Abstract
Purpose
Mitochondrial trifunctional protein deficiency (TFPD) and isolated long chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) are two related defects of fatty acid β -oxidation. While NBS has decreased mortality, morbidity remains significant. Additionally, the relationship of genotype to clinical outcome remains unclear. To better understand these issues, we collected natural history data for these conditions by reviewing seven years of retrospective data from 45 cases of TFPD or LCHADD in the Inborn Errors of Metabolism – Information System.
Methods
Available data included age at database entry, last datapoint, and development of various complications. Data were analyzed by clinical assigned diagnosis (LCHADD or TFPD), subdivided by method of ascertainment (newborn screening-NBS, or other than by newborn screening-NNBS), then re-analyzed based on four genotype groups: homozygous c.1528GC (p.E510Q) (common LCHAD variant); heterozygous c.1528GC (p.E510Q), other HADHA variants; and HADHB variants.
Results
Forty-five patients from birth to 34 years of age were analyzed by assigned diagnosis (30 LCHADD and 15 TFPD) and method of ascertainment. Thirty had further analysis by genotype (22 biallelic HADHA variants and 8 biallelic HADHB variants). With regards to maternal complications, retinopathy, cardiomyopathy and hypoglycemia, patients with biallelic HADHA variants (with or without the common LCHAD variant) manifest a traditional LCHADD phenotype, while those with HADHB gene variants more commonly reported neuromusculoskeletal type TFPD phenotype. While retinopathy, rhabdomyolysis and peripheral neuropathy tended to present later in childhood, many features including initial report of cardiomyopathy and hypoglycemia presented across a wide age spectrum.
Conclusion
This study demonstrates the utility of genotypic confirmation of patients identified with LCHADD/TFPD as variants in the HADHA and HADHB genes lead to different symptom profiles. In our data, biallelic HAHDA variants conferred a LCHADD phenotype, regardless of the presence of the common LCHAD variant.
Keywords: Inborn errors of metabolism, Genetics, Pediatrics, Fatty acid oxidation disorders, LCHAD, TFP, MTFP, Trifunctional protein, Mitochondrial trifunctional protein
1. Introduction
Fatty acid β-oxidation (FAO) is a critical physiologic process that allows utilization of fat as a source of energy during times of stress and fasting. Inborn errors of fatty acid metabolism affect ~1:5000–10,000 live births in the United States [1]. Three enzymatic steps and a carnitine molecule are required for transport of long chain fatty acids into mitochondria following their activation to acyl-CoAs in the cytoplasm [2]. In the mitochondrial matrix, one cycle of β-oxidation requires four sequential steps resulting in release of an acetyl-CoA moiety, two reducing equivalents that can enter the respiratory chain to generate ATP, and an acyl-CoA shortened by two carbons [3]. The second through fourth steps of long chain fatty acid oxidation in the mitochondrial matrix are located in a trifunctional protein (TFP) that contains the three sequential enzyme functions for long chain fats: 2,3-enoyl-CoA hydratase (ECH), 3-hydroxyacyl-CoA dehydrogenase (LCHAD), and 3-ketoacyl-CoA thiolase (LKAT) (Supplemental Fig. 1). Autosomal recessive defects in this enzyme complex can lead to isolated loss of long chain 3-hydroxyacyl-CoA dehydrogenase deficiency activity (LCHADD), or to deficiency of all three components of the TFP (TFPD) [4] [5]. Traditionally these two disorders (LCHADD and TFPD) were viewed as presenting with similar biochemical and clinical phenotypes, with high rates of morbidity and mortality prior to institution of tandem mass spectrometry-based newborn screening in the United States [6].
Mitochondrial TFP is composed of 4α subunits containing the ECH and LCHAD activities encoded by the HADHA gene, and 4β subunits containing the LKAT activity encoded by the HADHB gene [7]. A single common c.1528 G > C (p.E510Q) variant in the HADHA gene (heretofore referred as the LCHAD common variant) accounts for essentially all cases of isolated LCHADD, and occurs with estimated frequency of 1:110,000–150,000 [8]. This variant inactivates a catalytic residue in the substrate binding pocket of the LCHAD moiety of the α-subunit of TFP [9]. There is some controversy regarding the classification of other variants in HADHA as TPFD or isolated LCHADD. Variants in the HADHB gene typically result in TFPD, which is more rare [10].
LCHADD and TFPD share many clinical features including neonatal liver dysfunction and hypoglycemia, neonatal or later onset cardiomyopathy, and recurrent rhabdomyolysis [11]. Irreversible retinal disease has been reported uniquely in up to 30–50% of isolated LCHADD cases, while peripheral neuropathy was originally thought to be a unique characteristic of TFPD. It has been hypothesized that these findings are related to the specific pattern of acyl-CoA or acylcarnitine intermediates that accumulate in each disorder [11]. Two rare maternal complications, HELLP (hemolysis, elevated liver enzymes, and low platelet syndrome) and AFLP (acute fatty liver of pregnancy) [12], have been reported in obligate heterozygote women carrying fetuses predominantly with LCHADD but also other disorders of fatty acid oxidation [10]. Current treatment options for LCHAD/TFP deficiencies are limited, including dietary restriction of long chain fat with supplementation with medium chain triglycerides, prevention of fatty acid oxidation by frequent feeding, and anaplerotic therapy with the odd chain medium chain triglyceride triheptanoin [6] [13]. FAO disorders (FAOD) are now routinely identified by newborn screening (NBS) in the United States and many developed nations, resulting in significantly decreased morbidity and mortality [12].
The Inborn Errors of Metabolism-Information System (IBEM-IS) is a federally funded database designed to collect longitudinal clinical data on patients with metabolic disorders identifiable by newborn screening [14]. IBEM-IS data are de-identified, thus analysis is limited to the data as entered by the patient's center. We reviewed the IBEM-IS database for retrospective natural history data on patients with TFP and isolated LCHAD deficiencies to better understand genotype-phenotype correlations for common clinical problems in these disorders, including maternal/neonatal complications, long term neurodevelopment, and development of cardiomyopathy, retinopathy, neuropathy, and episodes of rhabdomyolysis. Evaluation of these longitudinal clinical data will help elucidate the differences between TFPD and LCHADD and provide better information for developing endpoints for future studies of novel therapies.
2. Materials and methods
We reviewed all cases of TFPD/LCHADD enrolled in the IBEM-IS, database from March 2007 to November 2014. Patients were ascertained by abnormal NBS, or by a method other than NBS (NNBS) (i.e. clinical presentation in the patient or a family member with this condition). Diagnosis was entered by the treating metabolic center and originally 30 patients with LCHADD and 16 with TFPD were included. One patient (labeled as TFPD) was dropped from further evaluation due to lack of sufficient information. The remaining 45 patients were analyzed in two ways: first by the diagnosis assigned by the entering provider in IBEM-IS and subdivided by method of ascertainment, and then by genotype. Specific outcome variables of interest included diagnostic method, age at enrollment to the study, genotypes, presence of maternal or neonatal complications, and initial and interval evaluations of cardiac, neurologic, and retinal function, hypoglycemia, as well as episodes of rhabdomyolysis. Data were de-identified and limited to that available in the database. An exemption was granted for this study by the University of Pittsburgh Institutional Review Board.
2.1. Analysis by assigned diagnosis
All 45 patients were included in the analysis and divided into two groups: a reported diagnosis of isolated LCHADD further subdivided into diagnosis by NBS (n = 16) or NNBS (n = 14), or a reported diagnosis of TFPD subdivided into diagnosis by NBS (n = 6) or NNBS (n = 9) (Supplemental Table 1). Eight subjects with unknown diagnostic method were grouped into the NNBS groups. For many patients, the information necessary to confirm the diagnosis by enzyme assay or genotyping was not available in the dataset. Although HADHA variants other than the common LCHAD variant are conventionally regarded as affording a TFPD phenotype, many patients are assigned diagnosis in the absence of molecular analysis. In our study population, many with biallelic variants other than the common LCHAD variant were assigned a diagnosis of LCHADD. Genotypic analysis was designed to more specifically define genotype-phenotype relationships, as well as to determine the accuracy of the initially assigned diagnosis.
2.2. Genotypic analysis
30 of the 45 patients had genotype data available. The patients were divided into five groups based on their genotype. Group I (n = 5): patients homozygous for the common LCHAD variant in the HADHA gene; Group II (n = 7): heterozygous for the common variant and a second variant in the HADHA gene; Group III (n = 10): biallelic for other HADHA variants; Group IV (n = 8): biallelic HADHB variants. Group V (n = 15) consisted of the remaining patients with no genotype available. It is important to note that, among subjects with known genotypes, all subjects with a variant in the HADHA gene had been assigned diagnosis of isolated LCHADD by their entering institution, while all subjects with HADHB variant were assigned the diagnosis of TFPD by their entering institutions (Table 2).
Table 2.
Demographics and incidence of common complications of patients undergoing genotypic analysis.
| Group |
I |
II |
III |
IV |
V |
|---|---|---|---|---|---|
| Genotype | Common 1528G > C HADHA Homozygous | Common 1528G > C HADHA Heterozygous | Other HADHA Variants | HADHB variants | Not genotyped |
| Assigned diagnosis | LCHAD | LCHAD | LCHAD | TFPD | LCHAD/TFPD |
| n | 5 | 7 | 10 | 8 | 15 |
| Age at enrollment (y) | 8.79 (0.37–34.47) |
13.08 (0.3–20.29) |
4.13 (0.03–17.55) |
7.79 (0.56–16.35) |
8.00 (0.04–19.32) |
| Age at last data point (y) | 10.60 (0.37–34.47) |
13.62 (1.51–21.11) |
7.50 (2.58–21.88) |
8.57 (0.56–17.26) |
10.02 (0.04–25.72) |
| Maternal HELLP/AFLP | 2 of 5 | 2 of 7 | 3 of 10 | 0 of 8 | 1 of 15 |
| Neonatal complication | 4 of 5 | 4 of 7 | 6 of 10 | 0 of 8 | 5 of 15 |
| Cardiomyopathy | 4 of 5 | 3 of 7 | 3 of 10 | 0 of 8 | 4 of 15 |
| Age onset cardiomyopathy (y) | 2.43 (0.37–5.96) |
14.96 (11.53–18) |
2.57 (0.66–4.35) |
⁎n/a | 7.88 (2.7-15.95) |
| Hypoglycemia | 1 of 5 | 2 of 7 | 6 of 10 | 0 of 8 | 1 of 15 |
| Age onset hypoglycemia (y) |
⁎n/a (5.96) |
⁎n/a (1.50, 17.59) |
5.34 (0.09–17.55) |
⁎n/a |
⁎n/a (15.95) |
| Retinopathy | 2 of 5 | 2 of 7 | 4 of 10 | 0 of 8 | 1 of 15 |
| Age onset retinopathy (y) |
⁎n/a (5, 6.90) |
⁎n/a (14.80-20.29) |
7.17 (3.4–17.69) |
⁎n/a |
⁎n/a (20.91) |
| Rhabdomyolysis | 2 of 5 | 2 of 7 | 4 of 10 | 2 of 8 | 7 of 15 |
| Age onset rhabdomyolysis (y) |
⁎n/a (3.16, 20) |
⁎n/a (17.01, 20.29) |
7.52 (0.7–17.69) |
⁎n/a (3, 7.90) | 7.50 (0.04–20.87) |
| Other neuromuscular | 2 of 5 | 2 of 7 | 3 of 10 | 6 of 8 | 5 of 15 |
| Age onset other neuromuscular (y) |
⁎n/a (5.96, 34.47) |
⁎n/a (18.76, 20.29) |
4.29 (0.51–7.66) |
9.87 (4.33–16.35) |
12.42 (3.49–19.32) |
| Peripheral neuropathy | 2 of 5 | 3 of 7 | 1 of 10 | 2 of 8 | 1 of 15 |
| Age onset peripheral neuropathy (y) |
⁎n/a (3.16, 20) |
18.64 (17.01–21.11) |
⁎n/a (7.66) |
⁎n/a (7.90, 3.00) |
⁎n/a (19.04, 19.32) |
y = In years.
Values listed as: Mean (range- range) or Mean (individual data points for 2 or fewer data points).
mean/range values were not calculated with 2 or fewer data points; individual data points were listed.
2.3. Phenotypic definitions
In this study, subjects were considered positive for cardiomyopathy if there was a specific mention in their database entries of cardiomyopathy, ventricular dilation, or hypertrophy. Patients were considered as having a history of hypoglycemia when there was a specific mention of the condition or laboratory results (blood glucose <60) in their database record. Retinopathy was defined as any cause of loss of visual acuity due to retinal changes, including retinitis pigmentosa, retinal pigment change, or retinopathy. Rhabdomyolysis was defined as muscular symptoms with creatinine kinase level > 2000 international units/L. Patients were also considered positive for rhabdomyolysis if there was a specific mention of rhabdomyolysis in their database record. Neuromuscular symptoms other than rhabdomyolysis were grouped in a single category, including muscle weakness, club foot, hypertonia, hypotonia, myalgia, decreased strength, decreased balance, abnormal gait, spastic gait, neuromuscular weakness, myopathy, decreased muscle mass, deterioration of muscle function, or use of a wheelchair. Peripheral neuropathy was considered present if there was mention of neurologic deficits including peripheral neuropathy, hyporeflexia, or overflow signs and alternating movements. The data did not allow determination whether some neuromuscular symptoms might be due to pre-existing peripheral neuropathy.
2.4. Statistical analysis
The wide age variation among the patients ascertained by NBS vs. other methods complicated the analysis of age of onset of various complications. It is likely some of the NNBS patients died without diagnosis or before study initiation, and many NBS ascertained patients were still very young and might not yet have developed some complications. Therefore, the NBS and NNBS patients were analyzed separately by Kaplan-Meier time to event. The sample size of the genotype analysis was not large enough to perform this analysis for that group. Chi-square analysis or Fisher's exact test was performed for comparisons as indicated. Where multiple comparisons were made (comparing outcomes in those having HADHA vs HADHB variants, or between those in the HADHA groups having two, one or no copies of the common LCHAD variant a p value of p < 0.01 was required for significance.
3. Results
3.1. Demographics in patients assigned by clinical diagnosis and genotype
Table 1 reports the demographics of study subjects by their initially assigned diagnoses, as well as the incidence of the most common complications. The subjects were divided by their assigned clinical diagnosis of LCHADD or TFPD, then by method of ascertainment (NBS or NNBS). In the LCHADD NBS group, 16 patients had been diagnosed with LCHADD by the entering provider. In LCHADD NNBS group, 14 patients carried a diagnosis of LCHADD. In the TFPD NBS group, six patients were diagnosed by the entering provider. In the TFPD NNBS group, nine were diagnosed by other means. Not surprisingly, patients in the NBS ascertained groups were younger at the time of enrollment than the NNBS ascertained group.
Table 1.
Demographics and incidence of common complications of patients undergoing analysis by given diagnosis.
| Group | LCHAD NBS | LCHAD NNBS | TFPD NBS |
TFPD NNBS |
|---|---|---|---|---|
| Given Diagnosis | LCHADD | LCHADD | TFPD | TFPD |
| n | 16 | 14 | 6 | 9 |
| Age at enrollment (y) | 2.44 (0.03–11.53) |
12.72 (0.5–34.47) |
3.05 (0.04–7.16) |
13.21 (2.66–19.32) |
| Age at last data point (y) | 4.66 (0.37–11.53) |
14.57 (0.6–34.47) |
3.81 (0.04–11.69) |
15.65 (2.66–25.72) |
| Maternal complication | 4 of 16 | 8 of 14 | 0 of 6 | 1 of 9 |
| Neonatal complication | 10 of 16 | 7 of 14 | 1 of 6 | 2 of 9 |
| ⁎⁎Cardiomyopathy | 5 of 16 | 6 of 14 | 0 of 6 | 3 of 9 |
| Age onset cardiomyopathy (y) | 4.26 (0.37–11.53) |
9.49 (0.66–18) |
⁎n/a | 3.9 (2.7-5) |
| Other cardiac anomalies | 3 of 16 | 2 of 14 | 1 of 6 | 0 of 9 |
| ⁎⁎⁎Hypoglycemia | 6 of 16 | 6 of 14 | 0 of 6 | 0 of 9 |
| Age onset hypoglycemia (y) | 1.70 (0.09–3.44) |
11.04 (1.5–17.59) |
⁎n/a | ⁎n/a |
| Retinopathy | 3 of 16 | 6 of 14 | 0 of 6 | 0 of 9 |
| Age onset retinopathy (y) | 3.67 (3.4–3.94) |
14.27 (5–20.91) |
⁎n/a | ⁎n/a |
| Rhabdomyolysis | 4 of 16 | 7 of 14 | 2 of 6 | 4 of 9 |
| Age onset rhabdomyolysis (y) | 2.63 (0.7–4.03) |
12.27 (0.87–20.29) |
⁎n/a (0.04, 7.9) |
9.07 (3–20.87) |
| ⁎⁎⁎⁎Other neuromuscular | 3 of 16 | 6 of 14 | 2 of 6 | 7 of 9 |
| Age onset other neuromuscular (y) | 2.9 (0.51–4.7) |
17.18 (5.96–34.37) |
⁎n/a (4.33, 7.90) |
12.80 (2.66–19.32) |
| Peripheral neuropathy | 1 of 16 | 5 of 14 | 1 of 6 | 3 of 9 |
| Age onset peripheral neuropathy (y) |
⁎n/a (3.16) |
16.72 (7.66–21.11) |
⁎n/a (7.9) |
18.08 (15.89–19.32) |
y = In years
Values listed as: Mean (range- range) or Mean (individual data points with 2 or fewer data points)
Mean/range values were not calculated with 2 or fewer data points; individual data points were listed.
Overall incidence of CM before age 11 = 0.22; Incidence of CM after age 11 = 0.21.
Oldest age at recurrence LCHADD NBS = 6.9 years old, LCHADD NNBS = 20.9 years old.
Other neuromuscular problems = 9 of 18 patients with other neuromuscular problems had age onset after 11 years old.
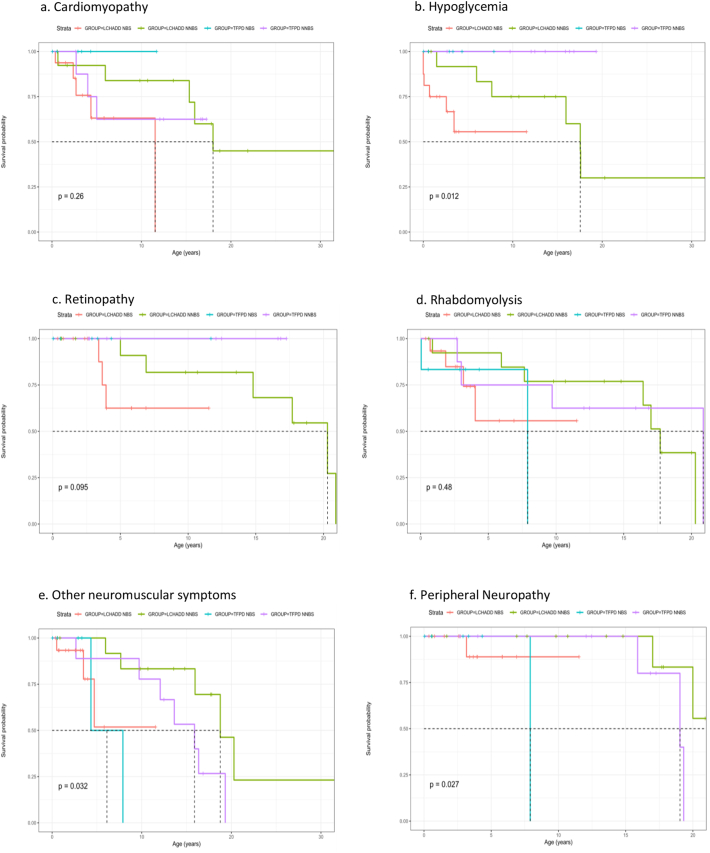
Fig. 1. shows Kaplan–Meier survival curves for adverse outcomes for patients by their original diagnosis stratified by the methods of ascertainment (NBS vs. NNBS). In this case “survival” denotes patients who did not develop outcome of interest and thus remained at risk of developing the outcome. By applying Peto's log-rank test, differences in survival functions were observed for hypoglycemia (p < 0.02), retinopathy (p < 0.01), neuromuscular symptoms (p < 0.04), and neuropathy (p < 0.03). For hypoglycemia, for example, at age 0 (birth), all were at risk for potential hypoglycemia. By 10 years of age, many patients in the NBS groups have already been diagnosed with hypoglycemia, while those in the NNBS group received the diagnosis at a later age.
Fig. 1.
Kaplan-Meier time to event analysis.
Table 2 shows the demographics of study subjects by their genotype, as well as the incidence of the most common complications. The supplemental table 1 provides details of each patient's genotype as indicated in the original dataset. Unfortunately, in the original dataset, variants other than HADHA common LCHAD variant were specified only as “other”. Thus, genotype/phenotype correlations were limited to grouping patients with HADHA variant only by the number of common LCHAD variants (as two, one, or none), vs HADHB variants. Subjects were thus divided into five groups. Group I: patients homozygous for the HADHA common LCHAD variant (n = 5); Group II: patients heterozygous for one copy of the HADHA common LCHAD variant and one other variant in the HADHA gene (n = 7); Group III: patients with biallelic other variants in the HADHA gene (n = 10); Group IV: patients with biallelic variants in the HADHB gene (n = 8). Group V consisted of patients with no genotype reported (n = 15) and were not further analyzed.
3.2. Maternal and neonatal complications
Neonatal complications included prematurity, respiratory distress, hypoglycemia, hypothermia, congenital anomalies, and bowel perforation. In analysis of patients by assigned diagnosis, 17 of 30 diagnosed with LCHADD by the referring center reported a neonatal complication vs. 3 of 15 with TFPD (Table 1); (p < 0.02). In the genotypic analysis, 14 of 22 patients with biallelic variants in HADHA reported a neonatal complication compared to none with HADHB variants (p < 0.003) (Table 2). Maternal complications reported included AFLP, HELLP syndrome, hypoglycemia, placenta previa, preterm labor, or postpartum hemorrhage. In analysis by reported diagnosis, 12 of 30 with a diagnosis of LCHADD reported a maternal complication, vs. 1 of 15 in the TFPD group (p < 0.021). In the genotypic analysis, there was a non-significant trend noting 7 of 22 with HADHA variants reported maternal HELLP/AFLPP, compared to 0 of 8 with HADHB variants (p < 0.14). In those with HADHA variants, there was no difference in the rate of neonatal or maternal complications when stratified by two, one or zero copies of the HADHA common variant.
3.3. Common complications
3.3.1. Cardiomyopathy
Cardiomyopathy (CM) has been reported in both isolated LCHADD and combined TFPD. In our cohort of 45 subjects, the median age of first report of CM was 4.18 years (range 0.37 to 18 years). We evaluated the time to first diagnosis of cardiomyopathy and other outcomes based on the clinically assigned diagnosis using Kaplan-Meier time to event curves. Fig. 1a demonstrates the age in years at development of cardiomyopathy in each of the four groups. Notably, cardiomyopathy occurred across the age spectrum. Early onset cardiomyopathy (defined as incidence prior to age 11) was reported across all groups and across a wide range of ages with an incidence of 0.22 before age 11 years and 0.21 after age 11. No one with TFPD identified by NBS reported CM; however the patients were young, the sample size was small, and patients were likely undergoing some metabolic therapy. Only the isolated LCHADD groups reported onset of CMs after the first decade of life, with initial appreciation as late as age 18 years in the LCHADD NNBS group. It is possible that younger patients born before NBS died without ascertainment, while older patients in the NBS group were protected by post-diagnostic management changes. Overall, cardiomyopathy was reported in 11 of 30 with LCHADD, and 3 of 15 with TFPD (not significant). In genotypic analysis, CM was reported in 10 of 22 individuals with variants in the HADHA gene [having two, one or no copies of the HADHA common LCHAD variant] but was not reported in individuals with variants in the HADHB gene (p < 0.03). While the latter group was small, it suggests a possible differential risk for developing CM based on genotype. Among those with HADHA variants, there was no significant difference in occurrence of cardiomyopathy when stratified by number of common HADHA variants. Other cardiac anomalies including patent foramen ovale and valvular anomalies occurred sporadically across different subgroups (Table 1) and were not included in the analysis.
3.3.2. Hypoglycemia
Hypoglycemia is a cardinal sign of long chain FAODs and has been reported to decrease with age [15]. In our cohort, the median age of first report of hypoglycemia was 3.01 years and range 0 to 17.6 years. Overall, hypoglycemia was only reported with a diagnosis of LCHADD or variants in the HADHA gene (Table 1, Table 2). In the analysis by assigned clinical diagnosis, 12 of 20 patients with LCHADD reported hypoglycemia, compared to 0 of 15 with TFPD (p < 0.004). Like that of CM, the younger LCHAD NBS group showed earlier incidence of hypoglycemia compared to the older LCHAD NNBS groups, which reported both early and late onset hypoglycemia (Table 1, Fig. 1b). The oldest age at recurrence was 20.91 years old in group LCHADD NNBS. These data suggest hypoglycemia can occur at any age in isolated LCHADD, though fasting prevention and supplementation with intravenous glucose might have prevented recurrence in NBS ascertained patients. In the genotypic analysis, hypoglycemia was identified in 11 of 22 patients with all HADHA variants combined, but not in patients with HADHB variant (p < 0.015) (Table 2). In those with HADHA variants, when stratified by two, one or no copies of the HADHA common LCHAD variant, there was no significant difference in incidence of hypoglycemia.
3.3.3. Retinopathy
Retinopathy is commonly viewed as a problem in isolated LCHADD rather than combined TFPD [11], and retinopathy was reported in 9 of 30 LCHADD patients but no TFP patients. In our cohort, the median age of first report of retinopathy was 6.9 years (range 3.4 to 20.91 years). In the analysis by assigned diagnosis, retinopathy indeed was only observed in patients with clinical diagnosis of LCHADD, and not in patients carrying a clinical diagnosis TFPD. The higher reported frequency of early onset retinopathy compared to the literature is likely explained by early ophthalmology exams in patients in the NBS cohort. The observation was reinforced by the report of later identification of retinopathy in individuals belonging to NNBS group (Fig. 1c). In the genotypic analysis, retinopathy occurred in all stratifications of the HADHA variants, with no significant difference among patients having two, one or no copies of the common LCHAD variant (Table 2).
3.3.4. Rhabdomyolysis and other neuromuscular complications
Rhabdomyolysis is the most frequent complication reported in both isolated LCHADD and combined TFPD, especially in older children and adults [15]. In our cohort, the median age of first report of rhabdomyolysis was 5.96 years but the range was very wide (0.04 to 20.87 years). In analysis by assigned diagnosis, there was no difference in the incidence of rhabdomyolysis between the LCHADD group (11 of 30) or the TFP group (6 of 15). Unlike hypoglycemia, which was less likely to recur with aging, most individuals continued to have episodes of rhabdomyolysis as they aged. Individuals who reported late onset rhabdomyolysis all belonged to NNBS groups, likely the result of early under-reporting due to a late diagnosis (Table 1, Fig. 1d). In the genotypic analysis, rhabdomyolysis also was reported across all genotype groups (Table 2), with no significant difference when stratified by number of HADHA common LCHAD variants.
Other neuromuscular problems including muscle weakness, club foot, hypertonia, hypotonia, myalgia, decreased strength, decreased balance, abnormal gait, spastic gait, neuromuscular weakness, myopathy, decreased muscle mass, deterioration of muscle function, and wheelchair use were also reported. In total, 18 patients had neuromuscular issues other than rhabdomyolysis. They were identified across the spectrum of the groups with a wide range of age of onset but they were more predominant in older patients. The TFPD NNBS group had the highest incidence of neuromuscular symptoms, 78% reporting symptoms, followed by 43% in LCHADD NNBS group, both cohorts containing mostly older individuals (Table 1). In the genotype analysis, 7 of 22 with biallelic variants in HADHA reported other neuromuscular concerns, compared to 6 of 8 of those with HADHB variants (p < 0.05). When stratified by number of HADHA common LCHAD variants in, no differences were significant.
3.3.5. Peripheral neuropathy
Peripheral neuropathy (PN) has traditionally been described in TFPD though milder symptoms are reported in LCHADD, especially with increasing age [11]. Though the least frequently reported symptom in our cohort, PN was observed in all assigned diagnosis groups and genotypes (Table 1, Table 2). The median age at presentation was 17.41 years, and the range was 3.16 to 21.11 years. There was no significant difference between the incidence of PN between the LCHADD group and the TFPD group, nor between the HADHA and the HADHB groups. Thus, PN was not limited to TFPD in our data.
4. Discussion
Life expectancy of newborns with isolated LCHAD and combined TFP deficiency has been poor due to acute on chronic symptoms, but is improving thanks to early detection by NBS, which allows opportunities for symptomatic and preventive intervention as well as improvements in treatment. However, newborn screening also poses a challenge as genotype/phenotype correlations have only been described in limited populations [16],[17]. Thus, longitudinal studies investigating long-term complications are crucial to appropriate counseling and long-term therapeutic planning for patients. This study highlights 7 years of disease history in 45 patients with TFP/LCHAD deficiencies ages 0 to 34 years (with the oldest patient ascertained by newborn screening being 11 years of age). Data captured include information on neonatal and maternal complications, cardiomyopathy, hypoglycemia, retinopathy, peripheral neuropathy, and rhabdomyolysis. We were also able to compare phenotypes of individuals with variants in the HADHA and HADHB genes to better address the correlation of genotype with clinical symptoms.
Traditionally having homozygosity for the common LCHAD variant (c.1528G > C [p.E510Q]) in the HADHA gene has been regarded as causing the LCHADD phenotype, while other biallelic variants in HADHA or HADHB genes have been regarded as causing the TFPD phenotype. The most noteworthy findings in these data reveal that patients with biallelic variants in the HADHA gene, regardless of whether they have the common LCHAD variant or other HADHA variants demonstrated a phenotype that resembles LCHADD more than TFPD. Specifically, retinopathy was found in all HADHA variant genotypes groups, even those with no copies of the HADHA common LCHAD variant. In addition, patients with HADHA variants (even in those with no copies of the LCHADD common variant) were much more likely to present with maternal HELLP or AFLP. The data also suggest trends that hypoglycemia and cardiomyopathy may be more common in patients with biallelic HADHA variants compared to those with HADHB variants, regardless of whether the patient is heterozygous, compound heterozygous, or has no copies of the HADHA common LCHAD variant. However, this analysis is limited by the small number of genotyped TFPD patients (n = 8).
Cardiomyopathy is a well-documented complication of long chain fatty acid defects, reported in the neonatal period with high infant mortality, as well as later in life [16], [18]. Incidence of CM in older patients of our clinical cohorts showed that risk for this finding persists, even into the teen years. The fact that no individuals with variants in the HADHB gene reported CM was somewhat surprising and may suggest a possible differential risk for developing CM based on genotype, though ascertainment bias and/or random chance due to a small cohort size are also possible.
Hypoglycemia is common to all the fatty acid oxidation disorders and is typically described as being more frequent in infancy than in late childhood or adulthood [15]. Concordant with this observation, the onset of hypoglycemia was more common in the first decade of life regardless of assigned diagnosis group or method of diagnosis. However, patients in NBS group was diagnosed with hypoglycemia earlier than those in NNBS group. This is likely accounted for by several reasons, including many NNBS patients may have died before diagnosis so that early onset was missed, that newborn diagnosis with early surveillance led to earlier identification of concerns, or that early post-diagnostic management prevented later onset of some complications. The lack of reported hypoglycemia in the TFPD population or individuals with variant in HADHB gene may also reflect the relatively small number of TFPD patients in the database. Larger population studies are clearly warranted.
Irreversible retinal disease and peripheral neuropathy are findings unique to LCHAD/TFP deficiencies among the LC-FAODs, and these findings have been speculated to be related to the accumulation of specific acyl-CoA/carnitine intermediates in these disorders [11,19]. Traditionally, retinal changes are much more common in LCHADD compared to TFPD [11] which was indeed demonstrated by our study but may be due to small number of TFPD patients in the database.
Rhabdomyolysis and other neuromusculoskeletal problems were the most common and recurrent symptoms across all diagnostic and genotypic groups.
Peripheral neuropathy has traditionally been more frequently described in TFPD [11]. However, we observed this finding across all assigned diagnosis groups and genotypes. Age of onset of this complication was late in most cases, supporting the hypothesis that this complication develops over time. It should be noted that most entries regarding peripheral neuropathy in this population were based on symptoms rather than formal nerve conduction testing. Given the multiple care providers across study centers, there is certainly significant inter-reporter variability. However, this assumption should be true for all genotypes.
Assigned diagnosis as reported in the original IBEM-IS data posed an interesting challenge in our analysis due to discrepancy from the current understanding that isolated LCHADD is almost exclusively caused by homozygous c.1528G > C variant, and any other variants cause TFPD. Unfortunately, there was no information regarding enzymatic testing to support these diagnoses, nor could it be determined from the dataset why the clinical diagnoses were assigned. However, the analysis by genotype was entirely consistent over numerous outcome measures, and mirrored the assigned diagnosis made by the entering metabolic center. Overall, the correlation of genotype with clinical diagnosis in our population supported a more consistent profile consisting of maternal complications, cardiomyopathy, retinopathy and/or hypoglycemia in individuals with any HADHA variants, regardless of the variant. Without prospective data, it is not clear how the clinical assignments were influenced ultimately by genotypic data. Our findings are also consistent with the previous understanding that newborns with TFPD who survive the infancy might have milder forms of TFPD and thus present with recurrent rhabdomyolysis and myopathy with peripheral neuropathy +/− cardiomyopathy, as opposed to more severe forms of TFPD with severe dilated cardiomyopathy, Reye like syndrome, and neonatal death [10,19,20].
Finally, our finding that 7 of 8 newborns whose mothers reported AFLP or HELLP had variants in the HADHA gene (the eighth did not have genotype available) is consistent with prior reports that variants in this gene are highly associated with development of maternal liver dysfunction, again possibly due to long chain 3-hydroxyacylcarnitines (Table 2).
5. Limitations
Limitations of this study included relatively small numbers, retrospective data collection, and the availability of only de-identified data. We were unable to query individual centers for missing data points for additional information. The phenotypic analysis depended on the diagnosis assigned by the enrolling center, and where no genotypic data were available could not be verified.
6. Conclusion
In summary, we report the outcomes of 45 patients with LCHADD or TFPD enrolled in the IBEM-IS database over a 7-year period. Our data suggest that biallelic variants in HADHA gene are more likely to present with the LCHADD phenotype, which is at odds with the “conventional” belief that only the HADHA common LCHAD variant causes the LCHADD phenotype. Maternal and neonatal complications appear most commonly, but not exclusively, associated with all varieties of HADHA variants. The most-life threatening complication, cardiomyopathy, is not limited to the newborn period and can occur at any age. Historically, severe, neonatal, dilated cardiomyopathy has been reported more commonly in patients with TFPD, especially in patients with low levels of HADHB gene expression, but we did not substantiate this finding [7]. In contrast, variants in HADHB appear more likely to lead to milder forms of TFPD phenotype, with predominance of neuromuscular symptoms peripheral neuropathy. However, this observation is limited by low sample size. Furthermore, significant overlap in the symptoms occur, consistent with the notion that LCHADD and TFPD are ends of a clinical spectrum. It will be informative to see if the two more distinctive clinical phenotypes (retinopathy and neuropathy) are more apparent in older patients in either group.
Data availability
Tables and figures were generated using IBEM-IS database.
Author information
Chelsey Chaehee. Lim – Formal analysis, Investigation, Methodology, Visualization, Writing – original draft.
Jerry Vockley – Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Georgianne L. Arnold – Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – review & eediting.
Mathew J. Edick – Data curation , Writing - review.
Susan A. Berry – Data curation , Writing - review.
Otobo Ujah – Formal analysis.
Russell S. Kirby – Formal analysis.
Ethics declaration
IRB# REN19080059/PRO11010175.
Patient data used in this study were de-identified.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), National Institutes of Health under award number 5R01HD069039 to SB. JV was supported in part by NIH grant RO1 DK1099078.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2022.100884.
Appendix A. Supplementary data
Supplementary material
References
- 1.Watson M.S., Mann M.Y., Lloyd-puryear M.A., Rinaldo P. Executive summary. Genet. Med. 2006;8:1–11. [Google Scholar]
- 2.Longo, N., Frigeni, M. & Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta, Mol. Cell Res. 1863, 2422–2435 (2016). [DOI] [PMC free article] [PubMed]
- 3.Houten S.M., Violante S., Ventura F.V., Wanders R.J.A. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu. Rev. Physiol. 2016;78:23–44. doi: 10.1146/annurev-physiol-021115-105045. [DOI] [PubMed] [Google Scholar]
- 4.Ibdah J.A., Dasouki M.J., Strauss A.W. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: variable expressivity of maternal illness during pregnancy and unusual presentation with infantile cholestasis and hypocalcaemia. J. Inherit. Metab. Dis. 1999;22:811–814. doi: 10.1023/a:1005506024055. [DOI] [PubMed] [Google Scholar]
- 5.Strauss A.W., et al. Inherited long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and a fetal-maternal interaction cause maternal liver disease and other pregnancy complications. Semin. Perinatol. 1999;23:100–112. doi: 10.1016/s0146-0005(99)80044-5. [DOI] [PubMed] [Google Scholar]
- 6.De Biase I., et al. Diagnosis, treatment, and clinical outcome of patients with mitochondrial trifunctional protein/long-chain 3-hydroyxy acyl-CoA dehydrogenase deficiency. JIMD Rep. 2012;4:113–116. doi: 10.1007/8904_2016_558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagher R., Massie R., Gentil B.J. MTP deficiency caused by HADHB mutations: pathophysiology and clinical manifestations. Mol. Genet. Metab. 2021;133:1–7. doi: 10.1016/j.ymgme.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Thangavelu S. Fatty acid oxidation disorders. Indian J. Pract. Pediatr. 2010;12:181–183. [Google Scholar]
- 9.Xia C., Fu Z., Battaile K.P., Kim J.J.P. Crystal structure of human mitochondrial trifunctional protein, a fatty acid β-oxidation metabolon. Proc. Natl. Acad. Sci. U. S. A. 2019;116:6069–6074. doi: 10.1073/pnas.1816317116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiekerkoetter U., Khuchua Z., Yue Z., Bennett M.J., Strauss A.W. General mitochondrial trifunctional protein (TFP) deficiency as a result of either α- or β-subunit mutations exhibits similar phenotypes because mutations in either subunit Alter TFP complex expression and subunit turnover. Pediatr. Res. 2004;55:190–196. doi: 10.1203/01.PDR.0000103931.80055.06. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher A.L., Pennesi M.E., Harding C.O., Weleber R.G., Gillingham M.B. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol. Genet. Metab. 2012;106:18–24. doi: 10.1016/j.ymgme.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rector R.S., Ibdah J.A. Fatty acid oxidation disorders: maternal health and neonatal outcomes. Semin. Fetal Neonatal Med. 2010;15:122–128. doi: 10.1016/j.siny.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Gillingham M.B., et al. Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial. J. Inherit. Metab. Dis. 2017;40:831–843. doi: 10.1007/s10545-017-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry S.A., et al. Inborn errors of metabolism collaborative (IBEMC): large scale data collection about long-term follow-up for newborn-screened conditions for the inborn errors of metabolism collaborative * HHS public access. Joyanna Hansen Michigan Public Heal. Inst Genet Med. 2016;18:1276–1281. doi: 10.1038/gim.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vockley J. Long-chain fatty acid oxidation disorders and current management strategies. Suppl. Featur. Publ. 2020;26 doi: 10.37765/ajmc.2020.88480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiekerkoetter U., et al. Management and outcome in 75 individuals with long-chain fatty acid oxidation defects: results from a workshop. J. Inherit. Metab. Dis. 2009;32:488–497. doi: 10.1007/s10545-009-1125-9. [DOI] [PubMed] [Google Scholar]
- 17.Marsden D., Bedrosian C.L., Vockley J. Impact of newborn screening on the reported incidence and clinical outcomes associated with medium- and long-chain fatty acid oxidation disorders. Genet. Med. 2021;23:816–829. doi: 10.1038/s41436-020-01070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathur A., et al. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation. 1999;99:1337–1343. doi: 10.1161/01.cir.99.10.1337. [DOI] [PubMed] [Google Scholar]
- 19.Moczulski D., Majak I., Mamczur D. 2009. An Overview of b -Oxidation Disorders; pp. 266–277. [PubMed] [Google Scholar]
- 20.Spiekerkoetter U., Sun B., Khuchua Z., Bennett M.J., Strauss A.W. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to β-subunit mutations. Hum. Mutat. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Tables and figures were generated using IBEM-IS database.