesion formation. The aim of this study was to highlight and characterize materials used for prevention of postoperative surgical adhesions in both animal and human studies.
Methods
A systematic review was performed of all original research articles presenting data related to the prevention of postoperative adhesions using a barrier agent. All available observational studies and randomized trials using animal models or human participants were included, with no restrictions related to type of surgery. PubMed and Embase databases were searched using key terms from inception to August 2019. Standardized data collection forms were used to extract details for each study and assess desirable characteristics of each barrier and success in animal and/or human studies.
Results
A total of 185 articles were identified for inclusion in the review, with a total of 67 unique adhesion barrier agents (37 natural and 30 synthetic materials). Desirable barrier characteristics of an ideal barrier were identified on review of the literature. Ten barriers achieved the primary outcome of reducing the incidence of postoperative adhesions in animal studies followed with positive outputs in human participants. A further 48 materials had successful results from animal studies, but with no human study performed to date.
Discussion
Multiple barriers showed promise in animal studies, with several progressing to success, and fulfilment of desirable qualities, in human trials. No barrier is currently utilized commonly worldwide, but potential barriers have been identified to reduce the burden of postoperative adhesions and associated sequelae.
In this systematic review, we evaluated barrier materials utilized for the prevention of postoperative adhesions. We assessed 67 adhesions barriers from 185 animal and human studies in the published literature, with each barrier material was assessed based on achieving the primary goal of postoperative adhesion reduction and on the potential ability to achieve a standardized set of desirable characteristics. Multiple barriers showed promise in animal studies, with several progressing to success in and fulfilment of desirable qualities in human trials.
Introduction
Postoperative adhesions are scar tissue resulting from trauma of the peritoneal surface and have been documented in 79–90 per cent of individuals after open abdominal or pelvic surgery1–3. Postoperative adhesions are a leading cause of long-term morbidity following surgery4–6, with 27 per cent of patients being re-admitted following abdominal or pelvic surgery for disorders directly related to adhesions within 5 years6. Adhesions are associated with significant morbidity including small bowel obstruction (SBO), chronic pain, infertility, and requirement for a repeat procedure4,7,8; in addition to the socioeconomic implications7, including the significant financial burden with cumulative direct hospital care costs estimated at 2.3 billion dollars in 2011 in the USA alone9. Postoperative adhesions are characteristically difficult to treat4, with the severity of formed adhesions and rate of iatrogenic bowel injury during adhesiolysis increasing exponentially with each additional operation7. Adhesive disease has no specific laboratory or radiological finding that are currently in use in common practice, although cine-MRI has shown potential in providing information related to extent, location, and strength of intra-abdominal adhesions10. Prevention or reduction of adhesion formation is a key priority.
A wide range of materials have been evaluated in animal and/or human studies as physical barriers to separate the wound from surrounding tissue in an effort to reduce the rate and severity of postoperative adhesions9,11,12; however, despite constructive efforts and the obvious necessity, no specific barrier remains widely utilized in clinical practice to prevent postoperative adhesion formation13. Animal studies remain critical to advancing clinical research, as they are biologically similar to humans, susceptible to similar health issues, and have a shorter life cycle allowing testing over a life span and successive generation14. However, animal welfare and economic funding must be central to any decision to progress with research. The European Union (EU) Directive 2010/63/EU on the protection of animal welfare was produced to harmonize standards of animal research across the EU15. Research using animal models must be carefully designed and relevant, with animal welfare remaining a central concern14. Furthermore, a comprehensive listing of studied barriers in animal and human studies is lacking in systematic reviews to date9,11,12, prompting the need to investigate the breadth of barriers previously published, including those whose investigation was halted after the animal investigation phase.
The aim of this study was to characterize the strengths and shortcomings of each barrier, comparing tissue adherence (traumatized and oozing tissue); applicability through a laparoscope; safety for the patient; ease of application; postoperative pain; and overall efficacy to reduce the rate and severity of postoperative adhesions. Utilizing the information above, the aim is to identify whether an ideal solution exists or whether a pre-existing barrier shows promise for advancement to further research, and also to assess the pre-existing barriers in terms of their readiness for the market: success in animal study; progression to human study and the outcomes; and product on the market.
Methods
Selection criteria
A systematic review was performed according to published guidelines from the Cochrane Collaboration16 and is reported according to the PRISMA guidelines17. A study protocol (Appendix S1) was developed to include original research articles presenting data related to the prevention of postoperative adhesions using a barrier agent. Studies involving physical barrier agents and non-physical barriers were included. Studies of non-resorbable barriers (such as polytetrafluoroethylene), where a further interventional procedure would be necessary for removal, were excluded. All published observational studies and randomized trials were included if they met the following criteria: contained original data, used animal or human participants, or evaluated an adhesion barrier(s) in abdominal and/or pelvic adhesions. No date restrictions were applied and there was no restriction on the type of surgery.
Search strategy
A systematic search of the literature was performed in two databases (PubMed and Embase). The databases were searched from inception to August 2019. The search was performed using key terms: (Surg*(Title/Abstract)) AND (adhesion*(Title/Abstract)) AND (prevent*(Title/Abstract)) AND (barrier*(Title/Abstract)). Two reviewers (M.W. and C.J.) independently screened titles and abstracts using the Rayyan web application for systematic review screening18. Full texts were sourced for relevant articles. Inclusion criteria were assessed independently (M.W. and C.J.), and the final list was agreed by consensus with a third reviewer (L.F.). The reference lists of similar review articles were also screened. The systematic review was performed in accordance with the pre-specified protocol, which was prospectively registered on PROSPERO, the international prospective register of systematic reviews (ID CRD42020125090).
Data extraction
Three standardized data collection forms for animal and human studies respectively were used (Appendix S2). For each study, the title, year of publication, barrier type (natural or synthetic), barrier category (categories were finalized after data extraction), generic and brand name (where applicable), and whether the barrier contained a combination of agents, were extracted. The animal model (such as rat, chicken, or rabbit) for animal studies, and the type of surgery performed (such as abdominal or pelvic) for human studies, were recorded. Reviewers (M.W., C.J., and L.F.) independently extracted data, compared for inconsistencies, and merged into a final data set. Discrepancies were resolved following discussion under supervision of the lead author (M.O.H.).
Appraisal of studies
Additionally, desirable barrier characteristics (Appendix S3) including adherence to traumatized tissue, adherence to oozing tissue, application laparoscopically, safety for the patient, cost-effectiveness, postoperative pain, and ease of application were extracted from full-text articles. Pathway to the market characteristics were extracted as listed in Appendix S4. Successful barriers were those where positive outputs have been reported for each of the desirable characteristics in previous literature and potentially successful barriers were those that had positive outcomes but a number of desirable characteristics required further research.
Results
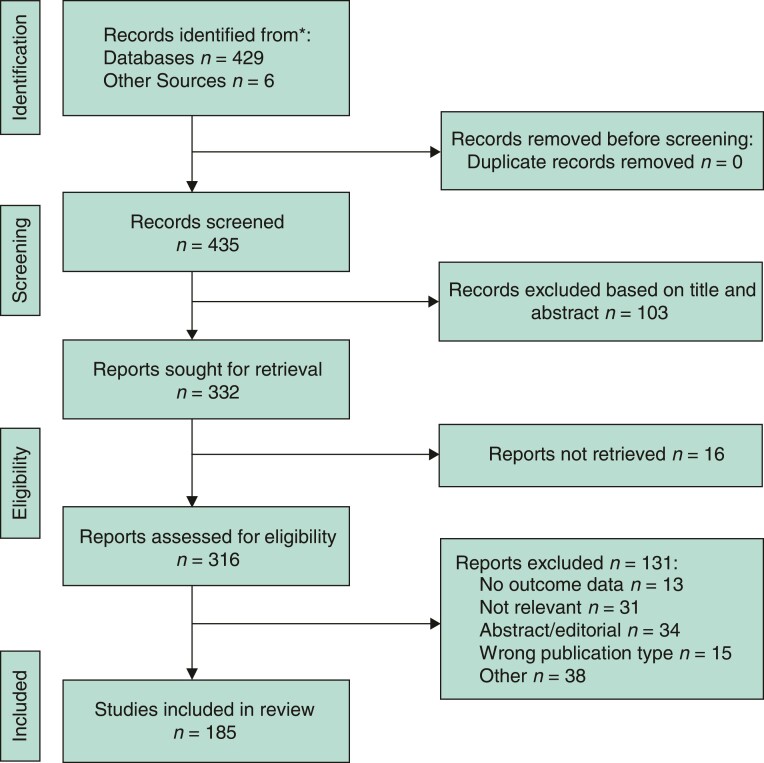
The search of PubMed and Embase databases identified 429 unique articles, with a further six identified from a review of reference listings. A total of 103 articles were excluded on review of titles and abstracts. Sixteen reports could not be retrieved and a further 131 records were removed after full-text review, with 185 remaining for inclusion in the review (Fig. 1).
Fig. 1.
PRISMA flow diagram
Characteristics of included studies
The 185 included studies comprised 51 human studies (38 randomized clinical trials and 13 observational studies) and 134 animal studies. The type of surgery or animal respectively, and relative success of the barrier material are described in Appendix S5. Some 96 animal studies were in rat or mouse, 32 in rabbit, four in chicken, and two in pig. Human studies consisted of 26 gynaecological and 25 abdominal surgeries. Full details are described in Appendix S6.
Characteristics of barrier agents
A total of 67 unique adhesion barriers materials were identified, comprising 16 barrier categories. The barrier materials included 37 natural and 30 synthetic products. The characteristics of the 67 barrier agents based on the eight distinctive properties are summarized in Table 1 and described in detail in Appendix S7.
Table 1.
Characteristics of promising barrier materials
| Adherence to traumatized tissue | Adherence to oozing tissue | Safety | Laparoscopic applicability | Ease of application | Postoperative pain | Cost-effectiveness | |
|---|---|---|---|---|---|---|---|
| ORC | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CMC/HA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| cHA | ✗ (Liquid) | ✗ (Liquid) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Icodextrin | ✗ (Liquid) | ✗ (Liquid) | ✓ | ✓ | ✓ | ✓ | ✓ |
| PEG | ✗ (Liquid) | ✗ (Liquid) | ✓ | ✓ | ✓ | ✓ | ✓ |
| HA hydrogel | ✗ (Liquid) | ✗ (Liquid) | ✓ | ✓ | ✓ | ✓ | ? |
| PLA/PEG | ✓ | ✗ | ? | ✓ | Mixed | Mixed | ? |
| Poloxamer 407/alginate | ✓ | ? | ✓ | ✓ | ✓ | ? | ? |
| Dextran 70 | ✓ | ✓ | ? | ✓ | ? | ? | ? |
| Polyester/collagen | ✓ | ✓ | ✓ | ✗ | ? | ? | ? |
?, no data available. ORC, oxidized regenerated cellulose; CMC, carboxymethylcellulose; HA, hyaluronic acid; cHA, crosslinked HA; PEG, polyethylene glycol; PLA, polylactic acid.
Natural barriers
Algae
Alginate and alginate/hyaluronic acid both had success in animal studies19–22. No human studies were found for any of the materials. The alginate barrier had a higher efficacy compared with a commercialized barrier Interceed in an animal study19. Safety concerns for agar films were identified in an animal study, where there was an increased rate of adverse events23.
Cellulose
Oxidized regenerated cellulose (ORC) and a combination of carboxymethylcellulose (CMC)/hyaluronic acid (HA) had successful animal24–48 and human studies (de novo, reformation, elective, and emergency surgery) after both open and laparoscopic approaches8,49–77. ORC showed greater efficacy compared with control in reducing de novo adhesions during laparoscopic myomectomy52 but was inferior to poloxamer 407 in a comparator study26, although poloxamer 407 is only compatible on a completely haemolysed surface. ORC, modified xyloglucan hydrogel, and CMC/HA have very good safety profiles, low levels of postoperative pain, and score highly on ease of application64,73,78.
Chitosan
Six barriers identified had successful animal studies79–88 but had no human studies performed thus the safety profiles, cost-effectiveness, and levels of postoperative pain remain unknown.
Glycoprotein
Four barrier materials were identified as having successful findings in animal studies36,39,40,89–95, with only a single human study for fibrin, which was not successful in preventing de novo adhesions after open surgery96.
Hyaluronic acid
Three barriers were identified which were successful in animal studies97–109, with HA hydrogel achieving positive results in preventing de novo adhesions following laparoscopic surgery in a single human study110,111. It can be applied laparoscopically with low levels of postoperative pain111, although cost-effectiveness remains unknown.
Icodextrin
Icodextrin had positive outcomes in both animal29,101,112 and human studies (de novo and elective surgery)113–117. It can be applied laparoscopically and has positive outputs in terms of safety, cost-effectiveness, levels of postoperative pain, and ease of use114,115,117.
Starch
Sterile hydrophilic starch and dextrin had positive results in animal studies29,118–120, but neither material was successful in human studies121,122. Positive outputs have been reported for sterile hydrophilic starch in terms of safety, levels of postoperative pain, and ease of application118–121.
Miscellaneous
Twelve barriers in the group were identified with successful animal studies102,123–136; however, only Dextran 70 progressed to have a single successful human study (de novo and laparoscopic surgery). Each of the 12 barriers reported were easy to apply102,117,124,126,132,136; however, safety, cost-effectiveness, and levels of postoperative pain remain unknown for each barrier.
Synthetic barrier
Polycaprolactone
Four barriers had successful animal studies137–144, with no human studies identified. Polycaprolactone/polyhydroxybutyrate, and polycaprolactone/polyethylene glycol (PEG) can be applied laparoscopically and demonstrated good usability141,145.
Polyethylene glycol
Four barriers had successful animal studies26,38,146–155, with positive outcomes reported in human studies for PEG (de novo, reformation, and elective surgery) and poloxamer 407/alginate (de novo) in laparoscopic surgery156–165. No human studies were identified for poloxamer 407. PEG has had positive outputs in terms of patient safety, cost-effectiveness, and level of postoperative pain157,159,160. Poloxamer 407 alginate has been shown to have a high level of patient safety165, but cost-effectiveness, and postoperative pain are unknown.
Polyglycolic acid
The polyglycolic acid barrier had no successful animal study166 and no human studies have been identified.
Polylactic acid
Two barriers identified had successful animal studies38,167–172, with one successful human study performed analysing polylactic acid (PLA)/PEG barrier173. PLA/PEG had reports of high level of patient safety, mixed reports related to postoperative pain, and ease of application169–171,173.
Polypropylene
Polypropylene/omega-3 had a single successful animal study174, whereas the remaining three barriers in the category had unsuccessful animal studies118,174. No human studies were identified for any of the materials. Each of the barriers requires sutures to adhere to traumatized and oozing surfaces.
Polyvinyl alcohol
Polyvinyl alcohol hydrogel and polyvinyl alcohol/CMC had successful animal studies175–181, but no human studies were identified. Characteristics including patient safety, cost-effectiveness, and postoperative pain are unknown for the two barriers.
Silicone
Polysiloxane had no successful animal studies182 and no human studies have been performed to date118,174,183.
Miscellaneous
Eight further identified barriers except for polyester/collagen had successful animal studies118,136,174,184–190. No human studies were identified for any of the materials. Polyester/collagen has a poor level of safety reported in animal studies118,174, with unknown level of the ease of barrier application. Patient safety and ease of application are unknown for the remaining barriers.
Pathway to market
The market potential for each barrier is described in Table 2, based on outcomes from animal and human studies. Six barriers with successful animal and human studies, which are currently available on the market were identified. A further 52 barrier materials with positive outcomes, where further research is required (success in both animal and human studies or success in animal studies without progression to human study) were identified. Fourteen barrier materials with negative outcomes were noted.
Table 2.
Pathway to the market characteristics
| Barrier type | Pathway status | Successful animal test | Followed by human test | Positive outputs | On the market |
|---|---|---|---|---|---|
| Category | |||||
| Barrier name | |||||
| Natural | |||||
| Algae | |||||
| Alginate |

|
Yes | No | No | No |
| Agar films |

|
No | No | No | No |
| Alginate/hyaluronic acid |

|
Yes | No | No | No |
| Cellulose | |||||
| Oxidized regenerated cellulose |

|
Yes | Yes | Yes | Yes |
| Modified xyloglucan hydrogel |

|
Yes | No | No | No |
| Carboxymethylcellulose |

|
Yes | No | No | No |
| Carboxymethylcellulose/hyaluronic acid |

|
Yes | Yes | Yes | Yes |
| Carboxymethylcellulose/polyethylene glycol |

|
No | No | No | No |
| Chitosan | |||||
| N,O-carboxymethyl chitosan |

|
Yes | No | No | No |
| Chitosan |

|
Yes | No | No | No |
| Chitosan/carboxymethylcellulose/collagen |

|
Yes | No | No | No |
| N,O-carboxymethyl chitosan/hyaluronic acid |

|
Yes | No | No | No |
| Chitosan/gelatin |

|
Yes | No | No | No |
| N,O-carboxymethyl chitosan/dextran |

|
Yes | No | No | No |
| Chitosan/polyglycolic acid |

|
Yes | No | No | No |
| Glycoprotein | |||||
| Fibronectin derivative |

|
Yes | No | No | No |
| Lactoferrin |

|
Yes | No | No | No |
| Fibrin |

|
Yes | No | No | No |
| Gelatin/polyglycan |

|
Yes | No | No | No |
| Gelatin/proteoglycan |

|
Yes | Yes | Yes | No |
| Hyaluronic acid | |||||
| Hyaluronic acid hydrogel |

|
Yes | Yes | Yes | No |
| Crosslinked hyaluronic acid |

|
Yes | Yes | Yes | Yes |
| Hyaluronic acid membrane |

|
Yes | Yes | Yes | No |
| Icodextrin | |||||
| Icodextrin |

|
Yes | Yes | Yes | Yes |
| Miscellaneous | |||||
| Dextran 70 |

|
Yes | No | No | No |
| Phosphorylcholine |

|
Yes | No | No | No |
| Silk |

|
Yes | No | No | No |
| Ancrod |

|
Yes | No | No | No |
| Bromelain |

|
Yes | No | No | No |
| Xanthan gum |

|
Yes | No | No | No |
| Pectin |

|
Yes | No | No | No |
| Modified pullulan |

|
Yes | No | No | No |
| Liquid paraffin |

|
Yes | No | No | No |
| Galls ethyl acetate |

|
Yes | No | No | No |
| Ethyl pyruvate |

|
Yes | No | No | No |
| Tongfu xiere enteroclysis mixture |

|
Yes | No | No | No |
| Starch | |||||
| Sterile hydrophilic starch |

|
Yes | No | No | No |
| Dextrin |

|
Yes | No | No | No |
| Synthetic Polycaprolactone | |||||
| Polycaprolactone/polyhydroxybutyrate |

|
Yes | No | No | No |
| polycaprolactone/hyaluronic acid |

|
Yes | No | No | No |
| Polycaprolactone/polyethylene glycol |

|
Yes | No | No | No |
| Polycaprolactone/gelatin |

|
Yes | No | No | No |
| Polyethylene glycol | |||||
| Polyethylene glycol |

|
Yes | Yes | Yes | Yes |
| Polyethylene glycol/collagen/glycerol |

|
Yes | Yes | Yes | No |
| Poloxamer 407 |

|
Yes | No | No | No |
| Poloxamer 407/alginate |

|
Yes | Yes | No | No |
| Polyglycolic acid | |||||
| Polyglycolic acid |

|
Yes | No | No | No |
| Polylactic acid | |||||
| Polylactic acid |

|
Yes | No | No | No |
| Polylactic acid/polyethylene glycol |

|
Yes | Yes | No | No |
| Polylactic acid/polycaprolactone |

|
Yes | No | No | No |
| Poly(l-lactic acid)/modified mesoporous silica/ibuprofen |

|
Yes | No | No | No |
| Polypropylene | |||||
| Polypropylene |

|
No | No | No | No |
| Polypropylene/glycolide/polycaprolactone |

|
Yes | No | No | Yes |
| Polydioxanone/polypropylene/carboxymethylcellulose |

|
Yes | No | No | No |
| Polypropylene/titanium |

|
No | No | No | No |
| Polypropylene/omega 3 |

|
Yes | No | No | No |
| Polyvinyl alcohol | |||||
| Polyvinyl alcohol hydrogel |

|
Yes | No | No | No |
| Polyvinyl Alcohol/carboxymethylcellulose |

|
Yes | No | No | No |
| Silicone | |||||
| Polysiloxane | No | No | No | No | |
| Polyesterurethane/polydimethylsiloxane |

|
Yes | Yes | Yes | No |
| Miscellaneous | |||||
| Chitosan/poly(d,l-lactic-co-glycolic acid)/polyethylene oxide |

|
Yes | No | No | No |
| Polyester/collagen |

|
No | No | No | No |
| N-isopropylacrylamide |

|
Yes | No | No | No |
| C17 glycerin ester |

|
Yes | No | No | No |
| Methylene blue |

|
Yes | No | No | No |
| Dimethyl-sulfoxide |

|
Yes | No | No | No |
| Polyhydroxyethylmethacrylate |

|
Yes | No | No | No |
| Poly(lactic-co-glycolic acid)/epigallocatechin-3-O-gallate |

|
Yes | No | No | No |
Green, on the market; orange, positive outcomes in animal and human study (but not on the market) or successful animal study with no human study to date; red, negative results from animal and/or human studies.
Discussion
Ten barriers were identified (HA hydrogel, PLA/PEG, poloxamer 407/alginate, and Dextran 70 in addition to the six commercially available barriers) that achieved the primary outcome of preventing adhesions in both animal and human studies, with varying success in attaining each of the optimal characteristics. Furthermore, 48 additional barriers achieved positive outcomes in animal studies but never successfully progressed to a human study. The remaining nine barriers were those with unsuccessful human studies following positive animal studies and those with no successful in animal studies.
Animal models have been the basis of many great discoveries in modern biomedical research14; however, animal welfare must remain a central consideration. The large number of barriers achieving positive outcomes in animal subjects yet failing to progress to human trials questions the investigators’ intentions on progression, appropriateness of model utilized, study design, and reliability of results. Currently, there are six barriers available commercially in Europe comprising ORC (Interceed, Ethicon, Somerville, New Jersey, USA), CMC/HA (Seprafilm, Sanofi, Paris, France), crosslinked HA (cHA) (Hyalobarrier, Nordic group, Paris, France), polyester/collagen (Parietex, Medtronic, Watford, UK), icodextrin 4 per cent solution (Adept, Baxter, Deerfield, Illinois, USA), and PEG (Sprayshield, Integra, LifeSciences, Plainsboro, New Jersey, USA).
The capacity to adhere to traumatized tissue is a fundamental requirement for any barrier to envelope the damaged tissue and partition the aggregated fibrin surface, thereby diminishing adhesion formation4. Overall, only three natural (ORC, CMC/HA, and HA) and two synthetic (PLA/PEG and poloxamer 407/alginate) barriers that were successful in adhesion reduction in animal and human studies demonstrated adequate ability to adhere to traumatized tissue. The barrier was a liquid preparation, except for the PLA/PEG barrier, which requires sutures to impede migration. The PLA/PEG barrier has only been utilized in a single human study of cardiac patients with positive outcomes173; however, previous studies have shown that the additional use of sutures entails a heightened opportunity for adhesion formation173,191.
Barrier attachment to oozing surfaces is an important factor to ensure the anti-adhesion effect is maintained, particularly during surgeries that include a high risk of bleeding148. Overall, natural barriers seem to maintain more effective anti-adhesion effects on oozing surfaces. HA hydrogel and CMC/HA both highlighted their capabilities in human studies; however, the ORC barrier is of limited effectiveness in the presence of blood or peritoneal fluid192. Interestingly, chitosan-based (CS) barriers exhibit haemostatic effects193. This prophylactic property, in addition to the ability of the agent to be applied to oozing surfaces, highlights promise as a barrier constituent; however, although positive outputs were achieved in animal studies utilizing CS in combination79–88,194,195, no successful human study exists.
Patient safety is of utmost importance, balancing the utility risks of a barrier with the current standard of care (no barrier). Patients who suffer postoperative adhesions have a longstanding augmented risk of a number of discrete clinical sequelae, including chronic pain, small bowel adhesive disease, increased operating time, increased duration of hospital stay, female infertility, opioid dependency, and reduced quality of life9,196. While, any potential barrier candidate should aim to alleviate or reduce potential patient risks, it is important that the barrier itself does not pose further patient safety concerns or augment postoperative pain. Overall, the nine barriers achieving the primary endpoint of reducing the extent and severity of postoperative adhesions scored highly on the Likert safety scale. Five barriers achieved positive results regarding extent of postoperative pain, with PLA/PEG barrier having mixed results, whereas poloxamer/alginate and Dextran 70 barriers had no reported outcomes.
The application of ORC during gynaecological surgery decreases the incidence and severity of postoperative adhesions without any significant adverse events76. Concerns have been raised that a single adhesion band produced from incomplete cover or on the periphery of a barrier may result in an augmented risk of strangulated SBO; however, the available evidence contradicts these concerns, highlighting that extensive adhesive disease as opposed to isolated areas correlates with incidence of SBO7. The CMC/HA barrier has been demonstrated to reduce the rate of SBO in several controlled trials56,59. Furthermore studies have found a reduction in the incidence of chronic abdominal pain8 and duration of procedure77. Despite predominantly positive outputs for the barrier, safety concerns have been highlighted with augmented risk of abdominal abscess formation on application of the barrier to the region of anastomoses56,62.
The utilization of a laparoscopic approach, where feasible, has consistently demonstrated improved patient outcomes relative to open surgery. Krielen and colleagues analysed a retrospective cohort study of 72 270 patients with adhesion-related readmissions following abdominal surgery, comprising open (n = 50 751) and laparoscopic (n = 21 519) approaches. The study interval encompassed hospital readmissions from 2009 to 2011 utilizing the validated population data for the Scottish National Health Service with a 5-year follow-up. They recorded a statistically significant reduction in the number of readmissions directly related to adhesions (1.7 per cent versus 4.3 per cent; P < 0.0001) and those possibly related to adhesions (16.0 per cent versus 18.2 per cent; P < 0.005) in the laparoscopic group6. Of the nine barriers highlighted, each can be applied laparoscopically except for PLA/PEG, where it is unknown and mixed results are reported regarding its ease of application. No studies to date have reported the ease of application of Dextran 70. ORC and CMC/HA are solid membrane barriers and therefore present an augmented challenge in laparoscopic application compared with alternative barriers, which are liquid, gel, or spray preparations. ORC has also been associated with elevated handling issues in comparison with the other preparations.
Postoperative adhesions and related complications accrue substantial healthcare costs, both directly and indirectly. Cost-effectiveness analysis of widespread utilization is an essential prerequisite for any barrier considered for introduction by policymakers. No such analysis assessing the overall cost-effectiveness of a barrier was identified in this systematic review.
The primary strength of the present study is that independent screening and abstraction for both animal and human studies was performed, resulting in the largest systematic review on the topic to date. Ideal characteristics for each barrier were independently reviewed and extracted, allowing potential barriers to be highlighted for further investigation; however, limitations including publication bias and small study bias exist as with all systematic reviews. Additional limitations rely on heterogenous reporting of characteristics and study success. Furthermore, animal models and human clinical indications were heterogenous. It was not possible to assess the long-term safety and efficacy data of the majority of barriers, as most only included short-term data.
Meticulous surgical technique and increasing performance of minimally invasive procedures have reduced the incidence and severity of the complication, but adhesions remain a significant global burden. Despite a concerted effort and vast investigation over the past two decades, there remains no specific barrier agent in widespread use internationally with only five agents licenced for use in the EU. Positive long-term data on efficacy and safety have been demonstrated for Seprafilm8; however, these remain sparse overall. Future research should concentrate on assessing the safety and confirming efficacy observed in animal studies, ensuring that all research is well designed, relevant, and takes into account issues on animal welfare. Outcomes should be reported in a uniform manner based on location of adhesions (such as the modified American Fertility Society endometriosis scale for gynaecology adhesions). Effects on quality of life seem to have been poorly explored to date and require evaluation. Furthermore, before the production of novel barriers, researchers must first ensure compliance with the EU Directive guidance, which puts a clear and explicit obligation on researchers to replace, reduce, and refine studies with animal involvement15. Additionally, alignment with clinically based surgeons to identify and assess reluctance and possible concerns with utilization of commercially available barriers, including Seprafilm, is required, and the long-term efficiency and safety data of successful barriers requires evaluation in future research8.
Funding
The authors have no funding to declare.
Supplementary Material
Acknowledgements
The corresponding author certifies that no other persons have made substantial contributions to the research and/or manuscript. The systematic review was performed in accordance with the pre-specified protocol, which was prospectively registered on PROSPERO, the international prospective register of systematic reviews (ID CRD42020125090). M.W., C.J., and L.F. were responsible for data collection. All authors contributed to data interpretation and critical revision of the report.
Disclosure. The authors declare no conflict of interest.
Contributor Information
Michael Gerard Waldron, Translational Medical Device Laboratory, National University of Ireland Galway, Galway, Ireland.
Conor Judge, Translational Medical Device Laboratory, National University of Ireland Galway, Galway, Ireland.
Laura Farina, Translational Medical Device Laboratory, National University of Ireland Galway, Galway, Ireland.
Aoife O’Shaughnessy, Translational Medical Device Laboratory, National University of Ireland Galway, Galway, Ireland.
Martin O’Halloran, Translational Medical Device Laboratory, National University of Ireland Galway, Galway, Ireland.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data that support the findings of this study are available from the corresponding author, M.W., upon reasonable request.
References
- 1. Stommel MWJ, ten Broek RPG, Strik C, Slooter GD, Verhoef C, Grünhagen DJ et al. Multicenter observational study of adhesion formation after open-and laparoscopic surgery for colorectal cancer. Ann Surg 2018;267:743–748 [DOI] [PubMed] [Google Scholar]
- 2. Menzies D, Ellis H. Intestinal obstruction from adhesions–how big is the problem? Ann R Coll Surg Engl 1990;72:60–63 [PMC free article] [PubMed] [Google Scholar]
- 3. Diamond MP. Clinical implications of postsurgical adhesions. Hum Reprod Update 2001;7:567–576 [DOI] [PubMed] [Google Scholar]
- 4. Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH. Abdominal adhesions: a practical review of an often overlooked entity. Ann Med Surg 2017;15:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parker MC, Wilson MS, Menzies D, Sunderland G, Clark DN, Knight AD et al. The SCAR-3 study: 5-year adhesion-related readmission risk following lower abdominal surgical procedures. Colorectal Dis 2005;7:551–558 [DOI] [PubMed] [Google Scholar]
- 6. Krielen P, Stommel MWJ, Pargmae P, Bouvy ND, Bakkum EA, Ellis H et al. Adhesion-related readmissions after open and laparoscopic surgery: a retrospective cohort study (SCAR update). Lancet 2020;395:33–41 [DOI] [PubMed] [Google Scholar]
- 7. ten Broek RPG, Issa Y, van Santbrink EJP, Bouvy ND, Kruitwagen RFPM, Jeekel J et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and meta-analysis. BMJ 2013;347:f5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Wal JBC, Iordens GIT, Vrijland WW, van Veen RN, Lange J, Jeekel J. Adhesion prevention during laparotomy: long-term follow-up of a randomized clinical trial. Ann Surg 2011;253:1118–1121 [DOI] [PubMed] [Google Scholar]
- 9. Strik C, Wever KE, Stommel MWJ, Goor Hv, ten Broek RPG. Adhesion reformation and the limited translational value of experiments with adhesion barriers: a systematic review and meta-analysis of animal models. Sci Rep 2019;9:18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang RA, Buhmann S, Hopman A, Steitz H-O, Lienemann A, Reiser MF et al. Cine-MRI detection of intraabdominal adhesions: correlation with intraoperative findings in 89 consecutive cases. Surg Endosc 2008;22:2455–2461 [DOI] [PubMed] [Google Scholar]
- 11. Robb WB, Mariette C. Strategies in the prevention of the formation of postoperative adhesions in digestive surgery: a systematic review of the literature. Dis Colon Rectum 2014;57:1228–1240 [DOI] [PubMed] [Google Scholar]
- 12. Li J, Feng X, Liu B, Yu Y, Sun L, Liu T et al. Polymer materials for prevention of postoperative adhesion. Acta Biomater 2017;61:21–40 [DOI] [PubMed] [Google Scholar]
- 13. Arung W. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol 2011;17:4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barré-Sinoussi F, Montagutelli X. Animal models are essential to biological research: issues and perspectives. Future Sci OA 2015;1:FSO63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The European Parliament . Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010. OJEU 2010;33–79 [Google Scholar]
- 16. Green SHJ. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (eds Higgins JPT, Green S). The Cochrane Collaboration, 2011.
- 17. Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264. [DOI] [PubMed] [Google Scholar]
- 18. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho WJ, Oh SH, Lee JH. Alginate film as a novel post-surgical tissue adhesion barrier. J Biomater Sci Polym Ed 2010;21:701–713 [DOI] [PubMed] [Google Scholar]
- 20. Chaturvedi AA, Lomme RMLM, Hendriks T, van Goor H. Prevention of postsurgical adhesions using an ultrapure alginate-based gel: alginate-based gel for prevention of adhesions. Br J Surg 2013;100:904–910 [DOI] [PubMed] [Google Scholar]
- 21. Back JH, Cho WJ, Kim JH, Park IK, Kwon SW. Application of hyaluronic acid/sodium alginate-based microparticles to prevent tissue adhesion in a rabbit model. Surg Today 2016;46:501–508 [DOI] [PubMed] [Google Scholar]
- 22. Chaturvedi AA, Lomme RMLM, Hendriks T, van Goor H. Ultrapure alginate anti-adhesion gel does not impair colon anastomotic strength. J Surg Res 2014;192:432–439 [DOI] [PubMed] [Google Scholar]
- 23. Mirkheshti N, Mamoudieh M, Alavi SA. Evaluation of agar films in prevention of postoperative peritoneal adhesions in animal model. Turk J Trauma Emerg Surg 2011;17:108–112 [DOI] [PubMed] [Google Scholar]
- 24. Temiz A, Ozturk C, Bakunov A, Kara K, Kaleli T. A new material for prevention of peritendinous fibrotic adhesions after tendon repair: oxidised regenerated cellulose (Interceed), an absorbable adhesion barrier. Int Orthop 2008;32:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grow DR, Seltman HJ, Coddington CC, Hodgen GD. The reduction of postoperative adhesions by two different barrier methods versus control in cynomolgus monkeys: a prospective, randomized, crossover study. Fertil Steril 1994;61:1141–1146 [DOI] [PubMed] [Google Scholar]
- 26. Rice VM, Shanti A, Moghissi KS, Leach RE. A comparative evaluation of Poloxamer 407 and oxidized regenerated cellulose (Interceed [TC7] to reduce postoperative adhesion formation in the rat uterine horn model. Fertil Steril 1993;59:901–906 [PubMed] [Google Scholar]
- 27. Balbinotto RP, Muller AL, Nunes AG, Silva RD, Meyer FS, Cerski CS et al. Barrier methods used to prevent pelvic adhesions in videolaparoscopy: experimental study in female rabbits. Surg Endosc 2011;25:2637–2642 [DOI] [PubMed] [Google Scholar]
- 28. Demir U, Mihmanli M, Coskun H, Dilege E, Kalyoncu A, Altinli E et al. Comparison of prosthetic materials in incisional hernia repair. Surg Today 2005;35:223–227 [DOI] [PubMed] [Google Scholar]
- 29. Poehnert D, Grethe L, Maegel L, Jonigk D, Lippmann T, Kaltenborn A et al. Evaluation of the effectiveness of peritoneal adhesion prevention devices in a rat model. Int J Med Sci 2016;13:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genc A, Taneli F, Yılmaz O, Turkdogan P, Alp Arslan O, Sencan A et al. Effect of adhesion barrier (interceed TC7) on two-stage orchidopexy operation. Scand J Urol Nephrol 2004;38:401–404 [DOI] [PubMed] [Google Scholar]
- 31. Ates U, Ata B, Ortakuz S, Seyhan A, Urman B. Prevention of adhesion formation following ovarian surgery in a standardized animal model: comparative study of interceed and double layer surgicell. J Obstet Gynaecol Res 2008;34:12–17 [DOI] [PubMed] [Google Scholar]
- 32. Coelho Junior ER, Costa LO, Alencar AV, Barbosa AP, Pinto FC, Aguiar JL. Prevention of peritoneal adhesion using a bacterial cellulose hydrogel, in experimental study. Acta Cir Bras 2015;30:194–198 [DOI] [PubMed] [Google Scholar]
- 33. Haney AF, Doty E. Murine peritoneal injury and de novo adhesion formation caused by oxidized-regenerated cellulose (Interceed [TC7]) but not expanded polytetrafluoroethylene (Gore-Tex surgical membrane). Fertil Steril 1992;57:202–208 [DOI] [PubMed] [Google Scholar]
- 34. Burns JW, Skinner K, Colt MJ, Burgess L, Rose R, Diamond MP. A hyaluronate based gel for the prevention of postsurgical adhesions: evaluation in two animal species. Fertil Steril 1996;66:814–821 [PubMed] [Google Scholar]
- 35. Greenawalt KE, Colt MJ, Corazzini RL, Krauth MC, Holmdahl L. A membrane slurry reduces postoperative adhesions in rat models of abdominal surgery. J Surg Res 2011;168:e25–e30 [DOI] [PubMed] [Google Scholar]
- 36. Oncel M, Remzi FH, Senagore AJ, Connor JT, Fazio VW. Application of Adcon-P or Seprafilm in consecutive laparotomies using a murine model. Am J Surg 2004;187:304–308 [DOI] [PubMed] [Google Scholar]
- 37. Kim YI. Comparative study for preventive effects of intra-abdominal adhesion using cyclo-oxygenase-2 enzyme (COX-2) inhibitor, low molecular weight heparin (LMWH), and synthetic barrier. Yonsei Med J 2013;54:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gruber-Blum S, Petter-Puchner AH, Brand J, Fortelny RH, Walder N, Oehlinger W et al. Comparison of three separate antiadhesive barriers for intraperitoneal onlay mesh hernia repair in an experimental model. Br J Surg 2011;98:442–449 [DOI] [PubMed] [Google Scholar]
- 39. Hellebrekers BWJ, Trimbos-Kemper GCM, van Blitterswijk CA, Bakkum EA, Trimbos JBMZ. Effects of five different barrier materials on postsurgical adhesion formation in the rat. Hum Reprod 2000;15:1358–1363 [DOI] [PubMed] [Google Scholar]
- 40. Arnold PB, Green CW, Foresman PA, Rodeheaver GT. Evaluation of resorbable barriers for preventing surgical adhesions. Fertil Steril 2000;73:157–161 [DOI] [PubMed] [Google Scholar]
- 41. Erpek H, Tuncyurek P, Soyder A, Boylu S. Hyaluronic acid/carboxymethylcellulose membrane barrier versus taurolidine for the prevention of adhesions to polypropylene mesh. Eur Surg Res 2006;38:414–417 [DOI] [PubMed] [Google Scholar]
- 42. Medina M, Paddock HN, Connolly RJ, Schwaitzberg SD. Novel antiadhesion barrier does not prevent anastomotic healing in a rabbit model. J Invest Surg 1995;8:179–186 [DOI] [PubMed] [Google Scholar]
- 43. Kelekci S, Yilmaz B, Oguz S, Zergeroglu S, Inan I, Tokucoglu S. The efficacy of a hyaluronate/carboxymethylcellulose membrane in prevention of postoperative adhesion in a rat uterine horn model. Tohoku J Exp Med 2004;204:189–194 [DOI] [PubMed] [Google Scholar]
- 44. Sheldon HK, Gainsbury ML, Cassidy MR, Chu DI, Stucchi AF, Becker JM. A sprayable hyaluronate/carboxymethylcellulose adhesion barrier exhibits regional adhesion reduction efficacy and does not impair intestinal healing. J Gastrointest Surg 2012;16:325–333 [DOI] [PubMed] [Google Scholar]
- 45. Hammer RA, Morse AN, Cornella JL, Keller RS, Hentz J, McDonald JA et al. Bringing molecular biology to bear on adhesion prevention: postsurgical adhesion reduction using intraperitoneal inoculation of hyaluronic acid–inducing adenoviral vector in a murine model. J Gynecol Surg 2006;22:7–18 [Google Scholar]
- 46. Bahadir I, Oncel M, Kement M, Sahip Y. Intra-abdominal use of taurolidine or heparin as alternative products to an antiadhesive barrier (Seprafilm®) in adhesion prevention: an experimental study on mice. Dis Colon Rectum 2007;50:2209–2214 [DOI] [PubMed] [Google Scholar]
- 47. Lim R, Morrill JM, Lynch RC, Reed KL, Gower AC, Leeman SE et al. Practical limitations of bioresorbable membranes in the prevention of intra-abdominal adhesions. J Gastrointest Surg 2009;13:35–42 [DOI] [PubMed] [Google Scholar]
- 48. Ryan CK, Sax HC. Evaluation of a carboxymethylcellulose sponge for prevention of postoperative adhesions. Am J Surg 1995;169:154–160 [DOI] [PubMed] [Google Scholar]
- 49. Stawicki SP, Green JM, Martin ND, Green RH, Cipolla J, Seamon MJ et al. Results of a prospective, randomized, controlled study of the use of carboxymethylcellulose sodium hyaluronate adhesion barrier in trauma open abdomens. Surgery 2014;156:419–430 [DOI] [PubMed] [Google Scholar]
- 50. Greenblatt EM, Casper RF. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: lack of correlation with pregnancy rate. Fertil Steril 1993;60:766–770 [PubMed] [Google Scholar]
- 51. Li TC, Cooke ID. The value of an absorbable adhesion barrier, Interceed, in the prevention of adhesion reformation following microsurgical adhesiolysis. Br J Obstet Gynaecol 1994;101:335–339 [DOI] [PubMed] [Google Scholar]
- 52. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod 1995;10:3133–3135 [DOI] [PubMed] [Google Scholar]
- 53. Saravelos H, Li T-C. Post-operative adhesions after laparoscopic electrosurgical treatment for polycystic ovarian syndrome with the application of Interceed to one ovary: a prospective randomized controlled study. Hum Reprod 1996;11:992–997 [DOI] [PubMed] [Google Scholar]
- 54. Tinelli A, Malvasi A, Guido M, Tsin DA, Hudelist G, Hurst B et al. Adhesion formation after intracapsular myomectomy with or without adhesion barrier. Fertil Steril 2011;95:1780–1785 [DOI] [PubMed] [Google Scholar]
- 55. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm adhesion study group. Fertil Steril 1996;66:904–910 [PubMed] [Google Scholar]
- 56. Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG et al. Reduction in adhesive small-bowel obstruction by Seprafilm® adhesion barrier after intestinal resection. Dis Colon Rectum 2006;49:1–11 [DOI] [PubMed] [Google Scholar]
- 57. Hayashi S, Takayama T, Masuda H, Kochi M, Ishii Y, Matsuda M et al. Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: a randomized clinical trial. Ann Surg 2008;247:766–770 [DOI] [PubMed] [Google Scholar]
- 58. Inoue M, Uchida K, Miki C, Kusunoki M. Efficacy of Seprafilm for reducing reoperative risk in pediatric surgical patients undergoing abdominal surgery. J Pediatr Surg 2005;40:1301–1306 [DOI] [PubMed] [Google Scholar]
- 59. Kusunoki M, Ikeuchi H, Yanagi H, Noda M, Tonouchi H, Mohri Y et al. Bioresorbable hyaluronate-carboxymethylcellulose membrane (Seprafilm) in surgery for rectal carcinoma: a prospective randomized clinical trial. Surg Today 2005;35:940–945 [DOI] [PubMed] [Google Scholar]
- 60. Park C-M, Lee WY, Cho YB, Yun HR, Lee W-S, Yun SH et al. Sodium hyaluronate-based bioresorbable membrane (Seprafilm®) reduced early postoperative intestinal obstruction after lower abdominal surgery for colorectal cancer: the preliminary report. Int J Colorectal Dis 2009;24:305–310 [DOI] [PubMed] [Google Scholar]
- 61. Salum M, Wexner SD, Nogueras JJ, Weiss E, Koruda M, Behrens K et al. Does sodium hyaluronate- and carboxymethylcellulose-based bioresorbable membrane (Seprafilm) decrease operative time for loop ileostomy closure? Tech Coloproctol 2006;10:187–191 [DOI] [PubMed] [Google Scholar]
- 62. Vrijland WW, Tseng LNL, Eijkman HJM, Hop WCJ, Jakimowicz JJ, Leguit P et al. Fewer intraperitoneal adhesions with use of hyaluronic acid–carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg 2002;235:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Merle M, Lallemand B, Lim A, Gantois G. Experimental and clinical evaluation of an absorbable biomaterial inducing an anti-adhesive barrier (Divide®). Eur J Orthop Surg Traumatol 2008;18:255–263 [Google Scholar]
- 64. Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm® adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum 2003;46:1310–1319 [DOI] [PubMed] [Google Scholar]
- 65. Kawamura H, Yokota R, Yokota K, Watarai H, Tsunoda Y, Yamagami H et al. A sodium hyaluronate carboxymethylcellulose bioresorbable membrane prevents postoperative small-bowel adhesive obstruction after distal gastrectomy. Surg Today 2010;40:223–227 [DOI] [PubMed] [Google Scholar]
- 66. Suresh A, Celso BG, Awad ZT. Seprafilm slurry does not increase complication rates after laparoscopic colectomy. Surg Endosc 2011;25:2661–2665 [DOI] [PubMed] [Google Scholar]
- 67. Hong M-K, Ding D-C. Seprafilm® application method in laparoscopic surgery. JSLS 2017;21:e2016.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ota K, Sato K, Ogasawara J, Takahashi T, Mizunuma H, Tanaka M. Safe and easy technique for the laparoscopic application of Seprafilm® in gynecologic surgery. Asian J Endosc Surg 2019;12:242–245 [DOI] [PubMed] [Google Scholar]
- 69. Kohanzadeh S, Lugo L, Long JN. Safety of antiadhesion barriers in hand surgery. Ann Plast Surg 2013;70:527–529 [DOI] [PubMed] [Google Scholar]
- 70. Bristow RE, Montz FJ. Prevention of adhesion formation after radical oophorectomy using a sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier. Gynecol Oncol 2005;99:301–308 [DOI] [PubMed] [Google Scholar]
- 71. Nordic Adhesion Prevention Study Group . The efficacy of interceed(TC7)* for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril 1995;63:709–714 [PubMed] [Google Scholar]
- 72. Franklin R. Reduction of ovarian adhesions by the use of Interceed. Obstet Gynecol 1995;86:335–340 [DOI] [PubMed] [Google Scholar]
- 73. Mais V, Ajossa S, Marongiu D, Peiretti R, Guerriero S, Benedettomelis G. Reduction of adhesion reformation after laparoscopic endometriosis surgery: a randomized trial with an oxidized regenerated cellulose absorbable barrier. Obstet Gynecol 1995;86:512–515 [DOI] [PubMed] [Google Scholar]
- 74. Wallwiener D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: an explanation for the controversy on the use of autologous and alloplastic barriers? Fertil Steril 1998;69:132–137 [DOI] [PubMed] [Google Scholar]
- 75. Keckstein J, Ulrich U, Sasse V, Roth A, Tuttlies F, Karageorgieva E. Reduction of postoperative adhesion formation after laparoscopic ovarian cystectomy. Hum Reprod 1996;11:579–582 [DOI] [PubMed] [Google Scholar]
- 76. Naito M, Ogura N, Yamanashi T, Sato T, Nakamura T, Miura H et al. Prospective randomized controlled study on the validity and safety of an absorbable adhesion barrier (Interceed®) made of oxidized regenerated cellulose for laparoscopic colorectal surgery: adhesion barrier for colorectal surgery. Asian J Endosc Surg 2017;10:7–11 [DOI] [PubMed] [Google Scholar]
- 77. Dupré A, Lefranc A, Buc E, Delpero JR, Quenet F, Passot G et al. Use of bioresorbable membranes to reduce abdominal and perihepatic adhesions in 2-stage hepatectomy of liver metastases from colorectal cancer: results of a prospective, randomized controlled phase II trial. Ann Surg 2013;258:30–36 [DOI] [PubMed] [Google Scholar]
- 78. Zhang E, Li J, Zhou Y, Che P, Ren B, Qin Z et al. Biodegradable and injectable thermoreversible xyloglucan-based hydrogel for prevention of postoperative adhesion. Acta Biomater 2017;55:420–433 [DOI] [PubMed] [Google Scholar]
- 79. Krause TJ, Zazanis GA, McKinnon RD. Prevention of postoperative adhesions with the chitin derivative N-O-carboxymethylchitosan. Wound Repair Regen 1996;4:53–57 [DOI] [PubMed] [Google Scholar]
- 80. Wei C-Z, Hou C-L, Gu Q-S, Jiang L-X, Zhu B, Sheng A-L. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials 2009;30:5534–5540 [DOI] [PubMed] [Google Scholar]
- 81. Zhang E, Guo Q, Ji F, Tian X, Cui J, Song Y et al. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater 2018;74:439–453 [DOI] [PubMed] [Google Scholar]
- 82. Cheng F, Wu Y, Li H, Yan T, Wei X, Wu G et al. Biodegradable N, O-carboxymethyl chitosan/oxidized regenerated cellulose composite gauze as a barrier for preventing postoperative adhesion. Carbohydr Polym 2019;207:180–190 [DOI] [PubMed] [Google Scholar]
- 83. Cai X, Hu S, Yu B, Cai Y, Yang J, Li F et al. Transglutaminase-catalyzed preparation of crosslinked carboxymethyl chitosan/carboxymethyl cellulose/collagen composite membrane for postsurgical peritoneal adhesion prevention. Carbohydr Polym 2018;201:201–210 [DOI] [PubMed] [Google Scholar]
- 84. Li L, Wang N, Jin X, Deng R, Nie S, Sun L et al. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid-based hydrogels for postoperative adhesion prevention. Biomaterials 2014;35:3903–3917 [DOI] [PubMed] [Google Scholar]
- 85. Chen C-H, Chen S-H, Mao S-H, Tsai M-J, Chou P-Y, Liao C-H et al. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr Polym 2017;173:721–731 [DOI] [PubMed] [Google Scholar]
- 86. Shahram E, Sadraie SH, Kaka G, Khoshmohabat H, Hosseinalipour M, Panahi F et al. Evaluation of chitosan–gelatin films for use as postoperative adhesion barrier in rat cecum model. Int J Surg 2013;11:1097–1102 [DOI] [PubMed] [Google Scholar]
- 87. Lin L-X, Luo J-W, Yuan F, Zhang H-H, Ye C-Q, Zhang P et al. In situ cross-linking carbodiimide-modified chitosan hydrogel for postoperative adhesion prevention in a rat model. Mater Sci Eng C Mater Biol Appl 2017;81:380–385 [DOI] [PubMed] [Google Scholar]
- 88. Falabella CA, Melendez MM, Weng L, Chen W. Novel macromolecular crosslinking hydrogel to reduce intra-abdominal adhesions. J Surg Res 2010;159:772–778 [DOI] [PubMed] [Google Scholar]
- 89. Nilsson E, Björn C, Sjöstrand V, Lindgren K, Münnich M, Mattsby-Baltzer I et al. A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann Surg 2009;250:1021–1028 [DOI] [PubMed] [Google Scholar]
- 90. Oh J, Kuan KG, Tiong LU, Trochsler MI, Jay G, Schmidt TA et al. Recombinant human lubricin for prevention of postoperative intra-abdominal adhesions in a rat model. J Surg Res 2017;208:20–25 [DOI] [PubMed] [Google Scholar]
- 91. Sato D, Takahara M, Narita A, Yamakawa J, Hashimoto J, Ishikawa H et al. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J Hand Surg 2012;37:1356–1363 [DOI] [PubMed] [Google Scholar]
- 92. Komatsu K, Fujii A, Higami T. Haemostatic fleece (TachoComb®) to prevent intrapleural adhesions after thoracotomy: a rat model. Thorac Cardiovasc Surg 2007;55:385–390 [DOI] [PubMed] [Google Scholar]
- 93. Kuschel TJ, Gruszka A, Hermanns-Sachweh B, Elyakoubi J, Sachweh JS, Vázquez-Jiménez JF et al. Prevention of postoperative pericardial adhesions with TachoSil. Ann Thorac Surg 2013;95:183–188 [DOI] [PubMed] [Google Scholar]
- 94. Kim E-H, Kim J-W, Han G-D, Noh S-H, Choi J-H, Choi C et al. Biocompatible, drug-loaded anti-adhesion barrier using visible-light curable furfuryl gelatin derivative. Int J Biol Macromol 2018;120:915–920 [DOI] [PubMed] [Google Scholar]
- 95. De Clercq K, Schelfhout C, Bracke M, De Wever O, Van Bockstal M, Ceelen W et al. Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal adhesions: in vitro and in vivo characterization. Biomaterials 2016;96:33–46 [DOI] [PubMed] [Google Scholar]
- 96. Osada H, Tanaka H, Fujii T, Tsunoda I, Yoshida T, Satoh K. Clinical evaluation of a haemostatic and anti-adhesion preparation used to prevent post-surgical adhesion. J Int Med Res 1999;27:247–252 [DOI] [PubMed] [Google Scholar]
- 97. Liu J, Ni B, Zhu L, Yang J, Cao X, Zhou W. Mitomycin C-polyethylene glycol controlled-release film inhibits collagen secretion and induces apoptosis of fibroblasts in the early wound of a postlaminectomy rat model. Spine J 2010;10:441–447 [DOI] [PubMed] [Google Scholar]
- 98. Özgenel GY, Şamli B, Özcan M. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J Hand Surg 2001;26:332–339 [DOI] [PubMed] [Google Scholar]
- 99. Tanaka T, Zhao C, Sun Y-L, Zobitz ME, An K-N, Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg 2007;32:876–881 [DOI] [PubMed] [Google Scholar]
- 100. Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 2006;27:4698–4705 [DOI] [PubMed] [Google Scholar]
- 101. Wallwiener M, Brucker S, Hierlemann H, Brochhausen C, Solomayer E, Wallwiener C. Innovative barriers for peritoneal adhesion prevention: liquid or solid? A rat uterine horn model. Fertil Steril 2006;86:1266–1276 [DOI] [PubMed] [Google Scholar]
- 102. Kataria H, Singh VP. Liquid paraffin vs hyaluronic acid in preventing intraperitoneal adhesions. Indian J Surg 2017;79:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ozmen MM, Aslar AK, Terzi MC, Albayrak L, Berberoğlu M. Prevention of adhesions by bioresorbable tissue barrier following laparoscopic intraabdominal mesh insertion. Surg Laparosc Endosc Percutan Tech 2002;12:342–346 [DOI] [PubMed] [Google Scholar]
- 104. Liu Y, Li H, Shu X, Gray S, Prestwich G. Crosslinked hyaluronan hydrogels containing mitomycin C reduce postoperative abdominal adhesions. Fertil Steril 2005;83:1275–1283 [DOI] [PubMed] [Google Scholar]
- 105. Yeo Y, Adil M, Bellas E, Astashkina A, Chaudhary N, Kohane DS. Prevention of peritoneal adhesions with an in situ cross-linkable hyaluronan hydrogel delivering budesonide. J Controlled Release 2007;120:178–185 [DOI] [PubMed] [Google Scholar]
- 106. Mitchell J, Lee R, Neya K, Vlahakes G. Reduction in experimental pericardial adhesions using a hyaluronic acid bioabsorbable membrane. Eur J Cardiothorac Surg 1994;8:149–152 [DOI] [PubMed] [Google Scholar]
- 107. Tsai S-W, Fang J-F, Yang C-L, Chen J-H, Su L-T, Jan S-H. Preparation and evaluation of a hyaluronate-collagen film for preventing post-surgical adhesion. J Int Med Res 2005;33:68–76 [DOI] [PubMed] [Google Scholar]
- 108. Kato T, Haro H, Komori H, Shinomiya K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J 2005;5:479–488 [DOI] [PubMed] [Google Scholar]
- 109. Kuo SM, Chang SJ, Wang H-Y, Tang SC, Yang S-W. Evaluation of the ability of xanthan gum/gellan gum/hyaluronan hydrogel membranes to prevent the adhesion of postrepaired tendons. Carbohydr Polym 2014;114:230–237 [DOI] [PubMed] [Google Scholar]
- 110. Hagberg L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: a controlled clinical trial. J Hand Surg 1992;17:132–136 [DOI] [PubMed] [Google Scholar]
- 111. Koninckx PR, Corona R, Timmerman D, Verguts J, Adamyan L. Peritoneal full-conditioning reduces postoperative adhesions and pain: a randomised controlled trial in deep endometriosis surgery. J Ovarian Res 2013;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tepetes K, Asprodini EK, Christodoulidis G, Spyridakis M, Kouvaras E, Hatzitheofilou K. Prevention of postoperative adhesion formation by individual and combined administration of 4 per cent icodextrin and dimetindene maleate. Br J Surg 2009;96:1476–1483 [DOI] [PubMed] [Google Scholar]
- 113. Trew G, Pistofidis G, Pados G, Lower A, Mettler L, Wallwiener D et al. Gynaecological endoscopic evaluation of 4% icodextrin solution: a European, multicentre, double-blind, randomized study of the efficacy and safety in the reduction of de novo adhesions after laparoscopic gynaecological surgery. Hum Reprod 2011;26:2015–2027 [DOI] [PubMed] [Google Scholar]
- 114. Kössi J, Grönlund S, Uotila-Nieminen M, Crowe A, Knight A, Keränen U. The effect of 4% icodextrin solution on adhesiolysis surgery time at the Hartmann’s reversal: a pilot, multicentre, randomized control trial vs lactated Ringer’s solution. Colorectal Dis 2009;11:168–172 [DOI] [PubMed] [Google Scholar]
- 115. di Zerega GS, Verco SJ, Young P, Kettel M, Kobak W, Martin D et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod 2002;17:1031–1038 [DOI] [PubMed] [Google Scholar]
- 116. Catena F, Ansaloni L, Di Saverio S, Pinna AD, on behalf of the World Society of Emergency Surgery . P.O.P.A. study: prevention of postoperative abdominal adhesions by icodextrin 4% solution after laparotomy for adhesive small bowel obstruction. A prospective randomized controlled trial. J Gastrointest Surg 2012;16:382–388 [DOI] [PubMed] [Google Scholar]
- 117. Deus C, Kropf S, Kleinstein J. Comparison of 2 different barrier solutions (icodextrin 4% vs. dextran 70) used as adhesion-prevention agents after microsurgical adnexal operations. J Endometr 2014;6:127–134 [Google Scholar]
- 118. Winny M, Maegel L, Grethe L, Lippmann T, Jonigk D, Schrem H et al. Adhesion prevention efficacy of composite meshes Parietex®. Proceed® and:4D. ryField® PH covered polypropylene meshes in an IPOM rat model. Int J Med Sci 2016;13:936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Poehnert D, Abbas M, Kreipe H-H, Klempnauer J, Winny M. Evaluation of 4DryField® PH as adhesion prevention barrier tested in an optimized adhesion model in rats. Eur Surg Res 2015;55:341–351 [DOI] [PubMed] [Google Scholar]
- 120. Kai M, Maeda K, Tasaki M, Kira S, Nakamura S, Chino N et al. Evaluation of a spray-type, novel dextrin hydrogel adhesion barrier under laparoscopic conditions in a porcine uterine horn adhesion model. J Minim Invasive Gynecol 2018;25:447–454 [DOI] [PubMed] [Google Scholar]
- 121. Blumhardt G, Haas M, Polte S. Effect of 4DryField® PH, a novel adhesion barrier, on recurrence of intestinal adhesions after extensive visceral adhesiolysis. Case Rep Surg 2018;2018:9628742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kojima Y, Sakamoto K, Okuzawa A. Experience of using a spray-type anti-adhesion barrier in laparoscopic surgery for colorectal cancer. J Surg Case Rep 2019;2019:rjz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Konovalova MV, Markov PA, Popova GY, Nikitina IR, Shumikhin KV, Kurek DV et al. Prevention of postoperative adhesions by biodegradable cryogels from pectin and chitosan polysaccharides. J Bioact Compat Polym 2017;32:487–502 [Google Scholar]
- 124. Konar S, Guha R, Kundu B, Nandi S, Ghosh TK, Kundu SC et al. Silk fibroin hydrogel as physical barrier for prevention of post hernia adhesion. Hernia 2017;21:125–137 [DOI] [PubMed] [Google Scholar]
- 125. Chowdhury SM, Hubbell JA. Adhesion prevention with ancrod released via a tissue-adherent hydrogel. J Surg Res 1996;61:58–64 [DOI] [PubMed] [Google Scholar]
- 126. Sahbaz A, Aynioglu O, Isik H, Ozmen U, Cengil O, Gun BD et al. Bromelain: a natural proteolytic for intra-abdominal adhesion prevention. Int J Surg 2015;14:7–11 [DOI] [PubMed] [Google Scholar]
- 127. Song Z, Zhang Y, Shao H, Ying Y, Chen X, Mei L et al. Effect of xanthan gum on the prevention of intra-abdominal adhesion in rats. Int J Biol Macromol 2019;126:531–538 [DOI] [PubMed] [Google Scholar]
- 128. Giusto G, Vercelli C, Iussich S, Audisio A, Morello E, Odore R, et al. A pectin-honey hydrogel prevents postoperative intraperitoneal adhesions in a rat model. BMC Vet Res 2016;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Popov SV, Popova GY, Nikitina IR, Markov PA, Latkin DS, Golovchenko VV et al. Injectable hydrogel from plum pectin as a barrier for prevention of postoperative adhesion. J Bioact Compat Polym 2016;31:481–497 [Google Scholar]
- 130. Bang S, Lee E, Ko Y-G, Kim WI, Kwon OH. Injectable pullulan hydrogel for the prevention of postoperative tissue adhesion. Int J Biol Macromol 2016;87:155–162 [DOI] [PubMed] [Google Scholar]
- 131. Li X, Zou B, Zhao N, Wang C, Du Y, Mei L et al. potent anti-adhesion barrier combined biodegradable hydrogel with multifunctional Turkish galls extract. ACS Appl Mater Interfaces 2018;10:24469–24479 [DOI] [PubMed] [Google Scholar]
- 132. Artis T, Artis AS, Arslan E, Mutlu F, Akay A, Deniz K. Preventive effect of ethyl pyruvate on postoperative adhesion formation following abdominal surgery. J Invest Surg 2016;29:260–265 [DOI] [PubMed] [Google Scholar]
- 133. Moro-Oka T, Miura H, Mawatari T, Kawano T, Nakanishi Y, Higaki H et al. Mixture of hyaluronic acid and phospholipid prevents adhesion formation on the injured flexor tendon in rabbits. J Orthop Res 2000;18:835–840 [DOI] [PubMed] [Google Scholar]
- 134. Ishiyama N, Moro T, Ohe T, Miura T, Ishihara K, Konno T et al. Reduction of peritendinous adhesions by hydrogel containing biocompatible phospholipid polymer MPC for tendon repair. J Bone Joint Surg Am 2011;93:142–149 [DOI] [PubMed] [Google Scholar]
- 135. Soules MR, Dennis L, Bosarge A, Moore DE. The prevention of postoperative pelvic adhesions: an animal study comparing barrier methods with dextran 70. Am J Obstet Gynecol 1982;143:829–834 [DOI] [PubMed] [Google Scholar]
- 136. Chen YZ, Hao L, Yang HG, Wu JY, Pan YY, Lu WQ et al. Preventive effects of Tongfu Xiere Enteroclysis Mixture on postoperative intestinal adhesion. Int J Clin Exp Med 2016;9:3981–3987
- 137. Liu S, Zhao J, Ruan H, Tang T, Liu G, Yu D et al. Biomimetic sheath membrane via electrospinning for antiadhesion of repaired tendon. Biomacromolecules 2012;13:3611–3619 [DOI] [PubMed] [Google Scholar]
- 138. Chen C-H, Chen S-H, Shalumon KT, Chen J-P. Dual functional core–sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater 2015;26:225–235 [DOI] [PubMed] [Google Scholar]
- 139. Chen SH, Chen CH, Shalumon KT, Chen JP. Preparation and characterization of antiadhesion barrier film from hyaluronic acid-grafted electrospun poly(caprolactone) nanofibrous membranes for prevention of flexor tendon postoperative peritendinous adhesion. Int J Nanomedicine 2014;9:4079–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wu Q, Li L, Wang N, Gao X, Wang B, Liu X et al. Biodegradable and thermosensitive micelles inhibit ischemia-induced postoperative peritoneal adhesion. Int J Nanomedicine 2014;9:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fu SZ, Li Z, Fan JM, Meng XH, Shi K, Qu Y et al. Biodegradable and thermosensitive monomethoxy poly(ethylene glycol)-poly(lactic acid) hydrogel as a barrier for prevention of post-operative abdominal adhesion. J Biomed Nanotechnol 2014;10:427–435 [DOI] [PubMed] [Google Scholar]
- 142. He T, Zou C, Song L, Wang N, Yang S, Zeng Y et al. Improving antiadhesion effect of thermosensitive hydrogel with sustained release of tissue-type plasminogen activator in a rat repeated-injury model. ACS Appl Mater Interfaces 2016;8:33514–33520 [DOI] [PubMed] [Google Scholar]
- 143. Gong C, Yang B, Qian Z, Zhao X, Wu Q, Qi X et al. Improving intraperitoneal chemotherapeutic effect and preventing postsurgical adhesions simultaneously with biodegradable micelles. Nanomedicine 2012;8:963–973 [DOI] [PubMed] [Google Scholar]
- 144. Wu Q, Wang N, He T, Shang J, Li L, Song L et al. Thermosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci Rep 2015;5:13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Meier Bürgisser G, Calcagni M, Müller A, Bonavoglia E, Fessel G, Snedeker JG et al. Prevention of peritendinous adhesions using an electrospun DegraPol polymer tube: a histological, ultrasonographic, and biomechanical study in rabbits. BioMed Res Int 2014;2014:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hong JH, Choe JW, Kwon GY, Cho DY, Sohn DS, Kim SW et al. The effects of barrier materials on reduction of pericardial adhesion formation in rabbits: a comparative study of a hyaluronan-based solution and a temperature sensitive poloxamer solution/gel material. J Surg Res 2011;166:206–213 [DOI] [PubMed] [Google Scholar]
- 147. Mo F, Yue J, Zhang J, Howk K, Williams A. Evaluation of perivascular adhesion formation in New Zealand white rabbits using Oxiplex and DuraSeal Xact adhesion barrier system. Int J Spine Surg 2009;3:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ozbalci GS, Sulaimanov M, Hazinedaroğlu SM, Törüner A. The effects of hydrophilic polyethylene glycol-based adhesion barrier use to prevent intra-abdominal adhesions in intra-abdominal sepsis model. Indian J Surg 2015;77:398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dasiran F, Eryilmaz R, Isik A, Okan I, Somay A, Sahin M. The effect of polyethylene glycol adhesion barrier (spray gel) on preventing peritoneal adhesions. Bratisl Med J 2015;116:379–382 [DOI] [PubMed] [Google Scholar]
- 150. Elbert DL, Hubbell JA. Reduction of fibrous adhesion formation by a copolymer possessing an affinity for anionic surfaces. J Biomed Mater Res 1998;42:55–65 [DOI] [PubMed] [Google Scholar]
- 151. Gong CY, Wu QJ, Liao JF, Qi TT, Yang B, Wang YJ et al. Prevention of postsurgical cauterization-induced peritoneal adhesions by biodegradable and thermosensitive micelles. J Biomed Nanotechnol 2013;9:1984–1995 [DOI] [PubMed] [Google Scholar]
- 152. Oh SH, Kang JG, Lee JH. Co-micellized pluronic mixture with thermo-sensitivity and residence stability as an injectable tissue adhesion barrier hydrogel: co-micellized pluronic mixture as a tissue adhesion barrier. J Biomed Mater Res B Appl Biomater 2018;106:172–182 [DOI] [PubMed] [Google Scholar]
- 153. West JL, Hubbell JA. Comparison of covalently and physically cross-linked polyethylene glycol-based hydrogels for the prevention of postoperative adhesions in a rat model. Biomaterials 1995;16:1153–1156 [DOI] [PubMed] [Google Scholar]
- 154. Leach RE, Henry RL. Reduction of postoperative adhesions in the rat uterine horn model with poloxamer 407. Am J Obstet Gynecol 1990;162:1317–1319 [DOI] [PubMed] [Google Scholar]
- 155. Park JW, Bak KH, Cho TK, Chun H-J, Ryu JI. Effects of a temperature-sensitive, anti-adhesive agent on the reduction of adhesion in a rabbit laminectomy model. J Korean Neurosurg Soc 2016;59:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Banasiewicz T, Horbacka K, Karoń J, Malinger S, Antos F, Rudzki S et al. Preliminary study with SprayShieldTM adhesion barrier system in the prevention of abdominal adhesions. Wideochir Inne Tech Maloinwazyjne 2013;8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Johns DA, Ferland R, Dunn R. Initial feasibility study of a sprayable hydrogel adhesion barrier system in patients undergoing laparoscopic ovarian surgery. J Am Assoc Gynecol Laparosc 2003;10:334–338 [DOI] [PubMed] [Google Scholar]
- 158. Mettler L, Hucke J, Bojahr B, Tinneberg H-R, Leyland N, Avelar R. A safety and efficacy study of a resorbable hydrogel for reduction of post-operative adhesions following myomectomy. Hum Reprod 2008;23:1093–1100 [DOI] [PubMed] [Google Scholar]
- 159. Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil Steril 2004;82:398–404 [DOI] [PubMed] [Google Scholar]
- 160. Tjandra JJ, Chan MKY. A sprayable hydrogel adhesion barrier facilitates closure of defunctioning loop ileostomy: a randomized trial. Dis Colon Rectum 2008;51:956–960 [DOI] [PubMed] [Google Scholar]
- 161. ten Broek RPG, Kok-Krant N, Verhoeve HR, van Goor H, Bakkum EA. Efficacy of polyethylene glycol adhesion barrier after gynecological laparoscopic surgery: results of a randomized controlled pilot study. Gynecol Surg 2012;9:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tchartchian G, Hackethal A, Herrmann A, Bojahr B, Wallwiener C, Ohlinger R et al. Evaluation of SprayShieldTM adhesion barrier in a single center: randomized controlled study in 15 women undergoing reconstructive surgery after laparoscopic myomectomy. Arch Gynecol Obstet 2014;290:697–704 [DOI] [PubMed] [Google Scholar]
- 163. Mettler L, Audebert A, Lehmann-Willenbrock E, Jacobs VR, Schive K. New adhesion prevention concept in gynecological surgery. JSLS 2003;7:207–209 [PMC free article] [PubMed] [Google Scholar]
- 164. Mettler L. Pelvic adhesions: laparoscopic approach. Ann N Y Acad Sci 2003;997:255–268 [DOI] [PubMed] [Google Scholar]
- 165. Kim SG, Song KY, Lee HH, Kim EY, Lee JH, Jeon HM et al. Efficacy of an antiadhesive agent for the prevention of intra-abdominal adhesions after radical gastrectomy: a prospective randomized, multicenter trial. Medicine (Baltimore) 2019;98:e15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pihlajamäki H, Tynninen O, Karjalainen P, Rokkanen P. The impact of polyglycolide membrane on a tendon after surgical rejoining. A histological and histomorphometric analysis in rabbits. J Biomed Mater Res A 2007;81:987–993 [DOI] [PubMed] [Google Scholar]
- 167. Fukuhira Y, Ito M, Kaneko H, Sumi Y, Tanaka M, Yamamoto S et al. Prevention of postoperative adhesions by a novel honeycomb-patterned poly(lactide) film in a rat experimental model. J Biomed Mater Res B Appl Biomater 2008;86:353–359 [DOI] [PubMed] [Google Scholar]
- 168. Avital S, Bollinger TJ, Wilkinson JD, Marchetti F, Hellinger MD, Sands LR. Preventing intra-abdominal adhesions with polylactic acid film: an animal study. Dis Colon Rectum 2005;48:153–157 [DOI] [PubMed] [Google Scholar]
- 169. Jiang S, Zhao X, Chen S, Pan G, Song J, He N et al. Down-regulating ERK1/2 and SMAD2/3 phosphorylation by physical barrier of celecoxib-loaded electrospun fibrous membranes prevents tendon adhesions. Biomaterials 2014;35:9920–9929 [DOI] [PubMed] [Google Scholar]
- 170. Zong X, Li S, Chen E, Garlick B, Kim K, Fang D et al. Prevention of postsurgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann Surg 2004;240:910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ozpolat B, Gunal N, Pekcan Z, Ayva ES, Bozdogan O, Gunaydin S et al. Polylactic acid and polyethylene glycol prevent surgical adhesions. Bratisl Med J 2016;116:54–58 [DOI] [PubMed] [Google Scholar]
- 172. Hu C, Liu S, Zhang Y, Li B, Yang H, Fan C et al. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater 2013;9:7381–7388 [DOI] [PubMed] [Google Scholar]
- 173. Lodge AJ, Wells WJ, Backer CL, O’Brien JE, Austin EH, Bacha EA et al. A novel bioresorbable film reduces postoperative adhesions after infant cardiac surgery. Ann Thorac Surg 2008;86:614–621 [DOI] [PubMed] [Google Scholar]
- 174. Schreinemacher MHF, Emans PJ, Gijbels MJJ, Greve J-WM, Beets GL, Bouvy ND. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg 2009;96:305–313 [DOI] [PubMed] [Google Scholar]
- 175. Townsend KL, Race A, Keane M, Miller W, Dishaw L, Fisher ER et al. A novel hydrogel-coated polyester mesh prevents postsurgical adhesions in a rat model. J Surg Res 2011;167:e117–e124 [DOI] [PubMed] [Google Scholar]
- 176. Weis C, Odermatt EK. A-part gel: an efficient adhesion prevention barrier. J Biomed Mater Res B Appl Biomater 2007;82:174–182 [DOI] [PubMed] [Google Scholar]
- 177. Bae S-H, Son S-R, Kumar Sakar S, Nguyen T-H, Kim S-W, Min Y-K et al. Evaluation of the potential anti-adhesion effect of the PVA/gelatin membrane: PVA/gelatin membrane. J Biomed Mater Res B Appl Biomater 2014;102:840–849 [DOI] [PubMed] [Google Scholar]
- 178. Weis C, Odermatt EK, Kressler J, Funke Z, Wehner T, Freytag D. Poly(vinyl alcohol) membranes for adhesion prevention. J Biomed Mater Res B Appl Biomater 2004;70:191–202 [DOI] [PubMed] [Google Scholar]
- 179. Renz BW, Leitner K, Odermatt E, Worthley DL, Angele MK, Jauch K-W et al. PVA gel as a potential adhesion barrier: a safety study in a large animal model of intestinal surgery. Langenbecks Arch Surg 2014;399:349–357 [DOI] [PubMed] [Google Scholar]
- 180. Lang RA, Grüntzig PM, Weisgerber C, Weis C, Odermatt EK, Kirschner MH. Polyvinyl alcohol gel prevents abdominal adhesion formation in a rabbit model. Fertil Steril 2007;88:1180–1186 [DOI] [PubMed] [Google Scholar]
- 181. Lalountas M, Ballas KD, Michalakis A, Psarras K, Asteriou C, Giakoustidis DE et al. Postoperative adhesion prevention using a statin-containing cellulose film in an experimental model. Br J Surg 2012;99:423–429 [DOI] [PubMed] [Google Scholar]
- 182. Kalem M, Şahin E, Songür M, Zehir S, Armangil M, Demirtaş MA. Role of anti-adhesive barriers following rotator cuff repair surgery: an experimental study. Acta Orthop Traumatol Turc 2016;50:227–233 [DOI] [PubMed] [Google Scholar]
- 183. Tandon A, Shahzad K, Pathak S, Oommen C, Nunes Q, Smart N. ParietexTM Composite mesh versus DynaMesh®-IPOM for laparoscopic incisional and ventral hernia repair: a retrospective cohort study. Ann R Coll Surg Engl 2016;98:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ko JE, Ko Y-G, Kim WI, Kwon OK, Kwon OH. Nanofiber mats composed of a chitosan-poly(d, l -lactic-co-glycolic acid)-poly(ethylene oxide) blend as a postoperative anti-adhesion agent: chitosan-PLGA-PEO blend nanofibers. J Biomed Mater Res B Appl Biomater 2017;105:1906–1915 [DOI] [PubMed] [Google Scholar]
- 185. Chou P-Y, Chen S-H, Chen C-H, Chen S-H, Fong YT, Chen J-P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater 2017;63:85–95 [DOI] [PubMed] [Google Scholar]
- 186. Murakami T, Hijikuro I, Yamashita K, Tsunoda S, Hirai K, Suzuki T et al. Antiadhesion effect of the C17 glycerin ester of isoprenoid-type lipid forming a nonlamellar liquid crystal. Acta Biomater 2019;84:257–267 [DOI] [PubMed] [Google Scholar]
- 187. El-Sayed N, Galal S, El-Gowelli H, El-Khordagui L. Inhibition of postsurgical adhesions by methylene blue-loaded nanofibers versus cast film matrices. J Biomater Sci Polym Ed 2016;27:1029–1044 [DOI] [PubMed] [Google Scholar]
- 188. Gunay E, Abuoglu HH, Uzunoglu H, Sunamak O, Akyuz C. Efficacy level of dimethyl-sulfoxide (DMSO) in the prevention of peritoneal adhesions: an experimental rat model. Int J Clin Exp Med 2019;12:705–711 [Google Scholar]
- 189. Liu S, Pan G, Liu G, Neves Jd, Song S, Chen S et al. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release 2017;264:1–13 [DOI] [PubMed] [Google Scholar]
- 190. Shin YC, Yang WJ, Lee JH, Oh J-W, Kim TW, Park JC et al. PLGA nanofiber membranes loaded with epigallocatechin-3-O-gallate are beneficial to prevention of postsurgical adhesions. Int J Nanomedicine 2014;9:4067–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Whitfield RR, Stills HF, Huls HR, Crouch JM, Hurd WW. Effects of peritoneal closure and suture material on adhesion formation in a rabbit model. Am J Obstet Gynecol 2007;197:644.e1–644.e5 [DOI] [PubMed] [Google Scholar]
- 192. Sekiba K. Use of Interceed(TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. The Obstetrics and Gynecology Adhesion Prevention Committee. Obstet Gynecol 1992;79:518–522 [PubMed] [Google Scholar]
- 193. Chung Y-J, An S-Y, Yeon J-Y, Shim WS, Mo J-H. Effect of a chitosan gel on hemostasis and prevention of adhesion after endoscopic sinus surgery. Clin Exp Otorhinolaryngol 2016;9:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zhou J, Liwski RS, Elson C, Lee TDG. Reduction in postsurgical adhesion formation after cardiac surgery in a rabbit model using N, O-carboxymethyl chitosan to block cell adherence. J Thorac Cardiovasc Surg 2008;135:777–783 [DOI] [PubMed] [Google Scholar]
- 195. Li C, Wang H, Liu H, Yin J, Cui L, Chen Z. The prevention effect of poly(l-glutamic acid)/chitosan on spinal epidural fibrosis and peridural adhesion in the post-laminectomy rabbit model. Eur Spine J 2014;23:2423–2431 [DOI] [PubMed] [Google Scholar]
- 196. ten Broek RPG, Bakkum EA, Laarhoven CJHM, van Goor H. Epidemiology and prevention of postsurgical adhesions revisited. Ann Surg 2016;263:12–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.W., upon reasonable request.